Abstract
The T-cell immune response to Mycobacterium tuberculosis is critical in preventing clinical disease. While it is generally accepted that both major histocompatibility complex class I (MHC-I)-restricted CD8+ and MHC-II-restricted CD4+ T cells are important for the immune response to M. tuberculosis, the role of non-MHC-restricted T cells is still not clearly delineated. We have previously reported that CD1d−/− mice do not differ from CD1d+/+ mice in their survival following infection with M. tuberculosis. We now show that, although CD1d-restricted NKT cells are not required for optimum immunity to M. tuberculosis, specific activation of NKT cells by the CD1d ligand α-galactosylceramide protects susceptible mice from tuberculosis. Treatment with α-galactosylceramide reduced the bacterial burden in the lungs, diminished tissue injury, and prolonged survival of mice following inoculation with virulent M. tuberculosis. The capacity of activated NKT cells to stimulate innate immunity and modulate the adaptive immune response to promote a potent antimicrobial immune response suggests that α-galactosylceramide administration could have a role in new strategies for the therapy of infectious diseases.
The host immune response to Mycobacterium tuberculosis is critical in preventing clinically evident disease following infection. Cell-mediated immunity is particularly important, and it is well documented that people with defective T-cell responses are at a higher risk for developing primary or reactivation tuberculosis. Consequently, there is general interest in determining which T cells are involved in antimycobacterial immunity, delineating their effector functions, and evaluating whether protective T-cell-mediated immunity can be induced by vaccination.
During the last decade, T cells that recognize lipid antigens and that are restricted by the CD1 family of antigen-presenting molecules have been identified (2, 3, 43, 48, 49). The human CD1 locus encodes five proteins, CD1a, -b, -c, -d, and -e, which associate with β2-microglobulin and have an overall structural homology to class I major histocompatibility complex (MHC-I) proteins (47). However, in contrast to MHC, CD1 molecules present lipid and glycolipid antigens to T cells. Evidence that CD1-restricted T cells participate in antimycobacterial immunity is accumulating. The group 1 CD1 proteins (CD1a, -b, and -c) present mycobacterial lipids including mycolates, lipoarabinomannan (LAM), and phosphatidylinositolmannosides (PIM) to human T cells (2, 3, 43, 49). Not only do these T cells recognize the purified lipid antigens presented by CD1, but they also recognize these antigens after they are processed and presented by the CD1 pathway in macrophages infected with M. tuberculosis (53, 54). CD1-restricted T cells can lyse M. tuberculosis-infected macrophages, and some CD1-restricted T-cell clones have a bactericidal effect that appears to be mediated by granulysin (53). Last, CD1-restricted T-cell responses to mycobacterial glycolipid antigens can be detected in the peripheral blood of patients with active tuberculosis (44).
Mice lack group 1 CD1 molecules but retain the group 2 CD1 genes CD1D1 and CD1D2 in their genome (47). CD1d-restricted T cells are highly conserved in humans and mice and belong to a unique subset of T cells known as NKT cells, which are defined by their expression of NK cell markers and a T-cell receptor αβ (TCRαβ) of limited diversity (9). In contrast to T cells restricted by group 1 CD1, CD1d-restricted T cells that recognize microbial antigens such as LAM have not been described (unpublished data; 10). This is consistent with our previous report and the findings of others that the absence of CD1d, and by extension CD1d-restricted NKT cells, does not impair survival of mice following intravenous infection with M. tuberculosis (5, 18, 51a). Instead, many CD1d-restricted T cells recognize CD1d directly, most likely because of the presentation of endogenous self-lipid antigens (28).
Murine CD1d-restricted T cells have been divided into two subsets. One subset uses a diverse TCR repertoire; the other uses an invariant TCRα chain encoded by Vα14 and Jα281 and usually pairs with TCRβ chains belonging to the Vβ2, -7, and -8 families (4, 25). The latter NKT cell subset has been referred to as invariant NKT cells or classical NKT cells. Although it remains unclear whether these two T-cell subsets have different physiological roles, they differ significantly in their recognition of α-galactosylceramide (αGalCer), an antigen originally isolated from marine sponges and not generally thought to be produced by mammalian cells or microbial pathogens (35). The vast majority of diverse CD1d-restricted T cells do not recognize αGalCer, while, in contrast, both human and mouse invariant NKT cells recognize αGalCer presented by CD1d (4, 10, 28). This has been established by using clonal T cells, both with CD1d+ antigen-presenting cells (APC) and purified CD1d protein in APC-free systems (28, 35, 52). Following recognition of αGalCer in vitro, NKT cell clones and hybridomas are rapidly activated to proliferate, become cytotoxic, and secrete large amounts of cytokines, including gamma interferon (IFN-γ) and interleukin-4 (IL-4) (10, 52). The situation in vivo is more complex, as the behavior of NKT cells following activation differs from that of classical CD4+ and CD8+ TCRαβ+ T cells. Instead of undergoing clonal expansion, invariant NKT cells stimulated in vivo with αGalCer rapidly produce cytokines and express activation markers such as CD69 and then disappear from the tissues where they normally reside, apparently as a consequence of activation-induced cell death (20, 39-41, 46, 56). Following activation, NKT cells also down-regulate the NK1.1 antigen, which is a cell surface protein used to mark NKT cells in certain mouse strains (such as C57BL/6) but not others (including BALB/c and C3H/HeJ [C3H]) (15, 38). Nevertheless, NKT cells activated in vivo by αGalCer have potent immunomodulatory effects. Within 24 h after αGalCer injection, B cells, T cells, and macrophages express activation markers, NK cells produce IFN-γ, and elevated serum cytokines are detected (10, 19). Treatment with αGalCer in vivo can alter the Th1-Th2 balance of an immune response following antigen exposure. This secondary activation or transactivation of other immune cells is entirely dependent on the primary activation of NKT cells by αGalCer in a CD1d-dependent manner. In the absence of CD1d or invariant NKT cells, αGalCer does not induce any of the above phenomena. The ability of ligand-stimulated NKT cells to activate the various components of the immune system has led to the idea that CD1d-restricted NKT cells may function as immunoregulatory cells rather than as direct effector cells (11, 12).
For all of these reasons, we were particularly interested in the observation by Apostolou et al. that granulomas were elicited when deproteinized M. tuberculosis was injected subcutaneously in mice (1). Granuloma formation was dependent on invariant NKT cells, which accounted for the majority of infiltrating T cells. Purified mycobacterial cell wall glycolipids PIM and LAM were sufficient to induce these granulomas (1, 24, 42). The recruitment of NKT cells to granulomas induced by mycobacterial lipid antigens suggests that NKT cells could play a role in antimycobacterial immunity under certain circumstances. Therefore, we considered the possibility that activated NKT cells may enhance immunity to tuberculosis, even though their absence does not impair resistance to tuberculosis. To test this hypothesis, we treated genetically susceptible inbred strains of mice with α-GalCer, a specific activator of CD1d-restricted invariant NKT cells (10, 35, 52).
MATERIALS AND METHODS
Mice.
Six- to 8-week-old female C3H, C57BL/6, BALB/c (CD1d+/+), and BALB/c CD1d knockout (CD1d−/−) mice were obtained from Jackson Laboratories (Bar Harbor, Maine). All mice were housed in a biosafety level 3 facility under specific-pathogen-free conditions in the Animal Biohazard Containment Suite (Dana Farber Cancer Institute, Boston, Mass.) and used in a protocol approved by the institution. BALB/c CD1d−/− mice were bred at our institution (51).
Bacteria and infections.
Virulent M. tuberculosis (Erdman strain) was originally obtained from Barry Bloom (Harvard School of Public Health, Boston, Mass.). The bacteria were passed through mice and subsequently grown once in Middlebrook 7H9 supplemented with the oleic acid-albumin-dextrose complex (Difco, Detroit, Mich.) and stored at −80°C. Prior to injection into mice, an aliquot was thawed, sonicated twice for 10 s with a cup horn sonicator, and then diluted in 0.9% NaCl-0.02% Tween 80. Mice were infected intravenously via the lateral tail vein with 0.25 × 106 to 4.0 × 106 live mycobacteria. The size of the inoculum was confirmed by plating an aliquot onto 7H10 agar plates (Remel, Lenexa, Kans.).
Flow cytometry.
Lung mononuclear cells (MNC) were washed in fluorescence-activated cell sorter buffer (5% fetal bovine serum and 0.02% NaN3 in phosphate-buffered saline [PBS]). A total of 106 cells per condition were stained with an isotype-matched control immunoglobulin G or antibodies specific for mouse CD3, CD4, CD8, CD22, or NK1.1 conjugated to fluorescein isothiocyanate, phycoerythrin, or CyChrome (all from Pharmingen, San Diego, Calif.) for 20 min on ice. Cells were washed and fixed overnight at 4°C in 1% paraformaldehyde in PBS. After being washed twice, the cells were analyzed with a FACSort (Becton Dickinson). Intracellular flow cytometry was used to detect the production of IFN-γ by CD4+ or CD8+ T cells after a brief 3-h in vitro stimulation with phorbol myristate acetate (PMA) and ionomycin as previously described (13). The FlowJo software program (Tree Star, Inc., Stanford, Calif.) was used to analyze the data.
Treatment with αGalCer.
Kirin Pharmaceuticals generously provided αGalCer in a vehicle of 0.5% polysorbate 20. Mice were treated on days 1, 5, and 9 following inoculation with M. tuberculosis except where noted otherwise. Based on a dose of 100 μg/kg of body weight and an average body weight of 20 g, mice were injected intraperitoneally with 2 μg of αGalCer-0.5 ml of PBS or the equivalent amount of vehicle in 0.5 ml of PBS.
CFU determination.
For each time point, five infected animals per experimental group were analyzed. Lungs and spleens were aseptically removed from euthanized animals. Prior to removal, lungs were perfused by injecting 5 to 10 ml of sterile PBS into the right ventricle of the heart after severing the inferior vena cava. The left lung and half of the spleen from each animal were homogenized in 0.9% NaCl-0.02% Tween 80 with sterile Teflon homogenizers (Fisher, Pittsburgh, Pa.) or a Minibeadbeater-8 (Biospec Inc., Bartlesville, Okla.). To quantitate viable mycobacteria, organ homogenates were serially diluted 10-fold and plated onto 7H10 agar plates (Remel). Colonies were counted after incubation for 3 weeks at 37°C. Differences in the splenic or lung CFU among groups were determined by using a t test following log transformation.
In vitro restimulation assays.
Splenocytes or lung MNC were prepared as described elsewhere and cultured at 2.5 × 106 cells/ml in complete media with 1 μg of concanavalin A/ml, H37Ra M. tuberculosis sonicate (at 1:1,000, 1:5,000, and 1:25,000 dilutions), or media alone for 48 h at 37°C. Culture supernatants were assayed for cytokines by enzyme-linked immunosorbent assay using antibody pairs and cytokines from Pharmingen (6, 7, 13).
Histology.
Tissue was preserved in 10% buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin or stained for acid-fast bacilli (AFB) as previously described (5). Random lung sections from five or six individual mice per group were examined for each time point.
Survival studies.
Each experimental group consisted of approximately 10 mice (range, 7 to 15 mice), and, after inoculation with M. tuberculosis, the mice were monitored for survival. The results were analyzed by the method of Kaplan and Meier, and the survival curves for each group were compared by using the log rank test (Prism software package; GraphPad, San Diego, Calif.).
RESULTS
Administration of αGalCer prolongs the survival of mice infected with M. tuberculosis.
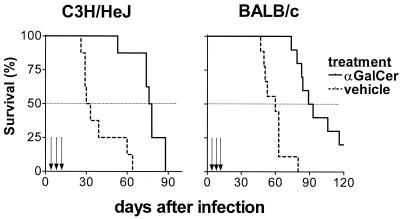
To determine whether activated NKT cells influence the response to M. tuberculosis infection, αGalCer was administered to inbred mouse strains known to be susceptible to tuberculosis. We previously determined that, following intravenous inoculation with 0.5 × 106 to 1.0 × 106 CFU of M. tuberculosis (Erdman), the mean survival time (MST) for C3H mice was 28 days and the MST for BALB/c mice was 60 days (13; our unpublished data). A dose of 100 μg of αGalCer/kg was administered intraperitoneally on days 1, 5, and 9 following infection because this regimen has been shown to be efficacious in reducing tumor metastases (36). Control mice were infected similarly but received an equivalent amount of the vehicle diluted in PBS. Administration of αGalCer prolonged the survival of both BALB/c and C3H mice (Fig. 1). The MST of BALB/c mice was increased to 91 days from 60 days (P < 0.0001), and αGalCer-treated C3H mice survived more than twice as long as controls (MST of 77 days compared with 31 days; P = 0.0002). These results demonstrated that αGalCer prolonged the survival of susceptible mice when administered following infection with virulent M. tuberculosis.
FIG. 1.
Treatment with αGalCer ameliorates the course of tuberculosis in susceptible mice. C3H (n = 18) or BALB/c (n = 16) mice were inoculated intravenously with M. tuberculosis and then treated with αGalCer or vehicle alone on days 1, 5, and 9 following infection. Mice treated with αGalCer had prolonged survival compared with vehicle-treated controls (P = 0.0002 for C3H mice and P < 0.0001 for BALB/c mice). Arrows, administration of αGalCer or vehicle.
αGalCer ameliorates tuberculosis by a CD1d-dependent mechanism.
The action of αGalCer has been shown to be dependent on CD1d and CD1d-restricted NKT cells both in vitro and in vivo (10, 36). We have previously shown that the absence of CD1d-restricted T cells had no impact on the survival of resistant C57BL/6 or susceptible BALB/c mice in this model of tuberculosis (5). We confirmed this finding for BALB/c CD1d−/− and CD1d+/+ mice, which survived similarly following infection with virulent M. tuberculosis following challenge with a range of inoculum sizes (Fig. 2a). It was likely that the beneficial effect of αGalCer on the outcome of infection was CD1d mediated. However, certain mycobacterial lipids are known to directly activate macrophages (14). To exclude the possibility that αGalCer was enhancing macrophage killing of intracellular M. tuberculosis, we treated M. tuberculosis-infected peritoneal macrophages with αGalCer but did not observe any reduction in CFU compared to mock treatment (data not shown). In contrast, the action of αGalCer was confirmed to be dependent on CD1d by infecting both CD1d−/− and CD1d+/+ mice followed by treatment with αGalCer. The survival of αGalCer-treated BALB/c CD1d+/+ mice was significantly prolonged compared to that of BALB/c CD1d−/− mice (Fig. 2b; P < 0.0001). Therefore, the ability of αGalCer to ameliorate disease in susceptible mice was dependent on CD1d. As αGalCer is a specific ligand of CD1d-restricted NKT cells, these data suggest that the protective effect of αGalCer is mediated by activated CD1d-restricted NKT cells.
FIG. 2.
The protective effect of αGalCer is CD1d dependent. (A) BALB/c CD1d−/− or CD1d+/+ mice were inoculated intravenously with 4 × 106, 1 × 106, or 0.25 × 106 CFU of M. tuberculosis. Each group contained five to eight mice, and there were no statistically significant differences in survival of the CD1d−/− mice compared to that of the CD1d+/+ mice. (B) BALB/c CD1d−/− and CD1d+/+ mice were inoculated intravenously with M. tuberculosis (3 × 106 CFU/mouse) and subsequently treated with αGalCer or vehicle on days 1, 5, and 9 following infection. Only BALB/c CD1d+/+ mice treated with αGalCer survived longer than the vehicle-treated mice (P < 0.0001). Arrows, administration of αGalCer or vehicle. WT, wild type.
Chronic administration of αGalCer provided no additional benefit.
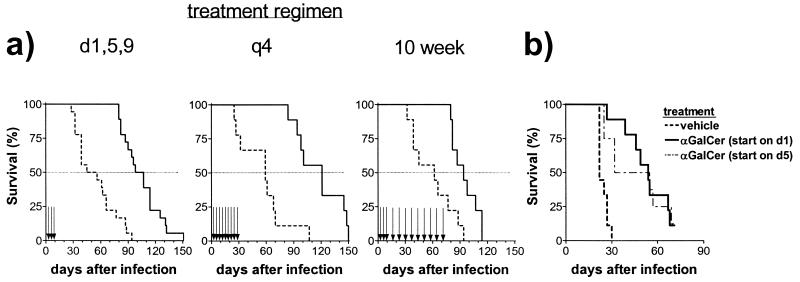
The dosing regimen employed in our studies was based on experiments showing that αGalCer reduced tumor metastases, an end point measured 2 weeks following tumor cell inoculation (16). As our experiments were much longer, we tested whether prolonged administration of αGalCer would result in a greater beneficial effect. The standard protocol (doses given on days 1, 5, and 9) was compared to two other regimens. In one, αGalCer was administered every 4 days for the first 30 days following infection (q4 protocol); in the other, αGalCer was administered on days 1, 5, and 9, followed by a weekly maintenance dose for 10 weeks (10-week protocol). The differences in survival among the regimens were minor compared to the differences between treated and untreated mice: αGalCer-treated mice survived longer than vehicle-treated controls with all three dosing regimens. However, longer treatment had less of a beneficial effect: mice treated with αGalCer according to the 10-week protocol had significantly decreased survival compared with that for the other dosing regimens (P = 0.042 versus doses on days 1, 5, and 9; P = 0.012 versus q4 protocol) (Fig. 3A).
FIG. 3.
Chronic administration of αGalCer provided no additional survival benefit. BALB/c mice were inoculated intravenously and then administered vehicle or αGalCer. (A) In the standard protocol, vehicle or αGalCer was administered on days 1, 5, and 9 (P < 0.0001); in the q4 protocol, vehicle or αGalCer was administered every 4 days for a total of nine doses (P = 0.0004); in the 10-week protocol, vehicle or αGalCer was administered on days 1, 5, and 9 and then once a week for 10 weeks (P = 0.0031). (B) BALB/c mice were inoculated intravenously with M. tuberculosis and then divided into groups of nine mice. Two groups were treated by the standard protocol and received αGalCer or vehicle on days 1, 5, and 9 following infection. Another group was treated with αGalCer on days 5, 9, and 13, and the fourth group was treated on days 8, 12, and 16. The groups that received αGalCer starting on day 1 (P < 0.0001) or day 5 (P = 0.0013) had a prolonged survival compared to vehicle-treated controls.
Relationship between inoculation and timing of αGalCer administration.
Entry of M. tuberculosis into macrophages occurs within hours following experimental inoculation. Following intravenous inoculation, the lymphoid organs are directly seeded with M. tuberculosis and the immune response is rapidly generated within 7 days (13). Preactivation of the immune system, necessary in αGalCer treatment of malaria or Cryptococcus neoformans infection, was not required for the beneficial effect of αGalCer in tuberculosis (26, 34). We were interested to determine the size of the window during which αGalCer could protect mice. The survival of the mice was prolonged if αGalCer administration was initiated on day 1 or day 5 following M. tuberculosis inoculation (Fig. 3B). In contrast, initiating treatment on day 8 did not provide any protection compared to treatment with vehicle alone (data not shown).
αGalCer treatment reduced the bacterial burden in the lung.
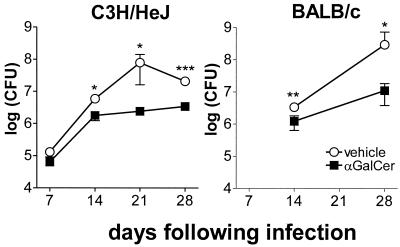
Mycobacterial replication in the lungs and spleens of infected mice was monitored following inoculation to determine whether αGalCer treatment led to a reduction in bacterial burden. A transient reduction in the bacterial burden in the lungs of αGalCer-treated mice compared to that in the lungs of vehicle-treated mice was observed. In BALB/c mice, this was most consistently observed between 21 and 35 days after infection (Fig. 4 and data not shown) and was observed in four of six experiments. In C3H mice, differences were observed as early as day 14 following infections (Fig. 4). No significant changes in the number of bacteria in the spleen were observed. These results suggest that the amelioration of disease by αGalCer is mediated in part by activation of bactericidal effector cells.
FIG. 4.
The mycobacterial burden is reduced in αGalCer-treated mice. Mice received αGalCer or vehicle on days 1, 5, and 9 following inoculation with M. tuberculosis. The inoculum for the experiments shown was 1.1 × 106/mouse. The CFU recoverable from the lung were determined at the indicated times after infection. Each data point is the mean bacterial counts from four to six mice ± standard deviation. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
Lung histology for αGalCer-treated mice.
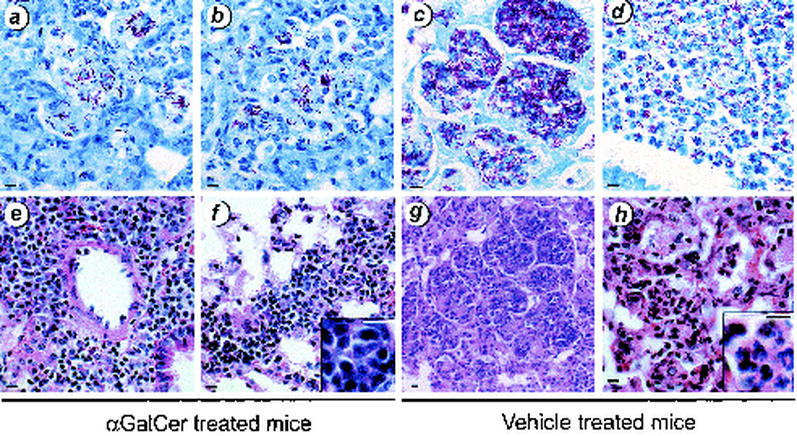
Following infection, the lungs of αGalCer-treated mice had few AFB; the lungs of vehicle-treated mice had noticeably more bacteria, especially in necrotic areas, mirroring the CFU data (Fig. 4 and 5a to d). Although large infiltrates were also observed in the lungs of αGalCer-treated mice, there was less necrosis and there tended to be greater numbers of lymphocytes in the inflammatory infiltrate. Both perivascular and interstitial lymphoid infiltrates were observed (Fig. 5e and f). The lymphoid nature of the infiltrates was more evident at higher power (Fig. 5f, inset). In contrast, vehicle-treated mice had large pulmonary infiltrates composed of mixed inflammatory cells. Although lymphocytes were also observed, these lesions were dominated by macrophages and polymorphonuclear leukocytes (Fig. 5g and h; see Fig. 5 h, inset, for a high-power picture of neutrophils in infiltrates). Areas of bronchopneumonia were clearly evident, and frankly necrotic areas were also observed.
FIG. 5.
αGalCer treatment reduced pulmonary injury. BALB/c mice were infected with M. tuberculosis and treated with αGalCer (a, b, e, and f) or vehicle (c, d, g, and h). After 4 weeks, mice were sacrificed and the lungs were removed and fixed in formalin. Paraffin-embedded sections were stained with Fite-Faracco stain for AFB (a to d) or with hematoxylin and eosin (e to h). The CFU for this experiment are depicted in Fig. 4. Although both groups of mice had significant pulmonary infiltrates 4 weeks after inoculation, the αGalCer-treated group tended to have greater numbers of lymphocytes within the lesions (e, f, and panel f inset), whereas the vehicle-treated mice had more areas of necrosis and accompanying polymorphonuclear leukocyte infiltrates (g, h, and panel h inset). Bar, 10 μm.
Immunological changes following αGalCer treatment.
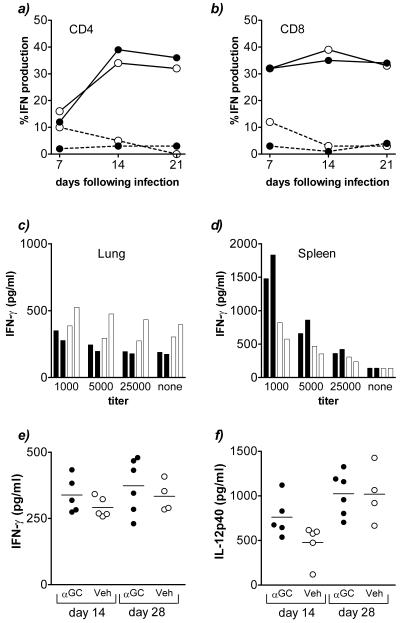
To examine whether the antituberculous immune response was altered following treatment with αGalCer, splenocytes and lung MNC were briefly stimulated in vitro with PMA and ionomycin and analyzed by intracellular cytokine flow cytometry. No difference in the percentage of IFN-γ-producing CD4+ or CD8+ lymphocytes between vehicle- and αGalCer-treated mice was observed (Fig. 6a and b). Furthermore, the splenocytes and lung MNC from vehicle- and αGalCer-treated mice produced similar amounts of IFN-γ following stimulation in vitro with crude M. tuberculosis sonicate (Fig. 6c and d). Similarly, there was no difference in the amounts of IFN-γ and IL-12 p40 detected in bronchoalveolar lavage fluid (Fig. 6e and f).
FIG. 6.
Immunological effects of αGalCer on the antituberculous immune response. Mice infected intravenously with M. tuberculosis and treated with αGalCer or vehicle were sacrificed, and various immunological parameters were measured. (a and b) Intracellular production of IFN-γ by CD4+ and CD8+ lymphocytes following brief ex vivo stimulation with PMA and ionomycin at various times after infection. Solid line, stimulated with PMA and ionomycin; broken line, unstimulated control. (c and d) IFN-γ production in vitro 48 h after stimulation of lung MNC or splenocytes with mycobacterial antigen. Each bar represents the results obtained by using cells pooled from three mice. The titer is the reciprocal dilution of the bacterial sonicate. Solid bar, αGalCer-treated mouse; open bar, vehicle-treated control. (e and f) Levels of IFN-γ and IL-12 p40 in the bronchoalveolar lavage fluid as determined 14 days after infection by enzyme-linked immunosorbent assay (P values [Student's t test] indicated that differences were not significant). Each symbol represents an individual mouse. Solid circle, αGalCer (αGC)-treated mouse; open circle, vehicle (Veh)-treated control.
DISCUSSION
Although the absence of CD1d did not adversely affect the mortality of mice following infection with M. tuberculosis, we found that administration of αGalCer, a treatment that specifically activates CD1d-restricted NKT cells, significantly prolonged their survival. This effect was dependent on CD1d, indicating that activated CD1d-restricted NKT cells can modulate the immune response to tuberculosis. We observed a reduction in lung CFU and an amelioration of lung pathology in αGalCer-treated mice. This result is remarkable as there are few compounds other than cytokines and vaccines that have been shown to enhance the resistance to tuberculosis through their modulation of the immune response (8, 27, 30-32, 45). Furthermore, these findings highlight the capacity of activated NKT cells to affect the outcome of infection with microbial pathogens (21, 26, 33).
In vivo administration of αGalCer is known to rapidly activate both the innate and adaptive immune systems, an effect that is dependent on CD1d-restricted NKT cells (11, 12). For example, NK cells produce IFN-γ within hours of αGalCer administration, and this effect is dependent upon the presence of CD1d and/or NKT cells (12). Similarly, αGalCer leads to the up-regulation of activation markers including CD69 and CD80/86 on T cells and B cells. In vivo administration of αGalCer to uninfected mice also induces the production of large amounts of both IL-4 and IFN-γ and can bias an antigen-specific immune response in favor of a Th1 or a Th2 phenotype (11, 17, 50). Other factors that regulate the Th1-Th2 balance following stimulation with αGalCer have not been completely delineated (11, 50). For example, when αGalCer activates NKT cells in the context of dendritic cells or macrophages, IL-12 production (CD40-40L dependent) shifts the immune response toward a Th1 bias (29, 37). Presentation of αGalCer by B cells leads to the activation of NKT cells in the absence of IL-12, and, under these conditions, the effects of IL-4 may dominate, leading to a Th2-biased immune response (11, 17, 50). Although one could envision a scenario in which αGalCer worsened the course of infection because a protective Th1 response was converted into a Th2-dominated response, this has not been observed. Instead, an improvement in microbial end points following infection with four different pathogens has been observed (21, 26, 33, 34).
The use of αGalCer in other mouse models of infection is associated with a beneficial antimicrobial effect dependent on CD1d-restricted NKT cells (26, 33). Treatment with αGalCer 1 to 2 days prior to inoculation with the sporozoite form of malaria reduces the parasite load in both blood and liver (26). The reduction in parasitemia is dependent on IFN-γ but is independent of IL-12, tumor necrosis factor alpha (TNF-α), perforin, fas, and NK cells (26). In the cryptococcus model, αGalCer treatment starting on the day of infection enhances IFN-γ production by CD4+ cells and results in a concomitant reduction in yeast CFU, which is dependent on IFN-γ but independent of NK cells (34). Viral immunity is also enhanced following treatment with αGalCer. Exley et al. demonstrated that CD1d-reactive T cells mediate resistance against the acute, diabetogenic encephalomyocarditis virus and that αGalCer protects mice against end organ damage (21). Kakimi et al. showed that a single injection of αGalCer inhibits viral replication in hepatitis B virus transgenic mice and that inhibition is dependent on IFN-γ and the IFN-α/β receptor but independent of TNF-α and inducible nitric oxide synthase 2 (33). Enhanced antigen-specific immunity is observed following αGalCer treatment in these models and is associated with a reduction in microbial burden. The ability of αGalCer to enhance the protective immune response to four very different pathogens demonstrates the pervasive ability of activated NKT cells to enhance antimicrobial immunity.
The immune response in mice following infection by M. tuberculosis is strongly Th1 biased, even in mouse strains that are inherently susceptible to tuberculosis (13). Therefore, we were surprised not see an enhancement of T-cell-mediated immunity, as measured by cytokine production in bronchoalveolar lavage fluid, in vitro recall responses to M. tuberculosis antigens, or intracellular cytokine production following stimulation with PMA and ionomycin. It may be that M. tuberculosis is such a strong Th1 stimulus that IFN-γ production is already maximal, consistent with the finding that administration of exogenous IFN-γ to susceptible mice results in little or no improvement in their survival (22, 23). Therefore, activated NKT cells may act through other pathways to influence the outcome of infection, such as by modulating the innate immune response or by acting directly as effector cells.
Our previous finding that the absence of CD1d did not impair immunity to tuberculosis suggests that, in the absence of αGalCer administration, NKT cells do not become activated during M. tuberculosis infection. The reason for this is elusive. Mycobacterial cell wall lipids such as PIM can induce granuloma formation and recruit NKT cells into the inflammatory lesions. We have shown that NKT cells are present, albeit in low numbers, in the lung both before and after infection (unpublished data). The processing of lipid antigens from the cell wall of intact M. tuberculosis may not occur efficiently enough for presentation by CD1d to activate NKT cells. Down-regulation of CD1d expression by infected macrophages, as has been reported for human CD1, may also decrease the effectiveness of antigen presentation to NKT cells (55). Given that αGalCer activation of NKT cells leads to a dramatic protective effect, a better understanding of these processes could lead to strategies to exploit the therapeutic potential of αGalCer in the treatment and prevention of tuberculosis and other infectious diseases.
Acknowledgments
This work was supported by National Institutes of Health grants HL64540 and AI49093 and an award from the Potts Memorial Foundation to S.M.B. A.C. was supported by T32 AI 07306.
We thank Steve Jean, Linda Callahan, and the staff of the Animal Biohazard Containment Suite at the Dana Farber Cancer Institute for their help in facilitating these experiments.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Apostolou, I., Y. Takahama, C. Belmant, T. Kawano, M. Huerre, G. Marchal, J. Cui, M. Taniguchi, H. Nakauchi, J. J. Fournie, P. Kourilsky, and G. Gachelin. 1999. Murine natural killer T (NKT) cells contribute to the granulomatous reaction caused by mycobacterial cell walls. Proc. Natl. Acad. Sci. USA 96:5141-5146. (Erratum, 96:7610.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckman, E. M., A. Melian, S. M. Behar, P. A. Sieling, D. Chatterjee, S. T. Furlong, R. Matsumoto, J. P. Rosat, R. L. Modlin, and S. A. Porcelli. 1996. CD1c restricts responses of mycobacteria-specific T cells. Evidence for antigen presentation by a second member of the human CD1 family. J. Immunol. 157:2795-2803. [PubMed] [Google Scholar]
- 3.Beckman, E. M., S. A. Porcelli, C. T. Morita, S. M. Behar, S. T. Furlong, and M. B. Brenner. 1994. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature 372:691-694. [DOI] [PubMed] [Google Scholar]
- 4.Behar, S. M., and S. Cardell. 2000. Diverse CD1d-restricted T cells: diverse phenotypes, and diverse functions. Semin. Immunol. 12:551-560. [DOI] [PubMed] [Google Scholar]
- 5.Behar, S. M., C. C. Dascher, M. J. Grusby, C. R. Wang, and M. B. Brenner. 1999. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J. Exp. Med. 189:1973-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behar, S. M., T. A. Podrebarac, C. J. Roy, C. R. Wang, and M. B. Brenner. 1999. Diverse TCRs recognize murine CD1. J. Immunol. 162:161-167. [PubMed] [Google Scholar]
- 7.Behar, S. M., S. A. Porcelli, E. M. Beckman, and M. B. Brenner. 1995. A pathway of costimulation that prevents anergy in CD28− T cells: B7-independent costimulation of CD1-restricted T cells. J. Exp. Med. 182:2007-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bekker, L. G., P. Haslett, G. Maartens, L. Steyn, and G. Kaplan. 2000. Thalidomide-induced antigen-specific immune stimulation in patients with human immunodeficiency virus type 1 and tuberculosis. J. Infect. Dis. 181: 954-965. [DOI] [PubMed] [Google Scholar]
- 9.Bendelac, A., M. N. Rivera, S. H. Park, and J. H. Roark. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15:535-562. [DOI] [PubMed] [Google Scholar]
- 10.Burdin, N., L. Brossay, Y. Koezuka, S. T. Smiley, M. J. Grusby, M. Gui, M. Taniguchi, K. Hayakawa, and M. Kronenberg. 1998. Selective ability of mouse CD1 to present glycolipids: alpha-galactosylceramide specifically stimulates V alpha 14+ NK T lymphocytes. J. Immunol. 161:3271-3281. [PubMed] [Google Scholar]
- 11.Burdin, N., L. Brossay, and M. Kronenberg. 1999. Immunization with alpha-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur. J. Immunol. 29:2014-2025. [DOI] [PubMed] [Google Scholar]
- 12.Carnaud, C., D. Lee, O. Donnars, S. H. Park, A. Beavis, Y. Koezuka, and A. Bendelac. 1999. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 163:4647-4650. [PubMed] [Google Scholar]
- 13.Chackerian, A. A., T. V. Perera, and S. M. Behar. 2001. Gamma interferon-producing CD4+ T lymphocytes in the lung correlate with resistance to infection with Mycobacterium tuberculosis. Infect. Immun. 69:2666-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatterjee, D., A. D. Roberts, K. Lowell, P. J. Brennan, and I. M. Orme. 1992. Structural basis of capacity of lipoarabinomannan to induce secretion of tumor necrosis factor. Infect. Immun. 60:1249-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, H., H. Huang, and W. E. Paul. 1997. NK1.1+ CD4+ T cells lose NK1.1 expression upon in vitro activation. J. Immunol. 158:5112-5119. [PubMed] [Google Scholar]
- 16.Cui, J., T. Shin, T. Kawano, H. Sato, E. Kondo, I. Toura, Y. Kaneko, H. Koseki, M. Kanno, and M. Taniguchi. 1997. Requirement for valpha14 NKT cells in IL-12-mediated rejection of tumors. Science 278:1623-1626. [DOI] [PubMed] [Google Scholar]
- 17.Cui, J., N. Watanabe, T. Kawano, M. Yamashita, T. Kamata, C. Shimizu, M. Kimura, E. Shimizu, J. Koike, H. Koseki, Y. Tanaka, M. Taniguchi, and T. Nakayama. 1999. Inhibition of T helper cell type 2 cell differentiation and immunoglobulin E response by ligand-activated Valpha14 natural killer T cells. J. Exp. Med. 190:783-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Souza, C. D., A. M. Cooper, A. A. Frank, S. Ehlers, J. Turner, A. Bendelac, and I. M. Orme. 2000. A novel nonclassic beta2-microglobulin-restricted mechanism influencing early lymphocyte accumulation and subsequent resistance to tuberculosis in the lung. Am. J. Respir. Cell Mol. Biol. 23:188-193. [DOI] [PubMed] [Google Scholar]
- 19.Eberl, G., and H. R. MacDonald. 2000. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur. J. Immunol. 30:985-992. [DOI] [PubMed] [Google Scholar]
- 20.Eberl, G., and H. R. MacDonald. 1998. Rapid death and regeneration of NKT cells in anti-CD3epsilon- or IL-12-treated mice: a major role for bone marrow in NKT cell homeostasis. Immunity 9:345-353. [DOI] [PubMed] [Google Scholar]
- 21.Exley, M. A., N. J. Bigley, O. Cheng, S. M. Tahir, S. T. Smiley, Q. L. Carter, H. F. Stills, M. J. Grusby, Y. Koezuka, M. Taniguchi, and S. P. Balk. 2001. CD1d-reactive T-cell activation leads to amelioration of disease caused by diabetogenic encephalomyocarditis virus. J. Leukoc. Biol. 69:713-718. [PubMed] [Google Scholar]
- 22.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flynn, J. L., M. M. Goldstein, K. J. Triebold, J. Sypek, S. Wolf, and B. R. Bloom. 1995. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J. Immunol. 155:2515-2524. [PubMed] [Google Scholar]
- 24.Gilleron, M., C. Ronet, M. Mempel, B. Monsarrat, G. Gachelin, and G. Puzo. 2001. Acylation state of the phosphatidylinositol mannosides from Mycobacterium bovis bacillus Calmette-Guerin and ability to induce granuloma and recruit natural killer T cells. J. Biol. Chem. 276:34896-34904. [DOI] [PubMed] [Google Scholar]
- 25.Godfrey, D. I., K. J. Hammond, L. D. Poulton, M. J. Smyth, and A. G. Baxter. 2000. NKT cells: facts, functions and fallacies. Immunol. Today 21:573-583. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Aseguinolaza, G., C. de Oliveira, M. Tomaska, S. Hong, O. Bruna-Romero, T. Nakayama, M. Taniguchi, A. Bendelac, L. Van Kaer, Y. Koezuka, and M. Tsuji. 2000. Alpha-galactosylceramide-activated Valpha 14 natural killer T cells mediate protection against murine malaria. Proc. Natl. Acad. Sci. USA 97:8461-8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greinert, U., M. Ernst, M. Schlaak, and P. Entzian. 2001. Interleukin-12 as successful adjuvant in tuberculosis treatment. Eur. Respir. J. 17: 1049-1051. [DOI] [PubMed] [Google Scholar]
- 28.Gumperz, J. E., C. Roy, A. Makowska, D. Lum, M. Sugita, T. Podrebarac, Y. Koezuka, S. A. Porcelli, S. Cardell, M. B. Brenner, and S. M. Behar. 2001. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity 12:211-221. [DOI] [PubMed] [Google Scholar]
- 29.Hayakawa, Y., K. Takeda, H. Yagita, L. Van Kaer, I. Saiki, and K. Okumura. 2001. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J. Immunol. 166:6012-6018. [DOI] [PubMed] [Google Scholar]
- 30.Holland, S. M., E. M. Eisenstein, D. B. Kuhns, M. L. Turner, T. A. Fleisher, W. Strober, and J. I. Gallin. 1994. Treatment of refractory disseminated nontuberculous mycobacterial infection with interferon gamma. A preliminary report. N. Engl. J. Med. 330:1348-1355. [DOI] [PubMed] [Google Scholar]
- 31.Johnson, B., L. G. Bekker, S. Ress, and G. Kaplan. 1998. Recombinant interleukin 2 adjunctive therapy in multidrug-resistant tuberculosis. Novartis Found. Symp. 217:99-106. [DOI] [PubMed] [Google Scholar]
- 32.Juffermans, N. P., J. C. Leemans, S. Florquin, A. Verbon, A. H. Kolk, P. Speelman, S. J. van Deventer, and T. van der Poll. 2002. CpG oligodeoxynucleotides enhance host defense during murine tuberculosis. Infect. Immun. 70:147-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kakimi, K., L. G. Guidotti, Y. Koezuka, and F. V. Chisari. 2000. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J. Exp. Med. 192:921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawakami, K., Y. Kinjo, S. Yara, Y. Koguchi, K. Uezu, T. Nakayama, M. Taniguchi, and A. Saito. 2001. Activation of Vα14+ natural killer T cells by α-galactosylceramide results in development of Th1 response and local host resistance in mice infected with Cryptococcus neoformans. Infect. Immun. 69:213-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, H. Koseki, and M. Taniguchi. 1997. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 278:1626-1629. [DOI] [PubMed] [Google Scholar]
- 36.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, H. Sato, E. Kondo, M. Harada, H. Koseki, T. Nakayama, Y. Tanaka, and M. Taniguchi. 1998. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc. Natl. Acad. Sci. USA 95:5690-5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitamura, H., K. Iwakabe, T. Yahata, S. Nishimura, A. Ohta, Y. Ohmi, M. Sato, K. Takeda, K. Okumura, L. Van Kaer, T. Kawano, M. Taniguchi, and T. Nishimura. 1999. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 189:1121-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koo, G. C., and J. R. Peppard. 1984. Establishment of monoclonal anti-Nk-1.1 antibody. Hybridoma 3:301-303. [DOI] [PubMed] [Google Scholar]
- 39.Laloux, V., L. Beaudoin, C. Ronet, and A. Lehuen. 2002. Phenotypic and functional differences between NKT cells colonizing splanchnic and peripheral lymph nodes. J. Immunol. 168:3251-3258. [DOI] [PubMed] [Google Scholar]
- 40.Leite-de-Moraes, M. C., A. Herbelin, C. Gouarin, Y. Koezuka, E. Schneider, and M. Dy. 2000. Fas/Fas ligand interactions promote activation-induced cell death of NK T lymphocytes. J. Immunol. 165:4367-4371. [DOI] [PubMed] [Google Scholar]
- 41.Matsuda, J. L., O. V. Naidenko, L. Gapin, T. Nakayama, M. Taniguchi, C. R. Wang, Y. Koezuka, and M. Kronenberg. 2000. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192:741-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mempel, M., C. Ronet, F. Suarez, M. Gilleron, G. Puzo, L. Van Kaer, A. Lehuen, P. Kourilsky, and G. Gachelin. 2002. Natural killer T cells restricted by the monomorphic MHC class 1b CD1d1 molecules behave like inflammatory cells. J. Immunol. 16:8365-8371. [DOI] [PubMed] [Google Scholar]
- 43.Moody, D. B., B. B. Reinhold, M. R. Guy, E. M. Beckman, D. E. Frederique, S. T. Furlong, S. Ye, V. N. Reinhold, P. A. Sieling, R. L. Modlin, G. S. Besra, and S. A. Porcelli. 1997. Structural requirements for glycolipid antigen recognition by CD1b− restricted T cells. Science 278:283-286. [DOI] [PubMed] [Google Scholar]
- 44.Moody, D. B., T. Ulrichs, W. Muhlecker, D. C. Young, S. S. Gurcha, E. Grant, J. P. Rosat, M. B. Brenner, C. E. Costello, G. S. Besra, and S. A. Porcelli. 2000. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature 404:884-888. [DOI] [PubMed] [Google Scholar]
- 45.Moreira, A. L., L. Tsenova-Berkova, J. Wang, P. Laochumroonvorapong, S. Freeman, V. H. Freedman, and G. Kaplan. 1997. Effect of cytokine modulation by thalidomide on the granulomatous response in murine tuberculosis. Tuber. Lung Dis. 78:47-55. [DOI] [PubMed] [Google Scholar]
- 46.Osman, Y., T. Kawamura, T. Naito, K. Takeda, L. Van Kaer, K. Okumura, and T. Abo. 2000. Activation of hepatic NKT cells and subsequent liver injury following administration of alpha-galactosylceramide. Eur. J. Immunol. 30:1919-1928. [DOI] [PubMed] [Google Scholar]
- 47.Porcelli, S. A. 1995. The CD1 family: a third lineage of antigen-presenting molecules. Adv. Immunol. 59:1-98. [DOI] [PubMed] [Google Scholar]
- 48.Prigozy, T. I., P. A. Sieling, D. Clemens, P. L. Stewart, S. M. Behar, S. A. Porcelli, M. B. Brenner, R. L. Modlin, and M. Kronenberg. 1997. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity 6:187-197. [DOI] [PubMed] [Google Scholar]
- 49.Sieling, P. A., D. Chatterjee, S. A. Porcelli, T. I. Prigozy, R. J. Mazzaccaro, T. Soriano, B. R. Bloom, M. B. Brenner, M. Kronenberg, and P. J. Brennan. 1995. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science 269:227-230. [DOI] [PubMed] [Google Scholar]
- 50.Singh, N., S. Hong, D. C. Scherer, I. Serizawa, N. Burdin, M. Kronenberg, Y. Koezuka, and L. Van Kaer. 1999. Cutting edge: activation of NK T cells by CD1d and alpha-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 163:2373-2377. [PubMed] [Google Scholar]
- 51.Smiley, S. T., M. H. Kaplan, and M. J. Grusby. 1997. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science 275:977-979. [DOI] [PubMed] [Google Scholar]
- 51a.Sousa, A. O., R. J. Mazzaccaro, R. G. Russell, F. K. Lee, O. C. Turner, S. Hong, L. Jan Kaer, and B. R. Bloom. 2000. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc. Natl. Acad. Sci. USA 97:4204-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spada, F. M., Y. Koezuka, and S. A. Porcelli. 1998. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J. Exp. Med. 188:1529-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stenger, S., D. A. Hanson, R. Teitelbaum, P. Dewan, K. R. Niazi, C. J. Froelich, T. Ganz, S. Thoma-Uszynski, A. Melian, C. Bogdan, S. A. Porcelli, B. R. Bloom, A. M. Krensky, and R. L. Modlin. 1998. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282:121-125. [DOI] [PubMed] [Google Scholar]
- 54.Stenger, S., R. J. Mazzaccaro, K. Uyemura, S. Cho, P. F. Barnes, J. P. Rosat, A. Sette, M. B. Brenner, S. A. Porcelli, B. R. Bloom, and R. L. Modlin. 1997. Differential effects of cytolytic T cell subsets on intracellular infection. Science 276:1684-1687. [DOI] [PubMed] [Google Scholar]
- 55.Stenger, S., K. R. Niazi, and R. L. Modlin. 1998. Down-regulation of CD1 on antigen-presenting cells by infection with Mycobacterium tuberculosis. J. Immunol. 161:3582-3588. [PubMed] [Google Scholar]
- 56.Takeda, K., Y. Hayakawa, L. Van Kaer, H. Matsuda, H. Yagita, and K. Okumura. 2000. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc. Natl. Acad. Sci. USA 97:5498-5503. [DOI] [PMC free article] [PubMed] [Google Scholar]