Abstract
CymA, the outer membrane component of the cyclodextrin (CD) uptake and metabolism system of Klebsiella oxytoca, was reconstituted into lipid bilayer membranes. The channel properties of this unusual porin were studied in detail. The binding of CDs to the channel resulted in its complete block for ion transport. This result allowed the detailed investigation of carbohydrate binding, and the stability constants for the binding of cyclic and linear carbohydrates to the binding site inside the channel were calculated from titration experiments of the membrane conductance with the carbohydrates. Highest stability constant was observed for α-cyclodextrin (α-CD; K = 32,000 1/M) followed by β-cyclodextrin (β-CD; K = 1970 1/M) and γ-cyclodextrin (γ-CD; K = 310 1/M). Linear maltooligosaccharides bound also to CymA but with much smaller stability constants as compared to cyclic ones. The noise of the current through CymA in multi- and single-channel experiments was investigated using fast Fourier transformation. The current through the open channels had a rather high spectral density, which was a Lorentzian function of the frequency up to 2000 Hz. Upon addition of cyclic dextrins to the aqueous phase the spectral density decreased in a dose-dependent manner, which made it impossible to evaluate the binding kinetics. Experiments with single CymA-channels demonstrated the channel is highly asymmetric concerning channel flickers and current noise.
INTRODUCTION
Ten different gene products are involved in cyclodextrin (CD) metabolism by Klebsiella oxytoca (Fiedler et al., 1996), which allow the growth of this organism on different circularly shaped oligosaccharides made up of six (α-CD), seven (β-CD), or eight (γ-CD) glucose units. The different genes are designated cymA to cymJ. Four of them are constituents of a periplasmic binding protein-dependent uptake system (Fiedler et al., 1996), from which CymE is the binding protein (Pajatsch et al., 1998), CymF and CymG are the integral inner membrane components, and CymD is the ATP-splitting enzyme. There is convincing evidence that α- and β-CDs are taken up as intact entities (Fiedler et al., 1996; Pajatsch et al., 1998) and that they are linearized in the cytoplasm by the product of the cymH gene which is a cyclodextrinase (Feederle et al., 1996). CymE, CymF, CymG, and CymD are homologs of the components of the paradigmatic maltose uptake system, MalE, MalF, MalG, and MalK, respectively (Fiedler et al., 1996; Schwartz, 1987). The metabolism of γ-CDs is dependent on the activity of the cyclodextrin-glucanotransferase (Fiedler et al., 1996), so γ-CDs have to be converted into α- or β-CDs or linearized (Bender, 1980; 1982) to be taken up.
Important for the transport of CDs across the cell wall is CymA, an outer membrane protein, which has a molecular mass of 39 kDa and is presumably active as a monomer in contrast to the trimeric general diffusion pores and specific porins of Escherichia coli outer membrane (Benz, 2000; Pajatsch et al., 1999). The primary sequence of CymA possesses a high content of antiparallel β-sheet, typical for outer membrane porins (Benz, 1994; Pajatsch et al., 1999). Incorporation of purified CymA into lipid bilayers and conductance measurements revealed that it forms ion-permeable channels, which exhibit a substantial current noise and channel flicker in contrast to the stepwise increase of most outer membrane porins (Pajatsch et al., 1999; Benz, 1994). α-cyclodextrin binds to CymA and the binding leads to a block of the channel for the permeation of ions with a half-saturation constant of ∼28 μM (Pajatsch et al., 1999), indicating that the carbohydrate-specific binding site is located in the interior of the channel. LamB, the carbohydrate-specific outer membrane channel of enteric bacteria, is not able to transport the cyclic dextrins, but CymA is able to replace LamB for maltooligosaccharide transport across the outer membrane of E. coli (Pajatsch et al., 1999).
In this article we studied the binding of different carbohydrates to CymA in detail. We could demonstrate that not only the bulky α-CD, β-CD, and γ-CD, i.e., diameters of 1.37 (α-CD), 1.53 nm (β-CD), and 1.69 nm (γ-CD), bind to CymA but also linear maltooligosaccharides. Furthermore, the noise of the current through CymA, was studied. These experiments did not allow measurement of binding kinetics but allowed interesting insight into the properties of the CymA-channel. The analysis was performed using a similar treatment proposed previously for the kinetics of nerve channels (Conti and Wanke, 1975; Conti et al., 1980), of gramicidin (Kolb et al., 1975), and of the analysis of ameloride-induced block of frog epithelial sodium channels (Lindemann and Van Driessche, 1977; Van Driessche and Lindemann, 1979). Single-channel studies of CymA demonstrated that this channel is highly asymmetric.
MATERIALS AND METHODS
Growth of bacteria and purification of CymA
CymA was isolated and purified essentially as has been described previously (Pajatsch et al., 1999). In brief: CymA was overproduced using E. coli K-12 strain JM109, which was transformed with plasmid pCYMA (Yanisch-Perron et al., 1985; Pajatsch et al., 1999). Cells were grown aerobically at 37°C overnight to an A600 of 1.2–1.7 in Luria-Bertani medium and harvested by centrifugation. Cells were suspended in 50 mM potassium phosphate pH 7.5 containing 1 mM phenylmethylsulfonylfluoride and broken by three passages through a French pressure cell at 16,000 psi. Unbroken cells were removed by centrifugation at 6000 × g for 45 min. The cell envelopes were pelleted by centrifugation at 100,000 × g for 90 min. The outer membrane was obtained by solubilization of the cytoplasmic membrane components by repetitive treatment with 0.5% sarcosyl (Sigma Chemicals, Deisenhofen, Germany), followed by centrifugation. To facilitate the solubilization of the outer membrane components, the pellet was resuspended in 50 mM potassium phosphate pH 7.5 containing 100 μg/ml lysozyme and 3 mM NaN3, and stirred overnight at 37°C followed by centrifugation. The outer membrane components were solubilized by resuspending the pellet for 1 h at room temperature in 50 mM potassium phosphate pH 7.5, 5 mM EDTA, and 5% of octyl-polyoxyethylene (OPOE; Bachem Biochemica, Heidelberg, Germany). The supernatant of the subsequent centrifugation step was dialyzed against 10 mM potassium phosphate pH 7.5, 0.6% OPOE, and was subjected to different chromatographic steps. Final purification of CymA was achieved by ion-exchange chromatography across a Mono Q-HR 5/5 column. The fractions containing highly enriched CymA were pooled, concentrated by ultrafiltration to a concentration of 1 mg/ml, supplied with 3 mM NaN3, and stored at 4°C or at −20°C (Pajatsch et al., 1999).
Lipid bilayer experiments
Multichannel experiments were formed as described previously (Benz et al., 1978). The instrumentation consisted of a Teflon chamber with two aqueous compartments connected by a small circular hole. The hole had a surface area of 0.5 mm2. Membranes were formed by painting onto the hole a 1% solution of diphytanoyl phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL) in n-decane. The aqueous salt solutions (Merck, Darmstadt, Germany) were used in most experiments unbuffered and had a pH of ∼6. The aqueous phase was buffered with 10 mM MES and 10 mM Tris for the measurements at pH 5 and pH 8, respectively. The cyclic dextrins were obtained from Sigma-Aldrich (Steinheim, Germany). CymA was reconstituted into the lipid bilayer membranes by adding concentrated stock solutions to the aqueous phase one side (the cis-side) of a membrane in the black state. The temperature was kept at 20°C for most of the experiments reported here. Some other experiments were performed within the temperature range between 4°C and 40°C.
Some single-channel experiments were performed using folded membranes made from diphytanoyl-phosphatidylcholine dissolved in hexane/chloroform 9:1 and formed across a small hole (diameter, 60-μm) in a thin Teflon foil (Goddfellow, Cambridge, UK). The Teflon cell with a 60-μm diameter aperture in the 25-μm-thick Teflon partition and Ag/AgCl electrodes with agarose bridges have previously been described (Bezrukov and Vodyanoy, 1993). These membranes have a much smaller surface, resulting in a much smaller background noise. The folded membranes allow the detection and the study of small events through single CymA-channels and the study of its asymmetric properties.
For the multichannel experiments the membrane current was measured with a pair of Ag/AgCl electrodes with salt bridges switched in series with a battery-operated voltage source and a homemade current-to-voltage converter made using a Burr Brown operational amplifier with a three-pole filter (Texas Instruments, Dallas, TX). The feedback resistors of the current amplifier were between 0.01 and 10 GΩ. The amplified signal was monitored with an oscilloscope and recorded with a strip chart recorder to measure the absolute magnitude of the membrane current and to calculate the stability constant for carbohydrate binding (Benz et al., 1987).
Noise analysis
For the noise measurements the amplified output signal of the current-to-voltage converter was passed through a Krohn-Hite filter (model 113340, Nucletron Electronic, Munich, Germany). The amplified AC-component of the signal was fed into an A/D converter and stored in a PC. The PC performed fast Fourier transformation of the current noise using different time-domain windows as has been described previously (Nekolla et al., 1994; Andersen et al., 1998). The spectra were composed of either 200 or 400 points and they were averaged either 128 or 256 times. The further analysis of the power density spectra was also performed with the same PC using the program EasyPlot. The noise of the single channels was measured using an Axopatch 200B current amplifier (Axon Instruments, Foster City, CA). Its output signal was fed into a Lecroy digital oscilloscope (LT342, Lecroy, Geneva, Switzerland) that also performed fast Fourier transformation. The current noise density spectra were transferred into a PC and analyzed using the programs EasyPlot and Analysis.
Theory
In recent publications the properties of substrate-gated or ligand-gated channels has been studied in detail (Benz et al., 1987; Nekolla et al., 1994; Jordy et al., 1996; Bachmeyer et al., 2001). It has been demonstrated that the minimum requirements for the binding of substrates, such as carbohydrates to the channel, can be described by on- and off-rate constants from both sides of the channel similar to carbohydrate transport through a simple one-site, two-barrier channel, such as LamB (Läuger, 1973; Benz et al., 1987; Benz and Hancock, 1987) or CymA (Pajatsch et al., 1999). This model assumes in principle a binding site for the substrate in the center of the channel with symmetrical barriers for the on-rate constants of substrate binding. The second-order rate constant k1 describes the jump of the substrate from the aqueous phase (concentration c) to the binding site, whereas the inverse movement is described by the rate constant k−1.
The stability constant of the binding between a substrate and the binding site inside the channel is K = k1/k−1. Furthermore, we assume that only one substrate molecule can bind to the binding site at a given time (Benz et al., 1987). This means that a substrate molecule can bind to the channel only when the binding site is free. The substrate-gated channel (given by P) is open when no substrate L is bound, and closed when it is occupied to form the non- or low-conducting substrate-channel complex PL:
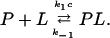 |
(1) |
The probability, p, that the binding site is occupied by a substrate molecule, and that the channel does not conduct ions, or is in the low-conductance state, is given by
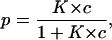 |
(2) |
and that it is free, and the channel is in its high-conductance state, is given by
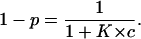 |
(3) |
The conductance, G(c) = Im/Vm, of a CymA-containing membrane in the presence of a substrate with the stability constant, K, and a ligand concentration, c, is given by the probability that the binding site is free,
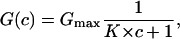 |
(4) |
where Gmax is the membrane conductance before the start of the addition of the ligand to the aqueous phase. Eq. 4 may also be written as:
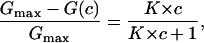 |
(5) |
which means that the titration curves can be analyzed using Lineweaver-Burke plots as has been shown in previous publications (Benz et al., 1987; Andersen et al., 1995). The half-saturation constant, KS, is given by the inverse stability constant.
RESULTS
Evaluation of the stability constant of substrate-binding to CymA
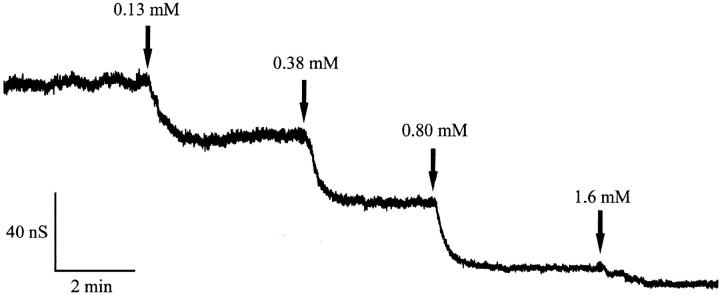
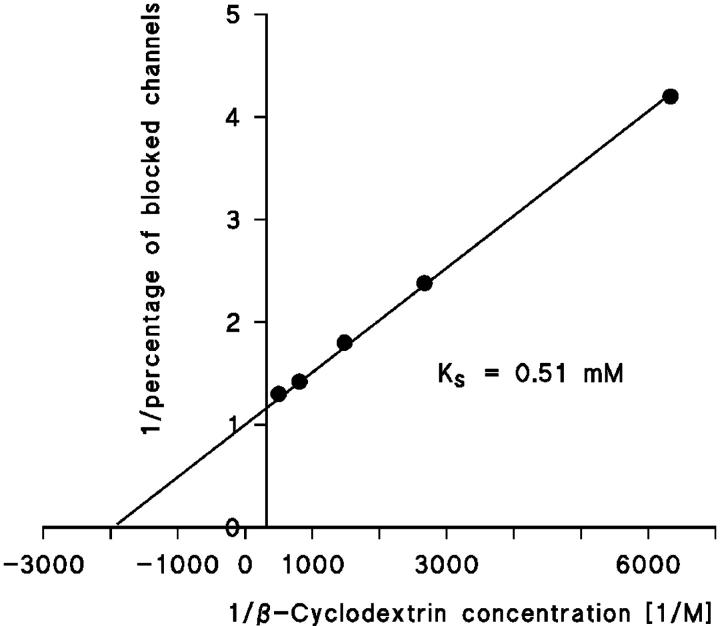
In a previous investigation we have demonstrated that the CymA-mediated ion current through lipid bilayer membranes can be blocked by the addition of α-cyclodextrin because of a binding site inside the channel, which leads to block of ion flux when it is occupied (Pajatsch et al., 1999). Here we report experiments with other cyclodextrins and linear maltooligosaccharides that are structurally related to β-cyclodextrin (see Fig. 1). An example for this type of measurement is shown in Fig. 2. After incorporation of 250 CymA-channels in a lipid bilayer membrane, increasing concentrations of β-cyclodextrin were added to the aqueous phase while stirring to allow equilibration. The membrane current decreased in a dose-dependent manner. The titration curve given in Fig. 2 could be analyzed using a Lineweaver-Burke plot (compare Eq. 5), from which a stability constant, K, of ∼2,000 1/M (half-saturation constant KS = 500 μM) was calculated for the binding of β-cyclodextrin to the CymA-channels in the experiment of Fig. 2 (see Fig. 3). The stability constant K is given by the ratio of the on-rate constant k1 divided by the off-rate constant k−1 for ligand binding. Similar analyses were also performed in this study with α-CD, β-CD, and also the linear maltooligosaccharides maltotriose, maltopentaose, and maltoheptaose. The linear maltooligosaccharides had a smaller affinity to CymA, which means that the stability constant was much smaller as compared to CD binding. Interestingly, it increased slightly with increasing number of glucose residues, whereas it decreased for increasing numbers within the CD. The addition of carbohydrates resulted in an almost complete block of the CymA channels. The results are included in Table 1 together with the stability constants that have been derived previously for the binding of the maltooligosaccharides to the LamB-channel (Benz et al., 1987) that results also in its complete block for ion translocation.
FIGURE 1.
Structure of the cyclic dextrins used in this study.
FIGURE 2.
Titration of CymA-induced membrane conductance with β-cyclodextrin. The membrane was formed from diphytanoyl phosphatidylcholine/n-decane. The aqueous phase contained 20 ng/ml CymA, 1 M KCl, and β-cyclodextrin at the concentrations shown at the top of the figure. The temperature was 20°C and the applied voltage was 20 mV. Note that the current noise decreased for increasing concentration of β-cyclodextrin.
FIGURE 3.
Lineweaver-Burke plot of the inhibition of CymA-induced membrane conductance by β-cyclodextrin. The data were taken from Fig. 2 and were analyzed using Eq. 5. The straight line corresponds to a stability constant, K, for β-cyclodextrin binding of 1960 1/M. For further explanations, see text.
TABLE 1.
Stability constants of CD binding to CymA as derived from titration experiments similar to that shown in Fig. 2, using Lineweaver-Burke plots (Fig. 3)
| Sugar | Maltoporin E. coli | CymA K. oxytoca pH 6 | Saturation | pH 5 | pH 8 | T = 6°C |
|---|---|---|---|---|---|---|
| α-cyclodextrin | N.D. | 32,000 | 82% | 16,970 | 28,610 | 84,750 |
| β-cyclodextrin | N.D. | 1970 | 82% | N.D. | N.D. | N.D. |
| γ-cyclodextrin | N.D. | 310 | 94% | N.D. | N.D. | N.D. |
| Maltotriose | 2800 | 20 | 85% | N.D. | N.D. | N.D. |
| Maltopentaose | 14,000 | 75 | 85% | N.D. | N.D. | N.D. |
| Maltoheptaose | 17,000 | 91 | 88% | N.D. | N.D. | N.D. |
The membranes were formed of diphytanoyl phosphatidylcholine dissolved in n-decane. The aqueous solutions were unbuffered and had a pH of 6 unless otherwise indicated. The applied voltage was 20 mV and the temperature was 20°C unless otherwise indicated. The data represent the means of at least three individual titration experiments. The standard deviation was typically <10% of the mean value.
To check whether the single CymA-channels follow the same block as in multichannel experiments we performed single-channel conductance experiments with CymA in the presence of concentrations of α-cyclodextrin increasing from 0.03 to 0.3 mM. The results (summarized in Table 2) indicate that the pure CymA protein does not contain a channel-forming impurity because the conductance of CymA was found to be dependent on the presence of α-cyclodextrin. Analysis of the single-channel conductance as a function of the α-cyclodextrin concentration using Eq. 5 resulted in a similar half-saturation constant as derived above from the titration experiments (KS = 25 μM; K = 39,930 1/M). This result indicates that α-cyclodextrin completely blocks the CymA-channel. Similarly, a complete block was observed for β-CD, γ-CD, and the linear maltooligosaccharides maltotriose, maltopentaose, and maltoheptaose despite their partially smaller size and different structure as compared to the cyclodextrins (see Fig. 1).
TABLE 2.
Single channel conductance of CymA channels in presence of α-cyclodextrin
| α-CD [mM] | Single-channel-conductance [pS] |
|---|---|
| 0 | 550 |
| 0.03 | 250 |
| 0.1 | 150 |
| 0.3 | 100 |
The membranes were formed of diphytanoyl phosphatidylcholine dissolved in n-decane. The aqueous solutions that contained 1M KCl were unbuffered and had a pH of 6. The applied voltage was 20 mV and the temperature was 20°C. The average single-channel conductance was calculated from at least 100 single events.
The influence of different parameters on α-CD binding to CymA was studied in further experiments. The pH of the aqueous phase had a relatively small influence on the stability constant when the pH was increased to pH 8 (see Table 1). The effect of decreasing pH on K was more substantial and it decreased from 32,000 1/M to 17,000 1/M, when the pH was lowered to pH 5. Similarly, the temperature also had a strong influence on α-cyclodextrin binding to CymA. When the temperature was decreased to 6°C the stability constant increased by a factor of ∼3 compared to the situation at 20°C. This result indicated that decreasing the temperature increased the stability of the CymA-α-cyclodextrin complexes (Table 1).
Analysis of the current noise through the CymA-channel
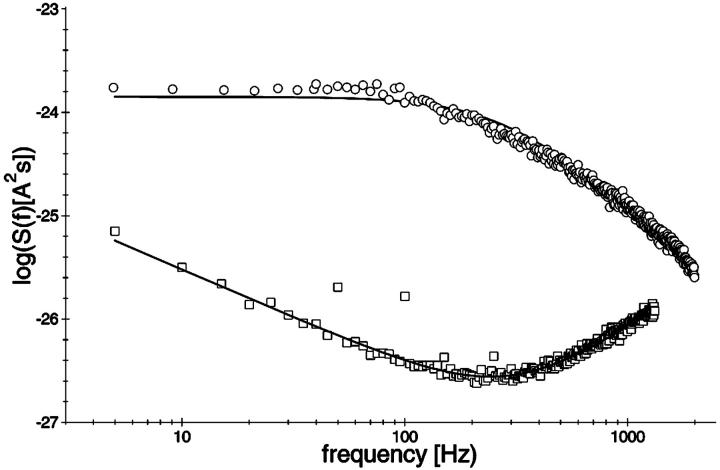
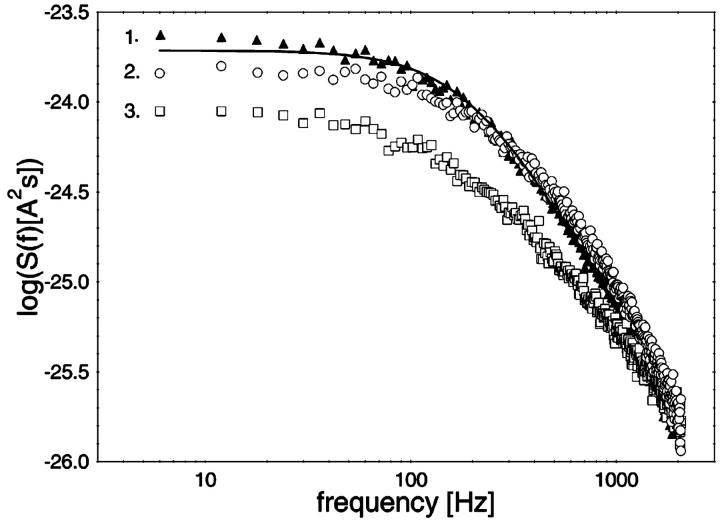
In previous publications we investigated the carbohydrate-induced current noise of porin channels of the LamB family (Nekolla et al., 1994; Andersen et al., 1995, 1998; Jordy et al., 1996). The open LamB- or ScrY-channels exhibit 1/f noise that is probably related to the structure of the porin trimers and may be caused by short flickers of the current through the channels (Nekolla et al., 1994; Wohnsland and Benz, 1997; Bezrukov and Winterhalter, 2000). The addition of carbohydrates leads to a complete change of the current noise, and its spectral density, S(f), as a function of the frequency f is given by a so-called Lorentzian function,
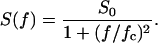 |
(6) |
S0 is the plateau value of the spectral density at small frequency and the corner frequency fc is dependent on the time constant, τ, of the chemical reaction given by the expression
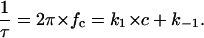 |
(7) |
In this study a similar analysis was performed with CymA to measure the kinetics of cyclodextrin binding. Surprisingly, the open CymA channel did not exhibit 1/f noise similar to other porins including the carbohydrate-specific LamB-channel (see the open squares in Fig. 4). Instead, the open CymA-channel showed Lorentzian type of noise as the open circles of Fig. 4 clearly demonstrate. This is probably caused by the rapid fluctuations of the current through the open channel; i.e., in contrast to most outer membrane porins, the conductance increase does not increase in stepwise fashion, which has already been observed in a previous study (Pajatsch et al., 1999). The power density of the spectrum of the membrane containing the CymA-channels was much higher than that of the open LamB-channel despite the fact that the membrane in the experiment with LamB contained more channels than that with CymA. The power density spectrum of the current noise through the CymA-channels in Fig. 4 could be fitted to Eq. 6. The corner frequency, fc, of the Lorentzian was 160 1/s, which corresponds to a time constant, τ, of the underlying chemical reaction of ∼0.99 ms. This result indicates that CymA does not represent a normal porin channel as has been demonstrated for open porins, because it does not show 1/f noise (Wohnsland and Benz, 1997). It seems, moreover, that CymA undergoes molecular fluctuations that modulate the current and are not random, but are instead controlled by a chemical reaction similar to a switch with uneven open and closed probabilities (Conti and Wanke, 1975). In such a case, the equation for the time constant, τ, has the form
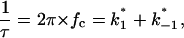 |
(8) |
where 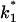 and
and 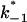 are the on- and off-rate constants of the first-order reaction that modulates the current through the open CymA-channel.
are the on- and off-rate constants of the first-order reaction that modulates the current through the open CymA-channel.
FIGURE 4.
Power density spectra of a membrane containing ∼450 open LamB-channels (open squares; lower trace) and another membrane containing ∼200 open CymA-channels (open circles; upper trace). The membranes were formed from diphytanoyl phosphatidylcholine/n-decane. Note that the open LamB-channels showed 1/f-noise, whereas the power density spectrum of the open CymA-channels could be explained by a Lorentzian function using the following parameters: τ = 0.99 ms; S0 = 1.33 × 10−24 A2s; T = 20°C; and Vm = 20 mV.
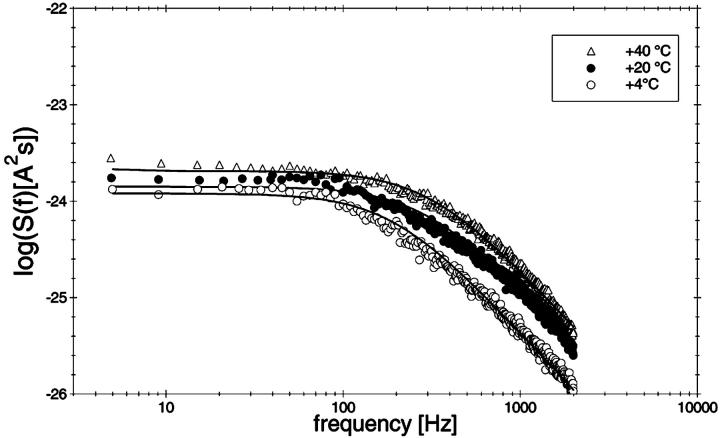
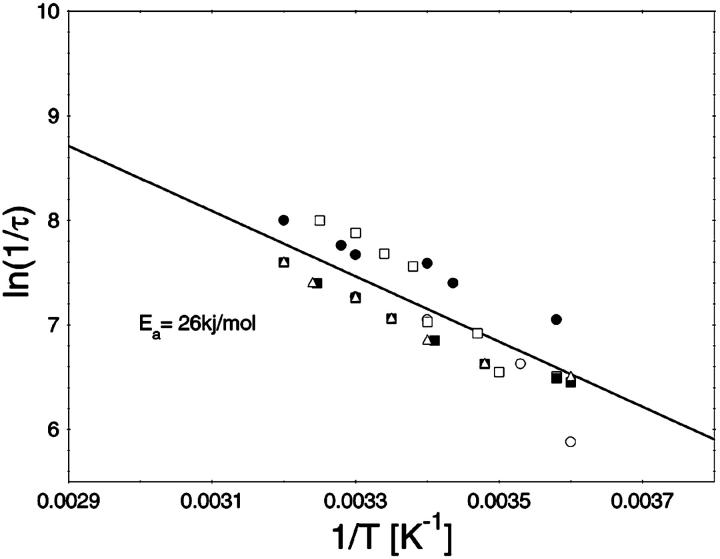
To study the current fluctuations mediated by molecular changes of the CymA-channel in more detail, experiments were performed with membranes with the same number of channels, but the temperature of the membrane cell was changed between 4°C and 40°C. In these experiments the power density spectra of the current noise was calculated using fast Fourier transform. Fig. 5 shows an example of these measurements. The plateau value, S0, of the spectral density of the current noise was more or less independent of temperature, indicating that the noise is caused by the single channels. However, the corner frequency of the Lorentzians was found to be dependent on temperature and increased from 131 1/s at 4°C to 340 1/s at 40°C (average of five measurements). Simultaneously, the time constant, τ, decreased within the same temperature range from 1.2 ms to 0.47 ms, indicating the rate constants of the current modulating process increased. Fig. 6 shows an Arrhenius plot of 1/τ vs. 1/T, which allows the calculation of its activation energy. We obtained 26 kJ/mol (corresponding to 6.2 kilocalories/mol) for the sum of the rate constants 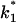 and
and 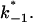 This activation energy is within the range of the free energy differences of multiple hydrogen bonds between uncharged residues in an aqueous solution (0.5–1.5 kcal/mol; Fersht et al., 1985).
This activation energy is within the range of the free energy differences of multiple hydrogen bonds between uncharged residues in an aqueous solution (0.5–1.5 kcal/mol; Fersht et al., 1985).
FIGURE 5.
Power density spectra of a membrane containing ∼220 open CymA-channels taken at different temperatures between 4°C and 40°C. The membrane was formed from diphytanoyl phosphatidylcholine/n-decane. The aqueous phase contained 1 M KCl and 20 ng/ml CymA. The power density spectra of the control, i.e., of the membranes before the addition of CymA was subtracted from the Lorentzians. The power density spectra of the open CymA-channels could be explained by a Lorentzian function using the following parameters: τ = 1.66 ms, S0 = 1.2 × 10−24 A2s (T = 4°C); τ = 1.08 ms, S0 = 1.4 × 10−24 A2s (T = 20°C); and τ = 0.51 ms, S0 = 2.0 × 10−24 A2s (T = 40°C).
FIGURE 6.
Temperature-dependence of the current modulating process in CymA. The ln(1/τ) values of five independent measurements were plotted as functions of the inverse temperature in Arrhenius plots. The activation energy was 26.6 kJ/mol (6.2 kcal/mol).
Effect of cyclodextrins on CymA-mediated current noise
In additional experiments we studied the effect of the cyclic dextrins on CymA-mediated current noise. Fig. 7 shows three power density spectra obtained after addition of different concentrations of α-CD. The upper trace (trace 1) shows a Lorentzian, taken when the reconstitution of the CymA-channels had slowed down (250 channels). Then 10 μM and 630 μM of α-CD were added to both sides of the membrane and two noise spectra were taken (trace 2 and trace 3, respectively). The spectral density of the current noise decreased upon addition of α-CD and the noise spectra could no longer be fitted as a single Lorentzian curve because the slope was no longer −2. This result indicated that the binding of the substrate decreased the current noise through the CymA-channel in contrast to the measurements with LamB and the sucrose-specific ScrY, where the addition of the carbohydrates and their binding to the channel always resulted in an increase of the current noise (Nekolla et al., 1994; Andersen et al., 1998). The decrease of the current noise may indicate that the molecular switch that produces the current noise of the open CymA-channel is switched off during binding of the cyclodextrins, i.e., it is involved in substrate binding. This means also that it was not possible to evaluate the kinetics of cyclodextrin binding to CymA in a similar way as has been performed with LamB and ScrY (Nekolla et al., 1994; Jordy et al., 1996; Andersen et al., 1998).
FIGURE 7.
Power density spectra of a membrane containing ∼250 CymA-channels after addition of different concentrations of α-CD. The membrane was formed from diphytanoyl phosphatidylcholine/n-decane. The upper trace (trace 1) shows a Lorentzian, taken in the absence of α-CD (τ = 0.84 ms; S0 = 1.9 × 10−24 A2s). Trace 2 and trace 3 show the power density spectra in the presence of 10 and 630 μM of α-CD, respectively, added to both sides of the membrane. The aqueous phase contained 1 M KCl (pH 6). T = 20°C; Vm = 20 mV.
Single-channel studies of CymA
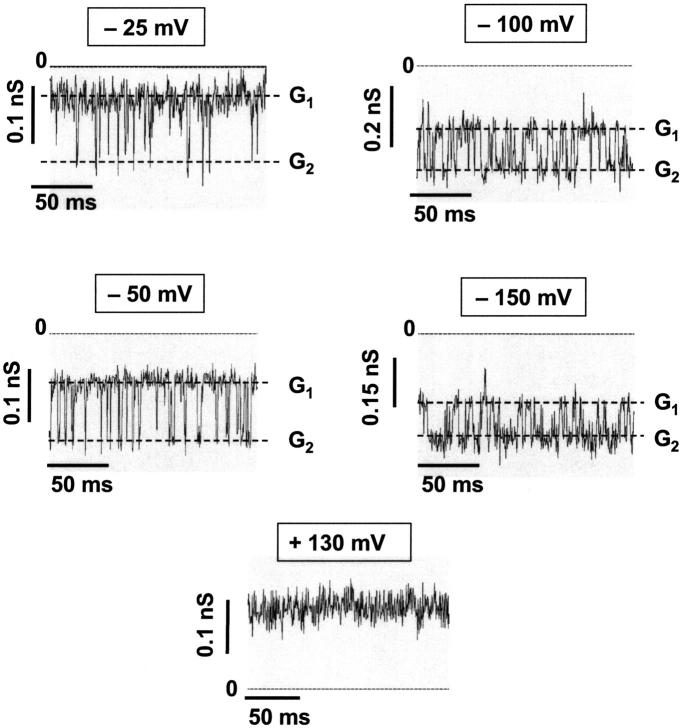
The orientation of the CymA-channels could not be studied in solvent-containing membranes because the reconstitution of the channels was presumably random, and because it was very difficult to reconstitute only one channel. To overcome this problem, we performed some experiments with folded membranes, which have a much smaller surface and allow easy access to the study of membranes containing only one CymA-channel. Furthermore, membranes of small surface allow a higher time resolution of the membrane current. Fig. 8 shows single-channel recordings of one single CymA-channel reconstituted in a folded membrane. The traces were recorded at different voltages. They clearly demonstrate that the lifetime of the open channel varied as a result of magnitude and polarity of the applied voltage. The current recordings of Fig. 8 demonstrate that the CymA-channel has basically two conductance states, when the polarity was negative at the trans-side of the membrane, the opposite side of the addition of the protein. Only for very high negative potentials at the trans-side was an additional conductance state was observed. For positive potentials at the trans-side, the channel was always in its open configuration (see Fig. 8).
FIGURE 8.
Single-channel recordings of one single CymA-channel reconstituted in a folded membrane made of diphytanoyl phosphatidylcholine. G1 and G2 correspond to the two conductance states of CymA. The traces were recorded at different voltages. The aqueous phase contained 1 M KCl (pH 6), T = 20°C. Experiments were performed using folded membranes.
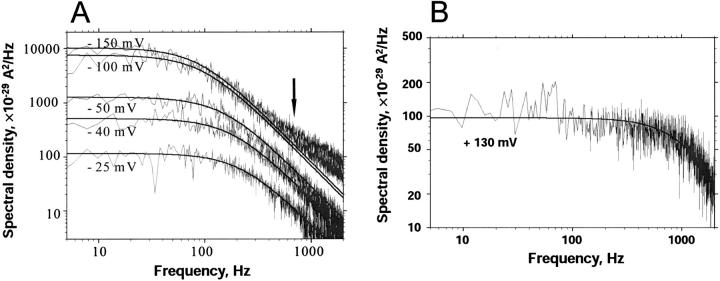
Folded membranes exhibit a higher time resolution in noise measurements than solvent-containing membranes because of the smaller capacity of the former. We took advantage of the higher time resolution to study the current noise as a function of the applied voltage in single-channel experiments. Fig. 9 A shows power density spectra of a single CymA-channel when increasing negative potentials were applied to the trans-side. The corner frequencies of the spectra agreed with those derived above from multichannel experiments and were only little dependent on the applied voltage. Only for very high negative voltages at the trans-side was an additional dispersion with a corner frequency of ∼2000 1/s observed. Fig. 9 B shows the power density spectrum when a positive potential was applied to the trans-side of the same membrane. The corner frequency was shifted to higher values as compared to the experiments with negative potential at the trans-side. Furthermore, the spectral density was ∼50 times lower than compared to the conditions of Fig. 9 A (negative potential at the trans-side). This result suggests that the CymA-channel is highly asymmetric. A comparison of the data derived from the single-channel experiments with those obtained above from the multichannel measurements suggests that the power density spectra of the latter measurement are controlled by the current noise that is related to Fig. 9 A. That of Fig. 9 B could probably not be detected in the multichannel measurements because of its small spectral density and because of its high corner frequency. We tried also to measure the kinetics of cyclodextrin binding to CymA in the single-channel experiments. It was not possible, however, since the addition of cyclodextrin had only a small influence on the corner frequencies but reduced the value of the spectral density in a similar way to that described above for multichannel experiments (data not shown).
FIGURE 9.
(A) Power density spectra of a single CymA-channel with increasing negative potentials applied to the trans-side. The experimental power density spectra were fitted to Lorentzian curves (see Eq. 6) with parameters: fc = 200 Hz, S0 = 115 × 10−29 A2/Hz at −25 mV; fc = 160 Hz; S0 = 510 × 10−29 A2/Hz at −40 mV; fc = 130 Hz, S0 = 1250 × 10−29 A2/Hz at −50 mV; fc = 100 Hz, S0 = 7460 × 10−29 A2/Hz at −100 mV; and fc = 95 Hz, S0 = 10,140 × 10−29 A2/Hz at −150 mV. (B) Power density spectra of a single CymA-channel with an applied voltage of +130 mV to the trans-side. The aqueous phase contained 1 M KCl (pH 6), T = 20°C. Folded membranes were used in A and B.
DISCUSSION
Binding of linear and cyclic dextrins to CymA
In a previous study it has been demonstrated that CymA is an outer membrane channel with a similar function as the carbohydrate-specific channels of enteric bacteria such as LamB or ScrY (Luckey and Nikaido, 1980; Benz et al., 1986; Schülein et al., 1991; Pajatsch et al., 1999). The CymA protein of K. oxytoca is required for growth of the organism on α-CD, β-CD, or γ-CD as sole carbon source. Here we investigated the binding of cyclic dextrins (CD) to the binding site inside the CymA-channel in detail. This was done by measuring the inhibition of the ionic current through the channel with increasing concentrations of α-CD, β-CD, and γ-CD, and by assuming that a simple model is valid for the movement of the carbohydrates through the CymA-channel (Benz et al., 1987). Implicit also in the model is that no competition exists between CD and ions for the binding site—which is justified by the single-channel experiments at different salt concentrations suggesting that no binding site exists for ions (Pajatsch et al., 1999). It is unlikely that CymA forms trimers. However, this is not a serious restriction because the stability constant derived from the titration experiments corresponds either to that of the binding to an individual channel or to the binding site inside one of the three channels in a trimer, assuming that no cooperativity exists between them. Highest stability constant for binding was obtained for α-CD with six glucose residues (K = 32,000 1/M) followed by β-CD (seven residues; K = 1970 1/M) and γ-CD (eight residues; K = 310 1/M). This means presumably that the bulky α-CD wheels fit best in the hydrophilic pocket within CymA, followed by the other CDs. This can be concluded from the structure of the CDs: they are hydrophilic and readily soluble in the aqueous phase, whereas the cavity of the cylinder is hydrophobic—a property which is used commercially for the inclusion of hydrophobic chemicals to render them soluble (Gu et al., 2001).
In vivo experiments have shown that CymA can replace LamB for growth on maltooligosaccharides (Pajatsch et al., 1999). Mutants with deletions of cymA lose this capacity but they are still able to grow on linear maltodextrins that enter the periplasm via the LamB maltoporin. A defect in lamB abolishes this capacity (Wandersman et al., 1979); if such a lamB mutant is provided with a functional cymA gene, growth on maltodextrins is restored. Therefore, CymA has a role in the uptake of CDs and linear maltodextrins whereas LamB only accepts linear maltodextrins, which means that K. oxytoca possesses both systems. Titration experiments with the linear maltooligosaccharides maltotriose, maltopentaose, and maltoheptaose reveals that CymA binds also linear maltooligosaccharides in agreement with the in vivo situation, albeit with much smaller affinity as compared to the CDs. The stability constant for binding of the linear maltooligosaccharides increased slightly with their number of glucose residues from 20 1/M (maltotriose) to 91 1/M (maltoheptaose). This result suggests that the linear maltooligosaccharides can adopt, in the binding pocket, a structure similar to that of CDs. Nevertheless, the binding of the relatively small maltotriose leads still to complete block of CymA for ion flux.
The current noise of the open CymA-channel can be explained by a Lorentzian function
We tried to measure the binding kinetics of CD binding to CymA in a similar way to what we had done earlier, using the analysis of current noise generated by the opening and closing of the LamB- or ScrY-channels caused by carbohydrate binding (Nekolla et al., 1994; Andersen et al., 1995; Andersen et al., 1998). Surprisingly, the open CymA-channel did not exhibit 1/f-noise as all porins investigated to date do (Nekolla et al., 1994; Wohnsland and Benz, 1997; Bezrukov and Winterhalter, 2000). The current noise was of Lorentzian type similar to that observed for opening and closing of nerve channels (Conti and Wanke, 1975; Conti et al., 1980), the ameloride-induced block of epithelial sodium channels (Lindemann and van Driessche, 1977; Lindemann, 1980), and the carbohydrate-induced block of LamB (Nekolla et al., 1994). Furthermore, the power density of the current noise was much higher than that observed with open porin channels (Wohnsland and Benz, 1997). This result indicates that the structure of the CymA-channel is substantially different to that of other porins. CymA obviously contains a section, which is mobile and can block the channel at least partially. Normal porins—so far as their three-dimensional structure is known—have a solid structure without mobile elements, and only amino acid residues at the third external loop that folds inside the β-barrel cylinder may undergo the small structural motions that may be responsible for the 1/f-noise (Wohnsland and Benz, 1997). It is noteworthy that other open membrane channels also showed some Lorentzian noise. However, their amplitudes are much smaller than observed here for CymA (Sigworth, 1985; 1986). Single-channel measurements support that view of CymA. Whereas normal porins show a more stepwise reconstitution with small channel noise (Benz et al., 1978, 1980), the channels formed by CymA exhibit rapid flickers (compare Fig. 8) that are presumably responsible for the Lorentzians.
The current modulating process of CymA could be treated in a similar way to what has been done earlier for opening and closing of nerve channels (Conti and Wanke, 1975; Conti et al., 1980). This means that we were able to calculate 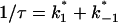 from the corner frequencies of the Lorentzians. The two rate constants could not be separated from the noise measurements. However, analysis of the single-channel data (Fig. 8) shows that the open and closed probabilities of CymA are approximately the same when a negative potential of ∼100 mV was applied to the trans-side. This suggests that both rate constants have almost the same magnitude and are of the order of 500 1/s under these conditions. For smaller voltages the closed probability is somewhat higher than the open one. The results can also be derived from Fig. 10, which shows the single channel conductance of the open and closed states of the CymA-channel (Fig. 10 top) and the rate constants for the transition between the open and closed state (
from the corner frequencies of the Lorentzians. The two rate constants could not be separated from the noise measurements. However, analysis of the single-channel data (Fig. 8) shows that the open and closed probabilities of CymA are approximately the same when a negative potential of ∼100 mV was applied to the trans-side. This suggests that both rate constants have almost the same magnitude and are of the order of 500 1/s under these conditions. For smaller voltages the closed probability is somewhat higher than the open one. The results can also be derived from Fig. 10, which shows the single channel conductance of the open and closed states of the CymA-channel (Fig. 10 top) and the rate constants for the transition between the open and closed state (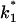 and
and 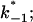 Fig. 10 bottom). Noise measurements with the same number of CymA channels and different temperatures allowed the evaluation of the activation energy, Ea, of the sum of the rate constants of the current modulating process to be ∼26.6 kJ/mol (6.2 kcal/mol). This activation energy is approximately half of the value that was derived for the on- and off-rate constants of maltooligosaccharide binding to the LamB-channel of E. coli (Ea = 46 kJ/mol; Andersen et al., 1995).
Fig. 10 bottom). Noise measurements with the same number of CymA channels and different temperatures allowed the evaluation of the activation energy, Ea, of the sum of the rate constants of the current modulating process to be ∼26.6 kJ/mol (6.2 kcal/mol). This activation energy is approximately half of the value that was derived for the on- and off-rate constants of maltooligosaccharide binding to the LamB-channel of E. coli (Ea = 46 kJ/mol; Andersen et al., 1995).
FIGURE 10.
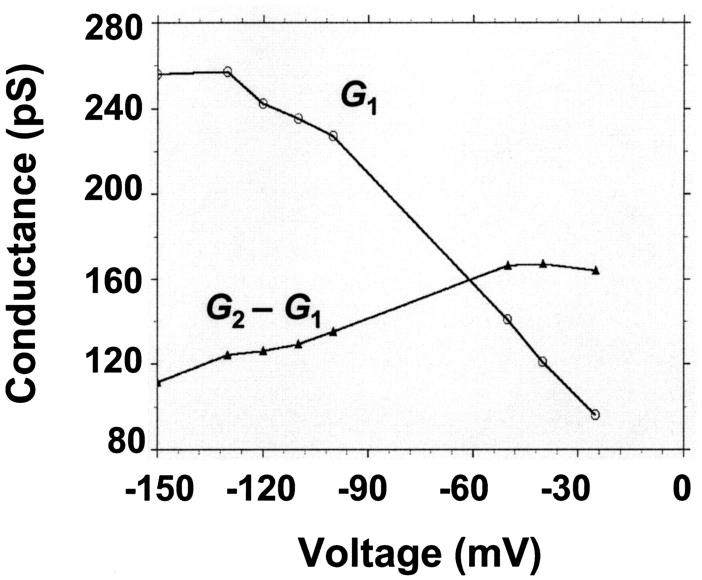
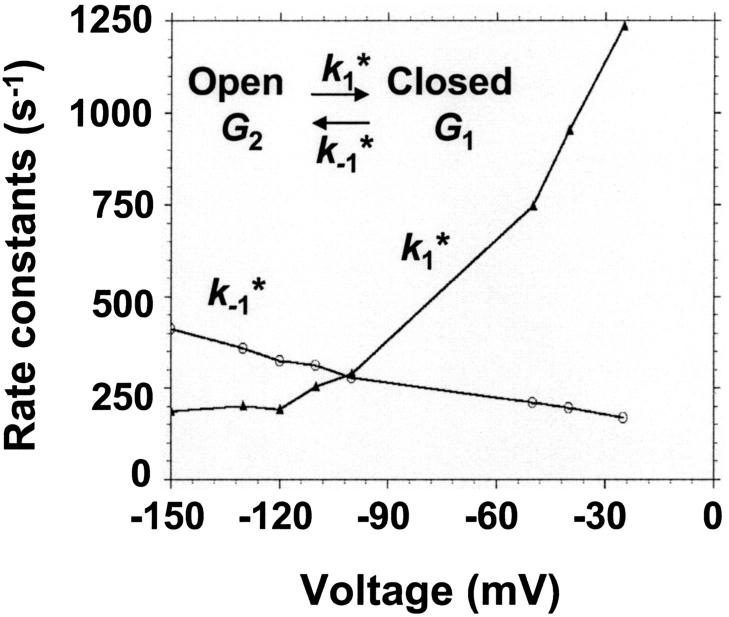
(Top) Plot of the two conductance substates G1 and (G2–G1) observed at the single channel level, versus the applied voltage. (Bottom) Voltage-dependence of the gating rate constants governing the open and closed lifetimes experienced by the substate (G2–G1). 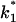 is the closure rate constant and
is the closure rate constant and 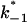 stands for the opening rate constant.
stands for the opening rate constant. 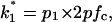 and
and 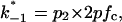 where p1 and p2 are the probabilities to be in the states of conductance G1 and G2, respectively, and fc is the corner frequency of the Lorentzian curves represented in Fig. 9 top. Interestingly, whereas
where p1 and p2 are the probabilities to be in the states of conductance G1 and G2, respectively, and fc is the corner frequency of the Lorentzian curves represented in Fig. 9 top. Interestingly, whereas 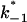 and
and 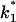 are voltage-dependent, the corner frequency,
are voltage-dependent, the corner frequency, 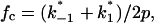 is only slightly affected by the external potential.
is only slightly affected by the external potential.
The current noise of the open CymA-channel decreases when CDs are added to the aqueous phase. This result suggests that the part of the channel that creates the Lorentzian noise is involved in the binding of CD and of maltooligosaccharides. This is a very interesting outcome of the noise measurements because it has not been observed for porins before. On the other hand, it makes the evaluation of the rate constants of carbohydrate transport impossible. The only conclusion from the noise experiments in the presence of the substrates is that the kinetics of substrate binding is probably within the range of the on- and off-rate constants of the channel flicker because no major change of the corner frequency of the Lorentzian was observed during the titration experiments, i.e., only S0 changed and not fc. The CD-mediated decrease of S0 was approximately the same as that of the single-channel conductance under the same conditions.
The CymA-channel is asymmetric concerning its voltage-dependence
Experiments with one single CymA-channel show that it is highly asymmetric, dependent on the polarity of the applied voltage. When the trans-side—the opposite side of the membrane to which the protein was added—is negative, the channel exhibited rapid flickers whose lifetime was highly voltage-dependent. For low negative potential at the trans-side, the channel was mostly in its closed configuration, which corresponds probably to the in vivo situation because the potential across the outer membrane is presumably small (Sen et al., 1988). For higher voltages, the open state became more and more predominant. When the potential was positive at the trans-side, the channel was predominantly in its closed configuration. The measurement of the current noise at the single-channel level agrees with this view. When the potential at the trans-side is negative the power density spectra have a corner frequency of ∼141 Hz with a high value for S0, which matches with the data of the multichannel experiments. For positive potential, S0 is much smaller and the corner frequency is within a range that cannot be detected in the multichannel experiments. This means that the CymA channel shows a much higher asymmetry than other porins, including LamB of E. coli, which exhibits only a small asymmetry regarding carbohydrate binding (Kullman et al., 2002; Orlik et al., 2002).
The experimental results suggest that the CymA-channel is gated. At low voltages it is in its closed configuration most of the time, which may represent an important feature of its function as an outer membrane channel for bulky substrates. The diameters of CDs range from 1.37 nm (α-CD) to 1.53 nm (β-CD) to 1.69 nm (γ-CD), which means that an open CymA-channel would create a considerable hole in the outer membrane. As a consequence the outer membrane would lose part of its function as a permeability barrier. The section of CymA that creates the channel flicker and the Lorentzian noise obviously restricts its channel size in a defined way and restores a low permeability, thus protecting the bacteria from the import of poisonous substances.
Acknowledgments
The authors thank Bettina Schiffler and Elke Maier for expert technical support.
This work was supported by grants from the Fonds der Chemischen Industrie (to A.B. and R.B.), and the Deutsche Forschungsgemeinschaft (Be 865/10 to R.B. and an Emmy Nöther Fellowship to C.A.).
References
- Andersen, C., R. Cseh, K. Schülein, and R. Benz. 1998. Study of sugar binding to the sucrose specific ScrY-channel of enteric bacteria using current noise analysis. J. Membr. Biol. 164:263–274. [DOI] [PubMed] [Google Scholar]
- Andersen, C., M. Jordy, and R. Benz. 1995. Evaluation of the rate constants of sugar transport through maltoporin (LamB) of Escherichia coli from the sugar-induced current noise. J. Gen. Physiol. 105:385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmeyer, C., R. Benz, H. Barth, K. Aktories, M. Gilbert, and MR. Popoff. 2001. Interaction of Clostridium botulinum C2 toxin with lipid bilayer membranes and Vero cells: inhibition of channel function by chloroquine and related compounds in vitro and intoxication in vivo. FASEB J. 15:1658–1660. [DOI] [PubMed] [Google Scholar]
- Bender, H. 1980. Kinetic studies of the (1 linked to 4)-alpha-D-glucopyranosyltransferase reaction catalyzed by cyclodextrin glycosyltransferase, particularly the cyclization with amylose, amylopectin and total starch as substrate. Carbohydr. Res. 78:133–145. [DOI] [PubMed] [Google Scholar]
- Bender, H. 1982. Effect of various acceptors on the rates of the cyclization and chain-shortening of amylose catalyzed by the cyclodextrin glycosyltransferase from Klebsiella pneumoniae M 5 a1. Improvement of new photometric assay methods. Carbohydr. Res. 101:279–285. [DOI] [PubMed] [Google Scholar]
- Benz, R. 1994. Solute uptake through bacterial outer membranes. In Bacterial Cell Wall. R. Hackenbeck, and J.-M. Ghuysen, editors. Elsevier, Amsterdam. pp.397–423.
- Benz, R. 2000. Porins—structure and function. In Microbial Transport Systems. G. Winkelmann, editor. Wiley-Verlag, Weinheim, Germany. pp.227–246.
- Benz, R., and R. E. W. Hancock. 1987. Mechanism of ion transport through the anion-selective channel of the Pseudomonas aeruginosa outer membrane. J. Gen. Physiol. 89:275–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz, R., K. Janko, W. Boos, and P. Läuger. 1978. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim. Biophys. Acta. 511:305–319. [DOI] [PubMed] [Google Scholar]
- Benz, R., K. Janko, and P. Läuger. 1980. Pore formation by the matrix protein (porin) of Escherichia coli in planar bilayer membranes. Ann. N. Y. Acad. Sci. 358:13–24. [DOI] [PubMed] [Google Scholar]
- Benz, R., A. Schmid, T. Nakae, and G. H. Vos-Scheperkeuter. 1986. Pore formation by LamB of Escherichia coli in lipid bilayer membranes. J. Bacteriol. 165:978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz, R., A. Schmid, and G. H. Vos-Scheperkeuter. 1987. Mechanism of sugar transport through the sugar-specific LamB-channel of Escherichia coli outer membrane. J. Membr. Biol. 100:12–29. [DOI] [PubMed] [Google Scholar]
- Bezrukov, S. M., and I. Vodyanoy. 1993. Probing alamethicin channels with water-soluble polymers. Effect on conductance of channel states. Biophys. J. 64:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezrukov, S. M., and M. Winterhalter. 2000. Examining noise sources at the single-molecule level: 1/f noise of an open maltoporin channel. Phys. Rev. Lett. 85:202–205. [DOI] [PubMed] [Google Scholar]
- Conti, F., B. Neumcke, W. Nonner, and R. Stämpfli. 1980. Conductance fluctuations from the inactivation process of sodium channels in myelinated nerve. J. Physiol. 308:217–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti, F., and I. Wanke. 1975. Channel noise in membranes and lipid bilayers. Quar. Rev. Biophys. 8:451–506. [DOI] [PubMed] [Google Scholar]
- Feederle, R., M. Pajatsch, E. Kremmer, and A. Bock. 1996. Metabolism of cyclodextrins by Klebsiella oxytoca M 5a1: purification and characterisation of a cytoplasmically located cyclodextrinase. Arch Microbiol. 165:206–212. [DOI] [PubMed] [Google Scholar]
- Fersht, A. R., J. P. Shi, J. Knill-Jones, D. M. Lowe, A. J. Wilkinson, D. M. Blow, P. Brick, P. Carter, M. M. Waye, and G. Winter. 1985. Hydrogen bonding and biological specificity analysed by protein engineering. Nature. 314:235–238. [DOI] [PubMed] [Google Scholar]
- Fiedler, G., M. Pajatsch, and A. Bock. 1996. Genetics of a novel starch utilisation pathway present in Klebsiella oxytoca. J. Mol. Biol. 256:279–291. [DOI] [PubMed] [Google Scholar]
- Gu, L. Q., S. Cheley, and H. Bayley. 2001. Capture of a single molecule in a nanocavity. Science. 291:636–640. [DOI] [PubMed] [Google Scholar]
- Jordy, M., C. Andersen, K. Schülein, T. Ferenci, and R. Benz. 1996. Rate constants of sugar transport through two lamB mutants of Escherichia coli: comparison to wild-type maltoporin and to LamB of Salmonella typhimurium. J. Mol. Biol. 259:666–678. [DOI] [PubMed] [Google Scholar]
- Kolb, H. A., P. Läuger, and E. Bamberg. 1975. Correlation analysis of electrical noise in lipid bilayer membranes. Kinetics of gramicidin A channels. J. Membr. Biol. 20:133–145. [DOI] [PubMed] [Google Scholar]
- Kullman, L., M. Winterhalter, and S. M. Bezrukov. 2002. Transport of maltodextrins through maltoporin: a single-channel study. Biophys. J. 82:803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger, P. 1973. Ion transport through pores: a rate-theory analysis. Biochim. Biophys. Acta. 311:423–441. [DOI] [PubMed] [Google Scholar]
- Lindemann, B. 1980. The beginning of fluctuation analysis of epithelial ion transport. J. Membr. Biol. 54:1–11. [DOI] [PubMed] [Google Scholar]
- Lindemann, B., and W. Van Driessche. 1977. Sodium-specific membrane channels of frog skin are pores: current fluctuations reveal high turnover. Science. 195:292–294. [DOI] [PubMed] [Google Scholar]
- Luckey, M., and H. Nikaido. 1980. Specificity of diffusion channels produced by lambda-phage receptor protein of Escherichia coli. Proc. Natl. Acad. Sci. USA. 77:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekolla, S., C. Andersen, and R. Benz. 1994. Noise analysis of ion current through the open and the sugar-induced closed state of the LamB-channel of Escherichia coli outer membrane: evaluation of the sugar binding kinetics to the channel interior. Biophys. J. 66:1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlik, F., C. Andersen, and R. Benz. 2002. Site-directed mutagenesis of tyrosine 118 within the central constriction site of the LamB (maltoporin) channel of Escherichia coli. II. Effect on maltose and maltooligosaccharide binding kinetics. Biophys. J. 83:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajatsch, M., C. Andersen, A. Mathes, A. Bock, R. Benz, and H. Engelhardt. 1999. Properties of a cyclodextrin-specific, unusual porin from Klebsiella oxytoca. J. Biol. Chem. 274:2559–2566. [DOI] [PubMed] [Google Scholar]
- Pajatsch, M., M. Gerhart, R. Peist, R. Horlacher, W. Boos, and A. Bock. 1998. The periplasmic cyclodextrin binding protein CymE from Klebsiella oxytoca and its role in maltodextrin and cyclodextrin transport. J. Bacteriol. 180:2630–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schülein, K., K. Schmid, and R. Benz. 1991. The sugar specific outer membrane channel ScrY contains functional characteristics of general diffusion pores and substrate-specific porins. Mol. Microbiol. 5:2233–2241. [DOI] [PubMed] [Google Scholar]
- Schwartz, M. 1987. The maltose regulon. In Escherichia coli and Salmonella typhimurium. F. C. Neidhardt, editor. ASM, Washington, DC. pp.1482–1502.
- Sen, K., J. Hellman, and H. Nikaido. 1988. Porin channels in intact cells of Escherichia coli are not affected by Donnan potentials across the outer membrane. J. Biol. Chem. 263:1182–1187. [PubMed] [Google Scholar]
- Sigworth, F. J. 1985. Open channel noise. I. Noise in acetylcholine receptor currents suggests conformational fluctuations. Biophys. J. 47:709–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth, F. J. 1986. Open channel noise. II. A test for coupling between current fluctuations and conformational transitions in the acetylcholine receptor. Biophys. J. 49:1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Driessche, W., and B. Lindemann. 1979. Concentration-dependence of currents through single-sodium selective pores in frog skin. Nature (Lond.). 282:519–521. [DOI] [PubMed] [Google Scholar]
- Wandersman, C., M. Schwartz, and T. Ferenci. 1979. Escherichia coli mutants impaired in maltodextrin transport. J. Bacteriol. 140:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohnsland, F., and R. Benz. 1997. 1/f-Noise of open bacterial porin channels. J. Membr. Biol. 158:77–85. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 133:103–119. [DOI] [PubMed] [Google Scholar]