Abstract
We calculated the implications of diffusion for the phosphoenolpyruvate:glucose phosphotransferase system (glucose-PTS) of Escherichia coli in silicon cells of various magnitudes. For a cell of bacterial size, diffusion limitation of glucose influx was negligible. Nevertheless, a significant concentration gradient for one of the enzyme species, nonphosphorylated IIAGlc, was found. This should have consequences because the phosphorylation state of IIAGlc is an important intracellular signal. For mammalian cell sizes we found significant diffusion limitation, as well as strong concentration gradients in many PTS components, and strong effects on glucose and energy signaling. We calculated that the PTS may sense both extracellular glucose and the intracellular free-energy state. We discuss i), that the effects of diffusion on cell function should prevent this highly effective bacterial system from functioning in eukaryotic cells, ii), that in the larger eukaryotic cell any similar chain of mobile group-transfer proteins can neither sustain the same volumetric flux as in bacteria nor transmit a signal far into the cell, and iii), that systems such as these may exhibit spatial differentiation in their sensitivity to different signals.
INTRODUCTION
Many cellular processes involve the movement of pathway components to and from a membrane. Kinetic descriptions of such metabolic systems and signal transduction pathways tend to ignore the diffusion process, implicitly assuming that the spatial diffusion is fast relative to the reaction kinetics. However, a relatively straightforward analysis of an imaginary eukaryotic “two-component system”, composed of a membrane-bound kinase and a cytoplasmic phosphatase, revealed that spatial gradients of phosphorylated and nonphosphorylated protein can be induced by signal-transducing flux within a mammalian cell (Brown and Kholodenko, 1999). These gradients might have significant control over the flux (Kholodenko et al., 2000). Essential to the phenomenon is the fact that here signal-transfer depends on the shuttling of a mobile protein and not on that of a metabolite.
Bacterial cells are much smaller than eukaryotic cells. A typical Escherichia coli B/r cell has dimensions of ∼1 × 3 μm (Nanninga, 1998), whereas a small eukaryote like baker's yeast (Saccharomyces cerevisiae) is already 4 μm in diameter (Sherman, 1991). Therefore, we wondered whether one should also expect an effect of diffusion on the functioning of important bacterial “protein chains”. Instead of studying an imaginary system, we investigated the behavior of the PTS. In many bacteria, the PTS is responsible for the uptake and phosphorylation of various carbohydrates (Postma et al., 1993) at a very high rate (van der Vlag et al., 1995). On top of that, the protein components of the system play a diverse yet central role in the regulation of cellular activity in most of these organisms (see Lee et al., 2000; Lux et al., 1999; Seok et al., 1997; Tanaka et al., 2000; and reviews by Postma et al., 1993; Saier et al., 1996; Stülke and Hillen, 1999). Due to its spatial organization, the functioning of the PTS depends on diffusion: a phosphoryl group derived from cytoplasmic phosphoenolpyruvate is transferred by cytoplasmic proteins to a membrane protein that imports and phosphorylates the carbohydrate. This implies that a spatial gradient in the concentration of some of the protein species (be it phosphorylated, nonphosphorylated, or complexed) should build up for the pathway to develop flux. The main issue addressed here is how large such a gradient should be and whether it might lead to substantial fractional changes in local concentrations of PTS components, eventually limiting flux or signaling (diffusion limitation). A second issue treated is whether a similar system in a eukaryotic cell should be expected to develop gradients and diffusion limitation.
We set out to calculate this for the glucose-PTS of E. coli in a comprehensive manner, taking into account the spatial separation of the pathway components and the diffusion processes that are responsible for the transport to and away from the membrane. The parameter values in our model were based on experimental data only, combining the kinetic description of the glucose-PTS by Rohwer et al. (2000) and diffusion measurements in live E. coli (Elowitz et al., 1999). We show how E. coli escapes diffusion limitation of the metabolic and transport flux through this system in ways that are not available to the larger mammalian cell.
METHODS
Mechanism
The glucose-PTS of E. coli (discovered by Kundig et al., 1964) consists of four proteins: the general PTS-proteins enzyme I (EI) and HPr and the carbohydrate-specific proteins IIAGlc and IICBGlc. The former three proteins are located in the cytoplasm and relay a phosphoryl-group derived from phosphoenolpyruvate (PEP) in a consecutive manner to the latter membrane-bound protein, which in turn imports glucose and concomitantly phosphorylates it (Meadow et al., 1990; Postma et al., 1993; Robillard and Broos, 1999). Using the in vivo uptake rate of α-d-methyl glucoside (a glucose analog) of ∼0.9 nmol s−1 per mg dry cell weight (reported by van der Vlag et al., 1995), an in vivo IICBGlc concentration of ∼10 μM and a cellular volume of ∼2.5 μl per mg dry weight (cf. Rohwer et al., 2000), we calculate that ∼37 molecules of glucose are imported and phosphorylated per molecule of IICBGlc per s. Rohwer et al. (2000) made an in silicon replica of the glucose-PTS, i.e., a precise model exclusively based on the available literature data on Km values, equilibrium constants, and association constants of the PTS-proteins for their substrates. Basic assumptions were that all elementary reactions in the pathway were bimolecular and that the reacting species were distributed homogeneously. Because the model ignored the spatiality of the pathway, we decided to make a new model that omits the latter assumption.
The explicit form of the modeled pathway is given in Fig. 1 and the model parameters in Table 1. The enzymes involved can occur in various states. Enzyme IIAGlc, for instance, can either be nonphosphorylated (IIAGlc), phosphorylated (IIAGlc-P) or complexed (IIAGlc-P-HPr and IICBGlc-P-IIAGlc). The term “enzyme species” will be used to refer to the different states throughout the text.
FIGURE 1.
Reaction mechanism of the glucose-PTS of E. coli. In reaction 5, glucose comes from the periplasm, is phosphorylated, and the resulting glucose-6-phosphate is released into the cytoplasm, making the PTS an active uptake system for the glucose moiety. IICBGlc is a cytoplasmic membrane protein. All other components of the reaction scheme are cytoplasmic.
TABLE 1.
Parameters of the reaction-diffusion model
| Concentration of PTS proteins and boundary metabolites in μM | |||
| [EI]tot, [HPr]tot: 5, 50 | PEP/Pyruvate: 2800/900 | ||
| [IIAGlc]tot, [IICBGlc]*tot: 40, 10 | glucose/ glc-6-P: 500/50 | ||
| Rate constants odd, − even: (μM−1 s−1); even, − odd: (s−1) | |||
| k1: 32.7 | k−1: 8000 | k6: 73.2 | k−6: 56.4 |
| k2: 1800 | k−2: 4.9 | k7: 14.7 | k−7: 14.7 |
| k3: 233.3 | k−3: 233.3 | k8: 44 | k−8: 16 |
| k4: 1400 | k−4: 56 | k9: 4.33 | k−9: 6.48 |
| k5: 366 | k−5: 366 | k10: 80 | k−10: 9 · 10−5 |
| Diffusion coefficients (μm2 s−1) | |||
| EI, EI-P(EP): 3.30 | IIA-P-HPr: 4.37 | ||
| HPr-P-EI: 3.15 | IIA, IIA-P: 5.00 | ||
| HPr, HPr-P: 6.30 | Infinite: 2000 | ||
The protein and boundary metabolite concentrations and rate constants were best estimates on the basis of the existing experimental data (see Rohwer et al., 2000). The tabulated diffusion coefficient of IIAGlc was based on that found for GFP in E. coli by Elowitz et al. (1999). The other values were calculated from that using a molecular mass of 63.5, 9.1, and 18.1 kDa for EI, HPr, and IIAGlc, respectively (de Reuse and Danchin, 1988).
The IICBGlc concentration is given as a volume concentration. To obtain the surface concentration (in μm μM), the number was multiplied by the surface/volume ratio of the cell, which in the case of a spherical cell equals 3/r.
Diffusion and reaction rates
We assumed that reacting species meet through passive diffusion and that the diffusion process for every molecular species could be characterized by a single constant. The behavior of the molecules was modeled using a continuous representation (the concentration). The local change in the concentration of protein species p in time was related to the net production rate and diffusion rate by the balance equation:
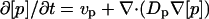 |
(1) |
Equation 1 implies that the increase with time in a local concentration must equal the local net production rate (vp) plus the net influx rate through diffusion. Convection was neglected because in the in vivo measurements of GFP movement, there were no indications of directional bias or enhanced apparent mobility (Elowitz et al., 1999; Dayel et al., 1999). Diffusion was parametrized by a protein specific diffusion constant Dp. ∇ is the operator for the spatial derivative and the precise formulation of ∇ · (Dp∇[p]) thus depends on the dimension of the system and the coordinate system. Here “local” indicates a certain position in the cellular space for the cytoplasmic enzyme species, or on the membrane surface for the species situated at the membrane boundary.
The net production rate vp represents the sum of all rates that lead to the production of p minus all rates that lead to the consumption of p. The rate equations were derived directly from the reaction scheme in Fig. 1 (see Blom and Peletier, 2000, 2002). Because the concentrations [IICBGlc], [IICBGlc-P], [IICBGlc-P- IIAGlc], and [glc-P-IICBGlc] are surface concentrations, their reaction rates were defined per unit of surface area.
For the mass balance of the cytoplasmic enzyme species, the reaction at the membrane represents a source/sink term. The corresponding boundary condition was obtained by equating the source/sink with the local flux near the membrane (direction represented by normal vector n). For IIAGlc and IIAGlc-P, the boundary condition yields Eqs. 2 and 3, and for the other cytoplasmic enzyme species Eq. 4:
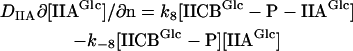 |
(2) |
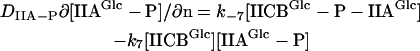 |
(3) |
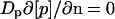 |
(4) |
The set of equations described in the above forms the core of our reaction-diffusion model.
Cell size, geometry, and numerical methods
E. coli cells are small cylinders with spherical poles. Depending on growth conditions and the strain, their diameter ranges from 0.5 to 1.5 μm and their length from 2 to 4 μm (Nanninga, 1998; Woldringh and Nanninga, 1985). One could describe the shape of the E. coli cell in various ways, such as cigar-like or rod-like. Because the latter term is used throughout biological literature, we will use rod or rod-like when describing the actual shape of the bacterial cell. We performed numerical experiments with a model rod cell with a diameter of 1.2 μm (r = 0.6 μm) and a length of 3 μm (see Blom and Peletier, 2002), and a spherical model cell with a radius (r) of 0.6 μm (see Blom and Peletier 2000). In all our calculations with both model cells, we found only a single steady state independent of the initial conditions and the lateral diffusion of the membrane component. Because the behavior of the system was essentially similar in both model cells, as described in the Appendix, we decided in this paper to treat the bacterial cell as if it were a sphere. This not only reduced the complexity of the description and the interpretation, but also made a straightforward comparison with a larger eukaryotic model cell possible. For a model mammalian cell, we assumed a sphere with a radius of 10 μm.
We considered all concentrations of cytoplasmic species to be functions of the spatial variable, and we confined the membrane-bound species to the boundary of the sphere. Because all solutions of our calculations were spherically symmetric, Eq. 1 can be reduced to the following system of reaction-diffusion equations:
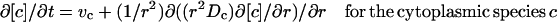 |
(1a) |
and
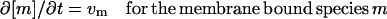 |
(1b) |
The concentrations of the cytoplasmic species were calculated using the resulting partial differential equations. For the membrane-bound species, these reduced to ordinary differential equations. The system was solved using the method of lines: the spatial derivatives were discretized on a computational grid and the resulting system of ordinary differential equations was integrated in time. We calculated the glucose flux (J) through the membrane with the aid of Eq. 5 when the system had reached a steady state:
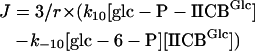 |
(5) |
In this relation [glc-6-P] is interpreted as the concentration of glc-6-P at the membrane. Because the concentrations of IICBGlc and glc-P-IICBGlc are surface concentrations, we normalized by the surface/volume ratio (i.e., (4πr2)/(4/3πr3)) to obtain the volume flux J. For a more detailed description of the numerical methods used, readers are referred to Blom and Peletier (2000, 2002).
Parameters and variables
Rate constants and enzyme concentrations were taken from Rohwer et al. (2000) and references cited therein. The membrane concentration of IICBGlc was calculated from the bulk-projected concentration by multiplying the bulk concentration with the volume/surface ratio. The PEP and pyruvate concentrations were taken from Hogema et al. (1998), who determined these in glucose-grown cells. Four different combinations of glucose and PEP concentrations were used to evaluate their influence. The lower PEP concentration was chosen such that it was below the Km (Km, PEP = 300 μM (Rohwer et al., 2000)). The glucose concentrations were chosen such to be either at saturation or below the Km (Km,glucose = 20 μM (Rohwer et al., 2000)).
In our calculations, the concentrations of the metabolites PEP, pyruvate, glucose-6-phosphate, and glucose were treated as constant parameters. In general the concentrations of intracellular metabolites are much higher than those of the intracellular proteins and are controlled by many processes. As a result, metabolite concentrations remain constant in the cell, at least for certain periods of time, independent of changes in the activity of one of the producing or consuming processes. Moreover, in our case, steady state was obtained within a fraction of a second, allowing only small eventual changes in metabolite concentrations. From a comparison of the cell density, the cellular volume, the diffusion rate of glucose in water (∼670 μm2 s−1 (Weast, 1975)), the glucose concentration, and the glucose uptake rate (van der Vlag et al., 1995), we do not expect any diffusion limitation of glucose on the outside of the model cells.
We assumed a diffusion constant for IIAGlc of 5 μm2 s−1. The diffusion rate of the GFP (27 kDa) in the cellular matrix of E. coli has been determined experimentally by Elowitz et al. (1999). It was measured in elongated E. coli cells by means of fluorescence recovery after photobleaching and the reported values range from 3.6 to 7.7 μm2 s−1. The lower rate was found in cells with a high GFP expression level and probably identifies the diffusion rate of GFP dimers (54 kDa). Protein diffusion in the cytoplasm of E. coli cells thus was two- to fourfold slower than in the eukaryotic endoplasmic reticulum or mitochondrion, respectively, and about five times slower than in eukaryotic cytoplasm (Dayel et al., 1999). To establish the possible effects of diffusion on the behavior of our bacterial transport system, we decided to use the lower number reported by Elowitz et al. (1999) because this value seems to present a lower limit to the rate of “free” protein diffusion in vivo.
The diffusion coefficients of the other PTS “enzyme species” were calculated from that of enzyme IIAGlc assuming that: i), all enzyme species are spheres, ii), the diffusion coefficient varies linearly with the inverse of the radius of that sphere, and iii), the volume of the sphere varies proportionally with the mass of the protein species. A situation in which all cytoplasmic enzyme species are distributed homogeneously was simulated by making the diffusion coefficient 2000 μm2 s−1 (∼infinite) for all species.
Signal
In the PTS, not only the protein components diffuse between membrane surface and cytoplasm, but also the phosphoryl-group. The latter does this by playing “piggyback” on the components of the PTS. Looking at the PTS as a signal relay chain, we define the local concentration of the PTS signal (σ) as the sum of local concentrations of the phosphorylated mobile PTS components minus the sum of the local concentrations of the nonphosphorylated mobile PTS components:
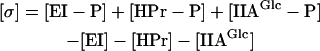 |
(6) |
For signal to transfer at steady state, a phosphorylated protein must diffuse to the membrane and a nonphosphorylated protein must diffuse back. Because the intermediary protein complexes can be considered to carry both a phosphorylated as well as a nonphosphorylated enzyme, diffusion of these complexes does not contribute to the signal diffusion.
RESULTS
Estimated concentration gradients
Brown and Kholodenko (1999) showed that in mammalian cells, gradients of active forms of signal transduction proteins may arise. Their approximate argument can be recalculated for the case of the E. coli PTS. If the signal protein is dephosphorylated at the plasma membrane and rephosphorylated in the center of the cell, a gradient of the nonphosphorylated form of the protein must exist to drive the diffusion. The concentration difference (Δ[p]) and the flux (J) are then related by Fick's first diffusion equation. For a spherical cell this reads:
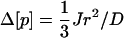 |
(7) |
Inserting a flux of 0.37 mM s−1 (Rohwer et al., 2000), a diffusion coefficient (D) of 5 μm2 s−1 and a radius (r) of 0.6 μm, one finds a concentration difference between cell center and membrane surface of 9 μM. Such a gradient should be quite significant for PTS proteins, which occur at concentrations between 5 and 50 μM (see Table 1). A cell radius of 10 μm leads, in the case of similar flux, to the enormous concentration gradient of ∼2.5 mM (over 10 μm) by the same calculation, and of ∼0.5 mM if diffusion is assumed to be five times faster, as prevalent in eukaryotic cytoplasm (Dayel et al., 1999). These estimated concentration differences are much higher than the total concentration of the PTS proteins, which amounts to only 0.1 mM. The difference in signal concentration, as expressed in [σ] (Eq. 6), between membrane surface and the cell center should be even double these numbers, because an inverse gradient will exist for the phosphorylated proteins. This indicates that in larger cells, diffusion should interfere with the functioning of a system such as the PTS.
The above estimations (cf. Fig. 3 A, open triangles) suggested that substantial gradients will be present in the active PTS. The PTS is a multicomponent system, however, in which both phosphorylated and nonphosphorylated forms of each protein may diffuse. The concentrations of these forms depend on the PTS activity. In addition, not all rephosphorylation of EI occurs in the center of the cell; PEP diffuses throughout the cytoplasm. Consequently, a more comprehensive calculation was required to see whether spatial limitations are indeed as prohibitive as suggested by the preliminary calculations described in this section.
FIGURE 3.
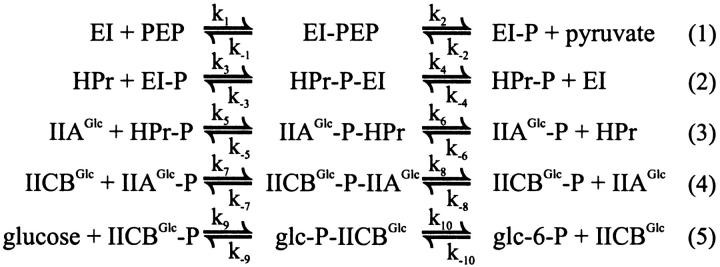
Cell size affects flux and concentration gradients. (A) Flux through the PTS (left ordinate, squares), and the difference in signal concentration between cell center and inner membrane as a function of cell radius (right ordinate, triangles). The open triangles represent a rough estimate based on Eq. 7 and the calculated flux, and the closed triangles represent the result of the precise calculation using Eq. 6 (note that the scale is logarithmic). (B) The concentration ratio of phosphorylated IIAGlc (closed circles, membrane/center) and of nonphosphorylated IIAGlc (open circles, center/membrane). The dark gray area is relevant for bacterial dimensions, the light gray area for eukaryotic cells.
Calculated gradients for bacterial cells
As a point of reference, we first calculated both the PTS flux and the concentration distribution of the four PTS enzymes over their different states in the case of extremely fast diffusion of the cytoplasmic species. The results are listed in column 2 of Table 2 and are identical to those reported by Rohwer et al. (2000).
TABLE 2.
Flux and distribution of the glucose-PTS protein species in cells of different radii
|
r = 0.3 μm
|
r = 0.6 μm
|
r = 10.0 μm
|
|||||
|---|---|---|---|---|---|---|---|
| 1. | 2. Dc = ∞ | 3. c | 4. m | 5. c | 6. m | 7. c | 8. m |
| EI | 0.27 | 0.27 | 0.27 | 0.27 | 0.27 | 0.27 | 0.27 |
| EI-PEP | 3.05 | 3.05 | 3.05 | 3.05 | 3.05 | 3.06 | 2.95 |
| EI-P | 1.19 | 1.19 | 1.19 | 1.20 | 1.19 | 1.25 | 1.01 |
| HPr-P-EI | 0.49 | 0.49 | 0.49 | 0.48 | 0.50 | 0.43 | 0.79 |
| HPr | 1.28 | 1.25 | 1.28 | 1.12 | 1.33 | 0.34 | 4.53 |
| HPr-P | 29.8 | 30.1 | 29.7 | 31.0 | 29.2 | 40.1 | 16.7 |
| IIA-P-HPr | 18.5 | 18.0 | 18.6 | 16.9 | 19.3 | 7.69 | 35.1 |
| IIA | 0.64 | 0.60 | 0.74 | 0.55 | 0.87 | 0.19 | 3.69 |
| IIA-P | 15.4 | 15.9 | 15.2 | 16.9 | 14.5 | 29.1 | 1.58 |
| IICB-P-IIA | 5.47 | 5.46 | 5.43 | 2.48 | |||
| IICB | 1.41 | 1.43 | 1.49 | 6.14 | |||
| IICB-P | 0.12 | 0.12 | 0.12 | 0.05 | |||
| glc-P-IICB | 2.99 | 2.99 | 2.97 | 1.33 | |||
| σ(signal) | 45.1 | 43.8 | 47.2 | 42.4 | 69.6 | 10.8 | |
| −Δ σ (m − c) | 1.3 | 4.8 | 58.8 | ||||
| σc/σm | 1.0 | 1.1 | 6.4 | ||||
| J | 240 | 239 | 237 | 106 | |||
Column 2 was obtained assuming a diffusion constant of 2000 μm2 s−1 for all species. For realistic diffusion coefficients, we arrived at the numbers of columns 3–8. For every radius, the first column gives the concentrations near the center of the cell and the second one those near the membrane boundary. The concentrations are given in μM, the flux in μM s−1.
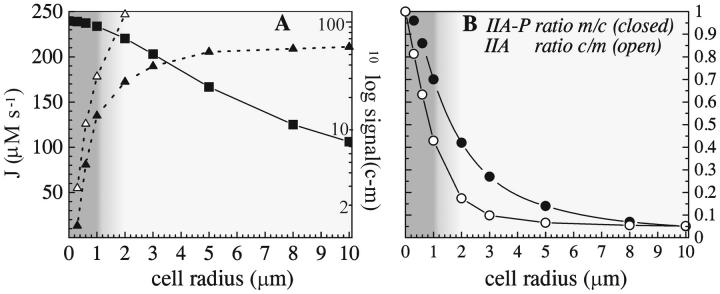
We then recalculated the flux and spatial distribution of the enzyme species for cells with a radius of 0.6 μm using realistic values for the diffusion coefficients. The results are shown in columns 5 and 6 of Table 2 and in Fig. 2. The effect of the less than infinitely fast diffusion on the flux was remarkably small, i.e., <1%. Likewise, the gradients that developed in the concentrations of components of EI of the PTS were small, concentration differences between the center of the cell and close to the membrane remaining below 4%. Defining the signal in terms of the concentration difference of phosphorylated and nonphosphorylated uncomplexed PTS protein (Eq. 6), the signal concentration difference between membrane surface and cell center was ∼5 μM. This was some four times smaller than the first-order estimate of 20 μM using Fick's diffusion equation (see earlier). An important reason for this difference is that rephosphorylation of EI occurred throughout the cell rather than being confined to the cell center. The signal concentration difference was mainly distributed over HPr and IIAGlc, the proteins present at higher concentrations.
FIGURE 2.
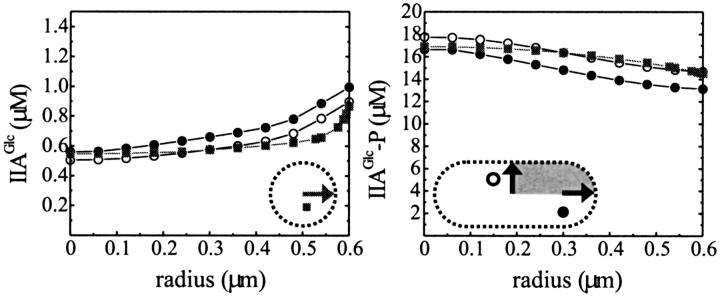
The concentration distribution of the IIAGlc-related (left panel) and nonphosphorylated cytoplasmic enzyme species (right panel) in a cell with radius 0.6 μm in the presence of both PEP (2.8 mM) and glucose (500 μM).
Although the concentration of both enzymes HPr and IIAGlc was high with respect to that of the signal, the phenomenon that each PTS protein was rather unevenly distributed over its subforms meant that some of those subforms could be subject to significant concentration gradients. Quite notably, this was the case for nonphosphorylated IIAGlc: its concentration was more than 35% higher near the membrane than in the cell center or than the cell average. The steady-state concentration of nonphosphorylated IIAGlc did not vary linearly with distance from the membrane, but increased sharply near the membrane (see Fig. 2, right). The ratio of IIAGlc-P/IIAGlc changed even more drastically.
We explored the role of the individual diffusion constants by reducing them one by one severalfold (not shown). When we looked at the effect on flux we saw that reduction in the movement of IIAGlc-P brought about a decrease in the flux: a 10-fold slowing down led to a 7% flux reduction. A 10-fold decrease of the other diffusion constants did not influence the flux markedly. Similar changes in the diffusion rate of the HPr and IIAGlc-related species led to significant concentration gradients for those species whose diffusion was being retarded and for nonphosphorylated IIAGlc, whereas for EI-related species nothing happened upon reduction of the diffusion rates. A detailed analysis of the diffusion control of glucose flux mediated by the PTS is described elsewhere (Francke et al., 2002).
Gradients and diffusion limitation in cells of various sizes
We next modified the radius of our model cell to 0.3 and 10 μm, keeping the volume-averaged concentrations of total EI, HPr, IIAGlc, and IICBGlc constant. We again calculated the flux through the pathway and the concentration distribution of the four enzymes over their different states and over the cellular space (cf. columns 3 and 4, and 7 and 8 of Table 2, respectively). As expected, decreasing the radius of the cell from 0.6 to 0.3 μm did not affect the flux through the glucose-PTS. The concentration difference between nonphosphorylated IIAGlc in the center of the cell and nonphosphorylated IIAGlc near the membrane lost significance by decreasing to 15%. This is in line with the squared dependence of the concentration gradients on the radius of the cell predicted by Eq. 7.
Clearly in the smallest cell considered, diffusion limitation lacked significance. This pinpointed that cell size might be a major factor determining whether or not concentration gradients and flux limitation by diffusion arise in the bacterial PTS. That this was indeed the case is illustrated by Fig. 3 A, where the PTS flux is represented as a function of the radius of our spherical model cell. The dark and light gray areas indicate the regions of the plot that may be relevant for bacterial and mammalian cells, respectively. Fig. 3 A also shows the corresponding signal concentration difference between membrane and bulk. Fig. 3 B shows the concentration of IIAGlc in the cell center relative to that near the membrane (open circles) and vice versa for IIAGlc-P (closed circles). From Fig. 3 it is apparent that whereas the cell radius only affected the flux due to diffusion limitation at the highest cell sizes, an effect on signal transduction through the concentrations of phosphorylated and nonphosphorylated IIAGlc (and their ratio) should already be expected for cells the size of E. coli or only slightly larger, or with only slightly hampered diffusion.
As can be seen in Table 2 and Fig. 3 B, in cells with a radius of 10 μm, nonphosphorylated IIAGlc was subject to a dramatic concentration gradient; its concentration at the membrane surface was almost 20 times that in the cell center. For HPr, a 13-fold, for HPr-P a 0.4-fold, for HPr-P-IIAGlc a 4.5-fold, and for IIAGlc-P a 0.05-fold concentration ratio was calculated between the membrane and the center of the cell. In these cells the flux was reduced by ∼55%. The difference in signal concentration between membrane surface and cell center amounted to 59 μM, getting closer to its theoretical maximum of 95 μM, i.e., the total cell-averaged concentration of PTS components. Again the diffusion gradients were distributed over more than one component and concentrated in components that were present at higher total concentrations. EI-related enzyme species were not subject to concentration gradients of any significance, with the exception of its complex with HPr. For IICBGlc, the amount of nonphosphorylated enzyme began to rise and the amount of phosphorylated species and complexes began to drop as the cell radius exceeded 0.6 μm. These changes correlated with the decrease in flux.
Phosphorylation state of IIAGlc: glucose and free-energy sensing
Enzyme IIAGlc and IIAGlc-P are considered sensors for glucose (Postma et al., 1993; Saier et al., 1996; Stülke and Hillen, 1999). We mimicked the absence of glucose by lowering its concentration from 500 to 0.5 μM. In case diffusion was infinitely fast and in the presence of PEP, the concentration of phosphorylated IIAGlc rose twofold and that of nonphosphorylated IIAGlc more than halved when glucose was removed, thus confirming that the concentration of these species responded significantly to the glucose signal. Changes in the cellular free-energy state, as reflected in the PEP concentration, induced a similar yet inverse response. The low PEP concentration applied (90 μM) has been found in cells ∼15 s after E. coli cells started importing glucose (Hogema et al., 1998).
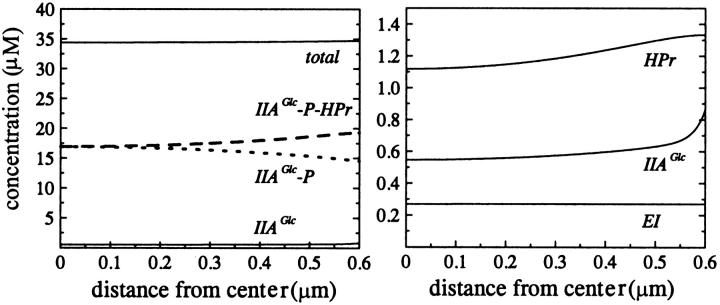
When we used realistic values for the diffusion constants, both nonphosphorylated and phosphorylated IIAGlc continued to respond to changes in extracellular glucose concentration as well as to changes in PEP levels (as depicted in Fig. 4, top). In addition, concentration gradients for both phosphorylated and nonphosphorylated IIAGlc were found. The same response was calculated for mammalian model cells but only within a micron distance from the membrane (depicted in Fig. 4, bottom). Further away from the membrane it looked as if the concentrations of nonphosphorylated and phosphorylated IIAGlc were influenced only by the concentration of PEP. Thus, in fact, in these larger cells, the glucose signal ceased to exist after ∼1 μm on its way into the cell. When we assumed diffusion to be five times faster, as is the case in eukaryotic cytoplasm (Dayel et al., 1999), this distance increased only to ∼1.5 μm (not shown). In the right panels of Fig. 4, a change in the relative ordering of the lines corresponding to low PEP concentration seems to have occurred. However, if one compares both graphs on the same spatial scale, i.e., one compares the top graph with the rightmost 0.6 μm of the bottom graph, then it becomes clear that the crossover in concentration occurs at too large a distance from the membrane to persist in the smaller cell of the top graph.
FIGURE 4.
Position dependent response to glucose and free energy. The concentration distribution of nonphosphorylated IIAGlc and phosphorylated IIAGlc in cells with radius 0.6 μm (top) and 10 μm (bottom) under various conditions of metabolite availability; 2800 μM PEP and 0.5 μM glucose (dashed line); 2800 μM PEP and 500 μM glucose (thin solid line, indicated by asterisk); 90 μM PEP and 500 μM glucose (dotted line); and 90 μM PEP and 0.5 μM glucose (thick solid line).
IIAGlc “senses” the concentration of PEP via two intermediate proteins, i.e., EI and HPr. We wondered what would happen should these be removed. The model was therefore slightly adjusted by fixing the concentrations of phosphorylated and nonphosphorylated HPr and setting their concentrations to 2.8 and 0.9 mM, respectively, identical to those of the original phosphoryl-donor PEP and pyruvate. Therewith our model became representative of a two-protein PTS. At the initial parameter settings, this system hardly phosphorylated glucose because of the relatively high affinity of IIAGlc-P for HPr (now pyruvate) and the 20-fold higher concentration of the latter, leaving nearly no free IIAGlc-P. Therefore we changed the rate constants of the association and dissociation reactions between PEP/pyruvate and IIAGlc so that they became equivalent to those between EI and PEP, i.e., k5, k−5, k6, and k−6 were replaced by k1, k−1, k2, and k−2, respectively (cf. Table 1). In this way, a high glucose phosphorylation flux was restored but now the concentrations of phosphorylated and nonphosphorylated IIAGlc became insensitive to the glucose concentration and only responded to changes in the PEP concentration. Thus IIAGlc would loose its signaling function for glucose. The reason for the insensitivity toward glucose might be the large concentration difference between the main actors (PEP and glucose) that influence the IIAGlc phosphorylation state.
Resensitization of the phosphorylation state of IIAGlc for glucose was achieved by decreasing the association rate constants of nonphosphorylated IIAGlc with PEP and phosphorylated IIAGlc with pyruvate, decreasing the dissociation rate constants of the formed IIAGlc-PEP complex, and simultaneously increasing the dissociation rate constants (k−7 and k8) of the IICBGlc-P-IIAGlc complex, all 100-fold. Indeed, such a decrease in the association and dissociation rate constants of the protein metabolite complex IIAGlc-PEP and an increase in the dissociation rate of the protein-protein complex IICBGlc-P-IIAGlc, rendered the phosphorylation state of the signaling protein IIAGlc sensitive to both glucose and PEP throughout the cell while maintaining a realistic glucose influx at both high and low PEP concentration. In this particular case, however, the association rate constants for nonphosphorylated and phosphorylated IIAGlc with PEP and pyruvate would be about two orders of magnitude smaller than the association rate constants for phosphorylated and nonphosphorylated IIAGlc with IICBGlc and IICBGlc-P. Such a difference in association rate constants between the protein-metabolite complex IIAGlc-PEP and the protein-protein complex IICBGlc-P-IIAGlc is highly unlikely considering the fact that the nature of the association in these phosphoryl-transfer complexes is nearly identical, with similar changes in free energy for the phosphoryl transfer, and the fact that the diffusion rate of the metabolites PEP and pyruvate is much higher than that of IIAGlc. Moreover, a large increase in the dissociation rate constants of the IICBGlc-P-IIAGlc protein-protein complex with respect to those we used, and which were determined in vitro, is rather improbable in the crowded environment of the cell because crowding reduces dissociation rates of protein-protein complexes instead of increasing them (Rohwer et al., 1998b).
DISCUSSION
Most models of metabolic and signal-transduction pathways describe the cell as a “well-stirred reactor”, its soluble components distributed homogeneously throughout. To investigate whether this is actually to be expected, we developed a reaction-diffusion model of the glucose-PTS of E. coli, a well-characterized multi component transport and signal transduction system exhibiting a very high membrane flux.
For a cell of bacterial size (diameter of 0.6 μm or smaller), we found that neither glucose flux nor the spatial distribution of most PTS enzyme species were affected when relaxing the usual assumption that diffusion was infinitely fast. This lack of effect was at first unexpected, because a simplified calculation suggested that a concentration gradient much in excess of the concentration of EI should arise. Our model revealed that the actual concentration gradient in signal was some three times smaller, still close to the total concentration of EI. However, most of the concentration gradient was carried by the more abundant enzymes HPr and IIAGlc. Apparently potential diffusion limitation in a phospho-relay chain can be prevented by having one or more of the participating proteins present at a sufficiently high concentration.
The relationship we found between flux and the total concentration of the different enzymes (not shown) was similar to that reported in vivo (van der Vlag et al., 1995) and obtained with the kinetic model of Rohwer et al. (2000) (cf. Fig. 1 of this reference). When we compared the individual enzyme species, we saw that the diffusion of EI, HPr, and related species hardly affected the behavior of the glucose-PTS. It seemed as if nonphosphorylated and phosphorylated IIAGlc performed nearly all of the diffusion labor (see also Francke et al., 2002). In this, nonphosphorylated IIAGlc was most apt to form concentration gradients and phosphorylated IIAGlc was by far the most important species sustaining the inward flux of glucose and the delivery of the phosphoryl groups toward the membrane. In fact, this role for IIAGlc was not without consequences. Already in cells with radius 0.6 μm, the concentration of nonphosphorylated IIAGlc was subject to diffusion limitations: An appreciable concentration difference developed between the membrane and the cell center due to the influx of glucose. Contrary to what is generally assumed (Postma et al., 1993; Saier et al., 1996), the calculations also suggest that in the presence of both glucose and PEP, there should be relatively little nonphosphorylated EI, HPr, and IIAGlc. The presence of nonphosphorylated IIAGlc at such relatively low concentrations (as compared to IIAGlc-P) made it more susceptible to concentration changes/gradients.
The effect of diffusion on the concentration of nonphosphorylated IIAGlc is potentially important, as it is this enzyme species that is responsible in E. coli for much of one of the most pleiotropic regulatory effects in bacteria, i.e., glucose or catabolite repression (Stülke and Hillen, 1999). Nonphosphorylated IIAGlc interacts with various non-PTS sugar transport proteins in a process called inducer exclusion (Misko et al., 1987; Nelson et al., 1982; Novotny et al., 1985; Osumi and Saier, 1982; Postma et al., 1984; Saier et al., 1983). Upon binding of IIAGlc, the activity of the transport protein is reduced and the import of several non-PTS carbon sources as well as the consequent induction of gene expression of genes related to their transport and metabolism is prevented (see Postma et al., 1993). Because these proteins are mostly, if not all (Voegele et al., 1993), located at the membrane, the effect of diffusion will be that it enhances the inhibition of these proteins under flux conditions by raising the concentration of nonphosphorylated IIAGlc near the membrane.
Normally the relative amounts of IIAGlc and proteins binding IIAGlc are such that the flux through the PTS is maximal. But on the rare occasion that the relative amount of IIAGlc is low, the flux can be reduced by its binding to non-PTS proteins, a phenomenon that is called “reverse inducer exclusion” (van der Vlag et al., 1994; Rohwer et al., 1998a). When the majority of the IIAGlc is bound, its effective diffusion coefficient should be reduced, and then even in very small cells, flux could cause concentration gradients.
Including the effects of macromolecular crowding on the PTS (as reported by Rohwer et al., 1998b) should lead to a further decrease in the concentrations of uncomplexed PTS components such as IIAGlc. Assuming then that nonphosphorylated IIAGlc, and not any of its complexes, is responsible for inducer exclusion, this should mean that the effects of diffusion limitation on inducer exclusion should be even stronger in crowded reality than in our uncrowded model.
Enzyme IIAGlc of E. coli mediates another signal transduction route also effecting glucose repression. At low glucose (and high PEP), many catabolic operons are activated by the action of cAMP (Botsford and Harman, 1992). The cAMP is produced by adenylate cyclase, and that enzyme is activated by phosphorylated IIAGlc (Saier et al., 1996). In our calculations the effects of diffusional limitation on the concentration of phosphorylated IIAGlc are much smaller, in relative terms, than the effects on nonphosphorylated IIAGlc. As a consequence diffusion limitation may affect the inducer exclusion mechanism of catabolite repression more strongly than the cAMP-mediated mechanism.
A point of concern might be that the results of our calculations depended to some extent on the diffusion coefficient that was applied. Because the effects of diffusion are inversely proportional to the square of the cell radius, increasing the diffusion coefficient is phenomenologically equivalent to reducing the cell radius. Behavior displayed by the PTS in a cell at a certain rate of diffusion will be observable in a proportionally bigger cell at a higher diffusion rate. The above implies that our findings will hold irrespective of the precise choice of the diffusion coefficient. In fact, the results appeared not very sensitive to the precise magnitude of the diffusion coefficient; only order of magnitude changes induced marked effects.
Our calculations show that in a bacterial cell, the phosphorylation state of IIAGlc is sensitive to both the presence of glucose and the free-energy status of the cell (in the form of PEP), and thus presents sort of a dual sensing mechanism. “Starved” E. coli cells have an elevated PEP concentration (Hogema et al., 1998). This “standby” condition is the one that gives rise to the dashed lines in Fig. 4. As a consequence of this condition, glucose repression should be minimal along either of its routes (e.g., mediated via inducer exclusion or via cAMP). In this way the cell is able to take up and metabolize any carbohydrate that happens to be available. Addition of glucose should activate both repression mechanisms somewhat, although incompletely, and this is illustrated by the thin solid lines (marked by an asterisk) of Fig. 4. Whereas in the cAMP-mediated glucose repression, effects of the cellular free-energy state have been invoked (e.g., through cAMP efflux at high free-energy states (Makman and Sutherland, 1965; Saier et al., 1975)), inducer exclusion was mainly perceived as a sugar-mediated repression mechanism and not as a free-energy checking mechanism (Postma et al., 1993; Saier et al., 1996; Stülke and Hillen, 1999). Consequently, the calculated result that free-energy (or rather PEP) depletion (dotted and thick solid lines) in itself should cause stronger inducer exclusion than glucose addition might come as a surprise. On the other hand, both the PEP/pyruvate ratio (Weigel et al., 1982) and the ATP/ADP ratio (Rohwer et al., 1996) were implied in the regulation of PTS activity. And Hogema et al. (1998) established a correlation between the phosphorylation state of IIAGlc and the PEP/pyruvate ratio. In fact, the strong inducer exclusion effect consequent to glucose addition to starved cells (Postma et al., 1993; Saier et al., 1996) may consist of a direct effect of glucose plus an indirect effect through a reduction in the intracellular PEP concentrations.
The right panel of Fig. 4, top, showed that also the phosphorylated form of IIAGlc, which directs the cAMP-mediated glucose repression mechanism, is sensitive to both glucose and loss of free energy in the form of PEP. In terms of absolute concentrations, the response of IIAGlc-P was some five times stronger than that of IIAGlc.
In larger cells, we observed rather curious phenomena, i.e., spatial differentiation of the dual sensing mechanism: close to the membrane IIAGlc and IIAGlc-P became primarily responsive to glucose, whereas away from the membrane both became exclusively responsive to the cellular free-energy state as projected into the PEP concentration. The explanation for this is the long diffusion path, semi-isolating the membrane part of the PTS from the cytosolic part. In cells of bacterial size, there is some tendency toward the same phenomena and any additional diffusion limitation due to, for instance, macromolecular crowding near that membrane should enhance the effect.
Sustenance of glucose and PEP sensing properties, although maintaining a realistic glucose influx, appeared difficult the moment we turned the four-enzyme PTS into a two-enzyme PTS, consisting of only enzymes IIAGlc and IICBGlc. This was probably due to incompatibility between the flux requirements and the large concentration differences between the metabolites and the enzymes. The outcome suggests that EI and HPr function as some sort of attenuator for the intracellular energy signal, such that IIAGlc can respond to concentration changes in both glucose and PEP in a concerted manner, rather than that it responds to PEP only. It is interesting in this respect that our calculations for the “normal” PTS also show that the concentrations of phosphorylated and nonphosphorylated EI react solely to changes in the PEP concentration, whereas Lux et al. (1999) suggest that the chemotactic response toward PTS sugars, which is supposedly triggered by dephosphorylation of EI (Lengeler and Jahreis, 1996), is an immediate effect of the presence of the sugar. Assuming our model is right, this can only be when PEP levels are compromised simultaneously.
According to our model, ordinarily the effects of diffusion on the PTS flux should be negligible in cells the size of E. coli. For cells with diameters exceeding 2 μm, however, the control by diffusion on flux became significant. Also many of the PTS proteins began to differ in concentration between membrane surface and cell center. Thus, considering the fact that most enzyme species are involved in other pathways, variable cellular activities can be envisaged in such cells, mediated by the large local concentration differences of these species. It may be telling that possibly the largest organism that contains a PTS, Bacillus megatherium, with an average size of 4 μm × 1.5 μm (Gordon, 1974), still falls within the range of cells that should not be subject to PTS flux limitation, or substantial gradients in all but one of the species.
Most eukaryotic cells are much larger than bacteria and completely lack the PTS. Until now it has remained unclear why these larger organisms lack this highly effective carbohydrate import system. For glucose uptake, many eukaryotes rely on a facilitated diffusion carrier, intracellular glucose carrying out the required diffusion. Perhaps the limitation by diffusion on PTS performance in larger cells is what prevents its effective use in eukaryotes. Here we should make two reservations: i), in our calculations, we increased the cell diameter while keeping the volumetric flux of the PTS constant, i.e., we increased the glucose influx per cell with the third power of the radius (in case diffusion was neglected); and ii), intracellular signaling of the external glucose concentration was impaired only over distances larger than, say, 0.3–0.5 μm, in other words the system would be capable of signaling both glucose and PEP over short distances within the eukaryotic cell.
The PTS has the triple function of catalyzing carbohydrate phosphorylation and uptake, as well as mediating glucose signaling. The former two processes require high flux capacity, the third much less so. Accordingly, it is only the flux function of the PTS that may be incompatible with eukaryotic cell size, not the signal transduction function. Indeed, whereas in eukaryotes metabolic flux and signaling seem to have been separated and metabolic phosphoryl-flux is mediated by low molecular mass carriers such as creatine and ADP rather than by proteins, signal transduction is still mediated by proteins. However, the mechanism of this signal transduction is catalytic in terms of activating kinases that take the signal phosphate from ATP. Only little of the amount of signal generated in, for instance, the nucleus actually diffuses from the plasma membrane through these kinase chains, whereas in phospho-relay chains such as the PTS, all the signal diffuses. Our results (cf. Fig. 4, bottom) make it clear that a PTS-like phospho-relay chain would fail miserably in transferring a signal from the membrane to the inside of a large cell, because already within a relatively short traveling distance (∼1 μm), the signal related to the status near the membrane should be lost and replaced by a signal that is representative of the status inside the cell. For quite a while, the function of the protein kinase cascades has been assumed to be signal amplification in terms of high response coefficients (Goldbeter and Koshland, 1982), but the rationale for this has recently been put into question (Ortega et al., 2002). Perhaps the primary functionality of the kinase cascade structure of these chains is their ability to prevent this loss of signal in a way that resembles action potential propagation or the signal transport along telephone networks.
Model calculations are only as valid as the underlying kinetic scheme and its assumed parameter values. Consequently, our results should be taken as indicative only for the implications of diffusion for signal transduction by phospho-relay chains such as the PTS. Yet, the results are as good as can be obtained with the current knowledge of this type of system, and the glucose-PTS of E. coli is one of the best studied systems of its sort. The parameter values we used did not result from fitting procedures, in line with the silicon cell philosophy (Westerhoff, 2001). Therefore, our results are the mere implications of the biochemical knowledge of the system that exists today. It should be noted that as our biochemical knowledge continues to progress, our results may develop further.
Acknowledgments
The authors thank Drs C.L. Woldringh, J.M. Rohwer, R. van Driel, K.J. Hellingwerf, B.N. Kholodenko, and the reviewers for thoughtful comments, and H. Brinkman for his contribution to the work.
The work was supported via the ICES-KIS II program.
APPENDIX: SPHERICAL VERSUS ROD-LIKE MODEL CELL
We modeled E. coli as a cylinder, with a diameter of 1.2 μm (r = 0.6 μm) and a length of 1.8 μm, closed on both sides by half a sphere, with radius 0.6 μm, and as a sphere with a radius of 0.6 μm (as described by Blom and Peletier, 2000, 2002). For the first rod-shaped cell we included lateral diffusion of the membrane components in the model. The diffusion coefficient for the lateral diffusion of the IICBGlc-related enzyme species was assumed to be 0.2 μm2 s−1 (Vrljic et al., 2002). The other parameter values were chosen as described in the main text. In the rod cell we assumed rotational symmetry around the long axis and mirror symmetry with respect to the plane through the center of the cell perpendicular to the long axis. The solutions in the spherical model cell showed spherical symmetry. To describe the behavior of the PTS in both model cells, we considered the concentrations of the enzyme species along a line with length equal to r perpendicular to the surface. At high glucose and PEP concentrations, we found that in the rod cell the concentration distribution of the different enzyme species along such lines varied slightly depending on the position in the rod, the difference in concentration gradients not amounting to more than 2% in most cases and being always smaller than 6%. The concentrations of the various enzyme species found along the radius of the spherical cell lay approximately in between the extremes found in the rod, with the exception of nonphosphorylated IIAGlc. The concentration gradients were therefore always somewhat smaller, but in general the deviations between the gradients found in the spherical cells and those in the rod-like cells were <2% and at most 11%, again in case of nonphosphorylated IIAGlc. In the rod, hardly any membrane gradients developed in IICBGlc-related species and the difference in glucose influx between the rod and the sphere was smaller than 1%. The above applied to all cases we studied, e.g., using other metabolite concentrations or membrane diffusion coefficients; the effect on the flux and concentration distribution of a hundredfold decrease in the membrane diffusion coefficient was <1‰. The difference between the gradients in the rod cell and the spherical cell at high flux are illustrated in Fig. 5, which shows the two cases with the largest deviations. It is clear from Fig. 5 that for the behavior of the PTS, the differences between a rod and a spherical cell are only marginal.
FIGURE 5.
The concentration distribution of nonphosphorylated (left) and phosphorylated IIAGlc (right) in a spherical cell with radius 0.6 μm (squares), and in a rod-like cell with length 3 μm. The distribution in the latter is represented along two lines of length 0.6 μm perpendicular to the membrane boundary. These lines represent the two extremes found in the rod, i.e., through the center of the cylindrical part (open circles) and the spherical part (closed circles), respectively. The arrows in the insets represent the direction of the lines. The metabolite concentrations were as depicted in Table 1.
Dedicated to the memory of Dr. Pieter W. Postma, who died on April 21, 2002.
Abbreviations used: PTS, phosphoenolpyruvate:carbohydrate phosphotransferase system; GFP, green fluorescent protein; glc, glucose; PEP, phosphoenolpyruvate.
References
- Blom, J. G., and M. A. Peletier. 2000. Diffusive gradients in the PTS system. Report MAS-R0020. CWI, Amsterdam (http://www.cwi.nl/cwi/publications_bibl/).
- Blom, J. G., and M. A. Peletier. 2002. The importance of being cigar-shaped. Report MAS-R0228. CWI, Amsterdam (http://www.cwi.nl/cwi/publications_bibl/).
- Botsford, J. L., and J. G. Harman. 1992. Cyclic AMP in prokaryotes. Microbiol. Rev. 56:100–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, G. C., and B. N. Kholodenko. 1999. Spatial gradients of cellular phospho-proteins. FEBS Lett. 457:452–454. [DOI] [PubMed] [Google Scholar]
- Dayel, M. J., E. F. Hom, and A. S. Verkman. 1999. Diffusion of green fluorescent protein in the aqueous-phase lumen of endoplasmic reticulum. Biophys. J. 76:2843–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Reuse, H., and A. Danchin. 1988. The ptsH, ptsI, and crr genes of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system: a complex operon with several modes of transcription. J. Bacteriol. 170:3827–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elowitz, M. B., M. G. Surette, P. E. Wolf, J. B. Stock, and S. Leibler. 1999. Protein mobility in the cytoplasm of Escherichia coli. J. Bacteriol. 181:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke, C., H. V. Westerhoff, J. G. Blom, and M. A. Peletier. 2002. Flux control of the bacterial phosphoenolpyruvate:glucose phosphotransferase system and the effect of diffusion. Mol. Biol. Rep. 29:21–26. [DOI] [PubMed] [Google Scholar]
- Goldbeter, A., and D. E. Koshland, Jr. 1982. Sensitivity amplification in biochemical systems. Q. Rev. Biophys. 15:555–591. [DOI] [PubMed] [Google Scholar]
- Gordon, R. E. 1974. The genus Bacillus. In Handbook of Microbiology, condensed edition. A. I. Laskin, and H. A. Lechevalier, editors. CRC Press, Cleveland, Ohio. 65–82.
- Hogema, B. M., J. C. Arents, R. Bader, K. Eijkemans, H. Yoshida, H. Takahashi, H. Aiba, and P. W. Postma. 1998. Inducer exclusion in Escherichia coli by non-PTS substrates: the role of the PEP to pyruvate ratio in determining the phosphorylation state of enzyme IIAGlc. Mol. Microbiol. 30:487–498. [DOI] [PubMed] [Google Scholar]
- Kholodenko, B. N., G. C. Brown, and J. B. Hoek. 2000. Diffusion control of protein phosphorylation in signal transduction pathways. Biochem. J. 350:901–907. [PMC free article] [PubMed] [Google Scholar]
- Kundig, W., S. Gosh, and S. Roseman. 1964. Phosphate bound to histidine in a protein as intermediate in a novel phosphotransferase system. Proc. Natl. Acad. Sci. USA. 52:1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. J., W. Boos, J. P. Bouche, and J. Plumbridge. 2000. Signal transduction between a membrane-bound transporter, PtsG, and a soluble transcription factor, Mlc, of Escherichia coli. EMBO J. 19:5353–5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler, J. W., and K. Jahreis. 1996. Phosphotransferase Systems or PTSs as Carbohydrate Transport and as Signal Transduction Systems. In Handbook of Biological Physics, Vol. 2. W. N. Konings, H. R. Kaback, and J. S. Lolkema, editors. Elsevier Science B.V., Amsterdam. 573–598.
- Lux, R., V. R. Munasinghe, F. Castellano, J. W. Lengeler, J. E. Corrie, and S. Khan. 1999. Elucidation of a PTS-carbohydrate chemotactic signal pathway in Escherichia coli using a time-resolved behavioral assay. Mol. Biol. Cell. 10:1133–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makman, R. S., and E. W. Sutherland. 1965. Adenosine 3′,5′-phosphate in Escherichia coli. J. Biol. Chem. 240:1309–1314. [PubMed] [Google Scholar]
- Meadow, N. D., D. K. Fox, and S. Roseman. 1990. The bacterial phosphoenolpyruvate:glycose phosphotransferase system. Annu. Rev. Biochem. 59:497–542. [DOI] [PubMed] [Google Scholar]
- Misko, T. P., W. J. Mitchell, N. D. Meadow, and S. Roseman. 1987. Sugar transport by the bacterial phosphotransferase system. Reconstitution of inducer exclusion in Salmonella typhimurium membrane vesicles. J. Biol. Chem. 262:16261–16266. [PubMed] [Google Scholar]
- Nanninga, N. 1998. Morphogenesis of Escherichia coli. Microbiol. Mol. Biol. Rev. 62:110–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, S. O., B. J. Scholte, and P. W. Postma. 1982. Phosphoenolpyruvate:sugar phosphotransferase system-mediated regulation of carbohydrate metabolism in Salmonella typhimurium. J. Bacteriol. 150:604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny, M. J., W. L. Frederickson, E. B. Waygood, and M. H. Saier, Jr. 1985. Allosteric regulation of glycerol kinase by enzyme IIIGlc of the phosphotransferase system in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 162:810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega, F., L. Acerenza, H. V. Westerhoff, F. Mas, and M. Cascante. 2002. Product dependence and bifunctionality compromise the ultrasensitivity of signal transduction cascades. Proc. Natl. Acad. Sci. USA. 99:1170–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi, T., and M. H. Saier, Jr. 1982. Regulation of lactose permease activity by the phosphoenolpyruvate:sugar phosphotransferase system: evidence for direct binding of the glucose-specific enzyme III to the lactose permease. Proc. Natl. Acad. Sci. USA. 79:1457–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma, P. W., W. Epstein, A. R. Schuitema, and S. O. Nelson. 1984. Interaction between IIIGlc of the phosphoenolpyruvate:sugar phosphotransferase system and glycerol kinase of Salmonella typhimurium. J. Bacteriol. 158:351–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57:543–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard, G. T., and J. Broos. 1999. Structure/function studies on the bacterial carbohydrate transporters, enzymes II, of the phosphoenolpyruvate-dependent phosphotransferase system. Biochim. Biophys. Acta. 1422:73–104. [DOI] [PubMed] [Google Scholar]
- Rohwer, J. M., R. Bader, H. V. Westerhoff, and P. W. Postma. 1998a. Limits to inducer exclusion: inhibition of the bacterial phosphotransferase system by glycerol kinase. Mol. Microbiol. 29:641–652. [DOI] [PubMed] [Google Scholar]
- Rohwer, J. M., P. W. Postma, B. N. Kholodenko, and H. V. Westerhoff. 1998b. Implications of macromolecular crowding for signal transduction and metabolite channeling. Proc. Natl. Acad. Sci. USA. 95:10547–10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwer, J. M., P. R. Jensen, Y. Shinohara, P. W. Postma, and H. V. Westerhoff. 1996. Changes in the cellular energy state affect the activity of the bacterial phosphotransferase system. Eur. J. Biochem. 235:225–230. [DOI] [PubMed] [Google Scholar]
- Rohwer, J. M., N. D. Meadow, S. Roseman, H. V. Westerhoff, and P. W. Postma. 2000. Understanding glucose transport by the bacterial phosphoenolpyruvate:glycose phosphotransferase system on the basis of kinetic measurements in vitro. J. Biol. Chem. 275:34909–34921. [DOI] [PubMed] [Google Scholar]
- Saier, M. H., Jr., B. U. Feucht, and M. T. McCaman. 1975. Regulation of intracellular adenosine cyclic 3′:5′ monophosphate levels in Escherichia coli and Salmonella typhimurium. Evidence for energy-dependent excretion of the cyclic nucleotide. J. Biol. Chem. 250:7593–7601. [PubMed] [Google Scholar]
- Saier, M. H., Jr., M. J. Novotny, D. Comeau-Fuhrman, T. Osumi, and J. D. Desai. 1983. Cooperative binding of the sugar substrates and allosteric regulatory protein (enzyme IIIGlc of the phosphotransferase system) to the lactose and melibiose permeases in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 155:1351–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier, M. H., Jr., T. M. Ramseier, and J. Reizer. 1996. Regulation of carbon utilization. In Escherichia coli and Salmonella cellular and Molecular Biology, 2nd ed, Vol 1. F. C. Neidhardt, editor. ASM press, Washington, D.C. 1325–1343.
- Seok, Y. J., M. Sondej, P. Badawi, M. S. Lewis, M. C. Briggs, H. Jaffe, and A. Peterkofsky. 1997. High affinity binding and allosteric regulation of Escherichia coli glycogen phosphorylase by the histidine phospho-carrier protein, HPr. J. Biol. Chem. 272:26511–26521. [DOI] [PubMed] [Google Scholar]
- Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3–21. [DOI] [PubMed] [Google Scholar]
- Stülke, J., and W. Hillen. 1999. Carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 2:195–201. [DOI] [PubMed] [Google Scholar]
- Tanaka, Y., K. Kimata, and H. Aiba. 2000. A novel regulatory role of glucose transporter of Escherichia coli: membrane sequestration of a global repressor Mlc. EMBO J. 19:5344–5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vlag, J., K. van Dam, and P. W. Postma. 1994. Quantification of the regulation of glycerol and maltose metabolism by IIAGlc of the phosphoenolpyruvate-dependent glucose phosphotransferase system in Salmonella typhimurium. J. Bacteriol. 176:3518–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vlag, J., R. van't Hof, K. van Dam, and P. W. Postma.1995. Control of glucose metabolism by the enzymes of the glucose phosphotransferase system in Salmonella typhimurium. Eur. J. Biochem. 230:170–182. [DOI] [PubMed] [Google Scholar]
- Voegele, R. T., G. D. Sweet, and W. Boos. 1993. Glycerol kinase of Escherichia coli is activated by interaction with the glycerol facilitator. J. Bacteriol. 175:1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrljic, M., S. Y. Nishimura, S. Brasselet, W. E. Moerner, and H. M. Mc Connell. 2002. Translational diffusion of individual class II MHC membrane proteins in cells. Biophys. J. 83:2681–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel, N., M. A. Kukuruzinska, A. Nakazawa, E. B. Waygood, and S. Roseman. 1982. Sugar transport by the bacterial phosphotransferase system. Phosphoryl transfer reactions catalyzed by enzyme I of Salmonella typhimurium. J. Biol. Chem. 257:14477–14491. [PubMed] [Google Scholar]
- Weast, R. C. (editor). 1975. Handbook of Chemistry and Physics. 55th ed. CRC Press, Cleveland, Ohio.
- Westerhoff, H. V. 2001. The silicon cell. Not dead but live! Metab. Eng. 3:207–210. [DOI] [PubMed] [Google Scholar]
- Woldringh, C. L., and N. Nanninga. 1985. Structure of nucleoid and cytoplasm in the intact cell. In Molecular Cytology of Escherichia coli. N. Nanninga, editor. Academic Press, London and New York. 161–197.