Abstract
Naturally occurring antibodies against [Gal α-1,3-Gal] structures (anti-Gal antibodies) are the primary effectors of human hyperacute rejection (HAR) of nonhuman tissue. Unlike most mammals, humans lack a functional α-1,3-galactosyltransferase (GalT) gene and produce abundant anti-Gal antibodies, putatively in response to GalT+ enteric bacteria. GalT knockout (KO) mice have been generated as a small animal model of HAR but inconsistently express anti-Gal antibodies. We hypothesized that enteric exposure of GalT KO mice to live GalT+ bacteria would produce cytolytic anti-Gal antibodies. Naive mice lacking anti-Gal antibodies were orally immunized with 1010 live GalT+ Escherichia coli O86:B7 bacteria and assayed for anti-Gal antibody titer, isotype, and cytolytic activity. Fecal samples were tested for E. coli O86:B7 prior to and after inoculation. In two separate experiments, 77 to 100% (n = 31) of mice developed serum anti-Gal immunoglobulin G (IgG; titer, 1:5 to 1:80) and/or anti-Gal IgM antibodies (titer, 1:5 to 1:1,280) 14 days postinoculation. Induced anti-Gal antibodies caused complement-mediated cytolysis of GalT+ target cells, with extensive cytolysis observed consistently at serum IgM titers of ≥1:320. Absorption with synthetic [Gal α-1,3-Gal] inhibited both antibody binding and cytolysis. E. coli O86:B7 was recovered from stool samples from 83 to 94% of inoculated mice but not from naive mice, thus confirming enteric exposure. These findings demonstrate that oral inoculation with E. coli O86:B7 is a novel and effective method to induce cytolytic anti-Gal antibodies in GalT KO mice and support the premise that enteric exposure to GalT+ bacteria induces anti-Gal antibodies in humans. These studies also suggest a role for GalT KO mice in elucidating anti-Gal responses in microbial immunity.
Naturally occurring human anti-Gal antibodies recognize cell surface [Gal α-1,3-Gal] glycoconjugates expressed abundantly on porcine and other mammalian cells. All mammals except humans, apes, and Old World monkeys (catarrhine species) exhibit [Gal α-1,3-Gal] glycoconjugates due to the activity of the α-1,3-galactosyltransferase (GalT) gene (8, 11). In catarrhine species the GalT gene exists only as a mutationally inactivated pseudogene and [Gal α-1,3-Gal] glycoconjugates are not expressed; they are instead antigenic and induce abundant serum anti-Gal antibodies (8, 9, 11, 16). Various enveloped viruses, bacteria, and protozoan parasites also express [Gal α-1,3-Gal] structures (2, 10, 26, 31).
It is accepted that enteric exposure to gram-negative bacteria expressing cell wall or lipopolysaccharide [Gal α-1,3-Gal] structures induces human anti-Gal antibody production, similar to the development of human antibodies against ABO blood group antigens (10). The [Gal α-1,3-Gal] glycoprotein is structurally similar, in fact, to the human blood group B antigen and was first reported as a “B-like” antigen on rabbit erythrocytes (7). Anti-Gal immunoglobulin M (IgM) antibodies appear after birth, correlating with neonatal microbial gut colonization and ABO antibody development, and are expressed throughout life (8, 22, 24). Human anti-Gal and anti-ABO antibodies, known as naturally occurring antibodies, are ubiquitous and not initially induced by classical peptide antigens. These antibodies are considered to result from humoral responses to polysaccharide antigens and to be comprised mainly of low-affinity, cold-agglutinating IgM (23).
Anti-Gal antibodies are the primary antibodies in humans that mediate hyperacute rejection (HAR) of nonhuman (i.e., porcine) organs by binding to mammalian [Gal α-1,3-Gal] glycoproteins (24, 28, 37). HAR occurs when preexisting antidonor antibodies induce rapid, complement-mediated graft destruction. Immune responses mediated by endogenous human anti-Gal antibodies thus pose major obstacles to the use of nonhuman organ donors in transplantation.
Catarrhine nonhuman primate species are the only naturally occurring mammalian models for human HAR. Wild-type mice express GalT and thus lack anti-Gal antibodies (11, 15). To provide a small animal model of HAR, GalT knockout (KO) mice have been generated by several groups and were initially reported to endogenously express anti-Gal antibodies (18, 33, 34).
However, we and others have found that GalT KO mice, in stark contrast to humans, fail to consistently express detectable anti-Gal antibodies (3, 21, 25; K. J. Posekany, H. K. Pittman, C. E. Haisch, and K. M. Verbanac, Proc. 24th Annu. Meet. Am. Soc. Transplant Surg. 1998, abstr. A-247, p. 549, 1998). To induce these antibodies and allow studies of anti-Gal immune responses in GalT KO mice, other investigators have employed immunization regimens involving injection of GalT+ eukaryotic cells such as rabbit erythrocytes, mouse splenocytes, and Leishmania major promastigotes (3, 17, 21, 25). These methods used multiple intraperitoneal immunizations of formalin-fixed cells or membrane lysates that clearly do not recapitulate the normal etiology by which human anti-Gal antibodies are induced.
In the present study, we hypothesized that immunization of naive GalT KO mice via oral inoculation with live GalT+ bacteria would induce production of cytolytic anti-Gal antibodies due to enteric exposure to bacterial [Gal α-1,3-Gal] antigens in a manner analogous to the natural development of human anti-ABO antibodies. Inoculation was performed by oral gavage with live GalT+ Escherichia coli O86:B7 bacteria. Inoculated GalT KO mice were tested for the development of serum anti-Gal antibodies and characterized as to anti-Gal titer, isotype, and cytolytic activity. Enteric exposure of orally inoculated mice was confirmed by testing fecal samples for the presence of live E. coli O86:B7.
MATERIALS AND METHODS
Mice.
GalT KO mice generated by J. B. Lowe were obtained and housed as breeder pairs (34). Progeny mice were caged separately and given tap water and commercial mouse chow. All studies were performed under appropriate institutional and Association for Assessment and Accreditation of Laboratory Animal Care guidelines. Mice were anesthetized by 0.5 to 3% Metafane (Mallinckrodt Veterinary, Mundelein, Ill.) inhalant anesthetic during retroorbital puncture. Mice were screened for endogenous anti-Gal antibody expression as described below.
Culture of Lewis lung carcinoma target cells.
A subclone of the murine Lewis lung carcinoma (LLCa) cell line (19) was kindly provided by C. J. Kovacs and M. Evans, Department of Radiation Biology and Oncology, East Carolina University School of Medicine. This subclone was derived from a bone marrow metastasis in an LLCa tumor-bearing mouse and used to provide GalT+ target cells for anti-Gal binding and cell cytolysis assays. Cells were grown in culture flasks in a humidified, 5% CO2 incubator with 300 mosM high-glucose Dulbecco modified Eagle medium (pH 7.2) supplemented with 10% fetal bovine serum (Gibco-BRL, Gaithersburg, Md.). Cells were harvested by using 0.25% trypsin (Gibco-BRL) and washed twice with culture media.
Culture of E. coli O86:B7 and inoculation of GalT KO mice.
E. coli O86:B7 was obtained from the American Type Culture Collection (Rockville, Md.). Stocks were frozen at −80°C as aliquots of 2 × 106 cells in 50% glycerol. Bacteria were plated for isolation on Luria agar, and colonies were selected, inoculated into 100 ml of Luria broth, grown at 37°C on a shaker rack, and harvested during exponential growth. The approximate cell number was determined by measuring the spectrophotometric absorbance at 600 nm. GalT KO mice were screened to confirm the absence of endogenous anti-Gal antibodies, and naive mice were split into control and test groups matched for age and sex. To optimize gut colonization with E. coli O86:B7, mice were treated for 72 h with 5 g of streptomycin sulfate (Gibco-BRL)/liter in drinking water and then given regular water for 24 h prior to oral gavage. Test mice were inoculated once by oral gavage with 1010 E. coli O86:B7 bacteria in 100 μl of sterile phosphate-buffered saline (PBS) by using a 22-gauge stainless steel animal feeding needle (Popper & Sons, Inc., New Hyde Park, N.Y.); control mice were not inoculated by gavage and received streptomycin treatment only.
Determination of GalT KO mouse and human anti-Gal antibody isotype and titer analysis by flow cytometry.
Mouse sera obtained by retroorbital puncture from control and test mice prior to and at day 14 postgavage were immediately stored at −80°C. The antibody titer was determined by serial dilution to give dilutions of 1:5 through 1:1,280. LLCa target cells (104) were incubated with heat-inactivated sera diluted in 100 μl of PBS containing 0.1% bovine serum albumin and 0.5% sodium azide for 10 min at room temperature and for 30 min at 4°C to facilitate the binding of both normal and cold-agglutinating antibodies. Target cells were washed, incubated with 50 μl of a 1:50 dilution of combined goat anti-mouse IgM-phycoerythrin (PE) and IgG-fluorescein isothiocyanate (FITC) antibodies (Jackson Immunoresearch Laboratories, West Grove, Pa.), washed, and stored fixed at 4°C in 250 μl of 1.85% formaldehyde-PBS prior to flow cytometric analysis. Target cells incubated with C57BL/6 mouse sera were used as controls for nonspecific fluorescence.
Human serum from healthy adult blood group B or AB donors was obtained by antecubital venipuncture according to appropriate institutional guidelines and assayed as described above for antibody titer and isotype with combined goat anti-human IgM-FITC and IgG-PE antibodies (Jackson Immunoresearch Laboratories). Using a FACSCAN flow cytometer (Becton Dickinson, Mountain View, Calif.), single-parameter logarithmic (log) red and log green geometric median channels of fluorescence intensity were obtained for each sample at each dilution. Target cells stained with secondary antibody only were used as controls for nonspecific fluorescence.
Control samples (C57BL/6 serum or secondary antibody only) were set at a geometric mean fluorescence intensity of 75 channels for each assay. A channel shift of 15 or more channels from the control was considered positive. An antibody titer was defined as the last dilution giving a positive channel shift.
Determination of serum cytolytic activity by flow cytometry.
Wild-type C57BL/6 mouse serum, GalT KO mouse serum, or normal human serum (NHS) was obtained as described above. Complement activity was maintained by rapidly freezing sera at −80°C or holding it at 4°C for immediate analysis. Exponentially growing LLCa cells (2.5 × 104) were incubated with a 1:5 dilution of fresh or frozen sera in Dulbecco modified Eagle medium for 60 min at 37°C. Samples were stained with a 10-μg/ml propidium iodide (PI) solution and immediately analyzed for PI uptake. Log red fluorescence was displayed versus linear side scatter, and the cell viability was determined by using Lysis software (Becton Dickinson) to calculate the percentage of dead (PI+) cells. LLCa cells treated with culture media only were used as controls for basal cell viability. LLCa cell cultures with basal viabilities of <95% were discarded.
Serum absorption with synthetic [Gal α-1,3-Gal] disaccharide.
Absorption studies were performed by preincubation of 20 μl of 1:5-diluted serum with 20 μl of 20 mM synthetic disaccharide (α-1-3-d-galactobiose; V-Labs, Covington, La.) for 18 h at 4°C prior to use in antibody-binding or cytolysis assays. Duplicate serum samples were incubated with 20 μl of 20 mM lactose [Gal β-1,4-Glc] (Sigma, St. Louis, Mo.) as a negative disaccharide control. Serum absorbed against either lactose or synthetic [Gal α-1,3-Gal] was assayed as described for anti-Gal antibody binding and cytolytic activity.
Identification and recovery of E. coli O86:B7 from GalT KO mouse stool specimens.
Fresh fecal pellets were obtained from control and test mice prior to streptomycin treatment and at 3 days postgavage. Pellets were mechanically dispersed in 200 μl of sterile PBS and streaked for isolation onto MacConkey agar plates. After overnight incubation at 37°C, lactose-fermenting colonies were selected. The BSI-B4 subunit of Bandeiraea simplicifiola lectin (IB4) has an exclusive affinity for terminal α-d-galactosyl residues and is widely used to detect surface [Gal α-1,3-Gal] structures (11). Colonies were split and either stained with IB4-FITC (Sigma) or submitted to the E. coli Reference Center, Pennsylvania State University, for serotypic analysis to confirm the presence of E. coli O86:B7.
Flow cytometric IB4-FITC staining to determine expression of surface [Gal α-1,3-Gal] structures.
LLCa cells were harvested as described, and 105 cells were stained with 10 μg of IB4-FITC/ml in PBS-bovine serum albumin-azide for 30 min at 37°C, washed, and stored refrigerated in 1.85% formaldehyde PBS for flow cytometric analysis. C57BL/6 or GalT KO mouse splenocytes were obtained by Ficoll-Hypaque density gradient centrifugation of mechanically dispersed splenic tissue and then stained with IB4-FITC as described above. E. coli O86:B7 and lactose-fermenting fecal isolate colonies were removed from plates and resuspended in PBS, and 105 cells were stained with IB4-FITC. Inhibition studies were performed by preincubating 50 μl of 10 μg of IB4-FITC/ml with 50 μl of 10 mM synthetic disaccharide for 18 h at 4°C prior to cell staining. Duplicate samples were incubated with 50 μl of 10 mM lactose as a negative disaccharide control.
RESULTS
Determination of cell surface [Gal α-1,3-Gal] structures.
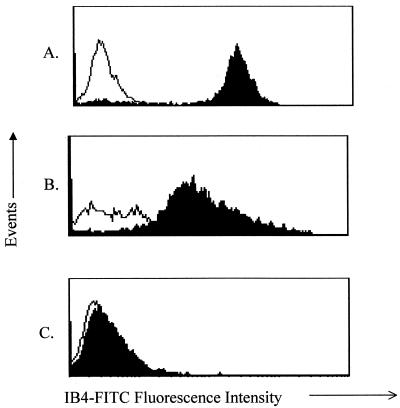
To confirm the appropriate expression of [Gal α-1,3-Gal] structures on the murine cells used in these studies, GalT KO mouse splenocytes, C57BL/6 splenocytes, and LLCa adenocarcinoma cells were stained with IB4-FITC, which binds surface [Gal α-1,3-Gal] structures. LLCa cells and C57BL/6 splenocytes were positive, a finding consistent with the reported species-restricted expression of the mammalian GalT gene (Fig. 1A and B). Staining was inhibited by absorption of lectin with synthetic [Gal α-1,3-Gal] disaccharide but not by lactose [Gal β-1,4-Glc], demonstrating that IB4-FITC staining was due to specific binding to [Gal α-1,3-Gal] structures (data not shown). As expected, splenocytes from GalT KO mice did not bind IB4-FITC (Fig. 1C).
FIG. 1.
Expression of surface [Gal α-1,3-Gal] structures on cells from GalT wild-type mice but not GalT KO mice. Open histograms show data for unstained cells. Filled histograms show data for IB4-FITC-stained cells. (A) Murine LLCa target cells. (B) GalT+ C57BL/6 splenocytes. (C) GalT KO splenocytes. Cells were stained with IB4-FITC to detect [Gal α-1,3-Gal] structures and then analyzed by flow cytometry as described. A second experiment yielded identical results.
Oral gavage with E. coli O86:B7.
Only 16% (n = 120) of mice tested in our colony demonstrated anti-Gal antibodies de novo, with no apparent correlation between antibody expression and age, sex, housing, or parentage. To determine whether oral immunization of naive GalT KO mice with live GalT+ bacteria would consistently induce serum anti-Gal antibodies, GalT KO mice lacking anti-Gal antibodies were orally inoculated with 1010 live E. coli O86:B7 bacteria. This is a wild-type strain of marginal pathogenicity and expresses repeating [Gal α-1,3-Gal] cell wall structures that bind human anti-Gal antibodies (10). Historical studies into the development of human ABO antibodies have shown that type A and O humans orally inoculated with E. coli O86:B7 developed increased blood group B antibodies (31).
All animals were treated with streptomycin in drinking water for 72 h and then given untreated water for 24 h. Test mice were then inoculated with E. coli O86:B7 by oral gavage; control GalT KO mice were not. No apparent morbidity or mortality occurred in any of the test or control KO mice due to streptomycin treatment and/or oral gavage with live E. coli O86:B7. Mice remained healthy and active, with no detectable weight loss, diarrhea, or observable abnormalities.
Recovery and identification of E. coli O86:B7 from mouse stool.
Studies were performed to confirm that oral inoculation with E. coli O86:B7 resulted in enteric exposure to microbial [Gal α-1,3-Gal] antigens. E. coli O86:B7 cultures and postgavage fecal isolates were characterized by their ability to ferment lactose (E. coli O86:B7 is a strong lactose fermenter), staining with IB4-FITC, and serotypic analysis. Since microbial isolates from a single fecal pellet are not representative of the entire gastrointestinal flora, the absence of E. coli O86:B7 in a specimen may be an artifact of sampling rather than a true negative.
In two separate experiments, lactose-fermenting fecal bacterial colonies were isolated from 88 to 91% of orally inoculated mice at day three postgavage. Bacterial suspensions from entire plates that exhibited lactose-fermenting colonies were stained with IB4-FITC and yielded 30 and 45% positive samples. No lactose-fermenting, IB4-FITC-positive fecal isolates were recovered from test mice prior to oral gavage or from control mice after streptomycin treatment. E. coli O86:B7 expression of surface [Gal α-1,3-Gal] structures was confirmed by IB4-FITC binding (Fig. 2C). Fecal isolates from mice inoculated by gavage with E. coli O86:B7 were also IB4-FITC positive (Fig. 2D), in contrast to isolates from noninoculated GalT KO mice (Fig. 2A and B). IB4-FITC staining of E. coli O86:B7 and postinoculation fecal isolates was inhibited by absorption with synthetic [Gal α-1,3-Gal] but not with negative control [Gal β-1,4-Glc] (Fig. 2E and F). Serotype E. coli O86:B7 was recovered in 83% (5 of 6 mice) and 94% (16 of 17 mice) of lactose-fermenting fecal samples analyzed. These results confirm both the specific expression of [Gal α-1,3-Gal] structures on E. coli O86:B7 and enteric exposure to 086:B7 in mice inoculated by gavage as demonstrated by the recovery of live E. coli O86:B7 in fecal isolates.
FIG. 2.
Absorption with synthetic [Gal α-1,3-Gal] inhibits IB4-FITC staining of E. coli O86:B7 and fecal bacterial isolates from orally inoculated GalT KO mice. Open histograms indicate results for unstained bacteria. Filled histograms indicate results for IB4-FITC-stained cells. (A) Fecal isolates from untreated GalT KO mice. (B) Fecal isolates from GalT KO mice treated only with streptomycin. (C) E. coli O86:B7 culture. (D) Fecal bacteria from inoculated GalT KO mice. (E and F) E. coli O86:B7 or fecal bacteria samples stained with IB4-FITC absorbed with synthetic [Gal α-1,3-Gal] disaccharide (filled histogram) or lactose (open histogram).
Titer and isotype of induced anti-Gal antibodies.
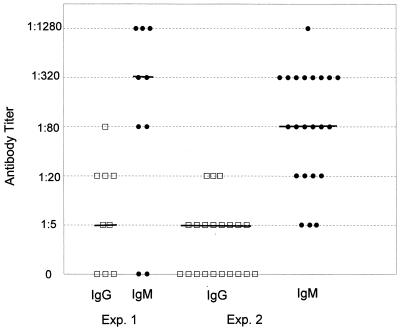
Sera were obtained from all mice 14 days postgavage and individually tested for anti-Gal antibodies by binding to GalT+ target cells. IgM and IgG antibody binding were compared in each test mouse before and after oral inoculation and in control GalT KO mice. In two separate experiments, antibodies were induced in 77% (n = 9) and 100% (n = 22) of test mice at day 14 postinoculation. IgG titers ranged from 1:5 to 1:80 (median titer of 1:5), whereas IgM titers ranged from 1:5 to 1:1,280 (median titers of 1:320 and 1:80). IgG and IgM titers for each test mouse are plotted in Fig. 3. In three subsequent studies, 88% (n = 12), 94% (n = 19), and 100% (n = 16) of orally inoculated mice developed antibodies of comparable titer and isotype at day 14 postgavage (data not shown), thus confirming the reproducibility of this method of immunization. None of the test GalT KO mice showed detectable antibody binding prior to oral inoculation, and none of the nonimmunized, control mice developed antibodies (data not shown). No significant binding to GalT+ target cells was found in sera from C57BL/6 or naive GalT KO mice, which lack anti-Gal antibodies (Fig. 4A). These results demonstrate that anti-Gal antibodies were induced specifically by inoculation with E. coli O86:B7 and not by alterations in endogenous gut flora.
FIG. 3.
Distribution of anti-Gal IgG and IgM titers in orally inoculated GalT KO mice at day 14 postgavage. Anti-Gal antibody binding titer and isotype were determined by incubating GalT+ LLCa target cells with serially diluted sera from immunized GalT KO mice. Target cells were then incubated with goat anti-mouse IgM-PE and IgG-FITC antibodies and analyzed by using a FACSCAN flow cytometer as described earlier. Each symbol represents a serum sample from one individual mouse. No antibody binding was detected in test mice prior to oral inoculation or in control mice after streptomycin treatment. Symbols: □, IgG titer; •, IgM titer. The bar indicates the median titer.
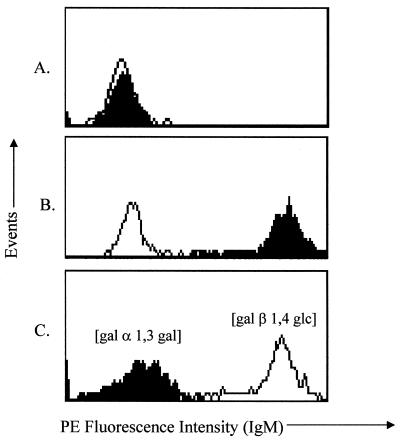
FIG. 4.
Antibodies induced in GalT KO mice by oral inoculation are specific for [Gal α-1,3-Gal]. Sera (1:5) were absorbed with 20 mM synthetic [Gal α-1,3-Gal] disaccharide for 18 h at 4°C. Duplicate samples were incubated with 20 mM lactose [Gal β-1,4-Glc] as a negative disaccharide control. Sera were then assayed for antibody binding to GalT+ target cells as described in the text. (A) Control mouse sera. The open histogram indicates results for the C57BL/6 serum. The filled histogram indicates results for the naive GalT KO serum. Target cells incubated only with secondary antibody yielded identical binding results. (B) Immunized GalT KO mice. The open histogram indicates results for the C57BL/6 serum control. The filled histogram indicates results for the serum from inoculated GalT KO mice. (C) Serum from inoculated GalT KO mice absorbed with 20 mM synthetic [Gal α-1,3-Gal] disaccharide (filled histogram) or 20 mM lactose (open histogram). Sera from nine representative immunized mice were assayed with similar results.
To confirm the specificity of induced antibodies for [Gal α-1,3-Gal] structures, sera from inoculated mice were absorbed with synthetic [Gal α-1,3-Gal] disaccharide or with lactose [Gal β-1,4-Glc] as a nonspecific disaccharide control. Sera from immunized GalT KO mice demonstrated strong antibody binding to GalT+ target cells (Fig. 4B). This binding was profoundly inhibited when sera were absorbed with [Gal α-1,3-Gal] disaccharide but not by absorption with lactose (Fig. 4C). These results show that the primary antibodies induced by oral immunization were specific for [Gal α-1,3-Gal] structures expressed on LLCa target cells.
Target cell cytolysis.
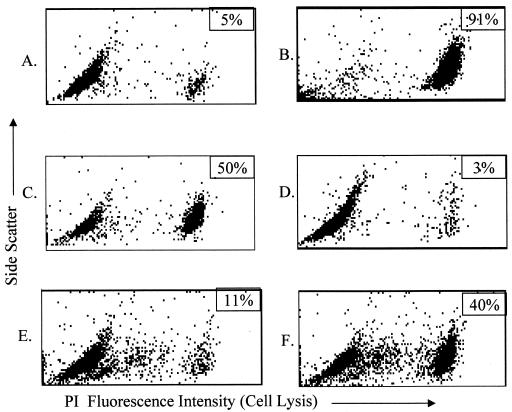
Serum cytolytic activity was assayed by measuring the PI uptake of GalT+ target cells exposed to GalT KO mouse sera. Anti-Gal antibodies induced by inoculation with E. coli O86:B7 caused complement-mediated cytolysis of LLCa target cells. Results from eight representative orally immunized mice with anti-Gal IgM titers of 1:320 or greater are presented. Cytolysis was negligible (≤5%) in target cells treated with media only or exposed to sera from antibody-negative C57BL/6 mice or nonimmunized, control GalT KO mice (Fig. 5A to C). In contrast, NHS (Fig. 5D) and sera from immunized GalT KO mice induced extensive (57 to 78%) target cell cytolysis (Fig. 5E and F). Cytolytic activity was completely removed from GalT KO sera by heat inactivation of complement and significantly inhibited by the absorption of serum samples with synthetic [Gal α-1,3-Gal] disaccharide (Fig. 6D and E) compared to untreated serum from immunized GalT KO mice (Fig. 6C). Absorption with lactose did not inhibit cytolysis (Fig. 6F). Again, treatment with media only did not induce cytolysis, whereas NHS induced extensive cytolysis (Fig. 6A and B). These results show that the cytolytic activity against LLCa cells in sera from orally immunized GalT KO mice is due to complement-fixing antibodies specific for [Gal α-1,3-Gal] structures.
FIG. 5.
Sera from inoculated GalT KO mice induce cytolysis of GalT+ target cells. LLCa cells were incubated with a 1:5 dilution of fresh sera for 60 min at 37°C and then assayed for viability by staining with 10 μg of PI/ml, followed by flow cytometric analysis of PI uptake as described in the text. (A and B) Media only and C57BL/6 sera used as negative controls. (C) Naive GalT KO sera. (D) NHS positive control. (E and F) Sera from immunized GalT KO mice. The percentage of dead (PI+) cells per sample is shown in the upper right histogram box. Sera from eight representative immunized GalT KO mice were assayed and yielded similar results.
FIG. 6.
Cytolytic activity of immunized GalT KO serum is inhibited by heat inactivation of complement or absorption with synthetic [Gal α-1,3-Gal] disaccharide. Serum from immunized GalT KO mice was heat inactivated or absorbed with synthetic [Gal α-1,3-Gal] prior to incubation with LLCa target cells. Cytolytic activity was assayed by measuring PI uptake as described in the text. (A) Media only. (B) NHS. (C) Intact serum from immunized GalT KO mice. (D) Heat-inactivated serum from immunized GalT KO mice. (E) Serum from immunized GalT KO mice absorbed with synthetic [Gal α-1,3-Gal] disaccharide. (F) Duplicate sample absorbed with lactose. The percentage of dead (PI+) cells per sample is shown in the upper right histogram box. Sera from three representative immunized GalT KO mice were assayed and yielded similar results.
Anti-Gal antibody titer, isotype, and cytolytic activity in NHS.
In order to determine whether immunized GalT KO mouse anti-Gal antibody titers, isotypes, and cytolytic activities were comparable to those in human sera, five normal blood group B or AB individuals were tested. Anti-Gal antibodies in blood group B or AB sera react only with [Gal α-1,3-Gal]; group A or O serum anti-Gal antibodies can cross-react with the blood group B antigen (8, 23). Anti-Gal antibody titers, isotypes, and cytolytic activity against LLCa target cells were assayed as described. Anti-Gal titers ranged from 1:5 to 1:320 for IgG (median titer of 1:80) and 1:80 to 1:1,280 for IgM (median titer of 1:320). All NHS samples induced 75 to 96% complement-mediated cytolysis of GalT+ LLCa target cells (mean, 85% lysis). Both antibody binding and cytolytic activity were inhibited by absorption with synthetic [Gal α-1,3-Gal] disaccharide but not by lactose (data not shown).
DISCUSSION
These studies demonstrate that oral inoculation with live E. coli O86:B7 efficiently induces cytolytic anti-Gal IgM antibodies in naive GalT KO mice by enteric exposure to microbial [Gal α-1,3-Gal] antigens. Induced antibodies were specific for [Gal α-1,3-Gal] cellular structures, since both antibody binding and cytolysis were inhibited by serum absorption with synthetic [Gal α-1,3-Gal]. Serum cytolytic activity against GalT+ target cells was also totally removed by serum heat inactivation to destroy complement.
Anti-Gal IgM titers and cytolytic activities were comparable in both immunized GalT KO mice and humans and were greater overall than the IgG titers in both. We found that IgM was the predominant anti-Gal isotype produced in our GalT KO mice by oral immunization. This finding is in agreement with that of Cretin et al., who reported that IgM antibodies comprise the majority of αGal-specific antibodies in GalT KO mice (4). Although the preponderance of anti-Gal IgM antibodies has been considered indicative of a T-cell-independent response to [Gal α-1,3-Gal] antigens, two recent studies of anti-Gal antibody induction in GalT KO mice have demonstrated the requirement for T-cell help in the production of both anti-Gal IgM and anti-Gal IgG murine antibodies (4, 32).
Cytolytic activity was slightly higher in NHS (75 to 96%; mean, 85%) than in sera from GalT KO mice (50 to 80%; mean, 68%) at similar binding titers. There is some evidence that humans and mice may differ in their ability to utilize antibody or complement systems in mediating tissue rejection (21, 25). The affinities of induced anti-Gal IgG and IgM in GalT KO mice have been reported to be 20 to 30% less than the affinities of human anti-Gal IgG and IgM (3). These factors could explain the slightly decreased cytolytic activity demonstrated by GalT KO sera compared to human sera of comparable IgM titer.
GalT KO mice do not consistently produce anti-Gal antibodies, a finding in stark contrast to the situation in humans, in whom anti-Gal antibody production is ubiquitous. This unanticipated finding may simply be due to differences in environmental exposure to strains of E. coli, Salmonella, Serratia, and Klebsiella bacteria that exhibit [Gal α-1,3-Gal] antigens (10). Various GalT+ inbred mouse strains housed in the East Carolina University animal facility demonstrated IB4-FITC-positive fecal bacteria and yet demonstrated no serum anti-Gal antibodies, confirming that GalT+ wild-type mice are immunotolerant to [Gal α-1,3-Gal] antigens. No systematic epidemiologic studies have compared the incidence of exposure to [Gal α-1,3-Gal]-positive pathogens in laboratory mice to that in humans.
Interestingly, the E. coli O86:B7 strain was originally isolated from a human infant (30) and is occasionally detected in contaminated ground meat for human consumption (unpublished data from Richard Wilson, E. coli Reference Center, Pennsylvania State University) and thus likely contributes to the development of anti-Gal antibodies in the human population. Studies in baboons have shown significant decreases in anti-Gal antibody production after elimination of endogenous gram-negative enteric bacteria (20), supporting the premise that anti-Gal antibodies are produced in response to bacteria expressing [Gal α-1,3-Gal] antigens.
Our studies only indirectly addressed the persistence of E. coli O86:B7 gut colonization and anti-Gal antibody production in orally inoculated mice. Antibody titers were found to peak at day 14 postgavage (data not shown). Of nine mice screened at 27 and 40 days postgavage, four demonstrated anti-Gal titers; three of these animals also had GalT+ fecal bacteria (data not shown). These findings suggest that continual or intermittent antigenic exposure is necessary for the lifelong production of anti-Gal antibodies.
Human anti-Gal antibodies have been studied most extensively in their biologic role as effectors of HAR. However, HAR is not a normal manifestation of the function of anti-Gal antibodies but rather an immunologic artifact occurring as a result of xenotransplantation (29, 38). It has been postulated that mutational inactivation of the GalT gene in humans and apes occurred during evolution as a result of selective pressure exerted by GalT+ pathogens against catarrhine species immunotolerant to [Gal α-1,3-Gal] antigens (6). Anti-Gal antibodies of IgG, IgM, and IgA isotypes are found in the sera and secretory fluids of all humans, and [Gal α-1,3-Gal] structures are present on the lipopolysaccharide or capsular polysaccharides of many gram-negative bacteria that normally colonize the human gut (10, 13, 14). The ubiquitous production of anti-Gal antibodies in humans and the expression of [Gal α-1,3-Gal] antigens on a variety of microbial pathogens argue that anti-Gal immune responses may have distinct functions in microbial immunity.
The incidence of E. coli septicemia in humans has been shown to be significantly higher in blood group B or AB individuals, suggesting a role for anti-Gal antibodies in protection against bacterial sepsis (39). Expression of [Gal α-1,3-Gal] structures on sequestered lipopolysaccharide molecules rather than the cell wall may protect certain bacterial strains from exposure to serum anti-Gal antibodies and complement, contributing to septicemic infections (10, 14). Binding of anti-Gal IgA to the pili of Neisseria meningitidis has been shown to compete with anti-Gal IgG binding and to inhibit complement-mediated cytolysis of piliated strains (12). Infection with E. coli and other gram-negative bacteria has been associated with elevated anti-Gal antibodies and autoimmune disease due to IgA immune complex deposition in children with Henoch-Schönlein purpura or IgA nephropathy (5).
Studies have also suggested a role for anti-Gal in host defense against parasitic infection. Trypanosoma, malarial, and Leishmania protozoan parasites all express surface [Gal α-1,3-Gal] antigens, and patients infected with these organisms show elevated anti-Gal antibody levels in serum. In vitro exposure to human anti-Gal antibodies inhibits growth of Plasmodium falciparum merozoites in culture (26). Pretreatment with human anti-Gal antibodies also inhibits the infectivity of Trypanosoma cruzi promastigotes in mice (2).
Human serum anti-Gal antibodies are believed to provide a species barrier against the transmission of mammalian enveloped viruses to humans. Mammalian C-type retroviruses express viral envelope [Gal α-1,3-Gal] glycoproteins derived from the host cell membrane and are inactivated by NHS anti-Gal antibodies and complement. However, human immunodeficiency virus and T-cell leukemia retroviruses are resistant to NHS (31). When human immunodeficiency virions are propagated in human HUT-78 cells transduced to express GalT, the retroviral particles express [Gal α-1,3-Gal] structures and are inactivated by human serum (27). The inactivation of other enveloped viruses such as lymphocytic choriomeningitis and Sindbis virus by NHS is also dependent on passage through GalT+ cell lines (38).
Interestingly, studies have shown that [Gal α-1,3-Gal] epitopes are expressed on human cervical cells infected with oncogenic, nonenveloped papillomavirus (35). Levels of anti-Gal antibody in serum are increased in patients with cervical intraepithelial lesions and carcinoma due to papillomavirus infection (36). Anti-Gal antibodies have also been shown to cross-react with aberrantly glycosylated mucin peptides expressed by certain human tumors (1, 29).
Our results with oral inoculation indicate that GalT KO mice may be useful in elucidating the roles of anti-Gal antibodies in human immunity to pathogens that express [Gal α-1,3-Gal] antigens. This study demonstrates that oral inoculation of GalT KO mice with E. coli O86:B7 induces anti-Gal IgM antibodies comparable in titer and cytolytic activity to human serum. These findings also suggest that this method of immunization recapitulates the normal etiology by which human anti-Gal antibodies are induced and support the hypothesis that enteric exposure to GalT+ bacteria accounts largely for the production of anti-Gal antibodies in humans.
Acknowledgments
This work was supported by a Sigma Xi Grant-in-Aid to support the research project “Evaluation of Lewis Lung Cell Carcinoma Tumor Growth and Metastasis in the α-1,3-Galactosyltransferase Knockout Mouse” and by ECU Faculty Research award 265531.
Editor: B. B. Finlay
REFERENCES
- 1.Apostolopoulos, V., M. S. Sandrin, and I. F. McKenzie. 1999. Carbohydrate/peptide mimics: effect on MUC1 cancer immunotherapy. J. Mol. Med. 77:427-436. [DOI] [PubMed] [Google Scholar]
- 2.Avila, J. L., M. Rojas, and U. Galili. 1989. Immunogenic Gal α1-3Gal carbohydrate structures are present on pathogenic American Trypanosoma and Leishmania. J. Immunol. 142:2828-2834. [PubMed] [Google Scholar]
- 3.Chiang, T. R., L. Fanget, R. Gregory, Y. Tang, D. L. Ardiet, L. Gao, C. Meschter, A. P. Kozikowski, R. Buelow, and W. M. Vuist. 2000. Anti-Gal antibodies in humans and 1,3 α-galactosyl-transferase knockout mice. Transplantation 27:2593-2600. [DOI] [PubMed] [Google Scholar]
- 4.Cretin, N., J. Bracy, K. Hanson, and J. Iacomini. 2002. The role of T-cell help in the production of antibodies specific for Gal α1-3Gal. J. Immunol. 168:1479-1483. [DOI] [PubMed] [Google Scholar]
- 5.Davin, J. C., M. Malaise, J. Foidart, and P. Malieu. 1987. Anti-alpha-galactosyl antibodies and immune complexes in children with Henoch-Schonlein purpura or IgA nephropathy. Kidney Int. 31:1132-1139. [DOI] [PubMed] [Google Scholar]
- 6.Galili, U., and P. Andrews. 1995. Suppression of α-galactosyl epitope synthesis and production of the natural anti-Gal antibody: a major evolutionary event in ancestral Old World primates. J. Hum. Evol. 29:433-442. [Google Scholar]
- 7.Galili, U., J. Buehler, S. B. Shohet, and B. A. Macher. 1987. The human natural anti-Gal IgG: the subtlety of immune tolerance in man as demonstrated by crossreactivity between natural anti-Gal and anti-B antibodies. J. Exp. Med. 165:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galili, U., M. R. Clark, S. B. Shohet, J. Buehler, and B. A. Macher. 1987. Evolutionary relationship between the natural anti-Gal antibody and the Gal α 1,3 Gal epitope in primates. Proc. Natl. Acad. Sci. USA 84:1369-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galili, U., and D. C. LaTemple. 1997. Natural anti-Gal antibody as a universal augmenter of autologous tumor vaccine immunogenicity. Immunol. Today 18:281-285. [DOI] [PubMed] [Google Scholar]
- 10.Galili, U., R. E. Mandrell, R. M. Hamadeh, S. B. Shohet, and J. M. Grifiss. 1988. Interaction between human natural anti-α-galactosyl immunoglobulin G and bacteria of the human flora. Infect. Immun. 56:1730-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galili, U., S. B. Shohet, E. Kobrin, C. L. M. Stults, and B. A. Macher. 1988. Man, apes, and Old World monkeys differ from other mammals in the expression of α-galactosyl epitopes on nucleated cells. J. Biol. Chem. 263:17755-17762. [PubMed] [Google Scholar]
- 12.Hamadeh, R. M., M. M. Estabrook, P. Zhou, G. A. Jarvis, and J. M. Griffiss. 1995. Anti-Gal binds to pili of Neisseria meningitidis: the immunoglobulin A isotype blocks complement-mediated killing. Infect. Immun. 63:4900-4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamadeh, R. M., U. Galili, P. Zhou, and J. M. Griffiss. 1995. Anti-α-galactosyl immunoglobulin A (IgA), IgG, and IgM in human secretions. 1995. Clin. Diagn. Lab. Immunol. 2:125-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamadeh, R. M., G. A. Jarvis, U. Galili, R. E. Mandrell, P. Zhou, and J. M. Griffiss. 1992. Human natural anti-Gal IgG regulates alternative complement pathway activation on bacterial surfaces. J. Clin. Investig. 4:1223-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joziasse, D. H., N. L. Shaper, D. Kim, D. H. van de Eijendent, and J. H. Shaper. 1992. Murine α 1,3 galactosyltransferase: a single gene locus specifies four isoforms of the enzyme by alternative splicing. J. Biol. Chem. 267:5534-5541. [PubMed] [Google Scholar]
- 16.Larson, R. D., C. A. Rivero-Marrero, L. K. Ernst, R. D. Cummings, and J. B. Lowe. 1990. Frameshift and nonsense mutations in a human genomic sequence homologous to a murine UDP-Gal:β-d-Gal (1,4)-d-GlcNac α (1,3) galactosyl transferase cDNA. J. Biol. Chem. 265:7055-7061. [PubMed] [Google Scholar]
- 17.LaTemple, D. C., J. T. Abrams, S. Y. Zhang, and U. Galili. 1999. Increased immunogenicity of tumor vaccines complexed with anti-Gal: studies in knockout mice for α1,3-galactosyltransferase. Cancer Res. 115:3417-3423. [PubMed] [Google Scholar]
- 18.Love, S. D., W. Lee, Y. C. Nakamura, J. L. Platt, R. R. Bollinger, and W. Parker. 2001. Unexpected anti-αGalNAc antibodies in α-galactosyl transferase-deficient mice: complex relationship between genotype and the natural antibody repertoire. Immunobiology 203:650-658. [DOI] [PubMed] [Google Scholar]
- 19.Mandelboim, O., E. Bar-Heim, E. Vadai, M. Fridken, and L. Eisenbach. 1997. Identification of shared tumor-associated antigen peptides between two spontaneous lung carcinomas. J. Immunol. 159:6030-6036. [PubMed] [Google Scholar]
- 20.Manez, R., F. Blanco, I. Diaz, A. Centeno, E. Lopez-Pelaez, M. Hermida, H. Davies, and A. Katapodis. 2001. Removal of bowel aerobic gram-negative bacteria is more effective than immunosuppression with cyclophosphamide and steroids to decrease natural α-galactosyl IgG antibodies. Xenotransplantation 8:15-23. [DOI] [PubMed] [Google Scholar]
- 21.McKenzie, I. F. C., A. D. Thall, M. Sandrin, K. Patton, and Y. Q. Li. 1998. A murine model of antibody-mediated hyperacute rejection by galactose-α (1,3) galactose antibodies in Gal o/o mice. Transplantation 66:754-763. [DOI] [PubMed] [Google Scholar]
- 22.Minanov, O., S. Itescue, F. Neethling, and A. Morganthaue. 1997. Anti-Gal antibodies in sera of newborn humans and baboons and its significance in pig xenotransplantation. Transplantation 63:182-186. [DOI] [PubMed] [Google Scholar]
- 23.Parker, W., K. Lundberg-Swanson, Z. E. Holzknecht, J. Lateef, S. A. Washburn, S. L. Braedehoeft, and J. L. Platt. 1996. Isohemagglutinins and xenoreactive antibodies: members of a distinct family of natural antibodies. Hum. Immunol. 45:94-104. [DOI] [PubMed] [Google Scholar]
- 24.Parker, W., P. B. Yu, Z. E. Holzknecht, K. Lundberg, R. H. Buckley, and J. L. Platt. 1997. Specificity and function of “natural” antibodies in immunodeficient subjects: clues to B cell lineage and development. J. Clin. Immunol. 17:311-321. [DOI] [PubMed] [Google Scholar]
- 25.Pearse, M. J., E. Witort, P. Mottram, W. Han, L. Murray-Segal, M. Romanella, E. Salvaris, T. A. Shinkel, D. J. Goodman, and A. J. d'Apice. 1998. Anti-Gal antibody-mediated allograft rejection in α1,3 galactosyl transferase gene knockout mice. Transplantation 66:748-754. [DOI] [PubMed] [Google Scholar]
- 26.Ramasamey, R., and R. Rajakanura. 1997. Association of malaria with inactivation of α1,3 galactosyl transferase in catarrhines. Biochim. Biophys. Acta 1360:241-246. [DOI] [PubMed] [Google Scholar]
- 27.Reed, J. L., X. Lin, T. D. Thomas, C. W. Birks, J. Tang, and R. P. Rother. 1997. Alteration of glycosylation renders HIV sensitive to inactivation by normal human serum. J. Immunol. 159:4356-4361. [PubMed] [Google Scholar]
- 28.Sandrin, M. S., H. A. Vaughan, P. L. Dabkowski, and I. F. C. McKenzie. 1993. Anti-pig IgM antibodies in human serum react predominantly with Gal α(1,3)Gal epitopes. Proc. Natl. Acad. Sci. USA 90:11391-11395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandrin, M. S., H. A. Vaughan, P. X. Xing, and I. F. McKenzie. 1997. Natural human anti-Gal α(1,3)Gal antibodies react with human mucin peptides. Glycoconjugate J. 14:97-105. [DOI] [PubMed] [Google Scholar]
- 30.Springer, G. F., and R. E. Horton. 1969. Blood group isoantibody stimulation in man by feeding blood-group active bacteria. J. Clin. Investig. 48:1280-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeuchi, Y., C. Porter, K. M. Strahan, A. F. Preece, K. Gustafsson, F. Cosset, R. A. Weiss, and M. K. L. Collins. 1996. Sensitization of cells and retroviruses to human serum by (α1,3) galactosyltransferase. Nature 379:85-88. [DOI] [PubMed] [Google Scholar]
- 32.Tanemura, M., Dengping, Y., Chong, A. S., and U. Galili. 2000. Differential immune responses to α-Gal epitopes on xenografts and allografts: implications for accommodation in xenotransplantation. J. Clin. Investig. 105:301-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thall, A. D., H. S. Murphy, and J. B. Lowe. 1996. α-1,3-Galactosyltransferase-deficient mice produce naturally occurring cytotoxic anti-Gal antibodies. Transplant. Proc. 28:556-557. [PubMed] [Google Scholar]
- 34.Thall, A. D., P. Maly, and J. B. Lowe. 1995. Oocyte Gal α 1,3 Gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J. Biol. Chem. 270:21437-21440. [DOI] [PubMed] [Google Scholar]
- 35.Tremont-Lukats, I. W., J. L. Avila, D. Hernandez, J. Vasquez, M. Teixeira, and M. Rojas. 1996. Antibody levels against α-galactosyl epitopes in sera of patients with squamous intraepithelial lesions and early invasive cervical carcinoma. Gynecol. Oncol. 64:207-212. [DOI] [PubMed] [Google Scholar]
- 36.Tremont-Lukats, I. W., J. L. Avila, F. Tapia, D. Hernandez, G. Caceres-Dittmar, and M. Rojas. 1996. Abnormal expression of galactosyl(α1-3) galactose epitopes in squamous cells of the uterine cervix infected by human papillomavirus. Pathobiology 64:239-246. [DOI] [PubMed] [Google Scholar]
- 37.Vaughan, H. A., B. E. Loveland, and M. Sandrin. 1994. Gal α(1,3) Gal is the major xenoepitope expressed on pig endothelial cells recognized by naturally occurring cytotoxic human antibodies. Transplantation 58:879-882. [DOI] [PubMed] [Google Scholar]
- 38.Welsh, R. M., C. L. O'Donnell, D. J. Reed, and R. P. Rother. 1998. Evaluation of the Gal α1,3 Gal epitope as a host modification factor eliciting natural humoral immunity to enveloped viruses. J. Virol. 72:4650-4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wittels, E. G., and H. C. Lichtman. 1986. Blood group incidence and Escherichia coli bacterial sepsis. Transfusion 6:533-535. [DOI] [PubMed] [Google Scholar]