Abstract
Na/K pump current (Ipump) and intracellular Na concentration ([Na]i) were measured simultaneously in voltage-clamped rabbit ventricular myocytes, under conditions where [Na]i is controlled mainly by membrane transport. Upon abrupt pump reactivation (after 10–12 min blockade), Ipump decays in two phases. Initially, Ipump declines with little [Na]i change, whereas the second phase is accompanied by [Na]i decline. Initial Ipump sag was still present at external [K] = 15 mM, but prevented by [Na]i ∼ 100 mM. Initial Ipump sag might be explained by subsarcolemmal [Na]i ([Na]SL) depletion produced by rapid Na extrusion and Ipump. Brief episodes of pump blockade allowed [Na]SL repletion, since peak postblockade Ipump exceeded Ipump at the end of previous activation (without appreciably altered global [Na]i). The apparent Km for [Na]i was higher for continuous Ipump activation than peak Ipump (14.1 ± 0.2 vs. 11.2 ± 0.2 mM), whereas that based on d[Na]i/dt matched peak Ipump (11.6 ± 0.3 mM). [Na]SL depletion (vs. [Na]i) could be as high as 3 mM for [Na]i ∼ 18–20 mM. A simple diffusion model indicates that such [Na]SL depletion requires a Na diffusion coefficient 103- to 104-fold below that expected in bulk cytoplasm (although this could be subsarcolemmal only). Ipump integrals and [Na]i decline were used to estimate intracellular Na buffering, which is slight (1.39 ± 0.09).
INTRODUCTION
In the heart, intracellular Na concentration ([Na]i) is very important in modulating the electrical and contractile activity. This is because [Na]i controls the intracellular Ca and H concentration via the Na/Ca exchanger (NCX) and Na/H exchanger, respectively, and both these ions are essential factors in excitation-contraction coupling (Bers, 2001). An increase in [Na]i shifts the balance of fluxes on the NCX to favor more Ca influx and less Ca efflux, resulting in larger Ca transients and therefore, enhanced contractility. Indeed, relatively small changes in [Na]i can have major effects on contractile force. For example, in cardiac Purkinje fibers, force can double with ∼1 mM rise of [Na]i (Lee and Dagostino, 1982).
The level of [Na]i is determined by a fine balance between Na influx and efflux. While there are many Na entry pathways, the Na/K pump is the main route for Na extrusion and therefore is essential in [Na]i regulation. The Na/K pump uses the energy derived from the hydrolysis of one ATP molecule to exchange three intracellular Na for two extracellular K ions. Not only does the pump control [Na]i, but [Na]i is a main regulator of the pump itself (Glitsch, 2001). This creates a feedback system, which tends to reduce the changes in [Na]i due to variations in the Na influx produced, for example, by changes in the heart rate or by ischemia.
The aim of this article is to investigate the relationship between [Na]i and the Na/K pump in rabbit ventricular myocytes. Generally, such studies are done by measuring the Na/K pump current (Ipump), using the whole-cell patch clamp technique, at various pipette Na concentrations and assuming that intracellular Na fully equilibrates with pipette Na (for a review, see Glitsch, 2001). However, this might not always be the case, especially when there is a net transmembrane Na flux—i.e., the cell is not in the steady state (Mathias et al., 1990). Furthermore, several reports indicate the presence of a transient Ipump component upon abrupt Na/K pump reactivation after a period of pump blockade (Bielen et al., 1991a; Su et al., 1998; Fujioka et al., 1998). Such transients could be due to either a [Na] gradient between the patch pipette and the cell or to a local, subsarcolemmal Na depletion brought about by the rapid Na extrusion upon Na/K pump activation. Here we used a different approach: we patched the cells with relatively high resistance electrodes so that [Na]i is mainly controlled by the membrane transporters and not by the pipette solution. Thus, we could manipulate [Na]i by changing the external conditions. The actual [Na]i was measured simultaneously with Ipump by using the fluorescent indicator, SBFI.
Simultaneous measurements of Ipump and [Na]i allowed us to 1), investigate whether rapid Na/K pump activation could generate intracellular Na gradients; 2), derive the [Na]i-dependence of Ipump in single cells; and 3), determine the level of endogenous Na buffering. That Na/K pump might lower [Na]i near sarcolemma ([Na]SL) below the level in the bulk cytosol has been suggested by the presence of a transient Ipump component upon Na/K pump activation (Bielen et al., 1991a; Su et al., 1998; Fujioka et al., 1998) and by the increase in the amplitude of Ca transients produced by rapid Na/K pump inhibition (Terracciano, 2001; Su et al., 2001). However, [Na]i has not been measured in these studies and one can argue that the effects are due to a change in global [Na]i rather than to a local variation in [Na]SL. If there is indeed a Na/K pump-dependent depletion of [Na]SL, this could affect the activity of all Na-dependent membrane transporters, including the pump itself, with important consequences on cell contractility.
To relate quantitatively the Na fluxes to changes in the free [Na]i, it is important to know the level of intracellular Na buffering. This is expected to be rather small but actual data are lacking. Here we determined the intracellular Na buffering as the ratio between the change in total [Na]i, calculated from the integral of Ipump, and the change in free [Na]i when the Na/K pump is reactivated after a period of pump inhibition.
MATERIALS AND METHODS
Myocytes isolation
The procedure for isolation of ventricular myocytes has been described previously (Bassani et al., 1994) and approved by the Loyola University Chicago animal welfare committee. Briefly, rabbits were anesthetized by IV injection of pentobarbital sodium (50–70 mg/kg) and hearts were excised quickly and placed on a Langendorff perfusion apparatus. Hearts were perfused for 5–7 min with nominally Ca-free Tyrode's solution, then perfusion proceeded with added collagenase (1 mg/ml) and albumin (0.05%) ([Ca] ≈ 36 μM) until the heart became flaccid (15–18 min). The left ventricular tissue was cut into small pieces for further incubation with 0.4 mg/ml collagenase. Incubations were stopped at 5–45 min by adding 200 μM Ca Tyrode's solution with 0.05% albumin and the tissue was filtered. The external Ca concentration was progressively increased to 2 mM. Isolated myocytes were plated on laminin-coated glass coverslips and mounted in perfusion chambers. The standard normal Tyrode's solution contained (in mM): 140 NaCl, 4 KCl, 1 MgCl2, 10 glucose, 5 HEPES, and 2 CaCl2 (pH = 7.4). All experiments were done at 35–37°C.
Na/K pump current measurements
Isolated ventricular myocytes were whole-cell voltage-clamped using patch electrodes made from borosilicate glass capillaries. The electrodes had a resistance of 3–5 MΩ when filled with the internal solution, resulting in a series resistance of 8–15 MΩ. After obtaining the whole-cell configuration, the cells were held at −30 mV to inactivate Na channels. Current signals were recorded using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA). Membrane capacitance and series resistance were calculated from a 5-mV voltage step. The standard pipette solution contained (in mM): 10 NaCl, 20 KCl, 100 K-aspartate, 20 TEA-Cl, 10 HEPES, 5 Mg-ATP, 0.7 MgCl2 (≈1 mM free Mg), 3 BAPTA, 1.15 CaCl2 (≈100 nM free Ca), and 1 SBFI tetraamonium salt (pH = 7.2). In the pipette solution containing 100 mM Na, K-aspartate was replaced by equimolar concentrations of aspartic acid and NaOH. The external solution contained (in mM): 136 NaCl, 5 NiCl2, 2 BaCl2, 1 MgCl2, 5 HEPES, 10 glucose, and 4 KCl (4 mM K solution) or Tris-Cl (0 mM K solution; pH = 7.4). For the 15-mM K solution, 11 mM KCl replaced 11 mM NaCl. Ipump was measured as the outward shift induced by switching from a K-free to a K-containing external solution.
[Na]i measurements
Myocytes were loaded with SBFI (Molecular Probes, Eugene, OR) via the patch pipette. Fluorescence was elicited by illumination with a 75-W Xenon lamp. Dual excitation measurements (at 340 and 380 nm, F340 and F380) were performed using an Optoscan monochromator (Cairn Research, Faversham, UK). The emitted fluorescence was recorded at 535 ± 20 nm with a photomultiplier. The whole system was controlled by the Optoscan microprocessor control unit (Cairn Research). Data acquisition was done in the integration mode, where for each wavelength the signal is integrated over 20 ms. For each cell, the background signal at both wavelengths was measured on seal formation and subtracted from the signals after dye loading. In situ calibration of SBFI was done at the end of each experiment as previously described (Despa et al., 2002a). Briefly, myocytes were exposed to divalent-free solutions with 0, 10, or 20 mM extracellular [Na], in the presence of 10 μM gramicidin and 100 μM strophanthidin.
Na/K-pump-mediated Na extrusion
The Na/K pump activity can also be described in terms of the rate of Na extrusion. This was derived from the rate of [Na]i decline (d[Na]i/dt) upon abrupt pump reactivation after a period of pump blockade (Despa et al., 2002b). The rate of [Na]i decline is given by:
 |
(1) |
where V is the cell volume and β is the intracellular Na buffering. Jpump is the rate (mmol/min) of the pump-mediated Na extrusion and is the dominant component of d[Na]i/dt, whereas Jleak and Jpip represent moderate corrections for the passive Na influx from the external solution and Na exchange between the patch pipette and the cell, respectively. We have shown previously (Despa et al., 2002b) that the cell volume does not change during our Na loading/recovery protocol. Mathias and co-workers (Mathias et al., 1990) showed that Na flux between the patch pipette and the cell is given by Jpip = ρDNa([Na]pip – [Na]i)/Rs, where ρ (60 Ω × cm) is the resistivity of the pipette solution, Rs is the series resistance, DNa (600 μm2/s; Kushmerick and Podolsky, 1969) is the diffusion coefficient for Na, and [Na]pip is Na concentration in the pipette solution. For Jleak, we used a [Na]i-independent value of 1.1 mM/min, as previously measured in rabbit myocytes (Despa et al., 2002b). Note that Jleak may include Na influx from multiple pathways (e.g., Na/Ca exchange, Na channels, or Na/H exchange).
We calculated d[Na]i/dt by numerical differentiation, and Jpump (transformed in units of mM/min) was determined from Eq. 1. For each cell, V was calculated from the membrane capacitance using a surface-to-volume ratio of 6.44 pF/pL cytosol (Satoh et al., 1996).
Measurement of intracellular Na buffering
This was done using the same protocol of abrupt Na/K pump activation described above. The changes of free [Na]i were obtained from the SBFI ratio. The amount of Na pumped out of the cell via the Na/K pump was calculated from the integral of Ipump. Corrections were made for the Na exchange between the cell and the patch pipette and for the passive Na influx from the external solution. The amount of Na diffusing into the patch pipette was calculated by integrating Jpip as defined above. The transsarcolemmal Na influx was assumed constant and given by the flux that will equilibrate Ipump and Jpip at steady state. Because in our experiments [Na]i ≈ [Na]pip at steady state, the amount of Na that passively enters the cell was calculated from the integral of steady-state Ipump.
Statistical analysis
Data are expressed as mean ± SE. Statistical discriminations were performed using the Student's unpaired t-test. Values of p < 0.05 were considered significant.
RESULTS
Simultaneous measurements of Ipump and [Na]i
We investigated the relationship between the Na/K pump and [Na]i in voltage-clamped rabbit ventricular myocytes. After reaching the whole-cell configuration, the cells were Na-loaded, at Em = −30 mV, by inhibiting the Na/K pump for 10–12 min in a K-free solution. Then, the pump was abruptly reactivated by adding back K (4 mM) and we monitored the membrane current and [Na]i (Fig. 1). Pump reactivation resulted in a rapid outward shift in the membrane current, followed by a decay which appears to be biphasic (see Fig. 1 A; see also below) and is accompanied by a decline in [Na]i (Fig. 1 B). Within 3–5 min, the cell reaches a steady state where both [Na]i and the current are constant. The protocol (including the Na-loading phase) was repeated in the same myocyte, this time with the pump blocked by 200 μM strophanthidin. In the presence of strophanthidin, external K did not elicit any change in the membrane current whereas [Na]i keeps increasing slightly (Fig. 1, right). This indicates that the outward shift in the membrane current is entirely due to activation of the Na/K pump.
FIGURE 1.
Time course of the membrane current (A) and [Na]i (B) upon abrupt Na/K pump reactivation after a period of pump blockade. The pump was reactivated by adding back 4 mM K after perfusing the cell for 12 min in a K-free solution. When both the current and [Na]i reached the steady state, the cell was incubated in K-free solution for another 12 min, this time in the presence of 200 μM strophanthidin. Then, the cell was perfused again with 4 mM K, in the continuous presence of strophanthidin. The myocyte was held at –30 mV throughout the experiment.
Fig. 2, A and B, show the first minute of a similar experiment (full curves are in the Inset). With this timescale, it becomes clear that, after abrupt activation, Ipump initially decays rapidly (phase a, Fig. 2 A), within 2–5 s, with little change in [Na]i. Then, Ipump declines at a slower rate (phase b) and this is paralleled by a decrease in [Na]i. The plot of Ipump versus [Na]i (Fig. 2 C) emphasizes the initial Ipump sag at practically constant [Na]i, followed by a Hill type [Na]i-dependence of Ipump.
FIGURE 2.

Ipump decay after abrupt activation is biphasic. (A) Ipump during the first minute of an experiment similar to that shown in Fig. 1 (full curve shown in inset). (B) [Na]i in the same experiment (full scale shown in inset). (C) The [Na]i-dependence of Ipump indicates that initially Ipump decreases at practically constant [Na]i (Ipump sag). The solid line represents the fit with a Hill equation Ipump = Ipumpmax/(1+(Km/[Na]i)n). Ipump sag was excluded from the fit.
Ipump sag and local [Na]SL depletion
Ipump is regulated by [Na]i near the sarcolemma, [Na]SL, as opposed to the bulk [Na]i. If the rate of Na extrusion upon the rapid activation of the pump is faster than the diffusion of Na between the subsarcolemmal space and the bulk cytosol, this will produce a local [Na]SL depletion, with minimal changes in the global [Na]i as measured by SBFI. Such a decrease in [Na]SL might reduce Ipump considerably, thus explaining the sag. However, alternative explanations are possible.
First, because Na/K pumps are located in both the external sarcolemma and the T-tubules of cardiac myocytes (McDonough et al., 1996), the Ipump sag could be caused by the depletion of external K in the T-tubules. To examine this possibility, we repeated the experiments at 15 mM external K (Fig. 3 A). This concentration is well above the Km for binding of external K to the pump (1–2 mM, Nakao and Gadsby, 1989; Bielen et al., 1991b), therefore a possible depletion of K in the T-tubules is not expected to affect Ipump. As Fig. 3 A shows, at 15 mM [K]o, Ipump displayed an even more prominent sag, suggesting that phase a is not a result of external K depletion. If anything, it may be enhanced by higher Ipump at near-maximal conditions for [K]o.
FIGURE 3.
(A) Membrane current (top) and [Na]i (bottom) upon Na/K pump reactivation with 15 mM external K. The pipette solution contained 10 mM Na. (B) Ipump (top) and the SBFI ratio (bottom) induced, in a different cell, by 4 mM K at [Na]pip = 100 mM.
Second, rapid activation of the Na/K pump might result in local ATP depletion near the sarcolemma. This is unlikely to affect Ipump significantly because the ATP concentration for half-maximal activation of the pump is in the range of 80–150 μM (Hilgemann et al., 1991; Friedrich et al., 1996) whereas the intracellular ATP concentration is ∼5 mM. Still, a combined effect of lower ATP and increased ADP near the sarcolemma might affect the pumping capacity of the Na/K pump. To rule out such an effect, we repeated the experiments with [Na]pip = 100 mM (Fig. 3 B). If Ipump sag is produced by an ATP depletion, it should be even more pronounced at higher [Na]i, because the pump rate is higher and therefore more ATP is hydrolyzed. On the other hand, [Na]SL depletion will have a minimal effect on Ipump because the pump is saturated with respect to internal Na. Fig. 3 B (top), shows that at high [Na]pip the Ipump sag disappears, which means that Ipump sag is not due to local ATP depletion. We did not perform a careful calibration of the SBFI signal at this high [Na]i, but a three-point calibration (0, 50, and 100 mM) indicated that [Na]i ≈ 50 mM. This large Na gradient between the cell and the patch pipette might be why [Na]i does not decrease upon pump activation.
To test whether local [Na]SL depletion is indeed the cause for Ipump sag, Na-loaded myocytes were exposed to several episodes of Na/K pump inhibition/reactivation (Fig. 4). In the first experiments, the pump was repeatedly activated for 5 s and then inhibited for 20 s (Fig. 4 A). This resulted in minimal changes in [Na]i and practically constant peak Ipump, which suggests that [Na]SL is completely replenished within the 20-s pump inhibition interval. To allow for [Na]i changes with this protocol, the duration of the pump activation was extended to 10 s in the next experiments (Fig. 4 B). Upon activation, Ipump decays, initially with little change in global [Na]i ([Na]SL depletion, see also Fig. 4 C). For each Na/K pump activation episode, the peak Ipump is higher than the Ipump at the end of the previous activation, despite a small decrease in the global [Na]i (Fig. 4, B and C). This is because [Na]SL was replenished during the pump inhibition, thus the [Na]SL sensed by the pump at the moment of the peak Ipump is higher than at the end of the previous activation. Because Na influx is rather slow, we can assume that with the Na/K pump blocked, diffusion will equilibrate [Na]i throughout the cell. This means that at the peak of Ipump, [Na]SL = [Na]i as measured by SBFI. Therefore, a plot of the peak Ipump versus [Na]i (Fig. 4 D) gives a more accurate description of the Na/K pump-dependence on [Na]SL than the continuous activation curve in Fig. 2 C.
FIGURE 4.
Ipump and [Na]i during interrupted Na/K pump reactivation, induced by switching the external solution from 0 to 4 mM K. Myocytes were perfused for 12 min in K-free solution before the first activation of the pump and held at –30 mV throughout the experiment. (A) Ipump (top) and [Na]i (bottom) in a cell where [Na]i did not change appreciably (pump activations and inhibitions were 5 and 20 s, respectively). (B) Ipump (top) and [Na]i (bottom) in a cell with longer pump activations (10 s). As more Na was extruded by the pump, [Na]i declined and this was paralleled by a decline in the peak of Ipump. (C) Ipump and [Na]i in the area indicated in B on an expanded scale, emphasizing Ipump sag (depletion) and the recovery after the brief pump inhibition (repletion). (D) The [Na]i-dependence of the peak Ipump. Data were fitted with a Hill expression.
Fig. 5 A shows average data for the [Na]i-dependence of Ipump in the presence (protocol from Figs. 1 and 2) and in the absence (protocol from Fig. 4) of [Na]SL depletion. The points were fit with a Hill equation to derive the maximum pump current (Ipumpmax), the [Na]i for half-maximal activation of the pump (Km), and the Hill coefficient, n (Table 1). The main effect of [Na]SL depletion is a shift in the apparent Km for internal Na from 11.2 mM to 14.1 mM (Table 1). Fig. 5 A can be used to estimate the amount of [Na]SL depletion produced by the pump. For example, for an [Na]i of 18 mM, Ipump measured in conditions that allow [Na]SL depletion is ∼1.0 A/F. The peak Ipump versus [Na]i curve (i.e., Ipump versus [Na]SL) shows that a similar current occurs at [Na]SL∼15 mM. Thus, the initial, abrupt activation of the pump lowers [Na]SL by ∼3 mM compared to the bulk [Na]i. This estimation is, of course, an upper limit, as it assumes that Ipump sag is entirely due to [Na]SL depletion. For each experiment, we integrated the initial current to calculate the amount of Na that is extruded from the cell during Ipump sag. Dividing this value by Δ[Na]SL determined as described above, we obtained a subsarcolemmal space volume of 10 ± 1% (n = 10) of the total cell volume. However, this is only a rough estimate because the subsarcolemmal space geometry and local Na buffering are not known.
FIGURE 5.

Ipump versus [Na]i. (A) Ipump in the presence (depletion) and in the absence (peak) of [Na]SL depletion. Solid lines are Hill expression fits (Table 1). Data in the presence of [Na]SL depletion are averages (n = 8) where the pump is continuously activated (protocol similar to Figs. 1 and 2). Initial Ipump sag was excluded from the plot and fit. Data without [Na]SL depletion are average of peak Ipump versus [Na]i (n = 7) from repeated episodes of pump inhibition/reactivation. (B) Difference between the curves in A.
TABLE 1.
[Na]i-dependence of Ipump, with (continuous activation) and without (peak) local [Na]SL depletion and of the pump-mediated Na extrusion
| With depletion (n = 8) | Peak (n = 7) | −d[Na]i/dt (n = 12) | |
|---|---|---|---|
| Vmax | 1.6 ± 0.2 A/F | 1.5 ± 0.2 A/F | 6.1 ± 0.5 mM/min |
| Km (mM) | 14.1 ± 0.2 | 11.2 ± 0.3* | 11.6 ± 0.3* |
| n | 2.3 ± 0.1 | 2.7 ± 0.1† | 3.2 ± 0.3† |
Significantly different from the continuously activated Ipump; *P < 0.0001; †P < 0.05.
Fig. 5 B shows that the difference in Ipump in Fig. 5 A (or extent of Ipump sag) is maximal around the Km for the pump. This makes sense because the [Na]i-dependence of Ipump is steepest there. One can also appreciate that this sag becomes negligible at low [Na]i (where Ipump is small) and where [Na]i ≫ Km as shown in Fig. 3 B (because even large D[Na]SL depletions near Vmax will not reduce Ipump appreciably).
Theoretical model for radial Na diffusion in the cell
The existence of an intracellular [Na]i gradient implies restricted diffusion with respect to the transport rate. To determine the diffusion coefficient for Na that could account for a [Na]SL depletion of the size inferred from the Ipump measurements, we developed a simple theoretical model for the radial diffusion of Na in the cell (see Appendix). Briefly, we divided the cell, considered as a long cylinder where diffusion is everywhere radial, into small circular shell compartments. The change in [Na]i in the compartment located immediately beneath the sarcolemma, i.e., [Na]SL, is determined by the Na extrusion via the Na/K pump, the passive transsarcolemmal Na influx, and the Na diffusion into the next compartment. In all other compartments, the [Na]i level is determined exclusively by Na diffusion into/from the adjacent compartments. Na extrusion via the Na/K pump was taken as a function of [Na]SL, using the parameters obtained from the plot of the peak Ipump versus [Na]i (Fig. 5, Table 1).
Experimental data indicate that in muscle cells DNa = 600 μm2/s; i.e., about one-half the value measured in aqueous solutions (Kushmerick and Podolsky, 1969). However, for this diffusion coefficient the model predicts no apparent differences between [Na]SL and bulk [Na]i (not shown). Next, we simulated a subsarcolemmal “fuzzy” space by assuming that Na diffusion is limited in a 100- to 500-nm space beneath the sarcolemma. Fig. 6 A shows that considerable [Na]i gradients could be induced upon abrupt Na/K pump activation if DNa in this 400-nm subsarcolemmal space is 0.12 μm2/s (i.e., bulk DNa/5000). In Fig. 6 A [Na]SL decreases by more than 2 mM within the first 1–2 s of pump activation, whereas average [Na]i that would be measured by SBFI stays almost constant (Fig. 6 A, inset). When we included interruptions in the Na/K pump activation (thus simulating the experiment shown in Fig. 4 B), the model predicted a rapid [Na]SL repletion during each episode of pump inhibition, with simulated Ipump recovery similar to the experimental data in Fig. 4 B (not shown). The [Na]i gradient is limited to the region with restricted Na diffusion (Fig. 6 B). Fig. 6 C shows how Ipump is expected to depend on the global [Na]i. The model predicts an Ipump sag similar to what we observed experimentally (Fig. 2 C), followed by a Hill-type dependence on global [Na]i. When we fitted Ipump versus global [Na]i (excluding Ipump sag) with a Hill expression, we derived a higher Km (14.6 mM) and a somewhat lower Hill coefficient (1.9) than the values used for Ipump versus [Na]SL (11.2 mM and 2.7, respectively). Our experimental data (Fig. 5, Table 1) show a similar behavior.
FIGURE 6.
[Na]i gradients predicted by a simple model assuming the existence of a subsarcolemmal space with restricted Na diffusion. (A) Time course of [Na]i in the compartment near the sarcolemma (SL), at the center of the cell (center) and the mean [Na]i in the cell as it would be measured by SBFI (Global). (Inset) [Na]i changes in these regions during the first 5 s upon pump reactivation on an expanded scale. (B) The radial distribution of [Na]i gradient at various moments after abrupt Na/K pump reactivation. (C) Predicted dependence of Ipump on the global [Na]i. Simulations were done considering a 400-nm subsarcolemmal space where the diffusion coefficient for Na is 0.12 μm2/s. In the bulk, DNa = 600 μm2/s. (D) Local DNa versus the size of the subsarcolemmal space that produces a [Na]SL depletion similar to Fig. 6 A.
The extent of [Na]SL depletion and Ipump sag depend critically on two parameters: the size of the subsarcolemmal space and the local DNa. Fig. 6 D shows that if we use a smaller subsarcolemmal space, the subsarcolemmal DNa must be reduced even more dramatically to obtain results similar to Fig. 6, A and C. We also performed simulations where DNa was reduced everywhere in the cell, not just in a limited subsarcolemmal region (not shown). To obtain a difference between [Na]SL and the bulk [Na]i similar to Fig. 6 A, global DNa had to be reduced to ∼0.6 μm2/s (versus the 0.12 μm2/s for the case in Fig. 6 A). Any Na buffering at the sarcolemma (Philipson et al., 1980) would also further reduce the DNa required to simulate our data. Thus, the upper limit of DNa which would explain our observations is 1000× smaller than that expected.
Ipump and the rate of pump-mediated Na efflux
The rate of [Na]i decline in the experiments shown in Figs. 1 and 2 can also be used to characterize the activity of the Na/K pump (Despa et al., 2002a,b). This provides an additional, independent, estimate to the Ipump data. The pump-mediated d[Na]i/dt was calculated by numerical differentiation of the [Na]i decline and corrected for the Na influx from the external solution and for the Na exchange with the patch pipette. Fig. 7 shows comparatively the [Na]i-dependence of Ipump and of the pump-mediated Na efflux. The Km for [Na]i of the Na/K pump-mediated Na extrusion was 11.6 ± 0.3 mM (n = 12), which is significantly lower than the Km derived from steadily activated Ipump (14.1 ± 0.2 mM), but similar to the value derived from peak Ipump in the absence of [Na]SL depletion (11.2 ± 0.3 mM), in experiments with repeated Na/K pump inhibition/activation episodes.
FIGURE 7.
The [Na]i-dependence of the Na/K pump in terms of Ipump (A) and the rate of pump-mediated [Na]i decline (B). Data are from an experiment similar to Fig. 1, after the initial Ipump sag is excluded. The rate of [Na]i decline was calculated by numerical differentiation and then corrected for non-pump-mediated Na fluxes between the cell and the patch pipette and external solution to derive the pump-mediated d[Na]i/dt (see Methods).
Intracellular Na buffering
The simultaneous measurements of Ipump and [Na]i decline during the abrupt Na/K pump activation that was described above allowed us to estimate the level of intracellular Na buffering (Fig. 8). Three processes contribute to the [Na]i change in this protocol: 1), Na extrusion via the Na/K pump; 2), passive Na influx across sarcolemma; and 3), Na exchange with the patch pipette. Fig. 8 A shows the calculation for the net change in total [Na]i due to Na fluxes across the sarcolemma, i.e., Na pumping out of the cell and passive Na entry. The contribution of transsarcolemmal and pipette fluxes to the change in total [Na]i is shown in Fig. 8 B. This shows that the membrane transport is the dominant factor in controlling the [Na]i. The change in [Na]i estimated from these fluxes adds up to a higher level than the change in free [Na]i measured by SBFI, indicating that some endogenous Na buffering exists (Fig. 8 C). However, this is rather slight (1.39 ± 0.09, n = 17).
FIGURE 8.

Estimation of endogenous Na buffering. (A) Ipump in a representative experiment where the Na/K pump was initially inhibited for 12 min in K-free solution and then abruptly reactivated by adding 4 mM K to the superfusing solution. The amount of Na pumped out of the cell via the Na/K pump was calculated from the integral of Ipump. To correct for the passive Na influx, this was assumed constant and given by the flux that will equilibrate Ipump at steady state (because in these experiments, [Na]i ≈ [Na]pip at steady state). Thus, the amount of Na that passively enters the cell was calculated from the integral of steady-state Ipump. (B) The contribution of Na fluxes across the sarcolemma and between the cell and the patch pipette to the [Na]i change. The pipette contribution was calculated by integrating the flux ρDNa([Na]pip − [Na]i)/RsV. (C) The change in free [Na]i as measured by SBFI versus total [Na]i (pipette + sarcolemma in B). The calculation was done assuming a surface-to-volume ratio of 6.44 pF/pL cytosol (Satoh et al., 1996).
DISCUSSION
This article characterizes the activity of the Na/K pump, presents data suggesting that abrupt pump activation could result in local [Na]SL depletion, and estimates the level of intracellular Na buffering in rabbit ventricular myocytes. Ipump and [Na]i were measured simultaneously using voltage-clamp and the fluorescent indicator, SBFI, respectively. Different from most studies, voltage-clamp experiments were done under conditions that minimized the effect of cell dialysis by the patch pipette on [Na]i while maximizing its control by the Na fluxes across sarcolemma. Thus, we selected large cells (average Cm ∼ 200 pF) and used relatively small electrodes (resulting in an Rs of 8–15 MΩ). In rabbit ventricular myocytes, [Na]i control by the membrane transporters versus the patch pipette is also favored by the fairly large surface-to-cytosol volume ratio (6.44 pF/pL; Satoh et al., 1996). This strategy allowed us to raise [Na]i significantly by simply blocking the Na/K pump, and then follow Ipump and [Na]i as the cell relaxes toward steady state upon pump reactivation.
Na/K pump and intracellular [Na]i gradients
We found that upon abrupt Na/K pump reactivation after a period of pump blockade, Ipump displays a transient peak that decays in two phases (Figs. 1 and 2). The first, rapid phase occurs with little change in [Na]i, whereas the second phase is accompanied by a [Na]i decline. The rapid phase is still present when the pump is reactivated by 15 mM K but disappears at high [Na]i. This rules out external K depletion in the T-tubular system and local ATP depletion as possible explanations for the Ipump sag. The fast Ipump decay might be due to a local subsarcolemmal [Na]i depletion produced by the pump itself. This hypothesis is supported by the behavior of Ipump when the cell is exposed to a series of Na/K pump inhibition/reactivation episodes (Fig. 4). For each pump activation episode, we observed the peak Ipump followed by the fast decay phase, whereas global [Na]i decline had slower kinetics. Every time, the peak Ipump was higher than Ipump measured at the end of the previous activation, with limited changes in global [Na]i. Thus, pump inhibition for a few seconds allows [Na]SL repletion.
The presence of an [Na]i gradient between the subsarcolemmal space and bulk cytosol in cardiac cells has been suggested by several studies (Bielen et al., 1991a; Carmeliet, 1992; Wendt-Gallitelli et al., 1993; Lipp and Niggli, 1994; Semb and Sejersted, 1996; Su et al., 1998, 2001; Terracciano, 2001). Transients in the Na/K pump current similar to those found here have been previously reported (Bielen et al., 1991a; Fujioka et al., 1998; Su et al., 1998), but [Na]i was not measured in these studies. Our approach has the advantage that it allows for quantitative considerations (see below). Su and co-workers (Su et al., 2001) showed that abrupt Na/K pump inhibition in mouse ventricular myocytes increases the efficacy of a given Ca current to trigger sarcoplasmic reticulum Ca release. This was interpreted as a prevention of Na extrusion in the junctional cleft (between the sarcoplasmic reticulum and sarcolemma) allowing local cleft [Na]i to rise and favor Ca entry via NCX. Abrupt Na/K pump inhibition was also shown to slow the decline of caffeine-induced Ca transients and INCX (Terracciano, 2001). Direct measurements of [Na]i gradients have also been reported (Wendt-Gallitelli et al., 1993; Silverman et al., 2002). Wendt-Gallitelli et al. (1993) observed an [Na]SL increase of up to 40 mM during stimulation in guinea-pig ventricular myocytes, with no change in the bulk [Na]i. This steep [Na]i gradient dissipated within minutes upon the cessation of stimulation. Silverman and co-workers (Silverman et al., 2002) found that in rabbit papillary muscle [Na]i is higher near the sarcolemma and falls to levels equal to that in the bulk cytosol within 200–300 nm of the membrane. This [Na]i gradient was similar whether measured in diastole or systole. However, both studies used electron probe x-ray microanalysis to measure [Na]i and this method measures total rather than free [Na]i. Thus, these data might reflect binding of Na in the subsarcolemmal space (Philipson et al., 1980), which could in turn alter Na diffusion.
The [Na]SL depletion affects the experimentally determined [Na]i-dependence of Ipump. The “true” activation curve can be obtained from the plot of the peak Ipump upon pump activation versus [Na]i. This is because [Na]SL is repleted during the brief episodes of pump inhibition, therefore [Na]SL is equal to the measured global [Na]i at the moment of pump reactivation. The main difference in the [Na]i-dependence of Ipump measured in the presence and in the absence of [Na]SL depletion is a shift of ∼3 mM in the apparent Km for intracellular Na (Fig. 5). Therefore, caution has to be taken when measuring this parameter under non-steady-state conditions. Surprisingly, d[Na]i/dt gives a closer estimate to the real Km than does the continuously activated Ipump. These Ipump-activation curves allow us to estimate quantitatively the amount of [Na]SL depletion. Thus, for an initial [Na]i of 20 mM, abrupt activation of the pump could lower [Na]SL by as much as 4 mM. When the pump is activated at 10 mM [Na]i, the depletion is reduced to 1.3 mM, and for an initial [Na]i of 4.5 mM, which is the resting [Na]i level in rabbit ventricular myocytes (Despa et al., 2002a), becomes negligible (<0.2 mM). This makes sense, because at lower [Na]i the pump rate is lower, causing less depletion. It is likely that the subsarcolemmal space is heterogeneous, thus different Na/K pumps might sense different [Na]SL. In this context, the Vmax, Km, and n values reported here reflect average values for all the pumps in the cell.
According to our model for radial Na diffusion, [Na]i gradients of such magnitude require that the diffusion coefficient for Na in an area extending 100–500 nm from the sarcolemma is 103 to 104 times lower than the values reported in the literature for Na diffusion into the cytoplasm (Kushmerick and Podolsky, 1969). One may expect that the mobility of ions is restricted near the sarcolemma due to interactions with the polar structures associated with the internal side of the membrane. The existence of a barrier to diffusion of Ca ions separating the bulk cytoplasm from a subsarcolemmal space has been suggested by a hysteresis between the intracellular Ca concentration and the transient inward current induced by spontaneous or caffeine-promoted Ca release from the sarcoplasmic reticulum (Trafford et al., 1995). Still, a diffusion coefficient several orders-of-magnitude lower than in the bulk cytoplasm is difficult to imagine in any simple physical sense. Although the main model predictions, including the Ipump sag and the shift in the Ipump versus [Na]i curve, were observed experimentally, the simple geometry used, especially the absence of the T-tubular system, might affect the results. Alternatively, the Ipump sag and the change in the [Na]i activation curve might reflect a yet-unknown regulatory mechanism, such as an external K-dependent inactivation, analogous to the Na-dependent inactivation of the Na/Ca exchanger (Hilgemann et al., 1992).
Intracellular Na buffering
The Ipump and [Na]i decline upon Na/K pump reactivation were used to calculate the intracellular Na buffering. This seems rather slight (1.39 ± 0.09), especially when compared with other ions, such as Ca (100:1), Mg (∼100:1), or H (>1000:1). The buffering measured here represents the sum of 1), the intrinsic Na buffering in the cytoplasm; 2), the buffering by internal organelles, especially mitochondria; and 3), the buffering provided by the added SBFI. To calculate the latter, we estimated the concentration of SBFI in the cell using the expression for the flux between the patch pipette and the cell given by Mathias and co-workers (Mathias et al., 1990), a surface-to-cytosol volume ratio of 6.44 pF/pL (Satoh et al., 1996) and an average Rs and Cm of 10 MΩ and 200 pF, respectively. This resulted in an SBFI concentration of 400–500 μM at the time of Ipump and [Na]i measurement (15–20 min postrupture). Assuming a dissociation constant of 11.3 mM (Haugland, 1996), the change in the concentration of Na bound to SBFI when the free [Na]i decreases from 20 to 10 mM (typical in our experiments) is ∼85 μM. Thus, Na buffering by the added SBFI is negligible (∼0.085).
In the calculation of Na buffering, the mitochondrial volume was excluded from the volume available to Na, by using the surface-to-cytosol volume ratio. This assumes that [Na] in mitochondria ([Na]m) does not change. However, in isolated heart mitochondria, [Na]m increases linearly with external Na (Jung et al., 1992). If this is also the case in intact myocytes, then the use of the surface-to-total cell volume ratio (4.58 pF/pL; Satoh et al., 1996) is more appropriate. With this ratio, the change in [Na]i estimated from Na fluxes becomes equal to the measured change in the free [Na]i. Thus, the intrinsic Na buffering power in the cytoplasm is negligible (e.g., less than twofold), when compared to the powerful 10- to 1000-fold cytosolic buffering of Ca, Mg, or H (Bers, 2001).
In summary, we investigated the relationship between the Na/K pump and [Na]i by simultaneous measurements of Ipump and [Na]i using voltage-clamp and SBFI. Our data suggest that rapid Na extrusion upon pump activation may result in local [Na]SL depletion. We designed a protocol that allowed us to characterize the [Na]i-dependence of Ipump in the absence of depletion and to estimate the extent of the [Na]SL depletion. A simple diffusion model indicated that such [Na]SL depletion requires that Na diffusion is 103 to 104 times slower near sarcolemma versus the bulk cytoplasm. However, a fully satisfying physical explanation is lacking at this point. We also estimated the intracellular Na buffering, which was found to be rather slight (1.39 ± 0.09).
Acknowledgments
We thank Jorge Acevedo for technical support.
This work was supported by National Institutes of Health grants HL-64098 and HL-64724 (to D.M.B.), and an American Heart Association postdoctoral fellowship 0225554Z (to S.D).
APPENDIX
We considered the cell as a long circular cylinder, with radius R, in which diffusion is everywhere radial. The model cell is divided into N small circular shell compartments of thickness Δr = R/N. [Na]i in the compartment at the sarcolemma is affected by Na influx and efflux across sarcolemma as well as Na diffusion into the next compartment, whereas in the internal compartments a change in [Na]i is brought about only by diffusion from the neighboring compartments. Thus, the change in [Na]i in the outer and subsequent shells per unit time (ΔCn/Δt) are given by
 |
(A1) |
where Vn and An are the volume and the area of the external surface of compartment n, Dn is the diffusion coefficient for Na in the compartment n, and Jleak and Jpump are the rates of passive Na influx and Na extrusion by the pump, respectively (in mole/time).
The Na/K-pump-mediated Na efflux is a function of [Na]i in the compartment near the sarcolemma (CN). According to the experimentally determined [Na]SL-dependence of Ipump (Figs. 3 and 4, and Table 1), Jpump can be written as
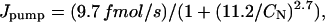 |
where we used a surface-to-volume ratio of 6.44 pF/pL cytosol (Satoh et al., 1996) and typical values for the cell diameter (20 μm) and length (100 μm), resulting in a cytosol volume of ∼32 pL. Jleak is calculated such that the cell is at steady state for an [Na]i of 8 mM, typical of what we observed in our measurements, thus
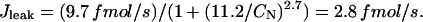 |
Because the rate of passive Na influx is rather small (Despa et al., 2002b), we assumed that Na is uniformly distributed within the cell at the moment of Na/K pump activation. The equations system in Eq. A1 was integrated numerically with an integration step of 50 μs. Generally, 10–50 shells were sufficient for convergence. In some cases, shells were of different thickness to more readily resolve the shape near the sarcolemma.
References
- Bassani, J. W. M., R. A. Bassani, and D. M. Bers. 1994. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J. Physiol. 476:279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers, D. M. 2001. Excitation-Contraction Coupling and Cardiac Contractile Force. Kluwer Academic Publishing, Dordrecht, The Netherlands.
- Bielen, F. V., H. G. Glitsch, and F. Verdonck. 1991a. Changes of the subsarcolemmal Na+ concentration in internally perfused cardiac cells. Biochim. Biophys. Acta. 1065:269–271. [DOI] [PubMed] [Google Scholar]
- Bielen, F. V., H. G. Glitsch, and F. Verdonck. 1991b. Dependence of Na+ pump current on external monovalent cations and membrane potential in rabbit cardiac Purkinje cells. J. Physiol. 442:169–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet, E. 1992. A fuzzy subsarcolemmal space for intracellular Na+ in cardiac cells. Cardiovasc. Res. 26:433–442. [DOI] [PubMed] [Google Scholar]
- Despa, S., M. A. Islam, S. M. Pogwizd, and D. M. Bers. 2002a. Intracellular [Na+] and Na+-pump rate in rat and rabbit ventricular myocytes. J. Physiol. 539:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despa, S., M. A. Islam, C. R. Weber, S. M. Pogwizd, and D. M. Bers. 2002b. Intracellular Na+ concentration is elevated in heart failure, but Na/K-pump function is unchanged. Circulation. 105:2543–2548. [DOI] [PubMed] [Google Scholar]
- Friedrich, T., E. Bamberg, and G. Nagel. 1996. Na+, K+-ATPase pump currents in giant excised patches activated by an ATP concentration jump. Biophys. J. 71:2486–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka, Y., S. Matsuoka, T. Ban, and A. Noma. 1998. Interaction of the Na+-K+ pump and Na+-Ca2+ exchange via [Na+]i in a restricted space of guinea-pig ventricular cells. J. Physiol. 509:457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch, H. G. 2001. Electrophysiology of the sodium-potassium-ATPase in cardiac cells. Physiol. Rev. 81:1791–1826. [DOI] [PubMed] [Google Scholar]
- Haugland, R. P. 1996. Handbook of Fluorescent Probes and Research Chemicals, 6th Ed. Molecular Probes Inc., Eugene, OR.
- Hilgemann, D. W., G. A. Nagel, and D. C. Gadsby. 1991. Na/K pump current in giant membrane patches excised from ventricular myocytes. In The Sodium Pump: Recent Developments. J. H. Kaplan and P. De Weer, editors. Rockefeller University Press, New York.
- Hilgemann, D. W., S. Matsuoka, G. A. Nagel, and A. Collins. 1992. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Sodium-dependent inactivation. J. Gen. Physiol. 100:905–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, D. W., L. M. Apel, and G. P. Brierley. 1992. Transmembrane gradients of free Na+ in isolated heart mitochondria estimated using a fluorescent probe. Am. J. Physiol. 262:C1047–C1055. [DOI] [PubMed] [Google Scholar]
- Kushmerick, M. J., and R. J. Podolsky. 1969. Ionic mobility in muscle cells. Science. 166:1297–1298. [DOI] [PubMed] [Google Scholar]
- Lee, C. O., and M. Dagostino. 1982. Effect of strophanthidin on intracellular Na ion activity and twitch tension on constantly driven canine cardiac Purkinje fibers. Biophys. J. 40:185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp, P., and E. Niggli. 1994. Sodium current-induced calcium signals in guinea-pig ventricular myocytes. J. Physiol. 474:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias, R. T., I. S. Cohen, and C. Oliva. 1990. Limitations of the whole-cell patch-clamp technique in the control of intracellular concentrations. Biophys. J. 58:759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough, A. A., Y. Zhang, V. Shin, and J. S. Frank. 1996. Subcellular distribution of Na pump isoform subunits in mammalian cardiac myocytes. Am. J. Physiol. 270:C1221–C1227. [DOI] [PubMed] [Google Scholar]
- Nakao, M., and D. C. Gadsby. 1989. [Na] and [K] dependence of the Na/K pump current-voltage relationship in guinea pig ventricular myocytes. J. Gen. Physiol. 94:539–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson, K. D., D. M. Bers, A. Y. Nishimoto, and G. A. Langer. 1980. The binding of calcium and sodium to sarcolemmal membranes: relation to the control of myocardial contractility. Am. J. Physiol. 238:H373–H378. [DOI] [PubMed] [Google Scholar]
- Satoh, H., L. M. Delbridge, L. A. Blatter, and D. M. Bers. 1996. Surface: volume relationship in cardiac myocytes studied with confocal microscopy and membrane capacitance measurements: species-dependence and developmental effects. Biophys. J. 70:1494–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semb, S. O., and O. M. Sejersted. 1996. Fuzzy space and control of Na+-K+ pump rate in heart and skeletal muscle. Acta Physiol. Scand. 156:213–224. [DOI] [PubMed] [Google Scholar]
- Silverman, B. D. Z., A. Warley, J. I. A. Miller, A. F. James, and M. J. Shattock. 2002. Is there a transient rise in sub-sarcolemmal Na and activation of Na/K pump current after activation of INa in ventricular myocardium? Cardiovasc. Res. 57:1025–1034. [DOI] [PubMed] [Google Scholar]
- Su, Z., A. Zou, A. Nonaka, I. Zubair, M. C. Sanguinetti, and W. H. Barry. 1998. Influence of prior Na+ pump activity on pump and Na+/Ca2+ exchange currents in mouse ventricular myocytes. Am. J. Physiol. 275:H1808–H1817. [DOI] [PubMed] [Google Scholar]
- Su, Z., K. Sugishita, M. Ritter, F. Li, K. W. Spitzer, and W. H. Barry. 2001. The sodium pump modulates the influence of INa on [Ca2+]i transients in mouse ventricular myocytes. Biophys. J. 80:1230–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano, C. M. N. 2001. Rapid inhibition of the Na+-K+ pump affects Na+-Ca2+ exchanger-mediated relaxation in rabbit ventricular myocytes. J. Physiol. 533:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafford, A. W., M. E. Diaz, S. C. O'Neil, and D. A. Eisner. 1995. Comparison of subsarcolemmal and bulk calcium concentration during spontaneous calcium release in rat ventricular myocytes. J. Physiol. 488:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt-Gallitelli, M. F., T. Voigt, and G. Isenberg. 1993. Microheterogeneity of subsarcolemmal sodium gradients. Electron probe microanalysis in guinea-pig ventricular myocytes. J. Physiol. 472:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]







