Abstract
Phosphatidylserine (PS), an anionic phospholipid of significant biological relevance, forms a multilamellar phase in water with net negative surface charge at pH 7.0. In this study we mixed dioleoylPS (DOPS) with reverse hexagonal (HII)-forming phosphatidylethanolamine (DOPE), and used x-ray diffraction and osmotic stress to quantify its spontaneous curvature (1/R0p) and bending modulus (Kcp). The mixtures were stable HII phases from 5 to 30 mol% PS, providing 16 wt% tetradecane (td) was also added to relieve chain-packing stress. The fully hydrated lattice dimension increased with DOPS concentration. Analysis of structural changes gave an apparent R0p for DOPS of +144 Å; opposite in sign and relatively flat compared to DOPE (−30 Å). Osmotic stress of the HII phases did not detect a significantly different bending modulus (Kcp) for DOPS as compared to DOPE. At pH ≤ 4.0, DOPS (with no td) adopted the HII phase on its own, in agreement with previous results, suggesting a reversal in curvature upon protonation of the serine headgroup. In contrast, when td was present, DOPS/td formed a lamellar phase of limited swelling whose dimension increased with pH. DOPS/DOPE/td mixtures formed HII phases whose dimension increased both with pH and with DOPS content. With tetradecane, estimates put 1/R0p for DOPS at pH 2.1 at zero. Tetradecane apparently affects the degree of dissociation of DOPS at low pH.
INTRODUCTION
Phosphatidylserine (PS) is one of the most prevalent naturally occurring negatively charged membrane phospholipids. It is known to be involved in physiologically important processes, such as membrane fusion (Papahadjopoulos et al., 1974) and activation of various phospholipases (Buckland and Wilton, 2000), protein kinase C (Nishizuka, 1984), and components of the blood coagulation process (Lentz, 1999). In addition, PS interacts with other components of the mammalian membrane such as sterols, cholesterol being the most important representative. Phospholipid, when hydrated, can aggregate in several different lyotropic phases. Increasingly, evidence suggests that the tendency of some specific phospholipids to form phases other than bilayers may have implications for biological functions (Chernomordik et al., 1995b; Cullis et al., 1985). The most common phases formed by naturally occurring phospholipids are the bilayer lamellar (Lα) phase and the reverse hexagonal phase (HII) (Chen and Rand, 1997; Epand, 1997, Rand and Fuller, 1994; Seddon, 1990). Lipids that form the most common of the nonlamellar phases, the HII phase, appear to be of fundamental relevance for many processes carried out by biomembranes (de Kruiff, 1987; Seddon, 1990). These lipids, incorporated within bilayers, appear to produce packing stresses that affect both membrane protein conformation and topological changes required in processes like membrane fusion and fission. Most recently, in engineered membranes for the application of drug and DNA delivery, the tendency of the lipid components to adopt the hexagonal phase was observed to correlate with fusion or transfection efficiency (Koltover et al., 1998; May et al., 2000).
The structural preference of lipid mixtures for the lamellar or hexagonal phase is dictated by the minimization of molecular free energies of the component molecules (May and Ben-Shaul, 1999). Remarkably, the total energy difference between the Lα and HII geometries can be extremely small (Kozlov et al., 1994b). Although some internal individual molecular constants contributing to the free energy of different lipid molecules cannot be measured directly (May and Ben-Shaul, 1999), the elastic constants of the lipid ensemble, monolayer spontaneous curvature (c0) and bending modulus (Kcp), can be determined and give some indication of phase preference. By convention, large negative curvature lipids tend to form HII phases, large positive curvature lipids form micelles or HI phases, and small curvature lipids form bilayers.
The structure and phase behavior of phosphatidylserines has been studied by several techniques (Browning and Seelig, 1980; de Kroon et al., 1990; Hope and Cullis, 1980; Portis et al., 1979). These studies have shown that at physiological or neutral pH, PS forms a lamellar phase upon hydration, and is capable of stabilizing nonbilayer forming lipids in a bilayer structure (Cullis and Verkleij, 1979). Thermodynamic properties of the negatively charged PS bilayers are not very different from zwitterionic phospholipids. Chain melting transition temperatures for pure diacyl PSs are only slightly higher than for the corresponding PCs, and lower than PEs. Transition enthalpies were found to be slightly larger. 2H and 31P NMR studies further show that the fatty acyl chain region of the PS bilayer is quantitatively similar to PC. In contrast, however, the headgroup order is more rigid than that of other phospholipids (Browning and Seelig, 1980), likely due to intermolecular electrostatic interactions (Roux et al., 1989) or hydrogen bonding (Boggs, 1987).
Under certain conditions, bilayer PS has been reported to undergo a transition to nonbilayer phases. Specifically, the HII phase has been observed in the anhydrous state (Hauser et al., 1982), in aqueous dispersions containing lithium ions (Cevc et al., 1985), and in aqueous dispersions at low pH (de Kroon et al., 1990; Hope and Cullis, 1980). This transition has led to the question of whether PS changes its curvature to highly negative upon neutralization of its charge. Significantly, in this specific case, Bezrukov et al. (1999) have shown that alamethicin channels change their gating properties in PS-containing membranes when the pH is changed. This is an explicit example of a protein's activity being modulated by the properties of its host lipid. We attempt here to measure some of these properties of PS. In this work we measure the spontaneous curvature of negatively charged dioleoylphosphatidylserine (DOPS) at neutral pH, and estimate the change in curvature at pH 2. A mixed lipid system, containing dioleoylphosphatidylethanolamine (DOPE) and various concentrations of DOPS, is used to give an HII phase. The radius of curvature of the equilibrium structure, in excess tetradecane (td) and excess water, is determined by x-ray diffraction. The contribution of DOPS to the negative curvature of DOPE/DOPS mixtures allows the spontaneous curvature of DOPS to be calculated. In addition, osmotic stress experiments (Leikin et al., 1996; Parsegian et al., 1986) are used to measure the effect of DOPS on the bending modulus of the DOPE monolayer.
MATERIAL AND METHODS
DOPE and DOPS (Na salt) were purchased from Avanti Polar Lipids Inc. (Alabaster, AL). Polyethylene glycol (PEG) 20,000 was purchased from Fluka (Switzerland) and used as received. n-Tetradecane was purchased from Sigma-Aldrich Canada Ltd. (Oakville, ON), and the water used in the experiments was double distilled.
Stock mixtures, each of different DOPE/DOPS composition, were prepared by mixing the lipids in chloroform solution, and then drying by rotary evaporation and vacuum desiccation. Unless otherwise stated, to relieve interstitial packing stresses, 16 wt% tetradecane was added to the dried lipid stocks and equilibrated as described previously (Leikin et al., 1996; Rand and Fuller, 1994; Rand et al., 1990). Aliquots from each stock were hydrated to varying degrees by either: 1), adding weighed amounts of distilled water; 2), adding excess amounts of PEG solutions of known osmotic pressure; or 3), adding excess amounts of various 100 mM buffer solutions at various pH values. The resulting equilibrated structures were investigated at 20°C by x-ray diffraction and their dimensions determined with a measuring error of ± 0.1 Å. Sample to sample variation including all experimental errors was a maximum of ± 0.5 Å. Thin-layer chromatography showed no degradation (<0.1%) of td-containing samples after equilibration. Degradation in td-free samples was <1%.
Structural analysis
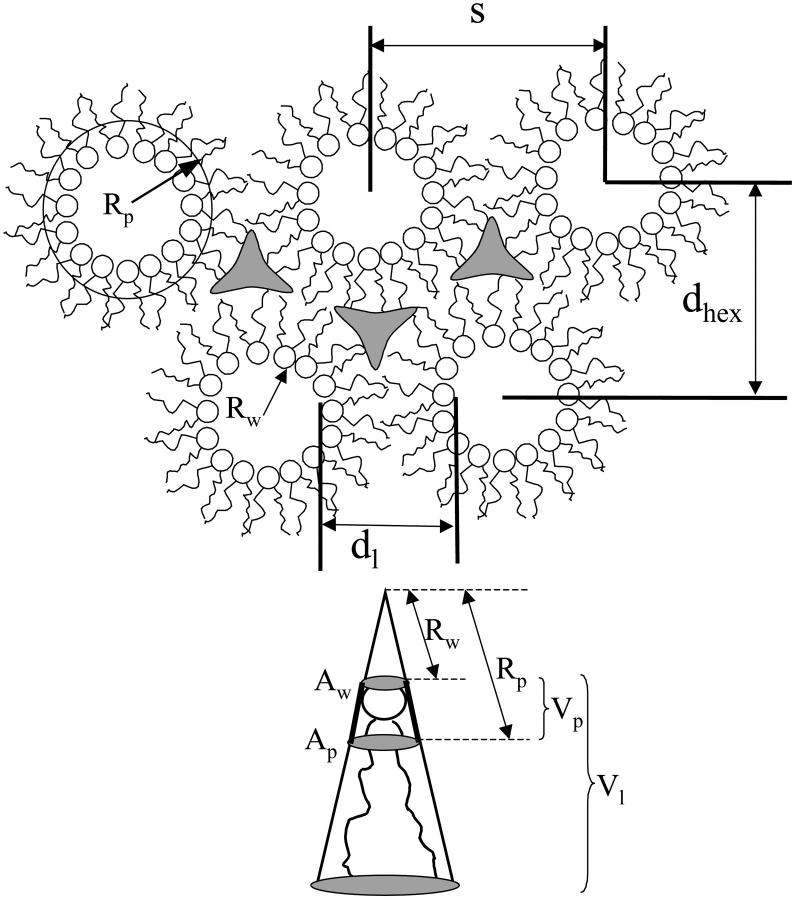
The DOPE/DOPS/td lipid mixtures (studied here from 0 to 30 mol% DOPS) with added tetradecane gave single, well-ordered, inverted hexagonal (HII) phases at all levels of hydration. The HII phase has been well characterized (Epand, 1997; Fuller and Rand, 2001; Rand and Fuller, 1994; Seddon, 1990), and is shown schematically in Fig. 1. Following the method originally introduced by Luzzati (Luzzati and Husson, 1962), the hexagonal lattice is divided by a cylindrical surface (the Luzzati plane) into separate compartments containing lipid and water. In this way, at known volume fractions of water (φw) and lipid (1 − φw), dimensions of the water cylinders and molecular dimensions of the lipid compartment can be determined (Eqs. 1–5, Fig. 1). We then follow a previously published procedure for determining structural and elastic characteristics of the HII phases.
FIGURE 1.
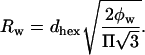 |
(1) |
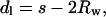 |
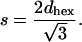 |
(2) |
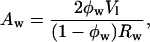 |
(3) |
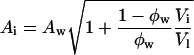 |
(4) |
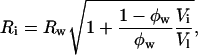 |
(5) |
Elastic energy of the hexagonal phase
Ideally, monolayer equilibrium elastic parameters are reported for one of two dividing surfaces within the lipid monolayer (Leikin et al., 1996): the neutral plane (where bending and compression are energetically uncoupled) (Kozlov and Winterhalter, 1991a; Kozlov and Winterhalter, 1991b)), or the pivotal plane (where the molecular area remains constant on bending) (Rand et al., 1990). Here, we analyze the experimental data with reference to the pivotal plane.
Given the following geometric relation,
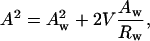 |
(6) |
between the area per molecule (A) at any cylindrical dividing surface inside the lipid monolayer, and the area per molecule at the water interface (Aw), (where Rw is the radius of the water cylinder), a diagnostic plot of Aw2 vs. Aw/Rw that gives a straight line, will verify that the system has a well-defined pivotal plane, and the slope of the line will determine its location in terms of V (the volume separating this plane and the Luzzati plane).
Using this volume, now called Vp, giving the position of the pivotal plane, the radius of curvature at the pivotal plane (Rp) can be calculated using another geometric relation:
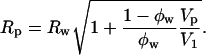 |
Knowing the radius of curvature at the pivotal plane, Rp, the elastic free energy, F, of the hexagonal phase (normalized per phospholipid molecule) can be approximated by the energy of bending (Helfrich, 1973; Kirk et al., 1984).
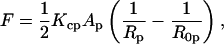 |
(7) |
where Kcp is the bending modulus, Ap is the molecular area, and R0p is the spontaneous radius of curvature, all at the pivotal plane (under equilibrium conditions of excess water and tetradecane).
Finally, by determining the elastic parameters of the lipid mixtures under conditions of osmotic stress (Gruner et al., 1986; Rand et al., 1990), we relate the elastic energy given by Eq. 7 with the osmotic work done by osmotic stress (Π):
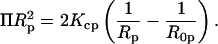 |
(8) |
A plot of (ΠRp2) vs. (1/Rp) gives, from the slope, the monolayer bending modulus (Kcp) (Gruner et al., 1986; Rand et al., 1990).
RESULTS
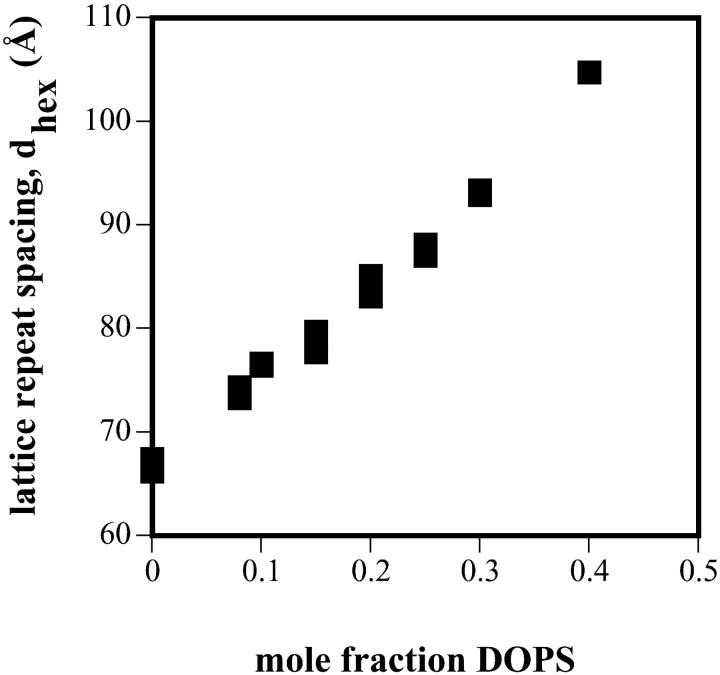
Fig. 2 shows that increasing amounts of DOPS result in an increase in the equilibrium, unstressed lattice dimension of the hexagonal phase formed by DOPE/DOPS mixtures. In addition to excess water, all samples contain 16 wt% tetradecane, which has been shown to relieve chain-packing stresses allowing large dimension hexagonal phases to form (Rand et al., 1990).
FIGURE 2.
Equilibrium lattice dimension, dhex, as it varies with water content for the hexagonal phases formed by DOPE/DOPS/td mixtures containing the indicated mol% DOPS.
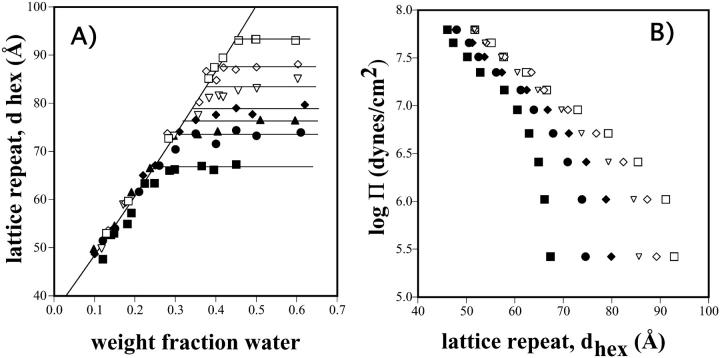
To determine molecular dimensions, gravimetric phase diagrams were constructed. Fig. 3 A shows the hexagonal dimension (dhex) as it varies with weight fraction of water for DOPE/td and six different DOPE/DOPS/td mixtures. At less than full hydration, all mixtures give the same dependence of dhex on water concentration. This dependence is used to determine water content of both the maximally swelled equilibrated mixtures in excess water (Table 1), and later, of the osmotically stressed samples from their measured lattice dimensions.
FIGURE 3.
(A) Lattice dimension, dhex, as it varies with water content, for the hexagonal phases formed by various DOPE/DOPS/td mixtures. Symbols are: □ 30%, ⋄ 25%, ▿ 20%, ♦ 15%, ▴ 10%, • 8%, and ▪ 0% DOPS. Horizontal lines at equilibrium dimensions were drawn using the relationship found in Fig. 2. (B) Lattice dimension, dhex, for the mixtures in A, as it varies with osmotic pressure Π, generated by PEG solutions of known osmotic pressure. Symbols as in A.
TABLE 1.
Equilibrium parameters for all mixtures of DOPE/DOPS/td
| Wt% DOPS | Equilibrium dhex (Å) | Equilibrium dl (Å) | Equilibrium wt. fraction water |
|---|---|---|---|
| 0 | 67.1 | 37.7 | 0.75 |
| 8 | 73.9 | 37.0 | 0.70 |
| 10 | 76.4 | 36.8 | 0.68 |
| 15 | 78.9 | 36.2 | 0.66 |
| 20 | 83.2 | 35.3 | 0.63 |
| 25 | 87.5 | 34.3 | 0.59 |
| 30 | 92.9 | 32.7 | 0.55 |
The effect of applied osmotic pressure on the lattice dimensions of the various DOPE/DOPS/td mixtures is shown is Fig. 3 B. Increasing osmotic pressure results in decreasing lattice dimension for all mixtures, as the lipid imbibes water only to the point where it is equilibrated with water whose chemical potential is set by the reservoir of PEG polymer solution. At any one osmotic pressure, increasing amounts of DOPS in the lipid mixture result in larger lattice dimensions.
Data analysis
Pivotal plane
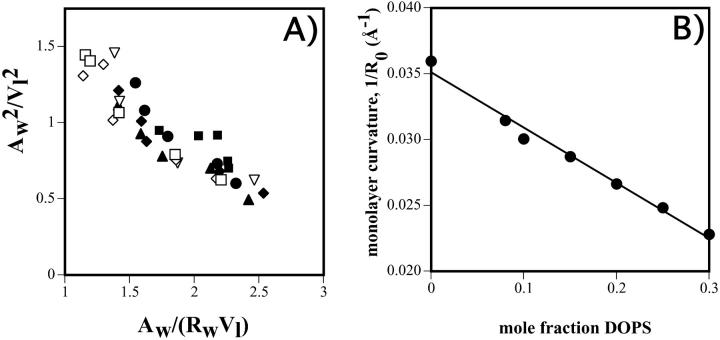
Molecular dimensions, including Aw and Rw, were calculated using the equations described in Fig. 1 for all samples in less than excess water where the volume fraction of water was known. To standardize these calculations for different DOPE/DOPS/td mixtures, an effective molecule is described, which is one DOPE plus an appropriate fraction of DOPS and a fraction of tetradecane. Diagnostic plots were constructed (Fig. 4 A) using normalized areas and volumes. Eq 6 can be rewritten:
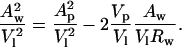 |
(9) |
FIGURE 4.
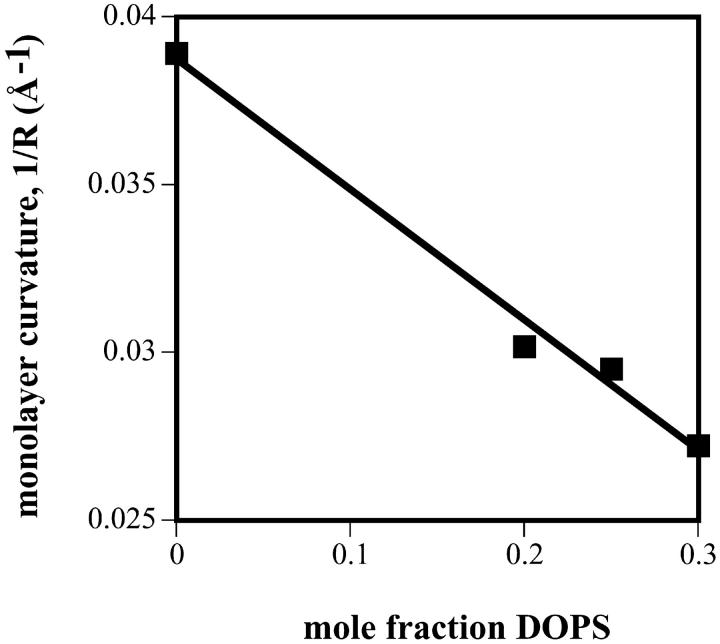
(A) “Diagnostic plots,” according to Eq. 9 of the text, for DOPE/DOPS/td mixtures. Symbols are: □ 30%, ⋄ 25%, ▿ 20%, ♦ 15%, ▴ 10%, • 8%, and ▪ 0% DOPS. Linearity indicates a pivotal plane, the slope (Vp/V1) gives its position, and the intercept (Ap/V1) gives the area of an effective molecule at this plane. (B) Plot of the monolayer curvature, (1/R0p) calculated at the pivotal plane determined in A, as it varies with DOPS concentration in the DOPE/DOPS/td mixtures. The equation of the best fit line gives R0pDOPS = +144 Å, and R0pDOPE = −30 Å.
The plot of (Aw/V1)2 vs. (Aw/V1)Rw gives a straight line with no dependence on DOPS concentration in the mixture, indicating that a well-defined pivotal plane exists, the position of which (Vp/Vl) is independent of the DOPE/DOPS ratio. The common linear fit to this line gives, from the slope, Vp/Vl = 0.3, a relative position of the pivotal plane consistent with many previous measurements for DOPE (Chen and Rand, 1997; Fuller and Rand, 2001; Leikin et al., 1996; Szule et al., 2002).
Spontaneous curvature and bending modulus
Knowing the position of the pivotal plane, the spontaneous radius of curvature (R0p) is calculated for each DOPE/DOPS/td mixture at the pivotal plane using Eq. 5 and the equilibrium conditions determined in Table 1. If the spontaneous curvature (1/R0p) of the mixtures is a linear function of DOPS molar fraction, then the apparent spontaneous radii of curvature for DOPS and DOPE can be determined from the best fit line to the data. This data is shown plotted in Fig. 4 B and yields: R0pDOPS = +144 Å and R0pDOPE = −30 Å.
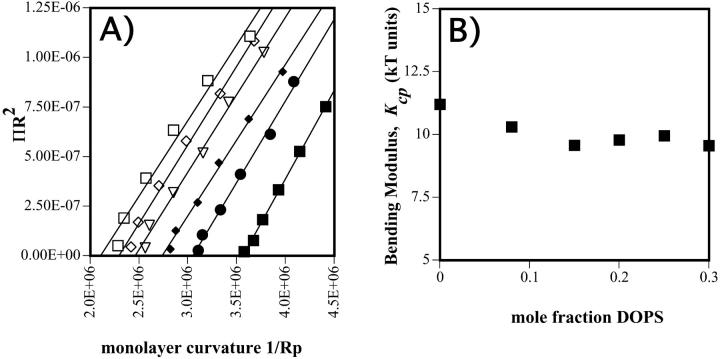
Osmotic pressure data (Fig. 3 B) can now also be analyzed in terms of the radius of curvature at the pivotal plane. Fig. 5 A shows this data replotted as described in the Materials and Methods section. The slope of each line in the ΠRp2 vs. 1/Rp plot gives the bending modulus (Kcp) for each mixture. Fig. 5 B shows the bending modulus as a function of DOPS content. It is evident that DOPS has no significant effect on the monolayer bending modulus previously determined for DOPE (Leikin et al., 1996).
FIGURE 5.
(A) Plots for various DOPE/DOPS/td mixtures, relating the osmotic work required to dehydrate the hexagonal phase with its change in monolayer curvature 1/Rp. The slope gives a measure of the bending modulus. Symbols are: □ 30%, ⋄ 25%, ▿ 20%, ♦ 15%, • 8%, and ▪ 0% DOPS. (B) Relation between the bending modulus determined in A and the mol fraction DOPS in the DOPE/DOPS/td mixtures.
The effect of pH on DOPE/DOPS mixtures
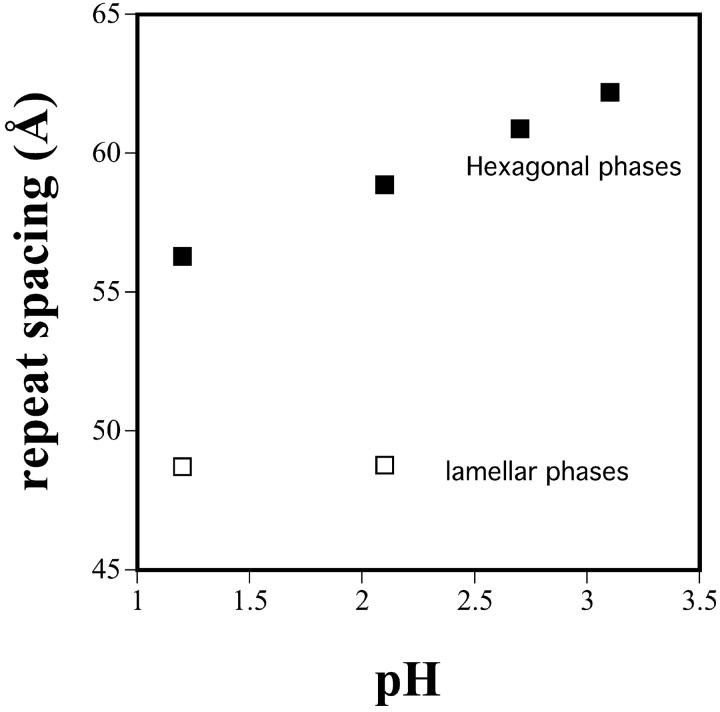
Our previous x-ray diffraction studies (Bezrukov et al., 1999) and NMR (de Kroon et al., 1990; Hope and Cullis, 1980) have shown that at low pH, fully hydrated pure DOPS with no tetradecane, forms an inverted hexagonal phase. These experiments were repeated as described in the methods, and the results confirmed in the present study (Fig. 6). From pH 1 to pH 4, an HII lattice, which is significantly smaller than that of DOPE, increases in spacing from ∼56 Å to 65 Å, and coexists with a small dimension (48 Å) lamellar phase at the lower pH values.
FIGURE 6.
Equilibrium phases and lattice dimensions as a function of pH for pure DOPS in large excess buffer reservoirs. For buffers at different pH values, 25 ml water was added to 25 ml of various mixed buffer reagents. Before dilution, these were 0.2 M HCl, 0.2 M KCl, 0.1 M glycine, 0.1 M citric acid, and 0.1 M Na citrate. For example, pH 2 buffer was 0.013 M HCl and 0.087 M KCl, and pH 3 buffer was 0.011M HCl and 0.05M glycine.
The picture is dramatically different with 16 wt% added tetradecane. Following the same protocol, DOPS/td and several of the DOPE/DOPS/td mixtures used in the above study of curvature and bending, were also examined at the low pH values previously shown to produce hexagonal phases for pure DOPS. Perhaps the most dramatic observation is that pure DOPS/td does not form a hexagonal phase at all but rather a large dimension lamellar phase whose equilibrium dimension increases from ∼95 Å at pH 2.1, to 140 Å at pH 3.2. In DOPS, tetradecane apparently reverses the effects of lowering pH.
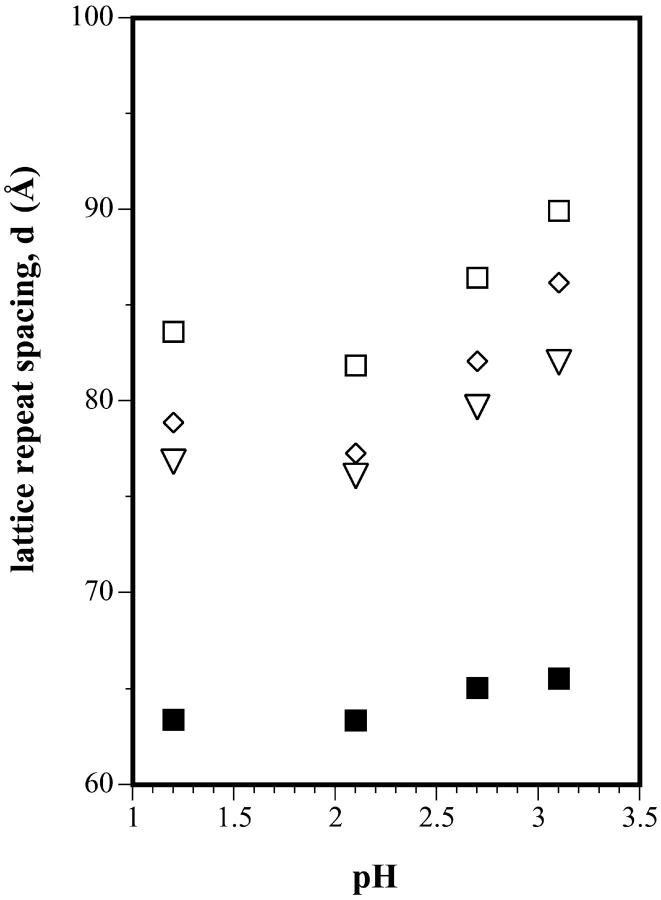
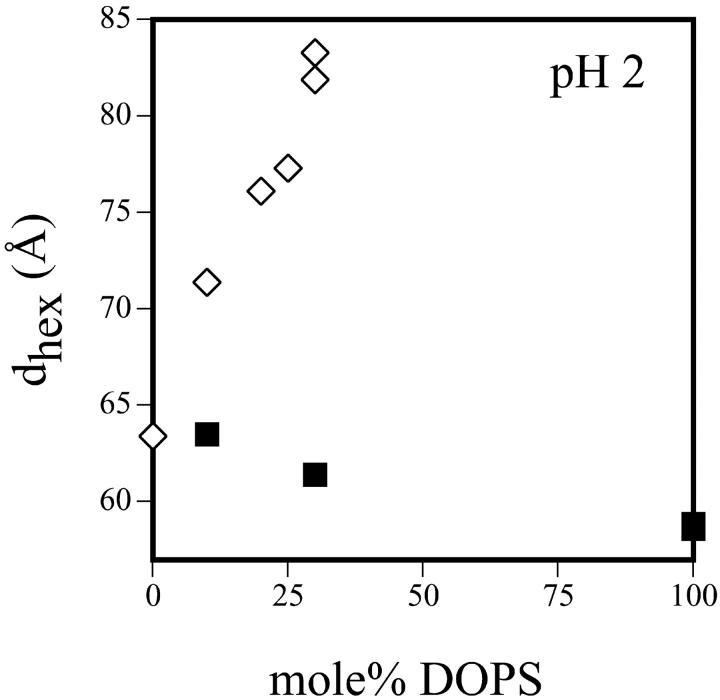
Fig. 7 shows the equilibrium dimensions of the tetradecane-containing hexagonal phases formed at low pH by DOPE/td, and by DOPS/DOPE/td mixtures containing 20, 25, and 30 mol% DOPS. The DOPE/DOPS/td mixtures all form HII phases that increase in dimension with pH. Unpredictably (because DOPS at low pH forms a smaller HII phase than DOPE), they are all larger than the HII phase formed by DOPE. As a first approximation, to get an estimate of R0p for DOPS under these low pH conditions, we use the equilibrium lattice dimension (large excess buffer reservoir) of the samples at pH 2, and calculate the volume fractions of lipid and water assuming lipid molecular dimension, dl, equal to that for the corresponding lattice dimension measured at neutral pH. Many previous measurements show that any changes in dl are very small compared to the changes we observe here in the repeat lattice dimension of the fully hydrated hexagonal phase. This can be seen in Table 1. Thus we estimate the spontaneous radius of curvature at the pivotal plane for DOPS in the same manner as described above. The results of these calculations are shown in Fig. 8. At pH 2, the observed dependence of curvature on DOPS concentration gives its apparent spontaneous radius of curvature as essentially infinite, i.e., flat. For comparison, using the same assumption for dl, the pure DOPS samples fully hydrated without tetradecane, equilibrate at an estimated radius of curvature of −23 Å at pH 2 (protonated), and at −50 Å in 1M NaCl (screened) (unpublished data). Again tetradecane apparently offsets the effects of protonation at low pH.
FIGURE 7.
Equilibrium lattice dimensions of hexagonal phases formed by DOPE/DOPS/td mixtures in large excess buffer reservoirs, as a function of pH. Symbols are: □ 30%, ⋄ 25%, ▿ 20%, and ▪ 0% DOPS. Buffer solutions were prepared as described in Fig. 6.
FIGURE 8.
Plot of the estimated monolayer curvature (1/R0p) calculated at the pivotal plane as it varies with DOPS concentration in the DOPE/DOPS/td mixtures at pH 2. The equation of the best fit line gives R0pDOPS = +“16666” Å, and R0pDOPE = −26 Å.
Further evidence that DOPS contributes a high negative curvature when protonated at low pH, comes from one additional experiment. DOPS/DOPE mixtures without tetradecane were equilibrated in buffer reservoirs at pH 2. Fig. 9 shows that in contrast to the samples that do contain td, whose lattice dimension increases with increasing DOPS, those without td, decrease in lattice dimension with increasing DOPS.
FIGURE 9.
Equilibrium lattice dimensions of hexagonal phases formed at pH 2 by various DOPE/DOPS mixtures; ⋄ with tetradecane, and ▪ without tetradecane.
DISCUSSION
The physical characteristics of phosphatidylserine are of interest because of the observed involvement of this lipid in many membrane-associated biological functions. In addition to its specific ionic interactions with divalent cations and acidic proteins, material properties of PS have been implicated both in membrane structural transitions leading to fusion (Cullis et al., 1985), and in affecting the activity of membrane-bound and associated proteins (Bezrukov et al., 1999). In this work, we have attempted to assign quantitative values to some lipid elastic constants that describe the phosphatidylserine molecule DOPS.
We have extended previous studies using DOPE/DOPS mixtures, designed to measure the effect of surface charge on monolayer curvature (Lerche et al., 1992). In this system, both lipids have the same hydrocarbon chains, and when salt screened, PS headgroups have been shown to have similar hydration properties to PE (Rand and Parsegian, 1989). The observed increase in the radius of curvature at the water interface, with increasing PS concentrations, was attributed to charge alone, and was in agreement with theoretical predictions (Kozlov et al., 1992).
The data presented here agree with and extend the previous data for DOPE/DOPS mixtures. Further analysis, using the procedure originally described by Leikin et al. (1996), allow the calculation of the position of a pivotal plane within the monolayer where the molecular area does not change on bending. The elastic constants, surface area (A0p), spontaneous curvature (1/R0p), and bending modulus (Kcp) were measured for the DOPS molecule at this plane. All samples used in this analysis (from 0 to 30 mol% DOPS) were pure hexagonal phases containing 16 wt% tetradecane. Tetradecane reduces chain-packing stresses or interstitial energy, and allows the monolayers to relax to their spontaneous radius of curvature (Kozlov et al., 1994a; Rand et al., 1990; Rand and Parsegian, 1997). This is important in determining spontaneous curvature because without added tetradecane, in mixtures of 15 mol% DOPS and above, the lamellar phase is the lowest energy phase (Lerche et al., 1992).
The position of the monolayer pivotal plane was determined to be independent of DOPS concentration and not detectably different from that of pure DOPE. The diagnostic plots (Fig. 4 A) emphasize experimental scatter in the data, but no systematic differences corresponding to DOPS concentration could be found. A value of Vp/Vl = 0.3 for the pooled data agrees with all previous measurements (Chen and Rand, 1997; Fuller and Rand, 2001; Leikin et al., 1996; Szule et al., 2002) and corresponds to a position close to the hydrocarbon polar group interface (∼2 CH2 groups on the hydrocarbon side).
The apparent spontaneous radius of curvature for DOPS was determined to be +144 Å. This represents the first positive curvature reported for a diacyl phospholipid, and likely is largely due to electrostatic interactions of the charged headgroup. By contrast, the apparent spontaneous radius of curvature of the highly hydrated but neutral lipid, DOPC, has been measured to be from −143 Å to −200 Å (Szule et al., 2002). Positively curved, charged DOPS confined in a flat membrane would be expected to exert packing stresses opposite to those of negatively curved lipids and so have opposite effects on membrane deformations or integral protein conformational changes. Such effects have been reported for lysoPC, which has been measured to have a positive radius of curvature from +38 Å to +58 Å. For example, lysoPC is reported to inhibit or promote different stages of membrane fusion depending on its location in either the proximal or distal monolayer (Chernomordik et al., 1995a,b; Chernomordik et al., 1993), and its effect on gramicidin channels is seen to increase channel formation and duration (Lundbaek and Anderson, 1994).
Interestingly, as previously reported, pure DOPS (without added tetradecane) undergoes a dramatic change in structure from lamellar to hexagonal under certain conditions including low pH. These results were confirmed here, and suggest a switch in curvature from positive to negative. Using an estimate for lipid thickness based on the common dehydrated data measured for all mixtures, the radius of curvature at the pivotal plane for this hexagonal phase would be approximately −23 Å (even smaller than DOPE). This apparent reversal in curvature from positive to highly negative is likely due to protonation of the PS headgroup at low pH and is consistent with the estimated pK value of 4.2 ± 0.2 found by Kroon et al. (1990) for the carboxyl group. Protonation would reduce electrostatic repulsion, increase hydrogen bonding, and decrease hydration of the headgroup, possibly resulting in a different orientation. All of this would change the balance of the energies stemming from headgroup interactions, chain-packing interactions, and surface tension associated with the water interface. Under these conditions, minimization of the energy results in the formation of this small dimension hexagonal phase (sometimes in equilibrium with a dehydrated lamellar phase). Because the dimension of this hexagonal phase is even smaller than that of DOPE, chain-packing stresses are believed to be at a minimum.
Following our normal routine or protocol, to get a measure of the spontaneous (relaxed) radius of curvature of HII phase lipids, low pH samples of DOPS and DOPE/DOPS mixtures were examined, not only in excess aqueous buffer, but also containing excess hydrocarbon solvent (tetradecane). Surprisingly, DOPS and DOPE/DOPS mixtures behave very differently at low pH when tetradecane is added. At pH 2, fully hydrated DOPE/DOPS/td mixtures form pure hexagonal phases, but the dimension of these phases is dependent on DOPS content in a manner that gives an estimate for DOPS curvature that is essentially flat. Indeed, consistent with this, pure DOPS at pH 2 with added tetradecane forms a lamellar phase of large dimension (93 Å) but limited swelling. This highly hydrated lamellar phase (the PS bilayer is only 39 Å thick) implies effective electrostatic charge repulsion, and is in sharp contrast with the extremely small dimension hexagonal phase formed without tetradecane. Clearly tetradecane appears to reduce the protonation of DOPS at low pH. Although more studies are necessary to determine the role of tetradecane in these systems, one interesting possibility is that tetradecane interaction somehow allows the hexagonal lattice to expand and take up more water, and/or affect the degree of polar group ionization. Cevc et al. (1985) has suggested a similar effect of hydrocarbon expansion by high temperature.
The application of osmotic stress technique (Leikin et al., 1996; Parsegian et al., 1986) using the DOPE/DOPS/td mixtures allowed us to measure the effect of increasing concentrations of DOPS on the bending modulus of the DOPE monolayer in the hexagonal phase. No significant effect of DOPS on bending modulus could be measured from our data, suggesting that Kcp for DOPS in the hexagonal phase is not significantly different from DOPE at ∼11 kT. Thus, our data could not detect a theoretical prediction (Kozlov et al., 1992, Winterhalter and Helfrich, 1988) that surface charge would influence rigidity in the monolayer.
Acknowledgments
We acknowledge the financial support of the Natural Sciences and Engineering Research Council of Canada (to N.F. and R.P.R.), and of FAPESP (The State of São Paulo Research Foundation; grant 00/03545-8 to C.R.B.).
References
- Bezrukov, S. M., R. P. Rand, I. Vodyanoy, and V. A. Parsegian. 1999. Lipid packing stress and polypeptide aggregation: alamethicin channel probed by proton titration of lipid charge. Faraday Discuss. 111:173–183. [DOI] [PubMed] [Google Scholar]
- Boggs, J. M. 1987. Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. Biochim. Biophys. Acta. 906:353–404. [DOI] [PubMed] [Google Scholar]
- Browning, J. L., and J. Seelig. 1980. Bilayers of phosphatidylserine: a deuterium and phosphorus nuclear magnetic resonance study. Biochemistry. 19:1262–1270. [DOI] [PubMed] [Google Scholar]
- Buckland, A. G., and D. C. Wilton. 2000. Anionic phospholipids, interfacial binding and the regulation of cell functions. Biochim. Biophys. Acta. 1483:199–216. [DOI] [PubMed] [Google Scholar]
- Cevc, G., J. M. Seddon, and D. Marsh. 1985. Thermodynamic and structural properties of phosphatidylserine bilayer membranes in the presence of lithium ions and protons. Biochim. Biophys. Acta. 814:141–150. [Google Scholar]
- Chen, Z., and R. P. Rand. 1997. The influence of cholesterol on phospholipid membrane curvature and bending elasticity. Biophys. J. 73:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik, L., A. Chanturiya, J. Green, and J. Zimmerberg. 1995a. The hemifusion intermediate and its conversion to complete fusion: regulation by membrane composition. Biophys. J. 69:922–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik, L., M. M. Kozlov, and J. Zimmerberg. 1995b. Lipids in biological membrane fusion. J. Membr. Biol. 146:1–14. [DOI] [PubMed] [Google Scholar]
- Chernomordik, L. V., S. S. Vogel, A. Sokoloff, H. O. Onaran, and E. Leikina. 1993. Lysolipids reversibly inhibit Ca2+-,GTP- and pH-dependent fusion of biological membranes. FEBS Lett. 318:71–76. [DOI] [PubMed] [Google Scholar]
- Cullis, P. R., M. J. Hope, B. de Kruijff, A. J. Verkleij, and C. P. S. Tilcock. 1985. Structural properties and functional roles of phospholipids in biological membranes. In Phospholipids and Cellular Regulation. J. F. Kuo, editor. CRC Press, Boca Raton, FL. 1–59.
- Cullis, P. R., and A. J. Verkleij. 1979. Modulation of membrane structure by Ca2+ and dibucaine as detected by 31P-NMR. Biochim. Biophys. Acta. 552:546–551. [DOI] [PubMed] [Google Scholar]
- de Kroon, A. I. P. M., J. W. Timmermans, J. A. Killian, and B. de Kruijff. 1990. The pH dependence of headgroup and acyl chain structure and dynamics of phosphatidylserine, studied by 2H-NMR. Chem. Phys. Lipids. 54:33–42. [DOI] [PubMed] [Google Scholar]
- de Kruiff, B. 1987. Polymorphic regulation of membrane lipid composition. Nature. 329:587–588. [DOI] [PubMed] [Google Scholar]
- Epand, R. M. 1997. Lipid polymorphism and membrane properties. In Current Topics in Membranes. D. M. Fambrough and D. J. Benos, editors. Academic Press, San Diego. 568.
- Fuller, N., and R. P. Rand. 2001. The influence of lysolipids on the spontaneous curvature and bending elasticity of phospholipid membranes. Biophys. J. 81:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner, S. M., V. A. Parsegian, and R. P. Rand. 1986. Directly measured deformation energy of phospholipid HII hexagonal phases. Faraday Discuss. Chem. Soc. 81:29–37. [DOI] [PubMed] [Google Scholar]
- Hauser, H., F. Paltauf, and G. G. Shipley. 1982. Structure and thermotropic behavior of phosphatidylserine bilayer membranes. Biochemistry. 21:1061–1067. [DOI] [PubMed] [Google Scholar]
- Helfrich, W. 1973. Elastic properties of lipid bilayers: theory and possible experiments. Zeitschrift Naturforschen. 28C:693–703. [DOI] [PubMed] [Google Scholar]
- Hope, M. J., and P. R. Cullis. 1980. Effects of divalent cations and ph on phosphatidylserine model membranes: a 31p nmr study. Biochem. Biophys. Res. Commun. 92:846–852. [DOI] [PubMed] [Google Scholar]
- Kirk, G. L., S. M. Gruner, and D. L. Stein. 1984. A thermodynamic model of the lamellar to inverse hexagonal phase transition of lipid membrane-water system. Biochemistry. 23:1093–1102. [Google Scholar]
- Koltover, I., T. Salditt, J. O. Radler, and C. R. Safinya. 1998. An inverted hexagonal phase of cationic liposome-dna complexes related to dna release and delivery. Science. 281:78–81. [DOI] [PubMed] [Google Scholar]
- Kozlov, M. M., S. Leikin, and R. P. Rand. 1994a. Bending, hydration and void energies quantitatively account for the hexagonal-lamellar-hexagonal reentrant phase transition in dioleoylphosphatidylethanolamine. Biophys. J. 67:1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov, M. M., S. Leikin, and R. P. Rand. 1994b. Energetics of the reentrant hexagonal-lamellar-hexagonal transition in phospholipids. Biophys. J. 66:A299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov, M. M., and M. Winterhalter. 1991a. Elastic moduli for strongly curved monolayers. Analysis of experimental results. Journal de Physique II. 1:1085–1100. [Google Scholar]
- Kozlov, M. M., and M. Winterhalter. 1991b. Elastic moduli for strongly curved monolayers. Position of the neutral surface. Journal de Physique II. 1:1077–1084. [Google Scholar]
- Kozlov, M. M., M. Winterhalter, and D. Lerche. 1992. Elastic properties of strongly curved monolayers. Effect of electric surface charges. Journal de Physique II. 2:175–185. [Google Scholar]
- Leikin, S., M. M. Kozlov, N. L. Fuller, and R. P. Rand. 1996. Measured effects of diacylglycerol on structural and elastic properties of phospholipid membrane. Biophys. J. 71:2623–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz, B. R. 1999. Lipids and liposomes can do more than carry drugs: phosphatidylserine as a regulator of blood coagulation. J. Liposome Res. 9:IX–XV. [Google Scholar]
- Lerche, D., N. L. Fuller, and R. P. Rand. 1992. Membrane curvature and structural transitions for charged/uncharged phospholipid mixtures. In The Structure and Conformation of Amphiphilic Membranes. R. Lipowsky, D. Richter, and K. Kremer, editors. Springer-Verlag, Berlin. 226–229.
- Lundbaek, J. A., and O. S. Anderson. 1994. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J. Gen. Physiol. 104:645–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzati, V., and F. Husson. 1962. The structure of the liquid-crystalline phases of lipid-water systems. J. Cell Biol. 12:207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, S., and A. Ben-Shaul. 1999. Molecular theory of lipid - protein interaction and the lα - hii transition. Biophys. J. 76:751–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, S., D. Harries, and A. Ben-Shaul. 2000. The phase behavior of cationic lipid-dna complexes. Biophys. J. 78:1681–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka, Y. 1984. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 308:693–698. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos, D., G. Poste, B. E. Schaeffer, and W. J. Vail. 1974. Membrane fusion and molecular segregation in phospholipid vesicles. Biochim. Biophys. Acta. 352:10–28. [DOI] [PubMed] [Google Scholar]
- Parsegian, V. A., R. P. Rand, N. L. Fuller, and D. C. Rau. 1986. Osmotic stress for the direct measurement of intermolecular forces. Methods Enzymol. 127:400–416. [DOI] [PubMed] [Google Scholar]
- Portis, A., C. Newton, W. Pangborn, and D. Papahadjopoulos. 1979. Studies on the mechanism of membrane fusion: evidence for an intermembrane Ca2+- phospholipid complex, synergism with Mg2+, and inhibition by spectrin. Biochemistry. 18:780–790. [DOI] [PubMed] [Google Scholar]
- Rand, R. P., and N. L. Fuller. 1994. Structural dimensions and their changes in a reentrant hexagonal-lamellar transition of phospholipids. Biophys. J. 66:2127–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand, R. P., N. L. Fuller, S. M. Gruner, and V. A. Parsegian. 1990. Membrane curvature, lipid segregation, and structural transitions for phospholipids under dual-solvent stress. Biochemistry. 29:76–87. [DOI] [PubMed] [Google Scholar]
- Rand, R. P., and V. A. Parsegian. 1989. Hydration forces between phospholipid bilayers. Biochim. Biophys. Acta. 988:351–376. [Google Scholar]
- Rand, R. P., and V. A. Parsegian. 1997. Hydration, curvature, and bending elasticity of phospholipid monolayers. Current Topics in Membranes. 44:167–189. [Google Scholar]
- Roux, M., J. Neuman, R. S. Hodges, P. F. Devaux, and M. Bloom. 1989. Conformational changes of phospholipid headgroups induced by a cationic integral membrane peptide as seen by deuterium magnetic resonance. Biochemistry. 28:2313–2321. [DOI] [PubMed] [Google Scholar]
- Seddon, J. M. 1990. Structure of the inverted hexagonal (HII) phase and non-lamellar phase transitions of lipids. Biochim. Biophys. Acta. 1031:1–69. [DOI] [PubMed] [Google Scholar]
- Szule, J., N. Fuller, and R. P. Rand. 2002. The effects of acyl chain length and saturation of diacylglycerols and phosphatidylcholines on membrane monolayer curvature. Biophys. J. 83:977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterhalter, M., and W. Helfrich. 1988. Effect of surface charge on the curvature elasticity of membranes. J. Phys. Chem. 92:6865–6867. [Google Scholar]