Abstract
Preclinical mouse infection models are widely used for Helicobacter vaccine development, but how well such models mimic important aspects of human infections is unknown. A comparison of Helicobacter pylori immunoproteomes of infected mice with previously reported patient data reveals a high agreement in the antigens recognized, suggesting that H. pylori in vivo protein composition and recognition by the host immune system are comparable in mice and humans. Murine Helicobacter models may thus be valid to screen antigens for human vaccination.
The gram-negative bacterium Helicobacter pylori is a major causative agent of chronic active gastritis as well as gastric and duodenal ulcers. Moreover, it contributes to the development of gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma. There has been extensive work to develop a Helicobacter vaccine. Most preclinical studies have been performed using mouse Helicobacter infection models because of the practical advantages of small-animal models (15). H. pylori does not normally colonize mice, but some mouse-adapted strains that can infect the murine stomach have been identified. Little work on in vivo gene expression of H. pylori has been done, but transcript levels of four important Helicobacter genes are similar for human biopsy samples and mouse samples (23). However, there is no detectable phase variation of Lewis antigen expression (27), and the important virulence factors CagA and VacA are lost during mouse colonization (11, 25). Pathological changes in the murine system include gastritis and in some cases follicle formation and even low-grade mucosa-associated lymphoid tissue lymphoma in the gastric mucosa (5, 17), whereas ulcer formation and adenocarcinoma have not been observed. Various vaccines that induce protective immunity against a Helicobacter challenge in the mouse model have been developed, but clinical trials have revealed a poor efficacy of such vaccines in humans (21), suggesting that murine Helicobacter models might be of limited value for vaccine development. The failure to transfer mouse vaccination strategies to humans could be due to potential differences in Helicobacter protein expression, antigen exposure to the host immune system, vaccine delivery, and protective immune mechanisms (8). To address the first two issues (protein composition and antigen exposure to the immune system,) which could particularly affect the screening of protective antigens, we compared the H. pylori immunoproteome in infected mice with previous data from infected human patients. Helicobacter antigens that induce specific antibody responses are obviously expressed in situ (4, 9, 26) and become exposed to the host immune system.
Female, 6- to 8-week-old, C57BL/6 mice were infected by three sequential oral inoculations of 5 × 109 H. pylori SS1 (16) cells as described previously (6). Mice were killed at 14 weeks postinfection, and H. pylori colonization was assessed by plating of stomach samples. Sera were obtained prior to infection and by terminal bleeding. Alternatively, mice were subcutaneously immunized with 550 μg of H. pylori P76 sonicate mixed with incomplete Freud's adjuvant and given two booster doses on days 14 and 28.
H. pylori SS1 proteins were resolved in two dimensions on small gels (7.0 by 8.5 cm) and blotted as described previously (12). The blots were incubated with mouse sera at a dilution of 1:200 and stained using a peroxidase-coupled polyvalent goat antibody to mouse immunoglobulins (Sigma) at a dilution of 1:10,000 and enhanced chemiluminescence detection (ECL kit; Amersham). To increase the detection range, films were exposed for 5 to 30 min. The 35 spots with the highest postinfection seroreactivity but low to nondetectable preinfection seroreactivity (specifically recognized) and the 14 spots with the highest preinfection seroreactivity (cross-reactive) were analyzed by matrix-assisted laser desorption ionization-mass spectrometry peptide mass fingerprinting using a minimum sequence coverage of 30% (12). Coomassie brilliant blue spot staining intensities were quantified using the gel analysis program TOPSPOT.
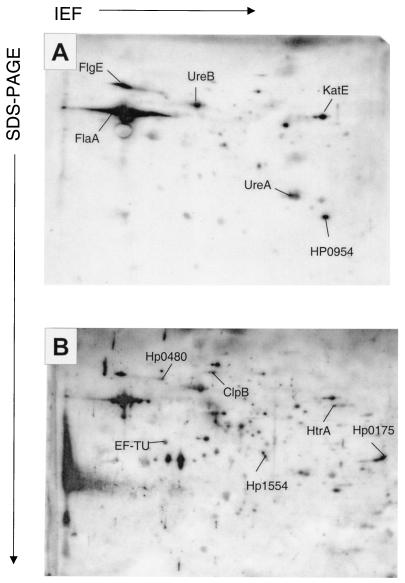
Among the several hundred detectable protein species of H. pylori strain SS1 (12), only a few were recognized by preinfection sera (Fig. 1A). Sera from the same individual mice obtained at 14 weeks postinfection reacted with a much larger number of proteins, and the overall staining intensity was higher (Fig. 1B), which agrees with previously published human data (7, 14, 19, 20). Despite the facts that all mice were from a genetically homogenous inbred background and all were infected with the same cultures of a single H. pylori strain, there were marked differences between the recognition patterns of the individual sera. Immunostaining intensities of each of the 587 recognized protein species were determined in mice using a semiquantitative scale and average values from eight infected mice. Of the 35 species that were most strongly recognized by sera from infected mice but not by preinfection sera (specific recognition), 31 species corresponding to 21 different proteins could be identified (Table 1). In addition to these specifically recognized proteins, 14 protein species that were already strongly recognized prior to infection (cross-reactive) were analyzed, resulting in the identification of 13 protein species that corresponded to 10 proteins (Table 1).
FIG. 1.
Typical immunoproteome of H. pylori strain SS1 as revealed by two-dimensional gel electrophoresis and immunoblotting with sera from the same mouse obtained either prior to infection (A) or 14 weeks after infection (B). The immunoblots were stained with a peroxidase-coupled antibody to mouse immunoglobulins followed by chemiluminescence detection. To enhance the detection of weakly cross-reactive antigens, film A was exposed for a longer time period (15 min) than film B (5 min). Abbreviations: SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; IEF, isoelectric focusing.
TABLE 1.
H. pylori antigens recognized by murine and human sera
| Antigen | Encoded proteina | Localizationb | Recognition
|
|||
|---|---|---|---|---|---|---|
| Mouse | Human
|
|||||
| A | B | C | ||||
| HP0010 | GroEL | ND | S | S | U | U |
| HP0011 | GroES | ND | - | - | S | - |
| HP0027 | Isocitrate dehydrogenase | ND | - | S | S | S |
| HP0072 | UreB | ND | U | U | U | U |
| HP0073 | UreA | Surf. | U | U | - | U |
| HP0109 | DnaK | ND | - | S | S | S |
| HP0115 | FlaB | ND | - | U | - | - |
| HP0153 | RecA | ND | - | - | S | - |
| HP0154 | Enolase | ND | - | S | - | - |
| HP0175 | Cell binding factor 2 | Sec., Surf. | S | S | - | - |
| HP0177 | EF-P | ND | - | - | S | - |
| HP0192 | FrdA | ND | S | - | - | S |
| HP0210 | HtpG | ND | S | - | - | - |
| HP0231 | Hypoth. protein | Sec., Surf. | - | - | - | S |
| HP0243 | NapA | ND | - | S | S | - |
| HP0264 | ClpB | ND | S | - | S | S |
| HP0305 | Hypo. protein | ND | - | - | - | U |
| HP0318 | Cons. hypo. protein | ND | U | - | S | U |
| HP0371 | FabE | ND | - | - | S | - |
| HP0399 | Rps1 | ND | S | - | - | - |
| HP0400 | LytB | ND | - | - | - | S |
| HP0410 | HpaA | Surf. | S | - | - | S |
| HP0480 | YihK | ND | S | - | - | - |
| HP0512 | GlnA | ND | - | - | S | - |
| HP0522 | Cag3 | ND | - | - | - | S |
| HP0537 | Cag16 | Surf. | - | S | - | U |
| HP0547 | CagA | ND | S | - | - | S |
| HP0589 | Ferredoxin oxidoreductase α | ND | - | U | - | - |
| HP0599 | HylB | ND | - | - | S | S |
| HP0601 | FlaA | ND | U | U | U | - |
| HP0632 | HydB | ND | S | - | - | - |
| HP0649 | AspA | ND | - | - | S | - |
| HP0691 | YxjD | ND | - | - | S | - |
| HP0752 | FliD | ND | - | - | S | - |
| HP0779 | AcnB | ND | - | - | S | - |
| HP0786 | SecA | ND | S | - | - | - |
| HP0794 | ClpB | ND | - | S | - | - |
| HP0795 | Trigger factor | ND | S | - | S | S |
| HP0829 | GuaB | ND | - | - | - | S |
| HP0870 | FlgE | Sec. | U | - | - | - |
| HP0875 | KatE | Surf. | U | S | - | U |
| HP0900 | HypB | ND | - | - | S | - |
| HP0912 | Omp20 | ND | - | S | - | - |
| HP0913 | Omp21 | ND | U | - | - | - |
| HP0954 | NAD(P)H nitroreductase | ND | U | - | - | - |
| HP1018 | Hypo. protein | ND | - | S | - | - |
| HP1019 | HtrA | Sec., Surf. | S | - | - | S |
| HP1037 | Cons. hypo. protein | ND | - | - | S | - |
| HP1098 | Cons. hypo. secreted protein | Sec., Surf. | - | - | - | U |
| HP1110 | Pyruvate ferredoxin oxidoreductase α | ND | - | S | S | - |
| HP1125 | Omp18 | ND | - | - | U | - |
| HP1132 | AtpD | ND | S | - | S | S |
| HP1134 | AtpA | ND | S | - | - | S |
| HP1152 | Ffh | ND | S | - | - | S |
| HP1173 | Hypo. protein | Sec. | S | - | - | - |
| HP1193 | Putative aldo-keto reductase | ND | U | - | - | - |
| HP1199 | RP L7/L12 | ND | - | U | U | U |
| HP1201 | RP L1 | ND | - | - | - | S |
| HP1205 | EF-TU | ND | S | U | U | S |
| HP1213 | Pnp | ND | S | - | - | - |
| HP1285 | Cons. hypo. protein | Surf. | - | - | - | U |
| HP1293 | RpoA | ND | - | - | S | U |
| HP1302 | RP S5 | ND | - | - | - | S |
| HP1307 | RP L5 | ND | - | - | - | S |
| HP1350 | Protease | Surf. | - | S | - | S |
| HP1379 | Lon | ND | S | - | - | - |
| HP1554 | RP S2 | ND | S | - | - | - |
| HP1555 | EF-Ts | ND | - | - | S | - |
| HP1563 | TsaA | ND | - | S | S | - |
| HP1564 | Outer membrane protein | Surf. | - | - | - | S |
| HP1582 | PdxJ | ND | U | - | - | U |
Among the total of 31 highly immunogenic proteins (21 specifically recognized and 10 cross-reactive) in the mouse model, 18 (58%) have been previously described as H. pylori antigens recognized by sera of infected patients, and similar levels of overlap are also observed for the specific and cross-reactive subsets, respectively (Table 1) (7, 14, 19, 20). Some differences between murine and human data sets are likely due to the different H. pylori strains. However, the level of agreement between the mouse data and the combined human data sets is still high compared to the rather large variation observed in the various human studies (only 34% of the seroreactive species were detected in more than one study). The majority of immunogenic Helicobacter antigens thus appears to be expressed both in infected murine and human stomachs and to be exposed to both immune systems (4, 9, 26).
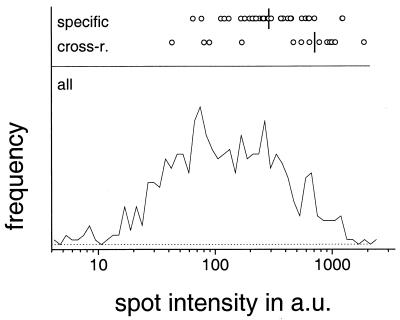
Abundant Helicobacter proteins are overrepresented among the seroreactive antigens (Fig. 2), suggesting that dose-dependent responses may influence the recognition pattern. An alternative explanation is that spots containing rather small amounts of antigen might bind to a small amount of antibody and thus escape detection. However, on the basis of the least abundant species that was strongly recognized on the immunoblots (spot 59, a protein species yet to be identified), it can be estimated that this technical limitation probably affects only a minority of rare species (Fig. 2).
FIG. 2.
Staining intensities of specifically recognized and cross-reactive (cross-r.) H. pylori antigens (top). The vertical lines represent the median values. For comparison, a histogram for the staining intensities of all detectable protein spots is shown at the bottom of the figure. Strongly immunogenic spots have higher staining intensities than those of all detectable spots (P < 0.001, t test), and cross-reactive species are significantly more intensely stained than specifically recognized species (P < 0.01, t test). a.u., arbitrary units.
The 32 identified secreted and/or surface-associated proteins (2, 24) represent only 2% of the total Helicobacter proteome but 13 (18%) of the 71 seroreactive antigens (Table 1), suggesting that antigen localization may influence antigenicity. Secreted proteins and surface-exposed proteins that are sequestered by vesicle budding (13) penetrate the mucosa (18) and may thus more easily gain access to inductive sites. Interestingly, a predominance of surface-associated proteins among seroreactive antigens has recently also been demonstrated for Staphylococcus aureus (4).
It was thought that immunoblotting would be a good way to select promising vaccine antigen candidates (10, 14, 19, 20), although cellular instead of humoral immune responses seem to be relevant for protection against H. pylori at least in the mouse model (1, 3, 22). Interestingly, the combined data from infected mice (this study) and differentially immunized mice (this study) (10) show that all known protective antigens can be recognized by antibodies (Table 2), supporting a correlation between seroreactivity and cellular immune responses which could be related to the fact that optimal antibody responses depend on help from T cells. Interestingly, many protective H. pylori antigens, including the well-characterized urease, are cross-reactive in noninfected mice and patients, suggesting that specificity is not a prerequisite for protective efficacy. A comparison between the recognition patterns of infected (nonprotected) versus lysate-immunized (protected) mice might yield interesting information about individual, potentially protective antigens, but our preliminary data indicate a large number of differentially recognized antigens, suggesting that relevant candidates might be difficult to identify (not shown).
TABLE 2.
Seroreactivity of protective Helicobacter antigens in mice and humans
| Antigen | Seroreactivitya
|
|||
|---|---|---|---|---|
| Mice
|
Humand | |||
| Infected | s.c.b | Oralc | ||
| Urease A | U | + | + | U |
| Urease B | U | + | + | U |
| Catalase | U | + | - | U |
| HspA | - | - | - | - |
| HspB | S | + | + | U |
| VacA | - | + | - | - |
| Lipoprotein Lpp20 | - | - | + | - |
| L7/L12 ribosomal protein | - | + | + | U |
| Hypothetical secreted protein HP1488 | - | - | + | - |
| Hypothetical secreted protein HP1117 | - | - | + | - |
| Hemolysin secretion protein precursor | - | + | + | S |
| Citrate synthase | - | + | - | - |
| NapA | - | + | - | S |
| CagA | S | + | - | S |
Seroreactivity of protective Helicobacter antigens in mice and humans. Abbreviations: U, unspecific; S, specific; +, positive (not analyzed for specificity); -, not detected.
Data are from a single subcutaneously immunized mouse.
Data are from orally immunized mice. Data are from reference 10.
In conclusion, the pattern of H. pylori proteins that are expressed in infected mice and become exposed to the mouse immune system appear to be similar to those in human Helicobacter infections, suggesting that the mouse infection model might be suitable for preclinical screening of antigen candidates.
Editor: E. I. Tuomanen
REFERENCES
- 1.Blanchard, T. G., S. J. Czinn, R. W. Redline, N. Sigmund, G. Harriman, and J. G. Nedrud. 1999. Antibody-independent protective mucosal immunity to gastric Helicobacter infection in mice. Cell. Immunol. 191:74-80. [DOI] [PubMed] [Google Scholar]
- 2.Bumann, D., S. Aksu, M. Wendland, K. Janek, U. Zimny-Arndt, N. Sabarth, T. F. Meyer, and P. R. Jungblut. 2002. Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect. Immun. 70:3396-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ermak, T. H., P. J. Giannasca, R. Nichols, G. A. Myers, J. Nedrud, R. Weltzin, C. K. Lee, H. Kleanthous, and T. P. Monath. 1998. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J. Exp. Med. 188:2277-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etz, H., D. B. Minh, T. Henics, A. Dryla, B. Winkler, C. Triska, A. P. Boyd, J. Sollner, W. Schmidt, U. von Ahsen, M. Buschle, S. R. Gill, J. Kolonay, H. Khalak, C. M. Fraser, A. von Gabain, E. Nagy, and A. Meinke. 2002. Identification of in vivo expressed vaccine candidate antigens from Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 99:6573-6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrero, R. L., J. M. Thiberge, M. Huerre, and A. Labigne. 1998. Immune responses of specific-pathogen-free mice to chronic Helicobacter pylori (strain SS1) infection. Infect. Immun. 66:1349-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez-Duarte, O. G., B. Lucas, Z. X. Yan, K. Panthel, R. Haas, and T. F. Meyer. 1998. Protection of mice against gastric colonization by Helicobacter pylori by single oral dose immunization with attenuated Salmonella typhimurium producing urease subunits A and B. Vaccine 16:460-471. [DOI] [PubMed] [Google Scholar]
- 7.Haas, G., G. Karaali, K. Ebermayer, W. G. Metzger, S. Lamer, U. Zimny-Arndt, S. Diescher, U. B. Goebel, K. Vogt, A. B. Roznowski, B. J. Wiedenmann, T. F. Meyer, T. Aebischer, and P. Jungblut. 2002. Immunoproteomics of Helicobacter pylori infection and relation to gastric disease. Proteomics 2:313-324. [DOI] [PubMed] [Google Scholar]
- 8.Hackett, C. J. 2000. Focus on the immunological basis of effective vaccines. Immunologist 5:171-174. [Google Scholar]
- 9.Handfield, M., L. J. Brady, A. Progulske-Fox, and J. D. Hillman. 2000. IVIAT: a novel method to identify microbial genes expressed specifically during human infections. Trends Microbiol. 8:336-339. [DOI] [PubMed] [Google Scholar]
- 10.Hocking, D., E. Webb, F. Radcliff, L. Rothel, S. Taylor, G. Pinczower, C. Kapouleas, H. Braley, A. Lee, and C. Doidge. 1999. Isolation of recombinant protective Helicobacter pylori antigens. Infect. Immun. 67:4713-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janvier, B., B. Grignon, C. Audibert, L. Pezennec, and J. L. Fauchere. 1999. Phenotypic changes of Helicobacter pylori components during an experimental infection in mice. FEMS Immunol. Med. Microbiol. 24:27-33. [DOI] [PubMed] [Google Scholar]
- 12.Jungblut, P. R., D. Bumann, G. Haas, U. Zimny-Arndt, P. Holland, S. Lamer, F. Siejak, A. Aebischer, and T. F. Meyer. 2000. Comparative proteome analysis of Helicobacter pylori. Mol. Microbiol. 36:710-725. [DOI] [PubMed] [Google Scholar]
- 13.Keenan, J., T. Day, S. Neal, B. Cook, G. Perez-Perez, R. Allardyce, and P. Bagshaw. 2000. A role for the bacterial outer membrane in the pathogenesis of Helicobacter pylori infection. FEMS Microbiol. Lett. 182:259-264. [DOI] [PubMed] [Google Scholar]
- 14.Kimmel, B., A. Bosserhoff, R. Frank, R. Gross, W. Goebel, and D. Beier. 2000. Identification of immunodominant antigens from Helicobacter pylori and evaluation of their reactivities with sera from patients with different gastroduodenal pathologies. Infect. Immun. 68:915-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, A. 2000. Animal models of gastroduodenal ulcer disease. Bailliere's Best. Pract. Res. Clin. Gastroenterol. 14:75-96. [DOI] [PubMed] [Google Scholar]
- 16.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 17.Lee, A., J. O'Rourke, and A. Enno. 2000. Gastric mucosa-associated lymphoid tissue lymphoma: implications of animal models on pathogenic and therapeutic considerations-mouse models of gastric lymphoma. Recent Results Cancer Res. 156:42-51. [DOI] [PubMed] [Google Scholar]
- 18.Mai, U. E., G. I. Perez-Perez, J. B. Allen, S. M. Wahl, M. J. Blaser, and P. D. Smith. 1992. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J. Exp. Med. 175:517-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McAtee, C. P., K. E. Fry, and D. E. Berg. 1998. Identification of potential diagnostic and vaccine candidates of Helicobacter pylori by “proteome” technologies. Helicobacter 3:163-169. [PubMed] [Google Scholar]
- 20.McAtee, C. P., M. Y. Lim, K. Fung, M. Velligan, K. Fry, T. Chow, and D. E. Berg. 1998. Identification of potential diagnostic and vaccine candidates of Helicobacter pylori by two-dimensional gel electrophoresis, sequence analysis, and serum profiling. Clin. Diagn. Lab. Immunol. 5:537-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michetti, P., C. Kreiss, K. L. Kotloff, N. Porta, J. L. Blanco, D. Bachmann, M. Herranz, P. F. Saldinger, I. Corthesy-Theulaz, G. Losonsky, R. Nichols, J. Simon, M. Stolte, S. Ackerman, T. P. Monath, and A. L. Blum. 1999. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology 116:804-812. [DOI] [PubMed] [Google Scholar]
- 22.Pappo, J., D. Torrey, L. Castriotta, A. Savinainen, Z. Kabok, and A. Ibraghimov. 1999. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect. Immun. 67:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rokbi, B., D. Seguin, B. Guy, V. Mazarin, E. Vidor, F. Mion, M. Cadoz, and M. J. Quentin-Millet. 2001. Assessment of Helicobacter pylori gene expression within mouse and human gastric mucosae by real-time reverse transcriptase PCR. Infect. Immun. 69:4759-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabarth, N., S. Lamer, U. Zimny-Arndt, P. R. Jungblut, T. F. Meyer, and D. Bumann. 2002. Identification of surface-exposed proteins of Helicobacter pylori by selective biotinylation, affinity purification, and two-dimensional gel electrophoresis. J. Biol. Chem. 277:27896-27902. [DOI] [PubMed] [Google Scholar]
- 25.Sozzi, M., M. Crosatti, S. K. Kim, J. Romero, and M. J. Blaser. 2001. Heterogeneity of Helicobacter pylori cag genotypes in experimentally infected mice. FEMS Microbiol. Lett. 203:109-114. [DOI] [PubMed] [Google Scholar]
- 26.Vytvytska, O., E. Nagy, M. Bluggel, H. E. Meyer, R. Kurzbauer, L. A. Huber, and C. S. Klade. 2002. Identification of vaccine candidate antigens of Staphylococcus aureus by serological proteome analysis. Proteomics 2:580-590. [DOI] [PubMed] [Google Scholar]
- 27.Wirth, H. P., M. Yang, R. M. Peek, Jr., J. Hook-Nikanne, M. Fried, and M. J. Blaser. 1999. Phenotypic diversity in Lewis expression of Helicobacter pylori isolates from the same host. J. Lab. Clin. Med. 133:488-500. [DOI] [PubMed] [Google Scholar]