Abstract
Tumor necrosis factor alpha (TNF-α) not only induces apoptotic signals but also causes antiapoptotic and regenerative responses in the liver. However, the molecular mechanism(s) of the latter events remains unclear. In the present study, we examined TNF-α-induced genes in Hc human normal (unsensitized) hepatocytes by cDNA microarray analysis. Interleukin-8 (IL-8) induction was the most pronounced of the upregulated genes. The IL-8 protein level was also increased. IL-8 belongs to the ELR-CXC chemokine family and appears to exert mitogenic and antiapoptotic functions in other cell systems. IL-8 expression by TNF-α was inhibited when two survival signals, nuclear factor κB (NF-κB) and phosphatidylinositol 3-kinase (PI3K)/Akt, were inhibited by a mutant form of inhibitor of NF-κB (IκB); by dominant negative (kinase-dead) Akt; or by treatment with LY 294002, an inhibitor of PI3K. TNF-α induced apoptosis in Hc cells that were sensitized by inhibition of NF-κB and PI3K activation. IL-8 administration protected mice against concanavalin A-induced hepatitis in vivo. IL-8 also rescued the sensitized Hc cells, at least in part, from TNF-α-induced apoptosis in vitro. TNF-α inhibited DNA synthesis in unsensitized Hc cells in the absence of serum. Exogenous IL-8 reversed, though anti-IL-8 neutralization antibody enhanced, growth inhibition by TNF-α. These results indicate that IL-8, the production of which is stimulated by TNF-α, inhibits apoptosis of sensitized hepatocytes and releases normal (unsensitized) hepatocytes from growth inhibition induced by TNF-α.
Tumor necrosis factor alpha (TNF-α) is a multifunctional cytokine that plays a role in inflammation, immunity, antiviral responses, and a variety of diseases. In the liver, TNF-α modulates hepatocyte responses, depending on the physiological circumstances. TNF-α is particularly important in the pathophysiology of hepatocytes, inducing viral hepatitis, alcoholic liver disease, and fulminant hepatitis (4). TNF-α activates a variety of components implicated in cellular signal transduction. Binding of TNF-α to the type 1 TNF receptor (TNFR-1) (p55) results in trimerization of its C-terminal cytoplasmic death domain and recruitment of some intracellular proteins involved in apoptotic signal transduction (22, 27, 34). However, hepatocytes are normally resistant to the cytotoxicity of TNF-α. Administration of TNF-α alone does not induce hepatocyte apoptosis in mouse liver in vivo (25, 29, 31) or in cultured rat RALA (40) and human Hc (30) hepatocytes, suggesting that TNF-α also activates molecules that protect cells against apoptosis. For example, TNF-α transmits antiapoptotic signals via nuclear factor κB (NF-κB) and phosphatidylinositol 3-kinase (PI3K)/Akt. Blockage of these signaling pathways results in sensitization to apoptosis induced by TNF-α (30). Pretreatment of mouse hepatocytes with TNF-α prevents subsequent TNFR-mediated apoptosis by a rapid defense mechanism induced by the activation of NF-κB (26). In addition to its capacity as an apoptosis inducer, TNF-α plays a role in hepatocyte proliferation (18, 42). These findings implicate the TNF-α molecule in a function(s) other than apoptosis. However, regarding the signals involved in cell responses other than apoptosis induced by TNF-α, only NF-κB and PI3K/Akt have been investigated. To date, little is known about other signaling molecules. Therefore, we adopted cDNA microarray analysis using Hc hepatocytes to identify genes whose expression levels are regulated by TNF-α. In the genes upregulated by the TNF-α treatment, interleukin-8 (IL-8) showed the highest increase in expression level compared to levels in untreated control cells.
IL-8, a member of the CXC family of chemokines, is a potent chemoattractant and activates neutrophils (3, 8), and it may be implicated in infiltration of neutrophils during acute alcoholic hepatitis (35). IL-8 is an ELR-containing chemokine that contains three amino acid residues, Glu-Leu-Arg (i.e., ELR). ELR-CXC chemokines, including IL-8, also exert mitogenic or antiapoptotic effects (6, 10, 11, 14, 36, 37). IL-8 is produced in response to stimulation by TNF-α (1, 12). However, the relationship between TNF-α and production of IL-8 in hepatocytes remains to be elucidated.
In the present study, we examined genes that were upregulated by TNF-α and found that IL-8 was remarkably produced in TNF-α-treated cells. TNF-α-induced IL-8 production correlated with activation of the NF-κB and PI3K/Akt pathways. Inhibition of these two pathways resulted in a blockage of TNF-α-inducible IL-8 production and also induced apoptosis in Hc cells. The synthesized IL-8 rescues sensitized hepatocytes from apoptosis and releases normal (unsensitized) hepatocytes from growth inhibition in the presence of TNF-α. We propose the hypothesis that the role of TNF-α in sensitized hepatocytes may be different from that in unsensitized hepatocytes.
MATERIALS AND METHODS
Materials.
Hc normal human fetal hepatocytes (Hc cells), which were obtained from six fetal liver tissues by elution following dispase digestion, were purchased from the Applied Cell Biology Research Institute (Kirkland, Wash.). Cell culture medium (CS-C complete) was from Cell Systems (Kirkland, Wash.). Seven-week-old male pathogen-free BALB/c mice were from Japan SLC (Shizuoka, Japan). Recombinant human IL-8 and mouse TNF-α immunoassay kits were from Genzyme techne (Minneapolis, Minn.). LY 294002 was from Alexis (San Diego, Calif.). Hoechst 33258 (bisbenzimide) staining dye was from Wako (Osaka, Japan). Fluorescein isothiocyanate (FITC)-conjugated annexin V was from Bender MedSystems (Vienna, Austria). [α-32P]dCTP was from ICN Biomedicals (Costa Mesa, Calif.). [methyl-3H]thymidine, Cy3 and Cy5 mono-reactive dye pack, anti-rabbit immunoglobulin G horseradish peroxidase-coupled secondary antibody, QuickPrep Micro mRNA purification kit, and the enhanced chemiluminescence Western blot detection system were from Amersham-Pharmacia Biotech (Little Chalfont, Buckinghamshire, United Kingdom). Antibodies against phosphorylated Akt (Ser-473) and Akt were from Cell Signaling Technology (Beverly, Mass.). Monoclonal anti-human IL-8 antibody for both Western blotting and neutralization and concanavalin A (ConA) were from Sigma (St. Louis, Mo.). Isogen was from Nippon Gene (Tokyo, Japan). Myc-tagged constitutively active or kinase-dead Akt plasmid (pUSE-myc-active Akt or pUSE-myc-dominant negative Akt) were obtained from Upstate Biotechnology (Lake Placid, N.Y.). Atlas glass fluorescent labeling kit and Atlas glass human 1.0 microarray were from Clontech Laboratories (Palo Alto, Calif.). Lipofectamine Plus was from Invitrogen (Carlsbad, Calif.). GF/C glass fiber filters were from Whatman (Maidstone, United Kingdom). The Centricon YM-10 apparatus was from Millipore (Bedford, Mass.). All other reagents used were of the highest analytical grade available.
Cell culture and treatment.
Hc cells are not transformed cells, unlike HepG2 and Huh-7 cells, which are often used as human hepatocyte models, and have recently been used in several studies (23, 30, 43). Hc cells were cultured in CS-C complete medium supplemented with antibiotics (penicillin and streptomycin). Cells (3 × 105, 5 × 105, and 3 × 106) were plated on 35-, 60-, and 100-mm-diameter dishes, respectively. After 12 h of incubation in the medium, the cells were washed twice with phosphate-buffered saline (PBS), and the medium was changed to serum-free RPMI 1640. Then, cells were incubated for another 24 h in the presence or absence of recombinant adenoviruses at a multiplicity of infection of 25. Prior to stimulation with TNF-α (20 ng/ml), the cells were washed twice with PBS and, if necessary, incubated for 1 h in serum-free RPMI 1640 containing 25 μM LY 294002. In some experiments, cells were pretreated with IL-8 (1 ng/ml) or anti-IL-8 neutralization antibody (5 μg/ml) immediately before TNF-α stimulation.
Expression of Akt.
To overexpress constitutively active or dominant-negative Akt, expression plasmids pUSE-activated Akt or pUSE-dominant negative Akt were transfected into Hc cells using Lipofectamine Plus according to the manufacturer's instructions. Stable transfectants were selected in growth medium containing 0.5 mg of G418 per ml. The clones expressing the highest amounts of Akt, as assessed by Western blotting, were used in this study.
Array analysis of TNF-α-induced genes.
An Atlas glass array was used according to the manufacturer's instructions to compare mRNA expression patterns between cells stimulated with TNF-α and with PBS. Total RNA was isolated using Isogen according to the manufacturer's instructions, and mRNA was purified with a QuickPrep Micro mRNA purification kit. mRNA was reverse transcribed and labeled with either Cy3 or Cy5 fluorescent probes using an Atlas glass fluorescent labeling kit before array. The cDNA was hybridized on a glass (Atlas glass human 1.0 microarray) containing 1,081 supplied clones, and human expression sequences were tagged according to the established protocols. Expression analyses were performed by GenePix 4000A and GenePix Pro 3.0 softwares (Axon Instruments, Inc. Union City, Calif.).
Northern blot analysis.
To confirm the differential gene expression observed by microarray, Northern blot analysis was performed. Total RNA (20 μg) was subjected to electrophoresis in 1% agarose-formaldehyde gel. RNA was transferred onto nylon membrane and hybridized with 32P-labeled probes. The probe, which specifically detects human IL-8 mRNA, was synthesized by reverse transcription (RT)-PCR using a set of primers (5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ and 5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′) based on the human IL-8 cDNA sequence.
Animal treatment.
The protocol was approved by the animal care committee of Gifu University School of Medicine. Seven-week-old male BALB/c mice were acclimated to our facility for at least 1 week before use. Food and water were available ad libitum until the experiments. ConA (800 μg/mouse) was intravenously administered in mice simultaneously with or without IL-8 (2.5 μg/mouse). After these treatments, animals were kept under fasting conditions but were allowed free access to water. Immediately after each animal was killed by withdrawal of blood from the inferior vena cava with heparin-coated syringes, the liver was removed and washed with PBS.
Assessment of hepatotoxicity.
Hepatocellular damage was monitored biochemically and histologically. Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities were measured using an automatic analyzer (type 726; Hitachi, Tokyo, Japan). The liver was excised and immediately fixed with 10% buffered formalin. The sections were cut at a thickness of 5 μm and stained with hematoxylin and eosin for light microscopic examination.
Measurement of TNF-α concentration in serum.
Levels of TNF-α in mouse serum were determined by a commercially available enzyme-linked immunosorbent assay kit according to the manufacturer's instructions.
Apoptosis assay.
For quantitation of apoptotic cells, cells were stained with Hoechst 33258 and nuclear morphological changes were examined. Briefly, harvested cells were fixed at 4°C for 30 min with 1% glutaraldehyde in PBS, stained with 1 mM Hoechst 33258 for 30 min, and examined under a fluorescent microscope (model BX60; Olympus, Tokyo, Japan) with excitation at 360 nm. In the second method for evaluating the fraction of cells undergoing apoptosis, exposure of phosphatidylserine to the cell surface was measured by FITC-conjugated annexin V. Cells were incubated for 10 min at room temperature with annexin V in binding buffer containing propidium iodide to exclude necrotic cells. FITC fluorescence was measured using a spectrofluorometer with excitation at 485 nm and emission at 535 nm.
Western blot analysis.
Total cellular protein extracts were used for the Western blot analysis of Akt, phospho-Akt, and IL-8. For the preparation of total cell proteins, cells were sonicated in RIPA buffer (50 mM Tris-HCl [pH 8.8], 150 mM NaCl, 10 mM EGTA, 1% Triton X, 0.1% sodium dodecyl sulfate [SDS], 1% deoxycholic acid, 0.3 mM phenylmethylsulfonyl fluoride, [l-3-trans-carboxyoxirane-2-carboryl]-l-leucyl-agmatine [E64] [30 μg/ml]). For measurement of released IL-8, the culture medium was concentrated using a Centricon apparatus. The proteins were separated by SDS-polyacrylamide gel electrophoresis and were electrophoretically transferred onto polyvinylidene difluoride membranes. The membranes were probed with the antibodies against Akt, phospho-Akt, and IL-8 and then incubated with the anti-rabbit immunoglobulin G horseradish peroxidase-coupled secondary antibody. Detection was performed with an enhanced chemiluminescence system.
Electrophoretic mobility shift assay.
Nuclear extracts were prepared as described previously (30). NF-κB-binding consensus single-strand oligonucleotide (5′-TAGTTGAGGGGACTTTCCCAGG-3′) was first annealed with the complement oligonucleotide (5′-TGCCTGGGAAAGTCCCCTCAACTA-3′). The annealed DNA fragment was labeled with [α-32P]dCTP by Klenow DNA polymerase. Nuclear proteins (20 μg) were incubated with 2.5 ng of 32P-labeled double-strand oligonucleotide probe for 30 min at room temperature. The mixture was electrophoresed on 4% polyacrylamide gels with 0.5× Tris-borate-EDTA buffer at 4°C. Gels were dried and exposed to film for autoradiography.
Cultured hepatocyte proliferation.
Cell proliferation was monitored by DNA synthesis as assessed by [3H]thymidine incorporation. Hc cells were plated at a density of 3 × 105 cells on a 35-mm-diameter dish in CS-C complete medium and incubated for 12 h. Cells were incubated for another 24 h in serum-free RPMI 1640 medium and then stimulated with TNF-α (20 ng/ml) in the presence or absence of IL-8 or anti-IL-8 neutralization antibody for 24 h, and [methyl-3H]thymidine (10 μCi/ml) was included for the last 4 h. Cells were then lysed with 0.05% SDS, and DNA was precipitated by trichloroacetic acid. The samples were filtered through the GF/C filter. The radioactivity retained on the filter was counted in a Beckman liquid scintillation counter (model LS-6500). The changes in thymidine incorporation were normalized based on total protein.
RESULTS
Gene expression profiling of TNF-α-modulated genes.
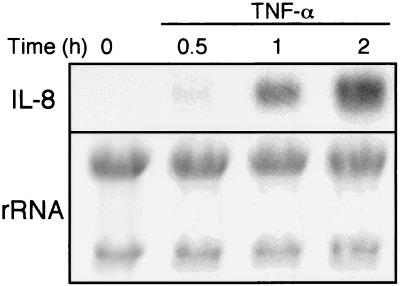
cDNA microarray analysis was performed to search for genes that are upregulated by TNF-α stimulation in Hc hepatocytes. A total of 1,081 genes were screened. The genes whose expression levels increased more than twofold were regarded as positive and are summarized in Table 1. These 19 genes account for ∼2% of the genes profiled. Among the genes induced by TNF-α, some are known for their roles in cell growth or cell death. For example, insulin-like growth factor 2 receptor (IGF2R), erbB2 CCND2, CCNE, EDG5, and hepatocyte growth factor (HGF)-like protein are related to cell growth, and IEX-1L, FLICE-like inhibitory protein (FLIP), TRAF5, c-rel, and BBP/53BP2 are implicated in cell apoptosis. Since IL-8 was found to show the highest induction, we focused on this molecule. To confirm the mRNA expression observed by microarray, Northern blot analysis was performed (Fig. 1). The mRNA expression of IL-8 was upregulated by TNF-α treatment in a time-dependent manner.
TABLE 1.
Gene expression profiling of TNF-α-stimulated Hc cells with microarraya
| Accession no. | Ratio | Description | Abbreviation |
|---|---|---|---|
| Y00787 | 14.10 | IL-8 precursor | IL-8 |
| AF003521 | 10.02 | jagged2 | JAG2 |
| M57730; M37476 | 5.03 | eph-related receptor tyrosine kinase ligand 1 precursor | EPGL1 |
| M90813; D13639 | 4.84 | Cyclin D2 | CCND2 |
| M92357 | 3.20 | B94 protein | |
| X14420 | 3.03 | Procollagen 3 alpha 1 subunit precursor | COL3A1 |
| AF039067; AF071596 | 2.63 | IEX-1L anti-death protein | |
| L20814 | 2.61 | Glutamate receptor 2 precursor | GLUR-2 |
| Y00285; J03528 | 2.41 | IGF2R | IGF2R |
| AF010127; Y14039; Y14040 | 2.40 | FLICE-like inhibitory protein | FLIP |
| M95667; M11730 | 2.34 | erbB2 proto-oncogene | |
| U02081 | 2.14 | Rho guanine nucleotide regulatory protein, alternate splice form | NET1 |
| M73812 | 2.14 | Cyclin E | CCNE |
| U14417 | 2.13 | RalGDSB | RALGEF |
| U69108 | 2.09 | TRAF5 | |
| AF011466 | 2.08 | G protein-coupled receptor EDG4 | |
| X75042 | 2.08 | c-rel proto-oncogene | |
| D49742; S83182 | 2.04 | HGF-like protein | |
| U58334 | 2.03 | Bcl2 and p53 binding protein | BBP/53BP2 |
Hc cells were treated with or without TNF-α for 1 h. mRNA was isolated, reverse-transcribed into fluorescent-labeled cDNA, and hybridized to glass microarray containing 1,081 genes. The data show 19 genes which are increased by two times above in expression ratios (post-TNF/no TNF).
FIG. 1.
Changes in IL-8 mRNA levels in Hc hepatocytes treated with TNF-α. Total RNA was prepared from Hc cells at the indicated time points after exposure to TNF-α (20 ng/ml). Twenty micrograms of RNA was separated by electrophoresis on 1% agarose gel and transferred to nylon membranes. The membranes were hybridized with the specific probe against IL-8 synthesized by RT-PCR. The results shown are representative of at least two independent experiments.
Protective effect of IL-8 against ConA-induced mouse liver injury and TNF-α-induced hepatocyte apoptosis.
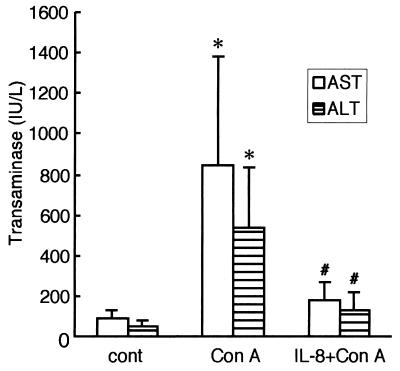
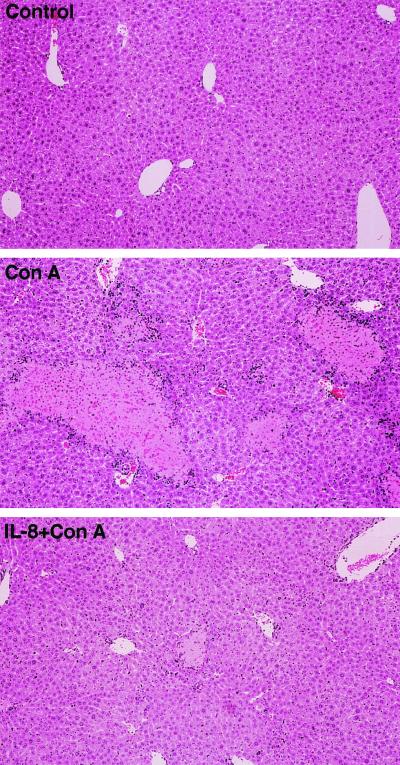
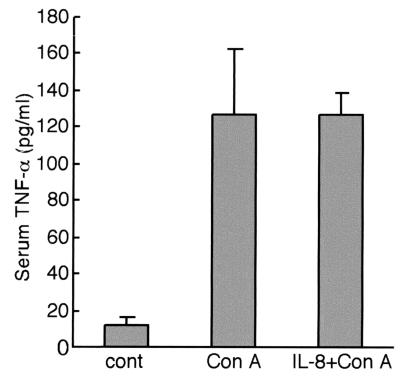
Since TNF-α induced genes are implicated in cell death or cell growth, the effect of IL-8 on TNF-α-mediated liver injury was first examined. ConA appears to cause liver injury through a TNF-α-dependent apoptosis pathway (21, 38). In BALB/c mice, ConA elicited liver damage, and the serum AST and ALT levels increased to ∼10-fold the control values at 48 h (Fig. 2). IL-8 treatment just before ConA administration reduced serum AST and ALT levels by ∼75% (Fig. 2). Histological analysis revealed that ConA injection resulted in pronounced hepatocyte destruction with a multifocal distribution in large areas of the liver (Fig. 3, middle panel). Consistent with the changes in serum transaminase activities, IL-8 treatment inhibited morphological changes in the liver at 48 h after ConA administration (Fig. 3, lower panel). To examine whether IL-8 administration affects TNF-α levels, serum TNF-α levels were measured (Fig. 4). Serum TNF-α levels elevated at 8 h after ConA injection. IL-8 pretreatment did not block elevation of TNF-α by ConA administration. Thus, the protective effect of IL-8 is not due to changing serum TNF-α levels.
FIG. 2.
Effect of IL-8 on ConA-induced liver damage. BALB/c mice were treated with (per mouse) 800 μg of ConA alone or 2.5 μg of IL-8 plus ConA for 48 h. Blood was removed from each mouse for AST and ALT activity determinations. Data are means + standard deviations (error bars) from seven independent experiments. Symbols for statistical significance (Student's t test): *, P < 0.01 compared to control; #, P < 0.01 compared to ConA alone.
FIG. 3.
Histological appearance of livers from mice treated with ConA or ConA plus IL-8. Mice were treated with (per mouse) 800 μg of ConA alone or 2.5 μg of IL-8 plus ConA for 48 h. Liver sections were stained with hematoxylin and eosin for light microscopic examination. The results shown are representative of three independent experiments.
FIG. 4.
Changes in serum TNF-α levels in mice treated with ConA or ConA plus IL-8. Mice were treated with (per mouse) 800 μg of ConA alone or 2.5 μg of IL-8 plus ConA for 8 h. Serum TNF-α levels were determined by enzyme-linked immunosorbent assay. Data are means + standard deviations (error bars) from four independent experiments. cont, control.
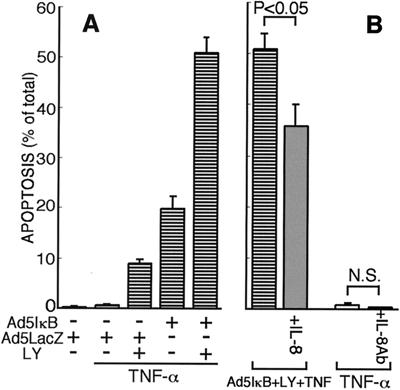
To further assess the role of IL-8 during hepatocyte apoptosis, its effect on TNF-α-mediated apoptosis was examined in Hc cells. It is known that TNF-α alone does not induce apoptosis of normal culture hepatocytes (30, 40). TNF-α (20 ng/ml) induced apoptosis of Hc cells only when two antiapoptotic signals, NF-κB and PI3K, were blocked (Fig. 5A), as previously reported (30). Less than 20% of cells were apoptotic as assessed by nuclear staining with Hoechst 33258 when Hc cells were infected with Ad5IκB (multiplicity of infection, 25) or pretreated with LY 294002 (25 μM) alone, but nearly 50% of cells were apoptotic when pretreated with both Ad5IκB and LY 294002. However, exogenously added IL-8 (1 ng/ml) significantly protected Hc cells against apoptosis induced by TNF-α in the presence of both Ad5IκB and LY 294002 (Fig. 5B). The protective effect, which was not prominent but significant (nearly 40% reduction, P < 0.01, n = 5), of IL-8 on TNF-α-induced apoptosis was confirmed by another method for evaluating apoptotic cells, annexin V staining (data not shown). To examine whether the inhibition of IL-8 production induced apoptosis in Hc cells after TNF-α treatment, anti-IL-8 neutralization antibody (5 μg/ml) was added to TNF-α-treated Hc cells. The antibody did not promote apoptosis in TNF-α-treated Hc cells (Fig. 5B). Therefore, IL-8 production was not necessarily required for survival of Hc hepatocytes after TNF-α treatment.
FIG. 5.
Effect of IL-8 on hepatocyte apoptosis induced by TNF-α. (A) Hc cells infected with Ad5IκB or Ad5LacZ were preincubated with 25 μM LY 294002 for 1 h and then treated with TNF-α (20 ng/ml) for 24 h. (B) Hc cells treated with the indicated agents were incubated for 24 h in the absence or presence of IL-8 (1 ng/ml) or anti-IL-8 neutralization antibody (5 μg/ml). Typical apoptotic cells stained with Hoechst 33258 were counted among more than 1,000 cells, and the percentage of apoptotic cell was determined. Data are means + standard deviations (error bars) from three independent experiments. Data were compared by the Student's t test.
Signal transduction pathways for induction of IL-8 expression.
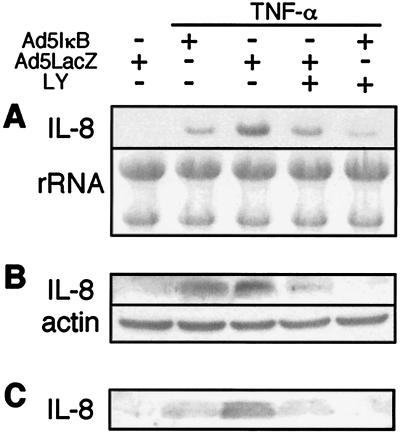
As described above, IL-8 protected hepatocytes against TNF-α-induced apoptosis, and activation of NF-κB and PI3K was necessary for survival of Hc cells after TNF-α treatment. To elucidate the involvement of these signaling molecules in IL-8 induction, the effects of Ad5IκB and LY 294002 on IL-8 expression were examined. Infection of Hc cells with Ad5IκB resulted in an inhibition of TNF-α-induced IL-8 mRNA expression (Fig. 6A). LY 294002, a PI3K inhibitor, also suppressed TNF-α-induced IL-8 mRNA expression. Moreover, treatment of cells with combined Ad5IκB and LY 294002 synergistically inhibited IL-8 mRNA expression. Consistent with Northern blot analysis, the protein level of IL-8 after TNF-α treatment in the cells (Fig. 6B) and in the culture medium (Fig. 6C) was reduced by Ad5IκB or LY 294002 and was reduced synergistically by their combination.
FIG. 6.
Effects of Ad5IκB and LY 294002 on IL-8 induction by TNF-α. Hc cells infected with Ad5IκB or Ad5LacZ were pretreated with or without 25 μM LY 294002 for 1 h prior to treatment with TNF-α (20 ng/ml). (A) Total RNA (20 μg) from Hc cells at 1 h after TNF-α administration was subjected to Northern blot analysis using the specific probe against IL-8 synthesized by RT-PCR. (B) Total cell lysate of Hc cells at 3 h after TNF-α administration was subjected to Western blotting with antibodies against IL-8 and actin. (C) At 5 h after TNF-α administration, the culture medium was harvested, concentrated, and subjected to Western blotting with antibody against IL-8. The results shown are representative of at least two independent experiments.
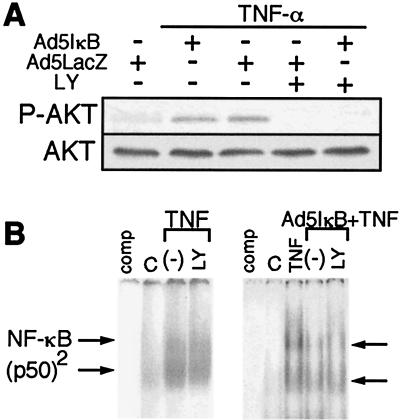
TNF-α treatment elicited phosphorylation of Akt in Hc cells (Fig. 7A). LY 294002 eliminated TNF-α-induced Akt activation, but Ad5IκB infection did not affect Akt activation (Fig. 7A). NF-κB activation induced by TNF-α was not prevented by LY 294002 (Fig. 7B), as previously reported (30). These results indicate the independence of NF-κB and PI3K/Akt signalings and the involvement of these two signals in IL-8 production.
FIG. 7.
Effects of Ad5IκB and LY 294002 on TNF-α-induced activation of NF-κB or Akt. (A) Hc cells infected with Ad5IκB or Ad5LacZ were pretreated with or without 25 μM LY 294002 for 1 h and then incubated with TNF-α (20 ng/ml) for 3 h. Extracted proteins were subjected to SDS-polyacrylamide gel electrophoresis, and immunoblotting was performed using anti-phosphorylated Akt and Akt antibodies. (B) Nuclear extracts were isolated from Ad5IκB-infected (right panel) or -uninfected (left panel) Hc cells which had been treated for 15 min with TNF-α alone or with LY 294002 plus TNF-α. Gel shift assays were performed as described in Materials and Methods. The positions of the p50/p65 NF-κB heterodimer and p50 homodimers are marked as NF-κB and (p50)2, respectively. For comp lanes, a sample of cells treated for 15 min with TNF-α was incubated with a 50-fold excess of unlabeled oligonucleotide. The results shown are representative of at least two independent experiments.
Effect of stably expressed Akt on IL-8 mRNA expression.
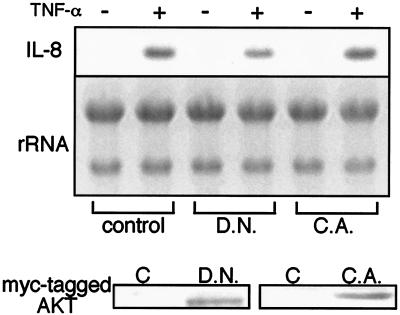
As described above, the PI3K inhibitor LY 294002 exerted a suppressive effect on TNF-α-induced IL-8 mRNA expression. In Hc cells, TNF-α appears to activate serine/threonine kinase Akt via PI3K (30). To further explore the role of the PI3K/Akt pathway in IL-8 expression, Hc cells were stably transfected with plasmids containing kinase-dead or constitutively active Akt. In cells expressing kinase-dead Akt, IL-8 mRNA expression by TNF-α was depressed (Fig. 8). In contrast, TNF-α-induced IL-8 response was slightly enhanced in cells expressing constitutively active Akt. IL-8 mRNA expression was not observed in constitutively active Akt-expressed cells in the absence of TNF-α treatment. These results indicate that the PI3K/Akt pathway is associated with but is insufficient by itself for IL-8 production. An additional signaling pathway, such as NF-κB, is necessary for IL-8 production.
FIG. 8.
Effects of dominant-negative and constitutively active Akt on IL-8 expression in Hc cells. Hc cells were transfected with a stable expression vector for dominant-negative (D.N.) or constitutively active (C.A.) Akt. After selection using G 418, the cells were incubated in serum-free medium and treated with or without TNF-α (20 ng/ml) for 1 h. Total RNA extracted from the cells was separated by electrophoresis on 1% agarose gels and transferred to nylon membranes. The membranes were hybridized with the specific probe against IL-8 synthesized by RT-PCR. The Akt expressed was assessed by Western blotting, as shown in the lower panel. The results shown are representative of at least two independent experiments.
Effect of IL-8 on hepatocyte proliferation.
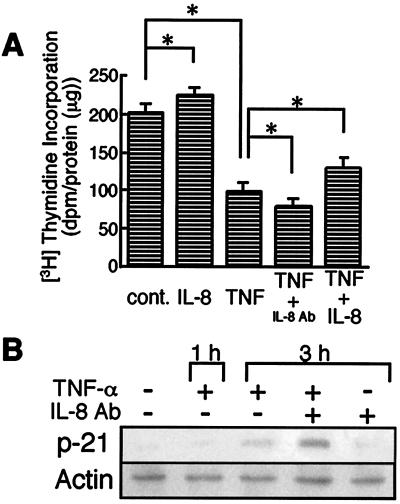
The TNF-α-induced genes implicated in cell growth in the microarray analysis as listed in Table 1. The cell proliferative effect of IL-8 was explored by measuring [3H]thymidine incorporation into DNA (Fig. 9A). Exogenous IL-8 (1 ng/ml) caused a slight increase of [3H]thymidine incorporation compared to that in cells exposed to PBS. On the other hand, TNF-α treatment markedly decreased [3H]thymidine incorporation. Anti-IL-8 neutralization antibody (5 μg/ml) further decreased DNA synthesis of TNF-α-treated cells, by 20%. In contrast, IL-8 administration to TNF-α-treated cells reversed the suppression of cell proliferation by 30%. These results suggested that TNF-α itself inhibited hepatocyte proliferation and that TNF-α-induced IL-8 production reversed the inhibitory effect of TNF-α.
FIG. 9.
Effects of TNF-α and IL-8 on proliferation of Hc cells. (A) Hc cells were treated with IL-8 alone (1 ng/ml) or TNF-α (20 ng/ml) in combination with or without IL-8 or anti-IL-8 neutralization antibody (5 μg/ml). DNA synthesis was measured by [3H]thymidine incorporation into acid insoluble fraction. Data are means + standard deviations (error bars) from at least six independent experiments. *, P < 0.01 using Student's t test. (B) Hc cells were treated with TNF-α (20 ng/ml) for the indicated periods of time in the absence or presence of anti-IL-8 neutralization antibody (Ab) (5 μg/ml). Total cell lysates were subjected to Western blotting with antibodies against p21 and actin. The results shown are representative of at least two independent experiments.
p21 protein is known to induce G1 arrest of the cell cycle in a variety of cells. In hepatocytes, p21 also participates in cell cycle arrest (13, 24). To examine whether the reduction of cell proliferation caused by TNF-α was associated with p21, protein expression levels of p21 were assessed by Western blot analysis (Fig. 9B). Expression of p21 was not observed in cells exposed to PBS. p21 was upregulated by TNF-α treatment in a time-dependent manner. Moreover, anti-IL-8 neutralization antibody exerted an additive effect on p21 expression in TNF-α-treated Hc cells, although it had no effect in untreated control cells.
DISCUSSION
Our present study demonstrates that TNF-α treatment induces IL-8 mRNA expression (more than a 10-fold increase over expression in the control) in hepatocytes. The production of IL-8 was mediated through NF-κB and Akt signaling cascades involved in TNF-α-induced signaling pathways, and this chemokine exerted antiapoptotic and mitogenic effects on hepatocytes.
CXC chemokines are widely recognized as inflammatory cell recruitment factors (37). Recently, the list of functions attributable to CXC chemokines has expanded, and newer biological effects of these mediators include angiogenesis (36) and mitogenesis (10). In particular, ELR-CXC chemokines containing IL-8 participate in the wound healing response (6, 14, 35). Previous studies have shown that increased levels of TNF-α in the damaged liver directly promote the generation of ELR-CXC chemokines (6, 7). ELR-CXC chemokines, including IL-8, protect the liver against damage induced by acetaminophen (14). In the present study, IL-8 was shown to rescue hepatocytes from damage induced by ConA, which is known to cause liver injury through a TNF-α-dependent apoptosis pathway (21, 38). The direct antiapoptotic effect of IL-8 on TNF-α-induced hepatocyte apoptosis was further examined in an in vitro model. TNF-α induces apoptosis of Hc hepatocytes when NF-κB and PI3K/Akt are inhibited (30). Exogenously added IL-8 efficiently suppressed hepatocyte apoptosis in this model. IL-8 appears to inhibit neutrophil apoptosis (11). Moreover, TNF-α-induced suppression of neutrophil apoptosis is mediated through IL-8 production (12). IL-8 also rescues human fetal astrocytes from Fas-mediated apoptosis (33). In contrast, the antisense IL-8 gene enhanced endothelial cell apoptosis (16). These findings and our results suggest that IL-8 directly acts on hepatocytes and promotes a protective effects against apoptosis.
IL-8 production is reported to be controlled by a variety of signals. There are several potential binding sites for nuclear transcription factors, including AP-1, NF-κB, and PEA3 in the IL-8 promoter region (15, 28, 39). In the HepG2 hepatoma cell line, which constitutively expresses IL-8 mRNA, AP-1 and PEA3 are shown to play important roles (15). In contrast, NF-κB has been shown to be essential for expression of IL-8 in several types of cells, and TNF-α has been known as an activator of NF-κB (29, 30). In the present study, IL-8 production by TNF-α was inhibited by either Ad5IκB or LY 294002. Therefore, transcription of IL-8 is located downstream of the NF-κB and PI3K signaling cascades in Hc hepatocytes. Moreover, Akt, a downstream effector of PI3K, is involved in IL-8 production, although its activation alone is not sufficient to induce IL-8 production. The involvement of PI3K/Akt as well as that of other kinases in IL-8 expression has been reported in other cell systems. For example, neurosin, a neuropentide in the gastrointestinal tract, leads to IL-8 secretion via extracellular signal-regulated kinase (ERK)- and NF-κB-dependent pathways in human colonocytes (45). HGF-induced activation of ERK kinase and PI3K was found to contribute to IL-8 expression in a human neck squamous cell cancer cell line (9). ERK and p38 mitogen-activated protein kinase are implicated in Fas-induced IL-8 expression in human glioma cells (5). However, the transcription factor(s) which locates at the downstream of PI3K/Akt is not clearly understood. To clarify the regulatory mechanism for IL-8 production, the transcription factor(s) should be identified and further investigation is under current progress in our laboratory.
The present results also indicated that TNF-α itself inhibited proliferation of normal hepatocyte and that TNF-α-induced IL-8 production reversed the inhibitory effect of TNF-α. p21 protein, a potent inhibitor of cyclin-dependent kinases (13), was induced in response to TNF-α. p21 is known to be associated with various cyclin/cyclin-dependent kinase complexes and inhibits their activations, causing G1 arrest in mammalian cells. The involvement of p21 in TNF-α-induced growth arrest at the G1 phase has been reported in several types of cells: MCF-7 cells (17), human colon cancer WiDr cells (20), and rat oligodendrocytes (44). Our data and these findings suggest that TNF-α induces growth arrest of Hc hepatocytes via p21. However, previous studies (32, 41, 42) have shown that TNF-α is implicated in hepatocyte regeneration. For example, mice lacking TNFR-1 were unable to exert a regenerative response after partial hepatectomy (42). TNF-α was also shown to stimulate mitogenic response of murine hepatocytes in culture (32). These results indicate that TNF-α plays a role in hepatocellular proliferation and liver mass homeostasis. Possible explanations for this discrepancy between our results and the prevailing hypothesis are numerous but unconvincing. For example, a proliferative effect of TNF-α was observed only when the hepatocytes were cultured in the medium containing fetal calf serum (i.e., not in its absence), indicating that TNF-α is not a complete mitogen for hepatocytes but, rather, that it acts by priming hepatocytes for replication (18). Indeed, TNF-α induced upregulation of mRNA levels of IGFR2, erbB2, and EDG4, which are receptors for mitogens in our microarray analysis. The induction of p21 is observed after 70% partial hepatectomy of the rat (2). Primary rat hepatocyte in culture demonstrated increased p21 expression after treatment with HGF (2). Thus, it seems quite reasonable to speculate that the induction of p21 is related with the priming events to stay hepatocytes at the G1 stage, ready to respond the following the challenge of mitogens (e.g., growth factors). TNF-α promotes hepatocyte proliferation, when other growth factor(s) is present. However, in the absence of the second challenge hepatocytes tend to stay at the G1 stage through activation of p21.
TNF-α activates both the NF-κB and PI3K/Akt pathways, and blockage of these signals sensitizes hepatocyte to TNF-α-induced apoptosis (30). As described above, TNF-α induced apoptosis in sensitized hepatocytes and growth inhibition in unsensitized cells. IL-8 protected sensitized hepatocytes against TNF-α-induced apoptosis. Moreover, IL-8 reversed the effects on cell growth arrest and p21 protein expression by TNF-α treatment in unsensitized hepatocytes. Therefore, the released IL-8 may reverse the growth inhibition of unsensitized hepatocytes and rescue sensitized hepatocytes from apoptosis induced by TNF-α. IL-8 is invariably found in the damaged liver (35) and has potent mitogenic effects on hepatocytes (6, 14). An increase in IL-8 secretion occurred after TNF-α treatment in unsensitized Hc cells and was correlated with the ability of the Hc cells to resist TNF-α-mediated apoptosis. However, administration of exogenous IL-8 resulted in a partial rescue of cells pretreated with Ad5IκB and LY 294002 from death induced by TNF-α. Moreover, anti-IL-8 neutralization antibody did not sensitize hepatocytes against TNF-α. Thus, downstream of NF-κB and PI3K/Akt, an additional molecule(s) is implicated in survival signaling pathways in TNF-α-stimulated hepatocytes.
IL-8 has antiapoptotic and mitotic effects on hepatocytes, although the mechanism(s) of these effects is unclear. Klein et al. (19) have reported that IL-8 inhibits polymorphonuclear neutrophil apoptosis by activation of the ERK- and PI3K-dependent pathways. However, IL-8, in the range of 1- to 100-fold concentrations, at which antiapoptotic and mitotic effects were observed (1 to 100 ng/ml), did not activate either ERK or Akt in Hc cells (data not shown). The elucidation of the intracellular signaling by IL-8 will provide a clearer picture of the mechanism of its antiapoptotic effect, and further investigation is under way.
In summary, we have shown here that TNF-α produces IL-8 through the NF-κB and PI3K mediating pathways in hepatocytes. Produced IL-8 rescues sensitized hepatocytes from apoptosis and releases normal (unsensitized) hepatocytes from growth inhibition, both of which are events induced by TNF-α itself.
Acknowledgments
This work was supported in part by a grant-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan (grant 10670462) and by the Research Group of Intractable Liver Diseases sponsored by the Ministry of Health and Welfare of Japan.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abreu-Martin, M. T., A. Vidrich, D. H. Lynch, and S. R. Targan. 1995. Divergent induction of apoptosis and IL-8 secretion in HT-29 cells in response to TNF-α and ligation of Fas antigen. J. Immunol. 155:4147-4154. [PubMed] [Google Scholar]
- 2.Albrecht, J. H., A. H. Meyer, and M. Y. Hu. 1997. Regulation of cyclin-dependent kinase inhibitor p21 (WAF1/Cip1/Sdi1) gene expression in hepatic regeneration. Hepatology 25:557-563. [DOI] [PubMed] [Google Scholar]
- 3.Baggiolini, M., A. Walz, and S. L. Kunkel. 1989. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Investig. 84:1045-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradham, C. A., J. Plumpe, M. P. Manns, D. A. Brenner, and C. Trautwein. 1998. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am. J. Physiol. 275:G387-392. [DOI] [PubMed] [Google Scholar]
- 5.Choi, C., X. Xu, J. W. Oh, S. J. Lee, G. Y. Gillespie, H. Park, H. Jo, and E. N. Benveniste. 2001. Fas-induced expression of chemokines in human glioma cells: involvement of extracellular signal-regulate kinase 1/2 and p38 mitogen-activated protein kinase. Cancer Res. 61:3084-3091. [PubMed] [Google Scholar]
- 6.Colletti, L. M., M. Green, M. D. Burdick, S. L. Kunkel, and R. M. Strieter. 1998. Proliferative effects of CXC chemokines in rat hepatocytes in vitro and in vivo. Shock 10:248-257. [DOI] [PubMed] [Google Scholar]
- 7.Colletti, L. M., S. L. Kunkel, M. Green, M. Burdick, and R. M. Strieter. 1996. Hepatic inflammation following 70% hepatectomy may be related to up-regulation of epithelial neutrophil activating protein-78. Shock 6:397-402. [DOI] [PubMed] [Google Scholar]
- 8.Desbaillets, I., A. C. Diserens, N. Tribolet, M. F. Hamou, and E. G. Van Meir. 1997. Upregulation of interleukin 8 by oxygen-deprived cells in glioblastoma suggests a role in leukocyte activation, chemotaxis, and angiogenesis. J. Exp. Med. 186:1201-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong, G., Z. Chen, Z. Y. Li, N. T. Yeh, C. C. Bancroft, and C. Van Waes. 2001. Hepatocyte growth factor/scatter factor-induced activation of MEK and PI3K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma. Cancer Res. 61:5911-5918. [PubMed] [Google Scholar]
- 10.Driscoll, K. E., D. G. Hassenbein, B. W. Howard, R. J. Isfort, D. Cody, M. H. Tindal, M. Suchanek, and J. M. Carter. 1995. Cloning, expression, and functional characterization of rat MIP-2: a neutrophil chemoattractant and epithelial cell mitogen. J. Leukoc. Biol. 58:359-364. [DOI] [PubMed] [Google Scholar]
- 11.Dunican, A. L., S. J. Leuenroth, A. Ayala, and H. H. Simms. 2000. CXC chemokine suppression of polymorphonuclear leukocytes apoptosis and preservation of function is oxidative stress independent. Shock 13:244-250. [DOI] [PubMed] [Google Scholar]
- 12.Dunican, A. L., S. J. Leuenroth, P. Grutkoski, A. Ayala, and H. H. Simms. 2000. TNF-α-induced suppression of PMN apoptosis is mediated through interleukin-8 production. Shock 14:284-289. [DOI] [PubMed] [Google Scholar]
- 13.El-Deiry, W. S., T. Tokino, V. E. Velculescu, D. B. Levy, R. Parsons, J. M. Trent, D. Lin, W. E. Mercer, K. W. Kinzler, and B. Vogelstein. 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75:817-825. [DOI] [PubMed] [Google Scholar]
- 14.Hogaboam, C. M., C. L. Bone-Larson, M. L. Steinhauser, N. W. Lukacs, L. M. Colletti, K. J. Simpson, R. M. Strieter, and S. L. Kunkel. 1999. Novel CXCR2-dependent liver regenerative qualities of ELR-containing CXC chemokines. FASEB J. 13:1565-1574. [DOI] [PubMed] [Google Scholar]
- 15.Iguchi, A., I. Kitajima, M. Yamakuchi, S. Ueno, T. Aikou, T. Kubo, K. Matsushima, N. Mukaida, and I. Maruyama. 2000. PEA3 and AP-1 are required for constitutive IL-8 gene expression in hepatoma cells. Biochem. Biophys. Res. Commun. 279:166-171. [DOI] [PubMed] [Google Scholar]
- 16.Inoue, K., C. G. Wood, J. W. Slaton, T. Karashima, P. Sweeney, and C. P. Dinney. 2001. Adenoviral-mediated gene therapy of human bladder cancer with antisense interleukin-8. Oncol. Rep. 8:955-964. [DOI] [PubMed] [Google Scholar]
- 17.Jeoung, D. I., B. Tang, and M. Sonenberg. 1995. Effects of tumor necrosis factor-α on antimitogenicity and cell cycle-related proteins in MCF-7 cells. J. Biol. Chem. 270:18367-18373. [DOI] [PubMed] [Google Scholar]
- 18.Kirillova, I., M. Chaisson, and N. Fausto. 1999. Tumor necrosis factor induces DNA replication in hepatic cells through nuclear factor κB activation. Cell Growth Differ. 10:819-828. [PubMed] [Google Scholar]
- 19.Klein, J. B., M. J. Rane, J. A. Scherzer, P. Y. Coxon, R. Kettritz, J. M. Mathiesen, A. Buridi, and K. R. McLeish. 2000. Granulocyte-macrophage colony-stimulating factor delays neutrophil constitutive apoptosis through phosphoinositide 3-kinase and extracellular signal-regulated kinase pathways. J. Immunol. 164:4286-4291. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi, N., Y. Takada, M. Hachiya, K. Ando, N. Nakajima, and M. Akashi. 2000. TNF-α induced p21WAF1 but not Bax in colon cancer cells WiDr with mutated p53: important role of protein stabilization. Cytokine 12:1745-1754. [DOI] [PubMed] [Google Scholar]
- 21.Ksontini, R., D. B. Colagiovanni, M. D. Josephs, C. K. Edwards III, C. L. Tannahill, C. C. Solorzano, J. Norman, W. Denham, M. Clare-Salzler, S. L. Mackay, and L. L. Moldawer. 1998. Disparate roles for TNF-α and Fas ligand in concanavalin A-induced hepatitis. J. Immunol. 160:4082-4089. [PubMed] [Google Scholar]
- 22.Liang, H., and S. W. Fesik. 1997. Three-dimensional structures of proteins involved in programmed cell death. J. Mol. Biol. 274:291-302. [DOI] [PubMed] [Google Scholar]
- 23.Matshusita-Nishiwaki, R., M. Okuno, S. Adachi, T. Sano, K. Akita, H. Moriwaki, S. L. Friedman, and S. Kojima. 2001. Phosphorylation of retinoid X receptor at serine 260 impairs its metabolism and function in human hepatocellular carcinoma. Cancer Res. 61:7675-7682. [PubMed] [Google Scholar]
- 24.Nagaki, M., A. Sugiyama, T. Naiki, Y. Ohsawa, and H. Moriwaki. 2000. Control of cyclin-dependent kinase inhibitors, p21 and p27, and cell cycle progression in rat hepatocytes by extracellular matrix. J. Hepatol. 32:488-496. [DOI] [PubMed] [Google Scholar]
- 25.Nagaki, M., A. Sugiyama, Y. Osawa, T. Naiki, S. Nakashima, Y. Nozawa, and H. Moriwaki. 1999. Lethal hepatic apoptosis mediated by tumor necrosis factor receptor, unlike Fas-mediated apoptosis, requires hepatocyte sensitization in mice. J. Hepatol. 31:997-1005. [DOI] [PubMed] [Google Scholar]
- 26.Nagaki, M., T. Naiki, D. A. Brenner, Y. Osawa, M. Imose, H. Hayashi, Y. Banno, S. Nakashima, and H. Moriwaki. 2000. Tumor necrosis factor-α prevents tumor necrosis factor receptor-mediated mouse hepatocyte apoptosis, but not fas-mediated apoptosis: role of nuclear factor-κB. Hepatology 32:1272-1279. [DOI] [PubMed] [Google Scholar]
- 27.Nagata, S. 1997. Apoptosis by death factor. Cell 88:355-365. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto, S., N. Mukaida, K. Yasumoto, N. Rice, Y. Ishikawa, H. Horiguchi, S. Murakami, and K. Matsushima. 1994. The interleukin-8 AP-1 and κB-like sites are genetic end targets of FK506-sensitive pathway accompanied by calcium mobilization. J. Biol. Chem. 269:8582-8589. [PubMed] [Google Scholar]
- 29.Osawa, Y., M. Nagak, Y. Banno, Y. Yamada, M. Imose, Y. Nozawa, H. Moriwaki, and S. Nakashima. 2001. Possible involvement of reactive oxygen species in D-galactosamine-induced sensitization against tumor necrosis factor-α-induced hepatocyte apoptosis. J. Cell. Physiol. 187:374-385. [DOI] [PubMed] [Google Scholar]
- 30.Osawa, Y., Y. Banno, M. Nagaki, D. A. Brenner, T. Naiki, Y. Nozawa, S. Nakashima, and H. Moriwaki. 2001. TNF-α-induced sphingosine 1-phosphate inhibits apoptosis through a phosphatidylinositol 3-kinase/Akt pathway in human hepatocytes. J. Immunol. 167:173-180. [DOI] [PubMed] [Google Scholar]
- 31.Osawa, Y., Y. Banno, M. Nagaki, Y. Nozawa, H. Moriwaki, and S. Nakashima. 2001. Caspase activation during hepatocyte apoptosis induced by tumor necrosis factor-α in galactosamine-sensitized mice. Liver 21:309-319. [DOI] [PubMed] [Google Scholar]
- 32.Rolfe, M., N. H. James, and R. A. Roberts. 1997. Tumour necrosis factor-α (TNF-α) suppresses apoptosis and induces DNA synthesis in rodent hepatocytes: a mediator of the hepatocarcinogenicity of peroxisome proliferators? Carcinogenesis 18:2277-2280. [DOI] [PubMed] [Google Scholar]
- 33.Saas, P., J. Boucraut, A. L. Quiquerez, V. Schnuriger, G. Perrin, S. Desplat-Jego, D. Bernard, P. R. Walker, and P. Y. Dietrich. 1999. CD95 (Fas/Apo-1) as a receptor governing astrocyte apoptotic or inflammatory responses: a key role in brain inflammation? J. Immunol. 162:2326-2333. [PubMed] [Google Scholar]
- 34.Schulze-Osthoff, K., P. H. Krammer, and W. Droge. 1994. Divergent signalling via APO-1/Fas and the TNF receptor, two homologous molecules involved in physiological cell death. EMBO J. 13:4587-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheron, N., G. Bird, J. Koskinas, B. Portmann, M. Ceska, I. Lindley, and R. Williams. 1993. Circulating and tissue levels of the neutrophil chemotaxin interleukin-8 are elevated in severe acute alcoholic hepatitis, and tissue levels correlate with neutrophil infiltration. Hepatology 18:41-46. [PubMed] [Google Scholar]
- 36.Strieter, R. M., P. J. Polverini, S. L. Kunkel, D. A. Arenberg, M. D. Burdick, J. Kasper, J. Dzuiba, J. V. Damme, A. Walz, D. Marriott, S. Y. Chan, S. Roczniak, and A. B. Shanafelt. 1995. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J. Biol. Chem. 270:27348-27357. [DOI] [PubMed] [Google Scholar]
- 37.Strieter, R. M., T. J. Standiford, G. B. Huffnagle, L. M. Colletti, N. W. Lukacs, and S. L. Kunkel. 1996. “The good, the bad, and the ugly.” The role of chemokines in models of human disease. J. Immunol. 156:3583-3586. [PubMed] [Google Scholar]
- 38.Trautwein, C., T. Rakemann, D. A. Brenner, K. Streetz, L. Licato, M. P. Manns, and G. Tiegs. 1998. Concanavalin A-induced liver cell damage: activation of intracellular pathways triggered tumor necrosis factor in mice. Gastroenterology 114:1035-1045. [DOI] [PubMed] [Google Scholar]
- 39.Wu, G. D., E. J. Lai, N. Huang, and X. Wen. 1997. Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin-8 promoter. The role of Oct-1 as a transcriptional repressor. J. Biol. Chem. 272:2396-2403. [PubMed] [Google Scholar]
- 40.Xu, Y., S. Bialik, B. E. Jones, Y. Iimuro, R. N. Kitsis, A. Srinivasan, D. A. Brenner, and M. J. Czaja. 1998. NF-κB inactivation converts a hepatocyte cell line TNF-α response from proliferation to apoptosis. Am. J. Physiol. 275:C1058-C1066. [DOI] [PubMed] [Google Scholar]
- 41.Yamada, Y., E. M. Webber, I. Kirillova, J. J. Peschon, and N. Fausto. 1998. Analysis of liver regeneration in mice lacking type 1 or type 2 tumor necrosis factor receptor; requirement for type 1 but not type 2 receptor. Hepatology 28:959-970. [DOI] [PubMed] [Google Scholar]
- 42.Yamada, Y., I. Kirillova, J. J. Peschon, and N. Fausto. 1997. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc. Natl. Acad. Sci. USA 94:1441-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamashita, Y., M. Shimada, E. Tsujita, S. Tanaka, H. Ikima, K. Nakazawa. R. Sakiyama, J. Fukuda, T. Ueda, K. Funatsu, and K. Sugimachi. 2001. Polyurethane foam/spheroid culture system using human hepatoblastoma cell line (Hep G2) as a possible new hybrid artificial liver. Cell Transplant. 10:717-722. [PubMed] [Google Scholar]
- 44.Yu, C., M. Takeda, and B. Soliven. 2000. Regulation of cell cycle proteins by TNF-α and TGF-β in cells of oligodendroglial lineage. J. Neuroimmunol. 108:2-10. [DOI] [PubMed] [Google Scholar]
- 45.Zhao, D., A. C. Keates, S. Kuhnt-Moore, M. P. Moyer, C. P. Kelly, and C. Pothoulakis. 2001. Signal transduction pathways mediating neurotensin-stimulated Interleukin-8 expression in human colonocytes. J. Biol. Chem. 276:44464-44471. [DOI] [PubMed] [Google Scholar]