Abstract
A comparison of extracellular proteins of virulent and avirulent Edwardsiella tarda strains revealed several major, virulent-strain-specific proteins. Proteomic analysis identified two of the proteins in the virulent strain PPD130/91 as flagellin and SseB, which are virulence factors in bacterial pathogens. PCR amplification and DNA sequencing confirmed the presence of the genes that encode these proteins. Our results clearly demonstrated the potency of the proteomic approach in identifying virulence factors.
Edwardsiella tarda is a gram-negative enteric bacterial pathogen of both animals (6) and humans (14). Fish mortality caused by E. tarda infection (28) led to severe losses in the aquaculture industry. The bacterium's virulence features include the ability to invade epithelial cells (16, 19), resistance to phagocytic killing (26), and production of enzymes such as hemolysins (5, 15) and chondroitinase (17). Functional genomic analysis is essential for a better understanding of the pathogenesis of E. tarda infections. The proteomic approach, which complements genomic research, has been employed to study the role of iron in regulating the pathophysiology of Mycobacterium tuberculosis (29) and the effect of environmental cues on gene expression within Salmonella pathogenicity island 2 (SPI2) in Salmonella enterica serovar Typhimurium (7). It was thus adopted here for the first time to compare and identify the secreted virulence factors of E. tarda.
Defining virulence.
Fourteen E. tarda strains were used for comparative proteomic analysis. The median 50% lethal doses (LD50s) of these strains were determined by using naïve blue gourami, Trichogaster trichopterus (Pallas), as described previously (19). Of the 14 strains used, 6 were described as virulent (LD50 of <106.5) and 8 were described as avirulent (LD50 of >107.0) (Table 1).
TABLE 1.
E. tarda strains used and their sources and characteristics
| Strain | Source, location of sourcea | LD50 | Reference |
|---|---|---|---|
| Avirulent | |||
| AL92448 | Unknown, Department of Fishery, Auburn University | >108.2 | This study |
| ATCC15947 | Human (type strain) | 107.7 | This study |
| ET82015 | Diseased Japanese eel, Shizuoka, Japan | 107.8 | This study |
| PPD499/84 | Diseased fish, AVA, Singapore | >107.5 | 19 |
| PPD453/86 | Arowana, AVA, Singapore | >107.2 | 19 |
| PPD76/87 | Sword tail, AVA, Singapore | >107.4 | 19 |
| PPD125/87 | Guppy, AVA, Singapore | >107.4 | 19 |
| PPD129/91 | Tilapia, AVA, Singapore | >107.3 | 19 |
| Virulent | |||
| AL9379 | Channel catfish, Department of Fishery, Auburn University | 105.9 | 19 |
| E381 | Tilapia, Niigata, Japan | 106.5 | This study |
| NE8003 | Japanese flounder, Nagasaki, Japan | 105.7 | This study |
| NUF251 | Japanese flounder, Nagasaki, Japan | 104.9 | This study |
| PPD130/91 | Serpae tetra, AVA, Singapore | 105.2 | 19 |
| SU226 | Eel culture pond water, Shizuoka, Japan | 106.0 | This study |
AVA, Agri-Veterinary Authority of Singapore.
Culture conditions.
Tryptic soy agar (TSA) medium (Difco) is used for growing E. tarda cultures in our laboratory. Brain heart infusion agar medium (Difco), which has also been used by others to culture E. tarda (27), was included for comparison. We first checked the extracellular protein (ECP) profiles of the two representative strains (virulent PPD130/91 and avirulent PPD125/87) cultured on TSA and brain heart infusion agar for 24 and 48 h at 25°C. ECP was prepared as described by Leung and Stevenson (18), with slight modifications. The final filtered ECP obtained was desalted and concentrated with a Millipore Biomax-5K column, and the protein concentration was determined by the Bio-Rad protein assay. One-dimensional (1D) polyacrylamide gel electrophoresis (PAGE) of the ECP was then performed according to standard procedures (22). Since the ECP production pattern was not affected by varying the culture media (data not shown), TSA was chosen for subsequent E. tarda cultures, and incubations of 24 instead of 48 h were used to avoid possible protein degradation due to a prolonged incubation period.
1D PAGE profile analysis.
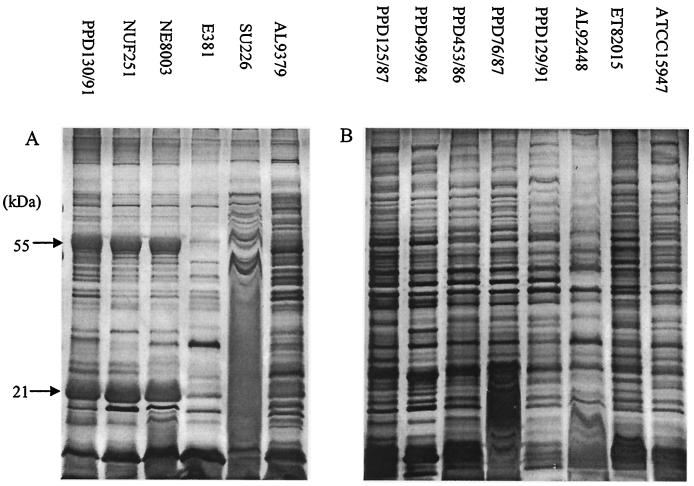
Once the culture conditions had been fixed, the ECP profiles of another five virulent and seven avirulent strains were surveyed (Fig. 1). Three virulent strains, PPD130/91, NUF251, and NE8003, exhibited very similar protein band patterns (Fig. 1A), with two unique major bands of approximately 55 and 21 kDa. The remaining three virulent strains, on the other hand, showed band profiles that were rather unique to each strain but that were different from those of the first three strains. As for the avirulent strains (Fig. 1B), their protein profiles were very similar, exhibiting multiple bands but no major bands as in the case of the virulent strains. Virulent strains in general shared background band profiles similar to those of the avirulent strains except for the virulent strains' additional major bands, which may thus be virulent-strain specific.
FIG. 1.
Survey of the ECP profiles of virulent (A) and avirulent (B) E. tarda strains (cultured for 24 h on TSA) with 1D PAGE (silver staining). The label at the top of each lane denotes the strain used in that lane.
2D PAGE profile analysis.
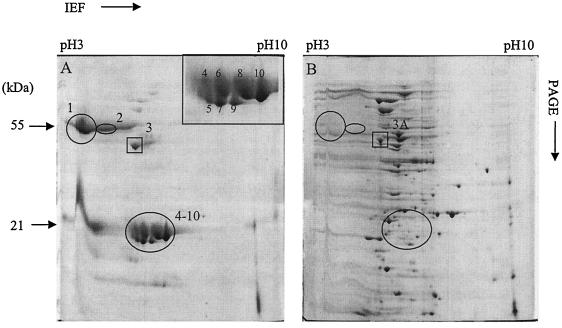
To obtain better protein resolution, two-dimensional (2D) PAGE (Bio-Rad) was employed to further resolve the ECP profiles of the two representative E. tarda strains. We used the following conditions for isoelectric focusing of the protein samples: 100 V for 4 h, 500 V for 2 h, 10,000 V for 3 h, and 10,000 V for 9 h. Nine prominent extra spots (Fig. 2A, spots 1 and 2 and 4 to 10), distributed from the acidic (pH 3) to the neutral pH range, were seen in the ECP of the virulent strain but not in that of the avirulent strain (Fig. 2B). Silver-stained gels (4) showed similar background patterns in both of the representative strains (data not shown). E. tarda NUF251 and NE8003, which shared 1D-gel band profiles similar to those of PPD130/91, were also found to have 2D-gel patterns similar to those of PPD130/91 (data not shown). Protein spot 3 (boxed) appeared to be common to both representative strains.
FIG. 2.
ECP profiles of E. tarda PPD130/91 (virulent) (A) and PPD125/87 (avirulent) (B) on a Coomassie blue-stained 2D gel with a broad range of pHs (3 to 10). Ten major protein spots (circled spots indicate proteins unique to the virulent strain; boxed spots [including spot 3A] indicate proteins common to both strains) were selected for MS analysis. The inset in panel A is an enlarged image of protein spots 4 to 10, and the number of each spot is as shown.
In a separate, concurrent experiment done in our laboratory, attenuated E. tarda PPD130/91 PhoA+ fusion mutants were found to have lost the extra major proteins in the ECP, indicative of these proteins' probable involvement in virulence (P. S. Srinivasa Rao and K. Y. Leung, unpublished data). These observations prompted us to further examine these proteins by mass spectrometry (MS). A common protein (spot 3) which appears to be up-regulated in the virulent strain compared to that in the avirulent strain (spot 3A) was also analyzed.
MS.
Ten protein spots of interest were excised from the 2D gel and digested with trypsin according to the procedure described by Shevchenko and coworkers (24). Mass spectra of each spot were acquired with a matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometer (Voyager-DE STR BioSpectrometry work station; Applied Biosystems) operating in the delayed-extraction reflectron mode. In addition, nanoflow electrospray ionization (ESI) tandem MS was performed for the purified tryptic digests (with Millipore Zip-Tip C18 pipette tips) with a quadrupole TOF mass spectrometer (Q-tof-2; Micromass), and partial amino acid sequences of the peptides were obtained.
The mass spectra obtained by both MALDI-TOF MS and ESI tandem MS (data not shown) revealed four basic peak patterns of the 10 spots analyzed, which categorized the spots into four groups: spots 1 and 2 in group I; spot 3 in group II; spots 5, 7, and 9 in group III; and spots 4, 6, 8, and 10 in group IV. The presence of more than one member in three of the groups and the close proximity of the spots was suggestive of probable protein isoforms. Peptide mass fingerprints (PMF) of the tryptic peptides from MALDI-TOF MS data on the representative spots (spots 1, 3, 7, and 8), together with the isoelectric points and molecular weights, were used to search the National Center for Biotechnology Information (NCBI) protein database with the programs Profound peptide mapping (ProteoMetrics) at http://129.85.19.192/profound_bin/WebProFound.exe and MS-Fit at http://prospector.ucsf.edu/ucsfhtml4.0/msfit.htm. The ESI tandem MS data obtained were subjected to the NCBI database search by the Mascot search engine at http://www.matrixscience.com.
Protein identification and Edman N-terminal sequencing.
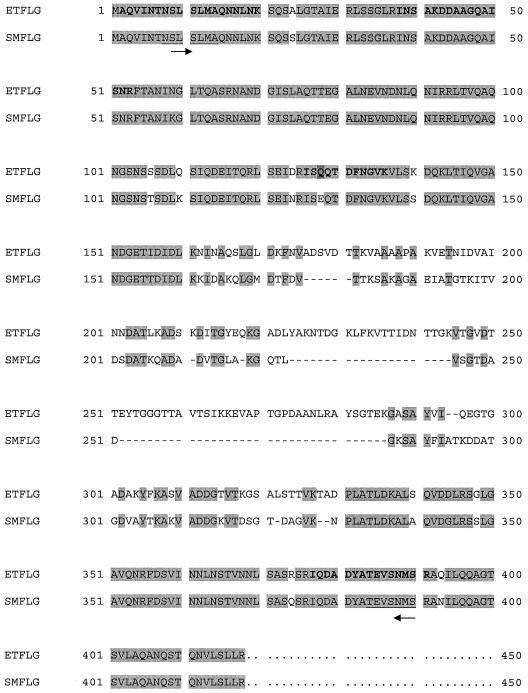
The database search with MALDI-TOF MS PMF data on the representative spots did not yield any positive protein identifications. When the ESI tandem MS data were subjected to the database search, only protein spot 3 was identified to be the flagellin protein. Results from the Mascot search of both the NCBI and bacterial databases showed that five peptides matched the Serratia marcescens 274 flagellin (accession no. P13713) (Fig. 3), with a significant total score of 160. Two of the peptides, DDAAGQAISNR and INSAKDDAAGQAISNR, appeared to be the same except for a missed cleavage in the fifth lysine residue (K) of the latter. For the peptide ISEQTDFNGVK, the third glutamic acid residue (E) was actually a glutamine (Q) residue according to the nucleotide sequence obtained. The residue alteration (from Q to E) in the tandem MS sequence is likely due to the occurrence of deamidation. A similar flagellin protein (spot 3A) was also identified in the ECP of avirulent E. tarda PPD125/87 by comparison of the MALDI-TOF MS PMF and by the recognition of at least 13 common flagellin peaks (data not shown).
FIG. 3.
Alignment of the E. tarda PPD130/91 (ET) and S. marcescens 274 (SM) flagellin (FLG) amino acid sequences. Lightly shaded sequences show conserved regions, and residues in bold indicate the matched peptides obtained by the Mascot database search with the ESI tandem MS data for protein spot 3. The residue (Q) highlighted with darker shading is obtained from the translation of the nucleotide sequence. Deamidation has occurred, which changed Q to E, and thus the residue matches the E residue in the S. marcescens sequence. Dashes indicate gaps in the sequence and were created for alignment. Underlined S. marcescens sequences denote regions of the matched peptides that were used for the design of primers to find the corresponding gene in E. tarda, and the arrows indicate the primer direction (5′ to 3′).
Since only one protein was identified by MS, Edman N-terminal sequencing of the proteins, which were either blotted onto a polyvinylidene difluoride membrane (spot 1) or purified by high-performance liquid chromatography (spots 7 and 8), was performed with a Procise model 494 pulsed-liquid-phase protein sequencer (Applied Biosystems). Nineteen, 55, and 80 N-terminal amino acids were obtained for spots 1, 7, and 8, respectively. The N-terminal sequences and/or the amino acid sequences obtained by ESI tandem MS were edited according to the rules provided by Shevchenko and coworkers (23) and subjected to a database search on the EMBL server, http://dove.embl-heidelberg.de/Blast2/, with the new MS-BLAST program for MS. Only protein spot 8 was identified to be the SseB protein, a member of the secretion system effector proteins of Salmonella serovar Typhimurium. Positive identification was claimed based on the fact that the sum of the scores for the high-scoring pair of the first two hits (110) was higher than the required confirmatory threshold score (102) (Fig. 4) (23). Protein spots 1 and 7, which did not exhibit any sequence homology to known proteins in the database, may be novel virulence-associated factors that require further characterization.
FIG. 4.
Alignment of the amino acid sequences of the E. tarda (ET) and Salmonella serovar Typhimurium (ST) SseB proteins (SSEB) and of enteropathogenic E. coli (EP) EspA protein (ESPA). Conserved regions are shaded, and the matched E. tarda partial peptides obtained by the MS-BLAST database search are given in bold. Underlined bold sequences were obtained by using both N-terminal and Q-TOF manual sequencing, while the two other matched peptides (bold) were obtained by using Q-TOF manual sequencing alone. The high-scoring pair (HSP) score for each matched peptide is given in parentheses, and the superscript number indicates the ranking of the hit. Dashes denote gaps in the sequence and were created for alignment. A degenerate primer was designed based on the N-terminal sequence (underlined) to work together with the adaptor primer to amplify the sseB gene in E. tarda. The direction of the degenerate primer (5′ to 3′) is indicated by the arrow.
Gene cloning and sequencing.
In order to confirm the presence of sseB-like and flagellin genes in E. tarda PPD130/91, PCR amplification was carried out with the Advantage 2 polymerase mix (Clontech) and the PCR fragments obtained were cloned in the pGEM-T Easy vector system (Promega) and transformed into E. coli TOP10F′.
For the flagellin gene, a pair of primers (GENSET; Singapore Biotech) was first designed based on the nucleotide sequences of two of the matched peptides flanking the front (5′-ACAGCCTGTCTCTGATGGCG-3′) and back (5′-CTCATGTTGGACACTTCGG-3′) portions of the flagellin homologue (Fig. 3) obtained from the Mascot search results. PCR was then performed using these two primers to amplify the relevant region in the E. tarda genomic DNA, with the following cycling conditions: 25 s at 94°C, seven cycles of 15 s at 94°C and 1 min at 72°C, 32 cycles of 15 s at 94°C and 1 min at 67°C, and 4 min at 67°C. For amplification of the sseB-like gene in E. tarda, a degenerate primer [5′-AA(C/T)AC(A/C/G/T)GA(C/T)TA(C/T)CA(C/T)GG(A/C/G/T)GG-3′] and the adaptor primer (Clontech) were used for PCR assay of the EcoRV genome-walking library, with the same cycling conditions. The PvuII and StuI libraries (Clontech) were also used for further genome walking to obtain the complete sequence. DNA sequencing, sequence assembly, and analysis were described previously (26).
Complete sequences of the flagellin (1,251-bp) and sseB-like (597-bp) genes, made up of 416 and 198 amino acids, respectively, were obtained. Alignment of the E. tarda flagellin homologue with the S. marcescens flagellin amino acid sequences (Fig. 3) showed that the N and C termini are well conserved, and the percentage of identity was 77.8%. Unlike flagellin, the SseB-like protein in E. tarda was found to have only 34% identity to the corresponding gene in Salmonella serovar Typhimurium (GenBank accession no. AAL20322). Compared to the EspA protein of enteropathogenic E. coli (accession no. AF022236), the percentage of identity was even lower (27%). The alignment pattern of these three proteins is shown in Fig. 4.
Putative roles of flagellin and SseB.
The flagellin protein identified in this study was homologous to that of S. marcescens strains 274 (9) and 8000 (1). The latter strain was earlier found to secrete a 37-kDa flagellin protein into the culture medium. E. tarda may likewise secrete a similar protein. Furthermore, Hirose and coworkers (13) recently identified a flagellin protein (Hag) from culture media while analyzing the extracellular proteins of Bacillus subtilis.
Although flagellin has been implicated in bacterial pathogenesis with regard to adhesion (2), motility (8), and/or in-phase variation (25), its exact role in E. tarda has not been studied. The isolation of a similar flagellin in the avirulent PPD125/87 strain may indicate an indirect role in the pathogenesis of E. tarda infections. Interestingly, a motility-deficient mutant of E. tarda, PPD130/91, that also lacks catalase production was found to be attenuated (20).
In Salmonella serovar Typhimurium, the SseB protein is a secretion system effector of the type III secretion system encoded by SPI2 (11, 12). A recent study showed that this protein, together with SseC and SseD, assembled into a translocon complex on the bacterial cell surface (21) to mediate other SPI2 effector protein translocations. Upon induction by acidic pH, SseB was rapidly secreted onto the bacterial cell surface (3). Due to its apparent surface localization, it appeared to be fairly prone to mechanical shearing, which allowed it to be isolated in the extracellular milieu (3, 21). In E. tarda, the secretion of this protein may not be pH dependent, as in the case of Salmonella serovar Typhimurium, since the cells were not subjected to pH changes prior to the isolation of ECP. However, subjection to mechanical forces such as washing and filtering in the ECP preparation procedure may have resulted in its release into the ECP supernatant if it is also surface localized as in Salmonella serovar Typhimurium.
Salmonella serovar Typhimurium SPI2 is important for systemic infection, intracellular survival, and replication (10). A mutation in sseB led to the attenuation of serovar Typhimurium cells and failure of the cells to accumulate within macrophages (12). Since E. tarda is biochemically similar to Salmonella (14) and it is known to survive and replicate within macrophages (26), it may possess an SPI2-like pathogenicity island involved in virulence, as indicated by the identification of the SseB-like protein. The isolation of this protein in the ECP may also be suggestive of a translocon role similar to that of SseB in serovar Typhimurium. Gene knockout experiments are essential for establishing the protein's exact in vivo function. Southern blot analysis performed to survey the distribution of the sseB-like gene in virulent and avirulent E. tarda strains showed that it is present in all six virulent strains and only one avirulent strain (ET82015) used in this study (data not shown). This survey thus suggests the probable involvement of this gene in E. tarda virulence.
Concluding remarks.
Comparison of ECP profiles from representative virulent and avirulent E. tarda strains led to the identification of two potential extracellular virulence-associated proteins out of the four categories of proteins isolated. The precise involvement of these two proteins will have to be established by gene knockout experiments in later studies.
Despite the lack of genome information, the work done here has clearly demonstrated the effectiveness of the comparative proteomic approach in the identification of important virulence factors. The understanding of the pathogenesis of E. tarda infections will be enhanced, and the knowledge gained in the present study will facilitate the identification of targets for the development of therapies against infections caused by this bacterium.
Nucleotide sequence accession numbers.
GenBank accession numbers for the sequences of the flagellin and sseB-like genes are AF487406 and AF498017, respectively.
Acknowledgments
We are grateful to the National University of Singapore for providing the research grant for this work.
We thank John Grizzle from Auburn University, Auburn, Ala.; H. Wakabayashi from the University of Tokyo, Tokyo, Japan; and T. T. Ngiam and H. Loh from the Agri-food and Veterinary Authority (AVA) of Singapore for providing us with E. tarda strains from the United States, Japan, and Singapore, respectively.
Editor: B. B. Finlay
REFERENCES
- 1.Akatsuka, H., E. Kawai, K. Omori, and T. Shibatani. 1995. Divergence of a flagellin protein in Serratia marcescens. Gene 163:157-158. [DOI] [PubMed] [Google Scholar]
- 2.Attridge, S. R., and D. Rowley. 1983. The role of flagellum in the adherence of Vibrio cholerae. J. Infect. Dis. 147: 864-872. [DOI] [PubMed] [Google Scholar]
- 3.Beuzon, C. R., G. Banks, J. Deiwick, M. Hensel, and D. W. Holden. 1999. pH-dependent secretion of SseB, a product of the SPI-2 type III secretion system of Salmonella typhimurium. Mol. Microbiol. 33:806-816. [DOI] [PubMed] [Google Scholar]
- 4.Blum, H., H. Beier, and H. J. Gross. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93-99. [Google Scholar]
- 5.Chen, J. D., and S. L. Huang. 1996. Hemolysin from Edwardsiella tarda strain ET16 isolated from eel Anguilla japonica identified as a hole-forming toxin. Fish. Sci. 62:538-542. [Google Scholar]
- 6.Cook, R. A., and J. P. Tappe. 1985. Chronic enteritis associated with Edwardsiella tarda infection in Rockhopper penguins. J. Am. Vet. Med. Assoc. 187:1219-1220. [PubMed] [Google Scholar]
- 7.Deiwick, J., and M. Hensel. 1999. Regulation of virulence genes by environmental signals in Salmonella typhimurium. Electrophoresis 20:813-817. [DOI] [PubMed] [Google Scholar]
- 8.Drake, D., and T. C. Montie. 1988. Flagella, motility, and invasive virulence of Pseudomonas aeruginosa. J. Gen. Microbiol. 134:43-52. [DOI] [PubMed] [Google Scholar]
- 9.Harshey, R. M., G. Estepa, and H. Yanagi. 1989. Cloning and nucleotide sequence of a flagellin-coding gene (hag) from Serratia marcescens 274. Gene 79:1-8. [DOI] [PubMed] [Google Scholar]
- 10.Hensel, M. 2000. Salmonella pathogenicity island 2. Mol. Microbiol. 36:1015-1023. [DOI] [PubMed] [Google Scholar]
- 11.Hensel, M., J. E. Shea, A. J. Bäumler, C. Gleeson, F. Blattner, and D. W. Holden. 1997. Analysis of the boundaries of Salmonella pathogenicity island 2 and the corresponding chromosomal region of Escherichia coli K-12. J. Bacteriol. 179:1105-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hensel, M., J. E. Shea, S. R. Waterman, R. Mundy, T. Nikolaus, G. Banks, A. Vazquez-Torres, C. Gleeson, F. C. Fang, and D. W. Holden. 1998. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol. Microbiol. 30:163-174. [DOI] [PubMed] [Google Scholar]
- 13.Hirose, I., K. Sano, I. Shioda, M. Kumano, K. Nakamura, and K. Yamane. 2000. Proteome analysis of Bacillus subtilis extracellular proteins: a two-dimensional protein electrophoretic study. Microbiology 146:65-75. [DOI] [PubMed] [Google Scholar]
- 14.Janda, J. M., and S. L. Abbott. 1993. Infections associated with the genus Edwardsiella: the role of Edwardsiella tarda in human disease. Clin. Infect. Dis. 17: 742-748. [DOI] [PubMed] [Google Scholar]
- 15.Janda, J. M., and S. L. Abbott. 1993. Expression of an iron-regulated hemolysin from Edwardsiella tarda. FEMS Microbiol. Lett. 111:275-280. [DOI] [PubMed] [Google Scholar]
- 16.Janda, J. M., S. L. Abbott, and L. S. Oshiro. 1991. Penetration and replication of Edwardsiella spp. in HEp-2 cells. Infect. Immun. 59:154-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janda, J. M., S. L. Abbott, S. Kroske-Bystrom, W. K. W. Cheung, C. Powers, R. P. Kokka, and K. Tamura. 1991. Pathogenic properties of Edwardsiella species. J. Clin. Microbiol. 29:1997-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung, K. Y., and R. M. W. Stevenson. 1988. Characteristics and distribution of extracellular proteases from Aeromonas hydrophila. J. Gen. Microbiol. 134:151-160. [Google Scholar]
- 19.Ling, S. H. M., X. H. Wang, L. Xie, T. M. Lim, and K. Y. Leung. 2000. Use of green fluorescent protein (GFP) to track the invasion pathways of Edwardsiella tarda in in vivo and in vitro fish models. Microbiology 146:7-19. [DOI] [PubMed] [Google Scholar]
- 20.Mathew, J. A., Y. P. Tan, P. S. Srinivasa Rao, T. M. Lim, and K. Y. Leung. 2001. Edwardsiella tarda mutants defective in siderophore production, motility, serum resistance and catalase activity. Microbiology 147:449-457. [DOI] [PubMed] [Google Scholar]
- 21.Nikolaus, T., J. Deiwick, C. Rappl, J. A. Freeman, W. Schröder, S. I. Miller, and M. Hensel. 2001. SseBCD proteins are secreted by the type III secretion system of Salmonella pathogenicity island 2 and function as a translocon. J. Bacteriol. 183:6036-6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 23.Shevchenko, A., S. Sunyaev, A. Loboda, A. Shevchenko, P. Bork, W. Ens, and K. G. Standing. 2001. Charting the proteomes of organisms with unsequenced genomes by MALDI-quadrupole time-of-flight mass spectrometry and BLAST homology searching. Anal. Chem. 73:1917-1926. [DOI] [PubMed] [Google Scholar]
- 24.Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 68:850-858. [DOI] [PubMed] [Google Scholar]
- 25.Silverman, M. J., and M. Simon. 1980. Phase variation: genetic analysis of switching mutants. Cell 19:845-854. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasa Rao, P. S., T. M. Lim, and K. Y. Leung. 2001. Opsonized virulent Edwardsiella tarda strains are able to adhere to and survive and replicate within fish phagocytes but fail to stimulate reactive oxygen intermediates. Infect. Immun. 69:5689-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strauss, E. J., N. Ghori, and S. Falkow. 1997. An Edwardsiella tarda strain containing a mutation in a gene with homology to shlB and hpmB is defective for entry into epithelial cells in culture. Infect. Immun. 65:3924-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thune, R. L., L. A. Stanley, and R. K. Cooper. 1993. Pathogenesis of gram-negative bacterial infections in warm water fish. Annu. Rev. Fish Dis. 3:37-68. [Google Scholar]
- 29.Wong, D. K., B.-Y. Lee, M. A. Horwitz, and B. W. Gibson. 1999. Identification of Fur, aconitase, and other proteins expressed by Mycobacterium tuberculosis under conditions of low and high concentrations of iron by combined two-dimensional gel electrophoresis and mass spectrometry. Infect. Immun. 67:327-336. [DOI] [PMC free article] [PubMed] [Google Scholar]