Abstract
The World Health Organization recommends Mycobacterium bovis BCG vaccination in areas of high tuberculosis prevalence. BCG's clinical and immune effects, not necessarily Mycobacterium tuberculosis specific, are unclear. BCG vaccine scarring often is used as a surrogate marker of vaccination or of effective vaccination. We evaluated BCG scarring status in relation to clinical findings and outcome in 700 hospitalized Malawians, of whom 32 had M. tuberculosis bloodstream infections (BSI) (10 of whom had cellular immune studies done) and of whom 48 were infants <6 months old and therefore recently vaccinated (19 of whom had immune studies). In the patients ≥6 months old, scarring was not related to the presence of pulmonary symptoms (35 versus 30%), chronic cough or fever, mortality, or M. tuberculosis BSI. In M. tuberculosis BSI patients, scarring was unrelated to mortality, vital signs, or clinical symptoms but those with scarring had higher proportions of memory and activated T cells and more type 2-skewed cytokine profiles. Infants with either BCG scarring (n = 10) or BCG lesional inflammation (n = 5) had no symptoms of sepsis, but 18 of 33 infants without BCG vaccination lesions did. Those with BCG lesions had localized infections more often than did those without BCG lesions. These infants also had lower median percentages of lymphocytes spontaneously making interleukin-4 (IL-4) or tumor necrosis factor alpha (TNF-α) and lower ratios of T cells spontaneously making IL-4 to T cells making IL-6. Thus, we found that, in older patients, BCG vaccine scarring was not associated with M. tuberculosis-specific or nonspecific clinical protection. Those with M. tuberculosis BSI and scarring had immune findings suggesting previous M. tuberculosis antigen exposure and induction of a type 2 cytokine pattern with acute reexposure. It is unlikely that this type 2 pattern would be protective against mycobacteria, which require a type 1 response for effective containment. In infants <6 months old, recent BCG vaccination was associated with a non-M. tuberculosis-specific, anti-inflammatory cytokine profile. That the vaccinated infants had a greater frequency of localized infections and lesser frequency of sepsis symptoms suggests that this postvaccination cytokine pattern may provide some non-M. tuberculosis-specific clinical benefits.
In 1995 alone, at least 180 million children <15 years old were infected with Mycobacterium tuberculosis worldwide and, of these, nearly 170,000 died (8). The public health impact of M. tuberculosis has become increasingly severe because of the concurrent human immunodeficiency virus (HIV) epidemic. M. tuberculosis and HIV have both clinical and in vitro synergism; active tuberculosis increases HIV-related immunodeficiency and mortality (14, 32). Because of the worsening mycobacterial and HIV epidemics, the World Health Organization recommends Mycobacterium bovis bacillus Calmette-Guerin (BCG) vaccination of newborns in areas with high tuberculosis prevalence and incidence (34, 35). BCG vaccination is now mandated in at least 64 countries and administered in at least 167 (20, 31).
Despite its widespread use, BCG vaccine's efficacy remains controversial (5, 13, 24). Some studies suggest that it protects against infantile M. tuberculosis meningitis and miliary, extrapulmonary, and glandular tuberculosis (2, 5, 10, 24, 36-38; V. Schwoebel, B. Hubert, and J. Grosset, Letter, Lancet 340:611, 1992). Its effects on pulmonary tuberculosis are less clear. At least one study suggests that repeated BCG vaccination might even increase the risk of pulmonary tuberculosis in HIV-positive persons (17). Efficacy rates vary greatly among various studies and by BCG strain, storage conditions, age at vaccination, and geographic region (5, 24). Indeed, the BCG vaccine may be more effective against Mycobacterium leprae than against M. tuberculosis, due to nonspecific stimulation of the immune system by the vaccine (3, 17, 19).
In studies of vaccine prevalence and/or efficacy, the presence or absence of a BCG vaccine scar routinely is used as a surrogate indicator of vaccination status, in place of or in addition to vaccination records (2, 7, 18, 29, 33, 36-38). Although not every immunized person scars, scarring occurs in most vaccinated individuals (12, 20) and is a stable phenomenon (11). Scarring generally is thought to at least represent “effective” vaccination, since it is associated with subsequent tuberculin skin test (TST) reactivity (4, 6, 26-28, 30; World Health Organization Expert Committee on Biological Standardization, Experimental data for the selection of a reference batch of BCG [W. H. O./BS/802.65]). TST is the most commonly used measure of antimycobacterial cellular, or type 1, reactivity. Type 1 reactivity is defined by the production of T-helper-1-like cytokines by CD8+ T cells, natural killer (NK) cells, natural T (NT) cells, monocytes, and other types of cell types, as well as by the CD4+ T cells for which T-helper-1 activity was classically defined (21). Cellular, or type 1, reactivity is considered essential in antimycobacterial, anti-HIV, and antisalmonella immunity (16, 21, 23).
In Malawi, Africa, BCG vaccination began in the 1970s, is strongly encouraged, and is offered within 3 days of birth and at routine clinic visits during infancy. We evaluated data from a cohort of hospitalized Malawian patients to examine the relationships between BCG vaccine scarring and the clinical histories and findings for all the patients (including their pulmonary symptoms, HIV status, types of infections, and outcome), the clinical and immune findings in patients with M. tuberculosis bloodstream infections, and the clinical and immune findings in infants <6 months of age (who, based on their age, were either unvaccinated or recently vaccinated). For these infants, we also examined relationships between inflammation (as opposed to scar healing) at the vaccine site and clinical and immune findings.
MATERIALS AND METHODS
Study population.
During three periods in 1997 and 1998, we enrolled all 497 febrile (oral temperature ≥ 38°C) persons ≥13 years old and all 245 acutely ill persons <13 years old admitted to the Lilongwe Central Hospital, a regional public hospital in Malawi into a study of bloodstream infections (16). All hospitalized children were included because infected children often do not present with fever. For each patient at admission, blood samples, epidemiologic data, and a medical history were obtained and a physical examination was performed by one of the investigators. A random subset of participants had immune studies done; this subset was comparable to the general study population (16). Currently, in Malawi, BCG vaccination with 0.05 ml of Pasteur Mérieux Connaught BCG vaccine, derived from strain 1077 and containing between 0.4 × 106 and 1.6 × 106 culturable particles per newborn dose, is offered within the first 3 days of life. If not given then, it is again offered at each subsequent routine health care visit.
The study protocol was approved by the institutional review boards of the Centers for Disease Control and Prevention and the Malawian Health Sciences Research Committee; informed consent was obtained from all participants and/or their guardians.
Clinical definitions.
An acute pulmonary history was positive if the patient or caretaker reported either cough or shortness of breath. Respiratory findings were considered positive when any of the following were present on physical examination: a respiratory rate ≥20 (adults) or ≥60 breaths/min (children), lung crackles, or auscultatory findings suggesting pulmonary consolidation. Diarrhea was defined as any quantity or frequency of watery stool. Sepsis was proven by a positive blood culture or suspected on the basis of leukocytosis, abnormal temperature, and organomegally. Medical history included the chronic (>1 month) presence or absence of fever or cough.
Laboratory procedures.
Blood cultures were performed as previously described (1); thick and thin malaria smears were done at admission and were considered positive if any Plasmodium falciparum asexual parasites were seen. HIV antibody testing was done at study enrollment by using enzyme-linked immunosorbent assay test kits (Murex Diagnostics Inc., Norcross, Ga.). HIV type 2 (HIV-2) has not been reported in Malawi. Plasma HIV-1 RNA levels for 81 participants were assessed (Monitor, version 1.5; Roche Diagnostics, Indianapolis, Ind.). Those with viral studies done tended to be younger than other participants but did not differ from other participants in regard to BCG scarring, infections, or mortality. Heparinized whole blood was either stimulated for 5 h at 37°C with phorbol 12-myristate 13-acetate (PMA; 200 ng/ml; Sigma Chemical Co., St. Louis, Mo.) and ionomycin (4 μg/ml) (Sigma) in the presence of brefeldin A (40 μg/ml) (Sigma) and RPMI 1640 with l-glutamine (induced or stimulated cytokine expression) or was retained in identical media without PMA and ionomycin but with brefeldin A (spontaneous or unstimulated cytokine expression) (16). No serum was added to the cultures. Cells were permeabilized and fixed with Permeafix (Ortho Diagnostic Systems, Inc., Raritan, N.J.) and then shipped at 4 to 8°C to the Centers for Disease Control and Prevention, stained with conjugated monoclonal antibodies, and processed for four-color flow-cytometric assessment by using a FACSort or FACSCalibur flow cytometer and CellQuest software (Becton Dickinson Immunochemistry Systems [BD], San Jose, Calif.). Isotype controls were obtained from BD. All antibodies used were pretested for stability to the permeabilization/fixation protocol. Cell identification and gating were done as previously described (16). Between 50,000 and 80,000 ungated events were collected from each tube in the panel.
Analytic techniques.
For each participant, analyses were done for all lymphocytes, CD3+ (T) lymphocytes, CD3+ CD8+ lymphocytes, CD3+ CD8− lymphocytes, CD3+ CD16/56+ lymphocytes (NT cells), CD3− CD16/56+ lymphocytes (NK cells), CD19+ (B) lymphocytes, and monocytes, depending on the tube configuration. Cytokine profiles were represented by the ratios of the percentages of CD3+ lymphocytes producing interleukin-10 (IL-10) or IL-4 to the percentages producing each of the other cytokines. IL-10 and IL-4 are both anti-inflammatory, regulatory, and type 2 (humoral) cytokines; they induce antibody maturation and production. IL-4 also induces the production of other type 2 cytokines (e.g., IL-5 and IL-13) and thus might also be considered “pro-type 2.” IL-10 inhibits the production of type 1 cytokines (e.g., gamma interferon [IFN-γ] and IL-12) and thus is “anti-type 1.” Tumor necrosis factor alpha (TNF-α), IL-6, and IL-8 are proinflammatory cytokines; IL-2, IFN-γ, and, possibly, TNF-α are type 1 cytokines, inducing and involved in cellular immunity, which is essential for immunity to intracellular organisms. We calculated similar ratios for monocytes in regard to IL-10 and IL-6, IL-8, and TNF-α.
Memory and activation markers.
CD45RO (a memory cell marker), CD45RA (a “naive” cell marker), HLA-DR (major histocompatibility complex antigen receptor that is induced with activation), CD38 (also induced with activation), and the percentages of cells expressing CD4 were assessed by using unstimulated cells. CD62L, often used to differentiate memory and naive cells, is not stable to our permeabilization/fixation protocol and therefore was not assessed.
Statistical techniques.
Analyses were done with statistical software from SAS Institute, Inc. (Cary, N.C.). Comparisons of continuous data between dichotomized categories were made by using Wilcoxon rank sum and, in some instances, also logistic regression analyses; trichotomous data were analyzed by using Kruskal-Wallis tests. For HIV+ persons with undetectable plasma HIV RNA levels, the analysis values were set at 200 copies/ml, half the lower detection limit of the assay. Proportions were compared by using Fisher's exact tests or chi-square tests. P values less than 0.05 were considered significant.
RESULTS
Clinical and demographic characteristics of all participants.
Of the patients enrolled, 474 adults (95% of adult participants) and 226 children (92%) had data concerning the presence or absence of BCG scarring recorded. Of those with scarring and clinical data recorded, 32 adults had M. tuberculosis isolated from their blood and 10 of these had immune studies done at admission; no child had mycobacteremia. No patient had active measles infection. Of the children <6 months old, 48 had BCG site scarring/inflammation status and clinical data recorded; 19 of these had immune studies done.
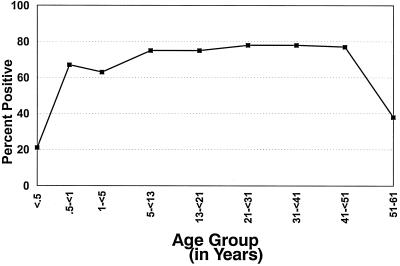
The proportion of patients with BCG scarring increased over the first 6 months of life; the proportion was stable for those ≥6 months through 50 years of age and was lower in the few participants 51 to 61 years of age (n = 13 with clinical data and n = 5 with immune data also) (Fig. 1). The trend seen in Fig. 1 is consistent with persons in Malawi being vaccinated in infancy and some persons in the oldest age stratum having been missed by the vaccination campaigns in previous decades. None of those in the two extreme-age strata (<6 months and 51 to 61 years of age) had mycobacteria isolated from their blood.
FIG. 1.
Median percentages of participants with BCG scars by age group. Numbers of participants in the different subgroups are as follows: <0.5 years old, 48; 0.5 to <1 year old, 42; 1 to <5 years old, 96; 5 to <13 years old, 40; 13 to <21 years old, 64; 21 to <31 years old, 181; 31 to <41 years old, 150; 41 to 51 years old, 53; and 51 to 61 years old, 13.
For those ≥6 months old, gender and age were not associated with BCG scarring (Table 1). Those with and without BCG scarring had similar rates of mycobacteremia, blood culture positivity, malaria smear positivity, HIV seropositivity, and mortality (Table 1). For HIV-seropositive persons, HIV-1 plasma viral titers did not vary by BCG scarring status and the percentages of lymphocytes expressing CD4 were low irrespective of scarring status. The distributions of bloodstream pathogens for both groups were similar and included M. tuberculosis (present in 23 of 479 participants with scarring and 9 of 173 without scarring), other Mycobacteria spp. (n = 3, all in persons with vaccine scarring), fungi or candida (present in 1.8% of those with scarring and 1.7% of those without scarring), gram-positive organisms (5.0 and 5.2% of those with and without scarring, respectively), and gram-negative organisms (11.2 and 11.6% of those with and without scarring, respectively). The proportions of participants with or without scarring who had positive acute pulmonary histories, pulmonary findings, or diarrhea were similar, as were the proportions who had chronic fever or cough.
TABLE 1.
Characteristics of participants ≥ 6 months of age, by BCG vaccine scarring status
| Characteristica | Value for participants with BCG scarringc:
|
|
|---|---|---|
| Present (n = 479) | Absent (n = 173) | |
| Male (%) | 50 | 51 |
| Age (yr) | ||
| Mean | 23.5 | 21.3 |
| Median | 25.0 | 22.0 |
| Range | 0.5-61 | 0.5-64 |
| Death (%) | 18 | 12 |
| Mycobacteremic (%) | 5 | 5 |
| Malaria positive (%) | 14 | 14 |
| Blood culture positive (%) | 24 | 24 |
| HIVb | ||
| Seropositive (%) | 63 | 56 |
| Median log10 viral titers/mm3 | 5.5 | 5.8 |
| Median % of lymphocytes expressing CD4 | 7.9 | 6.9 |
| % of participants with | ||
| Positive acute pulmonary history | 52 | 56 |
| Acute pulmonary findings | 35 | 30 |
| Acute diarrhea | 23 | 26 |
| History of chronic fever | 28 | 30 |
| History of chronic cough | 25 | 30 |
The indicated data were missing for the following numbers of individuals: gender, 8; HIV status, 10; mortality status, 118; malaria status, 29; history of chronic fever, 5; history of chronic cough, 4.
HIV seropositivity rates were for 574 participants ≥18 months old, for whom maternal antibody transmission was not a concern. HIV-1 plasma viral titers were evaluated for 78 individuals, excluding 3 HIV+ individuals <1.5 years old without detectable viral RNA. CD4 data were for 138 HIV+ individuals ≥1.5 years old.
None of the values differed significantly by BCG scarring status.
Participants with M. tuberculosis bloodstream infections.
Of these 32 patients, those with or without BCG scarring did not differ in demographic characteristics, vital signs, pulmonary symptoms or histories, malaria smear positivity, or mortality rates (data not shown). No mycobacteremic person with BCG scarring reported acute diarrhea, compared to three of nine without scarring (P = 0.004). Of the 10 patients with immune studies done, those with BCG scarring had higher proportions of memory T cells (CD45RO+), activated CD8+ T cells (CD38+ and/or HLA-DR+), T and B cells making the pro-type 2 cytokine IL-4, and NT cells (a small, immunoregulatory cell population) making both TNF-α and IFN-γ (Table 2). There was very little overlap in the distributions of these parameters for the two scarring groups, and the distributions for those with scarring were extremely skewed toward higher levels (Table 2). Scarring was not associated with differences in the proportions of non-NT cells making type 1 (IL-2 and IFN-γ) or proinflammatory cytokines (TNF-α and IL-8) (data not shown). Those with scarring had T-cell cytokine production skewed toward an anti-inflammatory profile (ratios of the percentages producing IL-4 or IL-10 to the percentages producing IL-8) (Table 2). None of these scar-related immune differences were found in those without M. tuberculosis bloodstream infections or, specifically, in the HIV+ participants without M. tuberculosis bloodstream infections (data not shown).
TABLE 2.
Immune differences between patients with and without BCG vaccine scarring and with M. tuberculosis bloodstream infections
| Parameter | Median value (range) for participants with BCG scarring:
|
P | |
|---|---|---|---|
| Present (n = 6) | Absent (n = 4) | ||
| % of cells making IL-4 | |||
| Lymphocytes (U)a | 5.0 (2.2-15.5) | 2.1 (1.8-4.3) | 0.042 |
| T cells (S) | 2.9 (1.4-7.2) | 0.9 (0.4-1.7) | 0.040 |
| B cells (U) | 2.7 (1.4-12.0) | 1.0 (0.7-1.3) | 0.014 |
| % of NT cellsb making both TNF-α and IFN-γ (U) | 0.5 (0.1-2.4) | 0 (0-0.3) | 0.030 |
| % of T cells making IL-4/% making IL-8 (S) | 3.0 (2.4-12.0) | 0.7 (0-1.2) | 0.014 |
| % of T cells making IL-10/% making IL-8 (S) | 1.6 (0.8-9.9) | 0.7 (0-0.9) | 0.043 |
| % of cells expressing CD45RO | |||
| T cells | 75.8 (47.7-89.6) | 47.5 (36.4-58.1) | 0.043 |
| CD8− T cells | 48.5 (30.1-50.9) | 26.5 (24.5-35.1) | 0.025 |
| CD8+ T cells | 74.7 (51.8-96.0) | 50.7 (41.1-58.3) | 0.043 |
| % of cells expressing CD45RAc | |||
| T cells | 27.9 (11.8-49.5) | 50.7 (40.8-65.5) | 0.043 |
| CD8+ T cells | 23.7 (9.2-38.8) | 45.6 (32.4-64.8) | 0.043 |
| % of cells expressing CD38 | |||
| T cells | 88.9 (77.7-92.9) | 63.6 (41.4-79.6) | 0.025 |
| CD8+ T cells | 92.2 (89.5-95.8) | 59.7 (4.5-83.8) | 0.014 |
| % of CD8+ T cells expressing both CD38 and HLA-DR | 28.5 (11.4-61.0) | 9.2 (1.3-12.3) | 0.025 |
U, without ex vivo stimulation; S, with ex vivo PMA and ionomycin stimulation.
Natural T (NT) cells defined as CD3+ CD16/56+ lymphocytes.
Differences in the proportions of CD8-negative T cells expressing CD45RA did not reach significance (medians: with scarring, 45.8%; without scarring, 60.3%; P = 0.070).
Participants <6 months old.
The age-related trend in Fig. 1 is consistent with children in Malawi being vaccinated in infancy. Therefore, we analyzed this age group separately to assess the possible effects of recent vaccination and also compared those with scarred lesions to those with inflamed BCG lesions, i.e., suppuration at the site of the inoculation occurring within the first few weeks postvaccination (Tables 3 and 4). Ten of 48 children <6 months old had a BCG vaccine scar, five had inflammation at the vaccine site, and 33 had no sign of vaccination (Table 3). Only one other person had inflammation at a BCG vaccination site: a 2.3-year-old HIV+ female with pneumonia and a negative blood culture and malaria smear who survived. In this age group, it is likely that the vast majority of those without any sign of scarring or inflammation had not been vaccinated, since full vaccination healing takes weeks. Supporting this probability, ages were lowest in those without scarring, followed by those with inflammation at the vaccination site, followed by those with scarring. Therefore, we examined three groups of infants: those with scarring postvaccination; those with inflammation at the site of vaccination, presumably representing recent or poorly healing vaccination; and those without evidence of vaccination (Table 3).
TABLE 3.
Characteristics of participants <6 months of age, by BCG vaccine scarring status
| Characteristic | Value for participants with BCG lesion
|
Pa | ||
|---|---|---|---|---|
| Scarred (n = 10) | Inflammed (n = 5) | Absent (n = 33) | ||
| Male (%) | 30 | 40 | 79 | 0.009 |
| Median age (yr) | 0.3 | 0.2 | 0.1 | 0.003 |
| Death (no. dead/no. with outcome data) | 1/9 | 0/4 | 4/23 | 0.625 |
| HIV seropositive (%)b | 22 | 60 | 19 | 0.144 |
| Blood culture positive (all Salmonella spp.) (no./total no.) | 0/10 | 0/5 | 8/33 | 0.158 |
| Proven or highly suspected sepsisc (no./total no.) | 0/10 | 0/5 | 18/33 | <0.001 |
| Localized infectiond (no./total no.) | 1/10 | 3/5 | 0/33 | <0.001 |
Kruskal-Wallis or chi-square tests.
HIV serostatus was not known for one infant with a BCG scar and two infants without BCG lesions.
Positive blood culture and/or leukocytosis, abnormal temperature, and organomegally.
Including bronchiolitis with possible pneumonia, acute otitis media with suspected meningitis, submandibular cellulitis, and HIV-associated oral candidiasis.
TABLE 4.
Immune findings differing by BCG vaccine scarring status of participants <6 months of age
| Finding | Value for participants with BCG lesion
|
Pa | ||
|---|---|---|---|---|
| Scarred (n = 6) | Inflammed (n = 3) | Absent (n = 10) | ||
| Median % of lymphocytes expressing CD4 | 34.2 | 30.8 | 36.2 | 0.341 |
| Median % of lymphocytes making IL-4 spontaneously | 0.6 | 0.6 | 2.7 | 0.013 |
| Median % of lymphocytes making induced IL-4 | 0.4 | 3.8 | 3.4 | 0.040 |
| Median ratio of the % of T cells making IL-4 spontaneously to the % making IL-6 spontaneously | 0 | 0 | 0.3 | 0.021 |
| Median ratio of the % of T cells making induced IL-10 to the % making induced IL-4 | 2.5 | 0.5 | 0.7 | 0.018 |
| Median ratio of the % of monocytes making induced IL-10 to the % making induced IL-6 | 0.6 | 0.1 | 0.1 | 0.034 |
| Median % of lymphocytes making TNF-α spontaneously | 0.4 | 0 | 1.3 | 0.008 |
Kruskal-Wallis tests.
None of these 48 infants had mycobacteremia. Two-thirds of all these infants were male; fewer males than females had BCG scarring (P = 0.022). Males tended to be younger than females, but the difference was not significant (0.1 versus 0.3 years). In a logistic model excluding those with inflammation, the interaction between age and gender made it impossible to determine which of these two variables, if either, had a predominant relationship with BCG scarring (only the interaction variable was significant). Sex was not related to HIV seropositivity rates (males, 22%; females, 25%), nor did males and females differ in admitting diagnoses, symptoms, blood culture positivity rates, or mortality rates.
The proportion of infants with inflammation at the vaccine site that were HIV seropositive was higher than the proportion of those without a lesion or with scarring (Table 3). Three of 5 infants with inflammation at the vaccine site were HIV seropositive (versus 8 of 40 with scarring or no visible BCG lesion); the HIV status of 3 infants was unknown. When age, gender, and the interaction between age and gender were taken into account, the relationship between HIV serostatus and inflammation was of borderline significance (P = 0.050), suggesting that those with HIV infection might have had difficulty with localized healing at the vaccination site. Consistent with this, those with BCG site inflammation had the highest rate of localized infections, followed by those with BCG scarring (Table 3). These localized infections included otitis media with suspected meningitis, submandibular cellulitis, bronchiolitis with possible pneumonia, and HIV-associated thrush. Of the 19 infants <6 month old with immune testing done, the percentages of lymphocytes expressing CD4 for those with scarring, with inflammation, and without scarring were not different (Table 4), nor were the percentages expressing any memory or activation markers (data not shown).
Relationships with either sign of vaccination (scarring or inflammation).
None of those with signs of vaccination (i.e., BCG scarring or inflammation) had documented sepsis or symptoms suggesting sepsis (Table 3). Eight infants without BCG scarring or inflammation had positive blood cultures (all Salmonella spp.), but of these, six were ≤1 month old. One 3-month-old male without a scar had a 4+ positive malaria smear (on a scale of 1+ to 4+). Those with either type of BCG lesion, scarring or inflammation, had lower median percentages of lymphocytes spontaneously making IL-4 or TNF-α and a lower ratio of the percentage of T cells spontaneously making IL-4 to the percentage of those spontaneously making IL-6. In logistic regression analyses with age as an additional independent variable, the percentages of lymphocytes spontaneously making IL-4 and the percentages spontaneously making TNF-α remained significantly related to vaccination status (for the former, beta coefficient [β] = −1.6, conditional odds ratio [OR] = 0.19, 95% confidence interval [CI] = 0.03 to 0.64, and P = 0.016; for the latter, β = −2.8, OR = 0.06, CI = 0.00 to 0.50, and P = 0.033).
Relationships with BCG vaccination site scarring.
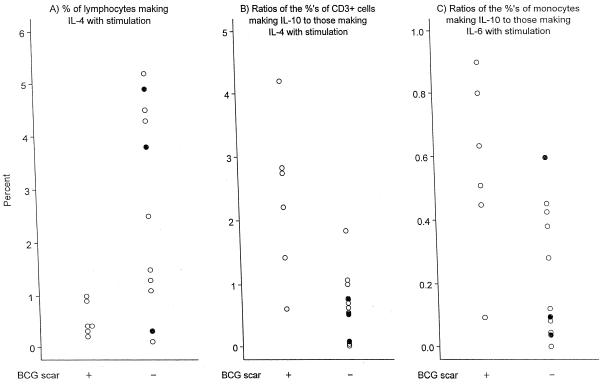
Scarring per se, compared to inflammation at the vaccine site or absence of a BCG-associated lesion, appeared to be associated with lower median proportions of lymphocytes making induced IL-4, a heightened median ratio of T cells making induced IL-10 to those making induced IL-4 (2.5 versus 0.6; P = 0.007), and a heightened median ratio of monocytes making induced IL-10 to those making IL-6 (Fig. 2). In logistic regression analyses with age as an additional independent variable, the T-cell and monocyte ratios remained significantly related to BCG scarring (for the former, β = +2.4, OR = 10.7, CI = 2 to 89, and P = 0.002; for the latter, β = +5.9, OR = 353, CI = 2 to 999, and P = 0.007).
FIG. 2.
Peripheral blood immune findings significantly associated with the presence or absence of a BCG vaccine scar in participants <6 months old. (A) Percentages of lymphocytes making IL-4 with stimulation; (B) ratios of the percentages of CD3+ cells making IL-10 to the percentages of those making IL-4 with stimulation; (C) ratios of the percentages of monocytes making IL-10 to the percentages of those making IL-6 with stimulation. Note that y axis scales differ among the panels. One outlier is excluded from the graph: for one child without a BCG scar, 23% of lymphocytes made IL-4 with stimulation. When this value is excluded from statistical calculations, results remain significant. Solid circles, individuals in the BCG scar-negative group who had inflammation at the BCG vaccination site but no scarring.
DISCUSSION
In this study of hospitalized Malawi patients, we examined the relationships between BCG vaccination scarring and acute clinical and culture findings for all patients, patients with M. tuberculosis bloodstream infections, and infants <6 months of age. Our study has several limitations, which lead us to be conservative in interpreting these data. First, although this is a cohort study, it was not a prospective vaccine study, nor was it designed to directly address the issue of BCG vaccine scarring. Related to this, for the infants, immune studies were not done at specific times after BCG vaccination. Second, the numbers of mycobacteremic patients and BCG-vaccinated infants with immune studies done were small, albeit larger than any numbers in studies previously published. Third, we did not have vaccination records for the participants in this study but instead compared those with and without scarring. This is consistent with most published studies on BCG vaccine efficacy; BCG vaccination scarring routinely is equated with effective BCG vaccination (2, 7, 18, 29, 33, 36-38). BCG vaccination scarring is related to TST reactivity, although the strength of this association varies among studies (4, 6, 26-28, 30; World Health Organization Expert Committee on Biological Standardization, Experimental data for the selection of a reference batch of BCG [W. H. O./BS/802.65]). TST is the most commonly used measure of antimycobacterial type 1, or cellular, reactivity, which is critical for the clearance or control of intracellular organisms.
We had four goals in this study. Our first goal was to examine whether BCG scarring was related to clinical or laboratory findings in the entire hospitalized cohort, in particular, pulmonary symptoms, chronic symptoms suggestive of M. tuberculosis infection, or blood culture positivity for M. tuberculosis. We could not assess the effectiveness of BCG vaccination against localized tuberculosis because lumbar punctures, chest roentgenograms, and gastric aspirates were not routinely done at this hospital. However, we found no difference in acute pulmonary symptoms or in chronic symptoms often associated with tuberculosis, including chronic cough and fever. Of those with localized infection, no infants and only 5 of 21 adults had lesions that could have even conceivably been mycobacterial, i.e., 1 patient with a mandibular mass, 1 with neck swelling, and 3 with submandibular abscesses. With the cohort size and scarring distribution in this study, we had at least a 90% probability of detecting a 15% difference between the scarred and unscarred participants if incidence ranges for the unscarred were anywhere between 5 and 50% (1 − α = 95%). These findings argue against scar-associated BCG vaccination protecting against disseminated or pulmonary mycobacteria in the Malawians receiving health care at this regional hospital. These results also rule against any long-term, systemic protective immune effect of BCG vaccination, except perhaps a gastrointestinal one, as suggested by our findings for mycobacteremic patients and by others (9).
Our second goal was to examine the relationships between the presence or absence of BCG scarring and the clinical and immune profiles of patients with proven M. tuberculosis bloodstream infection. Clinical presentation other than the presence or absence of acute diarrhea did not vary by scarring status, nor did mortality. Immune data indicated that BCG-scarred individuals with mycobacteremia had proportionately more memory T cells and more activated CD8+ T cells than did unscarred persons, consistent with the scarred persons having been previously exposed and responsive to BCG antigens. Those with scarring also had a relatively greater anti-inflammatory cellular cytokine profile, possibly as a regulatory, dampening response to the cellular activation. More important, they had a type 2 dominance, although mycobacterial infection is optimally handled with a type 1 response. These scar-related immune differences were not found in nonmycobacteremic patients. Thus, BCG vaccination was associated with distinct differences in the immune response to M. tuberculosis, but these differences were in a direction opposite to what would be desirable and were not clinically protective in a discernible way.
Third, we examined whether BCG vaccine scarring was in any way related to the severity of HIV infection. HIV requires a type 1 cytokine response, as does containment of mycobacteria, so if there were a long term, non-mycobacterium-specific effect of vaccination, it might affect the severity of HIV disease (i.e., percentages of T cells expressing CD4 and plasma HIV-1 titers). Further, we found that, within our study group, the vast majority of those with mycobacteremia had advanced HIV infection; thus we wanted to be certain that the findings above were associated with mycobacteremia and not with HIV infection. No relationships were found, i.e., for HIV-infected persons, there were no overall associations between BCG vaccination scarring and HIV serostatus, viral load, or the percentages of lymphocytes expressing CD4.
Our fourth goal was to examine early, rather than long-term, relationships between BCG vaccination and immunity, especially because BCG can produce, and has been used clinically to produce, generalized, non-M. tuberculosis-specific, adjuvant-like immune effects (15, 17, 19, 22, 25). As in the older patients in our cohort, in the infants <6 months old, recent vaccination, with either inflammation or scarring, was not associated with mortality. However, both those with BCG vaccine scarring and those with inflammation at the BCG vaccination site appeared to be better able to localize infections than were those without evidence of BCG vaccination. In particular, those with signs of vaccination did not have bacteremia due to Salmonella spp., organisms responsive to type 1 immunity (23), and were less likely to have signs of sepsis. Unlike adults with BCG scarring and mycobacteremia, infants with either scarring or inflammation had cytokine profiles that were less inflammatory and less pro-type 2 than did those infants without evidence of vaccination (lower median percentages of lymphocytes spontaneously making IL-4 or TNF-α and a lower ratio of the percentage of T cells spontaneously making IL-4 to those spontaneously making IL-6). These findings suggest the intriguing possibility that BCG vaccination may have some unplanned, non-mycobacterium-specific, short-term effects that might be beneficial for infants exposed to organisms controlled by type 1 immunity.
In summary, this study makes three important contributions to the BCG literature. First, in all nonmycobacteremic patients >6 months old, BCG scarring had no discernible relationship to any clinical parameter. In mycobacteremic patients, the only relationship found was with acute diarrhea. Second, in patients with confirmed M. tuberculosis bloodstream infections, BCG vaccine scarring was associated with evidence of T-cell activation consistent with prior M. tuberculosis antigen exposure and a relative type 2 cytokine profile, which would not be expected to be protective against mycobacteria, pathogens against which a type 1 response pattern is critical. Thus, the cytokine profile (type 1 versus type 2) induced by mycobacteremia infection may reflect the long-term, BCG-specific effects of vaccination in a given individual. Certainly one cannot assume that scarring implies that a long-term type 1 response pattern to mycobacteria has been conferred by vaccination. Rather, it might be useful to develop an in vitro system to determine whether various antimycobacterial vaccines produce type 1 versus type 2 cytokine responses to mycobacterial antigens and examine these responses in relation to clinical efficacy. Third, in infants <6 months old, scarring after Pasteur Mérieux Connaught BCG vaccine, derived from strain 1077, was associated with an inducible IL-10 dominance, consistent with the literature on the acute effects of BCG as an immunostimulant. In infants, a BCG lesion, be it scarring or inflammation, was associated with a relatively diminished spontaneous proinflammatory and IL-4 cytokine profile and lower rates of bloodstream infection and sepsis symptoms, suggesting an early, non-M. tuberculosis-specific, clinically protective effect of BCG vaccination.
Acknowledgments
We acknowledge the gracious and thoughtful graphing assistance of Timothy A. Green. We thank the nursing and medical staff of Lilongwe Central Hospital and the patients and their parents, who so generously cooperated in this study.
Use of all trade names and commercial sources is for identification only and does not imply endorsement by the Public Health Service or the U.S. Department of Health and Human Services.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Archibald, L. K., L. C. McDonald, R. M. Addison, C. M. McKnight, T. C. Byrne, O. Nwanyanwu, P. Kazembe, H. Dobbie, L. B. Reller, and W. R. Jarvis. 2000. A comparison of Myco-F-Lytic and Lysis-Centrifugation isolator systems for detection of bacteremia, mycobacteremia, and fungemia in febrile hospitalized patients, Lilongwe, Malawi. J. Clin. Microbiol. 38:2994-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awasthi, S., and S. Moin. 1999. Effectiveness of BCG vaccination against tuberculous meningitis. Indian Pediatr. 36:455-460. [PubMed] [Google Scholar]
- 3.Bloom, B. R., and P. E. M. Fine. 1994. The BCG experience: implications for future vaccines against tuberculosis, p. 531-537. In R. B. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. American Society for Microbiology, Washington, D.C.
- 4.Chhatwal, J., M. Verma, N. Thaper, and R. Aneja. 1994. Waning of post vaccinal allergy after neonatal BCG vaccination. Indian Pediatr. 31:1529-1533. [PubMed] [Google Scholar]
- 5.Colditz, G. A., C. S. Berkey, F. Mosteller, T. F. Brewer, M. E. Wilson, E. Burdick, and H. V. Fineberg. 1995. The efficacy of bacille Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analysis of the published literature. Pediatrics 96:29-35. [PubMed] [Google Scholar]
- 6.Collas, R., J. Wright, and J. P. Jardel. 1968. Comparison between tuberculin allergy and the reaction to BCG vaccination. Am. Rev. Respir. Dis. 1017-1031. [DOI] [PubMed]
- 7.Dhadwal, D., R. Sood, A. K. Gupta, S. K. Ahluwalia, A. Vatsayan, and R. Sharma. 1997. Immunization coverage among urban and rural children in the Shimla hills. J. Commun. Dis. 29:127-130. [PubMed] [Google Scholar]
- 8.Dolin, P. J., M. C. Raviglione, and A. Kochi. 1994. Global tuberculosis incidence and mortality during 1990-2000. Bull. W. H. O. 72:213-220. [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott, A. M., J. Nakiyingi, M. A. Quigley, N. French, C. F. Gilks, and J. A. G. Whitworth. 1999. Inverse association between BCG immunization and intestinal nematode infestation among HIV-1-positive individuals in Uganda. Lancet 354:1000-1001. [DOI] [PubMed] [Google Scholar]
- 10.Fine, P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 11.Fine, P. E. M., J. M. Ponnighaus, and N. Maine. 1989. The distribution and implications of BCG scars in northern Malawi. Bull. W. H. O. 67:35-42. [PMC free article] [PubMed] [Google Scholar]
- 12.Floyd, S., J. M. Ponnighaus, L. Bliss, D. K. Warndorff, A. Kasunga, P. Mogha, and P. E. M. Fine. 2000. BCG scars in northern Malawi: sensitivity and repeatability of scar reading, and factors affecting scar size. Int. J. Tuberc. Lung Dis. 4:1133-1142. [PubMed] [Google Scholar]
- 13.Gheorghiu, M. 1990. The present and future role of BCG in tuberculosis control. Biologicals 18:135-141. [DOI] [PubMed] [Google Scholar]
- 14.Imperiali, F. G., A. Zaninoni, L. LaMaestra, P. Tarsia, F. Blasi, and W. Barcellini. 2001. Increased Mycobacterium tuberculosis growth in HIV-1-infected human macrophages: role of tumour necrosis factor-α. Clin. Exp. Immunol. 123:435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson, A. M., A. B. Alexandroff, R. W. Kelly, A. Skibinska, K. Esuvaranathan, S. Prescott, G. D. Chisholm, and K. James. 1995. Changes in urinary cytokines and soluble intercellular adhesion molecule-1 (ICAM-1) in bladder cancer patients after bacillus Calmette-Guerin (BCG) immunotherapy. Clin. Exp. Immunol. 99:369-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jason, J., I. Buchanan, L. Archibald, O. C. Nwanyanwu, M. Bell, T. A. Green, A. Eick, A. Han, D. Razsi, P. N. Kazembe, H. Dobbie, M. Midathada, and W. R. Jarvis. 2000. Natural T, γδ, and NK cells in mycobacterial, Salmonella, and human immunodeficiency virus infections. J. Infect. Dis. 182:474-481. [DOI] [PubMed] [Google Scholar]
- 17.Karonga Prevention Trial Group. 1996. Randomized controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet 348:17-24. [PubMed] [Google Scholar]
- 18.Liard, R., M. Tazir, F. Boulahbal, and S. Perdrizet. 1996. Use of two methods of analysis to estimate the annual rates of tuberculosis infection in Southern Algeria. Int. J. Tuberc. Lung Dis. 77:207-214. [DOI] [PubMed] [Google Scholar]
- 19.Lima, M. C., G. M. Pereira, F. D. Rumjanek, H. M. Gomes, N. Duppre, E. P. Sampaio, I. M. Alvim, J. A. Nery, E. N. Sarno, and M. C. Pessolani. 2000. Immunological cytokine correlates of protective immunity and pathogenesis in leprosy. Scand. J. Immunol. 51:419-428. [DOI] [PubMed] [Google Scholar]
- 20.Lockman, S., J. W. Tappero, T. A. Kenyon, D. Rumisha, R. E. Huebner, and N. J. Binkin. 1999. Tuberculin reactivity in a pediatric population with high BCG vaccination coverage. Int. J. Tuberc. Lung Dis. 3:23-30. [PubMed] [Google Scholar]
- 21.Lucey, D. R., M. Clerici, and G. M. Shearer. 1996. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin. Microbiol. Rev. 9:532-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Donnell, M. A., Y. Luo, X. Chen, A. Szilvasi, S. E. Hunter, and S. K. Clinton. 1999. Role of IL-12 in the induction and potentiation of IFN-α in response to bacillus Calmette-Guerin. J. Immunol. 63:4246-4252. [PubMed] [Google Scholar]
- 23.Ottenhoff, T. H., T. de Boer, C. E. Verhagen, F. A. Verreck, and J. T. van Dissell. 2000. Human deficiencies in type 1 cytokine receptors reveal the essential role of type 1 cytokines in immunity to intracellular bacteria. Microbes Infect. 2:1559-1566. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues, L. C., V. K. Diwan, and J. G. Wheeler. 1993. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int. J. Epidemiol. 22:1154-1158. [DOI] [PubMed] [Google Scholar]
- 25.Sander, B., U. Skansen-Saphir, O. Damm, L. Hakansson, J. Andersson, and U. Andersson. 1995. Sequential production of Th1 and Th2 cytokines in response to live bacillus Calmette-Guerin. Immunology 86:512-518. [PMC free article] [PubMed] [Google Scholar]
- 26.Sedaghatian, M. R., and I. A. K. Shana'a. 1990. Evaluation of BCG at birth in the United Arab Emirates. Tubercle 71:177-180. [DOI] [PubMed] [Google Scholar]
- 27.Sedaghatian, M. R., and K. Kardouni. 1993. Tuberculin response in preterm infants after BCG vaccination at birth. Arch. Dis. Child. 69:309-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sučilienė, E., T. Rønne, A.-M. Plesner, B. Semėnaitė, D. Šlapkavskaitė, S. Olesen-Larsen, and K. Hasløv. 1999. Infant BCG vaccination study in Lithuania. Int. J. Tuberc. Lung Dis. 3:956-961. [PubMed] [Google Scholar]
- 29.Surekha, R. H., V. Vijayalakshmi, K. Sunil, K. A. Lakshmi, L. G. Suman, and K. J. R. Murthy. 1998. Cell-mediated immunity in children with scar-failure following BCG vaccination. Indian Pediatr. 35:123-127. [PubMed] [Google Scholar]
- 30.Tala-Heikkilä, M., T. Nurmela, O. Misljenovic, M. A. Bleiker, and E. Tala. 1992. Sensitivity to PPD tuberculin and M. scrofulaceum sensitivity in school children BCG vaccinated at birth. Int. J. Tuberc. Lung Dis. 73:87-93. [DOI] [PubMed] [Google Scholar]
- 31.Thayyil-Sudhan, S., A. Kumar, M. Singh, V. K. Paul, and A. K. Deorari. 1999. Safety and effectiveness of BCG vaccination in preterm babies. Arch. Dis. Child. 81:64F-66F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toossi, Z., H. Mayanja-Kizza, C. S. Hirsch, K. L. Edmonds, T. Spahlinger, D. L. Hom, H. Aung, P. Mugyenyi, J. J. Ellner, and C. W. Whalen. 2001. Impact of tuberculosis (TB) on HIV-1 activity in dually infected patients. Clin. Exp. Immunol. 123:233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vijayalakshmi, V., P. S. Devi, K. J. R. Murthy, D. V. Rao, and S. N. Jain. 1993. Cell-mediated immune responses in BCG-vaccinated children. Indian Pediatr. 30:899-903. [PubMed] [Google Scholar]
- 34.World Health Organization. 1995. W. H. O. statement on BCG revaccination for the prevention of tuberculosis. Bull. W. H. O. 73:805-806. [Google Scholar]
- 35.World Health Organization. 1987. Joint statement. Consultation on human immunodeficiency virus (HIV) and routine childhood immunization. Wkly. Epidemiol. Rec. 62:297-299. [Google Scholar]
- 36.Zodpey, S. P., B. R. Maldhure, A. G. Dehankar, and N. S. Shrikhande. 1996. Effectiveness of bacillus Calmette Guerin (BCG) vaccination against extra-pulmonary tuberculosis: a case-control study. J. Commun. Dis. 28:77-84. [PubMed] [Google Scholar]
- 37.Zodpey, S. P., S. N. Shrikhande, B. R. Maldhure, N. D. Vasudeo, and S. W. Kulkarni. 1998. Effectiveness of bacillus Calmette Guerin (BCG) vaccination in the prevention of childhood pulmonary tuberculosis: a case control study in Nagpur, India. Southeast Asian J. Trop. Med. Public Health 29:285-288. [PubMed] [Google Scholar]
- 38.Zodpey, S. P., B. R. Maldhure, S. N. Shrikhande, and P. R. Tiwari. 1996. Effectiveness of bacillus of Calmette-Guerin (BCG) vaccination against tuberculous meningitis: a case-control study. J. Indian Med. Assoc. 94:338-340. [PubMed] [Google Scholar]