Abstract
Epidemiological studies suggest that inflammatory bowel disease (IBD) is common in developed countries and rare in countries where intestinal nematode infections are common. T cells are critical in many immune responses, including those associated with IBD and nematode infection. Among the distinct T helper (Th) cell subsets, Th1-type immune response is predominantly associated with Crohn's disease, while many nematode infections generate a strong Th2 response. The reciprocal cross regulation between Th1 and Th2 cells suggests that generation of a Th2 response by nematodes could prevent or reduce the effects of Th1-mediated diseases. In the present study, we investigated the effect of polarizing the immune response toward the Th2 type, using intestinal nematode infection, on subsequent experimental colitis. Mice were infected with the intestinal nematode Trichinella spiralis and allowed to recover before colitis was induced with dinitrobenzene sulfonic acid. The mice were sacrificed postcolitis to assess colonic damage macroscopically, histologically, and by myeloperoxidase (MPO) activity and Th cytokines. Prior nematode infection reduced the severity of colitis both macroscopically and histologically together with a decreased mortality and was correlated with a down-regulation of MPO activity, Th1-type cytokine expression in colonic tissue, and emergence of a Th2-type immune response. These results indicate a protective role of nematode infection in Th1 cell-driven inflammation and prompt consideration of a novel therapeutic strategy in IBD based on immunological distraction.
Inflammatory bowel disease (IBD) is a chronic relapsing inflammatory condition of the gastrointestinal tract that manifests as ulcerative colitis or Crohn's disease (3, 4, 5, 34). IBD can begin relatively early in life and persist for long periods, leading to substantial morbidity and decreased quality of life (4). The causes of IBD are unknown, but epidemiological and laboratory work suggests that environmental and genetic factors are important in the pathogenesis of the IBD which is associated with dysregulation of the mucosal immune system. IBD is most common in highly industrialized temperate regions and is rare in tropical countries with poor sanitation and overcrowding (17). The rarity of IBD in tropical countries cannot be explained on the basis of genetics alone, as descendants of immigrants from such countries acquire the higher risk of IBD of the adopted developed country (32, 40). These observations underscore the importance of environmental factors in the expression of IBD and have led to the development of the “hygiene hypothesis” of IBD, that is, that IBD occurs more commonly in societies where the prevalence of chronic enteric infestation is low (17).
Parasitic infections, particularly those due to intestinal nematodes, are most common in warm climates and in areas of overcrowding and poor sanitation. Both Crohn's disease and ulcerative colitis are rare in developing countries of Asia and Africa, where nematode infections are endemic (46, 48). The prevalence of nematode infections in the United States has been declining for the past 60 years (17), except among new immigrants from developing countries (44).
T cells constitute an important part of many immune responses, including those associated with IBD and intestinal nematode infection. T helper (Th) cell-dependent immune responses are generally divided into two major subsets, Th1 and Th2 (37). Th1 cells secrete predominantly gamma interferon (IFN-γ) and interleukin-2 (IL-2), while Th2 cells secrete IL-4, IL-5, IL-9, IL-10, and IL-13. Th1 and Th2 cells cross regulate one another. IFN-γ secreted by Th1 cells directly suppresses IL-4 production and thus inhibits the differentiation of naïve Th cells into Th2 cells (12, 23). In contrast, IL-4 and IL-10 inhibit the secretion of IL-12 and IFN-γ, blocking polarization into Th1 cells (10, 38). The dichotomous split of Th lymphocytes into Th1 and Th2 cells has provided a convenient conceptual framework to characterize T-cell responses in different diseases.
The chronic inflammation of Crohn's disease is maintained by Th1-driven immune response. T cells isolated from the colons of patients suffering from Crohn's disease produce large amounts of IFN-γ and tumor necrosis factor alpha and little IL-4 or IL-10 (2, 5, 21). Many mouse models of experimental colitis are associated with a Th1-type immune response, reflected by infiltration of IFN-γ-producing T cells in the colon (3, 11). In these models, disease is prevented, or at least ameliorated, by treatment with IL-10, IL-4, or neutralizing antibody to IL-12 (1, 22, 28). In contrast to Crohn's disease, Th2 cells are important in host protective immunity to many helminths, including the intestinal nematode Trichinella spiralis (19, 27). Many intestinal nematodes survive for years within the intestinal tract and serve as potential sources of a Th2-type immune response. Considering the contrasting geographical distributions and immune responses of intestinal nematode infection and Crohn's disease, it has been hypothesized that environmental factors like nematode infection may have significant influence on the development of Crohn's disease (17).
To test this hypothesis, in the present study we investigated the effect on consequent experimental colitis in mice of distracting the immune response to Th2 by using nematode infection. Our results demonstrate that prior exposure of mice to T. spiralis infection effectively reduces the severity of dinitrobenzenesulfonic acid (DNBS) colitis. This is correlated with a down-regulation of myeloperoxidase (MPO) activity and IFN-γ expression and a persistence of the Th2-type immune response.
MATERIALS AND METHODS
Animals.
C57BL/6 mice were obtained from the Jackson Laboratories, kept in sterilized filter-topped cages, and fed autoclaved food in the animal facilities of McMaster University, Hamilton, Ontario, Canada. For the present study, only male mice were used at the age of 8 to 10 weeks. The protocols employed were in direct accordance with guidelines drafted by the McMaster University Animal Care Committee and the Canadian Council on the Use of Laboratory Animals.
Parasitological techniques.
The T. spiralis parasites used in the study originated in the Department of Zoology at the University of Toronto, and the colony was maintained through serial infections alternating between male Sprague-Dawley rats and male CD1 mice. The larvae were obtained from infected rodents 60 to 90 days postinfection (p.i.), using a modification (50) of the technique described by Castro and Fairbairn (7). The mice were infected orally with 375 infective larvae and were allowed to recover until day 21 p.i. before the induction of colitis.
Induction of colitis.
Colitis was induced by intracolonic administration of DNBS (ICN, Aurora, Ohio) as described before (41). In brief, a stock solution of DNBS was made by dissolving 60 mg of DNBS per ml of 50% ethanol. Mice anesthetized with 2% enflurane were injected in the distal 4 cm of the colon with 100 μl of this solution, containing 6 mg of DNBS, using a 1-ml tuberculin syringe (Becton Dickinson, Franklin Lakes, N.J.) and PE90 tubing (Clay Adams, Parsippany, N.J.). The mice were given 8% sucrose in 0.2% saline to prevent dehydration during the first week after DNBS administration. Control mice were injected intracolonically using the same procedure with 50% ethanol.
Assessment of colonic damage.
The colon was removed and opened longitudinally, and the damage was assessed macroscopically and histologically using previously published criteria (41). Briefly, the macroscopic criteria included macroscopic mucosal damage (assessed with a scale), thickening of the colonic wall, presence of adhesions between the colon and other intra-abdominal organs, consistency of fecal material (as an indicator of diarrhea), and presence of hyperemia. Microscopic criteria for damage and inflammation were investigated by light microscopy on hematoxylin- and eosin-stained histological sections obtained from gut segments taken from a region of the inflamed colon immediately adjacent to gross macroscopical damage. The histological criteria were based on the following: degree of mucosal architectural changes, cellular infiltration, goblet cell depletion, and presence of crypt abscess. Macroscopic and histological damage were recorded and scored for each mouse by two different investigators who were blinded to the treatment conditions.
MPO activity.
The degree of colonic inflammation was investigated by assay of MPO activity. Three days after intrarectal administration of DNBS, the colon was removed, snap frozen in liquid nitrogen, and stored at −70°C. Samples were weighed, and MPO was measured using a technique described before (47). MPO activity is reported as units of MPO per milligram of wet tissue. One unit of MPO is defined as the quantity of enzyme able to convert 1 μmol of hydrogen peroxide to water in 1 min at room temperature.
Detection of cytokines by reverse transcription-PCR.
Total RNA fractions were prepared from freshly isolated colon tissue based on a previously described guanidium isothiocyanate method (8). The concentration of RNA was determined by measuring absorbance at 260 nm, and its purity was confirmed using the ratio of absorbency at 260 nm to that at 280 nm. RNA was stored at −70°C until it was used for reverse transcription-PCR. mRNA was then reverse transcribed as described previously to yield cDNA, and the cDNA was amplified by PCR using gene-specific primers.
Fifty-nanogram aliquots of cDNA (0.1 μg) were then mixed with 20 pmol each of upstream (5′-CAT GGC TGT TTC TGG CTG TTA C-3′) and downstream (5′-TCG GAT GAG CTC ATT GAA TGC-3′) primers for IFN-γ (26). The glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) housekeeper gene was used as the positive control, and to detect it, upstream (5′-CCA TGG AGA AGG CTG GGG-3′) and downstream (5′-CAA AGT TGT CAT GGA TGA CC-3′) primers were used (25). PCR was performed in 50-μl volumes containing deoxynucleoside triphosphate (200 μM), MgCl2 (1.5 mM), and 2.5 U of Taq polymerase (Gibco BRL) with the corresponding buffer and distilled water. Messages for IFN-γ and GAPDH were coamplified using the following parameters: denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 60 s. The PCR products were loaded onto a 2.5% agarose gel and then visualized under UV light after ethidium bromide staining. The densities of the bands were determined for each sample (with each lane representing one mouse), and the ratio of IFN-γ gene expression to GADPH expression was calculated.
Production of cytokines from spleen cells.
Single-cell suspensions of spleen tissue were prepared in RPMI 1640 containing 10% fetal calf serum, 5 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 25 mM HEPES, and 0.05 mM 2-mercaptoethanol (all from Gibco-BRL). Cells (107/well) were plated in 24-well plates with or without 5 μg of concavalin A (ConA; Sigma)/ml. Culture supernatants were harvested after 48 h, and the cytokine levels were determined.
Blood collection for IL-12 measurement.
To investigate the levels of IL-12 in sera, we collected blood from the mouse retro-orbital plexus after intracolonic administration of DNBS.
Enzyme-linked immunosorbent assay (ELISA).
IL-4, IL-13, and IFN-γ concentrations in the culture supernatants and IL-12 levels in sera were measured by the enzyme immunoassay technique using commercially available kits purchased from R&D Systems (Minneapolis, Minn.) and Biosource (Camarillo, Calif.).
Statistical analysis.
Data were analyzed using Student's t test, with a P value of <0.05 considered significant. All results are expressed as the mean ± standard error of the mean (SEM).
RESULTS
Primary T. spiralis infection reduced the severity of DNBS-mediated colonic damage.
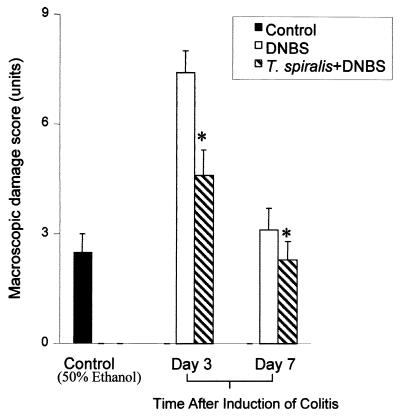
On day 3 after administration of 6 mg of DNBS, macroscopic evaluation of the colon revealed massive ulceration, thickening, and severe intra-abdominal adhesions. This was accompanied by a 16% mortality rate. In surviving DNBS-treated mice, there was a significant increase in the macroscopic score compared to that in ethanol-treated controls (Fig. 1). Mucosal damage in DNBS treatment was associated with thickening of the colonic wall, hyperemia, and the presence of adhesions between the colon and other organs. Infection of mice with T. spiralis prior to DNBS treatment resulted in a significant reduction in the mortality rate from 16 to 5%. This was accompanied by less mucosal damage, less thickening of the colonic wall, and fewer adhesions evident on day 3 postcolitis. The improvement in the macroscopic score was also evident on day 7 postcolitis.
FIG. 1.
DNBS-induced colitis in T. spiralis-infected and noninfected mice. In the T. spiralis-infected group, mice were infected orally with 375 larvae, and colitis was induced by DNBS (6 mg) administered intrarectally on day 21 p.i. Macroscopic damage was evaluated 3 and 7 days after DNBS administration. The control mice were sacrificed 3 days after intracolonic administration of 50% ethanol. Each bar represents the mean + SEM. ∗, significantly lower than DNBS-treated mice. n = 7 to 9 mice per group. Representative data from three experiments are shown.
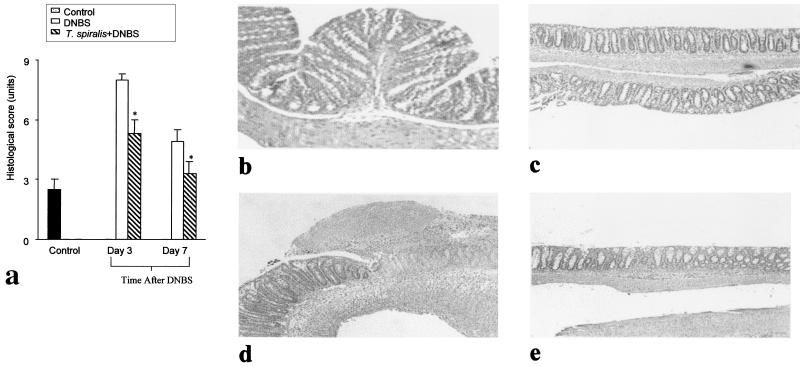
Histological examination of the colon 3 days post-DNBS administration revealed an intense granulocyte infiltrate extending throughout the mucosa and submucosa, often involving muscularis propria, which invariably appeared thickened. There was also marked mucosal damage associated with goblet cell depletion. Prior infection of mice with T. spiralis caused a significant improvement of the microscopic score on both days 3 and 7 post-DNBS administration (Fig. 2), and this was accompanied by less mucosal damage, goblet cell depletion, and cellular infiltration into the mucosa and submucosa. In mice which were infected with T. spiralis but did not receive DNBS, we observed large numbers of goblet cells and intact mucosal lining on day 21 p.i.
FIG. 2.
Histological assessment of the colon in DNBS colitis with or without T. spiralis infection. In the T. spiralis-infected group, mice were infected orally with 375 larvae, and colitis was induced by intracolonic administration of DNBS (6 mg) on day 21 p.i. Histological investigations were done on days 3 and 7 postinduction of colitis. The controls were mice which were sacrificed 3 days after intracolonic administration of 50% ethanol. (a) Histological damage score. (b) Light micrograph of hematoxylin- and eosin-stained colonic section from T. spiralis-infected mouse on day 21 p.i. (c) Light micrograph of hematoxylin- and eosin-stained colonic section of mouse administered 50% ethanol (control). (d) Light micrograph of hematoxylin- and eosin-stained colonic section of mouse 3 days after administration of DNBS. (e) Light micrograph of hematoxylin- and eosin-stained colonic section of T. spiralis-infected mouse 3 days after administration of DNBS. Each bar represents the mean + SEM. ∗, significantly lower compared to DNBS-treated mice. n = 7 to 9 mice per group. Representative data from three experiments are shown.
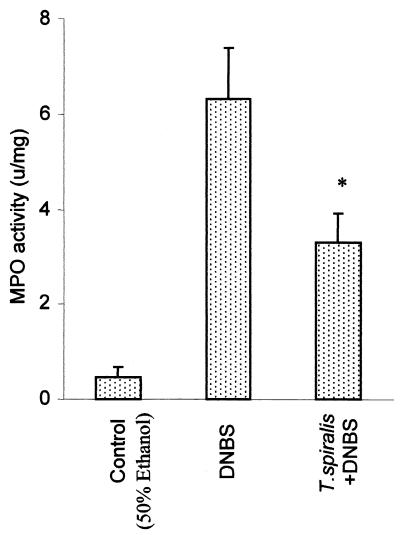
Reduction of MPO activity in DNBS colitis by the exposure of mice to T. spiralis infection.
The intracolonic administration of DNBS in noninfected mice caused a significant increase in MPO activity, as shown in Fig. 3. After 3 days of DNBS administration, MPO activity in the colons of noninfected mice was more than fivefold higher than that in ethanol-treated control mice. There was a significant suppression of MPO activity after DNBS administration in T. spiralis-infected mice.
FIG. 3.
Measurement of MPO activity of DNBS colitis in the colon with or without T. spiralis infection. In the T. spiralis-infected group, mice were infected orally with 375 larvae, and colitis was induced by DNBS (6 mg) administered intrarectally on day 21 p.i. MPO activity from the colon was evaluated on day 3 postadministration of DNBS. The controls were mice which were sacrificed 3 days after intracolonic administration of 50% ethanol. Each bar represents the mean + SEM of six animals. ∗, significantly lower compared to DNBS-treated mice.
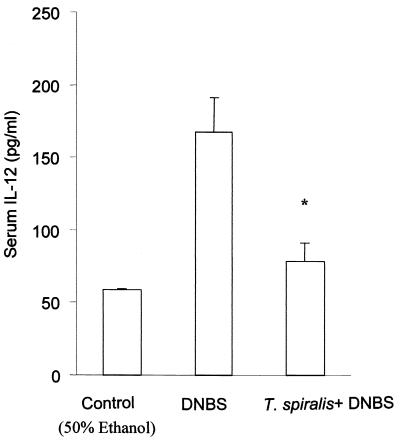
The serum IL-12 and expression of IFN-γ in colonic tissue increased after DNBS administration.
In noninfected mice, we observed an increase in the serum IL-12 levels on day 3 post-DNBS administration compared to that seen in 50% ethanol-treated control mice (Fig. 4). Although we had also detected IL-12 in the sera of DNBS-treated mice previously, when mice were infected with T. spiralis, the levels of IL-12 were significantly lower than that seen in colitic mice without prior nematode infection.
FIG. 4.
Serum IL-12 levels post-DNBS administration. Blood was collected from the retro-orbital plexus 3 days after the intracolonic administration of 6 mg of DNBS. The levels of IL-12 in sera were measured by ELISA. The controls were mice which were sacrificed 3 days after the intracolonic administration of 50% ethanol. Each bar represents the mean + SEM from four animals. *, significantly lower compared to DNBS-treated mice.
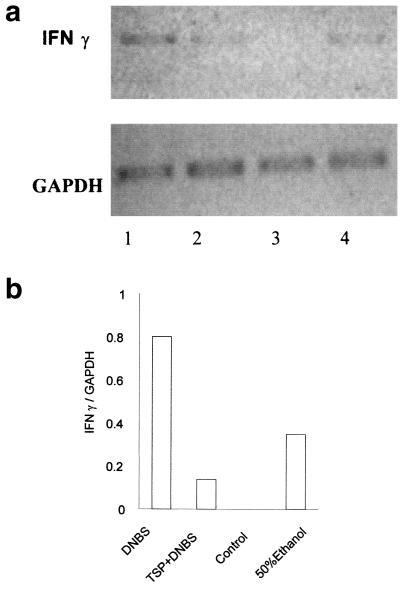
DNBS-induced colitis was accompanied by an up-regulation of IFN-γ expression in the colon on day 7 (Fig. 5). In contrast, there was no up-regulation of IFN-γ in DNBS-treated mice that had been previously infected with T. spiralis. Interestingly, we observed IFN-γ expression to a lesser extent in the colon tissue of mice which received only 50% ethanol.
FIG. 5.
(a) IFN-γ gene expression within colon tissue of mice after administration of DNBS with or without T. spiralis infection. In the T. spiralis-infected group, mice were infected orally with 375 larvae, and colitis was induced by DNBS (6 mg) administered intrarectally on day 21 p.i. The mice were killed 7 days after DNBS administration to investigate IFN-γ gene expression. GAPDH was used as a positive control. Lane 1, noninfected mice after DNBS administration; lane 2, T. spiralis-infected mice after DNBS administration; lane 3, T. spiralis-infected (day 21 p.i) mice; lane 4, control mice which received 50% ethanol. (b) Ratios of IFN-γ band densities compared to GAPDH band densities. Representative data from two experiments are shown.
Presence of Th2 cytokines is correlated with amelioration of colitis.
The measurement of in vitro cytokine production by ConA-stimulated spleen cells revealed a marked difference in Th2 cytokine production between infected and noninfected mice after DNBS administration. We observed a higher level of IL-4 and IL-13 production by spleen cells in T. spiralis-infected mice after the administration of DNBS on days 3 and 7 postinduction than in noninfected mice after DNBS administration (Table 1). In contrast, we observed IFN-γ production by spleen cells in noninfected mice after DNBS administration on day 7 postinduction.
TABLE 1.
Cytokine production by spleen cells after ConA stimulation
| Day | Challenge | Cytokine productiona
|
||
|---|---|---|---|---|
| IL-4 | IL-13 | IFN-γ | ||
| 3 | DNBS | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| T. spiralis + DNBS | 19.1 ± 1.1 | 88.1 ± 13.6 | 0 ± 0 | |
| 7 | DNBS | 0 ± 0 | 2.9 ± 2.9 | 31 ± 1.7 |
| T. spiralis + DNBS | 20.4 ± 1.7 | 33.9 ± 11.1 | 0 ± 0 | |
Values (in picograms per milliliter) are means ± SEM for three mice. DNBS (6 mg) was administered intrarectally to C57BL/6 mice with or without exposure to T. spiralis infection. The mice of the infected group were given 375 T. spiralis larvae orally and were given DNBS on day 21 p.i. The mice of both groups were killed on days 3 and 7 postinduction of colitis by DNBS, the spleen cells were stimulated with ConA for 48 h, and the levels of cytokines present in the supernatant were investigated by ELISA.
DISCUSSION
DNBS-induced colitis is a well-characterized transmural Th1-driven inflammation of the colon and may be considered a model of Crohn's disease, which is also characterized by transmural inflammation with the generation of Th1-type immune response. In the present study, we have shown that a prior Th2-mediated infection by T. spiralis reduced the mortality and severity of inflammation associated with DNBS-induced colitis.
Intestinal parasites are important because of their wide prevalence and their ability to cause human diseases. Highly prevalent in humans are nematode parasites, which infect approximately one-quarter of the world's population, particularly in developing countries (51). While the incidence of nematode infections has fallen substantially in the industrialized countries due to improved hygiene, the incidence of Crohn's disease has risen, and IBD currently affects ∼1 person in 1,000 in North America and Europe (34). Thus, the contrasting geographical distributions of nematode infection and IBD prompted consideration of a protective effect of intestinal parasitism against the development of IBD. The results of this study are consistent with the notion that nematode infection confers protection against an experimental form of IBD.
Although there are several studies of the association between bacterial infection by Helicobacter pylori and Crohn's disease, the rate of incidence of this infection in Crohn's disease varies considerably from report to report. Some investigators have claimed that the H. pylori-positive rate is lower in Crohn's disease patients than in healthy controls (18, 49), while others reported that it is comparable (13, 35). Therefore, the precise role of H. pylori infection in Crohn's disease is still not clearly known.
The beneficial effect of T. spiralis on DNBS-induced colonic inflammation was observed for all the parameters studied, including both the macroscopic and microscopic indices, as well as MPO activity, and was evident on both days 3 and 7 postcolitis.
MPO is an enzyme contained in the azurophilic granules of neutrophils, as well as other myeloid cells, and is commonly used as an index of neutrophil infiltration and inflammation (47). Previous studies have reported extensive accumulation of neutrophils and a significant increase of MPO in DNBS-induced colitis (41). Taken together, these results indicate that infection of mice with T. spiralis efficiently reduces the severity of gut inflammation.
The improvement in colonic damage in mice which had been previously infected with T. spiralis was accompanied by the emergence of a Th2 response, as reflected by the presence of IL-4 and IL-13 in the spleen cell culture supernatant stimulated with ConA. In contrast, we observed IFN-γ expression in DNBS-treated mice without prior infection. We interpret these findings to indicate that infection of mice with T. spiralis distracted the mucosal immune system response from a Th1 response toward a protective Th2 response with an attendant reduction in the severity of colonic damage.
Several factors influence the polarization of the immune response toward a Th1 or Th2 response, but the most important is the local cytokine environment during antigen presentation to the T cells (45). IL-12 produced by antigen-presenting cells (primarily macrophages and B cells) activates the differentiation of Th cells toward Th1-type responses (24) by stimulating T-cell and natural killer cell production of IFN-γ through the activation of signal transducers and activators of transcription factor 4 (Stat4). Conversely, IL-4 and IL-13 are important in the development of Th2 cells by activating the Stat6 pathway (29, 36). Previous work has shown that Stat6-dependent Th2 responses mediate intestinal motor activity and smooth-muscle hypercontractility and contribute to host protective immunity in T. spiralis infection (30). Very recently, it was also shown that IL-12 gene transfer by using adenovirus vector in nematode-infected mice shifts the protective Th2 immune response to a Th1 response, attenuates the intestinal physiological changes which accompany the infection, and prevents worm expulsion (31). This observation suggests that by manipulation of the immune response it is possible to modulate intestinal physiology, which may have therapeutic implications in gut dysfunction. In the present study, it seems likely that the presence of a Th2-type cytokine environment evoked by T. spiralis infection at the time of DNBS administration had an inhibitory effect on the development of Th1-mediated inflammation. Very recent work also showed a more severe colonic inflammatory response in DNBS colitis in Stat6-deficient mice (15), further suggesting a protective role of the Stat6-mediated Th2 response in this model of experimental colitis.
The Th-2 response induced by nematode parasites influences immune responses to other antigens. This includes attenuation of the Th1 response to mycobacterial antigen (39) and to tetanus toxoid (43). In addition, nematode infection has been shown to impair the rejection of kidney transplants in rats (33). Cross regulatory suppression of Th1 activity by a strong Th2 response has been considered to play an important role in delaying graft rejection. In addition, others have shown that infection with the helminth Heligmosomoides polygyrus in mice attenuated the Th-1 response, as well as the severity of the inflammatory response, to subsequent infection with Helicobacter felis (20). More recently, it has also been reported that tapeworm infection reduced the colonic epithelial ion transport irregularities observed in murine dextran sulfate sodium-induced colitis (42). Preliminary data have also been presented to show that infection of mice with Schistosoma mansoni attenuated the severity of subsequent colitis induced by intrarectal administration of trinitrobenzen sulfonic acid, and a therapeutic role for helminthic infection in IBD has been suggested (16). Our data provide further support for the therapeutic potential of nematode infection in IBD.
Other mechanisms may contribute to the modulation of inflammation by T. spiralis. For example, nematode infection influences the neuropeptide content of the gut (9), and it has been reported that infecting nematodes can modulate their environment by immunosuppression through the release of immunoreactive neuropeptides (14). It has also recently been reported that the 45-kDa glycoprotein derived from T. spiralis has an anti-inflammatory effect (6). In addition, goblet cell hyperplasia is a hallmark of T. spiralis infection (31). We also observed high numbers of goblet cells in the colons of mice which were infected with T. spiralis. Therefore, it seems likely that increased mucin production by the goblet cells locally as a consequence of T. spiralis infection may also play a role in protection against subsequent exposure to DNBS by maintaining barrier integrity.
In conclusion, the results of the present study demonstrate the therapeutic potential of a strategy of immune distraction in the treatment of immunologically driven intestinal inflammation. Prepolarization of the mucosal immune system to a Th2 response conferred protection against a subsequent Th1-mediated inflammatory process. This strategy may be applicable to the treatment of Crohn's disease by using parasites, as suggested by this study and that of Elliott et al. (16), or by manipulating the mucosal immune system using genes for cytokines, such as IL-4 (28).
Acknowledgments
We thank A. Hirotada, E. F. Verdu, and D. McKay for valuable discussions.
This study was supported by a grant to S.M.C. from the Canadian Institutes for Health Research (CIHR).
Editor: J. M. Mansfield
REFERENCES
- 1.Barbara, G., Z. Xing, C. M. Hogaboam, J. Gauldie, and S. M. Collins. 2000. Interleukin 10 gene transfer prevents experimental colitis in rats. Gut 46:344-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhan, A. K., E. Mizoguchi, R. N. Smith, and A. Mizoguchi. 1999. Colitis in transgenic and knockout animals as models of human inflammatory bowel disease. Immunol. Rev. 169:195-200. [DOI] [PubMed] [Google Scholar]
- 3.Blumberg, R. S., L. J. Saubermann, and W. Strober. 1999. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr. Opin. Immunol. 11:548-656. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg, R. S., and W. Strober. 2001. Prospects for research in inflammatory bowel disease. JAMA 285:643-647. [DOI] [PubMed] [Google Scholar]
- 5.Braegger, C. P., and T. T. Macdonald. 1994. Immune mechanisms in chronic inflammatory bowel disease. Ann. Allergy 72:135-141. [PubMed] [Google Scholar]
- 6.Bruschi, F., G. Carulli, A. Azzara, W. Homan, S. Minnucci, A. Rizzuti-Gullaci, S. Sbrana, and C. Angiolini. 2000. Inhibitory effects of human neutrophil functions by the 45-kD glycoprotein derived from the parasite nematode Trichinella spiralis. Int. Arch. Allergy Immunol. 122:58-65. [DOI] [PubMed] [Google Scholar]
- 7.Castro, G. A., and D. Fairbairn. 1969. Carbohydrates and lipids in Trichinella spiralis larvae and their utilization. J. Parasitol. 55:51-58. [PubMed] [Google Scholar]
- 8.Chomczynski, P., and N. Sacchi. 1987. Single step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 9.Collins, S. M. 1996. The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology 111:1683-1699. [DOI] [PubMed] [Google Scholar]
- 10.D'Andrea, A., M. Aste-Amezaga, N. M. Valiante, X. Ma, M. Kubin, and G. Trinchieri. 1993. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 178:1041-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson, N. J., M. M. Fort, W. Muller, M. W. Leach, and D. M. Rennick. 2000. Chronic colitis in IL-10 −/− mice: insufficient counter-regulation of a Th1 response. Int. Rev. Immunol. 19:91-98. [DOI] [PubMed] [Google Scholar]
- 12.Demeure, C. E., C. Y. Wu, U. Shu, P. V. Schneider, C. Heusser, H. Yssel, and G. Delespesse. 1994. In vitro maturation of human neonatal CD4 T lymphocytes. II. Cytokines present at priming modulate the development of lymphokine production. J. Immunol. 152:4775-4782. [PubMed] [Google Scholar]
- 13.D'Inca, R., G. Sturniola, M. Cassaro, C. di Pace, G. Longo, I. Callegari, et al. 1998. Prevalence of upper gastrointestinal lesions and Helicobacter pylori infection in Crohn's disease. Dig. Dis. Sci. 43:988-992. [DOI] [PubMed] [Google Scholar]
- 14.Duvaux-Miret, O., G. B. Stefano, E. M. Smith, C. Dissous, and A. Capron. 1992. Immunosuppression in the definitive and intermediate hosts of human parasite Schistosoma mansoni by release of immunoactive neuropeptides. Proc. Natl. Acad. Sci. USA 89:778-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Ashry, D., P. A. Blennerhasset, Y. Deng, and S. M. Collins. 2001. Modulation of experimental colitis in mice by the STAT6 pathway. Gastroenterology 120:A314. (Abstract.) [Google Scholar]
- 16.Elliott, D. E., J. Li, C. Crawford, A. Blum, A. Metwali, K. Qadir, J. F. Urban, and J. V. Weinstock. 1999. Exposure to helminth parasites protect mice from intestinal inflammation. Gastroenterology 116:A706. (Abstract.) [Google Scholar]
- 17.Elliott, D. E., J. F. Urban, C. K. Argo, and J. V. Weinstock. 2000. Does failure to acquire helminth parasites predispose to Crohn's disease? FASEB J. 14:1848-1855. [DOI] [PubMed] [Google Scholar]
- 18.El-Omar, E., T. Penman, G. Cruikshank, S. Dover, S. Banarjee, and C. Williams. 1994. Low prevalence of Helicobacter pylori in inflammatory bowel disease: association with sulfasalazine. Gut 35:1385-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Else, K. J., and F. D. Finkelman. 1998. Intestinal nematode parasites, cytokines and effector mechanisms. Int. J. Parasitol. 28:1145-1158. [DOI] [PubMed] [Google Scholar]
- 20.Fox, J. G., P. Beck, C. A. Dangler, M. T. Whary, T. C. Wang, H. Ning Shi, and C. Nagler-Anderson. 2000. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces Helicobacter-induced gastric atrophy. Nat. Med. 42:536-542. [DOI] [PubMed] [Google Scholar]
- 21.Fuss, I. J., M. Neurath, M. Boirivant, J. S. Klein, C. de la Motte, S. A. Strong, C. Fiocchi, and W. Strober. 1996. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. J. Immunol. 157:1261-1270. [PubMed] [Google Scholar]
- 22.Fuss, U., T. Marth, M. F. Neurath, G. R. Pearlstein, A. Jain, and W. Strober. 1999. Anti-interleukin-12 treatment regulates apoptosis of T helper 1 T cells in experimental colitis. Gastroenterology 115:1464-1475. [DOI] [PubMed] [Google Scholar]
- 23.Gajewski, T. F., and F. W. Fitch. 1988. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J. Immunol. 140:4245-4252. [PubMed] [Google Scholar]
- 24.Gately, M. K., L. M. Renzetti, J. Magram, A. S. Stern, L. Adorini, U. Gubler, and D. H. Presky. 1998. Interleukin-12/interleukin-12 receptor system: role in normal and pathological immune responses. Annu. Rev. Immunol. 16:495-521. [DOI] [PubMed] [Google Scholar]
- 25.Gendelman, H. E., R. M. Friedman, S. Joe, L. Baca, J. A. Turpin, G. Dvekslen, M. S. Meltzer, and C. W. Dieffenbach. 1990. A selective defect of IFN production in HIV-infected monocytes. J. Exp. Med. 72:1433-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray, P. W., and D. V. Goeddel. 1983. Cloning and expression of murine immune interferon cDNA. Proc. Natl. Acad. Sci. USA 80:5842-5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grencis, R. K., L. Hultner, and K. J. Else. 1991. Host protective immunity in Trichinella spiralis mice: activation of Th cell subsets and lymphokine secretion in mice expressing different response phenotypes. Immunology 74:329-332. [PMC free article] [PubMed] [Google Scholar]
- 28.Hogaboam, C. M., B. A. Vallance, A. Kumar, C. L. Addison, F. L. Graham, J. Gauldie, and S. M. Collins. 1997. Therapeutic effects of interleukin-4 gene transfer in experimental inflammatory bowel disease. J. Clin. Investig. 100:2766-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keegan, A. D., K. Nelms, L. Wang, J. H. Pierce, and W. E. Paul. 1994. Interleukin-4 receptor: signaling mechanisms. Immunol. Today 15:423-431. [DOI] [PubMed] [Google Scholar]
- 30.Khan, W. I., B. A. Vallance, P. A. Blennerhasset, Y. Deng, E. F. Verdu, K. I. Matthaei, and S. M. Collins. 2001. Critical role for signal transducer and activator of transcription factor 6 in mediating intestinal muscle hypercontractility and worm expulsion in Trichinella spiralis-infected mice. Infect. Immun. 69:838-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan, W. I., P. A. Blennerhasset, Y. Deng, J. Gauldie, B. A. Vallance, and S. M. Collins. 2001. IL-12 gene transfer alters gut physiology and host protective immunity. Am. J. Physiol. 281:G102-G110. [DOI] [PubMed] [Google Scholar]
- 32.Kurata, J. H., S. Kator-Fish, H. Frank, P. Godly, and C. M. Vadheim. 1992. Crohn's disease among ethnic groups in a large health maintenance organization. Gastroenterology 102:1940-1988. [DOI] [PubMed] [Google Scholar]
- 33.Ledingham, D. L., V. C. McAlister, H. N. Ehigiator, C. Giacomantario, M. Theal, and T. D. Lee. 1996. Prolongation of rat kidney allograft survival by nematodes. Transplantation 61:184-188. [DOI] [PubMed] [Google Scholar]
- 34.Macdonald, T. T. 1994. Inflammatory bowel disease in knockout mice. Curr. Biol. 4:261-263. [DOI] [PubMed] [Google Scholar]
- 35.Matsumura, M., T. Matsui, S. Hatakeyama, H. Matake, H. Uno, T. Sakurai, T. Yao, T. Oishi, A. Iwashita, and T. Fujioka. 2001. Prevalence of Helicobacter pylori infection and correlation between severity of upper gastrointestinal lesions and H. pylori infection in Japanese patients with Crohn's disease. J. Gastroenterol. 36:740-747. [DOI] [PubMed] [Google Scholar]
- 36.McKenzie, G. J., C. L. Emson, S. E. Bell, S. Anderson, P. G. Fallon, G. Zurawski, R. Murray, and A. N. J. McKenzie. 1998. Impaired development of Th2 cells in IL-13 deficient mice. Immunity 9:423-432. [DOI] [PubMed] [Google Scholar]
- 37.Mosmann, T. R., and R. L. Coffman. 1989. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 38.Ohmori, Y., and T. A. Hamilton. 1997. IL-4 induced STAT6 suppresses IFN-gamma stimulated STAT1 dependent transcription in mouse macrophages. J. Immunol. 159:5474-5482. [PubMed] [Google Scholar]
- 39.Pearlman, E., J. W. Kazura, F. E. Hazlett, and W. H. Boom. 1993. Modulation of murine cytokine responses to mycobacterium antigens by helminth induced T helper 2 cell response. J. Immunol. 151:4857-4864. [PubMed] [Google Scholar]
- 40.Probert, C. S., B. F. Warren, T. Perry, E. H. Mackay, J. F. Mayberry, and A. P. Corfield. 1995. South Asian and European colitics show characteristic differences in colonic mucus glycoprotein type and turnover. Gut 36:696-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu, B. S., B. A. Vallance, P. A. Blennerhasset, and S. M. Collins. 1999. The role of CD4+ T lymphocytes in the susceptibility of mice to stress-induced reactivation of experimental colitis. Nat. Med. 10:1178-1182. [DOI] [PubMed] [Google Scholar]
- 42.Reardon, C., A. Sanchez, C. M. Hogaboam, and D. M. McKay. 2001. Tapeworm infection reduces the epithelial ion transport abnormalities in murine dextran-sulfate sodium-induced colitis. Infect. Immun. 69:4417-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sabin, E. A., M. I. Arauja, E. M. Carvalho, and E. J. Pearce. 1996. Impairment of tetanus toxoid specific Th1 like immune responses in lumen infected with Schistosoma mansoni. J. Infect. Dis. 173:269-272. [DOI] [PubMed] [Google Scholar]
- 44.Salas, S. D., R. Heifetz, and E. Barrett-Connor. 1990. Intestinal parasites in central American immigrants in the United States. Arch. Intern. Med. 150:1514-1516. [PubMed] [Google Scholar]
- 45.Seder, R. A., and W. E. Paul. 1994. Acquisition of lymphokine producing phenotype by CD4+ T cells. Annu. Rev. Immunol. 12:635-673. [DOI] [PubMed] [Google Scholar]
- 46.Shapira, M., and A. Tamir. 1994. Crohn's disease in the Kinneret sub-district, Israel, 1960-1990: incidence and prevalence in different ethnic subgroups. Eur. J. Epidemiol. 10:231-233. [DOI] [PubMed] [Google Scholar]
- 47.Smith, J. W., and G. A. Castro. 1978. Relation of peroxidase activity in gut mucosa to inflammation. Am. J. Physiol. 235:R72-R79. [DOI] [PubMed] [Google Scholar]
- 48.Tan, C. C., J. Y. Kang, R. Guen, I. Yap, and H. H. Tay. 1992. Inflammatory bowel disease: an uncommon problem in Singapore. J. Gastroenterol. Hepatol. 7:360-362. [DOI] [PubMed] [Google Scholar]
- 49.Vare, P. O., B. Heikius, J. A. Silvennoinen, R. Kartunen, S. E. Niemela, J. K. Lehtola, and T. J. Kartunen. 2001. Seroprevalence of Helicobacter pylori infection in inflammatory bowel disease: is Helicobacter pylori infection a protective factor? Scand. J. Gastroenterol. 12:1295-1300. [DOI] [PubMed] [Google Scholar]
- 50.Vermillon, D. L., and S. M. Collins. 1988. Increased responsiveness of jejunal longitudinal muscle in Trichinella-infected rats. Am. J. Physiol. 254:G124-G129. [DOI] [PubMed] [Google Scholar]
- 51.Wakelin, D., W. Harnett., and M. E. Parkhouse. 1993. Nematodes, p. 496-526. In K. S. Warren (ed.), Immunology and molecular biology of parasitic infections. Blackwell Scientific Publications, Oxford, United Kingdom.