Abstract
Fructans are a group of fructose-based oligo- and polysaccharides, which appear to be involved in membrane preservation during dehydration by interacting with the membrane lipids. To get further understanding of the protective mechanism, the consequences of the fructan-membrane lipid interaction for the molecular organization and dynamics in the dry state were studied. POPC and DMPC were investigated in the dry state by 2H, 31P NMR, and Fourier transform infrared spectroscopy using two types of fructan and dextran. The order-disorder transition temperature of dry POPC was reduced by 70°C in the presence of fructan. Fructan increased the mobility of the acyl chains, but immobilized the lipid headgroup region. Most likely, fructans insert between the headgroups of lipids, thereby spacing the acyl chains. This results in a much lower phase transition temperature. The headgroup is immobilized by the interaction with fructan. The location of the interaction with the lipid headgroup is different for the inulin-type fructan compared to the levan-type fructan, since inulin shows interaction with the lipid phosphate group, whereas levan does not. Dextran did not influence the phase transition temperature of dry POPC showing that reduction of this temperature is not a general property of polysaccharides.
INTRODUCTION
Fructans are a group of fructose-based polysaccharides that are mainly produced by plants, fungi, and bacteria. Fructans are divided in different classes based on their glycosidic linkages; inulins containing exclusively β(2→1) linkages, and levans, containing mainly β(2→6) linkages.
Fructans function as carbohydrate storage in plants. However, they appear to have an additional function in drought and freezing tolerance of plants (Hendry, 1993; Konstantinova et al., 2002; Pontis, 1989; Vijn and Smeekens, 1999). For instance, a transgenic tobacco plant that accumulates bacterial levan shows enhanced drought tolerance compared to the wild control plants (Pilon-Smits et al., 1995). Since membranes are one of the primary targets of desiccation injury of cells (Crowe et al., 1992; Senaratna et al., 1984), it was hypothesized that fructans could protect the plant against drought by direct interaction with the membrane (Demel et al., 1998).
Evidence is accumulating that fructans indeed fulfill a role as a membrane protectant. It has been shown that different types of fructan, under fully hydrated conditions, insert between the lipid headgroups in mono- and bilayer systems, thereby stabilizing the Lα phase (Vereyken et al., 2001). During dehydration, it has been established that both inulin and levan are present between the lipid bilayers and stabilize the Lα phase during drying (unpublished results). At extremely low humidity the Lβ-Lα phase transition is shifted to lower temperature in the presence of inulin (Hincha et al., 2000, 2002). In addition, a direct interaction was observed for inulin with the phosphate group by a shift in IR frequency of a phosphate band ∼1240 cm−1 (Hincha et al., 2000, 2002). Furthermore, both inulin and levan protect the membrane barrier during air-drying, since both carbohydrates considerably reduce the amount of CF leakage from liposomes during a drying and rehydration cycle (Hincha et al., 2000, 2002; Vereyken, unpublished results).
Fructans appear to be special in their membrane protective action, since other polymers such as dextran and HES failed to protect the membrane barrier function during freeze-drying, whereas chicory inulin was able to retain CF (Crowe et al., 1994, 1997; Hincha et al., 2000). Furthermore, during air-drying both inulin- and levan-type fructan were more efficient protectants of the membrane barrier function than was dextran (Vereyken, unpublished results). In addition, neither HES nor dextran was able to influence the phase transition temperature of dry lipid systems (Crowe et al., 1994, 1997).
It is largely unknown how fructans interact with lipids and what the consequences of this interaction are for the molecular organization and dynamics of lipids. Yet this information is of importance to obtain insight into the mechanisms involved in membrane preservation during dehydration.
To answer these questions, different polysaccharide-lipid systems were studied under dry conditions by 31P NMR, 2H NMR using both headgroup and acyl chain labeled lipid, and Fourier transform infrared spectroscopy (FTIR). These techniques give complementary insight into the lipid organization and dynamics and have been successfully employed in the study of other carbohydrate-lipid systems (Crowe et al., 1984; Lee et al., 1989, 1986; Tsvetkova et al., 1998). The polysaccharides used were levan from Bacillus subtilis, inulin DP 10 from chicory root, and dextran.
Both fructan types caused a considerable decrease in order-disorder transition temperature, whereas dextran was ineffective. In addition, in the presence of fructan the acyl chains were much more mobile, whereas the headgroups were immobilized, most likely as a result of insertion of fructans into the lipid headgroup region.
MATERIALS AND METHODS
Material
POPC, lyso-PC, and 2H-DMPC were obtained from Avanti Polar Lipids. Dicyclohexylcarbodiimide and N, N-dimethyl-4-aminopyridine were purchased from Sigma. [11,11-2H2]-oleic acid was obtained as described by Chupin et al. (1987).
2H2-POPC was synthesized essentially as described by Gupta et al. (1977). The starting material was lyso-PC instead of glycero-phosphocholine. The synthesis yielded 1.66 mmol (79%) 2H POPC, which was more than 99% pure according to a thin-layer chromatography analysis on silica gel using CHCl4:MeOH:H2O 65:25:4 as the eluent.
Levan (DP 125, 25 kDa) was isolated from B. subtilis as described in Vereyken et al. (2001). Inulin DP 10, a kind gift of Sensus (Roosendaal, the Netherlands), had an average DP of 10 fructose units. Dextran (mol wt 37.9 kDa) was obtained from Sigma.
Preparation of lipid dispersions
Lipids dissolved in chloroform were dried using rotational evaporation. After storage of the lipid film for at least 2 h under high vacuum, the lipids were dispersed in water or in a solution containing the appropriate amount of polysaccharide facilitating access of the carbohydrate to the lipids. Heating the solution to 30°C and using mechanical agitation facilitated hydration of the lipids. The samples were dehydrated as described in “NMR experiments” and “FTIR experiments”.
NMR experiments
Lipid dispersions of either 35 μmol headgroup-labeled 2H4-DMPC or 35 μmol of acyl chain labeled 2H-POPC were prepared as described in Preparation of lipid dispersions. The samples were transferred into an NMR tube and were first dried using a stream of nitrogen for 24 h and afterwards put under high vacuum in the presence of anhydrous P2O5 for five days. The NMR tubes were sealed using a rubber stopper and teflon tape.
The NMR experiments were carried out on a Bruker Avance 500 WB NMR spectrometer. 31P NMR spectra were obtained at 202.5 MHz using either a SE or a CP technique (Davis, 1983; Frye et al., 1985). Spectra were recorded using broad band proton decoupling and a recycling time of 2 seconds. The 90° pulse was 3.45 μs. In the CP experiments the contact time was 1–3 ms. In the spin echo experiments τ (the echo time) was 30 μs. The number of scans varied between 5000 and 15,000.
2H NMR spectra were obtained using a quadrupolar echo technique (Davis et al., 1976). The recycling delay was 100 ms, the 90° pulse was 6.15 μs, and 50,000–100,000 scans were collected.
Before Fourier transformation, an exponential multiplication with a line broadening factor of 100 or 300 Hz was used dependent on the signal-to-noise ratio. All 2H NMR spectra were symmetrized. Chemical shifts in 31P NMR spectra were measured relative to the isotropic signal of 85% H3PO4.
Spectra were measured at different temperatures, and samples were allowed to equilibrate at each temperature for at least 10 min before data collection.
FTIR experiments
IR samples were obtained by drying 4 μl of a lipid dispersion containing 10 mg/ml POPC and the desired amount of polysaccharides on a circular CaF2 (13 × 2 mm diameter) window in a cabinet continuously purged with dry air (RH < 3%) at 24°C for 24 h. Subsequently, each sample was hermetically sealed by applying a second IR-window using a rubber O-ring in between and mounted on a temperature-controlled brass cell. These procedures were performed in the above described cabinet to prevent possible rehydration of dry samples. The entire protocol resulted in completely dry samples, similar to those dried over P2O5, as concluded on the basis of data on the phase transition temperature by Koster et al. (Koster et al., 2000; Koster et al., 1994).
IR spectra were recorded on a PerkinElmer 1725 Fourier transform IR-spectrometer (PerkinElmer, Beaconsfield, UK) equipped with an external beam facility to which a PerkinElmer IR microscope was attached. The microscope was equipped with a narrow-band liquid nitrogen-cooled mercury/cadmium/telluride detector. The temperature of the brass cell was regulated by a computer-controlled device that activated a liquid nitrogen pump in conjunction with a power supply for heating the cell (Wolkers et al., 1998).
Spectra were recorded every minute at temperature increments of 1.5°C/min. The optical bench was purged with dry CO2-free air (Balston, Maidstone, UK) at a flow rate of 25 L/min. The acquisition parameters were: 32 co-added interferograms and 3500–900 cm−1 wavenumber range.
The position of the symmetric CH2-stretching vibration band around 2852 cm−1 was determined from second-derivative spectra using a nine-point smoothing factor. The position was calculated as the average of 35 spectral positions at 70–85% of the total peak height. This procedure allows for a precise determination of the peak position, irrespective of noise. The gel-to-liquid crystalline phase transition temperature was estimated from possible discrete shifts in these band positions with temperature, taking the midpoint of the shift as the average transition temperature. The peaks from the phosphate asymmetric stretch vibrations around 1250 cm−1 of different samples were compared after normalizing the absorbance and baseline flattening of the entire spectrum.
RESULTS
NMR
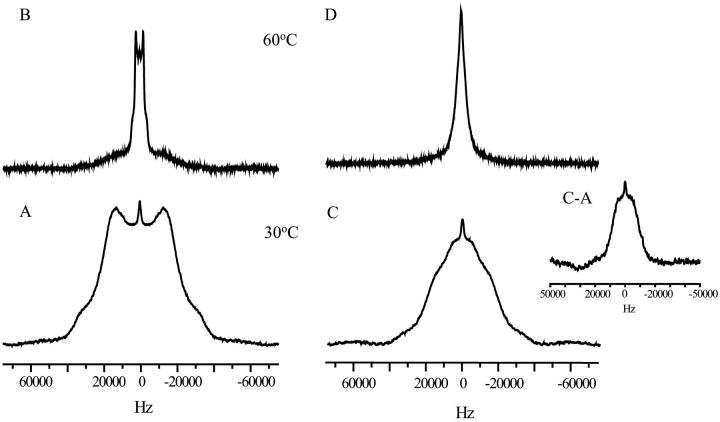
To obtain insight into the molecular organization and dynamics of dry lipid systems in the presence of polysaccharides 2H NMR experiments were conducted using labeled POPC. This lipid has two deuterons on the 11th carbon atom of the oleoyl chain. We chose POPC, since it was used in earlier studies on the effect of fructans on phase behavior and barrier properties during dehydration (Vereyken, unpublished results). The spectra were measured at different temperatures, however the observed effects are well illustrated by the spectra at 30°C and at 60°C (Fig. 1). The pure lipid at 30°C shows a well-defined spectrum with a quadrupolar splitting of 26 KHz. This is much larger than the value of 8 kHz obtained in a hydrated liquid-crystalline bilayer (Tilcock et al., 1982), but much less than the theoretical value of 128 kHz for a fully immobilized system (Davis, 1983; Davis et al., 1976). Thus, under dry conditions at 30°C the acyl chains have motional freedom, but are considerably restricted compared to those in the hydrated liquid-crystalline bilayer. The line shape showed characteristics of a gel phase spectrum (Davis, 1983; Davis et al., 1976). Increasing the temperature to 60°C resulted in a spectrum with a clearly defined quadrupolar splitting of 4 kHz, but the broad component observed at 30°C was still partially present. This change in spectral features indicates a phase transition for most of the lipids from an ordered gel state-like phase to a more disordered phase with a lipid packing similar to that of the liquid-crystalline phase.
FIGURE 1.
2H NMR spectra of acyl chain labeled POPC in the absence and presence of levan. Spectra were obtained at two different temperatures as shown in the figure. The lipid/polysaccharide mass ratio was 1:2. (A) POPC at 30°C, (B) POPC at 60°C, (C) POPC/Levan at 30°C, (D) POPC/Levan at 60°C. The inset shows the C-A difference spectrum at 30°C.
To study the effect of polysaccharides a 1:2 lipid/polysaccharide mass ratio was used, because the maximal protective effects of fructan in CF-leakage and fusion assays were obtained at this ratio (Vereyken, unpublished results). In the presence of levan at 30°C the spectrum appears to consist of two superimposed components. One component corresponded to the spectrum of pure POPC at 30°C on which a broad isotropic component was superimposed with a width at half height of 18 kHz (Fig. 1 C). This interpretation was supported by the difference spectrum C-A that is shown in the insert. The isotropic signal pointed to the presence of acyl chains with increased motional freedom. At 60°C only the isotropic signal remained (Fig. 1 D), but it was substantially narrowed compared to the situation at 30°C, which probably indicates that “melting” of the acyl chains also occurred in the presence of levan. The absence of a defined doublet suggests that the acyl chains undergo isotropic motion, also at higher temperature. For inulin DP10 the same results were obtained as for levan, but the complete isotropic signal was already reached at 45°C (data not shown). This demonstrated that the effect is not restricted to levan, but is observed for other fructans as well. In the presence of dextran the spectra resembled the spectra of pure POPC (data not shown), indicating that increasing the acyl chain mobility is not an intrinsic property of all polysaccharides.
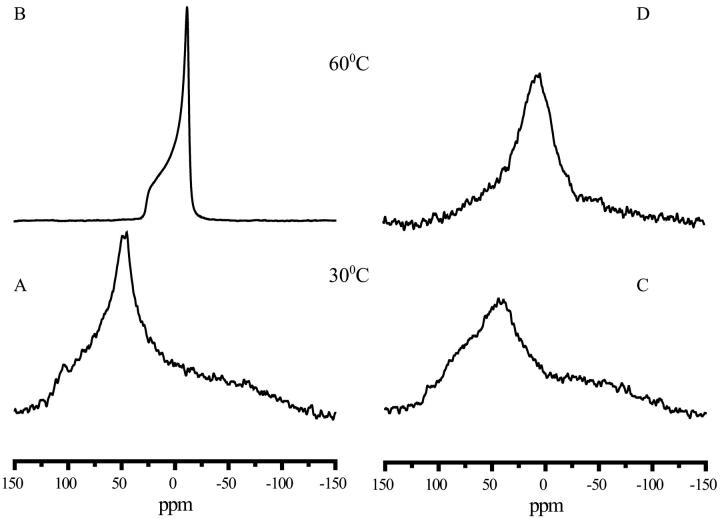
To get insight into the effect of polysaccharides on the phosphate region 31P NMR was used. The spectrum of pure POPC was recorded at 30°C using the cross polarisation technique, which is the method of choice to analyze immobile 31P containing samples. A broad spectrum was observed with a characteristic line shape of a powder spectrum showing the three components of the chemical shielding tensor (Fig. 2 A) (Smith and Ekiel, 1984). This demonstrated that in contrast to the acyl chains, the phosphate group is fully immobilized in the dry state. At 60°C (Fig. 2 B), the spectrum recorded with the conventional spin echo technique, showed a low-field shoulder and a high-field peak and a residual chemical shift anisotropy of 35.5 ppm. This is typical for a bilayer organization in which the lipid phosphate undergoes fast long axis rotation (Smith and Ekiel, 1984). That there was no broad component present as in the 2H NMR spectra is the result of the different NMR techniques used to record the spectra. In the presence of levan at 30°C a powder spectrum was observed, which was ∼200 ppm wide, as in the case of the pure lipid (Fig. 2 C). Increasing the temperature to 60°C narrowed this powder spectrum considerably (Fig. 2 D); however, the characteristic bilayer-type spectrum was not obtained, nor was it obtained at 65°C (data not shown). This indicated that the motional freedom of the phosphate group in the presence of levan is reduced, and this group cannot perform fast rotation along the long axis of the lipid molecule. The presence of inulin DP10 again gave rise to spectra similar to those seen with levan. In the presence of dextran, a spectrum resembling that of pure POPC was obtained, indicating that there is no interaction between the lipid and dextran.
FIGURE 2.
31P NMR spectra of POPC in the absence and presence of levan. Spectra were obtained at two different temperatures as shown in the figure. The lipid/polysaccharide mass ratio was 1:2. Spectra were recorded at 30°C using CP and at 60°C using SE technique. (A) POPC at 30°C, (B) POPC at 60°C, (C) POPC/Levan at 30°C, and (D) POPC/Levan at 60°C.
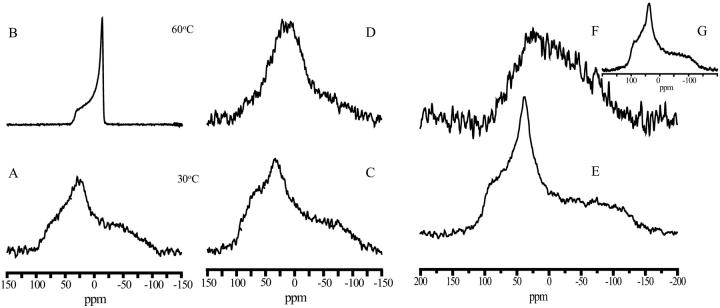
To investigate whether the immobilization of the phosphate moiety by fructans was a specific effect for POPC, DMPC was also tested. In addition, this gave the opportunity to study the choline group in more detail, because it was deuterated in both the α- and β-positions. For pure lipid (Fig. 3, A and B) and in the presence of fructan (Fig. 3, C and D) the 31P spectra were comparable to the spectra observed for POPC (Fig. 2), indicating that both lipids behave comparably in the presence and absence of fructan. However, for dextran a strikingly different effect was observed. The low-temperature spectrum (Fig. 3 E) had a shape similar to that of pure POPC. At a higher temperature, however, the spectrum recorded using the spin-echo technique (Fig. 3 F) suggested that the motion of this phosphate group was severely restricted, which was confirmed when the spectrum was recorded using the cross polarization technique (Fig. 3 G). In this case a typical spectrum of a fully immobilized phosphate was observed (Smith and Ekiel, 1984).
FIGURE 3.
31P NMR spectra of DMPC in the absence or presence of levan or dextran. Spectra were obtained at two different temperatures as shown in the figure. The lipid/polysaccharide mass ratio was 1:2. Spectra were recorded at 30°C using CP and at 60°C using SE technique. (A) DMPC at 30°C, (B) DMPC at 60°C, (C) DMPC/Levan at 30°C, (D) DMPC/Levan at 60°C, (E) DMPC/Dextran at 30°C, and (F) DMPC/Dextran at 60°C. The inset (G) shows the CP spectrum.
To test whether this effect is due to the mere presence of dextran in the sample or can be related to the distribution of the dextran inside the sample, dextran was added to the lipid dispersion after the lipid was hydrated instead of adding it before hydration of the lipid. In the latter protocol, in principle all lipids could be in contact with the carbohydrate, but in the former only the lipids on the outside are supposed to be in contact with dextran. The addition of dextran to the outside of the lipid dispersion resulted in 31P spectra that closely resembled the spectra of pure DMPC (data not shown). Therefore, the mere presence of this polysaccharide does not induce the observed effect; rather, dextran has to be present throughout the sample.
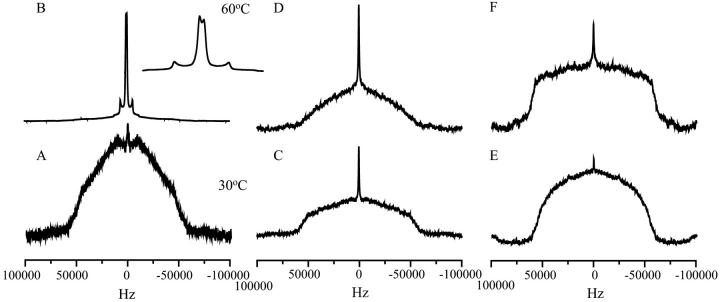
The effect of the different carbohydrates on the motional properties of the choline part of DMPC under dry conditions was studied using 2H-DMPC. At 30°C the 2H NMR spectrum of pure DMPC (Fig. 4 A) had an overall width of more than 100 kHz and lacked the characteristic double doublet typical for deuterons attached to lipids that undergo fast axial rotation. This signal was much broader than the signal obtained in the presence of 4 water molecules per lipid headgroup (Bechinger and Seelig, 1991; Ulrich and Watts, 1994), indicating that the choline moiety like the phosphate group is highly immobilized and confirming that virtually no water is present. Increasing the temperature to 60°C (Fig. 4 B) gave rise to a spectrum with clearly defined splittings. After the assignment of Bechinger and Seelig (Bechinger and Seelig, 1991) we attributed the signal with a splitting of 12 KHz to the α-deuterons and the splitting of 1 KHz to the β-deuterons. In the presence of levan at 30°C (Fig. 4 C) a featureless spectrum was observed, with a width of 125 KHz (Davis, 1983; Davis et al., 1976). Increasing the temperature to 60°C (Fig. 4 D) caused a slight narrowing of the spectrum, which remained much broader than the control spectrum. This indicated that the choline moiety of the DMPC headgroup is highly immobilized by levan. The presence of inulin DP10 resulted in similar spectra (data not shown), indicating that immobilization of the headgroup by fructans is a more general property of these carbohydrates. In the presence of dextran the same featureless spectrum was observed at 30°C as in the presence of levan (Fig. 4 E). Also at 60°C the choline group was largely immobilized in the presence of dextran (Fig. 4 F), which was in agreement with the 31P NMR spectra.
FIGURE 4.
2H NMR spectra of headgroup labeled DMPC in the absence and presence of levan or dextran. Spectra were obtained at two different temperatures as shown in the figure. The lipid/polysaccharide mass ratio was 1:2. (A) DMPC at 30°C, (B) DMPC at 60°C, (C) DMPC/Levan at 30°C, (D) DMPC/Levan at 60°C, (E) DMPC/Dextran at 30°C, and (F) DMPC/Dextran at 60°C. In the inset, a magnification of spectrum B is shown to illustrate the observed quadrupolar splitting.
FTIR
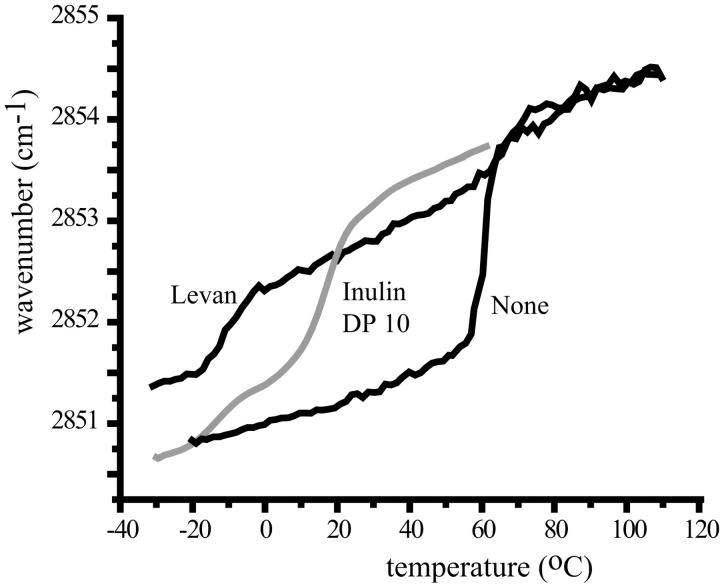
FTIR was used to get further insight into the molecular organization of the polysaccharide-lipid systems. The phase transition temperature of the lipids can be determined by studying the symmetric CH2-stretching vibration around 2850 cm−1, which abruptly changes with a clear jump in wavenumber upon chain melting (Mantsch and McElhaney, 1991). For pure POPC in the dry state (Fig. 5) an order-disorder transition was observed around 57°C, which is in agreement with earlier data (Koster et al., 2000, 1994). This transition temperature was much higher than for hydrated POPC (0°C) (Davis et al., 1981), which is consistent with the transition-lowering effect of hydration. The presence of levan had a large effect on the melting behavior of the lipids in the dry state. A substantial shift in wavenumber was detected around –10°C indicating that the majority of the lipid acyl chains melt at this temperature. Note that this temperature is even lower than that for fully hydrated POPC. A minor transition was detected at the temperature of dry POPC, probably indicating that not all POPC molecules are in contact with levan. Also, in the presence of inulin DP10 a substantial lowering of the phase transition temperature was observed. Again two phase transitions were measured, one around –15°C, which is again lower than for fully hydrated POPC, and a second around 15°C. The occurrence of two transitions can again be attributed to sample inhomogeneity. The results demonstrate that fructans lower the phase transition temperature considerably.
FIGURE 5.
Lipid melting curves of dry POPC samples in the absence or presence of either levan or inulin DP10 recorded by FTIR. The wavenumbers of the symmetric CH2-stretching vibrations are plotted as a function of temperature. The lipid/polysaccharide mass ratio was 1:2.
In the presence of dextran the phase transition temperature hardly changed compared to pure POPC (data not shown). Therefore, the lowering of the ordered to disordered phase transition temperature is not a property of all polysaccharides.
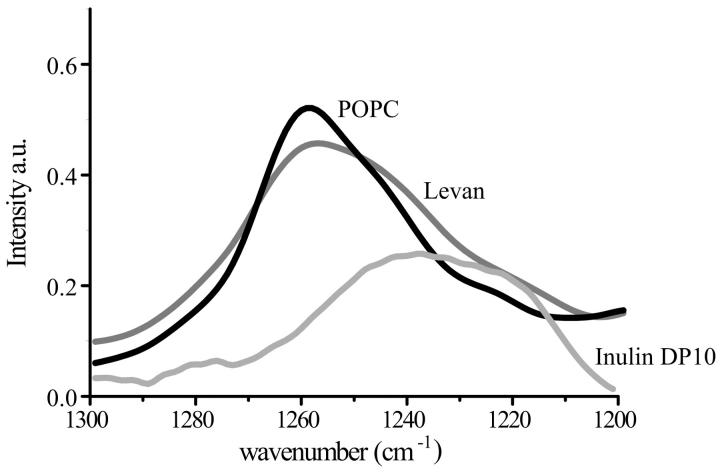
Using FTIR, the phosphate group can also be studied, since it has an asymmetric stretch vibration around 1240 cm–1. The position of this peak is sensitive to the interactions of the phosphate group with other molecules in the environment (Crowe et al., 1996; Hoekstra et al., 1997; Tsvetkova et al., 1998). Dried POPC showed a peak in the IR-absorption at 1256 cm−1 (Fig. 6), which is in accordance with literature data (Hoekstra et al., 1997). In the presence of levan hardly any change was observed in the phosphate vibration, indicating that there was no direct interaction with the phosphate group. However, in the presence of inulin DP10 a clear shift to a lower vibration frequency was observed. The peak was very broad and had its maximum at 1240 cm−1 and a shoulder at 1221 cm−1. These results indicate that there is a direct interaction between the inulin DP10 and the phosphate group of POPC.
FIGURE 6.
Infrared spectra of the phosphate asymmetric stretch region in POPC in the absence or presence of levan and inulin DP10. The lipid:polysaccharide mass ratio was 1:2.
DISCUSSION
The aim of this study was to investigate the consequences of the fructan-lipid interaction for the molecular organization and dynamics of lipids under dry conditions. It was found that fructans lowered the phase transition temperature by ∼70°C. In addition, in the presence of fructan the acyl chains showed more motional freedom, whereas the headgroup was immobilized. Not all polysaccharides have these effects on lipids: dextran was not able to influence the phase transition and molecular properties of POPC, although it did immobilize DMPC.
For dry POPC using FTIR it was found that the ordered-disordered phase transition was at ∼57°C. This melting behavior was also observed in the 2H and 31P NMR spectra. The ordered phase was characterized by a very broad, gel-like spectrum with a quadrupole splitting of 26 kHz for the 2H-labeled oleoyl chains and a powder pattern in 31P NMR characteristic for an immobilized phosphate di-ester. In the disordered phase at 60°C the 31P spectra and the 2H NMR spectra were typical for a liquid-crystalline lamellar phase.
In the presence of fructan, both the inulin and the levan-type, the phase transition temperature was lowered substantially to subzero temperatures. This reduction was not due to residual water, because even excess water will reduce the order-disorder transition temperature only to 0°C (Davis et al., 1981) and not to −10°C. These data correlate with the data that Hincha et al. (2002) found for smaller inulin molecules. The substantial lowering of the phase transition temperature was also observed in the 2H NMR spectra for the POPC acyl chains. However, an isotropic peak characterized the disordered phase, indicating that there was even more motional freedom than for pure POPC.
In contrast, the headgroup of PC was immobilized in the presence of fructans. From both 31P NMR and 2H NMR it became clear that the phosphate group and the choline moiety were immobilized in the presence of fructan even at 60°C. For the phosphate group this was measured for both DMPC and POPC, indicating that it was not dependent on the lipid type used.
How can the effect that fructan has on the lipids be understood? Earlier studies led to the conclusion that fructans insert between the headgroups of lipids under both hydrated and dehydrated conditions (Vereyken et al., 2001; and Vereyken, unpublished results). Through insertion fructans increase the lipid spacing, thereby preserving motional freedom for the acyl chains. This correlates with a lower phase transition temperature. During dehydration the fructans form a solid-like glassy structure (Hinrichs et al., 2001; Levine and Slade, 1988). Since the fructans are interacting with the lipid headgroup, glass formation will immobilize the headgroup.
These data provide an explanation for the earlier observed CF retention upon drying in the presence of fructan (Hincha et al., 2002; and Vereyken, unpublished results). The large reduction in transition temperature caused by fructan will prevent an ordered-disordered phase transition, which reduces the functioning of the membrane barrier. In addition, glass formation in the headgroup region will prevent fusion (Crowe et al., 1998, 1997), which also contributes to CF retention.
Although we emphasized the similarities between levan (DP125) and inulin (DP10), also differences were observed. The most important difference was that inulin DP10 interacted with the phosphate group, whereas levan did not, as revealed with FTIR. Both polysaccharides immobilized the lipid headgroup. This suggests that the location of interaction with the lipid headgroup is different from that of levan. Probably inulin inserts deeper into the membrane, thereby reaching the phosphate group, while levan interacts higher up in the headgroup region. In support of this hypothesis, it was found that inulin was somewhat more effective in stabilizing the Lα phase during dehydration (Vereyken, unpublished results) and also that under fully hydrated conditions inulin interacts more strongly with the membrane than levan (Vereyken, 2003). This is probably related to the greater flexibility of inulin, which might lead to a more profound membrane interaction (Vereyken et al., 2003).
Studies concerning the interaction of small carbohydrates with membranes have revealed that an interaction with the phosphate group is correlated with an increased CF retention and thereby with a better membrane barrier protection (Crowe et al., 1998; 1992). However, inulin and levan both showed a similar CF retention (Vereyken, unpublished results). From this, it can be concluded that the interaction with the phosphate group is not essential for membrane integrity preservation, which is in agreement with recent data (Luzardo et al., 2000).
Shifting the phase transition temperature is not a general property of polysaccharides, since dextran did not have an effect on POPC. Surprisingly, dextran influenced the dynamic properties of DMPC. Based on the 31P NMR and 2H NMR data, it can be concluded that dextran effectively immobilized the lipid headgroup, even at 60°C. The distribution of dextran throughout the sample is important, since DMPC in the presence of dextran behaved similarly to the DMPC sample in which the polysaccharide was added from the outside to the lipid dispersions. We favor the explanation that solidified dextran encloses small parts of the lipid system within a confined space. Chain melting, normally accompanied by expansion, is inhibited because of spatial limitations (Zhang and Steponkus, 1996). That POPC was not immobilized could be due to the presence of one unsaturated bond and therefore less ideal packing properties in the condensed phase. Therefore, chain melting will require less spatial expansion. We cannot exclude that dextran for unknown reasons distributes differently among the lipid stacks for DMPC and POPC.
How do our findings relate to the membrane protective effects of small carbohydrates and the known theories explaining the effects of carbohydrates on membranes? The effects of fructan on lipid organization and dynamics resemble those reported for trehalose on DPPC (Lee et al., 1989, 1986; Tsvetkova et al., 1998), with the exception that in the study of Tsvetkova et al. (1998) an increase in headgroup mobility was observed after the glass transition temperature was reached during a stepwise heating process. This apparent discrepancy might be due to the high glass transition temperature of both inulin and levan, which is most likely above 100°C (Hinrichs et al., 2001), a temperature not reached in the NMR experiment. Since the fructan stayed in the glass state, the headgroup interacting with the solid-like structure was immobilized.
The water replacement hypothesis is a theory that is often used to explain the effects carbohydrates have on membranes (Crowe et al., 1988, 1992). In this hypothesis, small carbohydrates replace water on the lipid headgroups, thereby inserting into the lipid layer. This results in spacing of the acyl chains and a much lower phase transition temperature. Our data and interpretation of the fructan-membrane interaction do fit closely with this theory. However, in the case of fructans it is probably not mere water replacement, since they also interact with the membrane under fully hydrated conditions (Vereyken et al., 2001, 2003). Therefore, some hydrophobic component is probably also involved in the interaction of fructans with lipids as indicated by earlier studies (Vereyken et al., 2001, 2003).
Abbreviations used: POPC, 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine; lyso-PC, 1-palmitoyl-sn-glycero-3-phosphocholine; 2H-DMPC, 1,2-dimyristoyl-sn-glycero-3-phospho-[1, 1′, 2, 2′-2H4]-choline; 2H2-POPC, 1-Palmitoyl-2-[11,11-2H2]-oleoyl-sn-glycero-3-phosphocholine; CF, carboxyfluorescein; CP, cross polarization; DP, degree of polymerization; HES, hydroxyethyl starch; SE, spin echo.
References
- Bechinger, B., and J. Seelig. 1991. Conformational changes of the phosphatidylcholine headgroup due to membrane dehydration. A 2H-NMR study. Chem. Phys. Lipids. 58:1–5. [DOI] [PubMed] [Google Scholar]
- Chupin, V., J. A. Killian, and B. de Kruijff. 1987. 2H-nuclear magnetic resonance investigations on phospholipid acyl chain order and dynamics in the gramicidin-induced hexagonal HII phase. Biophys. J. 51:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe, J. H., J. F. Carpenter, and L. M. Crowe. 1998. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 60:73–103. [DOI] [PubMed] [Google Scholar]
- Crowe, J. H., L. M. Crowe, J. F. Carpenter, A. S. Rudolph, C. A. Wistrom, B. J. Spargo, and T. J. Anchordoguy. 1988. Interactions of sugars with membranes. Biochim. Biophys. Acta. 947:367–384. [DOI] [PubMed] [Google Scholar]
- Crowe, J. H., L. M. Crowe, and D. Chapman. 1984. Infrared spectroscopic studies on interactions of water and carbohydrates with a biological membrane. Arch. Biochem. Biophys. 232:400–407. [DOI] [PubMed] [Google Scholar]
- Crowe, J. H., F. A. Hoekstra, and L. M. Crowe. 1992. Anhydrobiosis. Annu. Rev. Physiol. 54:579–599. [DOI] [PubMed] [Google Scholar]
- Crowe, J. H., F. A. Hoekstra, K. H. Nguyen, and L. M. Crowe. 1996. Is vitrification involved in depression of the phase transition temperature in dry phospholipids? Biochim. Biophys. Acta. 1280:187–196. [DOI] [PubMed] [Google Scholar]
- Crowe, J. H., S. B. Leslie, and L. M. Crowe. 1994. Is vitrification sufficient to preserve liposomes during freeze-drying? Cryobiology. 31:355–366. [DOI] [PubMed] [Google Scholar]
- Crowe, J. H., A. E. Oliver, F. A. Hoekstra, and L. M. Crowe. 1997. Stabilization of dry membranes by mixtures of hydroxyethyl starch and glucose: the role of vitrification. Cryobiology. 35:20–30. [DOI] [PubMed] [Google Scholar]
- Davis, J. H. 1983. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim. Biophys. Acta. 737:117–171. [DOI] [PubMed] [Google Scholar]
- Davis, J. H., K. R. Jeffrey, M. Bloom, M. I. Valic, and T. P. Higgs. 1976. Quadrupolar echo deuteron magnetic resonance spectroscopy in ordered hydrocarbon chains. Chem. Phys. Lett. 42:390–394. [Google Scholar]
- Davis, P. J., B. D. Fleming, K. P. Coolbear, and K. M. Keough. 1981. Gel to liquid-crystalline transition temperatures of water dispersions of two pairs of positional isomers of unsaturated mixed-acid phosphatidylcholines. Biochemistry. 20:3633–3636. [DOI] [PubMed] [Google Scholar]
- Demel, R. A., E. Dorrepaal, M. J. M. Ebskamp, J. C. M. Smeekens, and B. de Kruijff. 1998. Fructans interact strongly with model membranes. Biochim. Biophys. Acta. 1375:36–42. [DOI] [PubMed] [Google Scholar]
- Frye, J., A. D. Albert, B. S. Selinsky, and P. L. Yeagle. 1985. Cross polarization P-31 nuclear magnetic resonance of phospholipids. Biophys. J. 48:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, C. M., R. Radhakrishnan, and H. G. Khorana. 1977. Glycerophospholipid synthesis: Improved general method and new analogs containing photoactivable groups. Proc. Natl. Acad. Sci. USA. 74:4315–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry, G. A. F. 1993. Evolutionary origins and natural functions of fructans—a climatological, biogeographic and mechanistic appraisal. New Phytology. 123:3–14. [Google Scholar]
- Hincha, D. K., E. M. Hellwege, A. G. Heyer, and J. H. Crowe. 2000. Plant fructans stabilize phosphatidylcholine liposomes during freeze-drying. Eur. J. Biochem. 267:535–540. [DOI] [PubMed] [Google Scholar]
- Hincha, D. K., E. Zuther, E. M. Hellwege, and A. G. Heyer. 2002. Specific effects of fructo- and gluco-oligosaccharides in the preservation of liposomes during drying. Glycobiology. 12:103–110. [DOI] [PubMed] [Google Scholar]
- Hinrichs, W. L. J., M. G. Prinsen, and H. W. Frijlink. 2001. Inulin glasses for the stabilization of therapeutic proteins. Int. J. Pharm. 215:163–174. [DOI] [PubMed] [Google Scholar]
- Hoekstra, F. A., W. F. Wolkers, J. Buitink, E. A. Golovina, J. H. Crowe, and L. M. Crowe. 1997. Membrane stabilization in the dry state. Comp. Biochem. Physiol. 117A:335–341. [Google Scholar]
- Konstantinova, T., D. Parvanova, A. Atanassov, and D. Djilianov. 2002. Freezing tolerant tobacco, transformed to accumulate osmoprotectants. Plant Sci. 163:157–164. [Google Scholar]
- Koster, K. L., Y. P. Lei, M. Anderson, S. Martin, and G. Bryant. 2000. Effects of vitrified and nonvitrified sugars on phosphatidylcholine fluid-to-gel phase transitions. Biophys. J. 78:1932–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster, K. L., M. S. Webb, G. Bryant, and D. V. Lynch. 1994. Interactions between soluble sugars and POPC (1-palmitoyl-2-oleoylphosphatidylcholine) during dehydration: vitrification of sugars alters the phase behavior of the phospholipid. Biochim. Biophys. Acta. 1193:143–150. [DOI] [PubMed] [Google Scholar]
- Lee, C. W., S. K. Das Gupta, J. Mattai, G. G. Shipley, O. H. Abdel-Mageed, A. Makriyannis, and R. G. Griffin. 1989. Characterization of the L lambda phase in trehalose-stabilized dry membranes by solid-state NMR and X-ray diffraction. Biochemistry. 28:5000–5009. [DOI] [PubMed] [Google Scholar]
- Lee, C. W., J. S. Waugh, and R. G. Griffin. 1986. Solid-state NMR study of trehalose/1,2-dipalmitoyl-sn-phosphatidylcholine interactions. Biochemistry. 25:3737–3742. [DOI] [PubMed] [Google Scholar]
- Levine, H., and L. Slade. 1988. Principles of “cryostabilization” technology from structure/property relationships of carbohydrate/water systems—a review. Cryo. Lett. 9:21–63. [Google Scholar]
- Luzardo, M. C., F. Amalfa, A. M. Nuñez, S. Diaz, A. C. Biondi de Lopez, and E. A. Disalvo. 2000. Effect of trehalose and sucrose on the hydration and dipole potential of lipid bilayers. Biophys. J. 78:2452–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch, H. H., and R. N. McElhaney. 1991. Phospholipid phase transitions in model and biological membranes as studied by infrared spectroscopy. Chem. Phys. Lipids. 57:213–226. [DOI] [PubMed] [Google Scholar]
- Pilon-Smits, E. A. H., M. J. M. Ebskamp, M. J. W. Jeuken, P. J. Weisbeek, and S. C. M. Smeekens. 1995. Improved performance of transgenic fructan-accumulating tobacco under drought stress. Plant Physiol. 107:125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontis, H. G. 1989. Fructans and cold stress. J. Plant Physiol. 134:148–150. [Google Scholar]
- Senaratna, T., B. D. McKersie, and R. H. Stinson. 1984. Association between membrane phase properties and dehydration injury in soybean axes. Plant Physiol. 76:759–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, I. C. P., and I. H. Ekiel. 1984. Phosphorus-31 NMR of phospholipids in membranes. In Phosphorus-31 NMR: Principles and Applications. D. G. Gorenstein, editor. Academic Press, Orlando, FL. 447–478.
- Tilcock, C. P., M. B. Bally, S. B. Farren, and P. R. Cullis. 1982. Influence of cholesterol on the structural preferences of dioleoylphosphatidylethanolamine-dioleoylphosphatidylcholine systems: a phosphorus-31 and deuterium nuclear magnetic resonance study. Biochemistry. 21:4596–4601. [DOI] [PubMed] [Google Scholar]
- Tsvetkova, N. M., B. L. Phillips, L. M. Crowe, J. H. Crowe, and S. H. Risbud. 1998. Effect of sugars on headgroup mobility in freeze-dried dipalmitoylphosphatidylcholine bilayers: solid-state 31P NMR and FTIR studies. Biophys. J. 75:2947–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich, A. S., and A. Watts. 1994. Molecular response of the lipid headgroup to bilayer hydration monitored by 2H-NMR. Biophys. J. 66:1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereyken, I. J., V. Chupin, R. A. Demel, S. C. Smeekens, and B. de Kruijff. 2001. Fructans insert between the headgroups of phospholipids. Biochim. Biophys. Acta. 1510:307–320. [DOI] [PubMed] [Google Scholar]
- Vereyken, I. J., J. A. van Kuik, T. H. Evers, P. J. Rijken, and B. de Kruijff. 2003. Structural requirements of the fructan-lipid interaction. Biophys. J. 84:3147–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijn, I., and J. C. M. Smeekens. 1999. Fructan: More than a reserve carbohydrate? Plant Physiol. 120:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkers, W. F., A. Bochicchio, G. Selvaggi, and F. A. Hoekstra. 1998. Fourier transform infrared microspectroscopy detects changes in protein secondary structure associated with desiccation tolerance in developing maize embryos. Plant Physiol. 116:1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., and P. L. Steponkus. 1996. Proposed mechanism for depression of the liquid-crystalline-to-gel phase transition temperature of phospholipids in dehydrated sugar-phospholipids mixtures. Cryobiology. 33:21A. [Google Scholar]