Abstract
InsP3 is an important link in the intracellular information network. Previous observations show that activation of InsP3-receptor channels on the granular membrane can turn secretory granules into Ca2+ oscillators that deliver periodic trains of Ca2+ release to the cytosol (T. Nguyen, W. C. Chin, and P. Verdugo, 1998, Nature, 395:908–912; I. Quesada, W. C. Chin, J. Steed, P. Campos-Bedolla, and P. Verdugo, 2001, Biophys. J. 80:2133–2139). Here we show that InsP3 can also turn mast cell granules into proton oscillators. InsP3-induced intralumenal [H+] oscillations are ATP-independent, result from H+/K+ exchange in the heparin matrix, and produce perigranular pH oscillations with the same frequency. These perigranular pH oscillations are in-phase with intralumenal [H+] but out-of-phase with the corresponding perigranular [Ca2+] oscillations. The low pH of the secretory compartment has critical implications in a broad range of intracellular processes. However, the association of proton release with InsP3-induced Ca2+ signals, their similar periodic nature, and the sensitivity of important exocytic proteins to the joint action of Ca2+ and pH strongly suggests that granules might encode a combined Ca2+/H+ intracellular signal. A H+/Ca2+ signal could significantly increase the specificity of the information sent by the granule by transmitting two frequency encoded messages targeted exclusively to proteins like calmodulin, annexins, or syncollin that are crucial for exocytosis and require specific combinations of [Ca2+] “and” pH for their action.
INTRODUCTION
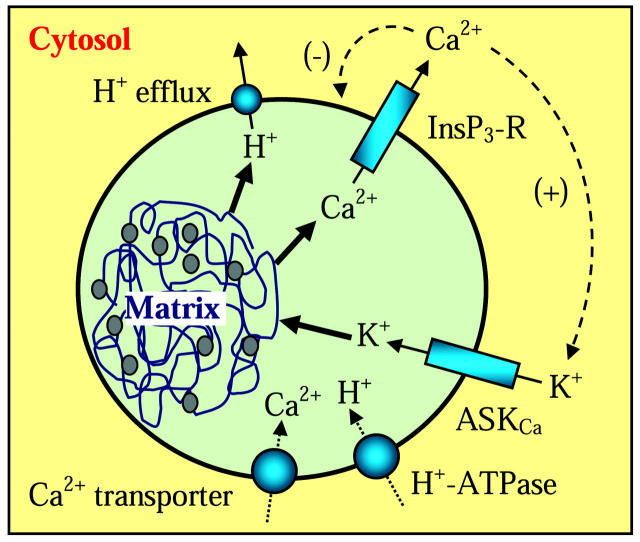
The dynamics of lumenal pH in the secretory pathway is critical for the proper function of a broad range of cell processes including protein sorting, enzyme activation, and biogenic amines loading (Bell-Parikh et al., 2001). In secretory granules, changes in the preexocytotic lumenal pH are thought to be necessary for the final steps of exocytosis (Williams and Webb, 2000; Barg et al., 2001; Han et al., 1999). The regulation of intralumenal pH (pHG) has been thought as a simple H+ influx/efflux equilibrium with pumps as H+ source and “leakage” to the cytosol as a H+ sink (Mitchell et al., 2001; Demaurex, 2002). However, the characteristic polyelectrolyte matrices present inside virtually all granules offer a novel alternative as intralumenal H+ sink/donors. The strong polyanionic properties of these polymer networks can function as efficient ion exchange resins controlling the bound/free turnover of intralumenal cations (Uvnas and Aborg, 1977, 1989; Verdugo, 1994; Nguyen et al., 1998; Quesada et al., 2001; Nanavati and Fernandez, 1993; Marszalek et al., 1997; Chin et al., 2002). Thus, the level of free ionized Ca2+, K+, and H+ inside the granule results not only from the influx/efflux equilibrium of these ions in/from the granule but also from their complex exchange with the secretory matrix. However, most of the work on the control of intralumenal cations, particularly Ca2+, remains focused almost exclusively on the action of pumps and export channels (Mitchell et al., 2001; Demaurex, 2002). The key role of the secretory matrix as cation sink/donor in the granule has been highlighted by recent observations that reveal that the granule can function as an intracellular Ca2+ oscillator, and that InsP3-induced intralumenal Ca2+ oscillations—and corresponding oscillatory release of Ca2+ to the cytosol—results from Ca2+/K+ exchange in the matrix (Nguyen et al., 1998; Quesada et al., 2001). According to this new model, the frequency-encoded Ca2+ signaling system of the granule results from the interplay between the Ca2+/K+ ion-exchange properties of the secretory matrix and two Ca2+-sensitive channels located at close proximity on the membrane of secretory vesicles: an ASKCa channel that mediates K+ entry into the vesicular lumen, and an InsP3-R channel that releases Ca2+ to the cytosol (see Fig. 1). Stimulation of the cell induces production of InsP3 leading to InsP3 binding to the InsP3-R channel, release of Ca2+ from the granule, and decrease of intralumenal [Ca2+]IL. Cytosolic Ca2+ ([Ca2+]C) increases around the granule, turning open nearby ASKCa channels and closing InsP3-R channels. K+ imported into the vesicular lumen exchange for Ca2+ in the polyanionic matrix and together with the closure of the InsP3-R channel results in an increase of [Ca2+]IL in the granule's lumen. As diffusion and cytosolic Ca2+-buffering restore the [Ca2+]C to lower levels, the InsP3-R channel opens again, starting a new cycle that recurs for as long as the InsP3-R remains activated (Nguyen et al., 1998; Quesada et al., 2001). In goblet cells, these [Ca2+]IL oscillations are accompanied by corresponding pHG oscillations that can increase the gain of the Ca2+/K+ exchange leading to increased Ca2+ unbinding and a rise in the flux of diffusion-driven Ca2+ release (Chin et al., 2002).
FIGURE 1.
Dynamics of H+ and Ca2+ in secretory granules. This model involves two pools of intralumenal cations: a pool of free ionized cations in equilibrium with a pool of cations bound to the granule polyanionic matrix that functions as a H+/K+ and Ca2+/K+ ion exchange network (Verdugo, 1994; Marszalek et al., 1997). The model further assumes that vesicular Ca2+ uptake is driven by an undefined Ca2+-ATPase (Mitchell et al., 2001), and that the activity of V-type H+-ATPases is responsible for H+ transport to maintain a steady-state intralumenal pH (Demaurex, 2002). Oscillations of intralumenal H+ and Ca2+ result from the interaction of the granule polyanionic matrix and two Ca2+-sensitive ion channels located in close proximity on the granular membrane: an ASKCa channel to mediate K+ flux into the granule, and an InsP3-R channel to release Ca2+ to the cytosol (Nguyen et al., 1998; Quesada et al., 2001). See text for further details.
Although the profusion of intracellular Ca2+-sensitive proteins explains the broad capacity of this cation to relay changes of functionality to intracellular sensor/effector molecules, it is insufficient to explain the specificity of the Ca2+ message. Here we show that InsP3 can turn the secretory vesicles of mast cells into a double ionic oscillator that broadcasts both Ca2+ and H+ signals, thereby constraining the granule's message exclusively to sensor/effector proteins that are sensitive to both Ca2+ and pH.
MATERIALS AND METHODS
Mast cell granule isolation and dye loading
Motion artifacts can be a critical problem when performing thin optical sections of secretory granules in intact cells. The advantages of the isolated mast cell granule preparation we used in these experiments are that, because of their large size, granules can be easily resolved by optical microscopy (Quesada et al., 2001; Monck et al., 1992), and they can be securely immobilized on poly-l-lysine coated glass. In these experiments, mast cells of beige mice (Bgj/Bgj) (Jackson Laboratory, Bar Harbor, ME) were isolated by peritoneal lavage (Quesada et al., 2001; Marszalek et al., 1997). Granules were labeled as previously described (Nguyen et al., 1998; Quesada et al., 2001). Briefly, cells were washed twice in a Ca2+-free Hanks' solution (pH = 7.2) and loaded for 30 min at 37°C with either 2 μM of LysoSensor Green DND-189 (LS) (pKa = 5.2; λexc = 443 nm, λem = 505 nm) to monitor pHG changes, or with 5 μM of Calcium Orange-5N (CO-5N) (Kd = 20 μM; λexc = 545 nm, λem = 580 nm) (Molecular Probes, Eugene, OR) for 45 min at 37°C, to monitor [Ca2+]IL (see Fig. 2). To remove any excess dye these two pools of cells were then washed and resuspended in an intracellular buffer solution containing 140 mM K+ glutamate, 20 mM HEPES, 5 mM MgSO4, 2 mM ATP, and 100 nM Ca2+ buffered with ethylene glycol bis(β-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), pH = 7.2. Secretory granules were extracted by sonication and separated by centrifugation at 10,000 rpm for 5 min. To detect extralumenal Ca2+ (Nguyen et al., 1998; Quesada et al., 2001; Belan et al., 1996), the granules were resuspended in intracellular buffer containing 10 μg ml−1 of low-diffusivity nonpermeant dextran-conjugated Calcium Green-1 (Kd = 190 nM; λexc = 506 nm, λem = 531 nm) or Calcium Crimson (Kd = 185 nM; λexc = 570 nm, λem = 610 nm). Changes of extralumenal pH were reported by 10 μg ml−1 of dextran-conjugated SNARF-1 (SN) (pKa = 7.5; λexc = 488 nm, λem = 587 nm) (Molecular Probes) diluted in intracellular buffer. Granule suspensions were then allowed to attach to poly-l-lysine-coated glass chambers for 5 min. The chambers were mounted and kept at 37°C on the thermoregulated stage of a Nikon inverted fluorescence microscope. Notice that our set-up allows detection of only one emission at a time. We can monitor two ions simultaneously if their fluorescent probes have similar spectral characteristics but are localized in different compartments. Our results report simultaneous measurements of fluctuations of intra- and extralumenal Ca2+, or intra- and extralumenal H+, or intralumenal H+ and extralumenal Ca2+. In all these cases we used probes that segregate in these two compartments.
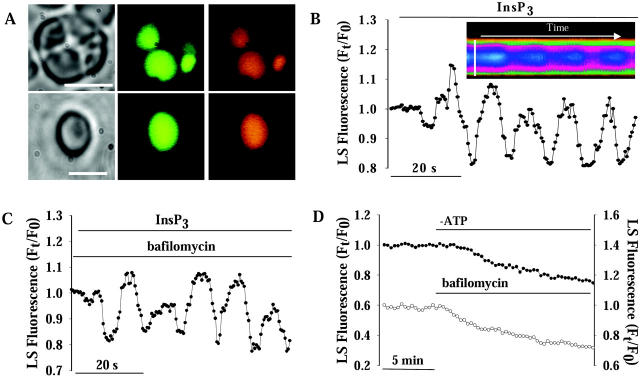
FIGURE 2.
InsP3-induced pHG oscillations. (A) Phase contrast and fluorescent images of intact mast cells (top panel) and isolated granules (bottom panel) loaded with the pH- and Ca2+-sensitive fluorescent probes LS (green) and CO-5N (orange), respectively. The large size of beige mast cell granules allows unequivocal intralumenal and extralumenal fluorescence measurements (Quesada et al., 2001). Scale bars: 5 μm. (B) Application of 3 μM InsP3 induced pHG oscillations of ∼0.1–0.12 Hz (n = 8). Notice that LS fluorescence increases with [H+], i.e., decreasing with pH. Inset displays a line-scan from a deconvoluted image of an isolated secretory granule (Nguyen et al., 1998; Quesada et al., 2001), showing intralumenal fluorescence changes resulting from pHG oscillations after exposure to InsP3. Scale bar: 3 μm. (C) InsP3-induced pHG oscillations were observed in isolated granules exposed to 0.5–1 μM bafilomycin (n = 5) or in the absence of ATP in the medium (not shown), ruling out the involvement of H+-ATPases in these oscillations. (D) Removal of ATP (filled circles, left axis; n = 3) or application of 500 nM bafilomycin (open circles; n = 5) caused pH alkalinization in isolated granules.
H+/K+ ion exchange
To investigate the H+/K+ ion exchange properties of the vesicular matrix, granules loaded with LS were equilibrated in ATP-free intracellular buffer containing heparin (100 μg ml−1) and apamin (100 nM). Under these conditions, resting [Ca2+]IL remains stable (∼25 μM), suggesting that the InsP3-R and the ASKCa channel were rendered inoperative (Nguyen et al., 1998; Quesada et al., 2001). To titrate the intralumenal [K+], the granules were exposed to the K+ ionophore valinomycin (20 μM) while [K+] in the intracellular buffer was increased from 0 to 140 mM. Ionic strength and osmolarity were kept constant by adjusting the concentration of monovalent organic cation NMG+.
Calibration of extralumenal pH
The pH/photon-count transfer function for SN emission was obtained by measuring the fluorescence in thin optical sections of solutions of SN similar to those used in experiments but in which the pH buffered with MES, HEPES, or Tris (20 mM) was progressively increased from 6 to 6.8, 7.2, 7.6, 8, and 9, yielding a pKa of 7.4.
Although the uncertainty of the quantum yield of LS in the intralumenal milieu prevented us from conducting absolute measurement of pH inside the granule, oscillations of intralumenal pH were readily reported by relative variations of LS photon count emission.
Optical sectioning
Granules were imaged with a Nikon Diaphot inverted fluorescence microscope using a 100 W mercury vapor epifluorescence source and a 100×, 1.4 NA oil-immersion objective. Images were formed on the 336 × 243 charge-coupled-device array of a thermoelectrically cooled, low dark noise (1.3 photoelectrons s−1 at −36°C) frame transfer digital camera with 16-bit resolution and 105 pixel s−1 maximum readout rate (Spectra Source Model 400, Westlake Village, CA). The camera was attached to the photoport of the microscope using a 20× relay lens, yielding a final resolution of 10 pixels μm−1. To avoid aliasing, we acquired three-line scans at a time, instead of the whole image, yielding a sampling rate of 3 scans s−1 with 300-ms exposure time and ∼25 samples/period of [Ca2+]IL or pHG oscillation. Scans sampled an area of 0.3 μm × 24 μm containing one or more granules and were accumulated in a memory buffer forming 50- to 60-s long sequential scan stacks (inset in Fig. 2 B). Optical sections of ∼0.2 μm for Ca2+ changes and extralumenal pH measurements and ∼2 μm for pHG were performed using a no-neighbors deconvolution algorithm (Nguyen et al., 1998; Quesada et al., 2001; Monck et al., 1992). Validation of the optical sectioning method has been published elsewhere (Nguyen et al., 1998; Quesada et al., 2001). The time course of average fluorescence intensity in photoelectron-counts per pixel s−1 inside and outside the secretory granules was measured from the line scans. Free [Ca2+] was calculated from the readouts of the line scans following published methods (Nguyen et al., 1998; Quesada et al., 2001; Kao, 1994).
RESULTS
InsP3 induces intralumenal pH oscillations in secretory granules
We performed experiments in isolated mast cell granules using pH-sensitive and Ca2+-sensitive fluorescent probes combined with digital sectioning (Nguyen et al., 1998; Quesada et al., 2001; Monck et al., 1992). Optical sections of isolated and in situ mast cell secretory granules loaded with the fluorescent probes LS and CO-5N are shown in Fig. 2 A. Isolated mast cell granules exposed to intracellular buffer containing 3 μM InsP3 exhibit periodic oscillations of pHG with a frequency of ∼0.12 Hz (Fig. 2 B). InsP3-induced pHG oscillations were blocked by exposure to intracellular buffer containing heparin (100 μg ml−1) (a blocker of InsP3-R channels) or apamin (100 nM) (a blocker of ASKCa channels) (Nguyen et al., 1998; Quesada et al., 2001), or by replacement of K+ by NMG+ (not shown), suggesting that activation of the InsP3-R and inflow of K+ into the granule are required for pHG oscillations. Notwithstanding the important role of H+ pumps in the control of pHG (Demaurex, 2002), removal of ATP from the intracellular solution or exposure of the granules to the H+ V-ATPase inhibitor bafilomycin (0.5 μM), failed to abolish the pHG oscillations, implying that they must result from a mechanism other than H+-pump activity (Fig. 2 C). However, in granules not treated with InsP3, the removal of ATP or exposure of isolated granules to bafilomycin (0.5 μM) resulted in intralumenal alkalinization (Fig. 2 D). This outcome is consistent with previous reports that secretory granules have a resting H+ permeability leading to continuous efflux of H+ to the cytosol (Demaurex, 2002; Wu et al., 2001). Replacement of K+ glutamate by equimolar concentrations of KCl rendered similar results (not shown).
H+/K+ exchange in the intralumenal matrix mediates pHG oscillations and oscillatory H+ efflux from the granule
The experimental validation that pHG oscillations can result from H+/K+ exchange was conducted in situ, in isolated granules loaded with LS, and in vitro, by titration of H+/K+ exchange in solutions of heparin. In valinomycin (20 μM) treated granules—in which both InsP3-R and ASKCa channels were blocked by heparin (100 μg ml−1) and apamin (100 nM), respectively—the increase of intralumenal K+ led to a concomitant acidification (Fig. 3). Heparin—the major constituent of the mast cell intralumenal matrix—had been shown to work as a histamine/K+ exchanger (Uvnas et al., 1989), and we found that it can function as a H+/K+ exchanger as well. In dilute solutions of heparin (6 mg ml−1), increasing [K+] decreased the pH (Fig. 3 B).
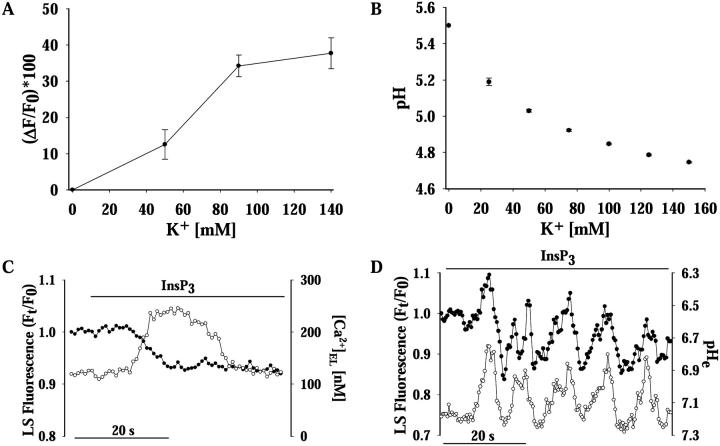
FIGURE 3.
Oscillatory efflux of H+ is driven by oscillations of ΔμH+ resulting from intralumenal H+/K+ exchange. (A) H+/K+ exchange was evaluated by equilibrating LS-loaded isolated granules in an intracellular medium containing 100 μg ml−1 heparin, 100 nM apamin to block InsP3–R and ASKCa channels, respectively, and 20 μM of the K+ ionophore valynomicin—to short-circuit the granular membrane to K+. Increasing the extralumenal [K+] led to corresponding increase of LS photon counts resulting from granule acidification (n = 6). Data are shown as the percentage of maximal fluorescence increase—in photon counts sec−1— with respect to the initial value. (B) Increasing the [K+] from 0 to 140 mM in a 6 mg ml−1 heparin solution results in an exponential decrease of pH (n = 6). (C) The time course of [H+]IL and [Ca2+]EL was studied in LS-loaded granules equilibrated in an intracellular medium containing 10 μg ml−1 of dextran-conjugated Calcium Green-1 and 100 nM apamin, to prevent K+ influx. InsP3-induced release of Ca2+ (open circles) was accompanied by a concomitant intralumenal alkalinization (filled circles; n = 4). (D) Intralumenal and extralumenal pH was simultaneously measured by equilibrating LS-loaded granules in an intracellular solution containing 10 μg ml−1 dextran-conjugated SN and 2 mM HEPES (pH = 7.2). SN fluorescence was captured at 587 nm. Addition of InsP3 (3 μM) led to extralumenal pH oscillations in the immediate vicinity of the granule (open circles, right axis). Notice that these extralumenal pH oscillations exhibit the same frequency (∼0.12 Hz) and are in phase with pHG changes (filled circles) (n = 6). In separate preparations in which granules were not loaded with LS, SN reported similar extralumenal pH oscillations in the perigranular space upon InsP3 application (not shown).
These observations suggest that K+ influx into the granule must drive both a Ca2+/K+ exchange process—responsible for [Ca2+]IL oscillations (Nguyen et al., 1998; Quesada et al., 2001)—and a H+/K+ exchange, that accounts for the periodic acidification of the granule during pHG oscillations (Fig. 1). The corresponding periodic alkalinization phases during pHG oscillations probably result from the release of Ca2+ through InsP3-R channels or from efflux of H+ from the granule. Since free Ca2+ and H+ are in equilibrium with their respective bound forms in the matrix, the release of Ca2+ through InsP3-R channels and the concomitant decrease of [Ca2+]IL may displace bound Ca2+ from the polyanionic network to restore the equilibrium with free Ca2+, leaving free negative sites which H+ could occupy, causing alkalinization. A similar competition for binding sites—in this case, cytosolic binding sites—between Ca2+ and H+ has been suggested to explain the formation of a secondary H+ signal in melanotrophs (Beatty et al., 1993). In fact, Fig. 3 C shows that InsP3–induced release of Ca2+ from granules in the presence of apamine, which prevents K+ influx, led to slight alkalinization. However, a more likely mechanism for intralumenal alkalinization is that the periodic increases of transmembrane pH gradient (ΔμH+) can result in higher efflux of H+, with periodic intralumenal alkalinization, and corresponding periodic acidification in the extralumenal side. This outcome is supported by our results and by several reports that have indicated that the major contributor to H+ export from the granule is an endogenous H+ permeability—or “leak”—driven by the transmembrane pH gradient (ΔμH+) (Demaurex, 2002; Wu et al., 2001; Schapiro and Grinstein, 2000; Farinas and Verkman, 1999). Although vesicular H+ “leakage” has been thought to probably take place via voltage-gated H+ channels (Demaurex, 2002; Schapiro and Grinstein, 2000), the specific mechanism of H+ efflux from the granule remains controversial (Wu et al., 2001; Schapiro and Grinstein, 2000). To test if secretory vesicles can produce extralumenal oscillations of pH, we equilibrated granules in an intracellular solution containing 10 μg ml−1 of dextran-conjugated SN, a nonpermeant, low diffusivity fluorescent pH sensor. When these granules were exposed to InsP3, the pH in the immediate periphery of the granule started to oscillate at the same frequency (∼0.12 Hz) and in phase with intralumenal pH oscillations (Fig. 3 D). Therefore, the intralumenal alkalinization we observed during pHG oscillations must result from H+ efflux to the cytosol.
Temporal relationship between intralumenal and extralumenal dynamics of Ca2+ and H+
To investigate the relationship between Ca2+ release from the granule and pHG, we equilibrated granules loaded with LS in an intracellular bathing solution (see Methods) containing 10 μg ml−1 of Calcium Crimson, a dextran-conjugated Ca2+ probe, to monitor [Ca2+]EL. The pH of the bathing solution was buffered at 7.2 by 40 mM of HEPES to prevent artifacts resulting from pH-dependent changes of quantum yield of Calcium Crimson. In agreement with previous results (Nguyen et al., 1998; Quesada et al., 2001), Fig. 4 shows that exposure of the granules to 3 μM InsP3 induced a train of [Ca2+]IL oscillations by triggering the release of Ca2+ with the corresponding rise of [Ca2+]EL and decrease of [Ca2+]IL. Similarly, InsP3 produced oscillations of [H+]IL of the same frequency but out of phase with the oscillations of [Ca2+]EL (Fig. 4 B), i.e., decreases of [H+]IL are accompanied by corresponding increases of [Ca2+] outside the granule. In isolated granules exposed to heparin (100 μg ml−1) and apamin (100 nM), [H+]IL was unaffected by raising the extralumenal [Ca2+] to 1 mM (not shown), ruling out the potential involvement of Ca2+/H+ exchangers on the granular membrane, in agreement with previous reports (Mitchell et al., 2001; Schapiro and Grinstein, 2000).
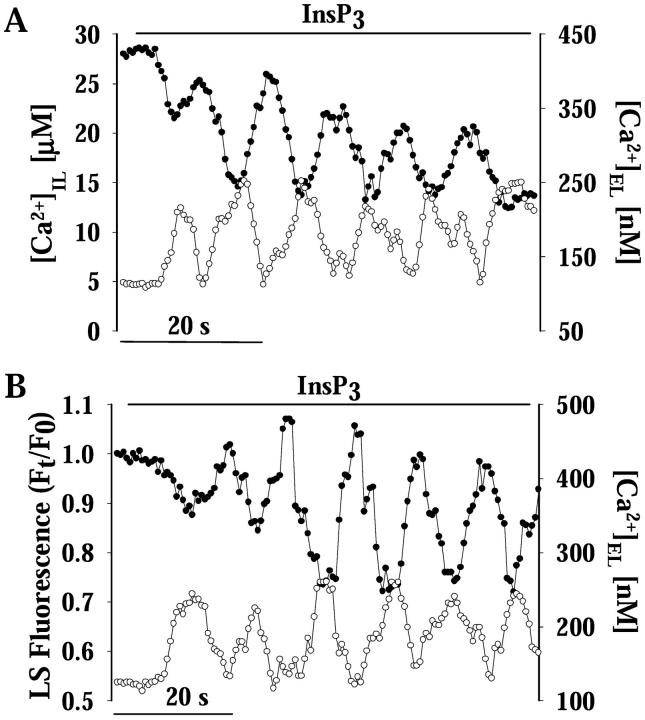
FIGURE 4.
Relationship between intralumenal and extralumenal H+ and Ca2+ oscillations. (A) The intralumenal and extralumenal changes of [Ca2+] were monitored in granules loaded with CO-5N and equilibrated in an intracellular solution containing 10 μg ml−1 Calcium Crimson. Application of 3 μM InsP3 provoked oscillations of [Ca2+]IL (filled circles, left axis) and [Ca2+]EL (open circles) of ∼0.12 Hz, which were ∼180° out of phase (n = 6). Periodic release of Ca2+ from the granules results in a corresponding increase of [Ca2+] outside the granule (Nguyen et al., 1998; Quesada et al., 2001). (B) Simultaneous monitoring of pHG and [Ca2+]EL was performed in granules loaded with LS and CG as in Fig. 3 C. InsP3 provoked oscillations of [H+]IL (filled circles, left axis) with the same frequency but ∼180° out of phase with the [Ca2+]EL oscillations (open circles; n = 6). The results in Fig. 3 D and Fig. 4, A and B, indicate that the release of Ca2+ and the efflux of H+ from the granule are 180° out of phase.
Notice that while the intralumenal and extralumenal oscillations of [Ca2+] are phase-shifted (Fig. 4 A), the oscillations of [H+]IL and [H+]EL are in phase (Fig. 3 D). To explain this outcome we need to consider that, although the intralumenal [Ca2+] and [H+] oscillations are both coupled to K+ influx, the oscillations of [Ca2+]IL are modulated by the open/close dynamics of both the InsP3–R and the ASKCa channels, while the oscillations of [H+]IL depend on the open/close dynamics of only the ASKCa channel and the leakage of this ion from the granule. In the case of Ca2+ (see model in Fig. 1), the InsP3-mediated Ca2+ efflux results in a transient decrease in [Ca2+]IL and an increase of [Ca2+]EL. The rise of [Ca2+]EL in the vicinity of the granule both closes the InsP3–R channel and turns on the ASKCa channel, activating the influx of K+ that results in Ca2+/K+ exchange and rebound of [Ca2+]IL. As Ca2+ around the granule dissipates by diffusion and buffering, the InsP3–R channel opens again and the cycle repeats for as long as the InsP3 remains bound to its receptor. In the case of H+ (see model in Fig. 1), the H+/K+ exchange in the matrix that increase [H+]IL steps in when ASKCa channels open and influx of K+ takes place. Since H+ efflux is driven by its intralumenal concentration, the oscillations of [H+] outside the granule are in phase with [H+]IL changes. During the closed time of the ASKCa channel, the H+/K+ exchange ceases but H+ still leaks out and [H+]IL decreases. An implication of these results is that the extralumenal [Ca2+] and [H+] must be out of phase. In addition, the rate of H+/K+ exchange from the heparin matrix must exceed the efflux of H+ leakage, otherwise efflux of H+ should result in increased [H+]EL but decreased [H+]IL. In the case of Ca2+, the conductance of the InsP3 channel in the open conformation must be higher than the rate of Ca2+ unbinding from the matrix as [Ca2+]IL rebounds only when the InsP3 channel closes and the influx of K+ exchanges for a new batch of Ca2+ from the matrix. We can also infer that oscillations of [Ca2+]IL and [H+]IL are probably in phase because: 1) [Ca2+]IL and [Ca2+]EL oscillations are out of phase, 2) [H+]IL and [H+]EL are in phase, and 3) [H+]IL and [Ca2+]EL are out phase (Fig. 4 A, Fig. 3 D, and Fig. 4 B, respectively).
Discussion
The polymer matrix found inside subcellular organelles—including the secretory granule—holds the answer to a highly significant set of questions in cell biology. From the polymer phase transition properties of the secretory matrix that allows the remarkable payload and efficient discharge of hormones and small molecules to the ion exchange properties of the intravesicular polymer networks, the granule offers one of the most elegant systems designed by evolution. The granule stores and releases material and signals its departure to the export machinery of the cell. Whereas the discovery of phase transitions of the granular matrix brought attention to storage and release in secretion (Verdugo, 1994; Marszalek et al., 1997), the study of the ion exchange properties of the matrix is shifting the focus to questions of signal transduction in secretory cells (Nguyen et al., 1998; Quesada et al., 2001). The H+ source/sink properties of the heparin matrix, and probably other secretory matrices, have a broad range of important implications, including pH regulation in subcellular organelles, phagosomal maturation, enzyme activation, protein packing, and sorting in the trans-Golgi network (Bell-Parikh et al., 2001; Reeves et al., 2002). However, the association of H+ release with the InsP3–induced Ca2+ signal from the granule, their oscillatory nature, and the presence of exocytic proteins sensitive to the joint action of Ca2+ and pH strongly suggest that Ca2+/H+ release from the secretory granule might encode a combined intracellular signal. According to our working model (Fig. 1), the activation of an extracellular receptor is relayed to the intracellular network by production of InsP3 (Berridge et al., 2000). The InsP3 signal is received by InsP3-R channels of nearby secretory granules, turning them into double ion oscillators that respond with two spatially and temporally constrained frequency-encoded signals of Ca2+ and H+. These oscillations are independent of ATP-mediated active uptake of Ca2+ or H+. Instead, they are brought about by the interaction of InsP3-R and ASKCa channels of the granule (Nguyen et al., 1998; Quesada et al., 2001; Gerasimenko et al., 1996; Yoo, 2000; Thevenod, 2002), with opposite gating sensitivities to Ca2+; the H+ “leakage” properties of the granular membrane (Demaurex, 2002; Wu et al., 2001); and the unique Ca2+/K+ and H+/K+ ion exchange properties of the heparin granular matrix (Uvnas and Aborg, 1977, 1989; Verdugo, 1994; Nguyen et al., 1998; Quesada et al., 2001; Nanavati and Fernandez, 1993; Marszalek et al., 1997; Chin et al., 2002).
In the space domain, the release of Ca2+ and H+ affects an exceedingly small cytosolic volume that probably scales to intermolecular distances not much farther than the local Debye potential field present in the cleft between plasma and granular membranes before membrane fusion. With these boundary conditions, diffusional distances become irrelevant, and the local concentration of Ca2+ and H+ in the cleft could very well mirror the intravesicular concentration of these ions. Because of the buffering properties of the cytosol, these signals should be time and space limited, reaching strictly confined domains in the cleft and preventing undesired cross talk with other receptor proteins not involved in membrane fusion.
In the time domain, the observed 0.1 Hz frequency of oscillation of Ca2+ and pH signals allows scanning of a broad range of cytosolic [Ca2+] and pH in 5-s periods. Diffusional delays are unlikely to occur because the sensor-effector proteins are already present in the cleft either in free form or anchored to the granule or plasma membranes (Sudhof, 1995), and the diffusion distance for Ca2+ and H+ to reach their targets across the cleft is extremely short. Thus, considering the typical μs-ms relaxation timescale of molecular conformational changes, effector proteins would have enough time to switch configuration (Subramaniam and Henderson, 2000; Rami and Udgaonkar, 2001). The preexocytic oscillations of Ca2+ and H+ in the narrow cleft existing between the two membranes exhibit broad overlapping. They scan a wide combination of concentrations of Ca2+ and H+ that could create multiple yet unique conditions, attuned to the specific optimal Ca2+/pH dependency of the different exocytic proteins, perhaps triggering their individual fusogenic properties in a well programmed sequence.
Several proteins implicated in exocytosis including calmodulin, syncollin, or Rab3a exhibit high interdependent sensitivity to Ca2+ and pH (An et al., 2000; Kiss and Korn, 1999; Kajio et al., 2001; Hudmon et al., 1996; Kennedy et al., 1983). The interaction of calmodulin with different substrates requires not only changes of pH and [Ca2+] but frequency-encoded signals of [Ca2+]C as well (De Koninck and Schulman, 1998). Protein kinase C is another protein involved in secretion that can also work as a decoder of oscillatory signals (Oancea and Meyer, 1998). However, the family of annexins gives the most striking case of combined Ca2+/pH dependence. These proteins are important mediators of exocytosis by means of their collective ability to fuse membranes in a Ca2+-dependent manner (Caohuy and Pollard, 2001; Konig et al., 1998). Remarkably, recent studies have demonstrated that the fusogenic efficiency of these proteins exhibits a critical sensitivity to pH, requiring an acidic environment of lower pH than the one found in the bulk cytosol. Depending on each specific annexin, different acidic pH values are required with slight variations of the synergy between Ca2+ and H+ (Langen et al., 1998; Isas et al., 2000; Caohuy and Pollard, 2002). Since the requirements of these proteins for both ions are much higher than those found in the bulk cytosol, several groups have proposed that membrane fusion induced by annexins is possible because of local signals that generate confined areas of high concentration of both Ca2+ and H+ (Langen et al., 1998; Isas et al., 2000; Caohuy and Pollard, 2002).
The present results are in agreement with observations in intact cells. Several groups have seen preexocytotic granular pH changes in pancreatic β-cells, mast cells, and neurons, postulating an active role of pH in priming granules for release (Williams and Webb, 2000; Barg et al., 2001; Han et al., 1999; Renstrom et al., 2002). The idea of a Ca2+/H+ signaling system is consistent with observations that both lumenal Ca2+ efflux and the maintenance of granular ΔμH+ are needed for vacuole and granule fusion (Peters and Mayer, 1998; Ungermann et al., 1999; Peters et al., 2001; Scheenen et al., 1998; Mundorf et al., 2000; Yang et al., 2002). Although the mechanisms of acidification remain uncertain, the idea that pH changes may facilitate secretion by affecting exocytotic proteins, making them more fusogenic, has also been considered (Barg et al., 2001, 2002; Yang et al., 2002; Renstrom et al., 2002).
The search for how specificity is encoded in intracellular signal transduction remains one of the most interesting and challenging topics in cell biology. Instances of built-in conditional arguments are present in the intracellular web of information (Beatty et al., 1993; Berridge et al., 2000; Susini et al., 2000). However, the formalization of simple principles of information theory in this field remains virtually unexplored. Although both Ca2+ and H+ can readily induce conformational changes, switching on/off functional conformations in proteins or other polyions present in the cell, the broad effect of these cations can decrease their specificity. The assignment of their combination in signaling could represent a heuristic model of Boolean conditional signaling whereby the granule can target a specific group of sensor/effector proteins involved in implementing exocytosis.
Acknowledgments
We thank Drs. Peter B. Detwiler, Albert M. Gordon, Thomas R. Hinds, and Garrett M. Odell.
This work was supported by a grant from the National Science Foundation Biocomplexity Program to P.V. and Florida State University First-Year Assistant Professor and Center Development Award to W.C.C. I.Q. is a recipient of a Spanish Ministry of Education and Culture postdoctoral fellowship.
Abbreviations used: InsP3, Inositol-1,4,5-trisphosphate; InsP3-R channel, InsP3-receptor channel; ASKCa channel, apamin-sensitive Ca2+-sensitive K+ channel; [Ca2+]IL, intralumenal Ca2+ concentration; [Ca2+]EL, extralumenal Ca2+ concentration; [H+]IL, intralumenal H+ concentration; [H+]EL, extralumenal H+ concentration.
References
- An, S. J., N. J. Hansen, A. Hodel, R. Jahn, and J. M. Edwardson. 2000. Analysis of the association of syncollin with the membrane of the pancreatic zymogen granule. J. Biol. Chem. 275:11306–11311. [DOI] [PubMed] [Google Scholar]
- Barg, S., L. Eliasson, E. Renstrom, and P. Rorsman. 2002. A subset of 50 secretory granules in close contact with L-type Ca2+ channels accounts for first-phase insulin secretion in mouse beta-cells. Diabetes. 51:S74–S82. [DOI] [PubMed] [Google Scholar]
- Barg, S., P. Huang, L. Eliasson, D. J. Nelson, S. Obermuller, P. Rorsman, F. Thevenod, and E. Renstrom. 2001. Priming of insulin granules for exocytosis by granular Cl− uptake and acidification. J. Cell Sci. 114:2145–2154. [DOI] [PubMed] [Google Scholar]
- Beatty, D. M., B. M. Chronwall, D. E. Howard, T. B. Wiegmann, and S. J. Morris. 1993. Calcium regulation of intracellular pH in pituitary intermediate lobe melanotropes. Endocrinology. 133:972–984. [DOI] [PubMed] [Google Scholar]
- Belan, P. V., O. V. Gerasimenko, D. Berry, E. Saftenku, O. H. Petersen, and A. V. Tepikin. 1996. A new technique for assessing the microscopic distribution of cellular calcium exit sites. Pflugers Arch. 433:200–208. [DOI] [PubMed] [Google Scholar]
- Bell-Parikh, L. C., B. A. Eipper, and R. E. Mains. 2001. Response of an integral granule membrane protein to changes in pH. J. Biol. Chem. 276:29854–29863. [DOI] [PubMed] [Google Scholar]
- Berridge, M. J., P. Lipp, and M. D. Bootman. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1:11–21. [DOI] [PubMed] [Google Scholar]
- Caohuy, H., and H. B. Pollard. 2001. Activation of annexin 7 by protein kinase C in vitro and in vivo. J. Biol. Chem. 276:12813–12821. [DOI] [PubMed] [Google Scholar]
- Caohuy, H., and H. B. Pollard. 2002. Protein kinase C and guanosine triphosphate combine to potentiate calcium-dependent membrane fusion driven by annexin 7. J. Biol. Chem. 277:25217–25225. [DOI] [PubMed] [Google Scholar]
- Chin, W. C., I. Quesada, T. Nguyen, and P. Verdugo. 2002. Oscillations of pH inside the secretory granule control the gain of Ca2+ release for signal transduction in goblet cell exocytosis. Novartis Found. Symp. 248:132–149. [PubMed] [Google Scholar]
- De Koninck, P., and H. Schulman. 1998. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 279:227–230. [DOI] [PubMed] [Google Scholar]
- Demaurex, N. 2002. pH homeostasis of cellular organelles. News Physiol. Sci. 17:1–5. [DOI] [PubMed] [Google Scholar]
- Farinas, J., and A. S. Verkman. 1999. Receptor-mediated targeting of fluorescent probes in living cells. J. Biol. Chem. 274:7603–7606. [DOI] [PubMed] [Google Scholar]
- Gerasimenko, O. V., J. V. Gerasimenko, P. V. Belan, and O. H. Petersen. 1996. Inositol trisphosphate and cyclic ADP-ribose-mediated release of Ca2+ from single isolated pancreatic zymogen granules. Cell. 84:473–480. [DOI] [PubMed] [Google Scholar]
- Han, W., D. Li, A. K. Stout, K. Takimoto, and E. S. Levitan. 1999. Ca2+-induced deprotonation of peptide hormones inside secretory vesicles in preparation for release. J. Neurosci. 19:900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon, A., J. Aronowski, S. J. Kolb, and M. N. Waxham. 1996. Inactivation and self-association of Ca2+/calmodulin-dependent protein kinase II during autophosphorylation. J. Biol. Chem. 271:8800–8808. [DOI] [PubMed] [Google Scholar]
- Isas, J. M., J. P. Cartailler, Y. Sokolov, D. R. Patel, R. Langen, H. Luecke, J. E. Hall, and H. T. Haigler. 2000. Annexins V and XII insert into bilayers at mildly acidic pH and form ion channels. Biochemistry. 39:3015–3022. [DOI] [PubMed] [Google Scholar]
- Kajio, H., S. Olszewski, P. J. Rosner, M. J. Donelan, K. F. Geoghegan, and C. J. Rhodes. 2001. A low-affinity Ca2+-dependent association of calmodulin with the Rab3A effector domain inversely correlates with insulin exocytosis. Diabetes. 50:2029–2039. [DOI] [PubMed] [Google Scholar]
- Kao, J. P. Y. 1994. Practical aspects of measuring [Ca2+] with fluorescent probes. In A Practical Guide to the Study of Calcium in Living Cells. R. Nucitelli, editor. Academic Press, San Diego. 155–181.
- Kennedy, M. B., T. McGuinness, and P. Greengard. 1983. A calcium/calmodulin-dependent protein kinase from mammalian brain that phosphorylates Synapsin I: partial purification and characterization. J. Neurosci. 3:818–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss, L., and S. J. Korn. 1999. Modulation of N-type Ca2+ channels by intracellular pH in chick sensory neurons. J. Neurophysiol. 81:1839–1847. [DOI] [PubMed] [Google Scholar]
- Konig, J., J. Prenen, B. Nilius, and V. Gerke. 1998. The annexin II-p11 complex is involved in regulated exocytosis in bovine pulmonary artery endothelial cells. J. Biol. Chem. 273:19679–19684. [DOI] [PubMed] [Google Scholar]
- Langen, R., J. M. Isas, W. L. Hubbell, and H. T. Haigler. 1998. A transmembrane form of annexin XII detected by site-directed spin labeling. Proc. Natl. Acad. Sci. USA. 95:14060–14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek, P. E., B. Farrell, P. Verdugo, and J. M. Fernandez. 1997. Kinetics of release of serotonin from isolated secretory granules. II. Ion exchange determines the diffusivity of serotonin. Biophys. J. 73:1169–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, K. J., P. Pinton, A. Varadi, C. Tacchetti, E. K. Ainscow, T. Pozzan, R. Rizzuto, and G. A. Rutter. 2001. Dense core secretory vesicles revealed as a dynamic Ca2+ store in neuroendocrine cells with a vesicle-associated membrane protein aequorin chimaera. J. Cell Biol. 155:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monck, J. R., A. F. Oberhauser, T. J. Keating, and J. M. Fernandez. 1992. Thin-section ratiometric Ca2+ images obtained by optical sectioning of fura-2 loaded mast cells. J. Cell Biol. 116:745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundorf, M. L., K. P. Troyer, S. E. Hochstetler, J. A. Near, and R. M. Wightman. 2000. Vesicular Ca2+ participates in the catalysis of exocytosis. J. Biol. Chem. 275:9136–9142. [DOI] [PubMed] [Google Scholar]
- Nanavati, C., and J. M. Fernandez. 1993. The secretory granule matrix: a fast-acting smart polymer. Science. 259:963–965. [DOI] [PubMed] [Google Scholar]
- Nguyen, T., W. C. Chin, and P. Verdugo. 1998. Role of Ca2+/K+ ion exchange in intracellular storage and release of Ca2+. Nature. 395:908–912. [DOI] [PubMed] [Google Scholar]
- Oancea, E., and T. Meyer. 1998. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell. 95:307–318. [DOI] [PubMed] [Google Scholar]
- Peters, C., M. J. Bayer, S. Buhler, J. S. Andersen, M. Mann, and A. Mayer. 2001. Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature. 409:581–588. [DOI] [PubMed] [Google Scholar]
- Peters, C., and A. Mayer. 1998. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 396:575–580. [DOI] [PubMed] [Google Scholar]
- Quesada, I., W. C. Chin, J. Steed, P. Campos-Bedolla, and P. Verdugo. 2001. Mouse mast cell secretory granules can function as intracellular ionic oscillators. Biophys. J. 80:2133–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rami, B. R., and J. B. Udgaonkar. 2001. pH-jump-induced folding and unfolding studies of barstar: evidence for multiple folding and unfolding pathways. Biochemistry. 40:15267–15279. [DOI] [PubMed] [Google Scholar]
- Reeves, E. P., H. Lu, H. L. Jacobs, C. G. Messina, S. Bolsover, G. Gabella, E. O. Potma, A. Warley, J. Roes, and A. W. Segal. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 416:291–297. [DOI] [PubMed] [Google Scholar]
- Renstrom, E., R. Ivarsson, and S. B. Shears. 2002. Inositol 3,4,5,6-tetrakisphosphate inhibits insulin granule acidification and fusogenic potential. J. Biol. Chem. 277:26717–26720. [DOI] [PubMed] [Google Scholar]
- Schapiro, F. B., and S. Grinstein. 2000. Determinants of the pH of the Golgi complex. J. Biol. Chem. 275:21025–21032. [DOI] [PubMed] [Google Scholar]
- Scheenen, W. J., C. B. Wollheim, T. Pozzan, and C. Fasolato. 1998. Ca2+ depletion from granules inhibits exocytosis. A study with insulin-secreting cells. J. Biol. Chem. 273:19002–19008. [DOI] [PubMed] [Google Scholar]
- Subramaniam, S., and R. Henderson. 2000. Crystallographic analysis of protein conformational changes in the bacteriorhodopsin photocycle. Biochim. Biophys. Acta. 1460:157–165. [DOI] [PubMed] [Google Scholar]
- Sudhof, T. 1995. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 375:645–653. [DOI] [PubMed] [Google Scholar]
- Susini, S., G. Van Haasteren, S. Li, M. Prentki, and W. Schlegel. 2000. Essentiality of intron control in the induction of c-fos by glucose and glucoincretin peptides in INS-1 beta-cells. FASEB J. 14:128–136. [PubMed] [Google Scholar]
- Thevenod, F. 2002. Ion channels in secretory granules of the pancreas and their role in exocytosis and release of secretory proteins. Am. J. Physiol. Cell Physiol. 283:C651–C672. [DOI] [PubMed] [Google Scholar]
- Ungermann, C., W. Wickner, and Z. Xu. 1999. Vacuole acidification is required for trans-SNARE pairing, LMA1 release, and homotypic fusion. Proc. Natl. Acad. Sci. USA. 96:11194–11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnas, B., and C. H. Aborg. 1977. On the cation exchanger properties of rat mast cell granules and their storage of histamine. Acta Physiol. Scand. 100:309–314. [DOI] [PubMed] [Google Scholar]
- Uvnas, B., and C. H. Aborg. 1989. Role of ion exchange in release of biogenic amines. News Physiol. Sci. 4:68–71. [Google Scholar]
- Uvnas, B., C. H. Aborg, L. Lyssarides, and L. G. Danielsson. 1989. Intracellular ion exchange between cytoplasmic potassium and granule histamine, an integrated link in the histamine release machinery of mast cells. Acta Physiol. Scand. 136:309–320. [DOI] [PubMed] [Google Scholar]
- Verdugo, P. 1994. Polymer gel phase transition in condensation-decondensation of secretory products. Advances in Polymer Science. 110:145–156. [Google Scholar]
- Williams, R. M., and W. W. Webb. 2000. Single granule pH cycling in antigen-induced mast cell secretion. J. Cell Sci. 113:3839–3850. [DOI] [PubMed] [Google Scholar]
- Wu, M. M., M. Grabe, S. Adams, R. Y. Tsien, H. P. Moore, and T. E. Machen. 2001. Mechanisms of pH regulation in the regulated secretory pathway. J. Biol. Chem. 276:33027–33035. [DOI] [PubMed] [Google Scholar]
- Yang, J., A. Hodel, and G. D. Holman. 2002. Insulin and isoproterenol have opposing roles in the maintenance of cytosol pH and optimal fusion of GLUT4 vesicles with the plasma membrane. J. Biol. Chem. 277:6559–6566. [DOI] [PubMed] [Google Scholar]
- Yoo, S. H. 2000. Coupling of the IP3 receptor/Ca2+ channel with Ca2+ storage proteins chromogranins A and B in secretory granules. Trends Neurosci. 23:424–428. [DOI] [PubMed] [Google Scholar]