Abstract
Infection by nematode parasites with a pulmonary migration in their life cycle and allergic asthma are two highly prevalent diseases in humans; therefore, one may expect both may occur concomitantly. There is a predominant and essential role of Th2 lymphocytes in the mechanisms underlying the control of parasite elimination as well as in the pathology observed in the asthmatic lung. The consequences of such situations have been explored, with controversial results, justifying the development of experimental models in which the relationship between allergic airway inflammation and helminth infection might be evaluated. The present work describes the inflammatory, humoral, and functional changes that occur in the lung of rats after single (subcutaneous inoculation of 1,500 L3 larvae) or multiple (five weekly subcutaneous inoculations of 1,500 L3 larvae) Strongyloides venezuelensis infections. The results show that the migration of S. venezuelensis larvae through the lungs of infected rats induces a local eosinophilic inflammation process which is mostly focal and parenchymal for rats infected a single time and which is peribronchial after multiple infections. The inflammatory process is accompanied by mucus hypersecretion, thickening of bronchial epithelial and muscle layers, and local increase in immunoglobulin E concentrations that peak after 5 to 7 days and are resolved after 12 days of single or multiple infections. The peak of lung immunopathologic changes observed in infected rats coincides with lung airway hyperresponsiveness (AHR), a key functional alteration in asthma. We propose that this experimental model is ideal to carry out further studies on immunoprotection against nematode infection versus immunopathology of allergic airway inflammation.
Helminth infections typically induce elevated levels in serum of immunoglobulin G1 (IgG1) and IgE in mice (IgG4 and IgE in humans), eosinophilia, intestinal mastocytosis, and goblet cell hyperplasia (2, 21). These responses are controlled mainly by interleukin-4 (IL-4), IL-13, IL-5, IL-3, and IL-10, which are indicative of Th2-cell activation in mice (1). The Th2 response observed during helminth infections appears to be associated with host protection in at least some experimental models, although the precise mechanisms of protection are still not clear (21, 29, 36). There is also strong experimental evidence indicating that airway inflammation, characterized by infiltration of Th2 cells, eosinophils, and mast cells, in addition to the genetic predisposition to develop an IgE response, have a central role in the pathophysiology of allergic diseases, such as asthma (7, 53). These alterations have been associated with the main physiological changes of the disease, namely, variable airflow obstruction and bronchial hyperresponsiveness (4, 19, 53).
Gastrointestinal nematode species which have a pulmonary migration in their life cycle, such as Necator americanus, Ancylostoma duodenalis, Strongyloides stercoralis, and Ascaris lumbricoides, are the most prevalent parasites in humans, infecting over one quarter of the world's population (6, 9). In a small proportion of patients, these nematode infections may induce an inflammatory process in the lung which, akin to asthma, is characterized by eosinophil infiltration and may be accompanied by asthma-like symptoms (16, 39, 42). However, most patients do not develop pulmonary symptoms; moreover, AHR and positive skin reaction against common allergens was not clearly associated with the nematode infections (16, 34, 52). The similarities in the immune response observed in asthma and nematode infection raise obvious questions about the relationship between allergic disorders and helminth infections. Epidemiological studies that address this association show conflicting results. Lynch and collaborators (31) reported a low prevalence of clinical atopic disorders among children living in a slum area of Caracas, Venezuela, in which gut helminth infection is highly prevalent. The low allergic reactivity was reverted in child groups that received regular anti-helminth treatment. In contrast, when researchers (32) selected asthmatic children living in the same location, anti-helminth treatment produced significant improvement in clinical symptoms of asthma but not in parameters of pulmonary function.
Therefore, it is necessary to establish experimental models in which the relationship between allergic airway inflammation and helminth infection may be studied. Mice infected with Nippostrongylus brasiliensis (15) have been used for this purpose. However, and in contrast to those of humans (22) and rats (20), mice eosinophils do not express cell surface receptors that bind IgE (18). In addition, and unlike that from the human system, IL-13 did not induce murine B cells to produce IgE independently of IL-4 (55). As eosinophils and IgE have been associated with development of asthma pathology and helminth protection, mice would not be an ideal model for such studies. Moreover, mice are not usually the natural host for N. brasiliensis or Strongyloides spp., parasites which have been tested in this species. Strongyloides venezuelensis is a nematode that was isolated from naturally infected wild rats (5). In experimental infection, S. venezuelensis larvae have an obligatory migration through the host lungs before establishment in the duodenal mucosa, and adult worms are eliminated spontaneously from the host after 5 weeks (50), inducing eosinophilia and intestinal mastocytosis (26, 27). Here we propose to use the infection of rats with S. venezuelensis as an experimental model to investigate the inflammatory, humoral, and functional changes that occur in the lung during single and multiple infections.
MATERIALS AND METHODS
Animals.
Male Wistar rats weighing 180 to 200 g were used in the experiments. All animals were fed with laboratory chow (Nuvilab, Colombo, PR, Brazil) and given tap water to drink ad libitum. Experimental procedures received prior approval from the local animal ethics committee.
Parasite and parasitological techniques.
S. venezuelensis, the intestinal nematode used in all the experiments, was isolated from Rattus norvegicus. The original isolation (5) recovered S. venezuelensis and Strongyloides ratti, which were later separated and which have been maintained at the Department of Parasitology, Universidade Federal de Minas Gerais, by serial passage in Wistar rats. For the experiments, S. venezuelensis infective filiform larvae (L3) were obtained from charcoal culture of infected-rat feces. Cultures were kept for 48 to 72 h at 28°C, and the infective larvae were collected and concentrated by using a Baermann apparatus. Subsequently, the larvae recovered were washed several times in phosphate-buffered saline (PBS) and were counted, and the concentration was adjusted to 5,000 L3 larvae/ml of PBS for the infection.
Rats were randomly divided in three experimental groups: the multiple-infection group that received five larvae inoculations 7 days apart; the single-infection group that received one larvae inoculation at the same time that the last inoculation was performed with the multiple-infection group; and the control group, which was not infected. For each infection, rats were inoculated subcutaneously with 1,500 infective larvae in 300 μl of PBS at the abdominal region.
Infectivity rates were determined by assessing fecal egg counts (with a modified Cornell McMaster method), number of larvae recovered from the lung, and number of worms recovered from the small intestine at 2, 5, 7, and 12 days after the infection of the single-infection rats and at 2, 5, 7, and 12 days after the fifth parasite inoculation of the multiple-infection rats. For worm recovery from the small intestine, the upper half of the small intestine was removed after sacrifice, washed, cut open longitudinally, and incubated in PBS at 37°C for 4 h. For worm recovery from the lung the organ was removed, fragmented in PBS, and incubated for 2 h at 37°C. Worms that emerged from each organ were quantified under stereo microscopy.
Infective larvae and adult worms were also used to produce total L3 antigen and total worm antigens. The infective larvae or worms were extensively washed with PBS and were resuspended in PBS containing protease inhibitor cocktail (1 tablet in 25 ml of PBS; Boehringer Mannheim, Indianapolis, Ind.). Parasite suspension was vortexed with glass beads (5 cycles of 1 min each) and the larvae mixture was transferred to another tube and completely disrupted with a cell sonic disrupter (PGC Scientific, Gaithersburg, Md.) by using 5 cycles of 1 min at the highest power allowed for the standard micro tip. After overnight extraction at 4°C, the homogenate was centrifuged at 400 × g for 30 min, the supernatant was removed, and the protein concentration was determined before aliquotting and storing at −20°C.
Determination of airway responsiveness to acetylcholine.
At 2, 5, 7, and 12 days after infection of the single-infection group or after the last infection of the multiple-infection group, four rats from each experimental group were anesthetized with thiopental sodium (40 mg/kg; Abbot Laboratories, São Paulo, Brazil) and the femoral artery, femoral vein, and trachea were cannulated. Acetylcholine (10 to 300 μg/kg in 100 μl; Sigma Chemical Co., Poole, Dorset, United Kingdom) was injected through a cannula inserted into the femoral vein. The mean arterial pressure was monitored via the cannula inserted into the femoral artery. A three-way connector was attached to the tracheostomy tube; a port was connected to the ventilator (a Harvard Apparatus with 10 ml of air/kg of body weight at a rate of 90 breaths/min) and a port was connected to a pressure transducer (Physiological Pressure Transducer; Ohmeda). Variation of intratracheal pressure after injection of increasing doses of acetylcholine was used as an indirect measurement of lung resistance. The signal from the transducers was digitized with an analogic digital board (System 1000 Power Supply; CWE Incorporated) connected to a computer for registration. Data are expressed as percentages of increase in intratracheal pressure compared to the baseline. There were no differences in baseline pressures in any of the groups analyzed (data not shown).
Collection of blood, bronchoalveolar lavage (BAL), and bone marrow.
After measurement of lung function parameters, anesthetized rats were bled via the abdominal aorta and blood samples were used to estimate circulating cell composition.
BAL was performed by intratracheal instillation of 5 ml of PBS containing 0.3% of bovine serum albumin (PBS-BSA; Sigma) and protease inhibitor cocktail (1 tablet in 50 ml of PBS; Boehringer Mannheim). The lavage fluid was centrifuged (200 × g for 7 min), and aliquots of the supernatant were kept at −70°C until further analysis (IgE and cytokine measurements). The cell pellet from the BAL fluid was resuspended in 1 ml of PBS-BSA.
Bone marrow cells were flushed from the right femur of each rat by injecting 5 ml of PBS containing heparin (50 IU/ml). The recovered solution was vortexed gently and centrifuged at 200 × g for 7 min. The cell pellet was resuspended in 1 ml of PBS-BSA and was counted.
Total leukocytes in blood, BAL fluid, and bone marrow lavage were estimated in a Neubauer chamber. Cytospin slides prepared from BAL fluid and bone marrow samples and blood smears were stained with May-Grünwald-Giemsa stain. Cells were differentiated into mononuclear cells, mature eosinophils, and mature neutrophils according to standard morphological criteria, and at least 200 cells were counted per slide under light microscopy.
Lung histopathology.
After BAL and at different periods after infection, the right lobe of the lungs from each animal was recovered for histological analysis. The lung was inflated via the tracheal cannula with 10% buffered formalin, fixed in the same solution, and embedded in paraffin and 5-μm sections were prepared for histology. Sections were stained with hematoxylin and eosin for the assessment of overall inflammatory response. Goblet cell and mucus production were analyzed with Alcian Blue-Safranin-stained slides.
EPO assay.
The eosinophil peroxidase (EPO) assay was used as an estimate of eosinophil numbers in lung tissue and BAL fluid (13). After flushing the pulmonary artery with 20 ml of PBS, the left lung was weighed, chopped, and homogenized in PBS (5% [wt/vol]) by using a tissue homogenizer (Power Gen 125; Fisher Scientific, Pittsburgh, Pa.). The homogenate was centrifuged (3,000 × g for 10 min), the red blood cells in the pellet were lysed, and cells were resuspended in PBS (pH 7.4) containing 0.5% hexadecyl trimethyl ammonium bromide (HTAB; Sigma). The cell solution was homogenized again, and the homogenates were then subjected to freeze/thaw three times in liquid nitrogen and were stored at −20°C until they were assayed. For the assay, samples of BAL and lung tissue were spun down and supernatant was diluted 1:3 in PBS/HTAB. The assay was carried out in 96-well plates (Nalge Nunc International Co., Naperville, Ill.). Each sample was tested in triplicate by adding 75 μl of the sample/well and 75 μl of OPD substrate (1.5 mM o-phenylalanine diamine [Sigma] and 6.6 mM hydrogen peroxide in 75 mM Tris-HCl, pH 8.0)/well. The reaction was carried out at 20°C for 30 min and was stopped with 4 M sulfuric acid solution. Plates were read at 492 nm on a microplate reader (Titter Tek Multiskan), and results are given in absorbance units.
Quantification of IgE in BAL fluid.
Total IgE concentrations in BAL fluid were estimated by an enzyme-linked immunosorbent assay (ELISA) method. Ninety-six-well plates (Nunc Maxisorp; Sigma) were coated with 5 μg of mouse monoclonal antibody (MAb) anti-rat IgE (clone MARE 1; Serotec, Oxford, England)/ml in 0.1 M carbonate buffer, pH 9.6, and blocked with 1% BSA in PBS buffer. Between each incubation step the plates were washed five times with PBS containing 0.05% Tween 20. BAL fluid samples (100 μl/well) diluted 1:8 in PBS containing 0.05% Tween 20 and 0.1% BSA or known concentrations of affinity-purified rat IgE (10 to 1,000 ng of IR-162/ml; kindly provided by Robin G. Bell, Cornell University, Ithaca, N.Y.) were added to the plate and were incubated for 2 h at room temperature. Bound IgE was detected by biotin-conjugated mouse anti-rat light chain MAb (clones RT-39 and RL-6; Sigma) diluted 1:1,000 in PBS containing 0.1% BSA followed by an alkaline phosphatase conjugate, streptavidin (Gibco BRL, Life Technologies, Gaithersburg, Md.), at 1:2,000 dilution. The enzyme activity of the bound conjugate was detected by 0.05% ρ-nitrophenyl phosphate (Sigma) in 0.1 M diethanolamine buffer, pH 9.8, and was measured at 405 nm.
The titer of S. venezuelensis filiform larvae (L3 antigen)-specific IgE in BAL fluid was also estimated by an ELISA method. Ninety-six-well plates (Nunc Maxisorp) were coated with 5 μg of total L3 antigen/ml in carbonate buffer and were blocked with 1% BSA in PBS. Serial dilutions of BAL fluid (1:10 to 1:1,280) collected from rats at different time points were then tested. The end point was the BAL fluid dilution in which the absorbance was equal to or more than twice the average absorbance observed with the uninfected BAL fluid. Bound specific IgE was detected with 2 μg of anti-rat IgE (MARE-1; Serotec)/ml followed by 1 μg of biotin-conjugated rabbit anti-mouse IgG/ml, F(ab)′2 fragment (clone STAR11B; Serotec), a 1:2,000 dilution of streptavidin conjugated to alkaline phosphatase, and substrate as described above. BAL fluid collected 5 days after multiple infections was also used to develop a nitrocellulose membrane containing total L3 or adult worm antigens. BAL IgE bound to parasite antigens was detected after incubation with mouse MAb anti-rat IgE-clone B5 (14), followed by biotin-conjugated rabbit anti-mouse IgG, F(ab)′2 fragment (clone STAR11B; Serotec), streptavidin conjugated to horseradish peroxidase (Gibco BRL), and diaminobenzidine substrate solution
Immunoprecipitation of IgE in BAL.
BAL fluid recovered 5 days after the last infection of multiple-infection group rats was immunoprecipitated with mouse MAb anti-rat IgE-clone A2 (14) conjugated to CNBr-activated Sepharose 4 Fast Flow (Amersham Pharmacia Biotech), as previously described by Negrão-Corrêa et al. (37). The A2-bound proteins were eluted by boiling the beads with electrophoresis sample buffer (10 mM Tris, 1 mM EDTA, 2.5% sodium dodecyl sulfate, bromophenol blue, pH 8.0), and the supernatants were collected after centrifugation to remove the Sepharose beads (200 × g for 5 min). A sample of the supernatant was loaded onto a sodium dodecyl sulfate-10% polyacrylamide electrophoresis gel, and the eluted proteins were electrophoretically separated by using the Laemmli gel method under nonreducing conditions and a constant current of 15 mA in a Hoefer mini VE electrophoresis and electrotransfer system (Amersham Pharmacia Biotech, Buckinghamshire, England). After electrophoretic separation the gel proteins were transferred (240 mA for 2 h at 4°C in a Hoefer mini VE) to nitrocellulose membranes. The membranes were blocked with PBS-BSA, and the presence of IgE was detected with mouse MAb anti-rat IgE-clone B5, as described above.
Quantification of cytokines in BAL.
Concentrations of IL-4, IL-6, IL-10, IL-1β, and tumor necrosis factor α (TNF-α) in BAL and in lung tissue homogenates were measured by an ELISA method described in previously published works (17, 48). Sheep anti-rat protein antibodies and standards have been kindly provided by Steve Poole, National Institute of Biological Standards and Control, Potters Bar, United Kingdom. Known concentrations of the recombinant proteins (recombinant TNF-α, IL-1β, IL-10, IL-6, and recombinant IL-4) were used to generate a standard curve to convert optical density readings of samples to picograms per milliliter.
Statistical analysis.
Data are reported as means ± standard errors of the means and were analyzed by using Students' t test (two groups) or one-way analysis of variance. In the latter analysis, P values were assigned by using a Student-Neuman-Keuls test. Differences in P values of <0.05 were considered significant.
RESULTS
Kinetics of S. venezuelensis infection.
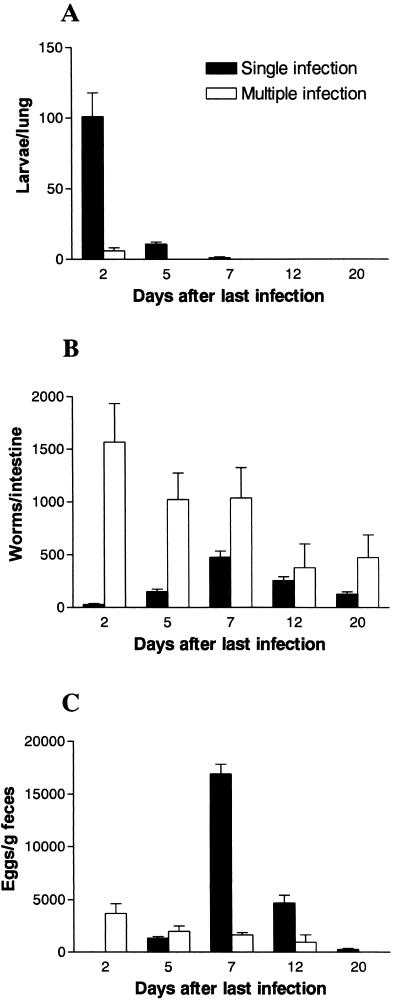
In order to determine the correlation of parasite migration through the lungs and arrival at the intestine with the pathological and functional changes in the lungs, initial studies evaluated the kinetics of S. venezuelensis infection under our experimental conditions. Most S. venezuelensis larvae migrated through the rat lung around 48 h after subcutaneous infection (Fig. 1A). In the single-infection group of rats, an average of 85.6 ± 16.5 live larvae were recovered from the lungs, and a small number of larvae were still recovered after 5 and 7 days postinfection (dpi). The number of live larvae recovered from the lungs of the multiple-infection rats was very low and was observed only at 2 days post-last infection (dpli).
FIG. 1.
Kinetics of S. venezuelensis infection following single (one subcutaneous inoculation of 1,500 L3 larvae) or multiple (five subcutaneous inoculations of 1,500 L3 larvae, 1 week apart) infections in rats. Shown are numbers of parasite larvae from lung (A), worms from small intestine (B), and eggs recovered from feces (C) at 2, 5, 7, 12, and 20 days after single infection or after the last infection of the multiple-infection rats. Each value represents mean ± standard error of the mean of five rats in each group. The experiment was repeated three times with similar results.
The first few worms were recovered from the small intestine at 2 dpi, reaching the maximum number around 7 dpi (475.3 ± 59.3) and starting to decline at 12 dpi (Fig. 1B). After multiple infection, rats showed higher numbers of adult worms in the small intestine at 2 and 5 dpli; however, the number of worms decreased faster than it did after single infection (Fig. 1B), and fecundity rates, as assessed by the number of eggs in the feces, were much lower (Fig. 1C).
Blood and bone marrow leukocytes.
In both groups of S. venezuelensis-infected animals there were only small changes in the concentration of total blood leukocytes (Table 1). However, the concentration of blood eosinophils increased significantly around 5 dpi (Table 1). Similarly, the concentration of bone marrow leukocytes changed little throughout the course of a single infection, but there was a significant increase in eosinophil numbers at 2 dpi (Table 1). After multiple infection, bone marrow had greater concentrations of total leukocytes and eosinophils than after single infection or no infection (Table 1). Indeed, the number of eosinophils in the bone marrow of rats submitted to multiple infection was at least 10 times greater than that for the noninfected controls. Moreover, the increase in eosinophil numbers was significantly greater and more persistent than that for rats receiving a single infection (Table 1).
TABLE 1.
Total number of leukocytes and eosinophils in blood, bone marrow, and BAL fluid after single and multiple S. venezuelensis infection of ratsa
| Cell type and days postinfection | No. after single infection
|
No. after multiple infection
|
||
|---|---|---|---|---|
| Leukocytes | Eosinophils | Leukocytes | Eosinophils | |
| Blood | ||||
| Uninfected | 795 ± 76 | 2 ± 1 | ||
| 2 | 789 ± 98 | 14 ± 4 | 1,070 ± 119 | 15 ± 6 |
| 5 | 806 ± 124 | 46 ± 5 | 807 ± 61 | 11 ± 3 |
| 7 | 929 ± 74 | 14 ± 5 | 925 ± 94 | 30 ± 12 |
| 12 | 458 ± 26.8 | 5.6 ± 2.5 | 1,038 ± 118 | 6 ± 3 |
| Bone marrow | ||||
| Uninfected | 261 ± 33 | 4.0 ± 1.4 | ||
| 2 | 342 ± 65 | 19.0 ± 4.5 | 1,310 ± 81 | 67.6 ± 3.0 |
| 5 | 162 ± 33 | 3.0 ± 1.3 | 892 ± 98 | 10.5 ± 6.3 |
| 7 | 400 ± 69 | 4.3 ± 0.4 | 542 ± 66 | 38.4 ± 11.0 |
| 12 | 357 ± 9.9 | 6.4 ± 1.3 | 1,140 ± 320 | 40.4 ± 29.0 |
| BAL | ||||
| Uninfected | 28.8 ± 4.9 | 0 | ||
| 2 | 133.2 ± 19.0 | 3.3 ± 1.1 | 77.7 ± 12.9 | 2.1 ± 1.4 |
| 5 | 125.7 ± 22.8 | 15.3 ± 4.7 | 47.7 ± 13.5 | 2.7 ± 1.4 |
| 7 | 106.0 ± 14.1 | 1.5 ± 0.5 | 33.1 ± 3.9 | 0.9 ± 0.4 |
| 12 | 25.9 ± 10.0 | 0 | 42.5 ± 10.2 | 0.2 ± 0.1 |
Each value represents the means ± standard errors of the means of 10 to 12 rats from three experiments. Bold numbers represent cell numbers statistically significant (P < 0.05) from the numbers observed in the uninfected rats. Results are given as 104 cells/ml of blood, 105 cells/marrow, and 104 cells in BAL.
Lung inflammation.
Larvae migration through the lungs produced an increase in the number of cells recovered in BAL fluid (Table 1). After single infection, the concentration of total leukocytes peaked at 2 dpi and at 5 to 7 dpi, with a trough between these two peaks. Leukocytes had returned to basal levels at 12 dpi (Table 1). After multiple infection, the concentration of leukocytes in BAL fluid also peaked at 2 dpli, but this peak was of lower intensity than that observed after single infection and cells returned to basal levels thereafter (Table 1). The cells recovered from BAL were mononuclear in their majority, but there was an increase in eosinophils observed at 5 dpi and at 2, 5, and 7 dpli (Table 1). Fluorescence-activated cell sorter analysis of leukocytes from a pool (n = 4 animals) of BAL fluids recovered 5 dpi showed that 5% of the mononuclear cells were B lymphocytes (labeled by MRC OX-33, mouse anti-rat CD45RA), 38% were T lymphocytes (CD3+ cells), and 32% were CD3+ CD4+ T lymphocytes. Over 97% of the CD4+ T lymphocytes did not express the CD45RC antigen (not labeled with MRC OX-22).
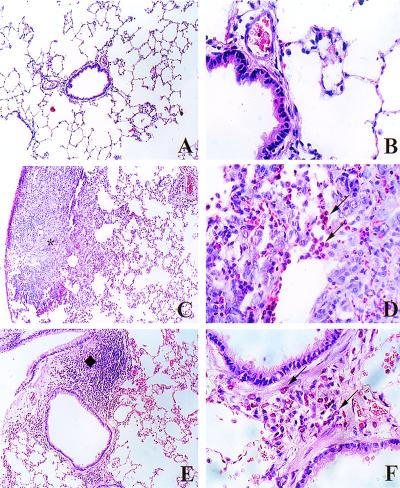
After single infection there were several foci of inflammatory cells at the pulmonary parenchyma (Fig. 2C), with noticeable eosinophil infiltration (Fig. 2D). In addition to the eosinophilic inflammation, migration of S. venezuelensis larvae through the lungs produced severe hemorrhage, pulmonary edema, and destruction of the alveolar wall (Fig. 2C) but produced little peribronchial inflammation. In animals submitted to multiple infections the reaction in the pulmonary parenchyma was a mild, diffuse cellular infiltration. In the latter group the cellular infiltration was especially intense around the bronchial tree, with a large number of eosinophils (Fig. 2F) and an increase in bronchial mucosa-associated lymphoid tissue (Fig. 2E). Infected rats also showed a thickening of the bronchial epithelial and muscle layer, a disruption and shedding of epithelial cells, an increase in the number of goblet cells, and an increase in mucus production, which was more evident after multiple infections (Fig. 3).
FIG. 2.
Histopathology of lungs after single or multiple infection of rats with S. venezuelensis. (A and B) Sections of lungs obtained from uninfected rats. Note in panels C and D the marked focal and eosinophilic inflammatory infiltrate in the lung parenchyma (indicated by an asterisk) at 5 days after single infection. Five days after the last infection of the multiple-infection rats (E and F) there were less inflammatory foci in the lung parenchyma, but there was a marked eosinophilic infiltrate around the bronchial tree and an increase in bronchial mucosa-associated lymphoid tissue (⧫). Arrows indicate eosinophils. Tissue was fixed with buffered formalin and was embedded in paraffin, and 5-μm sections were stained with hematoxylin-eosin (magnification of panels A, C, and E, ×100; magnification of panels B, D, and F, ×400).
FIG. 3.
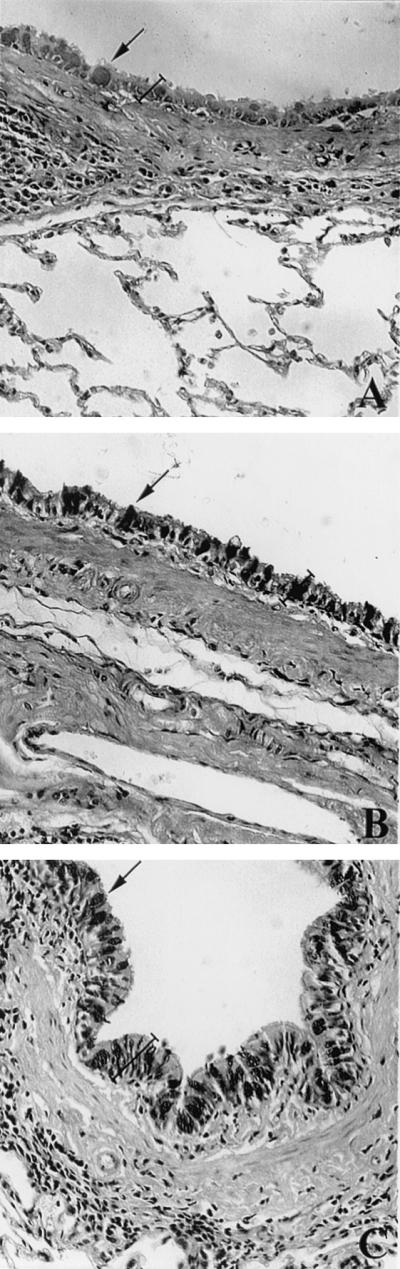
Goblet cells and mucus production at the bronchial epithelial layer of S. venezuelensis-infected rats. Sections of lung tissue from uninfected rats (A) and from rats 5 days after single infection (B) or 5 days after the last infection for multiple-infection rats (C). Tissue was fixed with buffered formalin and was embedded in paraffin, and 5-μm sections were stained with Alcian-Blue Safranin, pH 2.5. Note the increased number of goblet cells filled with mucus (dark areas indicated by arrows) on the epithelial layer and the epithelial layer (labeled with a bracket) after single and multiple infection with S. venezuelensis. Magnification, ×200.
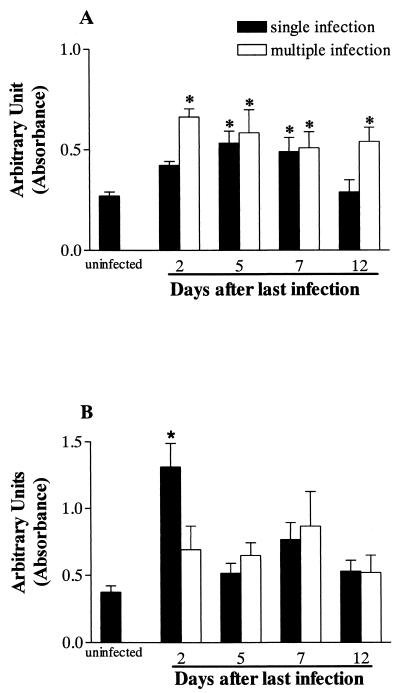
The increase in the number of eosinophils in lung tissue was confirmed by the measurement of EPO. Compared to that of uninfected animals there was a significant increase in EPO activity for single-infection animals at 5 and 7 dpi and in all time periods assessed after multiple infection (Fig. 4A). The concentration of free EPO in the BAL fluid, an index of eosinophil degranulation, is shown in Fig. 4B. There was a significant increase in EPO activity only after 2 dpi.
FIG. 4.
Levels of EPO in lung tissue (A) and BAL fluid (B) after single or multiple S. venezuelensis infection in rats. EPO was measured by using a colorimetric assay as described in Materials and Methods. Each data point represents the means ± standard errors of the means of 10 to 12 rats from three experiments. An asterisk indicates a P value of <0.05 compared to that of uninfected controls.
Total and parasite-specific IgE in BAL fluid.
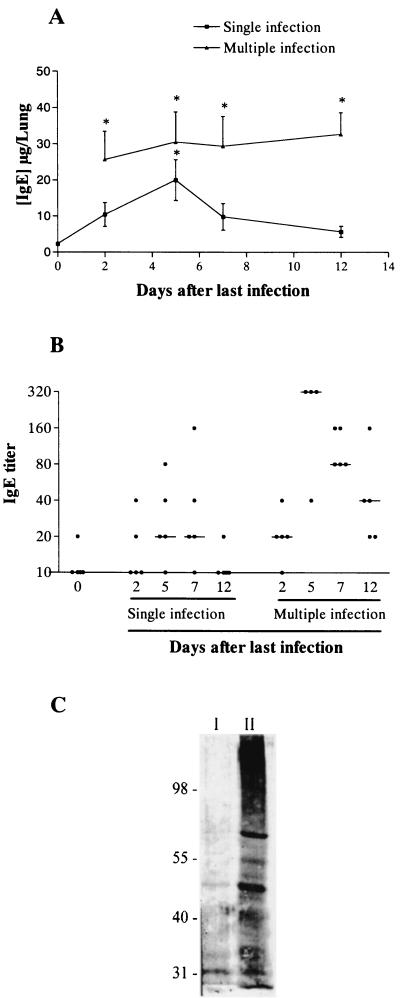
Total IgE was very low in BAL fluid of uninfected rats (Fig. 5A). After single infection with S. venezuelensis, there was a rapid increase in total IgE that reached maximum values at 5 dpi and returned to baseline at 12 dpi (Fig. 5A). After multiple infections, total IgE concentrations were greater than those found after single infection, and they persisted throughout the observation period (Fig. 5A). Immunoblotting analysis of BAL fluid from animals 5 dpli that was immunoprecipitated in an anti-rat IgE Sepharose column showed a protein band of approximately 190 kDa that coincided with the molecular size of intact rat IgE. Two other protein bands (around 60 and 30 kDa) were identified by anti-IgE antibodies in the immunoprecipitated sample (data not shown). The latter results confirm the presence of intact and degraded IgE in the BAL fluid of multiple-infection rats.
FIG. 5.
Total and specific IgE levels in BAL fluid after single or multiple S. venezuelensis infection of rats. (A) Concentration of total IgE in BAL fluid before and at 2, 5, 7, and 12 days after the single infection or the last infection of the multiple-infection rats. Each data point represents the means ± standard errors of the means of five rats in each group. The experiment was repeated three times with similar results. An asterisk indicates a P value of <0.05 compared to that of uninfected controls. (B) Titer of parasite-specific IgE in BAL fluid before and at 2, 5, 7, and 12 days after the single infection or the last infection of the multiple-infection rats. Each data point represents the value of one individual animal, and the line represents the median titer obtained at the same day of infection. (C) Proteins from S. venezu-elensis adult worm antigen (line I) and larvae antigen (line II) recognized by IgE from BAL fluid of 5-day multiple-infection rats. Parasite antigens were electrophoretically separated, transferred to nitrocellulose membrane, incubated with BAL fluid of infected rats, and developed with anti-rat IgE antibody, as detailed in Materials and Methods.
The titer of parasite-specific IgE as determined by ELISA showed a slight elevation between 5 and 7 days after single infection. After multiple infection, however, the titer of parasite-specific IgE was significantly elevated at 5 dpli. Antibody titers decreased but remained higher than those of the uninfected control at 7 and 12 dpli (Fig. 5B). Moreover, the IgE present in BAL fluid collected at 5 dpli strongly recognized proteins of different molecular weights of total S. venezuelensis larvae antigen but recognized much less from adult worm antigen (Fig. 5C).
Cytokines in BAL and lung.
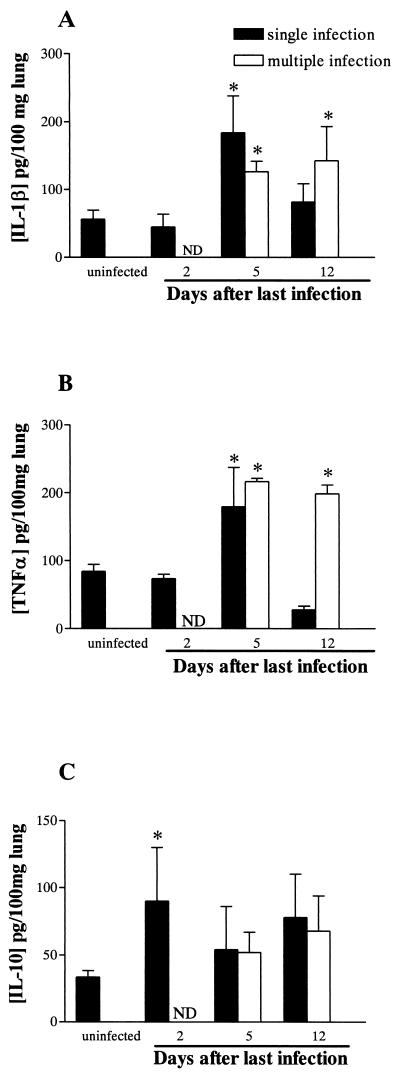
In BAL fluid, the concentration of cytokines after single or multiple infection was below the detection limit of the assay (data not shown). After single infection there was an increased concentration of IL-1β and TNF-α in lung tissue homogenates recovered 5 dpi that decreased to basal levels after 12 days. Moreover, after multiple infections the concentrations of IL-1β and TNF-α in lung tissue increased at 5 dpli and were still elevated at 12 dpli (Fig. 6A and B). In contrast, IL-10 concentrations were significantly elevated at 2 dpi and declined at 5 dpi or 5 dpli (Fig. 6C). The concentration of IL-4 was below the detection limit of the assay after single or multiple infection, and IL-6 levels in lung tissue were not altered during the course of infection or reinfection (data not shown).
FIG. 6.
Levels of IL-1β (A), TNF-α (B), and IL-10 (C) in lung tissue homogenate recovered before and at 2, 5, and 12 days after thesingle infection or the last infection of the multiple-infection rats. Cytokine levels were measured by ELISA as described in Materials and Methods. Each data point represents the means ± standard errors of the means of five rats. An asterisk indicates a P value of <0.05 compared to that of uninfected controls.
Evaluation of AHR.
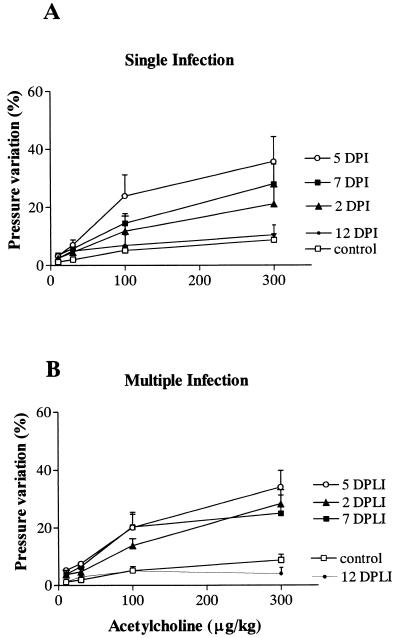
To determine whether lung inflammation induced by S. venezuelensis infection was associated with changes in lung function, the variation of intratracheal pressure in response to increasing doses of acetylcholine was evaluated after single and multiple infection. Five and 7 days after a single infection there was a significant leftward shift of the dose-response curve to acetylcholine compared to that for uninfected animals (Fig. 7A). At 2 dpi, responses were similar to those of uninfected rats, and the AHR had returned to baseline by 12 dpi (Fig. 7A). After multiple infections there was a slight shift in the dose-response curve to acetylcholine that started 2 dpli and peaked at 5 to 7 dpli. Similar to that for the single-infection group of animals, AHR also returned to baseline after 12 dpli (Fig. 7B).
FIG. 7.
AHR after single (A) or multiple (B) S. venezuelensis infection in rats. The change in intratracheal pressure in response to acetylcholine was used as a measurement of AHR in control and S. venezuelensis-infected rats (at 2, 5, 7, and 12 days after the last infection). Each data point represents the means ± standard errors of the means of 10 to 12 rats from three experiments. The values obtained at 5 days postinfection (A) and at 2, 5, and 7 days after reinfection (B) were statistically different (P ≤ 0.05) from values obtained from uninfected rats.
DISCUSSION
S. venezuelensis is a gastrointestinal nematode parasite that is natural in rats and has an obligatory migratory phase through the lungs before establishing itself in the small intestinal mucosa (50). Strongyloides spp.-infected rodents show increased levels of serum IgE (28), tissue and blood eosinophilia (41), and intestinal mast cell and goblet cell hyperplasia (24, 26, 35) that have been associated with host protection. However, much less is known of the pathological, immunological, and functional changes which occur in the lungs of rodents infected with S. venezuelensis.
Our data demonstrate that, after a single infection of rats with S. venezuelensis, there were pathological changes in the lungs that were typical of a Th2-predominant immune response. At 5 dpi, just after most of the parasite larvae had migrated through the lungs and reached the intestine, there was a marked pulmonary eosinophilic inflammatory process, mucus production, and increased levels of IgE in BAL fluid. Moreover, the majority of BAL CD4+ lymphocytes were CD4+ CD45RC−, an antigenic marker that subdivides the T-helper cells of rats into two functionally distinct subsets in which CD45RC− T cells (OX-22-negative T cells) have a typical Th2 function (43, 49).
Lung inflammation, bronchoalveolar leukocytosis with significant elevation of CD4+ CD45RC− T lymphocytes, and increased local IgE levels have also been reported for rats infected with N. brasiliensis, nematode parasites that possess an obligatory migration through the host lungs between 48 and 72 h after infection (44, 45, 46). However, the kinetics of the increase in the concentration of IgE and cellular infiltration detected in BAL fluid of N. brasiliensis-infected rats occurred at a later stage than that observed after S. venezuelensis infection. It is important to note that N. brasiliensis larvae reach the lungs through blood circulation (8), while S. venezuelensis larvae get into rat lungs by migrating through subcutaneous tissue (50). These different migratory routes may be relevant to and may explain the differences in lung immunopathology after infection by these two parasites.
After multiple infections, S. venezuelensis-infected rats showed no evident granulomatous reaction in the pulmonary parenchyma, as has been reported for rats after secondary N. brasiliensis infection (46). The cellular infiltration after multiple infections was more diffuse and was mainly peribronchial. Moreover, the number of eosinophils in lung tissue was elevated throughout the infection with evidence of degranulation, as assessed by the concentrations of EPO in lung tissue and BAL fluid. The different pattern of the inflammatory reaction after multiple infection could be explained by the repetitive antigenic stimulation resulting from weekly infections. Of interest, a similar peribronchial eosinophilic inflammation is characteristic of the pathological changes observed in lungs of asthmatic patients (19, 47). In addition, repetitive parasite infection also induced thickening of the bronchial epithelial layer and shedding of epithelial cells, pathological findings that also are observed in asthmatic patients.
In contrast to the total IgE levels, parasite-specific IgE titer was high only around 5 dpi. A sharp increase in parasite-specific IgE levels followed by fast decline was also observed in intestinal washes of Trichinella spiralis-infected rats (37, 38). The presence of IgE in BAL fluid of S. venezuelensis-infected rats might be explained by an increased leakage of serum contents, including immunoglobulin, or by the local production and secretion of IgE. Although a comparative study between serum versus BAL fluid is necessary to establish the actual source of IgE, the presence of large numbers of B cells (B220+) and the early appearance and high titers of larvae-specific IgE in BAL fluid are consistent with the local production and secretion of IgE. In this respect, nematode infection in rats induced early B-cell switches to IgE-producing cells in regional lymph nodes of gut and lungs (33) or in intestinal lamina propria (10). Moreover, there is also experimental evidence demonstrating that IgE present in intestinal washes of T. spiralis-infected rats (37) and in BAL fluid of N. brasiliensis-infected rats (44) was produced locally rather than systemically. For T. spiralis-infected rats, parasite-specific intestinal IgE has been associated with host protection (36, 38). Whether the IgE found in BAL fluid in our experimental system also plays a role in protection against S. venezuelensis infection in rats deserves further investigation. Of note, the number of live larvae recovered from the lungs after five larvae inoculations was significantly lower than that after single infection of rats, suggesting that the lung might be a site of larvae attrition.
Although the lung immunopathology observed in S. venezuelensis-infected (single or multiple infection) rats is typical of the Th2 type of response, we were unable to detect significant concentrations of IL-4 in BAL fluid or lung tissue homogenate by ELISA. One possibility to explain our inability to detect IL-4 could lie in the increase in IL-4 receptors on lung leukocytes. In this regard, we observed an increase in the numbers of Th2-like T cells in lung tissue. Further studies evaluating the expression of IL-4-positive cells in lungs of S. venezuelensis-infected rats are presently in progress in our laboratory.
The significant increase in IL-1β and TNF-α concentrations coincided with the peak of lung inflammation observed in infected rats. Increase in these proinflammatory cytokines has also been reported for allergic asthma, mainly as a consequence of mast cell stimulation (53). TNF-α is known to regulate the expression of adhesion molecules on vascular endothelium and leukocytes (40), and it may be involved in homing of Th2 cells to sites of allergic inflammation (12) and in regulating Th2 cytokine-mediated immune response at mucosal sites (3). The role of TNF-α in helminth infection is still unclear. However, for Trichuris muris-infected mice, treatment with anti-TNF-α MAb or TNF-α receptor gene-deficient mice showed that this cytokine is critical in mediating host protection to the parasite via an IL-13-dependent and IL-4-independent mechanism (3). In T. spiralis-infected mice, TNF-α was associated with the intestinal pathology but not with protective immune response against the parasite (29).
We observed a marked mucus production, as assessed histologically, in the lungs of animals after single or multiple infection, especially in the latter. Of interest, IL-1β (11) and TNF-α (30), cytokines shown to be elevated in our system, have been associated with mucus secretion by duodenal epithelial goblet cells and by human airway epithelial cells, respectively. More recently, Temann et al. (51) demonstrated that IL-4 enhanced the synthesis and release of mucus glycoprotein into the airway lumen by upregulating the MUC5AC gene. Production and secretion of mucins, such as sulfomucin, by gut goblet cells has been associated with the elimination of N. brasiliensis (25) and S. venezuelensis (24) from the gut. On the other hand, excessive production of airway mucus glycoproteins is also found in the lungs of asthmatic patients and has been associated with airflow limitation (19). Whether mucus production in our model is associated with protection or changes in lung function clearly deserves further investigation.
One interesting finding was the elevated concentration of IL-10 in lung tissue observed during the phase of larvae migration through the tissue (2 dpi). Of note, IL-10 has been shown to play an important role in the control of eosinophilic inflammation and AHR in animals with asthma (54). Whether the elevated levels of IL-10 observed in our model contribute functionally to the lack of increase of leukocytes, inflammatory cytokines and AHR when parasites are migrating through the lungs clearly deserve further investigation.
In addition to the inflammatory process observed in the lungs, single or multiple S. venezuelensis infection in rats resulted in reversible AHR. Of note, significant increase in AHR coincided with the peak of cytokine (TNF-α), BAL fluid IgE, and lung eosinophilia. Although after multiple infection animals had a more persistent antigenic stimulation with ensuing peribronchial inflammation, there did not appear to be a stronger or longer-lasting AHR compared to that of the rats that were infected once. Only two other studies have previously reported AHR following parasitic infection, and both were carried out with mice (15, 23). The limitations of studies of parasite and allergic inflammation in mice cannot be dismissed, as eosinophils from these species do not appear to express high-affinity IgE receptors and do not undergo IgE-dependent cytotoxicity (18). In Brugia malayi-immunized and -challenged mice (a nonnatural host of B. malayi), eosinophils comprised 84% of leukocytes recovered from the BAL fluid, and both eosinophilia and AHR were dependent on IL-5 (23). In this study, mice were immunized with dead parasites and then were challenged intravenously with live microfilariae (23). In N. brasiliensis-infected mice, IL-5 was responsible for the lung eosinophilia and damage but not for the AHR (15). In the latter experiments, lung inflammation was protracted and peaked 13 days after infection, a time long after the migration of parasites through the lungs, possibly reflecting the heterogeneity of the system, i.e., infection of mice with a nonnatural parasite. Thus, our results are the first to show that AHR can indeed occur after infection of a natural host (rat) with one of its natural helminth parasites (S. venezuelensis). In our model, AHR was coincident with the peak of lung eosinophilic inflammation, mucus and proinflammatory cytokine production, and local IgE increase. However, further studies are necessary to examine the precise role of each of these pathological and immunological alterations for the development of the functional changes in the airway. In this regard, although pathological changes typical of asthma were found more characteristically after multiple infections, significant AHR was also observed after a single infection.
Eosinophilic peribronchial inflammation of the lung, excess mucus production, local IgE, and bronchial hyperresponsiveness as induced by S. venezuelensis infection are characteristic changes of the lungs of asthmatic patients (4, 19, 53). There are many debates in the literature as to whether these pathological, immunological, and functional changes observed in the asthmatic population have evolved as a mechanism of protection against helminth parasites. For example, S. stercoralis-infected asthmatic patients showed decreases in IgE levels and eosinophilia and showed improvement in the number of episodes of bronchospasm after parasite treatment, but no difference was observed with spirometric parameters (16). Similarly, clinical improvement and no alteration in spirometric parameters were reported for an asthmatic population with a high prevalence of A. lumbricoides infection after albendazol treatment (32). In contrast, anti-helminth treatment of children living in areas where helminth infection is endemic resulted in increased levels of specific IgE in serum against environmental allergens and immediate-hypersensitivity skin tests (31). Therefore, there is a need for experimental models that examine the relevance of the allergic asthmatic reaction to the course of helminth infection. Moreover, there is a need to understand the precise mechanisms which lead (or do not lead) to changes in airway function following helminth infection. Due to the nature of the physiopathological alterations reported here, we propose that this experimental model is ideal to carry out further studies on immunoprotection against nematode infection versus immunopathology of allergic diseases, such as asthma.
Acknowledgments
We are grateful to the Fundação de Amparo a Pesquisa do Estado de Minas Gerais, Conselho Nacional de Desenvolvimento Científico e Tecnológico, Programa de Apoio ao Desenvolvimento Científico e Tecnológico, and the Wellcome Trust for financial support.
Editor: J. M. Mansfield
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T Lymphocytes. Nature (London) 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Allen, J. E., and R. M. Maizels. 1996. Immunology of helminth infection. Int. Arch. Allergy Immunol. 109:3-10. [DOI] [PubMed] [Google Scholar]
- 3.Artis, D., N. E. Humphreys, A. J. Bancroft, N. J. Rothwell, C. S. Potten, and R. K. Grencis. 1999. Tumor necrosis factor α is a critical component of interleukin 13-mediated protective T helper cell type 2 responses during helminth infection. J. Exp. Med. 190:953-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bousquet, J., P. K. Jeffery, W. W. Busse, M. Johnson, and A. M. Vignola. 2000. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am. J. Respir. Crit. Care Med. 161:1720-1745. [DOI] [PubMed] [Google Scholar]
- 5.Brener, Z., and G. Chaia. 1960. Isolamento e manutenção do Strongyloides ratti (Sandground, 1925) em condições de laboratório. Rev. Brasil. Biol. 20:447-451. [Google Scholar]
- 6.Bundi, D. A. 1994. Immunoepidemiology of intestinal helminthic infections. 1: The global burden of intestinal nematode disease. Trans. R. Soc. Trop. Med. Hyg. 88:259-261. [DOI] [PubMed] [Google Scholar]
- 7.Cara, D. C., D. Negrão-Corrêa, and M. M. Teixeira. 2000. Mechanisms underlying eosinophil trafficking and their relevance in vivo. Histol. Histopathol. 15:899-920. [DOI] [PubMed] [Google Scholar]
- 8.Clarke, K. R. 1967. The migration route of the third stage larvae of Nippostrongylus brasiliensis (Travassos, 1914). J. Helminthol. 41:285-290. [DOI] [PubMed] [Google Scholar]
- 9.Chan, M.-S. 1997. The global burden of intestinal nematode infections. Fifty years on. Parasitol. Today 13:438-443. [DOI] [PubMed] [Google Scholar]
- 10.Ching, H. W., E. M. Richards, and R. G. Bell. 1999. Rapid anti-helminthic response of B-lymphocytes in the intestinal mucosal tissues of rats. Cell. Immunol. 193:59-70. [DOI] [PubMed] [Google Scholar]
- 11.Cohan, V. L., A. L. Scott, and C. A. Dinarello. 1991. Interleukin-1 is a mucus secretagogue. Cell. Immunol. 136:425-434. [DOI] [PubMed] [Google Scholar]
- 12.Cohn, L., R. J. Homer, A. Marinov, J. Rankin, and K. Botmly. 1997. Induction of airway mucus production by T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J. Exp. Med. 186:1737-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins, P. D., S. Marleau, D. A. Griffiths-Johnson, P. J. Jose, and T. J. Williams. 1995. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J. Exp. Med. 182:1169-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conrad, D. H., E. Studer, J. Gervasoni, and T. Mohanakumar. 1983. Properties of two monoclonal antibodies directed against the Fc and Fab' regions of rat IgE. Int. Arch. Allergy Appl. Immunol. 70:352-360. [DOI] [PubMed] [Google Scholar]
- 15.Coyle, A. J., G. Köhler, S. Tsuyuki, F. Brombacher, and M. Kopf. 1998. Eosinophils are not required to induce airway hyperresponsiveness after nematode infection. Eur. J. Immunol. 28:2640-2647. [DOI] [PubMed] [Google Scholar]
- 16.Cremades Romero, M. J., C. Pellicer Ciscar, R. Menendez Villanueva, C. Ricart Olmos, A. Pastor-Guzman, F. Estelles Piera, R. Igual Adell, and M. J. Gilabert Bonet. 1997. Strongyloides stercoralis infection in patients with bronchial obstructive pathology. Arch. Bronconeumol. 33:384-388. [DOI] [PubMed] [Google Scholar]
- 17.Cunha, J. M., F. Q. Cunha, S. Poole, and S. H. Ferreira. 2000. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. Br. J. Pharmacol. 130:1418-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Andres, B., E. Rakasz, M. Hagen, M. L. McCormik, A. L. Mueller, D. Elliot, A. Metwali, M. Sandor, B. E. Britigan, J. V. Weinstock, and R. G. Lynch. 1997. Lack of Fc-ɛ receptors on murine eosinophils: implications for the functional significance of elevated IgE and eosinophils in parasitic infections. Blood 89:3826-3836. [PubMed] [Google Scholar]
- 19.Djukanovic, R., W. R. Roche, J. W. Wilson, C. R. W. Beasley, O. P. Twentyman, P. H. Howarth, and S. T. Holgate. 1990. Mucosal inflammation in asthma. Am. Rev. Respir. Dis. 142:434-457. [DOI] [PubMed] [Google Scholar]
- 20.Dombrowicz, D., B. Quatannens, J. P. Papin, A. Capron, and M. J. Capron. 2000. Expression of a functional Fc epsilon RI on rat eosinophils and macrophages. J. Immunol. 165:1266-1271. [DOI] [PubMed] [Google Scholar]
- 21.Finkelman, F. D., T. Shea-Donohue, J. Goldhill, C. A. Sullivan, S. C. Morris, K. B. Madden, W. C. Gause, and J. F. Urban, Jr. 1997. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu. Rev. Immunol. 15:505-533. [DOI] [PubMed] [Google Scholar]
- 22.Gounni, A. S., B. Lamkhioued, K. Ochial, Y. Tanaka, E. Delaporte, A. Capron, J. P. Kinet, and M. Capron. 1994. High-affinity IgE receptor on eosinophils is involved in defense against parasites. Nature (London) 367:183-187. [DOI] [PubMed] [Google Scholar]
- 23.Hall, L. R., R. K. Mehlotra, A. W. Higgins, M. A. Haxhiu, and E. Pearlman. 1998. An essential role for interleukin-5 and eosinophils in helminth-induced airway hyperresponsiveness. Infect. Immun. 66:4425-4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishikawa, N., B. B. Shi, A. I. Khan, and Y. Nawa. 1995. Reserpine-induced sulphomucin production by goblet cells in the jejunum of rats and its significance in the establishment of intestinal helminths. Parasite Immunol. 17:581-586. [DOI] [PubMed] [Google Scholar]
- 25.Ishikawa, N., Y. Horii, and Y. Nawa. 1993. Immune-mediated alteration of the terminal sugars of goblet cell mucins in the small intestine of Nippostrongylus brasiliensis-infected rats. Immunology 78:303-307. [PMC free article] [PubMed] [Google Scholar]
- 26.Khan, A. I., Y. Horii, Y. Tiuria, Y. Sato, and Y. Nawa. 1993. Mucosal mast cells and the expulsive mechanisms of mice against Strongyloides venezuelensis. Int. J. Parasitol. 23:551-555. [DOI] [PubMed] [Google Scholar]
- 27.Korenaga, M., Y. Hitoshi, N. Yamaguchi, Y. Sato, K. Takatsu, and I. Tada. 1991. The role of interleukin-5 in protective immunity to Strongyloides venezuelensis infection in mice. Immunology 72:502-507. [PMC free article] [PubMed] [Google Scholar]
- 28.Korenaga, M., Y. Nawa, and I. Tada. 1986. IgE response in Strongyloides ratti-infected rats with special reference to the life cycle of the parasite. Z. Parasitenk. 72:213-220. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence, C. E., J. C. M. Paterson, L. M. Higgins, T. T. MacDonald, M. W. Kennedy, and P. Garside. 1998. IL-4-regulated enteropathy in an intestinal nematode infection. Eur. J. Immunol. 28:2672-2684. [DOI] [PubMed] [Google Scholar]
- 30.Levine, S. J., P. Larivee, C. Logun, W. Angus, F. P. Ognibene, and J. H. Shelhmer. 1995. Tumor necrosis factor-alpha induces mucin hypersecretion and MUC-2 gene expression by human airway epithelial cells. Am. J. Respir. Cell. Mol. Biol. 12:192-204. [DOI] [PubMed] [Google Scholar]
- 31.Lynch, N. R., I. Hagel, M. Perez, M. C. DiPrisco, R. Lopez, and N. Alvarez. 1993. Effect of anthelmintic treatment on the allergic reactivity of children in a tropical slum. J. Allergy Clin. Immunol. 92:404-411. [DOI] [PubMed] [Google Scholar]
- 32.Lynch, N. R., M. Palenque, I. Hagel, and M. C. DiPrisco. 1997. Clinical improvement of asthma after anthelminthic treatment in a tropical situation. Am. J. Respir. Crit. Care Med. 156:50-54. [DOI] [PubMed] [Google Scholar]
- 33.Mayrhofer, G., H. Bazin, and J. L. Gowans. 1976. Nature of cells binding anti-IgE in rats immunized with Nippostrongylus brasiliensis: IgE synthesis in regional nodes and concentration in mucosal mast cells. Eur. J. Immunol. 6:537-545. [DOI] [PubMed] [Google Scholar]
- 34.McSharry, C., Y. Xia, C. V. Holland, and M. W. Kennedy. 1999. Natural immunity to Ascaris lumbricoides associated with immunoglobulin E antibody to ABA-1 allergen and inflammation indicators in children. Infect. Immun. 67:482-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mimori, T., Y. Nawa, M. Korenaga, and I. Tada. 1982. Strongyloides ratti: mast cell and goblet cell responses in the small intestine of infected rats. Exp. Parasitol. 54:366-370. [DOI] [PubMed] [Google Scholar]
- 36.Negrão-Corrêa, D. 2001. Importance of immunoglobulin E (IgE) in the protective mechanism against gastrointestinal namatode infection: looking at the intestinal mucosae. Rev. Inst. Med. Trop. S. Paulo 43:291-299. [DOI] [PubMed] [Google Scholar]
- 37.Negrão-Corrêa, D., L. S. Adams, and R. G. Bell. 1996. Intestinal transport and catabolism of IgE. A major blood-independent pathway of IgE dissemination during a Trichinella spiralis infection of rats. J. Immunol. 157:4037-4044. [PubMed] [Google Scholar]
- 38.Negrão-Corrêa, D., L. S. Adams, and R. G. Bell. 1999. Variability of the intestinal immunoglobulin E response of rats to infection with Trichinella spiralis, Heligmosomoides polygyrus or Nippostrongylus brasiliensis. Parasite Immunol. 21:287-297. [DOI] [PubMed] [Google Scholar]
- 39.Ogilvie, B. M., and D. de Savigny. 1982. Immune response to nematode, p. 715-757. In S. Cohen and K. S. Warren (ed.), Immunology of parasitic infections. Blackwell Scientific Publications, Oxford, England.
- 40.Osborn, L. 1990. Leukocyte adhesion to endothelium inflammation. Cell 62:3-6. [DOI] [PubMed] [Google Scholar]
- 41.Ovington, K. S., K. McKie, K. I. Matthaei, I. G. Young, and C. A. Behm. 1998. Regulation of primary Strongyloides ratti infections in mice: a role for interleukin-5. Immunology 95:488-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phills, J. A., A. J. Harrold, G. V. Whiteman, and L. Perelmutter. 1972. Pulmonary infiltrates, asthma and eosinophilia due to Ascaris suum infestation in man. N. Engl. J. Med. 286:965-970. [DOI] [PubMed] [Google Scholar]
- 43.Powrie, F., and D. Mason. 1988. Phenotypic and functional heterogeneity of CD4+ T cells. Immunol. Today 9:274-277. [DOI] [PubMed] [Google Scholar]
- 44.Ramaswamy, K., and D. Befus. 1993a. IgE antibody responses in bronchoalveolar spaces of rats infected with Nippostrongylus brasiliensis. Exp. Parasitol. 76:23-31. [DOI] [PubMed] [Google Scholar]
- 45.Ramaswamy, K., and D. Befus. 1993b. Pulmonary inflammation and immune responses during the course of Nippostrongylus brasiliensis infection: lymphocyte subsets in bronchoalveolar lavage fluids of rats. Parasite Immunol. 15:281-290. [DOI] [PubMed] [Google Scholar]
- 46.Ramaswamy, K., G. T. De Sanctis, F. Green, and D. Befus. 1991. Pathology of pulmonary parasitic migration: morphological and bronchoalveolar cellular responses following Nippostrongylus brasiliensis infection in rats. J. Parasitol. 77:302-312. [PubMed] [Google Scholar]
- 47.Roche, W. R. 1998. Inflammatory and structural changes in the small airways in bronchial asthma. Am. J. Respir. Crit. Care Med. 157:S191-S194. [DOI] [PubMed] [Google Scholar]
- 48.Souza, D. G., D. C. Cara, G. D. Cassali, S. F. Coutinho, M. R. Silveira, S. P. Andrade, S. Poole, and M. M. Teixeira. 2000. Effects of the PAF receptor antagonist UK74505 on local and remote reperfusion injuries following ischemia of the superior mesenteric artery in the rat. Br. J. Pharmacol. 131:1800-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spickett, G. P., M. R. Brandon, D. W. Mason, A. F. Williams, and G. R. Woollett. 1983. MRC OX-22, a monoclonal antibody that labels a new subset of T lymphocytes and reacts with the high molecular weight form of the leukocyte-common antigen. J. Exp. Med. 158:795-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takamure, A. 1995. Migration route of Strongyloides venezuelensis in rodents. Int. J. Parasitol. 25:907-911. [DOI] [PubMed] [Google Scholar]
- 51.Temann, U.-A., B. Prasad, M. W. Gallup, C. Basbaum, S. B. Ho, R. A. Flavell, and J. A. Rankin. 1997. A novel role for murine IL-4 in vivo: induction of MUC5AC gene expression and mucin hypersecretion. Am. J. Respir. Cell. Mol. Biol. 16:471-478. [DOI] [PubMed] [Google Scholar]
- 52.Wehner, J. H., C. M. Kirsch, F. T. Kagawa, W. A. Jensen, A. C. Campagna, and M. Wilson. 1994. The prevalence and response to therapy of Strongyloides stercoralis in patients with asthma from endemic areas. Chest 106:762-766. [DOI] [PubMed] [Google Scholar]
- 53.Wills-Karp, M. 1999. Immunologic bases of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 17:255-281. [DOI] [PubMed] [Google Scholar]
- 54.Zuany-Amorim, C., S. Haile, D. Leduc, C. Dumarey, M. Huerre, B. B. Vargaftig, and M. Pretolani. 1995. Interleukin-10 inhibits antigen-induced cellular recruitment into the airways of sensitized mice. J. Clin. Investig. 95:2644-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zurawski, G., and J. E. de Vries. 1994. Interleukin 13, an interleukin 4 like cytokine that acts on monocytes and B cells, but not on T cells. Immunol. Today 15:19-26. [DOI] [PubMed] [Google Scholar]