Abstract
Pneumolysin, neuraminidases A and B, and hyaluronidase are virulence factors of Streptococcus pneumoniae that appear to be involved in the pathogenesis of meningitis. In a murine model of meningitis after intracerebral infection using mutants of S. pneumoniae D39, only mice infected with a pneumolysin-deficient strain were healthier at 32 and 36 h, had lower bacterial titers in blood at 36 h, and survived longer than the D39 parent strain. Cerebellar and spleen bacterial titers, meningeal inflammation, and neuronal damage scores remained uninfluenced by the lack of any of the virulence factors.
Streptococcus pneumoniae meningitis frequently causes severe neurological sequelae and death (9, 15, 23). A number of pneumococcal proteins have been characterized as putative virulence factors, among them pneumolysin, the neuraminidases A and B, and hyaluronidase (7, 8, 16, 17, 21, 22, 27). Pneumolysin, a cytoplasmic protein, is released during autolysis of the bacterium and probably also via an autolysis-independent mechanism (3, 21). It interacts with cholesterol in the cell wall of host cells and forms transmembrane pores by oligomerization, leading to loss of membrane integrity of host cells. In sublytic concentrations it is capable of inhibiting respiratory burst, chemotaxis, and bactericidal activity of polymorphonuclear leukocytes (20). Furthermore, it leads to complement consumption (6), thereby reducing serum opsonic activity (1, 2). Neuraminidase activity has been indirectly linked to virulence in human pneumococcal meningitis on the basis of elevated cerebrospinal fluid concentrations of N-acetylneuraminic acid in patients with coma and bacteremia (19). A deficiency of neuraminidase A led to decreased virulence in a model of pneumococcal pneumonia (17). The role of hyaluronidase has not been studied extensively. Strains causing meningitis showed a higher in vitro expression of hyaluronidase (16). Intranasal instillation of pneumococci with addition of hyaluronidase to the inoculum was followed by meningitis in a model of pneumococcal pneumonia (28). We used a mouse model based on intracerebral infection (14, 26) to assess the role of pneumolysin, neuraminidases A and B, and hyaluronidase in meningitis by using mutants of an S. pneumoniae type 2 strain deficient in these putative virulence factors.
(This work was presented, in part, at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, 17 to 20 September 2000 [abstr. 433]).
Bacteria.
Mutant strains of S. pneumoniae D39 were generated by insertion duplication mutagenesis as described in detail before (30). In brief, internal gene fragments amplified from chromosomal DNA were ligated with the insertion vector pJDC9 by standard DNA techniques (12). The insertion was performed at position 547 of the 1,416-bp ply gene, position 605 of the 3,108-bp nanA gene, position 735 of the 2,094-bp nanB gene, and position 534 of the 2,850-bp hyl gene. Erythromycin-resistant transformants were selected with 1 μg of erythromycin/ml on Luria-Bertani agar containing 5% sheep blood. Insertion was demonstrated by PCR analysis and DNA sequencing. Functional deficiency of pneumolysin was confirmed by a hemolysis assay (4). The stability of mutants was confirmed by PCR testing of spleen isolates. In vitro growth rates were assessed in Todd-Hewitt broth supplemented with yeast extract.
Model of meningitis.
Three- to 5-month-old anesthetized (ketamine [100 mg/kg of body weight] and xylazine [10 mg/kg]) C57BL/6 mice were infected with 25 μl of 0.9% NaCl containing 104 CFU of the respective strain in the right frontal lobe (14). Mice were followed up at 12, 24, 32, and 36 h after infection by weighing, tightrope test, and a clinical score (26) as follows: appearing healthy (0); slightly lethargic (1); moderately lethargic but able to walk (2); severely lethargic and unable to walk (3); and dead (4). Mice with a clinical score of 3 were killed for ethical reasons. To determine in vivo growth rates, 36 h after infection mice were sacrificed by decapitation and blood was collected. The cerebellum and the ventral half of the spleen were homogenized in 0.9% saline (1/10 [wt/wt]) for determination of bacterial titers. The remaining brain was fixed in 4% paraformaldehyde. For a more detailed assessment of neuronal damage, tissue samples from mice infected with D39 wild-type and pneumolysin-deficient S. pneumoniae (n = 10 each) were perfused with phosphate-buffered saline and 4% paraformaldehyde and were embedded in paraffin. One-micrometer-thick coronary sections were stained with hematoxylin and eosin. Brain sections were scored semiquantitatively for inflammation and neuronal damage (14, 25). In a survival experiment mice were infected with either D39 wild-type strain or pneumolysin-deficient S. pneumoniae (n = 12 each). Animals were followed up at 8- to 12-h intervals for up to 14 days. In animals that were dead or were killed when having a clinical score of 3, blood bacterial titers were determined to ensure that infection was the cause of death. Surviving animals were killed after 14 days, and blood bacterial titers were determined to assess whether they had cleared the infection.
Data were expressed as means ± standard deviations if normally distributed. Groups were compared by analysis of variance (ANOVA) for independent samples followed by Dunnett's multiple-comparison test when overall P was < 0.05. In the absence of normal distribution, median and 25th and 75th percentiles were used. Then, groups were compared by Kruskal-Wallis test, followed by Dunn's multiple-comparison post hoc analysis when overall P was < 0.05. For neuronal damage scores data were compared by the Mann-Whitney U test. For survival analysis, the log rank test based on a Kaplan-Meier plot was used.
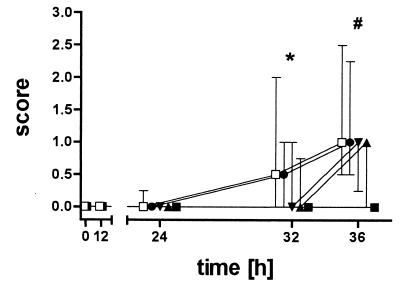
Not all mutant strains differed in their in vitro growth characteristics from the D39 wild-type strain. All mice lost weight during the course of meningitis. Although weight loss was least in mice infected with the pneumolysin-deficient strain, the differences were not significant between groups (32 h, P = 0.36; and 36 h, P = 0.10). Mice infected with the D39 wild-type strain started to become lethargic 24 h after infection (Fig. 1). At 32 h and 36 h animals infected with the pneumolysin-deficient mutant were significantly less lethargic than animals infected with the D39 wild-type strain (32 h, Kruskal-Wallis test [P = 0.02] and Dunn's multiple-comparison test [P < 0.05]; 36 h, P = 0.005; and posttest, P < 0.01 [Fig. 1]). The tightrope test reflected the differences in clinical scores but did not reach statistical significance due to the high variations in test performance (32 h, P = 0.33; 36 h, P = 0.04; and posttest, P > 0.05).
FIG. 1.
Clinical score of mice infected with the various strains (n = 11 each). S. pneumoniae D39 wild-type (□), pneumolysin-deficient (▪), neuraminidase A-deficient (▴), neuraminidase B-deficient (▾), and hyaluronidase-deficient (•) strains were used. Mice infected with the pneumolysin-deficient strain stayed clinically almost healthy throughout the 36 h of the experiment and performed well in the tightrope test as opposed to mice infected with the S. pneumoniae D39 wild type or any of the other deficient strains. *, P < 0.05; #, P < 0.01; Kruskal-Wallis test with Dunn's multiple-comparison test. Error bars denote 25th and 75th percentiles.
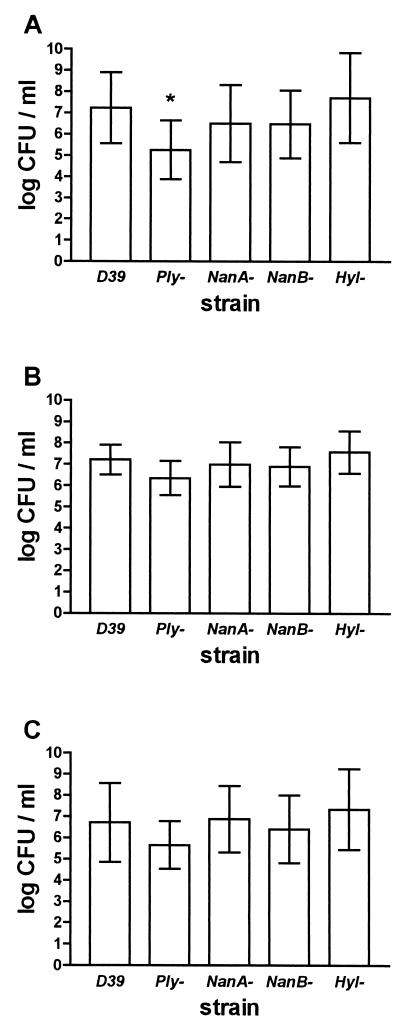
At 36 h, blood bacterial titers were almost 100 times lower in the group infected with the pneumolysin-deficient mutant (5.25 ± 1.39 versus 7.23 ± 1.67 log CFU/ml; ANOVA, P = 0.02; and posttest, P < 0.05 [Fig. 2]). Cerebellar and spleen bacterial titers were slightly but not significantly lower in mice infected with pneumolysin-deficient pneumococci (D39 versus pneumolysin-deficient mutant cerebellar titers, 7.21 ± 0.70 versus 6.36 ± 0.81 log CFU/ml; ANOVA, P = 0.04; post hoc tests, P > 0.05; and spleen titers, 6.71 ± 1.85 versus 5.65 ± 1.12 log CFU/ml; ANOVA, P = 0.18 [Fig. 2]). Direct comparison between cerebellar titers of mice infected with wild-type and pneumolysin-deficient bacteria by t test would result in a statistically significant difference (P = 0.015). Leukocyte recruitment into the subarachnoid space was evident on brain slices in all mice but again tended to be lower in the group infected with the pneumolysin-deficient mutant (inflammation score median [25th and 75th percentiles] for D39, 12 [4 and 17]; and for pneumolysin-deficient mice, 6 [4 and 11] [P = 0.16] [Fig. 3A]). Brain histology showed neuronal damage preferentially located in the hippocampal formation and neocortex (Fig. 3B to D). The neuronal damage score was not reduced in mice infected with pneumolysin-deficient bacteria (overall neuronal damage score median [25th and 75th percentiles] for D39, 4 [3 and 5]; and for pneumolysin-deficient bacteria, 3 [3 and 4.5] [P = 0.53]).
FIG. 2.
Bacterial titers in blood (A), cerebellar (B), and spleen (C) homogenates in the D39 wild type and the various deficient strains (n = 11 each). Ply, pneumolysin; NanA, neuraminidase A; NanB, neuraminidase B; and Hyl, hyaluronidase. Only the pneumolysin-deficient strain was significantly different from the D39 wild-type strain. Error bars show standard deviations. *, P < 0.05; ANOVA with Dunnett's multiple-comparison posttest.
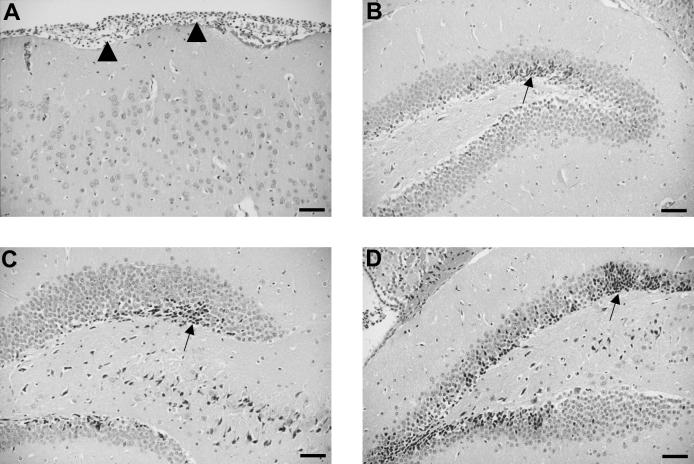
FIG. 3.
Hematoxylin-and-eosin-stained brain sections show a meningeal infiltrate (arrowheads) with polymorphonuclear neutrophils in a mouse infected with hyaluronidase-deficient pneumococci (A) and neuronal necrosis in the dentate gyrus of mice infected with pneumolysin-deficient (B), and neuraminidase A-deficient (C), and neuraminidase B-deficient (D) pneumococci. Note the clusters of necrotic granular cells (arrows). Scale bar, 50 μm.
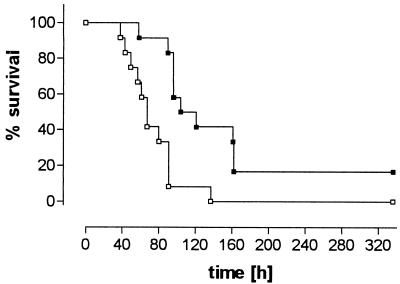
In the survival experiment, all mice infected with D39 wild-type bacteria died, whereas two mice infected with pneumolysin-deficient bacteria survived and were able to clear infection. Overall survival was significantly longer in the latter group (P = 0.0005 [Fig. 4]). Blood bacterial titers at time of death did not differ significantly between groups (8.34 ± 1.38 versus 7.43 ± 0.89 log CFU/ml; P = 0.09).
FIG. 4.
Cumulative survival (Kaplan-Meier plots) of mice infected with D39 wild-type (□) and pneumolysin-deficient (▪) S. pneumoniae (n = 12 each). P < 0.0005 (log rank test).
Pneumolysin-deficient pneumococci showed reduced virulence in this murine model of meningitis. Despite relatively high cerebellar bacterial titers, mice infected with this strain showed little signs of disease at 36 h. This was accompanied by a strongly reduced bacterial load in the blood, suggesting that the effect of a lack of pneumolysin on the clinical picture may be in part due to the less severe sepsis. The differences in cerebellar bacterial titers were less pronounced, just failing to reach statistical significance due to the adjustment of P for repeated testing. The probable reason is the immunocompromised state of the central nervous system, where pneumolysin may be less important for counteracting host defense.
Pneumolysin potentiates the proinflammatory activity of human neutrophils (13) and murine peritoneal macrophages (10). Meningeal inflammation scores in brain slices were slightly but not significantly reduced in these mice. Therefore, not only the number but also the activity of leukocytes in cerebrospinal fluid may be reduced in meningitis caused by pneumolysin-deficient bacteria, contributing to the mild clinical symptoms despite relatively high bacterial titers. The lack of pneumolysin was expected to result in reduced neuronal damage (11; A. K. Stringaris, F. Bergmann, J. Geisenhainer, M. Bähr, and R. Nau, Abstr. 54th Annu. Meet. Am. Acad. Neurol., abstr. P04.005, 2002). In our experiment the neuronal damage scores obtained (14), however, did not differ between groups. To detect minor differences, more sensitive modes of quantification may be necessary. Also, animals were not ill enough to produce serious damage. The median clinical score in the control animals at 36 h was only 1 (slightly lethargic). In accordance with this study, mice infected with pneumolysin-deficient strains of S. pneumoniae created by insertion-duplication mutagenesis showed increased survival after intranasal challenge, an increased 50% lethal dose after intraperitoneal inoculation, and reduced multiplication after intravenous injection compared to infection by the D39 wild-type strain (8). After intraperitoneal challenge of mice with various mutant strains of S. pneumoniae D39, Berry and Paton (7) found reduced virulence only for strains carrying a deletion mutation of pneumolysin, an insertion-duplication mutation of pneumococcal surface protein A, or of autolysin genes. Insertion-duplication mutations of neuraminidase A, hyaluronidase, or the choline-binding protein A gene did not result in reduced virulence. After intranasal inoculation there was a reduced virulence of mutants for pneumolysin and pneumococcal surface protein A (7).
Two main properties of pneumolysin are believed to be involved in the pathogenesis of pneumococcal infection. Firstly, it acts as a cytolysin (21, 24; Stringaris et al., Abstr. 54th Annu. Meet. Am. Acad. Neurol.). Secondly, it exerts complement-binding properties (1, 2). The reduced virulence of pneumolysin-deficient strains of S. pneumoniae in the murine peritonitis model appears to be linked to the cytolytic rather than the complement-binding properties (5). The induction of necrosis and apoptosis of neutrophilic granulocytes by pneumolysin interferes with the host response during the early stage of infection and promotes bacterial multiplication in vivo (29). In the present experiments the low blood bacterial titers of pneumolysin-deficient bacteria could reflect a decreased or delayed systemic spread and/or a higher clearance from the bloodstream. A role for pneumolysin in systemic spread of pneumococci is supported by increased levels of antipneumolysin immunoglobulin G in nonbacteremic infections compared to low levels in bacteremic pneumococcal infections (18).
The neuraminidases and hyaluronidase neither influenced the clinical course of disease nor had any effect on systemic bacterial spread and multiplication in our model. This is in agreement with the course of disease studied by using intraperitoneal infection (7). Similarly, neuraminidase A deficiency had no effect on hearing loss and cochlear damage in experimental meningitis in guinea pigs (27). The lack of effect of neuraminidase A (or B) deficiency on virulence may be partly explained by the compensatory action of neuraminidase B (or A) and possibly of another, similar enzyme, neuraminidase C (7). In confirmation of previous studies using intraperitoneal infection (7), hyaluronidase deficiency had no impact on virulence in our model. Our experiments do not, however, exclude a role for these virulence factors in the crossing of the mucosal or blood-brain barrier by pneumococci, an early pathogenetic step that is bypassed by direct intracerebral infection. In an infant mouse model of meningitis, addition of hyaluronidase to the nasal inoculum was necessary to cause invasive disease (28).
In conclusion, pneumolysin was the only virulence factor investigated whose absence had an attenuating influence on the clinical course after intracerebral infection. This effect may be partly caused by the less severe sepsis in these mice.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. Na 165/4-1).
Editor: V. J. DiRita
REFERENCES
- 1.Alcantara, R. B., L. C. Preheim, and M. J. Gentry. 1999. Role of pneumolysin's complement-activating activity during pneumococcal bacteremia in cirrhotic rats. Infect. Immun. 67:2862-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcantara, R. B., L. C. Preheim, and M. J. Gentry-Nielsen. 2001. Pneumolysin-induced complement depletion during experimental pneumococcal bacteremia. Infect. Immun. 69:3569-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balachandran, P., S. K. Hollingshead, J. C. Paton, and D. E. Briles. 2001. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J. Bacteriol. 183:3108-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benton, K. A., J. C. Paton, and D. E. Briles. 1997. Differences in virulence for mice among Streptococcus pneumoniae strains of capsular types 2, 3, 4, 5, and 6 are not attributable to differences in pneumolysin production. Infect. Immun. 65:1237-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry, A. M., J. E. Alexander, T. J. Mitchell, P. W. Andrew, D. Hansman, and J. C. Paton. 1995. Effect of defined point mutations in the pneumolysin gene on the virulence of Streptococcus pneumoniae. Infect. Immun. 63:1969-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry, A. M., A. D. Ogunniyi, D. C. Miller, and J. C. Paton. 1999. Comparative virulence of Streptococcus pneumoniae strains with insertion-duplication, point, and deletion mutations in the pneumolysin gene. Infect. Immun. 67:981-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry, A. M., and J. C. Paton. 2000. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect. Immun. 68:133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry, A. M., J. Yother, D. E. Briles, D. Hansman, and J. C. Paton. 1989. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 57:2037-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohr, V., O. B. Paulson, and N. Rasmussen. 1984. Pneumococcal meningitis. Late neurologic sequelae and features of prognostic impact. Arch. Neurol. 41:1045-1049. [DOI] [PubMed] [Google Scholar]
- 10.Braun, J. S., R. Novak, G. Gao, P. J. Murray, and J. L. Shenep. 1999. Pneumolysin, a protein toxin of Streptococcus pneumoniae, induces nitric oxide production from macrophages. Infect. Immun. 67:3750-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun, J. S., J. E. Sublett, D. Freyer, T. J. Mitchell, J. L. Cleveland, E. I. Tuomanen, and J. R. Weber. 2001. Pneumococcal pneumolysin and H2O2 mediate brain cell apoptosis during meningitis. J. Clin. Investig. 109:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, J. D., and D. A. Morrison. 1988. Construction and properties of a new insertion vector, pJDC9, that is protected by transcriptional terminators and useful for cloning of DNA from Streptococcus pneumoniae. Gene 64:155-164. [DOI] [PubMed] [Google Scholar]
- 13.Cockeran, R., A. J. Theron, H. C. Steel, N. M. Matlola, T. J. Mitchell, C. Feldman, and R. Anderson. 2001. Proinflammatory interaction of pneumolysin with human neutrophils. J. Infect. Dis. 183:604-611. [DOI] [PubMed] [Google Scholar]
- 14.Gerber, J., G. Raivich, A. Wellmer, T. Kunst, A. Werner, W. Brück, and R. Nau. 2001. A mouse model of Streptococcus pneumoniae meningitis mimicking several features of human disease. Acta Neuropathol. 101:499-508. [DOI] [PubMed] [Google Scholar]
- 15.Grimwood, K., V. A. Anderson, L. Bond, C. Catroppa, R. L. Hore, E. H. Keir, T. Nolan, and D. M. Roberton. 1995. Adverse outcomes of bacterial meningitis in school-age survivors. Pediatrics 95:646-656. [PubMed] [Google Scholar]
- 16.Kostyukova, N. N., M. O. Volkova, V. V. Ivanova, and A. S. Kvetnaya. 1995. A study of pathogenic factors of Streptococcus pneumoniae strains causing meningitis. FEMS Immunol. Med. Microbiol. 10:133-138. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell, T. J. 2000. Virulence factors and the pathogenesis of disease caused by Streptococcus pneumoniae. Res. Microbiol. 151:413-419. [DOI] [PubMed] [Google Scholar]
- 18.Musher, D. M., H. M. Phan, and R. E. Baughn. 2001. Protection against bacteremic pneumococcal infection by antibody to pneumolysin. J. Infect. Dis. 183:827-830. [DOI] [PubMed] [Google Scholar]
- 19.O'Toole, R. D., L. Goode, and C. Howe. 1971. Neuraminidase activity in bacterial meningitis. J. Clin. Investig. 50:979-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paton, J. C., and A. Ferrante. 1983. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity and migration by pneumolysin. Infect. Immun. 41:1212-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossjohn, J., R. J. C. Gilbert, D. Crane, P. J. Morgan, T. J. Mitchell, A. J. Rowe, P. W. Andrew, J. C. Paton, R. K. Tweten, and M. W. Parker. 1998. The molecular mechanism of pneumolysin, a virulence factor from Streptococcus pneumoniae. J. Mol. Biol. 284:449-461. [DOI] [PubMed] [Google Scholar]
- 22.Rubins, J. B., A. H. Paddock, D. Charboneau, A. M. Berry, J. C. Paton, and E. N. Janoff. 1998. Pneumolysin in pneumococcal adherence and colonization. Microb. Pathog. 25:337-342. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt, H., A. Tlustochowska, K. Stuertz, M. Djukic, J. Gerber, E. Schütz, U. Kuhnt, and R. Nau. 2001. Organotypic hippocampal cultures—a model of brain tissue damage in Streptococcus pneumoniae meningitis. J. Neuroimmunol. 113:30-39. [DOI] [PubMed] [Google Scholar]
- 24.Tuomanen, E. 1999. Molecular and cellular biology of pneumococcal infection. Curr. Opin. Microbiol. 2:35-39. [DOI] [PubMed] [Google Scholar]
- 25.Wellmer, A., J. Gerber, J. Ragheb, G. Zysk, T. Kunst, A. Smirnov, W. Brück, and R. Nau. 2001. Effect of deficiency of tumor necrosis factor alpha or both of its receptors on Streptococcus pneumoniae central nervous system infection and peritonitis. Infect. Immun. 69:6881-6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wellmer, A., C. Noeske, J. Gerber, U. Munzel, and R. Nau. 2000. Spatial memory and learning deficits after experimental pneumococcal meningitis in mice. Neurosci. Lett. 296:137-140. [DOI] [PubMed] [Google Scholar]
- 27.Winter, A. J., S. D. Comis, M. P. Osborne, M. J. Tarlow, J. Stephen, P. W. Andrew, J. Hill, and T. J. Mitchell. 1997. A role for pneumolysin but not neuraminidase in the hearing loss and cochlear damage induced by experimental pneumococcal meningitis in guinea pigs. Infect. Immun. 65:4411-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zwijnenburg, P. J., T. van der Poll, S. Florquin, S. J. van Deventer, J. J. Roord, and A. M. van Furth. 2001. Experimental pneumococcal meningitis in mice: a model of intranasal infection. J. Infect. Dis. 183:1143-1146. [DOI] [PubMed] [Google Scholar]
- 29.Zysk, G., L. Bejo, B. K. Schneider-Wald, R. Nau, and H. Heinz. 2000. Induction of necrosis and apoptosis of neutrophil granulocytes by Streptococcus pneumoniae. Clin. Exp. Immunol. 122:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zysk, G., B. K. Schneider-Wald, J. H. Hwang, L. Bejo, K. S. Kim, T. J. Mitchell, R. Hakenbeck, and H.-P. Heinz. 2001. Pneumolysin is the main inducer of cytotoxicity to brain microvascular endothelial cells caused by Streptococcus pneumoniae. Infect. Immun. 69:845-852. [DOI] [PMC free article] [PubMed] [Google Scholar]