Abstract
Extracellular ATP at millimolar concentrations inhibits growth of mycobacteria in human macrophages. Whether T cells can produce sufficient ATP is unknown. CD4+ and CD8+ T cells did not release sufficient ATP through either degranulation or lysis of bystander cells to restrict growth of Mycobacterium bovis BCG in monocytes.
It has been demonstrated that multiple T-cell subsets are involved in the restriction of mycobacterial growth in vitro (4, 9, 18, 19). How T cells mediate enhanced killing of Mycobacterium tuberculosis remains controversial. In vitro, ATP induces killing of mycobacteria in human macrophages by signaling through P2X7 receptors, which increases calcium and phospholipase D-dependent phagolysosome fusion (12, 15-17, 20). The concentrations of extracellular ATP required for mycobacterial growth restriction in macrophages are 1 to 3 mM. These are high concentrations for extracellular spaces, which normally contain nanomolar concentrations. Cytotoxic T cells could not raise extracellular ATP levels at sites of infection through either T-cell degranulation or the lysis of infected cells. In the present study, we aimed to determine whether the ATP released by T cells has a role in the control of mycobacterial growth in macrophages.
ATP-induced lysis in monocytes.
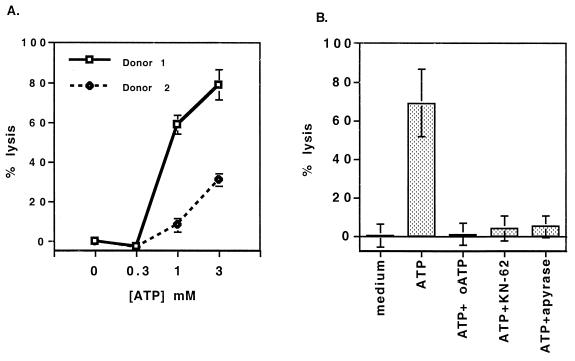
First, the concentration of extracellular ATP required to induce P2X7 signaling was determined by measuring ATP-induced cytolysis of blood monocytes (MN). Extracellular ATP induces apoptosis and lysis through P2X7 signaling in macrophages (3, 21). For all experiments, MN from normal donors were purified with immunomagnetic beads by negative selection (monocyte isolation kit; Miltenyi) and pretreated with gamma interferon (IFN-γ) (100 U/ml) for 2 days to upregulate P2X7 receptor expression, thereby increasing sensitivity to ATP. The degree of ATP-induced lysis was determined by release of 51Cr from MN 4 h after ATP exposure (Fig. 1A). Both donors responded maximally to 3 mM ATP, while exhibiting intermediate responses to 1 mM ATP and no response to 0.3 mM ATP. Thus, an ATP concentration range of 1 to 3 mM was found to be optimal for the killing of both Mycobacterium bovis BCG and M. tuberculosis in human macrophages (15, 17). ATP antagonists were found to completely inhibit the lysis of macrophages (Fig. 1B).
FIG. 1.
ATP induces lysis in monocytes. (A) IFN-γ-prestimulated MN from two donors were loaded with 51Cr and incubated with different concentrations of ATP for 4 h. (B) After 51Cr loading, macrophages were preincubated with oxidized ATP (oATP) (300 μM) and either KN-62 (2 μM) or apyrase (2 U/ml); ATP (3 mM) was then added. 51Cr release was measured by sampling the supernatant. The percentage of lysis was calculated on the basis of the maximal lysis induced by 1% sodium dodecyl sulfate. The error bars represent the standard deviations of values from triplicate wells.
Release of ATP during T-cell degranulation.
Whether the 1 to 3 mM concentrations of ATP required for macrophage apoptosis can be achieved following human T-cell degranulation is unknown. To quantitate ATP release from degranulating human CD4+ and CD8+ T cells, highly activated T-cell lines were generated by stimulating peripheral blood mononuclear cells with anti-CD3 and interleukin-2 for 7 to 14 days. CD4+ and CD8+ T cells were purified by positive selection with immunomagnetic beads (Dynal) and degranulation triggered by plate-bound anti-CD3. Assays were performed with serum-free assay buffer, as serum reduces ATP levels by 50% within 1 h (8). Supernatants from T cells with and without anti-CD3 stimulation were harvested for 1 h and analyzed for ATP, β-hexosaminidase (to measure the degree of degranulation), and lactate dehydrogenase, an indicator of nonspecific cell death (13). Plate-bound anti-CD3 induced a 10 to 43% degranulation of T cells with a nonspecific cytolysis of <3%. ATP and ADP plus AMP levels in supernatants were measured by a rephosphorylation assay using firefly luciferase (Table 1) (1). In half the experiments, statistically significant increases in ATP levels were measured after plate-bound anti-CD3 induced degranulation compared to what occurred in wells with no antibody (P < 0.05). These studies demonstrate that concentrations of ATP plus ADP plus AMP released by degranulating T cells (106/ml) were low, ranging from 2 to 92 nM.
TABLE 1.
Adenosine nucleotides released from degranulating T-cell linesa
| T cell | Expt | Concn of ATP (nM) with:
|
Total concn of ATP-ADP-AMP (nM) with:
|
||
|---|---|---|---|---|---|
| No MAb | Anti-CD3 | No MAb | Anti-CD3 | ||
| CD4+ | 1 | 2.7 | 2.2 | 5.9 | 2.2 |
| 2 | 2.7 | 4.0 | 15.7 | 24 | |
| 3 | 14 | 18b | 51 | 92 | |
| CD8+ | 4 | 0.53 | 1.2b | 1.4 | 1.5 |
| 5 | 1.1 | 1.8b | 6.9 | 4.5 | |
| 6 | 1.3 | 2.1 | 2.2 | 2.4 | |
CD4+ and CD8+ T cells (106/ml) were purified from anti-CD3- and interleukin-2-activated peripheral blood mononuclear cells and incubated in ATP assay buffer on anti-CD3-coated and -uncoated plates for 1 h. Wells were harvested and spun, and the supernatants were boiled. ATP alone and ADP plus AMP were measured by rephosphorylation assay. In all cases, the level of lactate dehydrogenase was <3% and levels of β-hexosaminidase were 10 to 20% for CD4+ T cells and 18 to 43% for CD8+ T cells of the sodium dodecyl sulfate-induced maximums for each. MAb, monoclonal antibody.
Significant increase in ATP over the level in wells not treated with MAb (P < 0.05).
ATP values reported here represent ATP concentrations after the degranulation of human T cells into a large extracellular space. The estimation of achievable concentrations in the local microenvironment of a pericellular space, such as the immunological synapse, requires the following calculations and assumptions. The maximum ATP concentration achieved in our studies (Table 1, experiment 3) was 18 nM, after 106 cells degranulated into 1 ml. This represents 18 pmol of ATP released. Several recent studies have estimated that the pericellular space in tightly packed tissues is only 10% of the cellular space (2, 5). One million T cells occupy about 1 μl of cellular space. If 18 pmol of ATP was released into 0.1 μl of pericellular space, ATP concentrations would reach 180 μM. This is a 5- to 10-fold-lower concentration than that required to inhibit mycobacterial growth. ATP is rapidly degraded by high levels of ecto-ATPase activity of activated T cells, which further reduces extracellular concentrations of ATP (10). Based on these assumptions and calculations, it is unlikely that a level of extracellular ATP sufficient to induce mycobacterial killing is generated locally by degranulating T cells.
These data do not exclude the possibility that vectorial ATP release into the small volume of an “immunologic synapse” can activate macrophages through P2X7 to kill mycobacteria. However, the low ATP concentrations are unlikely to be adequate for signaling the P2X7 receptor long enough, given the high ecto-ATPase activity on the surfaces of activated T cells. ATP antagonists, KN-62 and oxidized ATP, were found to have nonspecific inhibitory effects in 51Cr macrophage T-cell lysis assays and growth restriction assays. Thus, they could not be used to determine whether the small amounts of ATP after T-cell degranulation reached a concentration sufficient to signal the P2X7 receptor.
Bystander ATP does not inhibit BCG growth.
T cells may also generate extracellular ATP by lysing cells, which precipitates release of cytosolic ATP in the immediate vicinity of infected macrophages. As cytoplasmic ATP concentrations are 3 to 5 mM, bystander cells may be a significant source of ATP for signaling through purinergic receptors such as P2X7 (7, 11, 14).
Two experimental systems were established to determine if bystander ATP, which is released when T cells lyse target cells, affects BCG growth in MN. They were designed to generate extracellular ATP by lysis of bystander cells. The acute myeloid leukemia cell line KG-1 (American Type Culture Collection, Manassas, Va.), served as an ATP donor cell line because it has low ecto-ATPase activity (6). In the first experimental system, pretreatment of KG-1 cells with UV irradiation for 30 min induced lysis over the course of several hours. In the second, KG-1 cells were pretreated with the superantigen staphylococcal enterotoxin B (SEB), which binds their major histocompatibility complex class II molecules to label them as selective targets for activated T-cell lines. The maximum number of KG-1 cells that could be supported by the tissue medium was used. In each experimental system, KG-1 cells were in intimate contact with BCG-infected MN at the time of lysis to create a microenvironment where ATP concentrations would be highest locally.
Table 2 presents the concentrations of ATP and total concentrations of ATP plus ADP plus AMP detected in supernatants by these two experimental systems. Nucleotide levels were measured by rephosphorylation assays. By 3 h, UV-treated KG-1 cells were lysed by 50%, as measured by 51Cr release, and concentrations of total nucleotide (ATP plus ADP plus AMP) reached 2.6 μM. After incubation with T cells, 24 to 50% of SEB-treated KG-1 cells, measured by a 51Cr release assay, were lysed after 4 h. The lower nucleotide concentrations in cultures of T cells with SEB-treated KG-1 cells compared to those with KG-1 cells alone may have been due to the high ecto-ATPase activity of activated T cells.
TABLE 2.
Aderosine nucleotides released by KG-1 cells treated with UV irradiation or lysed with T cells
| Exptl system | Time of measure- ment (h) | Concn of ATP (nM) | Total concn of ATP-ADP-AMP (nM) |
|---|---|---|---|
| UV irradiation of KG-1 cells | 1 | 404 | 1,986 |
| No irradiation of KG-1 cells | 1 | 146 | 478 |
| UV irradiation of KG-1 cells | 3 | 293 | 2,609 |
| No irradiation of KG-1 cells | 3 | 88 | 442 |
| T cellsa + SEB-treated KG-1 cells | 1 | 2.8 | 2.8 |
| T cells + untreated KG-1 cells | 1 | 3.7 | 3.7 |
| T cells + SEB-treated KG-1 cells | 3 | 7.4 | 7.4 |
| T cells + untreated KG-1 cells | 3 | 7.1 | 3.8 |
In one group, 3 × 105 KG-1 cells per well were UV irradiated and placed in 200 μl. In a second group, 3 × 105 T cells per well and KG-1 cells labeled or not labeled with SEB were plated in 200 μl. At 1 and 3 h, supernatants were harvested and boiled, and ATP and ADP-AMP were measured by rephosphorylation assay. ATP results are representative of two experiments.
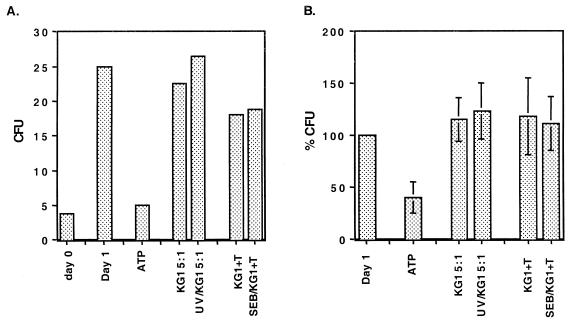
To determine the effect produced by the release of bystander cell ATP on mycobacterial growth, 105 IFN-γ-treated MN were infected with BCG at a 1:1 multiplicity of infection. After the cells were washed extensively, KG-1 cells, T cells, or ATP was added and the solution was briefly centrifuged to maximize cell contact. After overnight culture, cells were lysed with saponin (0.2%) and the number of CFU/well was measured. Figure 2A demonstrates that there were no reductions in the numbers of BCG CFU in experimental groups when lysed KG-1 cells served as an extracellular source of ATP. Figure 2B demonstrates the mean changes in the numbers of CFU in the experimental groups expressed as percentages relative to the number of CFU in infected MN alone. For the three donors, there was no statistically significant difference in numbers of CFU by paired t test between UV-treated and nontreated wells or between SEB-KG-1 cell-treated and nontreated wells. To constitute a positive control, BCG-infected MN were treated with extracellular ATP, which resulted in a significant reduction in growth.
FIG. 2.
Bystander ATP fails to induce killing of BCG. One hundred thousand IFN-γ-pretreated MN were infected with BCG at a multiplicity of infection of 1:1 and incubated overnight in round-bottom wells in two triplicate groups with either KG-1 cells (5 × 105) that were treated or not treated with UV, KG-1 cells (3 × 105) that were coated or not coated with SEB and activated T cells (3 × 105), or ATP (3 mM). (A) Results for one donor. Results are representative of three experiments performed identically on different donors. Values are expressed as mean numbers of CFU times 104 cells of duplicate cultures per milliliter. (B) Percentages of CFU for each condition relative to the number of BCG CFU in infected MN alone after overnight incubation. Values are the means and standard errors of the means of results from three different experiments.
On the basis of these studies, we conclude that human T cells release very low concentrations of ATP after degranulation and that bystander ATP release in the microenvironment of BCG-infected MN was insufficient to induce mycobacterial killing. Taken together, these findings suggest that T cells do not likely provide an adequate amount of ATP to induce mycobacterial growth restriction in MN.
Acknowledgments
W.H.B. and G.R.D. shared senior authorship in this work.
This work is funded by National Institutes of Health grants K08 AI 01581 (D.H.C.) and AI 27243 (W.H.B.) and Tuberculosis Research Unit grants AI 95383 and GM 36387 (G.R.D.) and HL 59858 (R.F.S.). This work was also supported in part by the Center for AIDS Research at Case Western Reserve/University Hospitals of Cleveland (grant AI-36219).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Beigi, R. D., and G. R. Dubyak. 2000. Endotoxin activation of macrophages does not induce ATP release and autocrine stimulation of P2 nucleotide receptors. J. Immunol. 165:7189-7198. [DOI] [PubMed] [Google Scholar]
- 2.Bjornaes, I., E. F. Halsor, A. Skretting, and E. K. Rofstad. 2000. Measurement of the extracellular volume of human melanoma xenografts by contrast enhanced magnetic resonance imaging. Magn. Reson. Imaging 18:41-48. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard, D. K., S. L. Hoffman, and J. Y. Djeu. 1995. Inhibition of extracellular ATP-mediated lysis of human macrophages by calmodulin antagonists. J. Cell. Biochem. 57:452-464. [DOI] [PubMed] [Google Scholar]
- 4.Canaday, D. H., R. J. Wilkinson, Q. Li, C. V. Harding, R. F. Silver, and W. H. Boom. 2001. CD4(+) and CD8(+) T cells kill intracellular Mycobacterium tuberculosis by a perforin and Fas/Fas ligand-independent mechanism. J. Immunol. 167:2734-2742. [DOI] [PubMed] [Google Scholar]
- 5.Chen, K. C., and C. Nicholson. 2000. Changes in brain cell shape create residual extracellular space volume and explain tortuosity behavior during osmotic challenge. Proc. Natl. Acad. Sci. USA 97:8306-8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clifford, E. E., K. A. Martin, P. Dalal, R. Thomas, and G. R. Dubyak. 1997. Stage-specific expression of P2Y receptors, ecto-apyrase, and ecto-5′-nucleotidase in myeloid leukocytes. Am. J. Physiol. 273:C973-C987. [DOI] [PubMed] [Google Scholar]
- 7.Cook, S., and E. McCleskey. 2002. Cell damage excites nociceptors through release of cytosolic ATP. Pain 95:41-47. [DOI] [PubMed] [Google Scholar]
- 8.Cowen, D. S., M. Berger, L. Nuttle, and G. R. Dubyak. 1991. Chronic treatment with P2-purinergic receptor agonists induces phenotypic modulation of the HL-60 and U937 human myelogenous leukemia cell lines. J. Leukoc. Biol. 50:109-122. [DOI] [PubMed] [Google Scholar]
- 9.Dieli, F., M. Troye-Blomberg, J. Ivanyi, J. J. Fournie, M. Bonneville, M. A. Peyrat, G. Sireci, and A. Salerno. 2000. Vgamma9/Vdelta2 T lymphocytes reduce the viability of intracellular Mycobacterium tuberculosis. Eur. J. Immunol. 30:1512-1519. [DOI] [PubMed] [Google Scholar]
- 10.Dombrowski, K. E., Y. Ke, K. A. Brewer, and J. A. Kapp. 1998. Ecto-ATPase: an activation marker necessary for effector cell function. Immunol. Rev. 161:111-118. [DOI] [PubMed] [Google Scholar]
- 11.Dubyak, G. R., and C. el-Moatassim. 1993. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am. J. Physiol. 265:C577-C606. [DOI] [PubMed] [Google Scholar]
- 12.Fairbairn, I. P., C. B. Stober, D. S. Kumararatne, and D. A. Lammas. 2001. ATP-mediated killing of intracellular mycobacteria by macrophages is a P2X(7)-dependent process inducing bacterial death by phagosome-lysosome fusion. J. Immunol. 167:3300-3307. [DOI] [PubMed] [Google Scholar]
- 13.Haddad, E. K., X. Wu, J. A. Hammer III, and P. A. Henkart. 2001. Defective granule exocytosis in Rab27a-deficient lymphocytes from Ashen mice. J. Cell Biol. 152:835-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jorgensen, N. R., Z. Henriksen, O. H. Sorensen, E. F. Eriksen, R. Civitelli, and T. H. Steinberg. 2001. Intercellular calcium signaling occurs between human osteoblasts and osteoclasts and requires activation of osteoclast P2X7 receptors. J. Biol. Chem. 276:52.. [DOI] [PubMed] [Google Scholar]
- 15.Kusner, D. J., and J. Adams. 2000. ATP-induced killing of virulent Mycobacterium tuberculosis within human macrophages requires phospholipase D. J. Immunol 164:379-388. [DOI] [PubMed] [Google Scholar]
- 16.Kusner, D. J., and J. A. Barton. 2001. ATP stimulates human macrophages to kill intracellular virulent mycobacterium tuberculosis via calcium-dependent phagosome-lysosome fusion. J. Immunol. 167:3308-3315. [DOI] [PubMed] [Google Scholar]
- 17.Lammas, D. A., C. Stober, C. J. Harvey, N. Kendrick, S. Panchalingam, and D. S. Kumararatne. 1997. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity 7:433-444. [DOI] [PubMed] [Google Scholar]
- 18.Silver, R. F., Q. Li, W. H. Boom, and J. J. Ellner. 1998. Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: requirement for CD4+ T cells in purified protein derivative-positive, but not in purified protein derivative-negative subjects. J. Immunol. 160:2408-2417. [PubMed] [Google Scholar]
- 19.Stenger, S., R. J. Mazzaccaro, K. Uyemura, S. Cho, P. F. Barnes, J. P. Rosat, A. Sette, M. B. Brenner, S. A. Porcelli, B. R. Bloom, and R. L. Modlin. 1997. Differential effects of cytolytic T cell subsets on intracellular infection. Science 276:1684-1687. [DOI] [PubMed] [Google Scholar]
- 20.Stober, C. B., D. A. Lammas, C. M. Li, D. S. Kumararatne, S. L. Lightman, and C. A. McArdle. 2001. ATP-mediated killing of Mycobacterium bovis bacille Calmette-Guerin within human macrophages is calcium dependent and associated with the acidification of mycobacteria-containing phagosomes. J. Immunol. 166:6276-6286. [DOI] [PubMed] [Google Scholar]
- 21.Zheng, L. M., A. Zychlinsky, C. C. Liu, D. M. Ojcius, and J. D. Young. 1991. Extracellular ATP as a trigger for apoptosis or programmed cell death. J. Cell Biol. 112:279-288. [DOI] [PMC free article] [PubMed] [Google Scholar]