Abstract
The sarcoplasmic reticulum channel (ryanodine receptor) from cardiac myocytes was reconstituted into planar lipid bilayers consisting of 1-palmitoyl-2-oleoyl-phosphatidylethanolamine (POPE) and 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC) in varying ratios. The channel activity parameters, i.e., open probability and average open time and its resolved short and long components, were determined as a function of POPE mole fraction (XPE) at 22.4°C. Interestingly, all of these parameters exhibited a narrow and pronounced peak at XPE ≈ 0.80. Differential scanning calorimetric measurements on POPE/POPC liposomes with increasing XPE indicated that the lipid bilayer enters a composition-driven transition from the liquid-crystalline state to the gel state at 22.4°C when XPE approaches 0.80. Thus, the peaking of the reconstituted channel activity at XPE ≈ 0.80 in the planar bilayer could result from the appearance of gel/liquid-crystalline domain boundaries at this POPE content. Lipid packing at domain boundaries is known to be looser as compared to the homogenous gel or liquid-crystalline state. We propose that the attractive potential of packing defects at lipid domain boundaries and entropic excluded-volume effects could result in the direct interactions of the transmembrane region of the channel protein with the lipid-packing defects at the lipid/protein interface, which could thus provide a favorable environment for the open state of the protein. The present findings indicate that the activity of the sarcoplasmic reticulum calcium channel could be modulated by lipid domain formation upon slight changes in membrane lipid composition in vivo.
INTRODUCTION
Ion channel proteins play an important role in the regulation of ion fluxes across cell and organelle membranes, e.g., the sarcoplasmic reticulum (reviewed by Fabiato, 1983 and Bers and Perez-Reyes, 1999). These membrane proteins undergo conformational changes during the ion transport process, and their transmembrane domains are located in a lipid bilayer composed of a variety of phospholipids and other lipids, mainly sterols (Fabiato, 1983). Thus, it is not surprising that the physical state of lipid bilayers can modulate the activity of ion channels as well as other membrane-associated proteins (Cheng et al., 1986; Huang, 1986; Keller et al., 1993; Chang et al., 1995a,b; Lundbaek et al., 1996; Somerharju et al., 1999). However, the molecular details of such lipid-associated modulation are still poorly understood. The lipid-associated parameters suggested to regulate channels or other membrane-associated proteins include bilayer surface hydration (Cheng et al., 1985; Cheng and Hui, 1986; Ho and Stubbs, 1992; Heller et al., 1997), curvature frustration (Gruner, 1985; Cheng and Hui, 1986; Cheng et al., 1986; Keller et al., 1993; Lundbaek et al., 1996; Scarlata and Gruner, 1997; Harroun et al., 1999), phase state (Menashe et al., 1986; Slater et al., 1994; Chang et al., 1995a; Romsicki and Sharom, 1998; Micol et al., 1999; Zhang and Kaback, 2000), bilayer thickness (Elliot et al., 1983), and lateral organization (Burack et al., 1997; Virtanen et al., 1998; Liu and Chong, 1999; Somerharju et al., 1999).
The ryanodine receptor (RyR) Ca2+-release channel from the heavy fraction of the sarcoplasmic reticulum (HSR) of muscle is a well-characterized membrane protein and shares many structural and functional properties with other ion channels (Bers and Perez-Reyes, 1999). This cardiac Ca2+-release channel is highly regulated by signaling pathways that control the excitation-contraction coupling for the proper function of the heart. Structurally, it is a homotetramer channel consisting of four 565-kDa subunits. The channel has a large cytoplasmic volume and a small transmembrane, or stalk region, which allows the channel to anchor on the lipid bilayer. (Orlova et al., 1998). The transmembrane part of RyR, which forms part of the ion pore, undergoes a significant conformational change during the gating event. In the open state, its four subunits are elongated and rotate 4° clockwise with respect to the closed state to form an opening of 20 Å2 (Orlova et al., 1998). These conformational changes in the transmembrane region can be expected to be sensitive to the physical state of the lipid environment in the bilayer. At present, the exact mechanisms of the modulation effect of lipid composition on cardiac calcium-release channels are still speculative. Recently, a modification of membrane composition in response to the activation of phospholipase A2 has been shown to affect the number of cardiac calcium-release channels during the late phase of sepsis (Dong et al., 2001). However, no systematic investigation of the direct effects of lipid compositions on the gating activity of single cardiac calcium-release channels has been reported.
In this study, we investigated how changes in bilayer properties due to varying lipid headgroup composition may affect the channel activity of reconstituted cardiac RyR. A single functional RyR/Ca2+ channel was reconstituted into a 1-palmitoyl-2-oleoyl-phosphatidylethanolamine (POPE)/1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC) bilayer separating two compartments, and various gating parameters were then measured as a function of the POPE mole fraction (XPE) at 22.4°C. A well-defined and narrow peak in all measured gating parameters was observed at XPE ≈ 0.80. This peak is probably due to the onset of composition-driven phase transition in the planar bilayer at XPE ≈ 0.80 as predicted from the gel-to-liquid-crystalline phase-transition temperatures (Tm) of neat POPC and POPE (∼6 and ∼25°C, respectively; Davis et al., 1981; Jaworsky and Mendelsohn, 1985) and deduced from calorimetric measurements of POPE/POPC liposomes. We propose that the peaking of the channel activity at XPE of 0.80 is due to 1), the appearance of lipid domain boundaries or packing defects due to coexistence of gel/liquid-crystalline domains at this composition; 2), interaction of the single RyR/Ca2+ channel with these defect regions; and 3), easier opening of the channel when located in such boundary defects as compared to homogenous gel or liquid-crystalline domains. Our results clearly demonstrated the effect of lipid phase transition, specifically the presence of lipid domains on the activity of the single cardiac calcium-release channel in vitro.
MATERIALS AND METHODS
Materials
Phospholipids POPE and POPC were purchased from Avanti Polar Lipids (Alabaster, AL). The lipids were mixed in chloroform at different molar ratios, and the solvent was evaporated under a nitrogen stream. Decane was then added to reach a total lipid concentration of 50 mg/ml. HSR microsomal membranes were isolated from rat ventricular muscle using standard differential centrifugation and discontinuous sucrose-gradient techniques as described earlier (Tate et al., 1985) and were stored at −80°C. Solvents and other chemicals were obtained from Sigma (St. Louis, MO).
Reconstitution of HSR calcium channels
The HSR calcium channels were reconstituted into preformed POPE/POPC planar lipid bilayers by an established method employing osmotic gradient-induced fusion (Fill et al., 1990). Briefly, a Delrin partition with an aperture of 150 μm in diameter was used to separate two concentric chambers termed cis and trans. Initially, both the cis and trans chambers were filled with 20 mM CsCH3SO3 and 10 mM HEPES, pH 7.4. A single lipid bilayer was then formed by painting the lipid mixtures across the aperture from the cis side. After a stable single bilayer had been formed, an osmotic gradient was established across the bilayer by increasing the CsCH3SO3 concentration of the cis side to 400 mM. HSR membranes were then pipetted into the cis chamber. The trans chamber was grounded, and the cis chamber was held at various voltages relative to ground. An incorporation protocol was used to monitor fusion of HSR membranes to the preformed POPE/POPC bilayer and the onset of channel-gating activity by measuring the transbilayer current while +50 to −50-mV voltage steps were applied at 1-s intervals. The appearance of sudden deflections from the baseline current was considered to indicate the successful reconstitution of channel protein to the planar bilayer and the presence of channel activity. To prevent further incorporation of channel protein, the osmotic gradient was removed by increasing the CsCH3SO3 concentration on the trans side to 400 mM. The steady-state activity of the channels was recorded by measuring the current response to +40 mV. The channel activity was measured using an AxoPatch 200A patch-clamp amplifier (Axon Instruments, Foster City, CA). In this study, Cs+ ion was employed, because the RyR, a cation-selective channel, has a larger conductance for monovalent cations (460 pS for Cs+) than for divalent cations (150 pS for Ca2+) as shown earlier (Williams, 1998). All experiments were performed at 22.4°C with bilayers that contained only a single channel. The temperature was monitored by a thermometer. The signal was filtered at 1 kHz using an eight-pole Bessel filter (Frequency Devices, Haverhill, MA) and was digitized at 5 kHz using pClamp 6.01 software (Axon Instruments).
The protein and phospholipid concentrations of HSR membrane preparation were ∼5 mg/ml and 13 mM, respectively, as determined using a modified Lowry protein assay (Stoscheck, 1990) and a phosphate assay (Litman, 1973; Cheng et al., 1985). Because RyR is the main protein component of HSR membranes (Tate et al., 1985), the phospholipid/RyR molar ratio of HSR could be estimated and was found to be ∼6.0 × 103. When a single RyR molecule is incorporated into the POPE/POPC bilayer, the amount of HSR-derived phospholipids in that bilayer is ∼10−20 mol. On the other hand, the amount of phospholipid in the phosphatidylethanolamine (PE)/phosphatidylcholine bilayer was calculated to be ∼10−13 mol based on the area of the aperture (1.78 × 10−8 m2) and the cross-sectional area of ∼50 × 10−20 m2 for a phospholipid molecule (Small, 1986). Accordingly, the amount of lipid associated with a single HSR channel is far too small to significantly affect the lipid composition of the bilayer.
Estimation of the lipid volume fraction in the planar bilayer by capacitance measurements
To estimate the fraction of the decane remaining in the planar bilayer, the specific membrane capacitance Cm was measured by analyzing the current response to voltage steps across the bilayer as described above. The lipid volume fraction (VL) in a solvent-containing membrane was then calculated from the specific capacitance Cm using the equation VL = 1.443 Cm + 0.123, which was derived from earlier data by Fettiplace et al. (1971) by linear regression analysis.
Kinetics analysis of channel gating
Analysis of the recorded single-channel current was performed using pClamp 6.01 and the accompanying module Pstat (Axon Instruments). The criterion for closed-to-open transition was set to 66% of the amplitude. The open probability (P) was calculated from the fraction of the total acquisition time the channel was in the open state. The open-time histograms were fitted with a double exponential decay function, 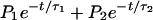 , to resolve the open short- and long-lifetime components (τ1 and τ2, respectively) using Marquardt-LSQ of Pstat. P1 and P2 are the relative open probabilities of the short and long components, respectively, with their sum normalized to 100%.
, to resolve the open short- and long-lifetime components (τ1 and τ2, respectively) using Marquardt-LSQ of Pstat. P1 and P2 are the relative open probabilities of the short and long components, respectively, with their sum normalized to 100%.
Differential scanning calorimetry
To prepare multilamellar liposomes, POPC and POPE in chloroform were mixed in appropriate ratios, and the solvent was evaporated under a nitrogen stream and further evaporated under high vacuum for 6 h. PEN buffer (5 mM phosphate, 1 mM EDTA, 50 mM NaCl) was added, and the dry lipids were dispersed by vortexing for 2 min, followed by a freeze-thaw cycle and bath sonication for 1 min at 25°C. This vortex/freeze-thaw/sonication procedure was repeated five times and was found necessary to obtain reproducible scans. Differential scanning calorimetry (DSC) measurements of the liposomes were performed on a model 6100 Nano II instrument (Calorimetry Sciences Corp., American Fork, UT) with a cell volume of 0.3 ml. The scan rate was 0.1°C/min, and the total lipid concentration was 3 mM.
RESULTS
Effect of POPE/POPC ratio on RyR/Ca2+-channel activity
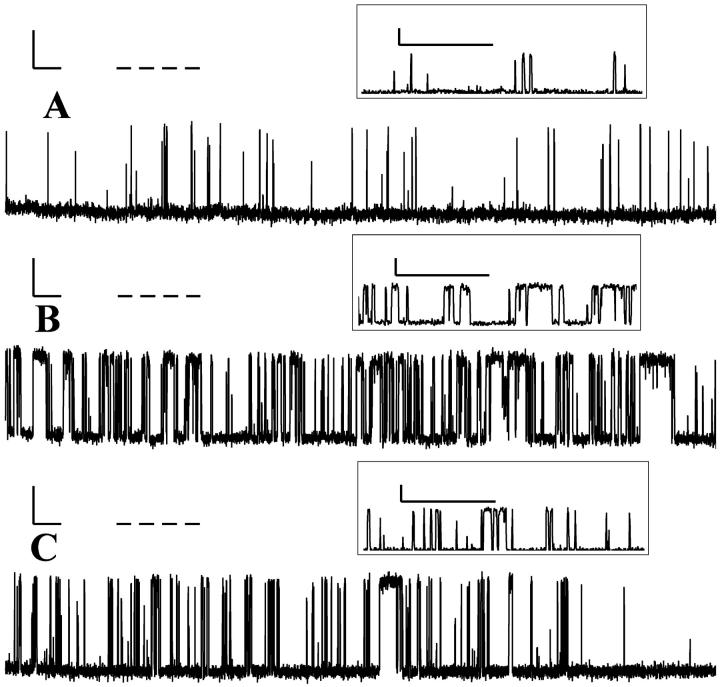
A single RyR/Ca2+ channel was reconstituted into a POPE/POPC bilayer as outlined in Materials and Methods, and the magnitude, duration, and frequency of openings were recorded as a function of XPE. Fig. 1 shows representative current traces for bilayers with XPE = 0.41, 0.81, and 0.90. Notably, the activity of the channel depended markedly on the lipid composition of the bilayer, i.e., XPE. The channel activity was similar for XPE of 0.41 and 0.90, but much higher for XPE of 0.81, i.e., the open events were far more frequent and their duration was greater as compared to the former compositions. The conductance of the channel was not affected by the lipid composition of the bilayer as evidenced by the similar profiles of the amplitude histograms for different lipid compositions (results not shown). This is in agreement with earlier findings on other channels (Keller et al., 1993; Chang et al., 1995a). The smaller-amplitude events (magnified in insets of Fig. 1) could represent openings that were too fast to be resolved by the instrument or, alternatively, could represent subconductance states that are independent of the lipid composition.
FIGURE 1.
Representative gating activity versus time plots for a single RyR/Ca2+ channel reconstituted into POPE/POPC bilayers with XPE values of 0.41 (A), 0.81 (B), and 0.90 (C). The scales for current and time are 10 pA and 50 ms, respectively. All measurements were performed at 22.4°C. Insets show magnified views of the regions highlighted by the dashed lines.
The lifetime profile of the reconstituted channel was visualized by plotting the channel open-time histograms as demonstrated in Fig. 2 for XPE = 0.41, 0.81, and 0.90. For these, as well as for all other compositions, the short-lived states were more frequent than the long-lived ones. The frequency of states with a long open time was found to be very similar for XPE of 0.41 (Fig. 2 A) and 0.90 (Fig. 2 C), with few events having an open time exceeding 2.0 ms. In contrast, there appeared to be more events exceeding 2.0 ms when XPE was 0.81 (Fig. 2 B). As shown in Table 1, the average open time (τ) for XPE = 0.81 was 2.15 ± 0.32 ms as compared with 0.59 ± 0.06 and 1.07 ± 0.43 ms for XPE of 0.41 and 0.91, respectively. The difference was statistically significant (p < 0.05).
FIGURE 2.
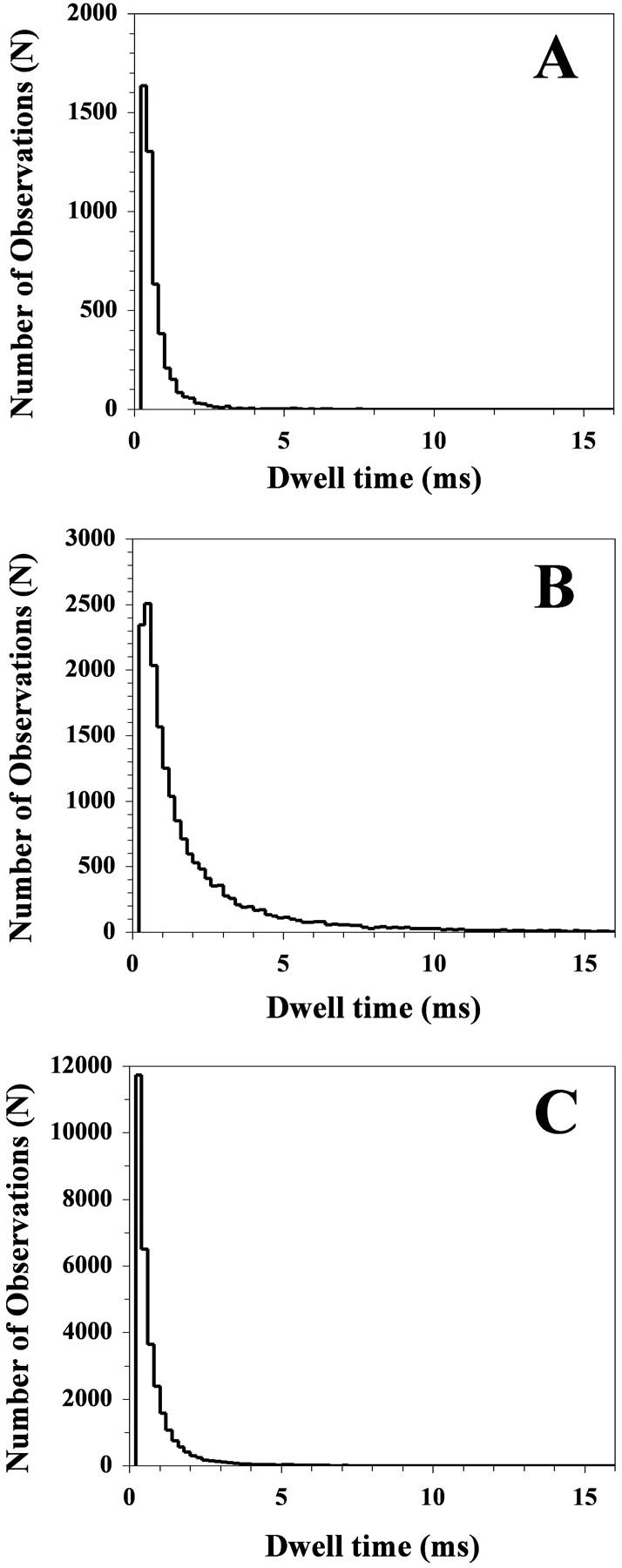
Representative channel open-time histograms for a single RyR/Ca2+ channel reconstituted into POPE/POPC bilayers with XPE values of 0.41 (A), 0.81 (B), and 0.90 (C).
TABLE 1.
Comparison of the channel-activity parameters of the reconstituted RyR/Ca2+ channel for lipid bilayer composition at XPE = 0.81 with those at XPE = 0.41, 0.71, 0.90, and 1.00
| XPE | n | P (%) | τ (ms) | τ1 (ms) | P1 (%) | τ2 (ms) |
|---|---|---|---|---|---|---|
| 0.81 | 5 | 18.44 ± 6.74 | 2.15 ± 0.32 | 0.71 ± 0.03 | 61.0 ± 1.3 | 3.02 ± 0.01 |
| 0.41 | 3 | 2.68 ± 0.85 (1.04 × 10−3) | 0.59 ± 0.06 (2.07 × 10−5) | 0.37 ± 0.14 (3.08 × 10−3) | 66.0 ± 4.0 (4.03 × 10−2) | 0.53 ± 0.10 (5.36 × 10−9) |
| 0.71 | 4 | 6.43 ± 1.01 (2.83 × 10−3) | 0.80 ± 0.04 (1.71 × 10−5) | 0.22 ± 0.10 (1.13 × 10−5) | 59.0 ± 1.2 (2.39 × 10−2) | 0.89 ± 0.03 (2.02 × 10−13) |
| 0.90 | 4 | 8.31 ± 0.27 (6.05 × 10−3) | 1.07 ± 0.43 (2.00 × 10−3) | 0.18 ± 0.19 (4.67 × 10−4) | 52.0 ± 1.0 (3.69 × 10−6) | 0.98 ± 0.04 (1.37 × 10−12) |
| 1.00 | 3 | 10.78 ± 8.86 (1.22 × 10−1)* | 0.70 ± 0.05 (3.13 × 10−5) | 0.30 ± 0.05 (6.77 × 10−6) | 77.0 ± 1.2 (1.05 × 10−6) | 1.07 ± 0.05 (4.64 × 10−10) |
Each activity parameter is represented by mean ± SD. The statistical significance of the difference between the mean of the activity-parameter peak for XPE = 0.81 and the same activity parameter for XPE = 0.41, 0.71, 0.90, or 1.00 is given by the p value (inside the parentheses) of a t-test with the null hypothesis of the difference of the two means being zero (Ferguson, 1971). The sample size, or number of successful reconstitution trials, is given by n. All p values, except the one marked with an asterisk (*), are <0.05.
Fig. 3 shows the systematic measurements of the values of P and τ of the reconstituted channel as a function of XPE. The results represent averages from data collected from 3–6 successful channel-reconstitution trials. The standard deviations of the averages, indicating the reproducibility of the data from different trials, are also shown. It is clear that P has a peak at XPE ≈ 0.80 (Fig. 3 A). Notably, a peak at XPE ≈ 0.80 was also found for τ (Fig. 3 B). As shown in Table 1, the peak in the P or τ versus XPE plot was found to be statistically significant by comparing the peak value at XPE = 0.81 with the adjacent “baseline” values at XPE = 0.71 and 0.90 on the left- and right-hand sides, respectively, of the activity peak using a standard t-test (Ferguson, 1971). As shown in Fig. 3 A, other than the peak of P around 18% at XPE ≈ 0.80, there appeared to be a general trend of an increase in the value of P from ∼3 to 10% as the PE content was increased from 0.4 to 1.0. Interestingly, the P value for the maximum XPE, i.e., 1.00, was statistically similar to the P value for XPE = 0.81 (p > 0.05), although the open time τ for XPE = 1.00 was still significantly smaller than that for XPE = 0.81 (p < 0.05).
FIGURE 3.
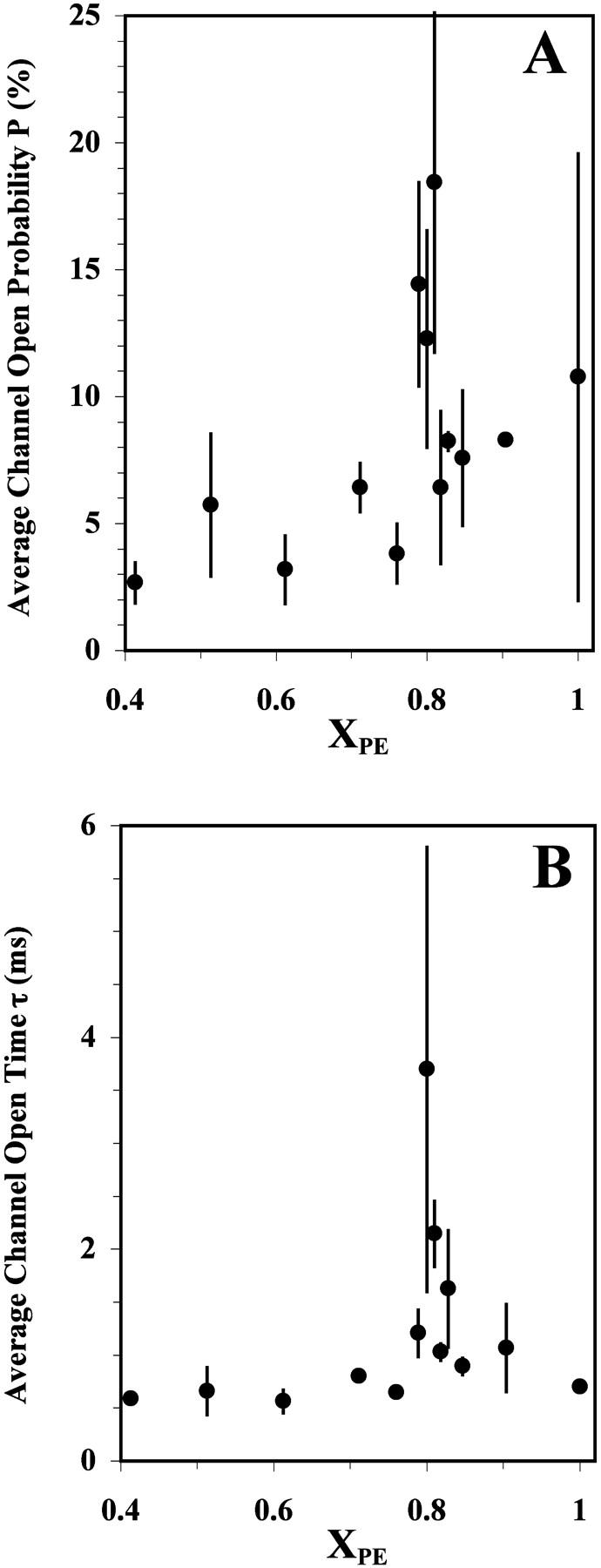
RyR/Ca2+-channel open probability P (A) and average open time τ (B) as a function of XPE. Each data point represents the average of independent measurements from 3–6 independent and successful reconstitution trials of the same lipid composition. Error bars represent the standard deviations.
Two distinct open lifetime components, i.e., one short (τ1) and one long (τ2), and their relative open probabilities (P1 and P2, respectively) were resolved by fitting a double exponential decay function (see Materials and Methods) to the open-time histogram. Fig. 4 shows the averages and standard deviations of the resolved τ1 and P1 as a function of XPE from 3–6 successful channel-reconstitution trials. Analogous plots for τ2 and P2 are shown in Fig. 5. The short component's lifetime τ1 peaked at XPE ≈ 0.80 (Fig. 4 A), and its relative probability P1 (Fig. 4 B) also reached a maximum close to this composition. The lifetime of the longer openings (τ2) displayed an even more prominent peak at XPE ≈ 0.80 (Fig. 5 A). The dip at XPE ≈ 0.80 for P2 (=1 − P1) as shown in Fig. 5 B reflects the peak of P1 at the same composition. As shown in Fig. 4 A and Table 1, the value of the τ1 peak at XPE ≈ 0.80 was ∼0.7 ms and was found to be significantly longer (p < 0.05) than the adjacent baseline values of 0.2–0.4 ms for other PE contents. Similarly, as shown in Fig. 5 A and Table 1, the value of the τ2 peak at XPE ≈ 0.80 was ∼3.5 ms and was found to be significantly longer (p < 0.05) than the baseline values of 0.5–1.5 ms for other PE contents. In comparison, the P1 peak was not as well defined as the τ1 or τ2 peaks. In summary, the channel activity data described above clearly demonstrate that the RyR/Ca2+-channel gating activity peaks sharply close to the XPE of 0.80.
FIGURE 4.
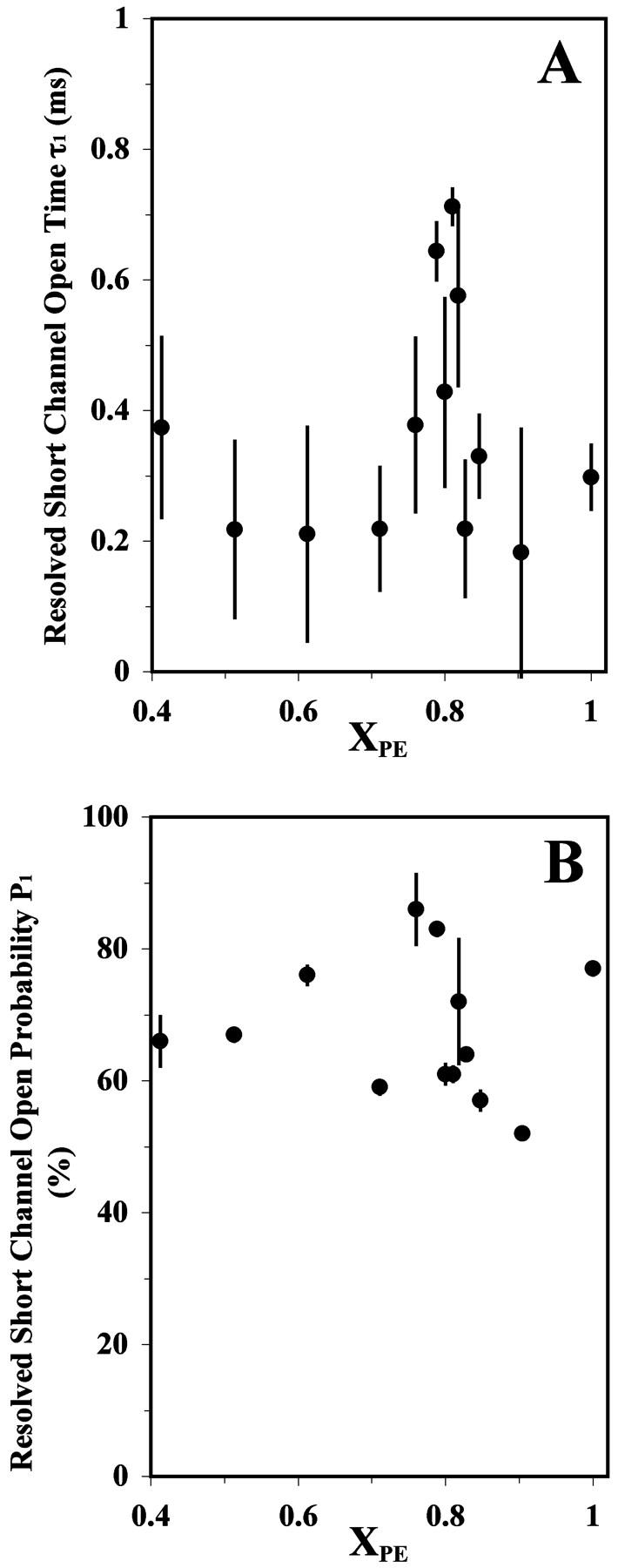
The resolved short open time τ1 (A) and its probability P1 (B) for a single RyR/Ca2+ channel reconstituted into a POPE/POPC bilayer as a function of XPE. See the legend of Fig. 3 for other details.
FIGURE 5.
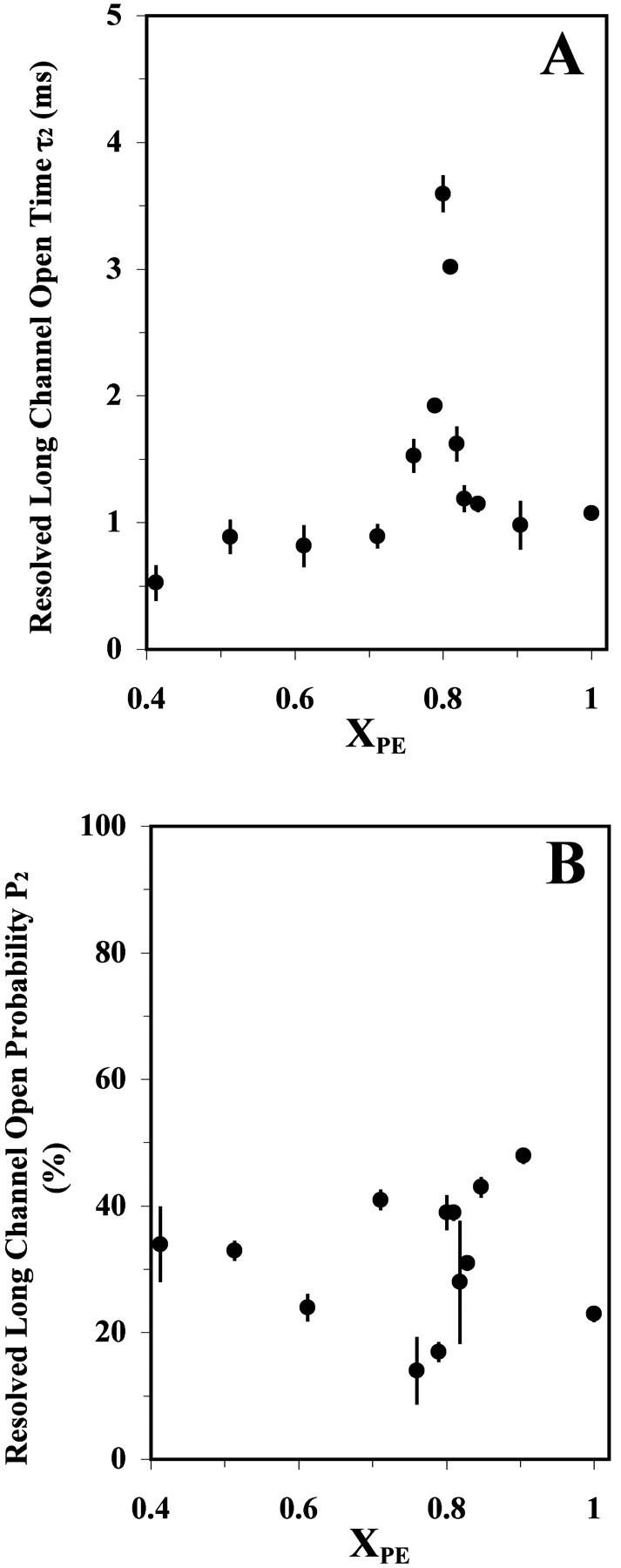
The resolved long open time τ2 (A) and its probability P2 (B) for a single RyR/Ca2+ channel reconstituted into a POPE/POPC bilayer as a function of XPE. See the legend of Fig. 3 for other details.
DSC measurements of POPE/POPC liposomes
Based on the considerations given in the Introduction, it seemed probable that the peaking of the channel activity close to XPE ≈ 0.80 is due to a composition-driven lipid phase transition. To study this, DSC measurements were carried out on pure POPE/POPC liposomes in the absence of decane with XPE varying from 0.61 to 1.0 with an increment of 0.01. Fig. 6 A shows some representative DSC scans, and the onset, peak, and completion temperatures of the gel-to-liquid-crystalline phase transition (Davis et al., 1981) as functions of XPE are plotted in Fig. 6 B. As can be seen, the peak temperature of the gel-to-liquid-crystalline transition increased progressively from 16 to 25°C as XPE increased from 0.61 to 1.00, whereas the width of the transition decreased markedly (from ∼7 to 1°C) over the same XPE range. The horizontal line in the figure marks the temperature (22.4°C) at which the channel activity was measured and crosses the onset, peak, and completion temperature lines at XPE ≈ 0.80, 0.85, and 0.92, respectively. These data indicate that the POPE/POPC bilayer was in the liquid-crystalline state for XPE < 0.80 and in the gel state for XPE > 0.92, whereas the liquid-crystalline and gel domains coexisted for 0.80 < XPE < 0.92. Accordingly, it is likely that the pronounced activity peak observed for the various gating parameters in Figs. 3–5 is due to a composition-driven transition of the POPE/POPC bilayer matrix at XPE ≈ 0.80.
FIGURE 6.
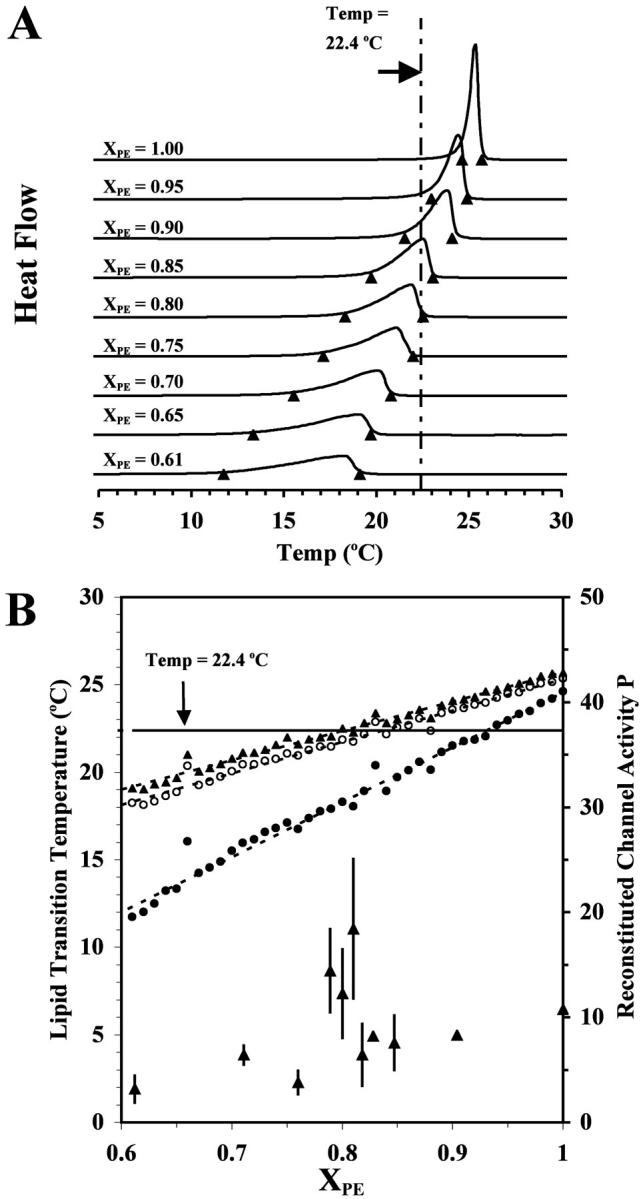
DSC analysis of neat POPE/POPC lipsomes with varying XPE values. (A) Representative DSC scans for POPE/POPC liposomes with XPE values of 0.61–1.00. Onset and completion temperatures (▴) were calculated from the intercepts of tangents at the half-widths of the peak with the baseline. The vertical line indicates the temperature of channel-activity measurement (22.4°C). (B) Partial phase diagram for liposomal POPE/POPC bilayers as determined by DSC. The onset (•), peak (○), and completion (▴) temperatures are shown. The horizontal line is plotted to visualize the bilayer composition at which the bilayer enters the domain-coexistence region at the temperature of measurement, i.e., 22.4°C. A channel-gating activity, i.e., the open probability P, is also plotted to highlight the match of single RyR/Ca2+-channel activity, which peaks at XPE ≈ 0.80 with the liquid-crystalline-to-gel phase boundary.
Estimation of the bilayer thickness from capacitance measurements
To assess the amount of decane remaining in planar POPE/POPC bilayers, the specific Cm was measured as a function of XPE (Fig. 7 A). Each data point represents the average from capacitance measurements of three or four independent planar bilayer samples of the same lipid composition. VL was calculated from Cm using the equation described in Materials and Methods and was plotted against XPE (Fig. 7 B). A recent work (Sonnleitner et al., 1999) on a solvent-free POPE/POPC bilayer with XPE of 0.70 reported a Cm value of 0.56 ± 0.10 μF/cm2, which corresponds to VL = 0.93 ± 0.27. These values are shown in Fig. 7 for the purpose of comparison. As can be seen, almost half of the values of VL fall into the range of solvent-free membrane.
FIGURE 7.
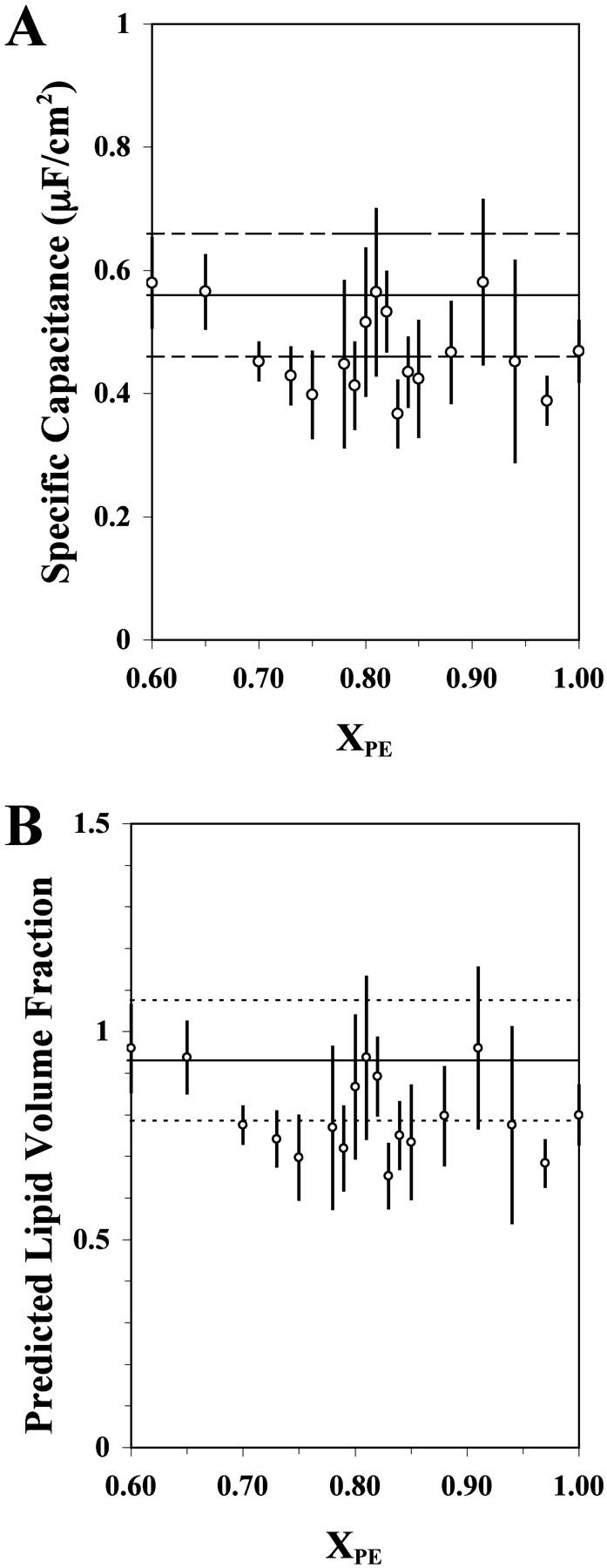
(A) Specific capacitance (Cm) of POPE/POPC planar bilayer as a function of XPE. Each data point represents the average from three or four different planar bilayers with the same lipid composition. (B) Predicted lipid volume fraction (VL) of POPE/POPC planar bilayer as a function of XPE. The value of VL was calculated from Cm as described in Materials and Methods. The horizontal solid line represents the value of Cm or VL at XPE = 0.70 in a solvent-free bilayer (Sonnleitner et al., 1999), and the corresponding upper and lower limits are indicated by the dashed lines. Error bars represent the standard deviations.
DISCUSSION
In this study, we have investigated the effect of composition-dependent physical properties of POPE/POPC membranes on the ion-gating activity of the RyR/Ca2+ channel. The transmembrane segments of this protein undergo significant conformational changes during gating (Orlova et al., 1998), and therefore, it was anticipated that gating would be sensitive to the physical state of the surrounding lipid bilayer. The present data show that both the average open probability and average open time (Fig. 3), as well as the resolved short and long open lifetimes (Figs. 4 and 5) of the reconstituted RyR/Ca2+ channel exhibit a narrow and pronounced maximum at XPE ≈ 0.80. At the same time, the DSC data (Fig. 6) showed that POPE/POPC liposomal bilayers undergo a composition-driven transition from the liquid-crystalline state to the gel state at XPE ≈ 0.80 at 22.4°C, i.e., the temperature at which the channel activity was recorded. Accordingly, it seems likely that the activity peak of the reconstituted channel close to XPE ≈ 0.80 is due to the planar bilayer containing the channel protein being in the liquid/gel-phase-coexistence state at this composition at 22.4°C. Previous studies have shown that a planar lipid bilayer can undergo a lipid phase transition leading to coexistence of gel and liquid-crystalline domains (Pagano et al., 1973; Boheim et al., 1980). In summary, these data provide significant evidence for regulation of the calcium-channel activity by a composition-driven liquid-to-gel phase transition of surrounding lipid bilayer. The most characteristic feature of the phase-transition region is the presence of phase or domain boundaries due to the coexistence of gel and liquid-crystalline domains (Sackmann, 1995). Thus, it seems likely that the activity peak at XPE ≈ 0.80 is due to 1), the RyR/Ca2+-channel protein migrates to those domain boundaries, and 2), its activity therein is significantly higher than either in the gel or liquid-crystalline domains.
Why would the reconstituted RyR/Ca2+-channel protein migrate to domain boundaries in the POPE/POPC model membranes? It is commonly thought that domain boundaries are bilayer regions where lipid packing is perturbed as compared to the (homogenous) gel or fluid regions (Sackmann, 1995). Such packing defects have an attractive potential (Sackmann, 1995) that could “pull” the channel molecules to domain boundaries. Another factor that could drive the protein molecule to domain boundaries is the depletion potential (also referred to as the entropic excluded-volume effect), a well-known phenomenon in colloid chemistry (Roth et al., 2000; Trokhymchuk et al., 2001). The depletion-potential model, as applied to the present case, proposes that the protein molecule becomes trapped at the domain boundaries because the Brownian collisions by fluid-phase lipids are more frequent than collisions by lipids in the gel phase. In other words, there is more “push” from the fluid phase than from the gel phase, since the lipids diffuse much faster in the former. However, further experiments are needed to substantiate these propositions.
The enhanced activity of the Ca2+ channel in domain boundaries is proposed to derive from the lipids that are more loosely packed in this region. Such looser packing would allow the channel protein to undergo the necessary conformational changes with less resistance than in the more tightly packed gel or liquid-crystalline phases (Hamill and Martinac, 2001). We assume that the activation energy for the opening of the channel would be lowered at the boundaries and thus would increase the frequency of openings. Also, the open time would increase due to a diminished energy difference between the open and closed states. Several previous studies have indicated that the appearance of domain boundaries can activate peripheral membrane enzymes. For instance, phospholipase A2 is most active at the gel-to-liquid-crystalline phase transition temperature (Op den Kamp et al., 1975; Wilschut et al., 1978; Upreti and Jain, 1980; Menashe et al., 1986; Burack et al., 1997) and under other conditions of domain coexistence (Liu and Chong, 1999). Also a lipid-transfer protein (Xu et al., 1983) and protein kinase C (Micol et al., 1999; Shen et al., 1999) have been found to be most active in the domain-coexistence region.
Besides the classical liquid-to-gel phase transition, domain boundaries could occur in a bilayer at particular compositions due to the tendency of the lipids to adopt different regular lateral distributions, as predicted by the membrane superlattice model (reviewed by Somerharju et al. (1999)). Notably, the channel activity peak at XPE ≈ 0.80 corresponds to a critical composition (XPE = 0.80) predicted by this model.
In addition to lipid domain coexistence, other phenomena could contribute to the activity peak of the Ca2+ channel close to XPE ≈ 0.80. For instance, it could be argued that bilayer thickness, which in the coexistence region should be intermediate between the gel and liquid phases, contributes to the increased channel activity. Matching of membrane thickness and the length of the transmembrane domain is important as indicated by data obtained for other proteins or peptides (Huang, 1986; Heller et al., 1997; Lundbaek et al., 1996). Also other bilayer properties such as surface hydration (Cheng et al., 1985, 1986; Cheng and Hui, 1986; Rand et al., 1998; Ho and Stubbs, 1992; Heller et al., 1997), acyl-chain order (Cullis et al., 1986), and intrinsic bilayer curvature (Keller et al., 1993; Chang et al., 1995a,b), each of which can vary with XPE, could contribute to the activity peaks close to XPE of 0.80. However, each of these properties is expected to change smoothly with XPE, and it is therefore unlikely that any of them is responsible for the narrow peak of activity. Accordingly, activation by domain boundaries appearing at a composition-driven lipid phase transition offers the most likely explanation for the activity peak observed at XPE of 0.80.
Decane was used here as the solvent to prepare the POPE/POPC bilayers for the channel activity measurement since channel reconstitution in a solvent-free bilayer is usually not favorable (Pantoja et al., 2001). It is known that some decane remains in the middle of the bilayer (Fettiplace et al., 1971) and could bias the data. For example, modest effects of decane and other solvents on the phospholipid Tm have been discovered previously (McIntosh et al., 1980). Although there are no simple and accurate methods to estimate the solvent content of planar bilyers, rough estimates can be obtained by measuring bilayer capacitance (Fettiplace et al., 1971). We found that within the experimental error, the capacitance value of the bilayer at XPE ≈ 0.65, 0.81, or 0.91 was quite similar (Fig. 7 A) to that obtained for a solvent-free bilayer reported previously (Sonnleitner et al., 1999). However, the protein activities at those XPE values were markedly different (Figs. 3–5). This suggests that the major deviation in channel activity occurring at XPE ≈ 0.80 is not related to the residual solvent remaining in the bilayer.
The marked activation of the RyR/Ca2+ channel by lipid domain boundaries indicated by the present in vitro study could be of biological relevance to the regulation of RyR in situ. Upon elevation of cytosolic Ca2+, RyRs mediate an extensive efflux of Ca2+ from the lumen of sarcoplasmic reticulum to the cytoplasm (Fabiato, 1983; Bers and Perez-Reyes, 1999). Ca2+ is capable of inducing a lateral segregation of phosphatidylserine and other acidic lipids in membranes (Papahadjopoulos et al., 1977; Haverstick and Glaser, 1988; Hinderliter et al., 1994), and this segregation can occur at [Ca2+] ≥ 10−7 M, corresponding to the Ca2+ concentrations in the cytoplasm after RyR/Ca2+-channel stimulation (Tokutomi et al., 1981). Accordingly, it is possible that release of Ca2+ to the cytoplasm leads to formation of domains rich in anionic lipids into the sarcoplasmic membrane. This would lead to formation of domain boundaries as well which, as implicated by the present data, would strongly stimulate the RyR/Ca2+-channel activity. Accordingly, it is possible that RyR/Ca2+ channels are regulated by an autocatalytic mechanism, and this could be important in muscle contraction and other events dependent on cytoplasmic Ca2+ spikes.
In this study, the RyR is assumed to be freely diffusible along the lateral direction of the planar bilayer. Recent studies of cardiac RyRs have indicated that the RyRs form clusters composed of ∼100 individual channels, which are strictly localized to the junctional membrane of sarcoplasmic reticulum (Franzini-Armstrong et al., 1999). Therefore, it is likely that the lateral movement of an individual RyR channel is restricted under normal physiological conditions (Takeshima et al., 2000; Scriven et al., 2002; Marks, 2002). The simultaneous activation of clustered RyR molecules is likely to cause marked elevation of local Ca2+ concentration due to the extensive burst of Ca2+ from the sarcoplasmic reticulum. This could lead to the proposed formation of domains and the associated packing defects in anionic lipids surrounding the channels. As mentioned in the Introduction, the cytoplasmic volume of RyR is larger than the transmembrane volume (Orlova et al., 1998). Therefore, the transmembrane regions of individual RyR channels within the RyR cluster should be freely accessible to the highly mobile lipid molecules. Thus, it would seem likely that the appearance of packing defects near the transmembrane regions of individual channels could regulate the gating activity of RyR channels within the relatively immobile RyR clusters in the junctional membrane.
Acknowledgments
This work was supported by Robert A. Welch Research Foundation (grant D-1158) to K.H.C.; National Institutes of Health (grant HL-52620) to S.G., Finnish Academy and Sigrid Juselius Foundation to P.S.; and Achievement Reward for College Scientists Foundation to B.C.
References
- Bers, D. M., and E. Perez-Reyes. 1999. Ca channels in cardiac myocytes: structure and function in Ca influx and intracellular Ca release. Cardiovasc. Res. 42:339–360. [DOI] [PubMed] [Google Scholar]
- Boheim, G., W. Hanke, and E. Eibl. 1980. Lipid phase transition in planar bilayer and its effects on carrier and pore-mediated ion transport. Proc. Natl. Acad. Sci. USA. 77:3403–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burack, W. R., A. R. Dibble, M. M. Allietta, and R. L. Biltonen. 1997. Changes in vesicle morphology induced by lateral phase separation modulate phospholipase A2 activity. Biochemistry. 36:10551–10557. [DOI] [PubMed] [Google Scholar]
- Chang, H., R. Reitstetter, and R. Gruener. 1995a. Lipid-ion channel interactions: increasing phospholipid headgroup size but not ordering acyl chains alters reconstituted channel behavior. J. Membr. Biol. 145:13–19. [DOI] [PubMed] [Google Scholar]
- Chang, H., R. Reitstetter, R. Mason, and R. Gruener. 1995b. Attenuation of channel kinetics and conductance by cholesterol: an interpretation using structural stress as a unifying concept. J. Membr. Biol. 143:51–63. [DOI] [PubMed] [Google Scholar]
- Cheng, K. H., and S. W. Hui. 1986. Correlation between the bilayer destabilization and activity enhancement by diacylglycerols in reconstituted Ca-ATPase vesicles. Arch. Biochem. Biophys. 24:382–386. [DOI] [PubMed] [Google Scholar]
- Cheng, K., J. Lepock, S. Hui, and P. Yeagle. 1986. The role of cholesterol in the activity of reconstituted Ca-ATPase vesicles containing unsaturated PE. J. Biol. Chem. 261:5081–5087. [PubMed] [Google Scholar]
- Cheng, K. H., T. Weidmer, and P. J. Sims. 1985. Fluorescence resonance energy transfer study of the membrane bound complexes of complement protein C5b-8. J. Immunol. 135:459–464. [PubMed] [Google Scholar]
- Cullis, P. R., M. J. Hope, and C. P. Tilcock. 1986. Lipid polymorphism and the roles of lipids in membranes. Chem. Phys. Lipids. 40:127–144. [DOI] [PubMed] [Google Scholar]
- Davis, P., B. Fleming, B. K. Coolbear, and K. Keough. 1981. Gel to liquid-crystalline transition temperatures of water dispersions of two pairs of positional isomers of unsaturated mixed-acid phosphatidylcholines. Biochemistry. 20:3633–3636. [DOI] [PubMed] [Google Scholar]
- Dong, L. W., L.-L. Wu, Y. Ji, and M.-S. Liu. 2001. Impairment of the ryanodine-sensitive calcium release channels in the cardiac sarcoplasmic reticulum and its underlying mechanism during the hypodynamic phase of sepsis. Shock. 16:33–39. [DOI] [PubMed] [Google Scholar]
- Elliott, J. R., D. Needham, J. P. Dilger, and D. A. Haydon. 1983. The effects of bilayer thickness and tension on gramicidin single-channel lifetime. Biochim. Biophys. Acta. 735:95–103. [DOI] [PubMed] [Google Scholar]
- Fabiato, A. 1983. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. Cell Physiol. 245:C1–C14. [DOI] [PubMed] [Google Scholar]
- Ferguson, G. A. 1971. Tests of significance: means. In Statistical Analysis in Psychology and Education, 3rd ed. McGraw-Hill, New York. 146–157.
- Fettiplace, R., D. Andrews, and D. Haydon. 1971. The thickness, composition and structure of some lipid bilayers and natural membranes. J. Membr. Biol. 5:277–296. [DOI] [PubMed] [Google Scholar]
- Fill, M., R. Coronado, J. R. Mickelson, J. Vilven, J. Ma, B. A. Jacobson, and C. F. Louis. 1990. Abnormal ryanodine receptor channels in malignant hyperthermia. Biophys. J. 57:471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong, C., F. Protasi, and V. Ramesh. 1999. Shape, size and distribution of Ca2+ release units and couplons in skeletal and cardiac muscles. Biophys. J. 77:1528–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner, S. 1985. Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc. Natl. Acad. Sci. USA. 82:3665–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill, O. P., and B. Martinac. 2001. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 81:685–740. [DOI] [PubMed] [Google Scholar]
- Harroun, T. A., W. T. Heller, T. M. Weiss, L. Yang, and H. W. Huang. 1999. Experimental evidence for hydrophobic matching and membrane-mediated interactions in lipid bilayers containing gramicidin. Biophys. J. 76:937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverstick, D. M., and M. Glaser. 1988. Visualization of domain formation in the inner and outer leaflets of a phospholipid bilayer. J. Cell Biol. 106:1885–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller, W., K. He, S. Ludtke, T. Harroun, and H. Huang. 1997. Effect of changing the size of lipid headgroup on peptide insertion into membranes. Biophys. J. 73:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinderliter, A. K., J. Huang, and G. W. Feigenson. 1994. Detection of phase separation in fluid phosphatidylserine/phosphatidylcholine mixtures. Biophys. J. 67:1906–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, C., and C. D. Stubbs. 1992. Hydration at the membrane protein-lipid interface. Biophys. J. 63:897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. W. 1986. Deformation free energy of bilayer membrane and its effects on gramicidin channel lifetime. Biophys. J. 54:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworsky, M., and R. Mendelsohn. 1985. Fourier-transform infrared studies of CaATPase partitioning in phospholipid mixtures of 1,2-dipalmitoylphosphatidylcholine-d62 with 1-palmitoyl-2-oleoylphosphatidylethanolamine and 1-stearoyl-2-oleoyl-phosphatidyl-choline. Biochemistry. 24:3422–3428. [DOI] [PubMed] [Google Scholar]
- Keller, S., S. Bezrukov, S. Gruner, M. Tate, I. Vodyanoy, and V. Parsegian. 1993. Probability of alamethicin conductance states varies with nonlamellar tendency of bilayer phospholipids. Biophys. J. 65:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman, B. 1973. Lipid model membranes. Characterization of mixed phospholipid vesicles. Biochemistry. 12:2545–2554. [DOI] [PubMed] [Google Scholar]
- Liu, F., and P. Chong. 1999. Evidence for a regulatory role of cholesterol superlattices in the hydrolytic activity of secretory phospholipase A2 in lipid membranes. Biochemistry. 38:3867–3873. [DOI] [PubMed] [Google Scholar]
- Lundbaek, J. A., P. Birn, J. Girsham, A. Hansen, and O. Andersen. 1996. Membrane stiffness and channel function. Biochemistry. 35:3825–3830. [DOI] [PubMed] [Google Scholar]
- Marks, A. R. 2002. Ryanodine receptors, FKBP12, and heart failure. Front. Biosci. 7:970–977. [DOI] [PubMed] [Google Scholar]
- McIntosh, T. J., S. A. Simon, and R. C. MacDonald. 1980. The organization of n-alkanes in lipid bilayers. Biochim. Biophys. Acta. 597:445–463. [DOI] [PubMed] [Google Scholar]
- Menashe, M., G. Romero, R. Biltonen, and D. Lichtenberg. 1986. Hydrolysis of DPPC small unilamellar vesicles by porcine pancreatic phospholipase A2. J. Biol. Chem. 261:5328–5333. [PubMed] [Google Scholar]
- Micol, V., P. Sanchez-Pinera, J. Villaliain, A. de Godos, and J. C. Gomez-Fernandez. 1999. Correlation between protein kinase C α activity and membrane phase behavior. Biophys. J. 76:916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdenkamp, J. A., M. T. Kauerz, and L. L. van Deenen. 1975. Action of pancreatic phospholipase A2 on phosphatidylcholine bilayers in different physical states. Biochim. Biophys. Acta. 406:169–177. [DOI] [PubMed] [Google Scholar]
- Orlova, E., I. Serysheva, S. Hamilton, W. Chiu, and M. van Heel. 1998. The skeletal muscle calcium-release channel visualized by electron microscopy. In The Structure and Function of Ryanodine Receptors. R. Sitsapesan, editor. Imperial College Press, London. 23–47.
- Pagano, R., R. Cherry, and D. Chapman. 1973. Phase transitions and heterogeneity in lipid bilayers. Science. 181:557–558. [DOI] [PubMed] [Google Scholar]
- Pantoja, R., D. Sigg, R. Blunck, F. Bezanilla, and J. R. Heath. 2001. Bilayer reconstitution of voltage-dependent ion channels using a microfabricated silicon chip. Biophys. J. 81:2389–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos, D., W. J. Vail, C. Newton, S. Nir, K. Jacobson, G. Poste, and R. Lazo. 1977. Studies on membrane fusion. III. The role of calcium-induced phase changes. Biochim. Biophys. Acta. 465:579–598. [DOI] [PubMed] [Google Scholar]
- Rand, R. P., N. Fuller, V. A. Parsegian, and D. C. Rau. 1998. Variation in hydration forces between neutral phospholipid bilayers: evidence for hydration attraction. Biochemistry. 27:7711–7722. [DOI] [PubMed] [Google Scholar]
- Romsicki, Y., and F. Sharom. 1998. The ATPase and ATP-binding function of P-glycoprotein modulation by interaction with defined phospholipids. Eur. J. Biochem. 256:170–178. [DOI] [PubMed] [Google Scholar]
- Roth, R., R. Evans, and S. Dietrich. 2000. Depletion potential in hard-sphere mixtures: theory and applications. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Topics. 62:5360–5377. [DOI] [PubMed] [Google Scholar]
- Sackmann, E. 1995. Biological membranes: architecture and function. In Structure and Dynamics of Membranes: From Cells to Vesicles. Handbook of Biological Physics, Vol. 1. R. Lipowsky and E. Sackmann, editors. Elsevier Science, New York. 1–63.
- Scarlata, S., and S. Gruner. 1997. Role of PE lipids in the stabilization of protein-lipid contacts. Biophys. Chem. 67:269–279. [DOI] [PubMed] [Google Scholar]
- Scriven, D. R. L., A. Klimek, K. L. Lee, and D. W. Moore. 2002. The molecular architecture of calcium microdomains in rat cardiomyocytes. Ann. N. Y. Acad. Sci. 976:488–499. [DOI] [PubMed] [Google Scholar]
- Shen, Y. M., O. I. Chertihin, R. L. Biltonen, and J. J. Sando. 1999. Lipid-dependent activation of protein kinase C-alpha by normal alcohols. J. Biol. Chem. 274:34036–34044. [DOI] [PubMed] [Google Scholar]
- Slater, S., M. Kelly, F. Taddeo, C. Ho, E. Rubin, and C. Stubbs. 1994. The modulation of protein kinase C activity by membrane lipid bilayer structure. J. Biol. Chem. 269:4866–4871. [PubMed] [Google Scholar]
- Small, D. M. 1986. The physical chemistry of lipids. In Handbook of Lipid Research. D. K. Hanaham, editor. Plenum Press, New York. 97–143.
- Somerharju, P., J. Virtanen, and K. H. Cheng. 1999. Lateral organization of membrane lipids: the superlattice view. Biochim. Biophys. Acta. 1440:32–48. [DOI] [PubMed] [Google Scholar]
- Sonnleitner, A., G. Schutz, and T. Schmidt. 1999. Free Brownian motion of individual lipid molecules in biomembranes. Biophys. J. 77:2638–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoscheck, C. M. 1990. Quantitation of protein. Methods Enzymol. 182:50–69. [DOI] [PubMed] [Google Scholar]
- Takeshima, H., S. Komazaki, M. Nishi, M. Iino, and K. Kangawa. 2000. Junctophilins: a novel family of junctional membrane complex proteins. Mol. Cell. 6:11–22. [DOI] [PubMed] [Google Scholar]
- Tate, C. A., R. J. Bick, A. Chu, W. B. Van Winkle, and M. L. Entman. 1985. Nucleotide specificity of canine cardiac sarcoplasmic reticulum. GTP-induced calcium accumulation and GTPase activity. J. Biol. Chem. 260:9618–9623. [PubMed] [Google Scholar]
- Tokutomi, S., R. Lew, and S. Ohnishi. 1981. Ca2+-induced phase separation in phosphatidylserine, phosphatidylethanolamine and phosphatidylcholine mixed membranes. Biochim. Biophys. Acta. 643:276–282. [DOI] [PubMed] [Google Scholar]
- Trokhymchuk, A., D. Henderson, A. Nikolov, and D. T. Wasan. 2001. Entropically driven ordering in a binary colloidal suspension near a planar wall. Phys. Rev. E Stat. Nonlin. Soft Matter. Phys. 64:012401. [DOI] [PubMed] [Google Scholar]
- Upreti, G. C., and M. K. Jain. 1980. Action of phospholipase A2 on unmodified phosphatidylcholine bilayers: organizational defects are preferred sites of action. J. Membr. Biol. 55:113–121. [DOI] [PubMed] [Google Scholar]
- Virtanen, J. A., K. H. Cheng, and P. Somerharju. 1998. Phospholipid composition of the mammalian red cell membrane can be rationalized by a superlattice model. Proc. Natl. Acad. Sci. USA. 95:4964–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, A. J. 1998. Ca2+ release channel/ryanodine receptor-L-type Ca2+ channel/dihydropyridine receptor interactions in skeletal muscle. In The Structure and Function of Ryanodine Receptors. R. Sitsapesan, editor. Imperial College Press, London. 95–111.
- Wilschut, J. C., J. Regts, H. Westenberg, and G. Scherphof. 1978. Action of phospholipases A2 on phosphatidylcholine bilayers. Effects of the phase transition, bilayer curvature and structural defects. Biochim. Biophys. Acta. 508:185–196. [DOI] [PubMed] [Google Scholar]
- Xu, Y. H., K. Gietzen, H. J. Galla, and E. Sackmann. 1983. Protein-mediated lipid transfer. The effects of lipid-phase transition and of charged lipids. Biochem. J. 213:21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W., and H. Kaback. 2000. Effect of the lipid phase transition on the lactose permease from Escherichia coli. Biochemistry. 39:14538–14542. [DOI] [PubMed] [Google Scholar]