Abstract
An approach is described using fluorescence resonance energy transfer (FRET) to detect inhomogeneity in lipid organization, on distance scales of the order of tens of nanometers or greater, in lipid bilayers. This approach compares the efficiency of energy transfer between two matched fluorescent lipid donors, differing in their affinities for ordered versus disordered regions of the bilayer, and an acceptor lipid that distributes preferentially into disordered regions. Inhomogeneities in bilayer organization, on spatial scales of tens of nanometers or greater, are detected as a marked difference in the efficiencies of quenching of fluorescence of the two donor species by the acceptor. Using a novel pair of 7-nitrobenz-2-oxa-1,3-diazol-4-yl (NBD)-labeled tetraacyl lipids as donor species with a rhodaminyl-labeled acceptor, this strategy faithfully reports homo- versus inhomogeneous mixing in each of several lipid bilayer systems whose organization on the FRET distance scale can be predicted from previous findings. Interestingly, however, the present FRET method reports clear evidence of inhomogeneity in the organization of mixtures combining sphingomyelin or saturated phospholipids with unsaturated phospholipids and physiological proportions of cholesterol, even at physiological temperatures where these systems have been reported to appear homogeneous by fluorescence microscopy. These results indicate that under physiological conditions, lipid mixtures mimicking the lipid composition of the outer leaflet of the plasma membrane can form domains on a spatial scale comparable to that inferred for the dimensions of lipid rafts in biological membranes.
INTRODUCTION
The plasma membrane, and at least some other membranes, of animal and fungal cells exhibit segregation of cholesterol- and sphingolipid-enriched domains (e.g., lipid rafts and caveolae) whose distinctness from other regions of the membrane appears to support important aspects of membrane function (for reviews see Simons and Ikonen, 1997; Anderson, 1998; Brown and London, 1998; Simons and Toomre, 2000; Kurzchalia and Parton, 1999; Anderson and Jacobson, 2002). Studies using model membranes have suggested that a central element in the formation of lipid rafts and related membrane microdomains is the tendency of sphingolipids, which carry mainly saturated acyl chains, to demix from unsaturated membrane phospholipids in the presence of cholesterol (Schroeder et al., 1994; Ahmed et al., 1997; Brown, 1998; Rietveld and Simons, 1998; Xu et al., 2001). Consistent with this observation, reduction of plasma membrane cholesterol levels in mammalian cells appears to perturb many aspects of raft organization and function (Furuchi and Anderson, 1998; Pike and Miller, 1998; Sheets et al., 1999; Roy et al., 1999; Ushio-Fukai et al., 2001; Pike and Casey, 2002; Matveev and Smart, 2002; Smart and Anderson, 2002).
Fluorescence measurements have previously been used to obtain evidence for an inhomogeneous lateral organization of lipids on different spatial scales in ternary mixtures combining cholesterol, phospholipids with low gel-to-liquid crystalline transition temperatures, and high-melting phospho- or sphingolipids. Fluorescence-microscopic studies (Dietrich et al., 2001a,b; Samsonov et al., 2001; Feigenson and Buboltz, 2001; Veatch and Keller, 2002, 2003) have revealed segregation of fluid-state domains of micron dimensions under certain conditions in vesicles with such compositions. However, in systems of this type that incorporate levels of cholesterol comparable to those found in mammalian cell plasma membranes, microscopically visible domains are typically not observed at physiological temperatures (Dietrich et al., 2001a; Veatch and Keller, 2002, 2003). Measurements of quenching of the fluorescence of membrane-incorporated probes by spin-labeled or brominated lipids (Silvius, 1992; Silvius et al., 1996; Ahmed et al., 1997; Wang and Silvius, 2000, 2001, 2003; Wang et al., 2000, 2001; Xu and London, 2000; Xu et al., 2001; London, 2002) have however indicated that even at physiological temperatures, similar systems exhibit an inhomogeneous lateral organization of lipids on a distance scale of the order of nearest-neighbor separations (≈1 nm).
In principle, fluorescence could be used to monitor inhomogeneity in the organization of bilayers on an intermediate scale of distances (≈tens of nanometers) through measurements of fluorescence resonance energy transfer (FRET). This possibility is attractive because lipid “raft” microdomains in biological membranes appear to have dimensions as small as a few tens of nanometers (Pralle et al., 2000; Varma and Mayor, 1998), and cholesterol-rich clusters with slightly smaller dimensions have also been proposed to exist in biological membranes (Radhakrishnan et al., 2000; Anderson and Jacobson, 2002). FRET has in fact been successfully employed to monitor lipid phase separations in binary mixtures of phospholipids (Pedersen et al., 1996; Stillwell et al., 2000; Leidy et al., 2001). However, to date FRET measurements have seen only very limited use to monitor the lateral organization of lipids in cholesterol-containing systems (Feigenson and Buboltz, 2001). This reflects at least in part the fact that it has proven difficult to design fluorescence probes that report consistently and in a straightforward manner the occurrence of inhomogeneity in the lateral organization of lipids in such systems (Stillwell et al., 2000).
This report describes the identification and use of new combinations of fluorescent probes that allow FRET-based detection of inhomogeneity in lipid lateral organization in a variety of cholesterol-containing systems. This approach reliably reports inhomogeneities in the lateral distributions of lipids in cholesterol-containing systems that have previously been shown by microscopy to exhibit large-scale segregation of fluid domains. However, the FRET assay also indicates that in such systems inhomogeneity in lipid distributions, on a spatial scale of the order of that reported for raft domains in biological membranes (tens of nanometers or greater), persists at physiological temperatures and cholesterol contents where large (micron-size) segregated domains have not been observed.
MATERIALS AND METHODS
Materials
Synthetic phospholipids, bovine brain sphingomyelin, and cholesterol were purchased from Avanti Polar Lipids (Alabaster, AL). 7-Nitrobenz-2-oxa-1,3-diazol-4-yl- (NBD-), lissamine rhodamine B sulfonyl- (Rho-), bimanylthioacetyl- (Bimta-), and fluorescein-labeled phosphatidylethanolamines (PEs) were synthesized as described previously (Monti et al., 1978; Struck et al., 1981; Wang et al., 2000). 1,2-Dioleoylphosphatidyl-N,N-dimethyl-N-(2,2,6,6-tetramethyl-y-piperidinyl)-ethanolamine (TEMPO-DOPC) and the bimane-labeled bis(phosphatidylethanolamido)mercaptosuccinyl conjugates DOBDO and DSBDS (Fig. 1) were also synthesized as discussed elsewhere (Wang et al., 2000). N-(7-dimethylaminocoumarinyl-4-acetyl)- (DMCA-) and fluorescein-labeled PEs were synthesized by reacting 5 μmol PE with 10 μmol each of triethylamine and either 7-dimethylaminocoumarin-4-acetic acid succinimidyl ester or 5-(and 6-)carboxyfluorescein succinimidyl ester in 0.25 ml 9:1 CH2Cl2 for 3 h at 25°C. The products of these reactions were purified by preparative thin-layer chromatography on silica gel 60 plates developed in 50:15:5:5:2 CH2Cl2/acetone/methanol/acetic acid/H2O.
FIGURE 1.
Structures of the tetraacyl NBD- and bimanyl-labeled phospholipid conjugates examined as potential FRET donor species in this study.
The NBD-labeled phosphatidylserine-phosphatidylethanolamine conjugates shown in Fig. 1 (DONDO and DSNDS) were synthesized as follows. Dioleoyl or distearoyl phosphatidylserine (PS) was converted to its N-t-butoxycarbonyl (Boc) derivative by reaction with (2-tert-butoxycarbonyloxyimino)-2-phenylacetonitrile in dry methylene chloride/triethylamine (99:1) for 6 h at 22°C (for dioleoyl PS) or 40°C (for distearoyl PS), respectively. After purification by preparative thin-layer chromatography (TLC) on silica gel 60 plates, developed in 50:12:4:4:1 CH2Cl2/acetone/methanol/acetic acid/H2O, the Boc-PS products were coupled to phosphatidylethanolamines with the same acyl chains in 3:1 dry CH2Cl2/dry dimethylformamide in the presence of 5, 5, 80, and 30 equivalents, respectively, of dicyclohexylcarbodiimide, N-hydroxysuccinimide, diisopropylethylamine, and trifluoroacetic acid (TFA). After incubation for 6 h at 25°C or 45°C, respectively, for the oleoyl- and stearoyl-substituted species, the reaction mixtures were partitioned between CH2Cl2 and 1:1 methanol/0.1 M aqueous HCOONa, pH 3, and the lower phases were washed twice with the same mixture of methanol and aqueous HCOONa and dried, first under N2 and then under high vacuum to remove residual dimethylformamide. The Boc-protected conjugates were purified by preparative TLC in 50:12:4:4:1 CH2Cl2/acetone/methanol/acetic acid/H2O, then N-deprotected by incubating in 50:50:3 (v/v/v) TFA/CH2Cl2/dimethylsulfide for 2 h at 0°C, adding the incubation mixture to a chilled mixture of CH2Cl2 and triethylamine (in quantities sufficient to neutralize the trifluoroacetic acid) and recovering by partitioning between CH2Cl2 and 1:1 methanol/0.1 M HCOONa as described above. The N-deprotected conjugates were finally labeled with NBD-fluoride (in 90:10:1 CH2Cl2/methanol/triethylamine, for 30 min at 20°C) and purified by preparative TLC as described for the corresponding Boc-protected conjugates.
Fluorescence assay of gel/fluid phase partitioning of lipid probes
Lipid samples (25 nmol total phospholipid, including 0.5 mol% fluorescent lipid) were prepared in 2:1 (v/v) CH2Cl2/CH3OH, then dried down under nitrogen with warming to 50–55°C. After further drying under high vacuum for 3–6 h, the mixtures were dispersed by vortexing at 55°C in 3 ml buffer (100 mM KCl, 10 mM 3-(N-morpholino)propanesulfonic acid, 1 mM ethylenediaminetetraacetic acid, pH 7.0). Samples were then sealed under argon, slowly cooled (at <0.2°C/min) from 55°C to 20°C, and incubated at this temperature for 96 h. Sample fluorescence was read at the latter temperature in a Perkin-Elmer LS-5 spectrofluorometer with a thermostatted cuvette holder, using the following excitation and emission wavelengths (slitwidths): 390/468 nm (10/10 nm) for bimane-labeled lipids, 400/460 nm (10/10 nm) for DMCA-labeled lipids, 470/538 nm (10/10 nm) for NBD-labeled lipids, 494/520 nm (5/5 nm) for fluorescein-labeled lipids, 525/596 nm (10/10 nm) for rhodamine-labeled lipids, and 517/563 nm (5/5 nm) for DiIC16(3) and DiIC18(3). After this initial fluorescence reading the samples were mixed with 150 μl 20% Triton X-100, heated to 55–60°C for 15 min, vortexed and bath-sonicated for 10 s each, and finally cooled to 20°C before remeasuring the fluorescence. Normalized fluorescence values FN were calculated by dividing the fluorescence of each sample by the fluorescence measured after Triton solubilization, with suitable blank corrections in each case. Similar results were obtained for vesicles prepared by ethanol dilution as described in the following section.
The relative affinities of fluorescent lipids for the gel versus the fluid phase in dipalmitoylphosphatidylcholine (DPPC)/TEMPO-DOPC bilayers at 20°C were determined by fitting the quenching curves (plots of normalized fluorescence versus the molar percentage of TEMPO-DOPC) within the region of phase separation to the equation
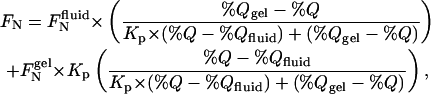 |
(1) |
where %Q is the molar percentage of TEMPO-DOPC in the bilayer, %Qgel and %Qfluid define the limiting compositions for the region of phase separation (roughly 5 mol% and 65 mol% TEMPO-DOPC, respectively), FNgel and FNfluid are the (fitted) values of FN for molar percentages of quencher equal to %Qgel and %Qfluid, respectively, and Kp is the gel/fluid phase partition coefficient.
FRET measurements of inhomogeneity in lipid bilayers
Dried cholesterol/phospholipid mixtures (25 nmol/sample) containing 0.3 mol% Rho-diphytanoyl PE and 0.5 mol% of either DSNDS or DONDO were prepared as described above. To compare optimally the fluorescence of DONDO and DSNDS in vesicles of a given composition, a quantity of lipids (including Rho-PE) sufficient to prepare six samples of a given composition, but without any NBD-labeled probe, was first mixed in solvent. Replicate aliquots of 25 nmol lipid were then dispensed from this mixture, adding DONDO to three of the samples and DSNDS to the others before drying. The dried lipid mixtures were redissolved at 55°C in 30 μl ethanol, then rapidly mixed (by vortexing) with 3 ml buffer prewarmed to 55°C. Samples were then sealed under argon, slowly cooled (at <0.2°C/min) from 55°C to the desired experimental temperature, and finally incubated at the latter temperature for 24 h (for samples examined at 37°C) or 48 h (for samples analyzed at lower temperatures). Vesicles prepared in this manner from DOPC/sphingomyelin/cholesterol mixtures (in varying proportions) and labeled with NBD-dioleoyl PE (1 mol%) typically showed a rapid quenching of fluorescence by 15–35% upon addition of the impermeant reducing agent sodium dithionite (5 mM). Since dithionite rapidly reduces (and hence quenches) only NBD-labeled lipid molecules exposed at the extravesicular surface (McIntyre and Sleight, 1991), these preparations thus appear to contain predominantly vesicles comprising from one to a few lamellae.
The ethanol-dilution method just outlined was used for the FRET experiments presented in this study to promote homogeneous incorporation of cholesterol into vesicles with even relatively high sterol contents (Feigenson and Buboltz, 2001). In a limited number of experiments (not shown), FRET measurements were carried out using vesicles (containing ≤33 mol% cholesterol) that were prepared by vortexing dried lipid films into buffer as described in the previous section. The results of these latter experiments were qualitatively very consistent with those obtained using vesicles prepared by ethanol dilution.
The normalized fluorescence of samples doubly labeled with DSNDS or DONDO and Rho-diphytanyol PE, prepared as just outlined, was determined from readings taken before and after Triton solubilization as described above. Normalized fluorescence values were determined similarly for control samples prepared without Rho-PE. The corrected fluorescence ratio (Rflcor) of DSNDS versus DONDO probes in bilayers of a given composition was determined from these data as
 |
(2) |
where the samples designated +Acc and −Acc are those containing the indicated donor species with or without acceptor, respectively. In most cases the normalized fluorescence values measured for DONDO versus DSNDS agreed to within 2–4% in bilayers of a given composition (the only significant exception is illustrated in the inset to Fig. 8 B, where for some compositions discrepancies of up to 7–8% were observed). FRET experiments were repeated from two to five times for each system examined; data shown are from representative experiments in each case.
FIGURE 8.
Corrected fluorescence ratios Rflcor (mean ± SD determined for triplicate sets of samples) measured for DONDO versus DSNDS in bilayers combining cholesterol with DOPC and phosphatidylcholines with higher gel/fluid transition temperatures. For each sample the content of DOPC is specified as its molar percentage in the total phospholipid fraction, i.e., as (100% × (moles DOPC)/(moles total phosphatidylcholine)). (A) Samples combining DPPC and DOPC with 33 mol% or (inset) 50 mol% cholesterol at 37°C. (B) Samples combining DMPC and DOPC with 33 mol% cholesterol at 20°C; (B, inset) identical samples prepared without the rhodaminyl-PE acceptor, showing the ratio of the normalized fluorescence values measured for DONDO and DSNDS. (C) Samples combining DMPC and DOPC with 50 mol% cholesterol or (inset) 0 mol% cholesterol at 37°C. (D) Samples combining DOPC and SOPC with 33 mol% cholesterol or (inset) DOPC and SOPC alone at 37°C.
RESULTS
The general strategy that was explored to monitor inhomogeneity of lipid distributions by FRET in cholesterol-containing systems is illustrated in Fig. 2. A matched pair of fluorescent donor lipids is utilized, differing only in the donor acyl chains, such that one donor species distributes with high selectivity into disordered domains within the bilayer whereas the second shows substantial affinity for ordered domains. These donor species are combined, in bilayers of a given composition, with an energy-transfer acceptor that partitions with high selectivity into disordered domains. In homogeneous lipid bilayers containing a given concentration of the acceptor species, quenching of the fluorescence of either donor species by the acceptor will be equally efficient, and the corrected fluorescence ratio Rflcor defined in Eq. 2 will have a value of unity. By contrast, in bilayers exhibiting inhomogeneous lateral distributions of the lipid species (on a spatial scale of tens of nanometers or greater), such that regions of greater and lesser lipid order coexist, the (disordered domain-preferring) acceptor will quench more efficiently the fluorescence of the donor species that prefers disordered domains. In this case the corrected fluorescence ratio Rflcor will show a value significantly less than unity.
FIGURE 2.

General strategy explored in this study to detect lateral inhomogeneity in lipid bilayer organization. An acceptor probe A, which shows a strong preference for less ordered regions of the bilayer, is combined in replicate samples with either of two matched donor species, D1 and D2. D1 and D2 are identical save for their acyl chains, which for probe D1 confer a strong preference for less ordered domains but for probe D2 confer a substantial affinity for more ordered regions of the bilayer. In bilayers where regions of greater and lesser order coexist, the donor species D1 and the acceptor species A will be coenriched in the less ordered regions of the bilayer (panel A). By contrast, a significant fraction of the donor species D2 will distribute into more ordered regions of the bilayer where the local concentration of acceptor species is very low (panel B). Heterogeneity in the lateral organization of the bilayer (specifically, the existence of more and less ordered regions with dimensions of the order of the Förster length Ro or greater) can thus be detected by comparing the relative efficiencies of energy transfer in replicate samples combining the acceptor species with donor D1 versus D2.
Successful exploitation of the above strategy requires first that the two matched donor species exhibit strongly differing affinities for ordered versus disordered lipid domains, and second that the acceptor species partitions with high selectivity into disordered domains. Initial experiments were thus undertaken to identify fluorescent-labeled lipids that fulfill these criteria.
Relative affinities of different fluorescent lipids for gel-state lipid domains
A key requirement of the above strategy is the use of paired fluorescent donor lipids, (only) one of which partitions with significant affinity into ordered lipid domains. To identify fluorescent-labeled lipids that can fulfill the latter criterion, the fluorescence-quenching approach developed by Feigenson and colleagues (London and Feigenson, 1981; Huang et al., 1988) was used to measure the affinities of various fluorescent molecules for gel versus fluid domains in phase-separated binary lipid mixtures. In this approach the normalized fluorescence for a given fluorescent species is measured in bilayers combining varying molar proportions of a gel phase-forming lipid (here, DPPC) and a fluid phase-forming quencher lipid (here, TEMPO-DOPC) that phase-separates from the saturated species at the experimental temperature. Examples of quenching curves thereby obtained are shown in Fig. 3 A. Quenching curves that show upward (/downward) concavity over the region of phase separation (ranging from ∼5 to 65 mol% TEMPO-DOPC at the experimental temperature of 20°C) indicate preferential partitioning into fluid- (/gel-) phase regions of the bilayer. As discussed previously (London and Feigenson, 1981; Huang et al., 1988) and as illustrated in the inset to Fig. 3 A, by fitting such experimental quenching curves in the region of phase separation, it is possible to determine the gel/fluid phase partition coefficient (Kp(gel/fluid)) for various fluorescent lipids.
FIGURE 3.
(A) Quenching curves showing the variation of the normalized fluorescence FN (defined in Eq. 1) with the molar percentage of TEMPO-DOPC in DPPC/TEMPO-DOPC bilayers at 20°C for (▴) DSNDS, (♦) NBD-distearoyl PE, and (○) DiIC18(3). Samples were prepared and incubated, and fluorescence readings taken subsequently, as described in Materials and Methods. Data shown are from a single representative experiment. (A, inset) Fits of the data from panel A to Eq. 1 to determine the gel/fluid phase partition coefficient Kp for the different fluorescent lipids. To facilitate comparison the fitted curves have been scaled to values of unity and zero, respectively, at the right- and left-hand boundaries of the region of phase separation (estimated respectively as 5 mol% and 65% TEMPO-DOPC). (B) Quenching curves for (▴) DSNDS, (♦) NBD-distearoyl PE, and (○) DiIC18(3) in sphingomyelin/TEMPO-DOPC/(33 mol% cholesterol) bilayers at 20°C. To facilitate comparison the quenching curves have been normalized to a value of unity for bilayers containing 0 mol% TEMPO-DOPC. The x axis represents the molar percentage of TEMPO-DOPC in the phospholipid fraction. Other experimental conditions were as for panel A.
In Table 1 are listed the values of Kp(gel/fluid) determined as just described for a variety of fluorescent lipids in the DPPC/TEMPO-DOPC system. A variety of headgroup-labeled fluorescent lipids and lipid analogs bearing two saturated 18-carbon chains, including bimane-, fluorescein-, NBD-, and rhodaminyl-PE, and indocyanine probes, partition preferentially into the fluid phase in this system. Probes with saturated 16-carbon chains show significantly lower affinity for gel-state domains, in agreement with previous observations (Spink et al., 1990; Wang et al., 2000). Increasing the acyl chain length of NBD-PE from di-18:0 to di-20:0 does not however enhance further affinity for the gel state (Table 1). This result is consistent with other findings that partitioning of lipid probes into ordered domains does not increase monotonically with increasing chain length (Spink et al., 1990; Wang et al., 2000). However, lipid conjugates bearing four stearoyl chains (DSNDS and DSBDS, with the general structures shown in Fig. 1) show a significantly greater affinity for the gel phase than do distearoyl species bearing the same fluorescent group (Table 1). Similar results were obtained using sphingomyelin/TEMPO-DOPC mixtures at 20°C (not shown). The tetrastearoyl lipid conjugates also appear to show a significant affinity for ordered domains in mixtures combining DOPC and sphingomyelin with 33 mol% cholesterol, as illustrated in Fig. 3 B (compare the almost zero concavity of the quenching curve for the DSNDS probe to the marked upward concavity of the quenching curves for NBD-distearoyl PE and DiIC18(3)). In contrast to the behavior of DSNDS, as expected the NBD-labeled tetraunsaturated species DONDO (structure shown in Fig. 1) is effectively excluded from the gel phase in these systems, as illustrated by the data in Table 1. The branched-chain species Rho-diphytanoyl PE also showed a very high preference for diphytanoyl fluid (liquid-disordered) over gel-phase domains (Table 1).
TABLE 1.
Values of gel/fluid phase partition coefficients (Kp) for fluorescent lipids in DPPC/TEMPO-DOPC bilayers at 20°C
| Fluorescent lipid | Kp* |
|---|---|
| Rho-distearoyl PE† | 0.25 ± 0.09 |
| DMCA-distearoyl PE† | 0.60 ± 0.25 |
| Fluoresceinyl-distearoyl PE† | 0.65 ± 0.05 |
| Bimta-distearoyl PE† | 0.65 ± 0.17 |
| NBD-dipalmitoyl PE† | 0.39 ± 0.04 |
| NBD-distearoyl PE† | 0.68 ± 0.08 |
| NBD-diarachidoyl PE† | 0.58 ± 0.09 |
| DiIC18(3) | 0.71 ± 0.19 |
| DiIC16(3) | 0.32 ± 0.07 |
| DSNDS | 1.75 ± 0.06 |
| DSBDS | 1.47 ± 0.36 |
| Rho-diphytanoyl PE† | 0.041 ± 0.006 |
| DONDO | 0.033 ± 0.011 |
Values of Kp were determined by fitting quenching curves to Eq. 1 as illustrated in Fig. 2 A and as described in the text. Values indicated represent the mean (± SD) of determinations in three independent experiments.
Table abbreviations: PE, phosphatidylethanolamine; Rho-PE, N-(lissamine rhodaminesulfonyl B)-PE; DMCA-PE, N-(7-dimethylaminocoumarinyl-4-acetyl)-PE; Bimta-PE, N-(bimanylthioacetyl)-PE; NBD-PE, N-(7-nitro-benz-2-oxa-1,3-diazol-4-yl)-PE. The structures of the tetraacyl species DONDO, DSNDS, and DSBDS are indicated in Fig. 1.
FRET measurements of inhomogeneity in cholesterol-containing bilayers
Based on the findings presented above, further experiments explored the use of the matched donor species DONDO/DSNDS and the acceptor Rho-diphytanoyl PE to probe for inhomogeneity of lipid distributions in mixed-lipid bilayers. As illustrated in Fig. 4, in homogeneous DOPC bilayers at 37°C the fluorescence of the two donor species is quenched with identical efficiency by the rhodamine-labeled acceptor. The same is true for these donor species in 2:1 (mol/mol) DOPC/cholesterol bilayers (Fig. 4, inset). Using the analysis described by Wolber and Hudson (1979), an apparent Förster energy-transfer length Ro of 69–71 Å was calculated for these donor/acceptor pairs on the assumption that energy transfer occurs between fluorescent lipids in the same bilayer leaflet. Considering potential quenching contributions from the trans leaflet of the bilayer (Fung and Stryer, 1978; Wolber and Hudson, 1979), the true value of Ro is estimated to be ∼60 Å for the combination of DONDO or DSNDS with Rho-diphytanoyl PE. This is similar to the Ro value of ∼56 Å estimated by Connor and Schroit (1987) for the combination of an NBD-labeled phosphatidylcholine and a rhodaminyl-labeled phosphatidylethanolamine in DOPC vesicles.
FIGURE 4.
Quenching of fluorescence of (•) DONDO and (○) DSNDS at 37°C in DOPC bilayers containing the indicated molar percentages of Rho-diphytanoyl PE. (Inset) Quenching of the fluorescence of the same species in 2:1 (mol/mol) DOPC/cholesterol bilayers at 37°C. Quenching curves have been scaled to a value of unity at 0 mol% Rho-PE; the unscaled quenching curves were likewise superimposable for DONDO and DSNDS in DOPC bilayers but were slightly discrepant (by roughly 2% at 0 mol% Rho-PE) in DOPC/cholesterol bilayers. Other experimental data were as for Fig. 2.
DONDO and DSNDS were used in combination with Rho-diphytanoyl PE to examine the homo- or inhomogeneity of lipid mixing at 37°C in bilayers combining brain sphingomyelin, dipentadecanoyl PC, or DOPC with increasing proportions of cholesterol. Lipid samples of a given composition were prepared containing 0.5 mol% of either DONDO or DSNDS together with either 0 or 0.3 mol% Rho-diphytanoyl PE. The normalized fluorescence intensities measured for these samples were used to calculate the corrected ratio of DONDO to DSNDS fluorescence (Rflcor) as described in Materials and Methods and defined in Eq. 2. As shown in Fig. 5 A, in sphingomyelin/cholesterol bilayers at 37°C, the value of Rflcor progressively decreases from unity as the proportion of cholesterol increases up to 30–35 mol%, then gradually increases again as the proportion of cholesterol increases further. Similar behavior was observed at 37°C (not shown) for mixtures of cholesterol and dipentadecanoyl PC (used in place of DPPC because it forms a fluid phase at 37°C even without cholesterol). This behavior indicates that cholesterol promotes an inhomogeneous lateral organization in bilayers in which it is combined with sphingomyelin or saturated PCs above their phase transition temperature, in agreement with the conclusions of previous studies using other experimental approaches (Vist and Davis, 1990; Huang et al., 1993; Sankaram and Thompson, 1990, 1991; McMullen and McElhaney, 1995). It appears that at least on a spatial scale of tens of nanometers, cholesterol induces a laterally inhomogeneous distribution of lipids in these systems over a wide range of sterol concentrations. By contrast, the value of the corrected fluorescence ratio does not vary significantly from unity in bilayers combining DOPC with varying proportions of cholesterol (Fig. 5 B).
FIGURE 5.
Corrected fluorescence ratios Rflcor, defined as in Eq. 2, measured for DONDO versus DSNDS at 37°C in bilayers combining brain sphingomyelin (A) or DOPC (B) with the indicated molar percentages of cholesterol. Rflcor values shown (mean ± SD) were determined for triplicate sets of samples, prepared with or without the Rho-diphytanoyl PE acceptor (0.3 mol%) and containing 0.5 mol% of either DONDO or DSNDS, as described in Materials and Methods.
Experiments illustrated in Fig. 6 were carried out to examine the homo- or inhomogeneity of lipid mixing in sphingomyelin/DOPC/cholesterol mixtures as a function of temperature and composition. As shown in Fig. 6 A, at 20°C in bilayers combining 33 mol% cholesterol with brain sphingomyelin and DOPC in varying relative proportions, the value of the corrected fluorescence ratio is substantially less than unity over a wide range of sphingomyelin contents (Fig. 6 A). At 30°C or 37°C the value of Rflcor is also substantially less than unity over a wide range of bilayer contents of sphingomyelin relative to DOPC (Fig. 6, B and C), although the magnitude of this divergence gradually decreases with increasing temperature. Previous fluorescence-microscopic experiments have shown that mixtures containing equimolar proportions of these three lipids show segregation of micron-size liquid domains at temperatures below ∼25°C but appear homogeneous at higher temperatures (Dietrich et al., 2001a; Veatch and Keller, 2003). By contrast, the present results indicate that the lateral organization of lipids in such mixtures remains markedly inhomogeneous on the finer scale of distances sampled by FRET, even at physiological temperatures. Raising the proportion of cholesterol to 50 mol% reduces but does not abolish the deviation of the corrected fluorescence ratio from unity in sphingomyelin/DOPC mixtures, even at 37°C (Fig. 6 D). By contrast, for cholesterol-free mixtures of sphingomyelin and DOPC at 37°C, the corrected fluorescence ratio is essentially equal to unity over the full range of compositions (Fig. 6 D, inset).
FIGURE 6.
Corrected fluorescence ratios Rflcor (mean ± SD determined for triplicate sets of samples) measured for DONDO versus DSNDS in sphingomyelin/DOPC/cholesterol bilayers. For cholesterol-containing samples, the content of DOPC is specified as its molar percentage in the total phospholipid fraction, i.e., as (100% × (moles DOPC)/(moles DOPC + moles sphingomyelin)). (A–C) Samples combining sphingomyelin and DOPC in varying proportions with 33 mol% cholesterol at 20°C (A), 30°C (B), or 37°C (C). (D) Samples combining sphingomyelin and DOPC in varying proportions with 50 mol% cholesterol at 37°C. (D, inset) Cholesterol-free samples combining sphingomyelin and DOPC in the indicated proportions at 37°C. Rflcor values shown (mean ± SD) were determined as described in the legend to Fig. 5.
The majority of phospholipids in mammalian cells carry one saturated and one unsaturated acyl chain. It was thus of interest to determine whether results similar to those just described would be observed in bilayers where the dioleoyl PC component was replaced by (saturated/unsaturated) mixed-acyl lipids. At 37°C mixtures combining sphingomyelin and 1-stearoyl-2-oleoyl phosphatidylcholine (SOPC) with 33 mol% cholesterol show inhomogeneous mixing over a wide range of sphingomyelin contents (Fig. 7 A). Mixtures combining sphingomyelin and 1-palmitoyl-2-linoleoyl PC with 33 mol% cholesterol also show inhomogeneous mixing at 37°C (Fig. 7 B).
FIGURE 7.
Corrected fluorescence ratios Rflcor (mean ± SD determined for triplicate sets of samples) measured for DONDO versus DSNDS in bilayers combining sphingomyelin and 33 mol% cholesterol with SOPC (A) or 1-stearoyl-2-linoleoyl PC (B). For each sample the content of DOPC is specified as its molar percentage in the total phospholipid fraction, i.e., as (100% × (moles DOPC)/(moles DOPC + moles sphingomyelin)). Rflcor values shown (mean ± SD) were determined as described in the legend to Fig. 5.
The above FRET approach was finally used to investigate the possible occurrence of laterally inhomogeneous lipid mixing in systems combining DOPC with various higher-melting phosphatidylcholines and cholesterol. Mixtures combining 33 mol% cholesterol and DOPC with DPPC show marked inhomogeneity of mixing at 37°C (Fig. 8 A). Inhomogeneity is still apparent in this system even at 50 mol% cholesterol (Fig. 8 A, inset), although the differences in the fluorescence of the two donor probes are reduced. Bilayers combining DOPC with 1,2-dimyristoylphosphatidyl-choline (DMPC) and containing 33 mol% cholesterol also show substantial inhomogeneity at 20°C (Fig. 8 B) or at 37°C (not shown). Even in the presence of 50 mol% cholesterol and at 37°C, DMPC/DOPC mixtures show inhomogeneous mixing (Fig. 8 C). By contrast, essentially homogeneous mixing is seen for DMPC/DOPC bilayers at 37°C in the absence of cholesterol (Fig. 8 C, inset). These findings provide evidence for cholesterol-promoted inhomogeneities in bilayer organization in the above systems (on a spatial scale of at least tens of nanometers) that persist even at physiological temperatures. Fluorescence-microscopic studies have reported formation of large (micron-size) segregated domains in DPPC/DOPC/cholesterol and DMPC/DOPC/cholesterol mixtures at 20°C, which however vanish at temperatures ≥30°C (Veatch and Keller, 2002). In contrast to the behavior observed here for mixtures of DOPC and cholesterol with saturated phospholipids, at 37°C, mixtures of DOPC and SOPC containing either 0 or 33 mol% cholesterol show apparently homogeneous mixing (Fig. 8 D and inset).
DISCUSSION
The FRET-based strategy used here was explored to allow detection of inhomogeneities in the organization of lipid bilayers (vesicles) on a spatial scale intermediate between that of fluorescence microscopy (micron or just-submicron dimensions) and that probed by methods based on quenching of bilayer-incorporated fluorophores by brominated or spin-labeled lipids (approaching nearest-neighbor distances, i.e., ∼1–2 nm). As noted previously (Feigenson and Buboltz, 2001), in models in which FRET acceptors are postulated to be excluded from the immediate vicinity of donor molecules within a surface, the efficiency of FRET is predicted to be most sensitive to the diameter of the exclusion domain when the latter is roughly 1–4 times the characteristic energy-transfer length Ro (Wolber and Hudson, 1979; Dewey and Hammes, 1980; Zimet et al., 1995). It can thus be (crudely) estimated that the DONDO/DSNDS/Rho-PE probe combinations examined here, with an Ro value of ∼6 nm, can detect inhomogeneities in the lateral organization of lipids within a bilayer when the spatial scale of such inhomogeneities is on the order of tens of nanometers or greater. This is similar to the dimensions suggested for lipid rafts in biological membranes (Varma and Mayor, 1998; Pralle et al., 2000) and for cholesterol-induced lipid clusters that have been hypothesized to play important roles in membrane organization and function (Anderson and Jacobson, 2002). At present, few experimental methods are available to characterize the organization of lipid bilayers on this spatial scale.
The FRET approach employed here correctly reports a homogeneous organization of the bilayer (on a spatial scale of tens of nanometers or greater) in several lipid systems in which homogeneous mixing of lipid species is observed even on small distance scales (Ahmed et al., 1997; Wang and Silvius, 2001; T.-Y. Wang and J. R. Silvius, unpublished results). These systems include DOPC, DOPC/cholesterol, and DOPC/SOPC/cholesterol bilayers as well as cholesterol-free bilayers combining DOPC with DMPC, sphingomyelin, or SOPC above their transition temperatures. By contrast, for mixtures of these lipids that have been shown to form micron-sized domains by fluorescence microscopy (bilayers combining equimolar proportions of cholesterol, DOPC, and either sphingomyelin or DMPC at temperatures ≤25°C (Dietrich et al., 2001a; Veatch and Keller, 2002)), the present approach correctly indicates a marked inhomogeneity in lipid lateral organization. For systems for which comparisons can be made with results obtained previously by other methods, the present approach thus appears to report faithfully the homo- or inhomogeneity of lipid organization (on a spatial scale of tens of nanometers or greater) within the bilayer. Importantly, in no case does the FRET approach give indications of an inhomogeneous lateral distribution of the lipids in systems whose components in fact mix homogeneously.
The major new finding provided by the present FRET experiments is that lipid bilayers whose compositions approximate that of the outer leaflet of mammalian cell plasma membranes can exhibit domain organization on a spatial scale similar to the inferred dimensions of lipid rafts (tens of nanometers or greater), even at physiological temperatures and cholesterol contents where large (micron-sized) segregated domains have not been observed. Fluorescence and atomic force microscopy have revealed segregation of fluid lipid domains under certain conditions in bilayers combining cholesterol, high-melting phospho- or sphingolipids, and low-melting phospholipids (Dietrich et al., 2001a,b; Feigenson and Buboltz, 2001; Milhiet et al., 2001; Rinia and de Kruijff, 2001; Rinia et al., 2001; Samsonov et al., 2001; Veatch and Keller, 2002, 2003). However, these studies have also reported that in bilayers with compositions (including cholesterol contents) that approximate those of the outer monolayer of mammalian cell plasma membranes, micron-sized domains typically disappear at physiological temperatures (Dietrich et al., 2001a; Veatch and Keller, 2002, 2003). Fluorescence-quenching studies have indicated that on a distance scale approaching nearest-neighbor separations, inhomogeneity in lipid distributions can still be detected in such systems at physiological temperatures (Ahmed et al., 1997; Wang et al., 2000; Wang and Silvius, 2003). It has not previously been determined, however, whether the inhomogeneity detected in these systems by the latter approach extends to spatial dimensions comparable to those estimated for lipid rafts. The present data indicate that this is in fact the case. At 37°C, for bilayers combining saturated phospho- or sphingolipids with unsaturated phospholipids and physiological proportions of cholesterol, the FRET assay gives strong indications of an inhomogeneous lateral organization (domain formation) on a spatial scale of tens of nanometers or greater. Since as already noted micron-sized domains have not been observed in such systems at 37°C, the segregated microenvironments detected by FRET in these systems would appear to exhibit dimensions comparable to those inferred for rafts in biological membranes, i.e., at least tens of nanometers but considerably less than one micron in size (Varma and Mayor, 1998; Pralle et al., 2000). The present results do not provide any information about the geometries of the domains formed in these systems, which as noted elsewhere (Feigenson and Buboltz, 2001) could for example exist as very elongated, ramifying structures as small as a few tens of nanometers in width rather than as compact, disc-shaped regions.
Feigenson and Buboltz (2001) have previously reported that for the DPPC/dilauroyl PC (DLPC)/cholesterol ternary system at 22°C, inhomogeneities in lipid lateral distributions can be detected by FRET at cholesterol contents up to ∼25 mol%, whereas domains visible by fluorescence microscopy disappear at somewhat lower cholesterol levels. This finding appears to agree with our observation that for several of the lipid systems examined here, inhomogeneities in lipid distributions can be detected by FRET under conditions (temperatures ≥30°C and/or high cholesterol contents) where formation of micron-sized domains has not been observed (Dietrich et al., 2001a; Veatch and Keller, 2002, 2003). For several of the systems examined here, however, inhomogeneity in lateral organization can still be observed by FRET for cholesterol contents ranging up to at least 50 mol%. These observations are not necessarily in conflict with those reported for the DPPC/DLPC/cholesterol system. First, of course, the detailed phase behavior of the latter system may differ significantly from that of the systems examined here. Second, and as Feigenson and Buboltz noted in their report, it is also possible that nanoscopic domains exist in DPPC/DLPC/cholesterol bilayers at sterol contents well above 25 mol% but were not clearly detected by the specific donor/acceptor probe combination used in that study. The findings of this study can thus be considered to agree at least qualitatively with those reported by Feigenson and Buboltz. These authors have previously noted the potential correlation between the “nanoscopic” segregation of different lipid environments in cholesterol-containing lipid bilayers and the formation of lipid rafts with small dimensions in biological membranes.
Like any probe-based methodology, the approach described here may carry potential limitations. However, these limitations are likely to result in failure to detect lateral inhomogeneity in lipid bilayers under certain circumstances, rather than to provide erroneous indications for such inhomogeneity in systems where it is absent. In some types of bilayers the two donor species could prove to be too similar in their partitioning between different lipid domains, or the acceptor species might not discriminate sufficiently in its partitioning between different domains, to allow the presence of such domains to be detected. As already discussed, the present FRET method is not well suited to detect inhomogeneities on scales of distances smaller than ∼10 nm. For such applications, fluorescence methods employing spin-labeled or brominated phospholipids are more appropriate (Silvius, 1992; Ahmed et al., 1997). A further, though lesser, caveat in the use of the present approach is that energy transfer between donor and acceptor molecules in opposite leaflets of the bilayer may attenuate somewhat (though not eliminate) the signature for inhomogeneity in bilayer organization in cases where the lateral organization of the two leaflets of the bilayer is uncorrelated. These latter effects are expected to be relatively modest, however, based on the estimates of Ro for the probes used here (Fung and Stryer, 1978; Wolber and Hudson, 1979) and the experimental findings of Connor and Schroit (1987) for closely related fluorescent probes. Moreover, such effects will be further reduced in cases where lipid lateral organization is correlated between the two halves of the bilayer, as has been observed in both cholesterol-containing and cholesterol-free systems (Korlach et al., 1999; Dietrich et al., 2001a).
The fluorescence-based approach utilized in this study differs from previously proposed FRET-based approaches, notably in its use of a matched pair of donor probes that bear four rather than two acyl chains to accentuate the differential partitioning of these species between different bilayer microenvironments. The fluorescence species employed here were designed specifically to examine systems combining saturated and unsaturated lipids with cholesterol, whose compositions resemble those of the outer leaflets of most mammalian cell plasma membranes (van Meer, 1989). However, other types of lipid mixtures, such as mixtures combining cholesterol and monounsaturated phospholipids with highly unsaturated phospholipids, have also been suggested to form microdomains of nanoscopic dimensions (Huster et al., 1998; Polozova and Litman, 2000; Brzustowicz et al., 2002). Combinations of donor and acceptor probes related to those examined here, but bearing for example different acyl chains, may prove useful to detect potential formation of microdomains in these latter systems as well.
Acknowledgments
This research was supported by an operating grant from the Canadian Institutes of Health Research (MOP-7776) to J.R.S.
References
- Ahmed, S. N., D. A. Brown, and E. London. 1997. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 36:10944–10953. [DOI] [PubMed] [Google Scholar]
- Anderson, R. G. 1998. The caveolae membrane system. Annu. Rev. Biochem. 67:199–225. [DOI] [PubMed] [Google Scholar]
- Anderson, R. G. W., and K. Jacobson. 2002. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 296:1821–1825. [DOI] [PubMed] [Google Scholar]
- Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111–136. [DOI] [PubMed] [Google Scholar]
- Brown, R. E. 1998. Sphingolipid organization in biomembranes: what physical studies of model membranes reveal. J. Cell Sci. 111:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzustowicz, M. R., V. Cherezov, M. Caffrey, W. Stillwell, and S. R. Wassall. 2002. Molecular organization of cholesterol in polyunsaturated membranes: microdomain formation. Biophys. J. 82:285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor, J., and A. J. Schroit. 1987. Determination of lipid asymmetry in human red cells by resonance energy transfer. Biochemistry. 26:5099–5105. [DOI] [PubMed] [Google Scholar]
- Dewey, T. G., and G. G. Hammes. 1980. Calculation of fluorescence resonance energy transfer on surfaces. Biophys. J. 32:1023–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich, C., L. A. Bagatolli, Z. N. Volovyk, N. L. Thompson, M. Levi, K. Jacobson, and E. Gratton. 2001a. Lipid rafts reconstituted in model membranes. Biophys. J. 80:1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich, C., Z. N. Volovyk, M. Levi, N. L. Thompson, and K. Jacobson. 2001b. Partitioning of Thy-1, GM1, and cross-linked phospholipid analogs into lipid rafts reconstituted in supported bilayer model membranes. Proc. Natl. Acad. Sci. USA. 98:10642–10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenson, G. W., and J. T. Buboltz. 2001. Tertiary phase diagram of dipalmitoyl-PC/dilauroyl-PC/cholesterol: nanoscopic domain formation driven by cholesterol. Biophys. J. 80:2775–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung, B. K.-K., and L. Stryer. 1978. Surface density measurements in membranes by fluorescence energy transfer. Biochemistry. 17:5241–5248. [DOI] [PubMed] [Google Scholar]
- Furuchi, T., and R. G. Anderson. 1998. Cholesterol depletion of caveolae causes hyperactivation of extracellular signal-related kinase (ERK). J. Biol. Chem. 273:21099–21104. [DOI] [PubMed] [Google Scholar]
- Huang, N. N., K. Florine-Casteel, G. W. Feigenson, and C. Spink. 1988. Effect of fluorophore linkage position of n-(9-anthroyloxy) fatty acids on probe distribution between coexisting gel and fluid phospholipid phases. Biochim. Biophys. Acta. 939:124–130. [DOI] [PubMed] [Google Scholar]
- Huang, T. H., C. W. Lee, S. K. Das Gupta, A. Blume, and R. G. Griffin. 1993. A 13C and 2H nuclear magnetic resonance study of phosphatidylcholine/cholesterol interactions: characterization of liquid-gel phases. Biochemistry. 32:13277–13287. [DOI] [PubMed] [Google Scholar]
- Huster, D., K. Arnold, and K. Gawrisch. 1998. Influence of docosahexaenoic acid and cholesterol on lateral lipid organization in phospholipid mixtures. Biochemistry. 37:17299–17308. [DOI] [PubMed] [Google Scholar]
- Korlach, J., P. Schwille, W. W. Webb, and G. W. Feigenson. 1999. Characterization of lipid bilayer phases by confocal microscopy and fluorescence correlation spectroscopy. Proc. Natl. Acad. Sci. USA. 96:8461–8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia, T. V., and R. G. Parton. 1999. Membrane microdomains and caveolae. Curr. Opin. Cell Biol. 11:424–431. [DOI] [PubMed] [Google Scholar]
- Leidy, C., W. F. Wolkers, K. Jorgensen, O. G. Mouritsen, and J. H. Crowe. 2001. Lateral organization and domain formation in a two-component lipid membrane system. Biophys. J. 80:1819–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London, E. 2002. Insights into lipid raft structure and formation from experiments on model membranes. Curr. Opin. Struc. Biol. 12:480–486. [DOI] [PubMed] [Google Scholar]
- London, E., and G. W. Feigenson. 1981. Fluorescence quenching in model membranes. An analysis of the local phospholipid environments of diphenylhexatriene and gramicidin A′. Biochim. Biophys. Acta. 649:89–97. [Google Scholar]
- Matveev, S. V., and E. J. Smart. 2002. Heterologous desensitization of EGF receptors and PDGF receptors by sequestration in caveolae. Am. J. Physiol. Cell Physiol. 282:C935–C946. [DOI] [PubMed] [Google Scholar]
- McIntyre, J. C., and R. G. Sleight. 1991. Fluorescence assay for phospholipid membrane asymmetry. Biochemistry. 30:11819–11827. [DOI] [PubMed] [Google Scholar]
- McMullen, T. P., and R. N. McElhaney. 1995. New aspects of the interaction of cholesterol with dipalmitoylphosphatidylcholine bilayers as revealed by high-sensitivity differential scanning calorimetry. Biochim. Biophys. Acta. 1234:90–98. [DOI] [PubMed] [Google Scholar]
- Milhiet, P. E., C. Domec, M. C. Giocondi, N. Van Mau, F. Heitz, and C. Le Grimellec. 2001. Domain formation in models of the renal brush border membrane outer leaflet. Biophys. J. 81:547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti, J. A., S. T. Christian, and W. A. Shaw. 1978. Synthesis and properties of a highly fluorescent derivative of phosphatidylethanolamine. J. Lipid Res. 19:22–28. [PubMed] [Google Scholar]
- Pedersen, S., K. Jorgensen, T. T. R. Baekmark, and O. G. Mouritsen. 1996. Indirect evidence for lipid-domain formation in the transition region of phospholipid bilayers by two-probe fluorescence energy transfer. Biophys. J. 71:554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike, L. J., and J. M. Miller. 1998. Cholesterol depletion delocalizes phosphatidylinositol bisphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. J. Biol. Chem. 273:22298–22304. [DOI] [PubMed] [Google Scholar]
- Pike, L. J., and L. Casey. 2002. Cholesterol levels modulate EGF receptor-mediated signaling by altering receptor function and trafficking. Biochemistry. 41:10315–10322. [DOI] [PubMed] [Google Scholar]
- Polozova, A., and B. J. Litman. 2000. Cholesterol dependent recruitment of di22:6-PC by a G protein-coupled receptor into lateral domains. Biophys. J. 79:2632–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralle, A., P. Keller, E. L. Florin, K. Simons, and J. K. Horber. 2000. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 148:997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan, A., T. G. Anderson, and H. M. McConnell. 2000. Condensed complexes, rafts, and the chemical activity of cholesterol in membranes. Proc. Natl. Acad. Sci. USA. 97:12422–12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld, A., and K. Simons. 1998. The differential miscibility of lipids as the basis for the formation of functional membrane rafts. Biochim. Biophys. Acta. 1376:467–479. [DOI] [PubMed] [Google Scholar]
- Rinia, H. A., and B. de Kruijff. 2001. Imaging domains in model membranes with atomic force microscopy. FEBS Lett. 504:194–199. [DOI] [PubMed] [Google Scholar]
- Rinia, H. A., M. M. E. Sniel, J. P. J. M. van der Enden, and B. de Kruijff. 2001. Visualizing detergent resistant domains in model membranes with atomic force microscopy. FEBS Lett. 501:92–96. [DOI] [PubMed] [Google Scholar]
- Roy, S., R. Luetterforst, A. Harding, A. Apolloni, M. Etheridge, E. Stang, B. Rolls, J. F. Hancock, and R. G. Parton. 1999. Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat. Cell Biol. 1:98–105. [DOI] [PubMed] [Google Scholar]
- Samsonov, A. V., I. Mihalyov, and F. S. Cohen. 2001. Characterization of cholesterol-sphingomyelin domains and their dynamics in lipid bilayers. 2001. Biophys. J. 81:1486–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaram, M. B., and T. E. Thompson. 1990. Interaction of cholesterol with various glycerophospholipids and sphingomyelin. Biochemistry. 29:10670–10675. [DOI] [PubMed] [Google Scholar]
- Sankaram, M. B., and T. E. Thompson. 1991. Cholesterol-induced fluid-phase immiscibility in membranes. Proc. Natl. Acad. Sci. USA. 88:8686–8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, R., E. London, and D. Brown. 1994. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc. Natl. Acad. Sci. USA. 91:12130–12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets, E. D., D. Holowka, and B. Baird. 1999. Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of FcepsilonRI and their association with detergent-resistant membranes. J. Cell Biol. 145:877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius, J. R. 1992. Cholesterol modulation of lipid intermixing in phospholipid and glycolipid mixtures. Evaluation using fluorescent lipid probes and brominated lipid quenchers. Biochemistry. 31:3398–3408. [DOI] [PubMed] [Google Scholar]
- Silvius, J. R., D. del Giudice, and M. Lafleur. 1996. Cholesterol at different bilayer concentrations can promote or antagonize lateral segregation of phospholipids of differing acyl chain length. Biochemistry. 35:15198–15208. [DOI] [PubMed] [Google Scholar]
- Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. [DOI] [PubMed] [Google Scholar]
- Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31–39. [DOI] [PubMed] [Google Scholar]
- Smart, E. J., and R. G. Anderson. 2002. Alterations in membrane cholesterol that affect structure and function of caveolae. Methods Enzymol. 353:131–139. [DOI] [PubMed] [Google Scholar]
- Spink, C. H., M. D. Yeager, and G. L. Feigenson. 1990. Partitioning behavior of indocarbocyanine probes between coexisting gel and fliud phases in model membranes. Biochim. Biophys. Acta. 1023:25–33. [DOI] [PubMed] [Google Scholar]
- Stillwell, W., L. J. Jenski, M. Zerouga, and A. C. Dumaual. 2000. Detection of lipid domains in docasahexaenoic acid-rich bilayers by acyl chain-specific FRET probes. Chem. Phys. Lipids. 104:113–132. [DOI] [PubMed] [Google Scholar]
- Struck, D. K., D. Hoekstra, and R. E. Pagano. 1981. Use of resonance energy transfer to monitor membrane fusion. Biochemistry. 20:4093–4099. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai, M., L. Hilenski, N. Santanam, P. L. Becker, Y. Ma, K. K. Griendling, and R. W. Alexander. 2001. Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J. Biol. Chem. 276:48269–48275. [DOI] [PubMed] [Google Scholar]
- van Meer, G. 1989. Lipid traffic in animal cells. Annu. Rev. Cell Biol. 5:247–275. [DOI] [PubMed] [Google Scholar]
- Varma, R., and S. Mayor. 1998. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 394:798–801. [DOI] [PubMed] [Google Scholar]
- Veatch, S. L., and S. L. Keller. 2002. Organization in lipid membranes containing cholesterol. Phys. Rev. Lett. 89:268101. [DOI] [PubMed] [Google Scholar]
- Veatch, S. L., and S. L. Keller. 2003. A closer look at the canonical “raft mixture” in canonical membrane studies. Biophys. J. 84:725–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vist, M. R., and J. H. Davis. 1990. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 29:451–464. [DOI] [PubMed] [Google Scholar]
- Wang, T.-Y., R. Leventis, and J. R. Silvius. 2000. Fluorescence-based evaluation of the partitioning of lipids and lipidated peptides into liquid-ordered lipid microdomains: a model for molecular partitioning into “lipid rafts”. Biophys. J. 79:919–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T.-Y., R. Leventis, and J. R. Silvius. 2001. Partitioning of lipidated peptide sequences into liquid-ordered domains in model and biological membranes. Biochemistry. 40:13031–13040. [DOI] [PubMed] [Google Scholar]
- Wang, T.-Y., and J. R. Silvius. 2000. Different sphingolipids show differential partitioning into sphingolipid/cholesterol-rich domains in lipid bilayers. Biophys. J. 79:1478–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T.-Y., and J. R. Silvius. 2001. Cholesterol does not induce segregation of liquid-ordered domains in bilayers modeling the inner leaflet of the plasma membrane. Biophys. J. 81:2762–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T. Y., and J. R. Silvius. 2003. Sphingolipid partitioning into ordered domains in cholesterol-free and cholesterol-containing lipid bilayers. Biophys. J. 84:367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolber, P. K., and B. S. Hudson. 1979. An analytic solution to the Förster energy transfer problem in two dimensions. Biophys. J. 28:197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X., R. Bittman, G. Duportail, D. Heissler, C. Vilchezel, and E. London. 2001. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal and disease-associated sterols and comparison of sphingomyelin, cerebrosides and ceramide. J. Biol. Chem. 276:33540–33546. [DOI] [PubMed] [Google Scholar]
- Xu, X., and E. London. 2000. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry. 39:8404–8409. [DOI] [PubMed] [Google Scholar]
- Zimet, D. B., B. J.-M. Thevenin, A. S. Verkman, S. B. Shohet, and J. R. Abney. 1995. Calculation of resonance energy transfer in crowded biological membranes. Biophys. J. 68:1592–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]









