Abstract
Within a Mycobacterium tuberculosis-induced granuloma, lymphocytes and macrophages work together to control bacterial growth and limit the spread of infection. Chemokines and chemokine receptors are involved in cell migration and are logical candidates for a role in granuloma formation. In the present study we addressed the role of CC chemokine receptor 2 (CCR2) in M. tuberculosis infection. In previous studies (W. Peters et al., Proc. Natl. Acad. Sci. USA 98:7958-7963, 2001), CCR2−/− mice were found to be highly susceptible to a moderate or high dose of H37Rv administered intravenously (i.v.). We have expanded those studies to demonstrate that the susceptibility of CCR2−/− mice is dose dependent. After low-dose aerosol or i.v. infection of CCR2−/− mice with M. tuberculosis, there was a substantial delay in cell migration to the lungs and delayed expression of gamma interferon and inducible nitric oxide synthase. The CCR2−/− mice had a severe and prolonged deficiency in the number of macrophages in the lungs and an early increase in the number of neutrophils. Despite these deficiencies in cell migration, the CCR2−/− mice did not have increased bacterial loads in the lungs compared to wild-type (C57BL/6) mice and successfully formed granulomas. This finding is in contrast to CCR2−/− mice infected with a high dose of M. tuberculosis administered i.v. These results indicate that with low-dose infection, a delay in immune response in the lungs does not necessarily have detrimental long-term effects on the progression of the disease. The fact that CCR2−/− mice survive with substantially fewer macrophages in the low-dose models implies that the immune response to low-dose M. tuberculosis infection in mice is more robust than necessary to control the infection. Finally, these data demonstrate that, in cases of infectious disease in knockout models, clear phenotypes may not be evident when one is solely evaluating bacterial numbers and survival. Functional assays may be necessary to reveal roles for components of the multifactorial immune system.
The hallmark of Mycobacterium tuberculosis infection is granuloma formation. After infection of alveolar and resident macrophages or dendritic cells in the lungs, monocytes and Mycobacterium-specific lymphocytes migrate from the blood to the lungs and form granulomas. Within the localized environment of the granuloma, the immune cells control the bacterial replication and limit the spread of infection within the lung and to other organs. In M. tuberculosis-infected hosts, CD4+ and CD8+ T lymphocytes produce gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), cytokines which aid in control of infection and macrophage activation (1, 7, 10, 11). Activated macrophages also produce TNF-α and reactive oxygen and nitrogen intermediates, which have antimycobacterial properties (5, 11). Identifying factors involved in migration of cells to the lungs and granuloma formation is a key to understanding the immune response to M. tuberculosis.
Chemokines and chemokine receptors are involved in cell migration and are logical candidates for a role in granuloma formation. The chemokines induced during M. tuberculosis infection have been studied to a limited degree. Gene expression of CCL3 (macrophage inflammatory protein 1α [MIP-1α]), CXCL2 (MIP-2), CXCL10 (IP-10), and CCL2 (monocyte chemoattractant protein 1 [MCP-1]) was increased in the lungs of infected mice (24). CCL2−/− mice did not demonstrate increased susceptibility to M. tuberculosis infection, but whether cell infiltration or histology was affected in these mice was not reported (18). However, transgenic mice overexpressing CCL2 were more susceptible to tuberculosis (25). In in vitro human studies, the chemokines CCL5 (RANTES), CCL2, CCL3, and CXCL8 (interleukin-8 [IL-8]) were released by alveolar macrophages upon infection with M. tuberculosis (26), and bronchoalveolar lavage fluid from pulmonary tuberculosis patients had increased levels of CCL5, CCL2, and CXCL8 compared to healthy controls (14, 26). Blood monocytes and lymph node cells from tuberculosis patients had increased levels of CCL2 protein compared to healthy controls (17).
There have been studies on the contribution of various chemokines to granuloma formation. Using “bead” instillation (purified protein derivative or schistosomal egg antigen beads) or injection of schistosomal eggs to induce granulomas in lungs of mice, roles for CCR1 and CCR2, as well as for CCL5 and CCL2, in granuloma formation were demonstrated (6, 23, 30, 32). CCR2 is present on monocytes, macrophages, and dendritic cells, as well as in activated T cells. This chemokine receptor is a logical candidate for monocyte/macrophage recruitment to the lungs because macrophages and monocytes are chemotactic toward CCR2 ligands, macrophage chemotactic protein CCL2, CCL7 (MCP-3), and CCL12 (MCP-5). Studies in CCR2−/− mice have indicated that this receptor plays a major role in mediating monocyte/macrophage recruitment in response to intracellular pathogens such as Listeria monocytogenes or Cryptococcus neoformans and to nonspecific inflammatory stimuli, such as thioglycolate or mycobacterial antigens (3, 15, 31), although resident macrophages were unaffected by the absence of CCR2 (15).
In the present study we addressed the requirement for CCR2 in various infection models of M. tuberculosis, including aerosol infection and both high- and low-dose intravenous (i.v.) infection. Previously we, along with coworkers, reported that CCR2−/− mice were highly susceptible to i.v. M. tuberculosis infection, with significantly higher bacterial loads by 14 days postinfection, severe pathology, and increased mortality (22). These mice demonstrated macrophage and dendritic cell migration deficiencies and delays in IFN-γ and inducible nitric oxide synthase (NOS2) expression (22). To determine whether the migration defects were evident after aerosol or i.v. infections with low doses of M. tuberculosis, we infected CCR2−/− mice and compared the development of the immune response to CCR2+/+ mice. Our results confirm that CCR2 plays a major role in migration of monocyte to the lung during M. tuberculosis infection. It also appears to affect early migration of T cells to the lung, which could result in the observed transient delay in IFN-γ and NOS2 expression. Surprisingly, the deficiencies in cell migration and cytokine expression did not noticeably affect the ability of CCR2−/− mice to control low-dose M. tuberculosis infection.
MATERIALS AND METHODS
Animals.
C57BL/6 female mice (Charles River Laboratories, Rockland, Mass.) and CCR2−/− (3) (8 to 14 weeks old) were used in all experiments. CCR2+/− or −/− mice (heterozygous breeding pair provided by I. Charo, University of California, San Francisco) were bred and genotyped by PCR on tail DNA and maintained under specific-pathogen-free conditions at the University of Pittsburgh Biotechnology Center. Only CCR2−/− mice were used. Mice were transferred to the Biomedical Science Tower Biosafety Level 3 animal facility at 7 to 8 weeks of age. All infected mice were maintained in the BSL3 animal laboratories and were routinely monitored for murine pathogens by means of serological and histological examinations. The University Institutional Animal Care and Use Committee approved all animal protocols employed in the present study.
Chemicals and reagents.
All chemicals were purchased from Sigma Chemical Co. (St. Louis, Mo.) unless otherwise noted. Middlebrook 7H9 liquid medium and 7H10 agar were obtained from Difco Laboratories (Detroit, Mich.). The antibodies used in flow cytometric analyses were obtained from Pharmingen (San Diego, Calif.). Pyrazinamide was purchased from Acros.
Mycobacteria and infection of mice.
To prepare bacterial stock, M. tuberculosis strain Erdman (Trudeau Institute, Saranac Lake, N.Y.) or H37Rv (John Belisle, Colorado State University, Fort Collins, Colo.) was used to infect mice, and then bacteria were harvested from their lungs, expanded in 7H9 liquid medium, and stored in aliquots at −80°C. Mice were infected via the aerosol route by using a nose-only exposure unit (In Tox Products, Albuquerque, N.M.), and exposed to M. tuberculosis (107 CFU/ml in the nebulizer chamber) for 20 min, followed by 5 min of air. This resulted in reproducible delivery of 50 to 100 viable CFU of M. tuberculosis as described previously (28), which was confirmed by CFU determination on the lungs of two to three infected mice 1 day postinfection. High-dose aerosol infection with 2 × 108 or 1 × 108 CFU in the nebulizer resulted in delivery of 1,500 or 4,000 viable CFU, respectively, of H37Rv M. tuberculosis in two different experiments and was confirmed by the number of day 1 CFU in the lungs. Mice were infected i.v. via tail vein with either 1 × 104 (low dose) or 2 × 105 (high dose) CFU in 100 μl of phosphate-buffered saline (PBS)-0.05% Tween 80. The tissue bacillary load was quantified by plating serial dilutions of the lung, liver, and spleen homogenates onto 7H10 agar as described previously (10).
Bone marrow-derived macrophages.
Macrophages were derived from C57BL/6 bone marrow based on adherence. The mice were euthanized, and their bone marrow was flushed out of the femur and tibia bones with Dulbecco modified Eagle medium as previously described (2). The bone marrow suspension was then washed twice with 2% fetal bovine serum in PBS, and the cells were counted. Then, 2 × 106 cells were plated on non-tissue culture-treated petri dishes (Labtek) in 25 ml of macrophage media (25% L-cell supernatant, 20% fetal bovine serum, 1% l-glutamine, 1% pyruvate, and 1% nonessential amino acids). After 4 days in culture the cells were fed with 10 ml of fresh macrophage media. On day 6 macrophages were infected with M. tuberculosis at a multiplicity of infection of 4. At 4 h postinfection the supernatant was removed, cells were washed, and fresh medium was added.
Flow cytometric analysis of lung cells.
To determine the amount of cellular infiltrate in the lung, lungs were removed for flow cytometric analysis at 10-day intervals. Lungs were subjected to a short period (25 min) of digestion with 1 mg of collagenase A and 25 U of DNase (both from Boehringer Mannheim, Mannheim, Germany)/ml at 37°C. The suspension was then pushed through a cell strainer as previously described (19). Red blood cells were lysed with red blood cell lysis buffer (NH4Cl-Tris solution), and the single-cell suspension was counted. The samples were stained with 0.2 μg of anti-CD4, anti-CD8, or anti-CD69 or with 0.15 μg of anti-Gr1 and 0.2 μg of anti-CD11b in fluorescence-activated cell sorting buffer (0.1% sodium azide, 0.1% bovine serum albumin, 20% mouse serum). After several washes, the cells were fixed in 4% paraformaldehyde for 1 h and collected on a FACSCaliber (Beckon Dickinson). Analysis was performed by using CellQuest software (Pharmingen).
Histopathology.
Tissue samples for histological studies were fixed in 10% normal buffered formalin, followed by paraffin embedment. For histopathological studies 5- to 6-μm sections were stained with Harris' hematoxylin and eosin.
RPA.
A multiprobe RNase protection assay (RPA) system (Pharmingen) was used to determine the levels of mRNA for genes of interest at 10-day intervals after aerosol infection in murine lungs or in infected macrophages in vitro. At the time of harvest, the lungs were snap-frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted by using Trizol reagent (Life Technology, Grand Island, N.Y.), followed by treatment with RNase free-DNase (Roche, Indianapolis, Ind.) and RNase inhibitor (Roche). Macrophage RNA was obtained from bone marrow-derived macrophages by treating ∼4 × 106 adherent cells with 1 ml of Trizol reagent. The RNA was subjected to RPA according to Pharmingen's protocol. In short, mRNA from macrophages or the lungs was hybridized overnight to [32P]UTP-labeled probes. The protected [32P]UTP-labeled RNA probes were resolved on a 6% polyacrylamide gel and analyzed by autoradiography. Cytokine analysis was performed by using a custom-made template set specific for NOS2, IL-4, IL-12p40, TNF-α, IL-1β, IL-1α, and IFN-γ. Chemokine analysis was performed by using either a custom-template set specific for CCL2, CCL7, CCL12, CCR2, CCL19 (MIP-3β) and CXCL10 (Pharmingen, provided by Joel Ernst, University of California at San Francisco) or an mCK5 multiprobe template set (Pharmingen). The expression of specific genes was quantified on a densitometer (ImageQuant Software; Molecular Dynamics, Sunnyvale, Calif.) relative to the abundance of the housekeeping gene, L32. We also performed phosphorimaging quantification on some samples but obtained results identical to those seen with densitometry; therefore, only the densitometric quantifications are shown.
Statistical analysis.
Three to four mice per group per time point were used for all studies. Statistical analysis was performed on the data by utilizing Prism Software for an unpaired t test. For bacterial numbers and cell numbers, log transformation was performed prior to statistical analysis.
RESULTS
Expression of CCR2 ligands, CCL2, CCL7, and CCL12 increases after infection.
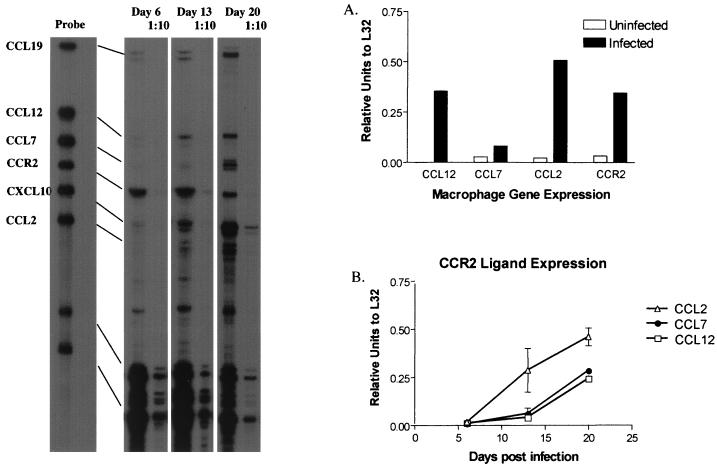
To determine whether expression of CCR2 ligands was enhanced after M. tuberculosis infection, RPA was performed on infected bone marrow-derived macrophages and the lungs of infected C57BL/6 mice. The expression of three major CCR2 ligands and CCR2 increased upon in vitro infection of macrophages (Fig. 1A). There was also enhanced expression of genes for CCL3, CCL4 (MIP-1β), CCL5, CXCL2, and CXCL10 in macrophages (data not shown). In the lungs, expression of the macrophage chemotactic proteins (CCL2, CCL7, and CCL12) increased after aerosol M. tuberculosis infection (Fig. 1B). The pattern of expression was similar to that of a number of other chemokines after infection in the lung, including CCL3, CCL4, CCL5, and CXCL10 (data not shown).
FIG. 1.
Expression of CCR2 ligands, CCL2, CCL7, and CCL12 increases after M. tuberculosis infection. RPA on mRNA isolated from bone marrow-derived macrophages (A) or C57BL/6 mice (B) reveals enhanced expression of CCL2, CCL7, and CCL12 after M. tuberculosis infection. Representative samples from the lung RPA are shown on the left.
CCR2−/− mice control low-dose infection with M. tuberculosis.
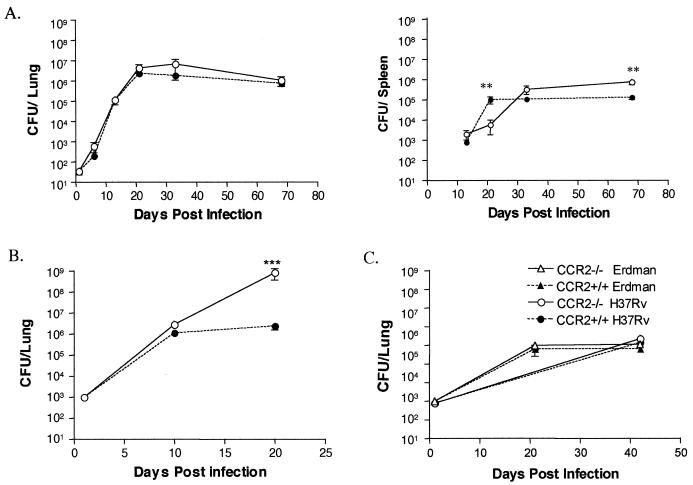
Mice were infected via the aerosol route (∼50 CFU) with M. tuberculosis and sacrificed to determine the bacillary burdens in the lung, liver, and spleen at various time points postinfection (Fig. 2A). The bacterial numbers in the lungs of CCR2−/− and wild-type mice were not significantly different at any time postinfection. There was no significant difference in bacterial dissemination to the spleen or liver between the mouse strains, although the initial growth of M. tuberculosis in the spleens of CCR2−/− was impaired compared to wild-type mice (P = 0.0019). However, at late time points (70 days) there were ∼10-fold more CFU in the CCR2−/− spleens compared to the wild type. No differences in the bacterial growth in the liver were observed (data not shown). No CCR2−/− mice succumbed to the aerosol infection during the course of the experiment; mice were maintained for up to 5 months. At 5 months postinfection the CCR2−/− mice had similar bacterial loads in the lungs (data not shown). The low-dose aerosol infections were performed with M. tuberculosis strains H37Rv (twice) and Erdman (twice), with similar results.
FIG. 2.
CCR2 deficient mice control low-dose infection with M. tuberculosis. CCR2−/− mice and wild-type C57BL/6 mice were infected via the aerosol route with ∼50 CFU of M. tuberculosis H37Rv (circles) (A), i.v. with 2 × 105 CFU of M. tuberculosis H37Rv (B), or i.v. with 104 CFU of M. tuberculosis Erdman (triangles) or H37Rv (C). CCR2−/− mice (open symbols) controlled growth of M. tuberculosis similar to wild-type (solid symbols) mice in the two lower-dose models (A and C) but had much higher bacterial numbers in the higher-dose model (B). Both lung and spleen CFU results are shown in panel A as indicated; only lung CFU are shown in panels B and C. Each time point represents three to four mice, and the bars represent the standard error. The aerosol route experiment was performed twice with H37Rv and twice with Erdman strain with similar results. The i.v. route experiments were performed twice.
These results were in sharp contrast to published results on CCR2−/− mice infected i.v. with 4 to 10 × 105 CFU (22), a study in which a majority of mice did not survive beyond 23 days. We repeated these studies with our standard i.v. dose of 2 × 105 CFU with strain H37Rv. This dose did not cause mortality in C57BL/6 mice in our study. As reported previously (22), the CCR2−/− mice infected with a relatively high dose of M. tuberculosis had 5-fold-higher bacterial numbers than wild-type mice by 10 days postinfection and 100-fold more CFU by 20 days postinfection (Fig. 2B). The CCR2−/− mice infected i.v. with 2 × 105 CFU did not survive past 23 days postinfection.
The decreased susceptibility of CCR2−/− mice to the aerosol infection could have been due to either the route used to infect the mice or the substantially lower dose that is delivered to the lungs by the aerosol (compared to the i.v.) infection. To distinguish between these possibilities, we infected CCR2−/− mice with a lower dose i.v., that is, with 104 CFU of H37Rv. This delivers <100 CFU to the lungs (4, 20; data not shown), which is similar to the dose delivered directly to the lung by our aerosol infection. CCR2−/− and wild-type mice infected i.v. with the lower dose were equally capable of controlling the infection, a finding similar to that seen in aerosol-infected mice (Fig. 2C). When a high dose of M. tuberculosis H37Rv was administered via aerosol (4,000 or 1,500 CFU), the CCR2−/− mice had significantly higher bacterial loads in their lungs than wild-type mice by 3 weeks postinfection (data not shown); both strains of mice succumbed by 4 weeks postinfection to these high doses of M. tuberculosis. Thus, the susceptibility of CCR2−/− mice is dependent on dose rather than on the route of infection.
Monocyte/macrophage migration deficiencies were observed even in low-dose infection.
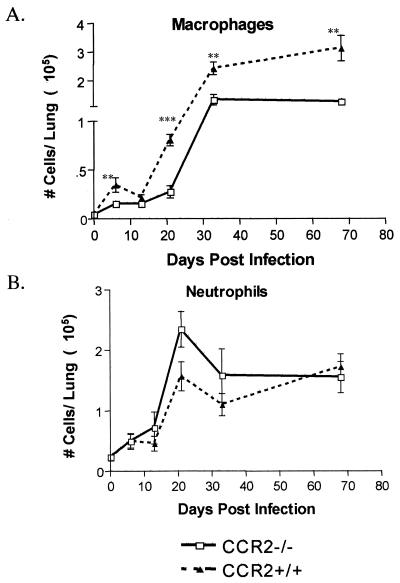
Murine macrophages express CCR2 and show chemotactic ability to CCR2 ligands, CCL2, CCL7, and CCL12. Monocyte/macrophage migration deficiencies have been observed in inflammatory models in CCR2−/− mice (3, 8, 15, 16, 21, 22, 31, 32). Since the CCR2−/− mice were capable of controlling low-dose M. tuberculosis infection, we examined whether the lungs of the CCR2−/− mice were also deficient in macrophages after low-dose M. tuberculosis infection. Antibody staining, followed by flow cytometric analysis, was performed on single-cell lung homogenates. A substantial macrophage deficiency was observed in the CCR2−/− mice infected with a low dose of M. tuberculosis. There was a 10-fold increase in macrophages by 32 days postinfection, but the number of macrophages in the CCR2−/− mice remained much lower than in wild-type mice (Fig. 3A), even up to 5 months postinfection (data not shown). In the low-dose i.v. model of M. tuberculosis infection (104 CFU), the CCR2−/− also exhibited at least twofold fewer macrophages in the lungs (Table 1).
FIG. 3.
Macrophages are present in fewer numbers throughout M. tuberculosis infection in the CCR2-deficient mice. The extent of migration of macrophages (A) and neutrophils (B) to the lung after aerosol infection (50 CFU) with M. tuberculosis H37Rv in CCR2−/− mice (□) and CCR2+/+ mice (▴) is charted. Flow cytometric analysis was performed on single-cell suspensions of the lung. Within the macrophage/granulocyte gate (determined by side-scatter versus forward-scatter parameters), macrophages were defined as CD11b+ Gr1− and neutrophils were defined as CD11b+ Gr1+. Each datum point represents three to four mice, and the bars represent the standard error. ✽, P ≤ 0.05; ✽✽, P ≤ 0.005; ✽✽✽, P ≤ 0.001. The experiment was repeated twice with similar results.
TABLE 1.
Numbers of immune cells within the lungs at 3 weeks postinfectiona
| Cellular infiltrate (105) | No. of immune cells (SEM)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Low dose
|
High dose
|
|||||||
| Aerosol
|
i.v.
|
Aerosol
|
i.v.
|
|||||
| WT | CCR2−/− | WT | CCR2−/− | WT | CCR2−/− | WT | CCR2−/− | |
| Macrophages | 0.81 (0.06) | 0.28 (0.06)∗∗ | 0.64 (0.10) | 0.36 (0.050)∗ | 8.30 (1.02) | 1.71 (0.33)∗∗∗ | 5.06 (0.50) | 0.7 (0.16)∗∗∗ |
| Neutrophils | 1.57 (0.24) | 2.35 (0.29) | 0.65 (0.29) | 1.70 (0.45) | 1.55 (1.23) | 45.9 (8.0)∗∗ | 2.16 (0.26) | 24.54 (7.15)∗∗∗ |
| CD4 T cells | 6.01 (0.88) | 3.72 (0.62)∗ | 5.04 (1.11) | 3.58 (0.66) | 21.0 (2.84) | 9.5 (2.81)∗ | 30.42 (5.31) | 16.81 (0.53)∗∗∗ |
| CD8 T cells | 3.84 (0.46) | 4.28 (0.28) | 3.25 (0.64) | 2.5 (0.36) | 11.8 (1.32) | 8.18 (1.88) | 16.46 (3.21) | 8.7 (0.53)∗∗∗ |
Numbers of macrophages, neutrophils, CD4+ lymphocytes, and CD8+ lymphocytes are represented from low-dose aerosol infections, low-dose i.v. infections, and high-dose aerosol and i.v. infections. Each value represents the mean of three to four mice. ∗, P ≤ 0.05; ∗∗, P ≤ 0.005; ∗∗∗, P ≤ 0.001. WT, wild-type mice; CCR2−/−, CCR2−/− mice.
These data and results previously reported (22) demonstrate that CCR2 plays an essential role in macrophage migration to the lung during M. tuberculosis infection, regardless of the dose, the route, or the strain of M. tuberculosis used for infection. In high-dose i.v. and aerosol infection models, the numbers of neutrophils increased to >50% of the total lung homogenate (Table 1) (22). An increase in neutrophils was evident in low-dose models of infection in CCR2−/− mice, but by 4 weeks postinfection the percentage of neutrophils was greatly reduced (Fig. 3B and Table 1).
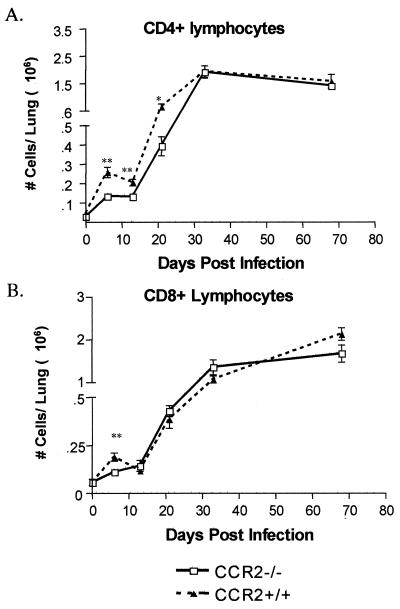
Lymphocyte migration to the lung after aerosol infection is affected.
Because CD4+ and CD8+ lymphocytes play important roles in M. tuberculosis infection (reviewed in reference 9) and can express CCR2, the migration of these cells to the lung after M. tuberculosis infection was evaluated by flow cytometry. The number of CD4+ T cells was significantly less in the CCR2-deficient mice compared to wild-type mice up to 50 days postinfection (Fig. 4A). There was a more modest delay in CD8+ lymphocyte migration to the lung, but this delay was overcome by 4 weeks postinfection (Fig. 4B). We assessed activation status of the lung T cells by staining for the early activation marker, CD69. There was no difference in CD69 expression on CD4+ T cells at any time point between wild-type and CCR2−/− mice. However, at 13 days postinfection there was a significantly higher percentage of CD8+ lymphocytes expressing CD69 in wild-type mice (P = 0.004); at other time points the expression of CD69 was not significantly different between CCR2−/− and CCR2+/+ mice (data not shown).
FIG. 4.
The migration of T lymphocytes to the lung was delayed after low-dose aerosol M. tuberculosis infection. Flow cytometric analysis with anti-CD4 (A) and anti-CD8 (B) antibodies was performed on single-cell suspensions of the lung at various time points postinfection. Each datum point represents three to four CCR2−/− mice (□) or CCR2+/+ mice (▴), and the bars represent the standard error. ✽, P ≤ 0.05; ✽✽, P ≤ 0.005. The experiment was repeated twice with similar results.
Histological analysis of CCR2-deficient mice.
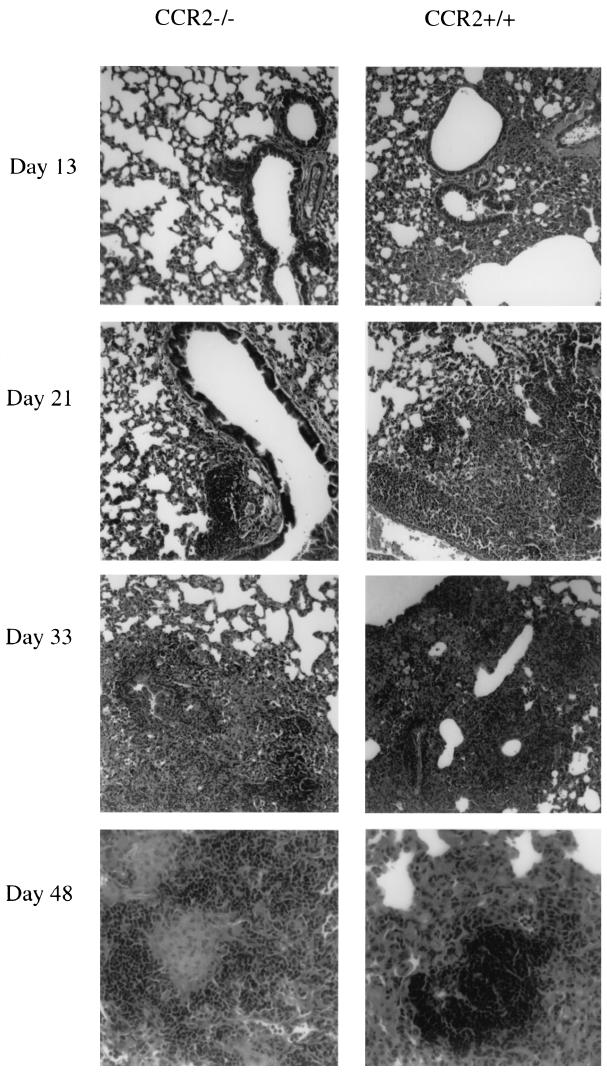
Granuloma formation parallels development of cell-mediated immunity in murine models of tuberculosis. This occurs in the M. tuberculosis-infected lung 3 to 4 weeks postinfection. The granuloma consists primarily of clusters of macrophages, including epithelioid macrophages, and lymphocytes. In human tuberculous granulomas, the lymphocytes typically surround the macrophages, which may fuse to form multinucleated giant cells. After aerosol infection, the CCR2−/− mice did form granulomas in the lungs, but the formation was delayed and the reduction in macrophages could clearly be observed in the overall granuloma structure and pathology of the lungs (Fig. 5). By 13 days postinfection, perivascular and peribronchial lymphocytic infiltrate was evident in wild-type mice and to a smaller extent in CCR2-deficient mice. This finding correlates with the delay in lymphocytic and macrophage infiltration evident in the flow cytometric analysis of the lung (Fig. 4). By 21 days postinfection the perivascular and peribronchial infiltrate was more noticeable in the CCR2-deficient mice, but in the wild-type mice the lymphocytes were infiltrating deeper into the parenchymal space. By 33 days postinfection granulomas were evident in both groups of mice but were more organized in wild-type mice with tighter lymphocytic clusters. By day 48 the granulomas were organized in the CCR2-deficient mice but, as a result of fewer macrophages in the lungs, the granulomas consisted of lymphocytic cuffs surrounding epithelioid macrophages rather than the lymphocytic aggregates within sheets of epithelioid macrophages as previously described in wild-type mice (12). Thus, granuloma formation in the lungs was delayed and ultimately the granulomas appeared to contain fewer macrophages in CCR2−/− mice compared to wild-type mice.
FIG. 5.
Granulomas form in the lungs with delayed kinetics in CCR2-deficient mice. Hematoxylin and eosin staining was performed on 5- to 6-μm formalin-fixed lung tissue sections at each time point postinfection. Magnifications: ×40, days 13, 21, and 33; ×100, day 48. Infiltration is delayed in CCR2−/− mice, but granulomas do form; however, these granulomas are histologically different from those found in CCR2+/+ mice.
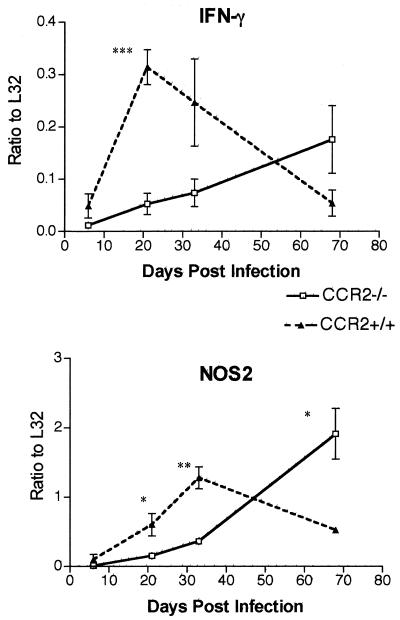
IFN-γ and NOS2 production is delayed in CCR2-deficient mice.
Mice deficient in CCR2 have transient delays in IFN-γ production (21, 27, 31). Since IFN-γ is required for the control of M. tuberculosis (7, 10, 13) and since the CCR2−/− mice control infection in the low-dose models, we examined whether the defect in IFN-γ production reported in the high-dose-infected mice (22) was evident in the aerosol model. In RPAs of whole-lung RNA preparations, there was a substantial delay in the IFN-γ gene expression (Fig. 6). Concomitant with the delay in IFN-γ was a delay in mRNA levels of the gene for inducible nitric oxide synthase (NOS2). RNA from the lungs of uninfected mice was also used in RPAs; the genes of interest were below the level of detection with this RNA (data not shown). The delayed NOS2 gene expression was confirmed at the protein level by immunostaining of lung tissue from the mice at each time point postinfection (data not shown). Expression of a number of other cytokines was examined by RPA, and no significant difference in the mRNA expression of IL-12, TNF-α, or IL-1 in CCR2−/− mice compared to wild-type mice was observed (data not shown).
FIG. 6.
Expression of IFN-γ and inducible nitric oxide synthase (NOS2) is delayed in the lungs of CCR2−/− mice infected with a low dose of M. tuberculosis H37Rv via aerosol. RPAs were performed on whole-lung RNA preparations as described in the text. Each datum point represents three to four CCR2−/− mice (□) or CCR2+/+ mice (▴), and the bars represent the standard error. ✽, P ≤ 0.05; ✽✽, P ≤ 0.005; ✽✽✽, P ≤ 0.001. The experiment was repeated once with similar results.
Memory response.
To determine whether CCR2 was required in the secondary immune or memory response to M. tuberculosis, we infected wild-type and CCR2−/− mice via aerosol (∼50 CFU/mouse) and, 4 months later, we treated the mice with isoniazid and pyrazinamide for 8 weeks, reducing bacillary loads below detection. After the mice were allowed to “rest” for 8 weeks, the mice were challenged via the aerosol route with ∼50 CFU M. tuberculosis. No CFU were detectable in the mice prior to challenge. After challenge, the bacterial loads were determined and cell migration was addressed by flow cytometric analysis. The macrophage defect in CCR2−/− mice was evident throughout the 6 weeks of observation, but again there was no sign of increased susceptibility (Table 2). There was no difference in T-cell migration to the lungs observed in these mice.
TABLE 2.
Cell migration to the lung after challenge of memory mice with aerosol M. tuberculosisa
| Days postchallenge | % CD4 + T cells (SEM)
|
% CD8 + T cells (SEM)
|
% Macrophages (SEM)
|
No. of CFU/lung (105) (SEM)
|
||||
|---|---|---|---|---|---|---|---|---|
| CCR2−/− | CCR2+/+ | CCR2−/− | CCR2+/+ | CCR2−/− | CCR2+/+ | CCR2−/− | CCR2+/+ | |
| 18 | 20.2 (2.6) | 27.6 (7.5) | 18.4 (2.4) | 16.0 (2.5) | ND | ND | 3.1 (0.25) | 5.0 (0.4) |
| 28 | 21.9 (1.5) | 22.5 (1.3) | 21.1 (0.9) | 17.3 (1.2) | 11.2 (0.8)∗∗ | 18.3 (1.14) | 5.8 (1.56) | 5.2 (1.66) |
| 49 | 16.4 (1.7) | 19.0 (1.4) | 18.4 (1.1) | 16.5 (0.9) | 8.2 (1.98)∗ | 16.5 (1.62) | 6.4 (1.27) | 6.8 (1.94) |
C57BL/6 mice and CCR2−/− mice were infected with M. tuberculosis H37Rv (50 CFU) via the aerosol route. At 4 months postinfection, the mice were drug treated for 2 months. The mice were then challenged with aerosol M. tuberculosis H37Rv (50 CFU), and flow cytometric analysis was performed at the times indicated in the table. ∗, P = 0.02; ∗∗, P = 0.001.
DISCUSSION
CCR2−/− mice are clearly deficient in monocyte/macrophage migration to an inflammatory site (3, 8, 15, 16, 21, 22, 31, 32). Here we confirmed the previous findings of impaired migration of cells to the lungs of CCR2−/− mice after M. tuberculosis infection. However, in contrast to CCR2−/− mice infected with a relatively high dose of M. tuberculosis, CCR2−/− mice infected with a low dose of M. tuberculosis controlled the infection, despite a substantially reduced macrophage population in the lungs. Thus, in a low-dose infection, a delayed or deficient immune response does not necessarily lead to higher bacterial numbers in the lungs.
The absence of CCR2 resulted in prolonged macrophage deficiency in the lungs in the low-dose model of infection. There were two- to fivefold fewer macrophages in the CCR2−/− lungs compared to the wild-type lungs. There was also a less pronounced but definite defect in macrophage migration to the lungs after challenge of previously infected and drug-treated (i.e., immune) mice. However, the level of macrophages was apparently sufficient to control the primary or secondary infection, suggesting that the macrophage response in wild-type mice to M. tuberculosis is more robust than necessary to control a low-dose infection. However, even a very robust response is insufficient to clear the infection in mice.
T-cell migration, particularly CD4 T cells, was also deficient in CCR2−/− mice during the early stages of the infection. This may be a direct effect of the absence of CCR2, since this receptor is found on activated T cells and may be important in T-cell trafficking to the lungs from the bloodstream. However, the delay in T-cell migration could also be an indirect effect of CCR2 deficiency. Macrophages produce additional chemokines that signal T-cell migration, and the reduction in macrophages in the lungs may result in altered chemokine levels contributing to reduced T-cell migration. Alternatively, priming of T cells by dendritic cells in the lymph nodes could also be affected by the absence of CCR2, and this would result in a delay in T-cell responses in the lungs. The T-cell migration delay was not observed in the memory response to challenge in the lungs, suggesting that chemokine responses for memory cells may differ from those required for a primary response or that priming of the T-cell response by dendritic cells may be the cause of the delay in the primary response.
A significant delay in IFN-γ gene expression was also observed in the lungs. This may be due to the delayed T-cell migration to the lungs or to reduced stimulation of the T cells that are present in the lungs because of macrophage deficiencies. Regardless of the mechanism responsible, the finding that a substantial delay in IFN-γ production does not necessarily lead to increased bacterial numbers is surprising. IFN-γ is an essential cytokine for control of M. tuberculosis infection, regardless of the dose or route of challenge (7, 10; data not shown). Nonetheless, it appears that IFN-γ levels substantially reduced from wild-type levels are sufficient to control low-dose infection, at least in the early stages of infection. This again implies that the immune response in the lungs is more robust than necessary for bacterial control. The delayed IFN-γ levels may be responsible for the reduced NOS2 gene expression, or this may also result from the deficiency of macrophages, the cells which produce NOS2, in the lungs. In any event, the control of bacterial growth in the lungs of CCR2−/− mice after low-dose infection suggests that the level of NOS2 production is sufficient; however, at higher doses, this level of macrophage activation was apparently not adequate to control bacterial growth.
One striking difference between the low-dose and high-dose infections in CCR2−/− mice was the neutrophilic infiltration to the lungs. The increased neutrophilic infiltration as a result of CCR2 deficiency was previously observed (22); the results in the low-dose-infected mice suggest that the continued infiltration of neutrophils may be due to uncontrolled bacterial replication in the high-dose model rather than a direct result of CCR2 deficiency. It is possible that the presence of large numbers of neutrophils in the lungs is damaging to the tissue and affects the ability of the macrophages to control infection. In the low-dose mice, the neutrophil infiltration is not significantly different at any time point, although there is a trend toward higher numbers of neutrophils in the CCR2−/− lungs. Coincident with the induction of cell-mediated immunity and control of bacterial growth in the lungs (at 3 to 4 weeks postinfection), the numbers of neutrophils decreased.
In summary, these studies confirm that CCR2 is an important chemokine receptor in the trafficking of macrophages to the lungs during M. tuberculosis infection. However, for low-dose infection, the prolonged paucity of macrophages and transient delay in T-cell recruitment and IFN-γ expression did not noticeably impair control of the infection in the lungs. These results imply that the wild-type immune response to M. tuberculosis in the lungs is more vigorous than necessary. Clearly, CCR2 is important for macrophage infiltration into the lungs after M. tuberculosis infection, and there is not a molecule that can replace CCR2 in this function. This striking phenotype was observed regardless of the infection dose, route, or strain of M. tuberculosis. The importance of macrophages to control of M. tuberculosis is well established. The ability of mice to control a low-dose, but not a relatively high-dose M. tuberculosis infection with a substantial deficiency in macrophage numbers in the lungs does not imply that CCR2 is not playing a role in the immune response to this pathogen. Instead, the ability of mice to control a low-dose infection with a minimal inflammatory response in the lungs, with respect to macrophages and T cells early in infection, can be observed in these studies. A higher-dose infection clearly overwhelms this reduced response, and the reduced subsequent IFN-γ and macrophage infiltration is not sufficient to gain control of the infection. The induction of a T-cell response in the lungs in the high-dose mice at ca. 2 to 3 weeks postinfection coincides with very high bacterial numbers and thus cannot contribute to the reduction of the bacterial load. This has been observed in other systems as well, including mice deficient in CD4 T cells (4). However, as with many immunologically deficient mice and tuberculosis, CD4 T-cell-deficient mice cannot control even low-dose aerosol infections (29). In contrast, in the low-dose infection, the minimal innate and inflammatory response that occurs in the absence of CCR2 is sufficient to control the infection until cell-mediated immunity is fully induced.
These studies have provided insight into the use of immunologic knockout mice to study M. tuberculosis. The use of CFU as the primary measure of the contribution of a specific immune component in the response to this infection can be misleading. In studies designed to unravel a complex system, such as cell migration and granuloma formation, the choice of model can be crucial to the outcome and interpretation of the results. The use of models, in vitro or in vivo, that are deficient in one component enables the assessment of a possible role for that component in cell migration or control of infection. However, the presence of a multitude of components can complicate the interpretation of the data, particularly in in vivo studies. An increase in bacterial numbers in a knockout mouse, compared to a wild-type animal, has been a standard for determining whether a particular component is essential in the immune response to M. tuberculosis. The infiltration of cells to the lungs and subsequent granuloma formation is clearly a complex process requiring a network of molecules and receptors. Nonetheless, although our data confirmed that CCR2 is clearly important in migration of macrophages to the lungs after infection, under certain infection conditions this deficiency does not impair control of bacterial growth in the lungs. Our interpretation of these data is that comparison of bacterial numbers is only one measure of the function of certain molecules. The data provided here demonstrate that CCR2 is an important molecule in monocyte/macrophage migration and also affects, perhaps indirectly, T-cell migration and IFN-γ expression. In the low-dose infection model, other immune components are sufficient to control the infection, whereas when a higher dose is administered to the mice, the absence of CCR2 results in lethal infection. Thus, there is a critical dose that can overwhelm the ability of macrophage-deficient lungs to control infection.
Acknowledgments
We are grateful to Amy Myers for excellent technical assistance and to Wendy Peters and Israel Charo for help in establishing our CCR2−/− breeding colony. We thank Joel Ernst for facilitating this collaboration and for providing a custom RPA probe set. We thank John Chan and the members of the Flynn laboratory for helpful discussions.
This work was supported by National Institutes of Health grants AI36990 and AI49157 (J.L.F), American Lung Association grant CI-016-N (J.L.F.), and T32 Molecular Microbial Persistence and Pathogenesis grant AI49820 (H.M.S).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Bean, A. G., D. R. Roach, H. Briscoe, M. P. France, H. Korner, J. D. Sedgwick, and W. J. Britton. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504-3511. [PubMed] [Google Scholar]
- 2.Bodnar, K. A., N. V. Serbina, and J. L. Flynn. 2001. Fate of Mycobacterium tuberculosis within murine dendritic cells. Infect. Immun. 69:800-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boring, L., J. Gosling, S. W. Chensue, S. L. Kunkel, R. V. Farese, H. E. Broxmeyer, and I. F. Charo. 1997. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Investig. 100:2552-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caruso, A. M., N. Serbina, E. Klein, K. Triebold, B. R. Bloom, and J. L. Flynn. 1999. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-γ, yet succumb to tuberculosis. J. Immunol. 162:5407-5416. [PubMed] [Google Scholar]
- 5.Chan, J., Y. Xing, R. S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chensue, S. W., K. S. Warmington, J. H. Ruth, P. S. Sanghi, P. Lincoln, and S. L. Kunkel. 1996. Role of monocyte chemoattractant protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation: relationship to local inflammation, Th cell expression, and IL-12 production. J. Immunol. 157:4602-4608. [PubMed] [Google Scholar]
- 7.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fife, B. T., G. B. Huffnagle, W. A. Kuziel, and W. J. Karpus. 2000. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J. Exp. Med. 192:899-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 10.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Juarrero, M., O. C. Turner, J. Turner, P. Marietta, J. V. Brooks, and I. M. Orme. 2001. Temporal and spatial arrangement of lymphocytes within lung granulomas induced by aerosol infection with Mycobacterium tuberculosis. Infect. Immun. 69:1722-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jouanguy, E., S. Lamhamedi-Cherradi, D. Lammas, S. E. Dorman, M. C. Fondaneche, S. Dupuis, R. Doffinger, F. Altare, J. Girdlestone, J. F. Emile, H. Ducoulombier, D. Edgar, J. Clarke, V. A. Oxelius, M. Brai, V. Novelli, K. Heyne, A. Fischer, S. M. Holland, D. S. Kumararatne, R. D. Schreiber, and J. L. Casanova. 1999. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nat. Genet. 21:370-378. [DOI] [PubMed] [Google Scholar]
- 14.Kurashima, K., N. Mukaida, M. Fujimura, M. Yasui, Y. Nakazumi, T. Matsuda, and K. Matsushima. 1997. Elevated chemokine levels in bronchoalveolar lavage fluid of tuberculosis patients. Am. J. Respir. Crit. Care Med. 155:1474-1477. [DOI] [PubMed] [Google Scholar]
- 15.Kurihara, T., G. Warr, J. Loy, and R. Bravo. 1997. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J. Exp. Med. 186:1757-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuziel, W. A., S. J. Morgan, T. C. Dawson, S. Griffin, O. Smithies, K. Ley, and N. Maeda. 1997. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Natl. Acad. Sci. USA 94:12053-12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin, Y., J. Gong, M. Zhang, W. Xue, and P. F. Barnes. 1998. Production of monocyte chemoattractant protein 1 in tuberculosis patients. Infect. Immun. 66:2319-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu, B., B. J. Rutledge, L. Gu, J. Fiorillo, N. W. Lukacs, S. L. Kunkel, R. North, C. Gerard, and B. J. Rollins. 1998. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med. 187:601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olszewski, M. A., G. B. Huffnagle, T. R. Traynor, R. A. McDonald, D. N. Cook, and G. B. Toews. 2001. Regulatory effects of macrophage inflammatory protein 1α/CCL3 on the development of immunity to Cryptococcus neoformans depend on expression of early inflammatory cytokines. Infect. Immun. 69:6256-6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orme, I. M., and F. M. Collins. 1994. Mouse model of tuberculosis, p. 113-134. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. ASM Press, Washington, D.C.
- 21.Peters, W., M. Dupuis, and I. F. Charo. 2000. A mechanism for the impaired IFN-gamma production in C-C chemokine receptor 2 (CCR2) knockout mice: role of CCR2 in linking the innate and adaptive immune responses. J. Immunol. 165:7072-7077. [DOI] [PubMed] [Google Scholar]
- 22.Peters, W., H. M. Scott, H. F. Chambers, J. L. Flynn, I. F. Charo, and J. D. Ernst. 2001. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 98:7958-7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu, B., K. A. Frait, F. Reich, E. Komuniecki, and S. W. Chensue. 2001. Chemokine expression dynamics in mycobacterial (type-1) and schistosomal (type-2) antigen-elicited pulmonary granuloma formation. Am. J. Pathol. 158:1503-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhoades, E. R., A. M. Cooper, and I. M. Orme. 1995. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect. Immun. 63:3871-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutledge, B. J., H. Rayburn, R. Rosenberg, R. J. North, R. P. Gladue, C. L. Corless, and B. J. Rollins. 1995. High-level monocyte chemoattractant protein-1 expression in transgenic mice increases their susceptibility to intracellular pathogens. J. Immunol. 155:4838-4843. [PubMed] [Google Scholar]
- 26.Sadek, M. I., E. Sada, Z. Toossi, S. K. Schwander, and E. A. Rich. 1998. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am. J. Respir. Cell Mol. Biol. 19:513-521. [DOI] [PubMed] [Google Scholar]
- 27.Sato, N., W. A. Kuziel, P. C. Melby, R. L. Reddick, V. Kostecki, W. Zhao, N. Maeda, S. K. Ahuja, and S. S. Ahuja. 1999. Defects in the generation of IFN-γ are overcome to control infection with Leishmania donovani in CC chemokine receptor (CCR) 5-, macrophage inflammatory protein-1α-, or CCR2-deficient mice. J. Immunol. 163:5519-5525. [PubMed] [Google Scholar]
- 28.Serbina, N. V., and J. L. Flynn. 2001. CD8+ T cells participate in the memory immune response to Mycobacterium tuberculosis. Infect. Immun. 69:4320-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serbina, N. V., V. Lazarevic, and J. L. Flynn. 2001. CD4+ T cells are required for the development of cytotoxic CD8+ T cells during Mycobacterium tuberculosis infection. J. Immunol. 167:6991-7000. [DOI] [PubMed] [Google Scholar]
- 30.Shang, X., B. Qiu, K. A. Frait, J. S. Hu, J. Sonstein, J. L. Curtis, B. Lu, C. Gerard, and S. W. Chensue. 2000. Chemokine receptor 1 knockout abrogates natural killer cell recruitment and impairs type-1 cytokines in lymphoid tissue during pulmonary granuloma formation. Am. J. Pathol. 157:2055-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traynor, T. R., W. A. Kuziel, G. B. Toews, and G. B. Huffnagle. 2000. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J. Immunol. 164:2021-2027. [DOI] [PubMed] [Google Scholar]
- 32.Warmington, K. S., L. Boring, J. H. Ruth, J. Sonstein, C. M. Hogaboam, J. L. Curtis, S. L. Kunkel, I. R. Charo, and S. W. Chensue. 1999. Effect of C-C chemokine receptor 2 (CCR2) knockout on type-2 (schistosomal antigen-elicited) pulmonary granuloma formation: analysis of cellular recruitment and cytokine responses. Am. J. Pathol. 154:1407-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]