Abstract
Transmission of Mycobacterium tuberculosis from one individual to another usually is associated with episodes of coughing. The bacteria leave the environment of the lung cavity of the infected person and travel in droplets to reach the recipient's respiratory tract. Therefore, at the time that the bacteria encounter alveolar cells (macrophages and epithelial cells) in the new host, they express virulence determinants that are regulated by the environmental conditions in the infected person. To determine if those environmental conditions encountered in the lung cavity (hyperosmolarity, acidic pH, and low oxygen tension, among others) would influence the uptake of M. tuberculosis by the recipient's alveolar macrophages, M. tuberculosis H37Rv was incubated under several conditions for different periods of time, washed at 4°C, and used to infect human monocyte-derived macrophages. While increased osmolarity had no effect on M. tuberculosis uptake compared to the uptake of bacteria grown on 7H10 Middlebrook medium, both acidic pH and anaerobiosis increased the uptake of the H37Rv strain four- to sixfold. Using anti-CD11b receptor blocking antibodies or mannoside to inhibit the uptake of M. tuberculosis by macrophages, we determined that while uptake of M. tuberculosis cultured on 7H10 medium was inhibited 77% ± 6% in the presence of anti-CD11b antibody, the antibody had no effect on the uptake of M. tuberculosis incubated at pH 6.0 and was associated with 27% inhibition of M. tuberculosis previously exposed to anaerobic conditions. The mannose receptor was also not involved with invasion after exposure to acidic conditions, and mannoside resulted in only 32% inhibition of uptake by macrophages of M. tuberculosis exposed to anaerobiosis. Uptake by macrophages also resulted in the secretion of significantly lower amounts of interleukin-12 and tumor necrosis factor alpha than that by macrophages infected with a strain cultured under laboratory conditions. M. tuberculosis cultured under the pH and oxygen concentration found in the granuloma expresses a large number of proteins that are different from the proteins expressed by bacteria grown under laboratory conditions. The results suggest that M. tuberculosis in vivo may be adapted to gain access to the intracellular environment in a very efficient fashion and may do so by using different receptors from the complement and mannose receptors.
Transmission of Mycobacterium tuberculosis from an infected individual to a naive host takes place by aerosol in the majority of cases (22, 23). The initial step in the infection process is the contact between the inhaled bacteria and alveolar epithelial mucosal cells and alveolar macrophages.
A number of studies have demonstrated that conditions present in the environment influence the gene expression and phenotype of virulent microorganisms. For instance, Yersinia pseudotuberculosis upregulates the expression of invasion-associated protein when exposed to acidic pH (15). Likewise, the Salmonella invasive phenotype is induced by low concentrations of oxygen in the environment, and virulence determinants in Bordetella pertussis are regulated by environmental conditions (17, 19). A recent study has shown that Mycobacterium avium invasion of intestinal mucosal cells is regulated by anaerobiosis and hyperosmolarity (5), which are both conditions encountered in the intestinal lumen.
The complement receptors have been linked with the uptake of mycobacteria by macrophages (6, 14, 25). In addition, the mannose receptor in macrophages was shown to bind to lipoarabinomannan (LAM) in both M. tuberculosis and M. avium (6, 24). In the lungs of infected individuals, M. tuberculosis can be found in granulomatous lesions with caseous centers, which when opened to the bronchial lumen can expel their contents in the airways, making the infected individual contagious (9). Therefore, a bacterium that is expelled from the lung of an infected host following an episode of coughing will come in contact with alveolar cells in the new host after being exposed to the cues existing within the granulomas, i.e., acidic pH, hyperosmolarity, and low oxygen tension (10, 20).
Because the conditions found in the granuloma are likely to influence the bacterial phenotype and consequently the manner that bacteria interact with cells in the new host, we investigated if exposure to the conditions encountered in granulomas of infected individuals would have any effect on the uptake of M. tuberculosis by macrophages.
MATERIALS AND METHODS
M. tuberculosis.
The M. tuberculosis H37Rv (ATCC 27294) and Erdman (ATCC 35801) strains were cultured as described previously (3). The bacterial inoculum was adjusted to 108 bacteria/ml prepared by passing the bacterial suspension through a 23-gauge needle 10 times and vortex agitating the suspension briefly. The suspension was allowed to rest for 5 min in a 15-ml polystyrene tube and then 5 ml of the top was removed and used as the inoculum. The inoculum was stained by the Ziehl-Neelson technique and observed by light microscopy. Only dispersed preparations were used in the assays. Viability of the bacteria was determined by the LIVE-DEAD assay (Molecular Probes, Eugene, Oreg.) and shown to be between 89 and 92% (4).
Monocyte-derived macrophages.
Blood was obtained from five healthy adult donors. Peripheral blood mononuclear cells were isolated from heparinized blood on a Ficoll-Histopaque (Sigma Chemicals, St. Louis, Mo.) gradient and seeded in 24-well tissue culture plates in the presence of RPMI 1640 supplemented with 20% autologous serum. Monocytes matured into macrophages after 3 to 4 days. Each well contained approximately 5 × 105 macrophages. Blood of all the donors was used to perform every type of assay. The results were not significantly different among the donors.
Reagents.
α-Methyl-mannoside was purchased from Sigma. The antibodies used were anti-CD11b OKM1 (immunoglobulin G2b [IgG2b]; American Type Culture Collection, Rockville, Md.), anti-CR1 (CD35; Becton Dickinson, Mountain View, Calif.), and anti-CD29 (Biosource, Camarillo, Calif.). Anti-integrin α2, α3, α5, and α6 chains were also purchased from Biosource International. IgG2b anti-Pseudomonas aeruginosa lipid A was purchased from List Biologicals Laboratory (Campbell, Calif.).
Uptake and binding assay.
The assay was carried out as previously described (4, 6). Briefly, macrophage monolayers (5 × 105 cells) were incubated with M. tuberculosis (approximately 5 × 105 or 5 × 106 bacteria/monolayer) in RPMI 1640 with 10% autologous serum for 1 h at 4°C (binding) or 37°C (uptake). For some experiments, heat-inactivated serum was used. Then the monolayers were washed vigorously to remove unbound extracellular bacteria and were lysed with sterile water and 0.02% sodium dodecyl sulfate for 10 min. The lysate was then diluted and plated for quantitation. To determine the degree of protein synthesis by M. tuberculosis, we pulsed 107 H37Rv cells with 1 μCi of [35S]methionine (specific activity, 1.032 mCi/mmol) in minimal medium for 2 h, exposed the bacteria to pH 6 for 5 h in the presence or absence of 0.5 μg of rifampin per ml, and measured incorporation of [35S]methionine as previously reported (7). A control in which unlabeled methionine was added prior to the addition of [35S]methionine was run in parallel. Some assays were performed with bacteria incubated for 4 h in the presence of 0.5 μg of rifampin per ml (subinhibitory concentration; MIC = 1 μg/ml) to inhibit transcription (12). To confirm the results obtained by the colony counts of intracellular bacteria, we used M. tuberculosis H37Rv expressing green fluorescent protein (GFP) constructed in a previously described plasmid (27) (kindly provided by Joel Ernst, University of San Francisco, San Francisco, Calif.). In this system, GFP expression in the bacterium is under control of the mycobacterial gene hsp-60. The internalized bacteria were quantitated by counting the number of intracellular organisms by optical microscopy and by quantitation fluorescent light emission by using a fluorimeter (18). Because the limit of detection by this method was determined to be approximately 5 × 105 bacilli for these specific GFP-labeled bacteria, we infected macrophage monolayers in 96-well tissue culture plates with 108 bacteria. The rest of the protocol was similar to that described above.
Environmental conditions.
In an attempt to reproduce the conditions encountered by M. tuberculosis in the site of infection in vivo, we incubated M. tuberculosis strains under the following conditions prior to the assays: 37°C, 20% O2, and pH 7.2. Differences in pH were established by using 0.1 N HCl and 0.1 N NaOH. The effect of increased osmolarity was tested aerobically in 7H9 broth supplemented with dextrose at equimolar concentrations (0.1, 0.2, and 0.3 M). Oxygen-deficient (anaerobic) conditions were obtained in an anaerobic jar (VWR, San Francisco, Calif.) as previously described (5). After the period of exposure to different conditions, bacteria were washed at 4°C and resuspended in RPMI 1640 prior to use in the assays. The number of bacteria in the inoculum after exposure to the different conditions was determined, as well as the degree of dispersion of the suspension and the viability of the inoculum, by using the LIVE-DEAD assay. No condition was shown to cause bacterial clumping or a decrease in viability.
Two-dimensional gel electrophoresis.
M. tuberculosis H37Rv was grown under the following conditions: (i) pH 6.0, high osmolarity (0.3 M dextrose), and anaerobiosis; (ii) pH 7.0, iso-osmolar medium, and anaerobiosis; (iii) pH 6.0, high osmolarity (0.3 M dextrose), and aerobiosis; and (iv) pH 7.0, iso-osmolarity, and aerobiosis. Differences in pH, osmolarity, and anaerobic condition were established as described previously (5). After 7 days of growing, cells were harvested, washed once with phosphate-buffered saline plus 0.05% Tween 80, and resuspended in sonication buffer (20 mM Tris-HCl, 1 mM EDTA, 5 mM β-mercaptoethanol, pH 8.0) supplemented with protease inhibitor (1 mM phenylmethylsulfonyl fluoride). Cells were disrupted with a Beat-beater (Biospe Products) six times for 30 s each, the supernatant was concentrated, and the protein concentration was determined by using the Coomassie blue assay (Bio-Rad). Two-dimensional gel electrophoresis was performed as described previously (16, 28). Protein samples of 40 μg each were dissolved in an equal volume of sample buffer containing 9.5 M urea, 2% Triton X-100, 5% β-mercaptoethanol, 1.6% Bio-lyte 5/7 ampholyte, and 0.4% Bio-lyte 3/10 ampholyte. The samples were centrifuged for 15 min, loaded onto 1 mm (internal diameter) by 8 cm (length) isofocusing tube gels, and then focused at 200 V for 16 h and 400 V for 2 h. The second dimensional gels were Tris-HCl-12% polyacrylamide gels focused at 200 V for 1 h. After that, the samples were electrotransferred from gels to polyvinylidene difluoride membranes at 75V for 2 h, and interesting spots were cut off and sent for protein sequencing.
ELISA.
Supernatants of macrophage monolayers infected with M. tuberculosis were obtained at several time points after infection with strain H37Rv exposed for 4 h to different conditions. At 24 h after uptake, supernatants were collected, filtered through a 0.22-μm-pore-size filter, and frozen. To determine the concentrations of interleukin-12 (IL-12) and tumor necrosis factor alpha (TNF-α) in the supernatant, we carried out enzyme-linked immunosorbent assay (ELISA) tests using kits purchased from Biosource International, according to directions suggested by the manufacturer.
Statistical analysis.
The assays were repeated at least three times and the results were analyzed by a two-tailed Student t test.
RESULTS
M. tuberculosis uptake by macrophages after exposure to different conditions.
M. tuberculosis H37Rv and Erdman were exposed for 1, 2, 4, and 24 h to different pHs (5.0, 6.0, 7.2, 8.0), anaerobiosis, and hyperosmolarity (0.1, 0.2, and 0.3 M dextrose). The data in Tables 1, 2 and 3 show that M. tuberculosis (because the results were similar, only the results with strain H37Rv are shown) exposed to acid pH and anaerobiosis but not hyperosmolarity was able to invade macrophages with significantly greater efficiency than M. tuberculosis grown under laboratory conditions (37°C, pH 7.2, 20% O2). The effect of pH and anaerobiosis in the uptake could be observed after 2 h of incubation and was maximal at 4 h. The influence of the environment on the bacterial phenotype is temporary, but it lasts longer than 1 h (4).
TABLE 1.
Uptake of M. tuberculosis by human monocyte-derived macrophages following exposure to different pHs
| Time of exposure to acid (h)a | Uptake (% of initial inoculum) of M. tuberculosis at pH:
|
Uptake (%) at pH 6 withb:
|
||||
|---|---|---|---|---|---|---|
| 5.0 | 6.0 | 7.2 | 8.0 | Serum (10%) | No serum | |
| 1 | 20 ± 4 | 23 ± 6 | 17 ± 6 | 15 ± 8 | ND | ND |
| 2 | 47 ± 6c | 49 ± 5c | 19 ± 4 | 16 ± 5 | ND | ND |
| 4 | 57 ± 14c | 59 ± 7c | 18 ± 6 | 20 ± 4 | 48 ± 5 | 49 ± 3 |
| 24 | 51 ± 9c | 48 ± 7c | 19 ± 5 | 20 ± 6 | ND | ND |
M. tuberculosis H37Rv in 7H9 broth was exposed to different conditions for 1, 2, 4, and 24 h. After exposure, the bacteria were centrifuged at 4°C and resuspended in RPMI 1640 with 10% heat-inactivated autologous serum, and the concentration was adjusted to 5 × 106 bacteria/ml (multiplicity of infection [MOI] of 10). Phagocytosis was carried out for 1 h. Results using an MOI of 1 were similar and are not shown. The experiment was repeated four times. Results are means ± standard deviations.
Bacteria (5 × 106) were incubated at pH 6 for 4 h prior to the assay. Then, bacteria were washed at 4°C and incubated with macrophages in the absence or presence of 10% autologous serum or were heat inactivated for 1 h. The uptake of bacteria cultured under laboratory conditions by macrophages increased from 16% ± 3% to 24% ± 4% of the inoculum (P < 0.05). ND, Not done.
P < 0.05 compared with uptake of M. tuberculosis incubated at pH 7.2 for the same period of time.
TABLE 2.
Uptake of M. tuberculosis by human monocyte-derived macrophages following exposure to anaerobiosis
| Time of exposure to anaerobiosis (h)a | Uptake (% of initial inoculum) of M. tuberculosis
|
Uptake (%) under anaerobiosisb with:
|
||
|---|---|---|---|---|
| Anaerobiosis | Control (20% O2) | 10% Serum | No serum | |
| 1 | 19 ± 6 | 16 ± 4 | ND | ND |
| 2 | 32 ± 4c | 17 ± 5 | ND | ND |
| 4 | 61 ± 8c | 20 ± 6 | 51 ± 5 | 53 ± 6 |
| 24 | 53 ± 12c | 21 ± 5 | ND | ND |
M. tuberculosis H37Rv cultured in 7H9 broth was exposed to anaerobiosis for 1, 2, 4, and 24 h. After exposure, the bacteria were centrifuged at 4°C and resuspended in RPMI 1640 with 10% heat-inactivated autologous serum, and the concentration was adjusted to 5 × 106 bacteria/ml (MOI of 10). Phagocytosis was carried out for 1 h. Results using an MOI of 1 were similar and are not shown. The experiment was repeated four times. Results are means ± standard deviations.
Bacteria (5 × 106) were incubated under anaerobiosis for 4 h prior to the assay. Then, the bacteria were washed at 4°C and incubated with macrophages in the absence or presence of 10% serum for 1 h. The uptake of bacteria cultured under laboratory conditions by macrophages increased from 16% ± 3% to 24% ± 4% of the inoculum (P < 0.05). ND, not done.
P < 0.05 compared with uptake of M. tuberculosis incubated under 20% oxygen tension.
TABLE 3.
Uptake of M. tuberculosis by human monocyte-derived macrophages following exposure to hyperosmolar conditions
| Time of exposure to osmolarity (h)a | Uptake (% of initial inoculum) of M. tuberculosis under conditions of osmolarity
|
Uptake (%) under conditions of osmolarity (0.3 M dextrose)b with:
|
||||
|---|---|---|---|---|---|---|
| Isoosmolarc | 0.1 M dextrose | 0.2 M dextrose | 0.3 M dextrose | 10% Serum | No serum | |
| 1 | 19 ± 6 | 17 ± 4 | 19 ± 2 | 19 ± 5 | ND | ND |
| 2 | 21 ± 4 | 19 ± 6 | 20 ± 4 | 23 ± 4 | ND | ND |
| 4 | 20 ± 6 | 19 ± 4 | 22 ± 3 | 22 ± 6 | 25 ± 5 | 18 ± 2 |
| 24 | 20 ± 3 | 21 ± 5 | 18 ± 5 | 21 ± 4 | ND | ND |
M. tuberculosis H37Rv cultured in 7H9 broth was exposed to different conditions for 1, 2, 4, or 24 h. After exposure, the bacteria were centrifuged at 4°C and resuspended in RPMI 1640 with 10% heat-inactivated autologous serum, and the concentration was adjusted to 5 × 106 bacteria/ml (MOI of 10). Phagocytosis was carried out for 1 h. Results using an MOI of 1 were similar and are not shown. The experiment was repeated four times. Results are means ± standard deviations.
Bacteria (5 × 106) were incubated under conditions hyperosmolarity for 4 h prior to the assay. Then, the bacteria were washed at 4°C and incubated with macrophages in the absence or presence of 10% serum for 1 h. The uptake of bacteria cultured under laboratory conditions by macrophages increased from 16% ± 3% to 24% ± 4% of the inoculum (P < 0.05). ND, not done.
P > 0.05 for all comparisons with uptake of M. tuberculosis incubated under isoosmolar conditions.
Some of the experiments were performed with M. tuberculosis expressing GFP, and uptake was determined by measuring emission of fluorescent light in a fluorimeter (481 nm for excitation and 507 nm for emission). The results obtained confirmed the observations measured in CFU. The uptake results were as follows: macrophage control, 32 ± 6 fluorescent units (FU); mycobacteria grown under laboratory conditions, 554 ± 20 FU; mycobacteria grown at pH 6, 1,752 ± 91 FU; mycobacteria grown under conditions of hyperosmolarity, 576 ± 42 FU; and mycobacteria grown under anaerobiosis, 1,590 ± 121 FU.
The combination of acidic pH and anaerobiosis was associated with uptake of 65 ± 6% of the inoculum at 1 h (P < 0.05 compared with each condition alone).
Role of complement in uptake.
To establish if complement would have any role in the uptake of bacteria exposed to different conditions, bacteria were prepared as described above and then incubated with macrophages in the presence or absence of 10% serum. Invasion after 1 h of exposure to macrophages was determined as described above and as described previously (4). The percentage of intracellular bacteria over the total was determined as described previously (4). The results shown in Tables 1, 2, and 3 suggest that complement and complement receptors have limited roles in the invasion of macrophages.
Effect of temperature on uptake by macrophages.
To determine if the ability of M. tuberculosis to enter macrophages was dependent on the host's temperature, the H37Rv and Erdman strains of M. tuberculosis were incubated at 23, 30, or 37°C for 2 or 4 h and then exposed to human macrophages for 1 h. As shown in Table 4, exposure of the bacterium to a temperature of 37°C prior to the invasion resulted in increased uptake by macrophages when compared with bacteria cultured at 23 and 30°C.
TABLE 4.
Effect of temperature on M. tuberculosis uptake by human macrophages
| Strain | Time of exposure (h)a | Uptake (% of initial inoculum) of M. tuberculosis at:
|
||
|---|---|---|---|---|
| 23°C | 30°C | 37°C | ||
| H37Rv | 2 | 6.1 ± 2 | 10 ± 3 | 16.8 ± 4b |
| 4 | 7.2 ± 4 | 12 ± 3 | 18 ± 5b | |
| Erdman | 2 | 7.4 ± 0.4 | 11.9 ± 4 | 19.7 ± 5b |
| 4 | 7.9 ± 3 | 14 ± 3 | 24 ± 6b | |
Bacteria were exposed to different temperatures for 2 or 4 h, washed at 4°C, and used to infect macrophages in the presence of 10% heat-inactivated autologous serum. Results are means ± standard deviations.
P < 0.05 compared with uptake of bacteria exposed to 23 or 30°C.
Effect of inhibition of complement receptors and mannose receptors on uptake.
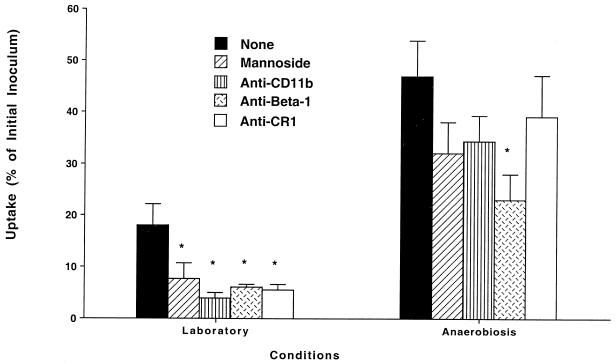
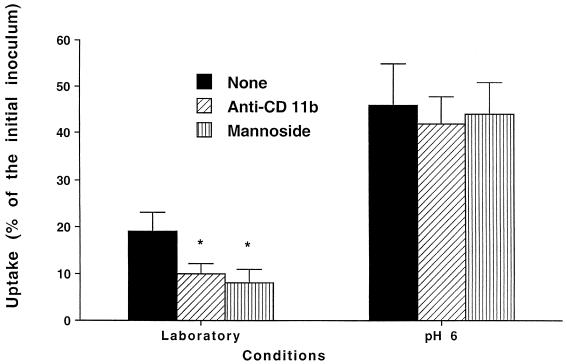
M. tuberculosis invades macrophages with increased efficiency after exposure to acidic pH and anaerobiosis but not to hyperosmolar conditions. Because previous studies have demonstrated that blocking of integrin receptors (CR3 and CR4) and CR1 or of the mannose receptor resulted in a partial decrease of M. tuberculosis uptake by macrophages, we sought to investigate if blocking of complement as well as mannose receptors had any impact on the uptake of strains exposed to conditions that resemble the granuloma environment. The use of blocking antibodies to the α chains (anti-α2, -α3, -α5, or -α6) of the integrin receptors had no effect on the binding of both bacteria grown under laboratory conditions (17% ± 4% of the inoculum) and bacteria grown under anaerobic conditions (52% ± 10% of the inoculum). Figure 1 shows that both anti-CD11b and an antagonist of the mannose receptor blocked uptake of both the laboratory strain and the strain grown under anaerobic conditions (clearly a trend; the power of the experimental design was 0.85), although the inhibition of uptake of the latter was not statistically significant and was smaller than the inhibition of the strain cultured under laboratory conditions. In contrast, the presence of anti-beta 1 antibody was associated with significant inhibition of uptake of M. tuberculosis grown under anaerobic conditions. Similarly, Fig. 2 shows that neither anti-CD11b antibody nor α-mannoside had any effect on the uptake of M. tuberculosis grown under acidic conditions by macrophages.
FIG. 1.
Effect of blocking integrins as well as mannose receptors on the uptake of M. tuberculosis grown under anaerobiosis by macrophages cultured in the presence of 10% autologous serum. Laboratory conditions were defined as 37°C, 20% O2, pH 7.2, and isoosmolarity; anaerobiosis was obtained by using an anaerobic jar as described in Materials and Methods. Antibodies were added to monolayers 1 h prior to the experiment at 23°C at concentrations of 30 μg/ml. Anti-P. aeruginosa lipid A monoclonal antibody (IgG) was used at 30 μg/ml as a nonrelevant antibody. No inhibition of uptake was observed. α-Methyl-mannoside was used at a concentration of 1 μg/ml. *, P < 0.05 compared with control.
FIG. 2.
Effect of blocking agents to the CR3 receptor and mannose receptor on the uptake of M. tuberculosis grown under pH 6.0 by macrophages cultured in the presence of 10% autologous serum. *, P < 0.05 compared with control. The antibodies used were anti-CD11b (30 μg/ml), nonrelevant IgG2b (30 μg/ml), and α-methyl-mannoside (1 μg/ml).
Uptake following inhibition of protein synthesis.
To investigate if the change in the efficiency of macrophage uptake of M. tuberculosis exposed to anaerobiosis and acidic pH required protein synthesis, H37Rv and Erdman strains were exposed to pH 6.0 and anaerobiosis for 4 h in the presence and absence (control) of 0.5 μg of rifampin per ml (subinhibitory concentration). Synthesis of proteins as measured by the incorporation of [35S]methionine was determined after exposure of the bacteria to pH 6 for 4 h. The results confirmed that treatment with a subinhibitory concentration of rifampin inhibited 96% ± 3% of the protein synthesis (incorporation of [35S]methionine in control bacteria, 2,850 ± 306 cpm/106 bacteria; in bacteria exposed to pH 6, 26,500 ± 1,740 cpm/106 bacteria; in bacteria exposed to pH 6 in the presence of rifampin, 3,730 ± 680 cpm/106 bacteria). As shown in Table 5, exposure to both anaerobiosis and acidic pH in the presence of a subinhibitory concentration of rifampin resulted in elimination of the effect of both acidic pH and anaerobiosis on uptake of M. tuberculosis by macrophages.
TABLE 5.
Effect of protein synthesis inhibition on M. tuberculosis invasion of macrophages
| M. tuberculosis strain | Conditiona | Uptake (% of inoculum) of M. tuberculosis in the presence or absence of antibioticb
|
|
|---|---|---|---|
| Absence | Presence | ||
| H37Rv | Laboratory | 18 ± 4 | 17 ± 3 |
| pH 6.0 | 56 ± 9 | 21 ± 4c | |
| Anaerobiosis | 57 ± 11 | 23 ± 5c | |
| Erdman | Laboratory | 23 ± 7 | 22 ± 2 |
| pH 6.0 | 61 ± 6 | 27 ± 5c | |
| Anaerobiosis | 58 ± 7 | 26 ± 4c | |
Bacteria were exposed to the conditions for 4 h and then incubated with macrophages in the presence of 10% heat-inactivated serum. Results are means ± standard deviations.
Bacteria were treated with rifampin (0.5 μg/ml) as described in Materials and Methods.
P < 0.05 compared with invasion without prior incubation with antibiotic.
Cytokine production.
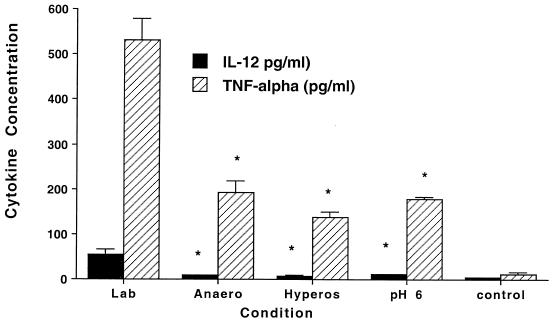
The production of IL-12 and TNF-α, two cytokines with an important role in the host defense against M. tuberculosis (8, 11), by macrophages was measured in macrophage monolayers infected with H37Rv exposed to laboratory conditions, anaerobiosis, hyperosmolarity (0.3 M dextrose), or pH 6.0 for 4 h and subsequently used to infect macrophages. Because the level of infection varied according to the condition to which the bacteria were exposed, we standardized to monolayers infected with 5 × 105 organisms. Supernatants were collected and the amount of cytokines was determined by ELISA. The results show that M. tuberculosis exposed to environmental conditions in the granuloma induced significantly less production of IL-12 and TNF-α by macrophages (Fig. 3). We then determined if the preopsonization of M. tuberculosis with 10% serum would have an effect of TNF-α and IL-12 production. The results shown in Table 6 indicate that preopsonization of the bacteria led to an increase in cytokine production, and it did not abolish the suppression of cytokine production by conditioned strains of M. tuberculosis.
FIG. 3.
Production of IL-12 and TNF-α by macrophages upon uptake of M. tuberculosis. Lab, laboratory conditions; Anaero, anaerobiosis; Hyperos, hyperosmolarity; pH 6, acidic pH; control, uninfected macrophage control; *, P < 0.05 compared to laboratory conditions; **, production at 24 h. Numbers represent the means ± standard deviations of four different experiments.
TABLE 6.
Effect of preopsonization on the induction of TNF-α and IL-12 production by macrophages
| Experimental group | Presence of opsonizationa | Production of cytokine (pg/ml)b
|
|
|---|---|---|---|
| TNF-α | IL-12 | ||
| Laboratory conditions | − | 430 ± 40 | 65 ± 10 |
| + | 505 ± 52 | 69 ± 4 | |
| Anaerobiosis | − | 110 ± 14c | 10 ± 2c |
| + | 293 ± 26c | 18 ± 3c | |
| pH 6.0 | − | 135 ± 37c | 8 ± 1c |
| + | 232 ± 20c | 17 ± 5c | |
M. tuberculosis was opsonized with 10% serum at 37°C for 30 min and then used to infect macrophages in the presence of 10% heat-inactivated serum.
Cytokine production was measured by ELISA. Results are means × standard deviations. The experiment was repeated three times. Results are means ± standard deviations.
P < 0.05 compared with laboratory conditions.
Two-dimensional gel electrophoresis.
We observed different expression patterns of protein under four different conditions. The expression of at least 10 proteins was induced and 3 proteins had their expression downregulated when incubated under the conditions of pH 6.0, iso-osmolarity, and anaerobiosis (pH 6.0AN) compared with cells incubated under conditions of pH 7.0, iso-osmolarity, and aerobiosis (pH 7.0A). Protein spots were either sent for sequencing or compared to the M. tuberculosis proteomics database (Max Planck Institute; www.mpiib/berlin.mpg.de). BLAST searches of the protein sequences yielded possible functional roles as summarized below.
Protein spots 3, 4, and 11 belong to the heat shock protein (Hsp) family. The Hsp family seems to act as chaperones that can protect other proteins to form large heterooligomeric aggregations under environmental stress (for example, low pH, heat and cold shock) at pH 6.0 and under anaerobic conditions. Spot 4, a 70-kDa Hsp, was overexpressed. Spot 3, a 14-kDa antigen which belongs to the Hsp20 family, was downregulated. An interesting phenomenon is spot 11, the 60-kDa chaperone II, which only appears at pH 7.0 and under aerobic conditions (Fig. 4). It might be the monooligomeric form of chaperone II and may cause a pI shift from 4.8 to 4.6.
FIG. 4.
Comparative evaluation of the protein profile by resolving M. tuberculosis proteins in a two-dimensional gel following exposure to pH 6 and anaerobiosis (A) or pH 7 and aerobiosis (B). At least 10 proteins showed upregulation of expression.
Protein spot 10, which belongs to the Ag85 complex, was overexpressed at pH 6.0 and under anaerobic conditions. The Ag85 complex is a major secreted antigen of M. tuberculosis and it is involved in the synthesis of mycolic acids. In addition, it has been shown to have high affinity for fibronectin (1, 2). Its upregulation might indicate a change in cell wall conformation. Spot 1 is 3-oxoacyl-(ACP)-reductase (fab G4; gene number Rv0242 [from the M. tuberculosis genome sequence]), involved in fatty acid synthesis.
Spot 5 is a chaperone protein similar to trigger factor, first identified in Bacillus subtilis. Trigger factor has been associated with protein folding transport. Spot 6 is the α-subunit of RNA polymerase (rpoA; Rv3457c) and contains ATP/GTP-binding site motif A. Spot 7 is a malase dehydrogenase (mdh; Rv1240) and is a component of the citric acid cycle glycoxylate pathway. Spot 8 is the antigen 84 (wag-31; Rv2145c), which has no known function. Spot 12 is the 50S ribosomal protein L7/L12 (rplL; Rv0652). Spot 13 is Rv3716, a protein of unknown function, similar to proteins in Escherichia coli and Haemophilus influenzae that are also of unknown function.
DISCUSSION
Several laboratories demonstrated in the past that the uptake of M. avium and M. tuberculosis by macrophages follows the binding of the bacteria to complement receptors as well as mannose receptors (6, 24, 25). Mycobacteria bind to the complement receptor in both the presence and absence of serum (6, 25). In the case of alveolar macrophages in the alveolar space, the role of complement (if any) has been linked to the ability of macrophages to produce several complement factors (26). It is important to consider, however, that in transmission by aerosol M. tuberculosis comes in contact with the recipient host's macrophage after being exposed to environmental conditions inside of the granuloma (and the caseum) of the infected individual for long periods of time. Our results confirmed the hypothesis that conditions encountered in the granuloma alter the phenotype of the bacterium with a consequent increase in invasiveness of macrophages. In addition, the uptake of this bacterial phenotype by macrophages is not mediated primarily by CR1, CR3, CR4, or mannose receptors. These findings might have a more direct correlation to what happens in vivo.
Bacterial phenotype is known to change according to the environment. There are several examples of pathogens that become more virulent under the conditions in which they are found in the host. For instance, the structure of Salmonella's peptidoglycan changes significantly depending on the environment that the bacterium is in inside the host, and the changes in peptidoglycan are assumed to be associated with the ability to survive and may constitute a sophisticated strategy for adapting to the host cells. In addition, the environment may influence the uptake of Salmonella by surrounding cells (13, 21). In the case of M. tuberculosis, the bacteria live in the infected host inside of a cavity filled with caseum (from the word “cheese”; dense material). The conditions in the center of the cavity are slightly acidic, with low oxygen tension and hyperosmolarity, and the exposure to these conditions in the host may influence the bacterial phenotype. Conversely, it is plausible to assume that the bacterial phenotype would not change during the relatively quick travel time between the infected host and recipient host. Nonetheless, it is important to consider that not all cases of tuberculosis are acquired by this route or mechanism. In fact, there are other forms of transmission of M. tuberculosis between two hosts, and under these other conditions, the bacterium may or may not show an invasive phenotype which might impact on its ability to infect the new host. It is quite possible that M. tuberculosis in different environments would not be exposed to conditions that would trigger the expression of the invasive phenotype. In addition, the content of the granuloma environment is certainly more complex than the conditions that we tested and may have further influence on the bacterial phenotype. One has to keep in mind, however, that although the conditions used are assumed to closely resemble the in vivo environment, they still are in vitro conditions and therefore are subject to limitations.
The observation that mannoside does not reduce uptake of bacteria exposed to pH 6 may have significant implications. Since LAM is the adhesin to the mannose receptor on macrophages (24), a possible implication of this finding is that incubation in an acidic environment either damages or alters the conformation of LAM, making it incapable of binding to the mannose receptor. Future studies will be needed to address these hypotheses.
Our data also suggest that a few hours of exposure to conditions existent in the granuloma are sufficient to trigger the change of phenotype. This observation confirms what has been observed in M. avium, another slow-growth mycobacterium, which is that it can synthesize new proteins and lipids in a short period of time (5). Our findings also suggest that putative significant changes in the cell wall of the bacterium are likely to be responsible for the observed difference in efficiency of invasion. The result obtained by comparing the two-dimensional electrophoresis pattern of bacteria incubated under the conditions encountered in the caseum in fact supports this hypothesis. At least 10 proteins were upregulated when bacteria were exposed to acidic pH and anaerobic conditions. The proteins belong to the group of stress proteins as well as to fatty acid synthesis and glyoxylate pathways. A couple of the proteins have unknown functions. Whether they have any participation in the increased binding or can influence the ability of M. tuberculosis to enter macrophages is currently unknown. In addition, it is possible that the lipid profile is altered as well. The results showing that invasion does not take place by using the mannose receptor suggest this possibility. The appearance of an invasive phenotype can be prevented by the incubation of the microorganism with subinhibitory concentrations of rifampin, which inhibits transcription and consequently has an impact on protein synthesis and, most likely, fatty acid synthesis. The fact that rifampin has a suppressive effect on environment-induced changes in phenotype indirectly implies that M. tuberculosis may be in an active metabolic state either early or even later during the granuloma stage of infection in the host.
The observation that the uptake of M. tuberculosis expressing the invasion phenotype by macrophages is associated with a significant decrease in the production of IL-12 and TNF-α raises the possibility that the host cell receptor(s) is not linked to the signal transduction pathway that would trigger both TNF-α and IL-12 or, conversely, may induce suppression. It certainly would represent an escape mechanism used by the bacterium to subvert the response of the host and suppress an effective innate immune response. It is interesting that LAM has been associated with the induction of TNF-α synthesis by M. tuberculosis, and as discussed above our data suggest that LAM is modified following incubation in acidic and low-oxygen-tension environments. Furthermore, the observation that opsonized bacteria induce levels of cytokines that are approximately 1.5 times the level of cytokines induced by bacteria in the absence of opsonization suggests that opsonization did not abolish the suppressive effect of preconditionment. The effect observed is probably due to the presence of serum and not a consequence of binding to a complement receptor.
In summary, we have shown that the host environment of the infected individual with tuberculosis affects the phenotype of M. tuberculosis and its efficiency to enter into macrophages. Future studies will aim to examine if the effect of environmental cues can be observed in an in vivo aerosol model of M. tuberculosis infection.
Acknowledgments
We thank Karen Allen for typing the manuscript and Joel Ernst for providing M. tuberculosis H37Rv expressing GFP.
This work was supported by funds from the Kuzell Institute and the Hedco Foundation.
Editor: J. D. Clements
REFERENCES
- 1.Abou-Zeid, C., T. L. Ratliff, H. G. Wiker, M. Harboe, J. Bennedsen, and G. A. Rook. 1988. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect. Immun. 56:3046-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belisle, J. T., V. D. Vissa, T. Sievert, K. Takayama, P. J. Brennan, and G. S. Besra. 1997. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 276:1420-1422. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez, L. E., and J. Goodman. 1996. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect. Immun. 64:1400-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez, L. E., A. Parker, and J. R. Goodman. 1997. Growth within macrophages increases the efficiency of Mycobacterium avium in invading other macrophages by a complement receptor-independent pathway. Infect. Immun. 65:1916-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudez, L. E., M. Petrofsky, and J. Goodman. 1997. Exposure to low oxygen tension and increased osmolarity enhance the ability of Mycobacterium avium to enter intestinal epithelial (HT-29) cells. Infect. Immun. 65:3768-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bermudez, L. E., L. S. Young, and H. Enkel. 1991. Interaction of Mycobacterium avium complex with human macrophages: roles of membrane receptors and serum proteins. Infect. Immun. 59:1697-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bermudez, L. E., L. S. Young, J. Martinelli, and M. Petrofsky. 1993. Exposure to ethanol up-regulates the expression of Mycobacterium avium complex proteins associated with bacterial virulence. J. Infect. Dis. 168:961-968. [DOI] [PubMed] [Google Scholar]
- 8.Cooper, A. M., J. Magram, J. Ferrante, and I. M. Orme. 1997. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J. Exp. Med. 186:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dannenberg, A. M., Jr. 1993. Immunopathogenesis of pulmonary tuberculosis. Hosp. Pract. 28:51-58. [DOI] [PubMed] [Google Scholar]
- 10.Dannenberg, A. M., Jr., and G. A. W. Rook. 1994. Pathogenesis of pulmonary tuberculosis: an interplay of tissue-damaging and macrophage-activating immune responses—dual mechanisms that control bacillary multiplication, p. 459-483. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection and control. ASM Press, Washington, D.C.
- 11.Flynn, J. L., M. M. Goldstein, K. J. Triebold, J. Sypek, S. Wolf, and B. R. Bloom. 1995. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J. Immunol. 155:2515-2524. [PubMed] [Google Scholar]
- 12.Gangadharam, P. R. J. 1988. Antimycobacterial drugs. In P. R. Peterson and F. Verhoef (ed.), The antimicrobial agents. Elsevier, New York, N.Y.
- 13.Guo, L., K. B. Lim, C. M. Poduje, M. Daniel, J. S. Gunn, M. Hackett, and S. I. Miller. 1998. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95:189-198. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch, C. S., J. J. Ellner, D. G. Russell, and E. A. Rich. 1994. Complement receptor-mediated uptake and tumor necrosis factor-alpha-mediated growth inhibition of Mycobacterium tuberculosis by human alveolar macrophages. J. Immunol. 152:743-753. [PubMed] [Google Scholar]
- 15.Isberg, R. R., A. Swain, and S. Falkow. 1988. Analysis of expression and thermoregulation of the Yersinia pseudotuberculosis inv gene with hybrid proteins. Infect. Immun. 56:2133-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, B. Y., and M. A. Horwitz. 1995. Identification of macrophage and stress-induced proteins of Mycobacterium tuberculosis. J. Clin. Investig. 96:245-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, C. A., and S. Falkow. 1990. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc. Natl. Acad. Sci. USA 87:4304-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limia, A., F. J. Sangari, D. Wagner, and L. E. Bermudez. 2001. Characterization and expression of secA in Mycobacterium avium. FEMS Microbiol. Lett. 197:151-157. [DOI] [PubMed] [Google Scholar]
- 19.Melton, A. R., and A. A. Weiss. 1989. Environmental regulation of expression of virulence determinants in Bordetella pertussis. J. Bacteriol. 171:6206-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poole, J. C. F., and H. W. Florey. 1970. Chronic inflammation and tuberculosis. In H. W. Florey (ed.), General pathology, 4th ed. W. B. Saunders, Co., Philadelphia, Pa.
- 21.Quintela, J. C., M. A. de Pedro, P. Zollner, G. Allmaier, and F. Garcia-del Portillo. 1997. Peptidoglycan structure of Salmonella typhimurium growing within cultured mammalian cells. Mol. Microbiol. 23:693-704. [DOI] [PubMed] [Google Scholar]
- 22.Riley, R. L., C. L. Mills, W. Nyka, N. Weistock, P. B. Storey, L. K. Sultan, M. C. Riley, and W. F. Wells. 1959. Aerial dissemination of pulmonary tuberculosis: a two year study of contagion in a tuberculosis ward. Am. J. Hyg. 70:185-195. [DOI] [PubMed] [Google Scholar]
- 23.Riley, R. L., and F. O'Grady. 1961. Airborne infection: transmission and control. The MacMillan Co., New York, N.Y.
- 24.Schlesinger, L. S. 1993. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J. Immunol. 150:2920-2930. [PubMed] [Google Scholar]
- 25.Schlesinger, L. S., C. G. Bellinger-Kawahara, N. R. Payne, and M. A. Horwitz. 1990. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J. Immunol. 144:2771-2780. [PubMed] [Google Scholar]
- 26.Strober, W., M. E. Lamm, J. R. McGhee, and S. P. James. 1988. Mucosal immunity and infections. Oxford University Press, New York, N.Y.
- 27.Valdivia, R. H., A. E. Hromockyj, D. Monack, L. Ramakrishnan, and S. Falkow. 1996. Applications for green fluorescent protein (GFP) in the study of host-pathogen interactions. Gene 173:47-52. [DOI] [PubMed] [Google Scholar]
- 28.Wong, D. K., B. Y. Lee, M. A. Horwitz, and B. W. Gibson. 1999. Identification of Fur, aconitase, and other proteins expressed by Mycobacterium tuberculosis under conditions of low and high concentrations of iron by combined two-dimensional gel electrophoresis and mass spectrometry. Infect. Immun. 67:327-336. [DOI] [PMC free article] [PubMed] [Google Scholar]