Abstract
Gramicidin is an antibiotic peptide that can be incorporated into the monolayers of cell membranes. Dimerization through hydrogen bonding between gramicidin monomers in opposing leaflets of the membrane results in the formation of an iontophoretic channel. Surrounding phospholipids influence the gating properties of this channel. Conversely, gramicidin incorporation has been shown to affect the structure of spontaneously formed lipid assemblies. Using small-angle x-ray diffraction and model systems composed of phospholipids and gramicidin, the effects produced by gramicidin on lipid layers were measured. These measurements explore how peptides are able to modulate the spontaneous curvature properties of phospholipid assemblies. The reverse hexagonal, HII, phase formed by dioleoylphosphatidylethanolamine (DOPE) monolayers decreased in lattice dimension with increasing incorporation of gramicidin. This indicated that gramicidin itself was adding negative curvature to the lipid layers. In this system, gramicidin was measured to have an apparent intrinsic radius of curvature, 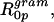 of −7.1 Å. The addition of up to 4 mol% gramicidin in DOPE did not result in the monolayers becoming stiffer, as measured by the monolayer bending moduli. Dioleoylphosphatidylcholine (DOPC) alone forms the lamellar (Lα) phase when hydrated, but undergoes a transition into the reverse hexagonal (HII) phase when mixed with gramicidin. The lattice dimension decreases systematically with increased gramicidin content. Again, this indicated that gramicidin was adding negative curvature to the lipid monolayers but the mixture behaved structurally much less consistently than DOPE/gramicidin. Only at 12 mol% gramicidin in dioleoylphosphatidylcholine could an apparent radius of intrinsic curvature of gramicidin
of −7.1 Å. The addition of up to 4 mol% gramicidin in DOPE did not result in the monolayers becoming stiffer, as measured by the monolayer bending moduli. Dioleoylphosphatidylcholine (DOPC) alone forms the lamellar (Lα) phase when hydrated, but undergoes a transition into the reverse hexagonal (HII) phase when mixed with gramicidin. The lattice dimension decreases systematically with increased gramicidin content. Again, this indicated that gramicidin was adding negative curvature to the lipid monolayers but the mixture behaved structurally much less consistently than DOPE/gramicidin. Only at 12 mol% gramicidin in dioleoylphosphatidylcholine could an apparent radius of intrinsic curvature of gramicidin 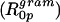 be estimated as −7.4 Å. This mixture formed monolayers that were very resistant to bending, with a measured bending modulus of 115 kT.
be estimated as −7.4 Å. This mixture formed monolayers that were very resistant to bending, with a measured bending modulus of 115 kT.
INTRODUCTION
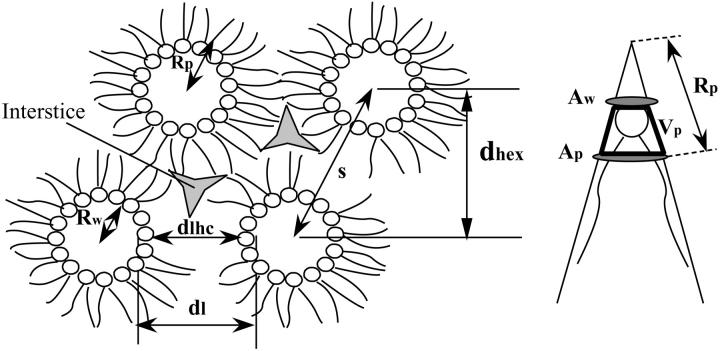
The vast repertoire of membrane function results from the complex composition and assembly of proteins and lipid species. The simple bilayer architecture contains many molecular species that spontaneously assemble into curved, nonbilayer structures when isolated and hydrated (Luzzati and Husson, 1962). These assemblies can be conceptualized as resulting from packing the projected shapes of the lipid molecules, where cylindrical lipids form the flat lamellar phase (Lα) and cone-shaped lipids form hexagonal phases with highly curved monolayers, reviewed by (Cullis and de Kruijff, 1979). The reverse hexagonal phase (HII) consists of indefinitely long tubes filled with water (Fig. 1).
FIGURE 1.
Schematic representation of the reverse hexagonal phase with the structural parameters shown. These variables are described and defined in the text.
Non-bilayer-prone lipids are thought to play a key role in reducing the energetic barriers of forming highly curved lipidic intermediates in the process of membrane fusion, reviewed by (Chernomordik et al., 1995). In addition, membrane protein conformations and activity are sensitive to the composition of their surrounding lipids, and many are affected by non-bilayer-prone lipids. Bilayers containing components that do not themselves pack into a flat surface, are likely to be stressed in a way that affect these two fundamental processes through lipid-lipid and lipid-protein interactions.
Quantifying the stresses introduced by individual lipid species can be achieved by studying purified lipids in model systems. When allowed to form unstressed assemblies, lipids form curved structures as a manifestation of their intrinsic curvature (Gruner, 1985). The intrinsic curvature and bending moduli of several lipids and mixtures of lipids have been measured (Chen and Rand, 1997; Epand et al., 1996; Leikin et al., 1996; Rand et al., 1990). Indeed lipid curvature has been shown to affect the gating properties of the peptide alamethicin (Keller et al., 1993; Bezrukov, 2000).
On the other hand the contribution of proteins to the curvature of membranes, important functionally (Bezrukov, 2000), has not yet been measured. Certainly it has been shown that peptides contribute to the phase structure of membrane lipids (Lindblom et al., 1988) and it has been shown that alamethicin induces the cubic phase (Keller et al., 1996). Here we attempt to quantify the curvature contribution of a peptide.
Gramicidin is a membrane peptide with antibiotic and channel-forming properties first isolated by Dubos (1939). We have used it in this first attempt to measure a protein's contribution to membrane curvature properties. It is small, can be easily modified, and its function is well documented. It has provided a model for i), the effects of peptide sequence on three-dimensional structure (Andersen et al., 1996), ii), the structure-function relationship of an iontophoretic channel (Koeppe and Andersen, 1996), (Busath, 1993), and iii), for lipid-protein interactions. Gramicidin has been used for studying the effects of the lipids on channel function (Andersen et al., 1998), and the effects of peptide inclusion on the packing properties of lipids (Killian, 1992). It has been shown to induce a transition from flat to nonflat lipid structures in model systems (Killian et al., 1986; Tournois et al., 1987; Van Echteld et al., 1982; Van Echteld et al., 1981). In this study we attempt to measure its intrinsic curvature and its effect on the membrane bending modulus, to aid estimates of its effect on membrane deformation energies (Helfrich and Jacobsson, 1990; Nielsen et al., 1998).
MATERIALS AND METHODS
Sample preparation
All phosphatidylcholines (PCs) and phosphatidylethanolamines (PEs) were purchased from Avanti Polar Lipids (Birmingham, AL) and stored under nitrogen at −18°C. Gramicidin D was purchased from Sigma Chemical Co. (St. Louis, MO) and stored under nitrogen at 2°C. Tetradecane (td) was purchased from Sigma Chemical Co. and stored at room temperature. Hereafter we refer to gramicidin D as gramicidin. Fluka Chemika polyethylene glycol 20,000 (PEG) crystals were purchased from Caledon Laboratories Ltd. (Georgetown, ON) and stored at room temperature. PEG solutions were prepared by mixing the desired weight of PEG crystals with double distilled water.
Appropriate dry weights of phospholipid and gramicidin D were dissolved in 3:1 chloroform:methanol and mixed together in solution. The solvent was removed by rotary evaporation followed by desiccation under vacuum. For selected samples, tetradecane was added to make up 16% of the dry weight of the total lipid mixture and allowed to equilibrate for 72 h at room temperature. Appropriate proportions of double-distilled water were added to the lipid mixtures by weight fraction and allowed to equilibrate for ∼5 days. Alternatively, hydration was achieved by immersing the lipid mixtures in ∼3 mL PEG solution and equilibrating for ∼5 days. After equilibration, the samples were examined by x-ray diffraction. Teflon shavings, providing an x-ray calibration line at 4.87 Å, and the hydrated lipid mixtures were placed into x-ray sample holders and sealed between mica windows 1 mm apart.
X-ray diffraction
A Rigaku rotating anode was used to generate x-rays where the CuKα1 line (wavelength of 1.540 Å) was isolated using a bent quartz crystal monochromator. The diffraction patterns were captured on film using Guinier x-ray cameras. Thermoelectric elements were used to maintain the desired temperature to ±0.5°C.
The x-ray diffraction pattern was used to characterize the phase formed by the lipid and its lattice dimension. Hexagonal phases gave spacings in the ratios of 1, 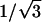 ,
,  ,
, 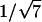 ,
, 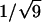 ,
, 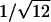 , etc. which were used to measure the lattice dimension dhex. The lamellar phase gave spacings in the ratios of 1, 1/2, 1/3, 1/4, etc. giving the lattice dimension dlam. The coexistence of independent sets of spacings indicated the coexistence of phases within the sample.
, etc. which were used to measure the lattice dimension dhex. The lamellar phase gave spacings in the ratios of 1, 1/2, 1/3, 1/4, etc. giving the lattice dimension dlam. The coexistence of independent sets of spacings indicated the coexistence of phases within the sample.
Experimental systems
The fully hydrated phases formed by increasing concentrations of gramicidin in dioleoylphosphatidylethanolamine (DOPE) and in dioleoylphosphatidylcholine (DOPC) were observed. This survey was performed on DOPE systems, without td, and DOPC systems, with td, to determine the composition range that would allow further structural analysis.
Phase diagrams relating lattice dimension to water content were constructed for mole fractions of 0.00, 0.01, 0.02, 0.03, and 0.04 gramicidin in DOPE. Increasing amounts of water were added gravimetrically to samples of each composition. Phase diagrams were also constructed for mole fractions of 0.07, 0.08, 0.10, 0.12, and 0.14 gramicidin in mixtures with DOPC in the presence of td.
Sensitivity of the lattice dimensions to osmotic stress was measured for mole fractions of 0.00–0.04 gramicidin in mixtures with DOPE, and mole fractions of 0.07–0.14 gramicidin in mixtures with DOPC, in the presence of td. Various concentrations of PEG 20,000 were used to exert known osmotic stresses on the samples (Parsegian et al., 1986).
STRUCTURAL ANALYSIS
HII phases are two-dimensional hexagonal lattices formed by the axes of indefinitely long and parallel regular prisms. Water cores, centered on the prism axes, are lined with the lipid polar groups, and the rest of the lattice is filled with the hydrocarbon chains.
For a hexagonal phase of known composition, the measured lattice can be divided into compartments, as shown in Fig. 1, each containing defined volume fractions of the lipid, peptide and water. This volume average division follows the method originally introduced by Luzzati (Luzzati and Husson, 1962) and depends only on the assumption of their linear addition. Specific data for the lipid components used in these systems can be found in Fuller and Rand (2001). Gramicidin's MW and density are taken as 1884 and 1.20 gm/cc.
In particular, we separate the water and nonwater compartments in the hexagonal lattice by an idealized cylindrical interface of radius Rw that encloses a volume equal to the volume of water in the HII phase (Fig. 1). This dividing surface is referred to as the Luzzati plane.
The radius of the water cylinder, Rw, is related to the first-order Bragg spacing in the hexagonal phase, dhex, and to the volume fraction of water in the sample, φw, as follows:
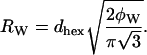 |
(1) |
The area per effective molecule at the Luzzati plane is given by
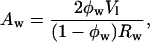 |
(2) |
where Vl is the volume of an effective lipid molecule and
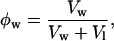 |
(3) |
where Vw is the volume of water added per effective lipid molecule in each sample.
For these calculations, we use the notion of an effective molecule that is one phospholipid + x gramicidin + y tetradecane molecules, where x is the molar ratio of gramicidin to phospholipid, and y is the molar ratio of tetradecane to phospholipid in the samples. The effective lipid molecular volume is
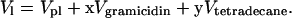 |
(4) |
Vpl, Vgramicidin, and Vtetradecane are the molecular volumes of DOPE, gramicidin, and tetradecane.
Elastic energy of the hexagonal phase
The monolayers of the HII phase can be described in terms of curvature, 1/Rp, measured at a pivotal plane where the molecular area remains constant (Rand et al., 1990; Leikin et al., 1996). The elastic free energy, F, of the hexagonal phase (normalized per effective molecule) can be approximated by the energy of bending (Helfrich, 1973, Kirk et al., 1984)
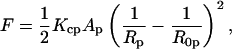 |
(5) |
where Kcp is the bending modulus, and Ap and R0p are the molecular area and the spontaneous radius of curvature at the pivotal plane.
The goal of this study is to find the position of any pivotal plane, and then measure the spontaneous curvature, molecular area, and bending moduli for different phospholipid/gramicidin mixtures. These structural parameters and elastic moduli (Leikin et al., 1996) are determined as follows:
- The molecular area, A, and radius of curvature, R, at any cylindrical dividing surface, separated by a volume V per lipid molecule from the Luzzati plane (Fig. 1) are related by
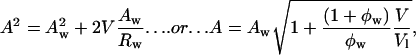
(6) 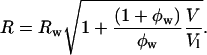
(7) - We verify whether the system has a well-defined pivotal plane by plotting
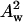 versus Aw/Rw, from Eq. 6, in the form
versus Aw/Rw, from Eq. 6, in the form
If the plot is a straight line, the system has a dividing surface of constant area, the pivotal plane. The slope and the intercept of the plot give both the position of the pivotal plane, Vp, which is the volume separating this plane and the Luzzati plane, and the molecular area of an effective molecule at that plane, Ap.
(8) From the position of the pivotal plane we calculate radii of curvature (Rp) using Eq. 7. For each mixture at equilibrium in excess water (determined from the phase diagrams) we determine the spontaneous radius of monolayer curvature (R0p).
- The relation between the spontaneous radius of curvature, and the molar fraction of gramicidin, mgram, can give an apparent spontaneous radius of curvature for each of the components if
is linear.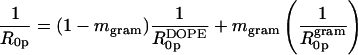
(9) - The elastic energy given by Eq. 5 can be related to the osmotic work done by the osmotic stress (Π):
A plot of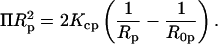
(10) 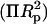 versus (1/Rp) gives, from the slope and intercept, the monolayer bending modulus (Kcp) (Gruner et al., 1986)(Rand et al., 1990) and the radius of spontaneous curvature.
versus (1/Rp) gives, from the slope and intercept, the monolayer bending modulus (Kcp) (Gruner et al., 1986)(Rand et al., 1990) and the radius of spontaneous curvature.
Temperature coefficients
Lattice dimensions were measured at 22°C, 30°C, and 40°C, where the sample was allowed to equilibrate at each temperature for at least 15 min before x-ray exposure. The temperature dependence, αs, of the lattice dimension, s,  (Fig. 1) is described by the following (Rand and Pangborn, 1973):
(Fig. 1) is described by the following (Rand and Pangborn, 1973):
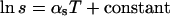 |
(11) |
where the slope of the ln s vs. T plot yields the temperature coefficient αs.
RESULTS
Structural characterizations for mixtures of gramicidin and DOPE
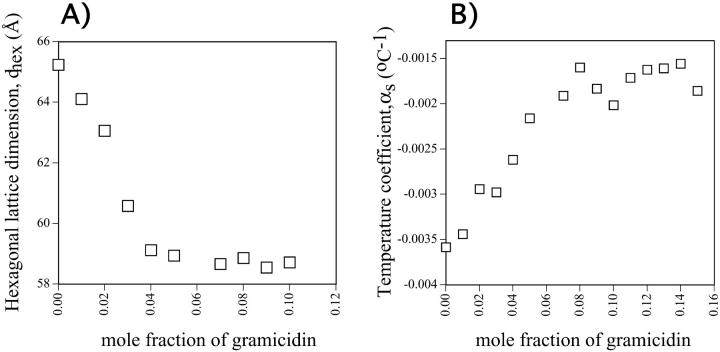
DOPE spontaneously forms the HII phase when hydrated and at a dimension very close to its intrinsic curvature (Chen and Rand, 1998). The lattice dimensions of HII phases formed by fully hydrated mixtures of gramicidin and DOPE, Fig. 2 A, show that dhex decreases systematically as gramicidin increases up to mole fraction ∼0.05, above which the dimensions change very little. The smaller changes may be due either to the exclusion of gramicidin from the HII phase or to different interactions between gramicidin and DOPE that do not affect the HII lattice dimensions.
FIGURE 2.
(A) The hexagonal lattice dimension dhex of the fully hydrated HII phases as they relate to the mole fraction of gramicidin in mixtures with DOPE. (B) The temperature dependence of the interaxial spacing s (see Fig. 1), of the HII phase as it relates to the mole fraction of gramicidin in mixtures with DOPE. The temperature coefficient, αs, for each composition was measured over a range of 20°C to 40°C.
From this experiment, it was concluded that the phases formed by mixtures of gramicidin and DOPE could be further analyzed for mole fractions up to 0.04 gramicidin.
Temperature coefficients of the HII lattice dimension
The temperature coefficient αs was measured in an attempt to determine whether gramicidin continued to be incorporated into the HII phase above a mole fraction of 0.05. As seen in Fig. 2 B, αs becomes less negative with increasing mole fraction gramicidin up to ∼0.07. Above this composition, αs does not change significantly (p < 0.05), suggesting that gramicidin is not incorporated beyond 0.07 mole fraction.
Phase diagrams for mixtures of gramicidin and DOPE
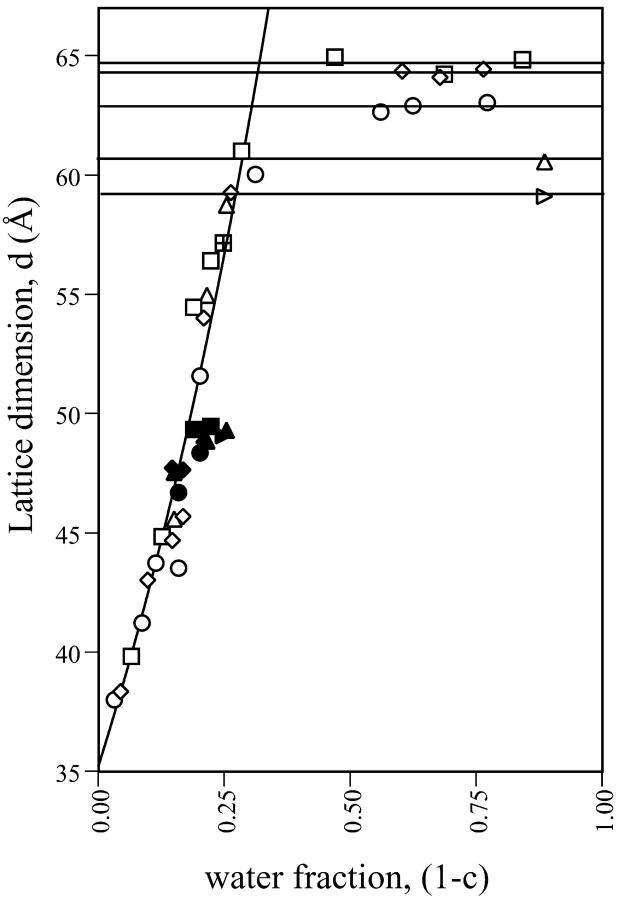
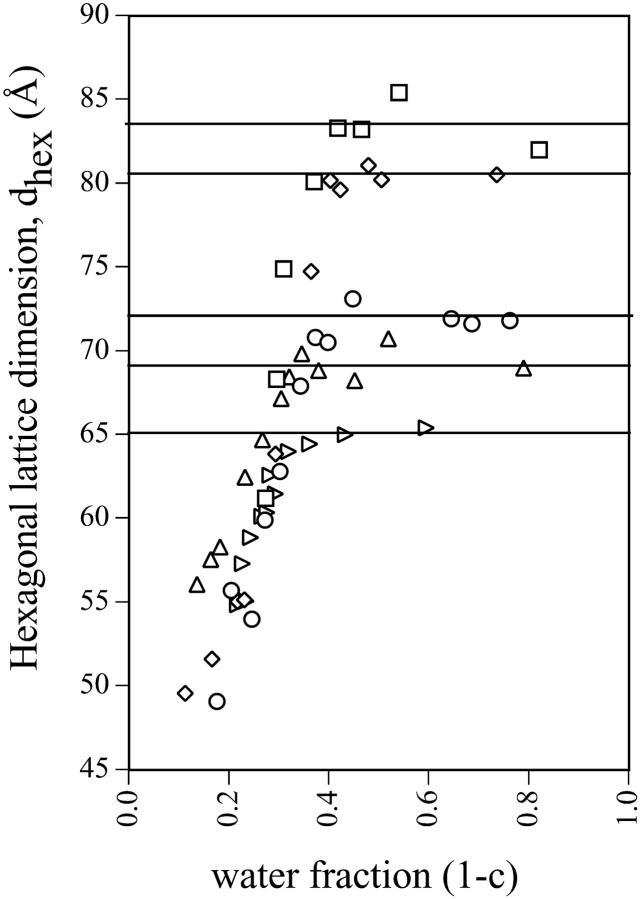
The HII and Lα lattice dimensions of phases formed by mixtures of gramicidin and DOPE were measured over a range of water content (Fig. 3). The lattice dimensions for all compositions measured increased as the weight fraction of water to lipid (1 − c) increased until a maximum spacing was reached at full hydration. The dehydrated data for all gramicidin contents were pooled and empirically fit with a common exponential curve. The maximum repeat spacings at full hydration were averaged and fitted with a horizontal line. As the content of gramicidin increased up to mole fraction 0.04, the maximum repeat spacings systematically decreased, as listed in Table 1 and consistent with Fig. 2 A. The minimal water contents required for each phase to achieve full hydration, as determined from the intersection point of the exponential curve and the horizontal line, is referred to as equilibrium hydration, and are also listed in Table 1. In the dehydrated regions of the phase diagrams, all compositions of gramicidin exhibited the appearance and disappearance of an Lα phase, previously described for pure DOPE as the reentrant HII-Lα-HII transition (Gawrisch et al., 1992; Kozlov et al., 1994). These two-phase samples were not used for further analysis because the composition of each phase was unknown. However, several dehydrated samples formed the HII phase only, and were used in the diagnostic analysis described below.
FIGURE 3.
The lattice dimensions, d, as they relate to the amount of water added to samples containing various mole fractions of gramicidin in mixtures with DOPE. Symbols are (□) 0.00, (⋄) 0.01, (○) 0.02, (▵) 0.03, and (▹) 0.04 mole fractions. Closed symbols are lamellar phases and open symbols are hexagonal phases. With increasing water fractions but still dehydrated, dhex increases until a maximum lattice dimension is reached at full hydration. The dehydrated data for all gramicidin contents were pooled and fitted with a common exponential curve. The maximum repeat spacings at full hydration were averaged and fitted with a horizontal line. Water fraction (1 − c) and dhex at the intersection points are listed in Table 1.
TABLE 1.
The equilibrium water fraction 1 − c required to achieve the maximum lattice dimensions, and dhex at full hydration, as determined from the phase diagrams (Fig. 3) for various contents of gramicidin in mixtures with DOPE
| Mole fraction of gramicidin | Equilibrium water fraction 1 − c | Maximum lattice dimensions dhex (Å) |
|---|---|---|
| 0.00 | 0.32 | 64.7 |
| 0.01 | 0.32 | 64.3 |
| 0.02 | 0.31 | 62.9 |
| 0.03 | 0.29 | 60.6 |
| 0.04 | 0.28 | 59.1 |
Structural parameters for mixtures of gramicidin and DOPE
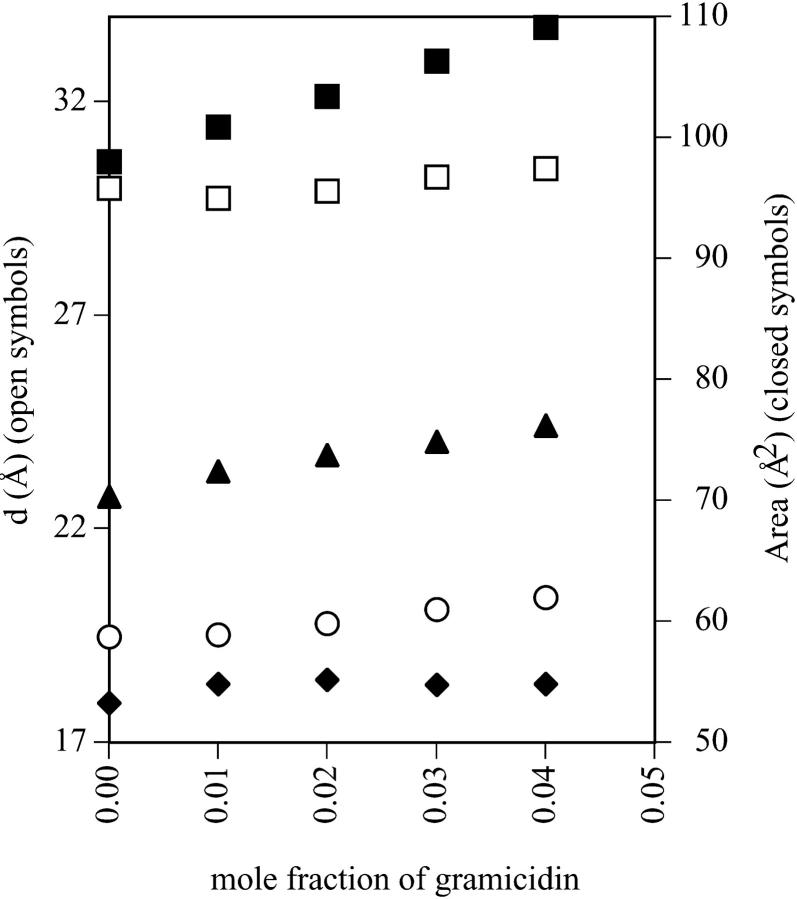
Structural dimensions within the fully hydrated HII phase, shown in Fig. 1, have been plotted as they relate to gramicidin content in Fig. 4. The thickness of an effective bilayer (dl) and the thickness of the hydrocarbon region of an effective bilayer (dlhc) in the interaxial direction of the HII phase both remain constant as the composition of gramicidin increases. The area of an effective molecule at the Luzzati plane (Aw) remains constant over the range of gramicidin composition. However, the areas of an effective molecule at the pivotal plane (Ap) and at the acyl chain terminals (At) both increase with increasing gramicidin content. These data will be used to discuss the structural effects of gramicidin on the HII phase.
FIGURE 4.
Through the geometrical relationships described in the Structural Analysis section, dimensions of the structural parameters shown in Fig. 1 are plotted as they relate to the mole fraction of gramicidin in mixtures with DOPE at equilibrium hydration. dl (□) is the thickness of an effective bilayer in the interaxial direction, and dlhc (○) is the thickness of the hydrocarbon region of an effective bilayer in the interaxial direction. The areas of an effective molecule are plotted at the Luzzati plane, Aw (♦), the pivotal plane, Ap (▴), and at the acyl chain terminals, At (▪).
Diagnostic plot for mixtures of gramicidin and DOPE
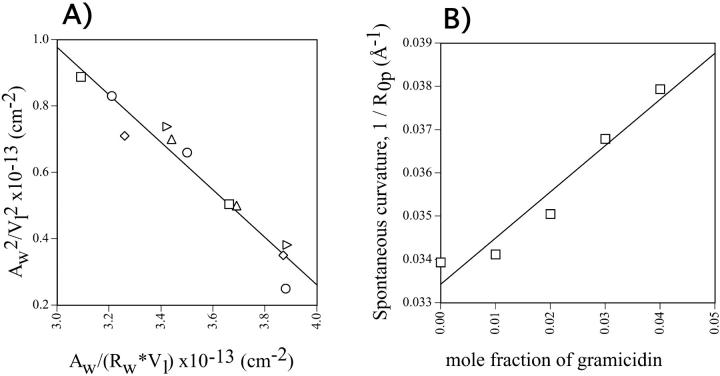
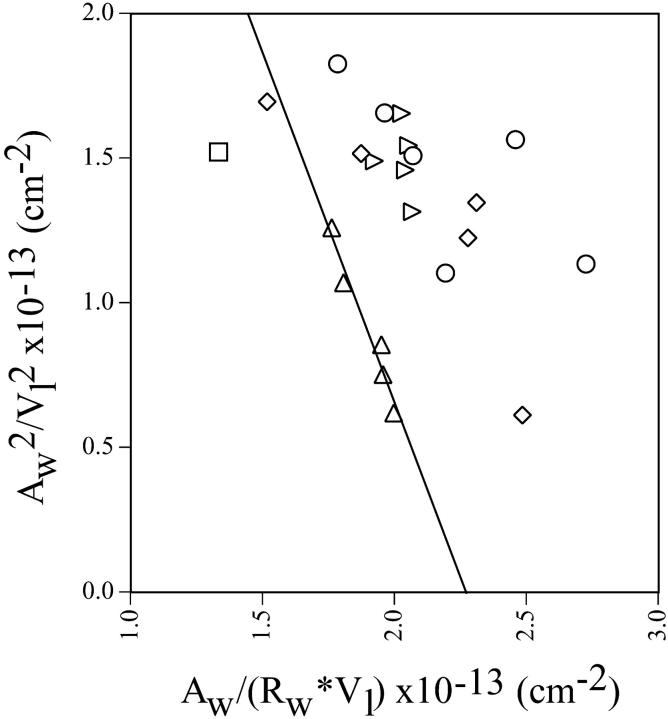
Structural parameters were determined for the single hexagonal phases formed by DOPE and gramicidin and used to construct a diagnostic plot (Eq. 8, Fig. 5 A). All mole fractions of gramicidin (0.00, 0.01, 0.02, 0.03, and 0.04) resulted in a common, linear plot. This indicates that a pivotal plane is well defined and exists at the same relative position, Vp/Vl = 0.36, for all gramicidin compositions examined. All radii of curvature are measured to this plane.
FIGURE 5.
(A) The diagnostic plot (Eq. 8) for various gramicidin/DOPE mixtures. Symbols are (□) 0.00, (⋄) 0.01, (○) 0.02, (▵) 0.03, and (▹) 0.04 mole fraction gramicidin. All compositions of gramicidin yield a common, linear relationship indicating that there is a well-defined, common pivotal plane at a position (Vp/Vl) of 0.36. (B) Spontaneous curvatures of fully hydrated mixtures of gramicidin and DOPE, measured to the pivotal plane as a function of gramicidin content.
Spontaneous curvature of monolayers containing gramicidin and DOPE
The radii of spontaneous curvature (R0p) for each gramicidin / DOPE mixture were determined from the fully hydrated structural parameters and the position of the pivotal plane according to Eq. 6. As the gramicidin content increased from mole fractions of 0.00–0.04, the spontaneous curvatures (1/R0p) increased linearly (Fig. 5 B). This relationship can be described in a least squares linear fit as 1/R0p = 0.107mgram + 0.033. From this equation, the intrinsic curvature of gramicidin itself, 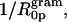 is −0.14 Å−1, and its radius of intrinsic curvature,
is −0.14 Å−1, and its radius of intrinsic curvature, 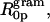 is −7.1 Å. The intrinsic curvature of DOPE
is −7.1 Å. The intrinsic curvature of DOPE 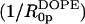 is −0.033 Å−1, with a corresponding radius of intrinsic curvature
is −0.033 Å−1, with a corresponding radius of intrinsic curvature 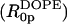 of −30.3 Å, in agreement with several previously measured values (Chen and Rand, 1997; Epand et al., 1996; Leikin et al., 1996; Rand et al., 1990).
of −30.3 Å, in agreement with several previously measured values (Chen and Rand, 1997; Epand et al., 1996; Leikin et al., 1996; Rand et al., 1990).
Osmotic stress exerted on mixtures of gramicidin and DOPE
Osmotic pressure exerted by PEG solutions on mixtures of gramicidin and DOPE alter the HII lattice dimensions. For mole fractions of 0.01, 0.02, and 0.03 gramicidin, there was a coexistence of Lα and HII phases at high osmotic pressures (Π), consistent with the HII-Lα-HII reentrant transition seen in Fig. 3. However, single HII phases exist at lower osmotic pressures. For all compositions of gramicidin examined, the HII lattice dimensions decreased as the osmotic pressure increased. This data was used to determine the bending moduli for mole fractions of 0.00, 0.01, 0.02, 0.03, and 0.04 gramicidin in DOPE as described by Eq.10 and shown in Fig. 6. These bending moduli ranged from 11 kT to 13 kT with no observable trend with gramicidin content.
FIGURE 6.
The osmotic force exerted by PEG 20,000 as it relates to monolayer curvature of the HII phase measured to the pivotal plane (Eq. 10). These plots are derived for mole fractions of (□) 0.00, (⋄) 0.01, (○) 0.02, (▵) 0.03, and (▹) 0.04 gramicidin in mixtures with DOPE. The bending moduli for these compositions were determined from the slopes of each.
Gramicidin in DOPC
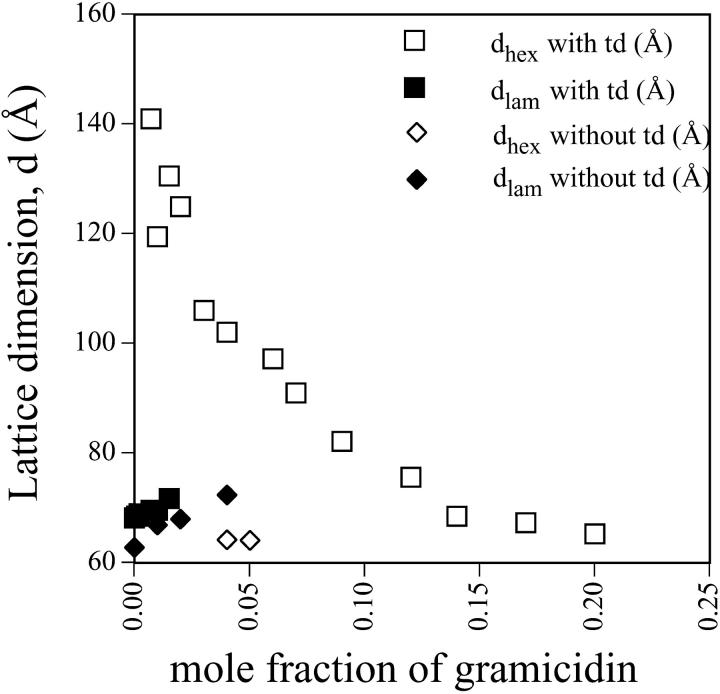
The phases, and their dimensions, formed by mixtures of gramicidin in DOPC at full hydration are shown in Fig. 7. Most samples were prepared with tetradecane to reduce interstitial stress and allow the HII phase to realize the lattice dimension of lowest free energy. Sjolund et al (Sjolund et al., 1989) have shown that alkanes alone can induce the hexagonal phase in certain conditions. In this system DOPC/td did not produce the hexagonal phase up to 60°C and so we take the transition we observe to be primarily due to gramicidin. Samples were also prepared without td to compare with other previous studies (Chupin et al., 1987; Killian et al., 1987; Killian and de Kruijff, 1985a,b; Killian et al., 1989, 1986; Van Echteld et al., 1982, 1981).
FIGURE 7.
The lattice dimensions, d, of the reverse hexagonal phase (open symbols) and the lamellar phase (closed symbols) in the presence and absence of td as they relate to mole fraction of gramicidin in fully hydrated mixtures with DOPC.
The results indicate that td has a qualitative and quantitative effect on the phase that forms by the mixture of DOPC and gramicidin. Without td the Lα phase persists and its lattice dimension increases up to mole fraction 0.04 gramicidin, at which point an HII phase appears with a very small dimension. With td however, very little gramicidin is required to induce an HII phase of large dimension that coexists with the Lα. At mole fractions of 0.03 gramicidin, the HII phase transition is complete and the lattice dimension dramatically decreases with increasing gramicidin content. At mole fractions greater than 0.15 gramicidin the lattice dimension appears to change very little. It was concluded that gramicidin / DOPC mixtures could be studied, in the presence of td, between mole fractions of 0.07 and 0.14.
Phase diagrams for mixtures of gramicidin and DOPC
The hexagonal lattice dimension dhex was determined as it varies with water content (Fig. 8). This was done for mole fractions of 0.07, 0.08, 0.10, 0.12, and 0.14 gramicidin in DOPC. Structural parameters were determined as for DOPE/gramicidin and are given in Table 2 for each content of gramicidin.
FIGURE 8.
The hexagonal lattice dimension, dhex, as it relates to the amount of water added to mole fractions of (□) 0.07, (⋄) 0.08, (○) 0.10, (▵) 0.12, and (▹) 0.14 gramicidin in mixtures with DOPC and td. With increasing hydration, but still limited water, dhex increases until an equilibrium hydration spacing is reached at full hydration. The average dhex value of each fully hydrated sample was fitted with a horizontal line for each composition. Water fraction (1 − c) and dhex at the intersection points (described in the text) are listed in Table 2 for each composition.
TABLE 2.
The equilibrium water fraction, 1 − c, required to achieve the maximum lattice dimensions, dhex, at full hydration, as determined from the intersection points on the phase diagrams (Fig. 8) for various compositions of gramicidin in mixtures with DOPC and td
| Mole fraction of gramicidin | Equilibrium water fraction 1 − c | Maximum lattice dimensions dhex (Å) |
|---|---|---|
| 0.07 | 0.41 | 83 |
| 0.08 | 0.40 | 81 |
| 0.10 | 0.38 | 72 |
| 0.12 | 0.33 | 69 |
| 0.14 | 0.30 | 65 |
In most previous studies (Chen and Rand, 1997; Fuller and Rand, 2001; Leikin et al., 1996; Szule et al., 2002), and in the DOPE system of the present study, the hexagonal lattice dimensions in the dehydrated regions were common for all compositions. This shows that the dehydrated lattice dimensions are not dependent on the amount of inclusion but only on the fraction of water present. However in contrast to this, various mixtures of gramicidin in DOPC do not yield common or systematically changing dehydrated dimensions (Fig. 8). Nevertheless these data can be used to determine several structural parameters (Fig. 9) and to construct a diagnostic plot (Eq. 8).
FIGURE 9.
Through the geometrical relationships described in the Structural Analysis section, the dimensions of the structural parameters shown in Fig. 1 are plotted as they relate to the mole fraction of gramicidin in mixtures with DOPC in the presence of td at equilibrium hydration. 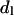 (□) is the thickness of an effective bilayer in the interaxial direction, and
(□) is the thickness of an effective bilayer in the interaxial direction, and 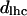 (○) is the thickness of the hydrocarbon region of an effective bilayer in the interaxial direction. The molecular areas of an effective molecule are plotted at the Luzzati plane,
(○) is the thickness of the hydrocarbon region of an effective bilayer in the interaxial direction. The molecular areas of an effective molecule are plotted at the Luzzati plane, 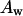 (♦), and at the acyl chain terminals,
(♦), and at the acyl chain terminals, 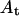 (▪).
(▪).
Structural parameters for mixtures of gramicidin and DOPC
Some structural dimensions within the HII phases, as shown in Fig. 1, have been plotted as they relate to the gramicidin content (Fig. 9). The thickness of an effective bilayer (dl), and the thickness of the hydrophobic region of an effective bilayer (dlhc) in the interaxial direction remain relatively constant as the mole fraction of gramicidin increases. As the composition of gramicidin increases, the area of an effective molecule at the Luzzati plane (Aw) increases slightly compared to the relatively large increase in area at the acyl chain terminals (At) (Fig. 9). These data will be used to discuss the structural effects of gramicidin on the HII phase.
Diagnostic plot of gramicidin in mixtures with DOPC
Different mole fractions of gramicidin in mixtures with DOPC did not yield a common plot (Fig. 10). Furthermore, the nonlinearity of these diagnostic plots for 0.07, 0.08, 0.10, and 0.14 mole fractions of gramicidin indicate that a well-defined pivotal plane does not exist for these compositions. On the other hand, 0.12 mole fraction of gramicidin does show a linear relationship which suggests the existence of a well-defined pivotal plane for this composition. If we accept this linearity as reflecting a true pivotal plane for this one composition, its position, (Vp/Vl), is 1.2. This ratio, greater than 1, means that the volume up to the pivotal plane (Vp) is greater than the volume of the effective molecule itself (Vl). By this, the pivotal plane exists outside the effective molecule, beyond the acyl chain terminals.
FIGURE 10.
Diagnostic plots for mole fractions of (□) 0.07, (⋄) 0.08, (○) 0.10, (▵) 0.12, and (▹) 0.14 gramicidin in mixtures with DOPC in the presence of tetradecane. The nonlinearity of the 0.07, 0.08, 0.10, and 0.14 compositions indicate that a pivotal plane does not exist. The linear relationship of the plot for the 0.12 composition indicates that a well-defined pivotal plane does exist at a position (Vp/Vl) of 1.2.
Spontaneous curvature of monolayers containing gramicidin and DOPC
From Eq. 9 and the calculated Vp/Vl, the spontaneous curvature (1/R0p) of monolayers made of 0.12 mole fraction of gramicidin in DOPC is determined to be −0.022 Å−1. Linear addition of component curvatures cannot be demonstrated in these mixtures. However its assumption can lead to an estimate of the curvature of gramicidin itself. The intrinsic curvature of DOPC determined from a previous study is −0.0066 Å−1 (Szule et al., 2002). Using this, the intrinsic curvature of gramicidin itself 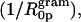 when mixed to 0.12 mole fraction in DOPC is determined to be −0.13 Å−1 making
when mixed to 0.12 mole fraction in DOPC is determined to be −0.13 Å−1 making 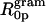 equal to −7.4 Å. In spite of this limited data and tenuous assumption of additive curvatures, this result is remarkably similar to that measured for gramicidin in mixtures of DOPE where linear addition of curvatures was shown to apply.
equal to −7.4 Å. In spite of this limited data and tenuous assumption of additive curvatures, this result is remarkably similar to that measured for gramicidin in mixtures of DOPE where linear addition of curvatures was shown to apply.
Osmotic stress on mixtures of gramicidin and DOPC
The lattice dimensions of the osmotically equilibrated samples showed the same anomalies and inconsistencies as they did in the phase diagrams. This all points to a degree of complexity of interactions between DOPC and gramicidin that precludes easy analysis.
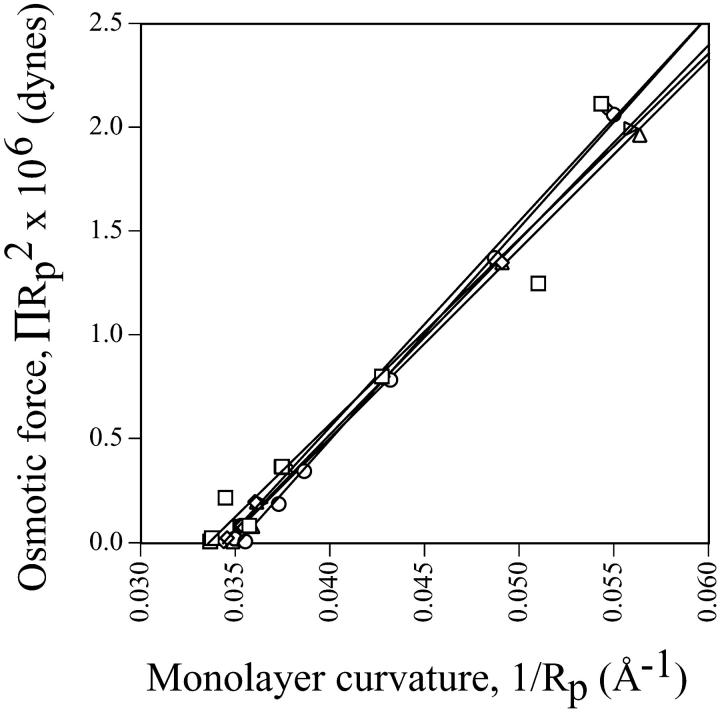
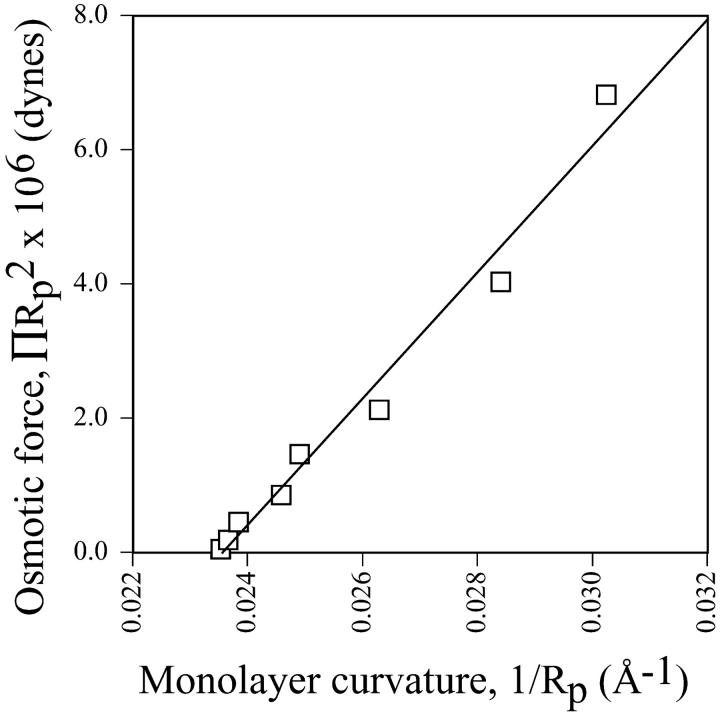
However, accepting that a well-defined pivotal plane appears to exist at a mole fraction of 0.12 gramicidin would allow an estimate of a bending modulus to be made for this composition (Fig. 11). A value of 115 kT suggests very stiff monolayers. Based upon the cross-sectional dimensions of gramicidin, (∼15 Å (Wallace, 1986)) and the lipids, (3.9 Å (Chen and Rand, 1998)), it is estimated that ∼8–9 lipids are required to surround a single gramicidin molecule. Contact between gramicidin molecules might therefore begin at ∼0.09–0.10 mole fraction, possibly leading to the observed monolayer stiffness.
FIGURE 11.
The osmotic force as it relates to monolayer curvature of HII phases measured to the pivotal plane (Eq. 10). This data is derived for the system composed of 0.12 mole fraction of gramicidin in mixtures with DOPC and td. The bending modulus, Kcp, could be determined to be 115 kT from the slope of this linear relationship.
DISCUSSION
We have attempted to measure for the first time the curvature parameters of a peptide inclusion in a phospholipid monolayer. Such parameters have been measured successfully for a range of more natural lipidic inclusions such as various phospholipid species, cholesterol and alpha tocopherol (Leikin et al., 1996; Fuller and Rand, 2001; Chen and Rand, 1997; Bradford, 2000). The peptide gramicidin was selected for its hydrophobic properties and the comprehensive characterization of its gating properties and how they are affected by its lipid host. Our attempts have been met with mixed success. In DOPE monolayers in the hexagonal phase, gramicidin is well behaved. In DOPC, it is not well behaved. Gramicidin has a variety of environment-dependent conformations that have a long history of controversy (reviewed by Ramachandran, 1975; Wallace, 1986; Killian, 1992; Andersen et al., 1996). Their conformation in the hexagonal phase is not known, and we can only treat it as an inclusion whose effects on dimensions we can accurately and systematically measure. In the case of DOPE, but not DOPC, we have determined a range of compositions over which gramicidin behaves uniformly and we proceed with analyzing the structural changes as we have for other inclusions. In the case of DOPC, the behavior is not uniform and our conclusions are limited.
Mixtures of gramicidin and DOPE
Between 0 and 0.04 mole fraction gramicidin, there is a systematic reduction of HII lattice dimension from that of pure DOPE, indicating that increased negative curvature is introduced by gramicidin. For compositions greater than 0.04 mole fraction gramicidin, dhex is small and remains constant, suggesting that gramicidin's curvature contribution is somehow altered or the peptide is excluded from the HII phase. Our interpretation of the temperature coefficients seen in Fig. 2 B is that gramicidin is not incorporated beyond mole fraction 0.06–0.07 in DOPE.
The different changes in molecular area along the length of the DOPE molecule (Fig. 4) show that gramicidin is contributing molecular area and volume deep in the hydrophobic regions of the monolayer and not at the polar group/water interface. This is consistent with the models of gramicidin gating, bound deep in the monolayer and sensitive to bilayer thickness. It is also consistent with the proposal that inter-PE hydrogen bonds interfere with interactions between the tryptophan residues of gramicidin and the polar regions (Scarlata and Gruner, 1997). Consequently, gramicidin would be more restricted to the hydrophobic regions of these monolayers.
The radius of intrinsic curvature for gramicidin measured in mixtures with DOPE 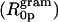 is −7.1 Å. This value should not be interpreted in terms of the effective shape of a molecule as is done for the lipids. Rather the value reflects that gramicidin behaves as if it contributes high negative curvature because it partitions largely into the hydrophobic part of the monolayer. Its effect is large considering that diacylglycerols and cholesterol, both powerful HII-phase inducing lipids, introduce less negative curvature into a membrane than does gramicidin (Szule et al., 2002; Leikin et al., 1996; Chen and Rand, 1997).
is −7.1 Å. This value should not be interpreted in terms of the effective shape of a molecule as is done for the lipids. Rather the value reflects that gramicidin behaves as if it contributes high negative curvature because it partitions largely into the hydrophobic part of the monolayer. Its effect is large considering that diacylglycerols and cholesterol, both powerful HII-phase inducing lipids, introduce less negative curvature into a membrane than does gramicidin (Szule et al., 2002; Leikin et al., 1996; Chen and Rand, 1997).
Gramicidin could not be observed to affect the bending modulus of DOPE monolayers. This could be due to the relatively small mole ratios of peptide to lipid, where the local effects of gramicidin may be too diluted to be observed. At these compositions, there are more than enough lipids to completely surround each gramicidin molecule, and our measurements may not reflect the local bending modulus around a gramicidin molecule. The very high bending modulus tenuously estimated for gramicidin in DOPC may better reflect that local bending, because the relative amount of lipid is less.
Mixtures of gramicidin and DOPC
Gramicidin in DOPC induces the lamellar-hexagonal phase transition indicating a large contribution of negative curvature. Typically, without td, the HII phase of gramicidin and DOPC mixtures have a small and invariant lattice dimension. Such small and invariant lattice dimensions have been observed for other mixtures of gramicidin and different phospholipids, (Chupin et al., 1987; Killian et al., 1987; Killian and de Kruijff, 1985a,b, Killian et al., 1989, 1986; Van Echteld et al., 1982, 1981) and are likely a result of chain stress, i.e., a requirement for the interstices to be filled. Such systems cannot reflect the spontaneous curvature of these mixtures. For curvature studies on systems of low spontaneous curvature, td is required to fill the interstices, allowing the monolayers to form the curvature of lowest free energy (Gruner, 1985). Our practice is to include td, allowing the small curvatures and large dimensions to be expressed. Under these conditions the Lα to HII phase transition is complete with as little as ∼0.03 mole fraction gramicidin in DOPC.
The structural parameters calculated and shown in Fig. 9 indicate that gramicidin in DOPC, as in DOPE, is mainly contributing area and volume to the hydrophobic region of an effective molecule, thereby adding negative curvature to the monolayers.
From the diagnostic plots, it is clear that a well-defined pivotal plane does not exist at most compositions. This is the first inclusion for which we have observed this. Nevertheless a well-defined pivotal plane appears to exist at 0.12 mole fraction gramicidin in DOPC. This composition is where each gramicidin can be surrounded with only one layer of lipid, were it uniformly distributed in the monolayers, and this may restrict structural rearrangements on dehydration. On this tenuous basis we proceeded with the curvature analysis which yielded a position for the pivotal plane at Vp/Vl greater than 1. This means that the monolayers are bending around a plane outside of the lipid molecules, beyond the acyl chain terminals. At all positions along the entire length of the effective molecule the area is changing in the same direction as the monolayers bend. This is qualitatively different from the bending of any other monolayer we have observed.
One can obtain an estimate of the intrinsic curvature of gramicidin itself in this mixture. This requires a), the intrinsic curvature of DOPC itself, which was determined from a previous study (Szule et al., 2002) and b), the unproved assumption that DOPC and gramicidin curvatures add linearly. On this basis gramicidin molecules are behaving as though they have an intrinsic radius of curvature, 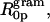 of −7.4 Å in DOPC. Remarkably, in spite of these severe assumptions, this value is very close to that obtained in the DOPE system, 7.1 Å. This suggests that gramicidin is contributing the same relative hydrophobic volume to the effective molecules in each lipid system.
of −7.4 Å in DOPC. Remarkably, in spite of these severe assumptions, this value is very close to that obtained in the DOPE system, 7.1 Å. This suggests that gramicidin is contributing the same relative hydrophobic volume to the effective molecules in each lipid system.
A bending modulus, Kcp could only be measured for this composition. A Kcp of 115 kT indicates that these monolayers are very stiff. They are an order of magnitude more resistant to change in curvature than monolayers composed of gramicidin and DOPE, and any other lipid system we have measured. Such stiffness may be a result of the high concentration of gramicidin found within the monolayers, higher than could be achieved with DOPE, and at a level where only one lipid is available to separate gramicidins. This may reflect bending moduli local to gramicidin more realistically. Lateral interactions between adjacent peptides, perhaps through interactions of tryptophans, may contribute to this observed stiffness (Killian and de Kruijff, 1986). Also, interaxial hydrogen bonds of single-stranded dimers may provide the phase structure with a more rigid backbone, as proposed by Van Echteld et al. (1981).
Why are the interactions of gramicidin with DOPE different from DOPC? PEs are able to form intermolecular hydrogen bonds, thereby reducing the effective size and hydration of the polar region. They also prevent hydrogen bonding between the tryptophan residues and the polar regions of the lipids (Scarlata and Gruner, 1997). It was postulated that gramicidin is excluded from this polar group, residing deeper in the hydrocarbon region. PCs on the other hand, do not form strong inter-headgroup associations, are more fully hydrated and allow hydrogen bonds between the tryptophan residues and the polar region (Scarlata and Gruner, 1997). This would position gramicidin closer to the polar groups of DOPC than DOPE. Such a looser polar group region in DOPC may allow the position of gramicidin in DOPC monolayers to change with dehydration and bending. Something like this may account for the variation in dimensions seen in the DOPC system.
Given these qualitative differences between PC and PE, one might expect to observe qualitative differences in the gating properties of gramicidin in these two different membranes. In PC/PE mixtures it is found that the channel lifetime decreases as the mole fraction of PE increases (Maer, A. M., J. A. Lundbæk and O. S. Andersen, personal communication). This provides hope of eventually connecting gating properties with such structural parameters as determined in this study.
Acknowledgments
Nola Fuller always provides valuable guidance in the laboratory and in discussion.
J.S. and R.P.R. gratefully acknowledge support of the Natural Sciences and Engineering Research Council of Canada, through research grants (R.P.R.) and Graduate Fellowships (J.S.)
References
- Andersen, O. S., C. Nielsen, A. M. Maer, J. A. Lundbaek, M. Goulian, and R. E. Koeppe. 1998. Gramicidin channels: molecular force transducers in lipid bilayers. Biol. Skr. Dan. Vid. Selsk. 49:75–82. [Google Scholar]
- Andersen, O. S., G. Saberwal, D. V. Greathouse, and R. E. Koeppe. 1996. Gramicidin channels - A solvable membrane “protein” folding problem. Indian J. Biochem. Biophys. 33:331–342. [PubMed] [Google Scholar]
- Bezrukov, S. M. 2000. Functional consequences of lipid packing stress. Curr. Opin. Coll. Inter. Sci. 5:237–243. [Google Scholar]
- Bradford, A. 2000. The Effect of Vitamin E on the Structure of Membrane Lipid Assemblies. Honours B.Sc. Thesis, Biological Sciences. Brock University, St. Catharines, Ontario.
- Busath, D. D. 1993. The use of physical methods in determining gramicidin channel structure and function. Annu. Rev. Physiol. 55:473–501. [DOI] [PubMed] [Google Scholar]
- Chen, Z., and R. P. Rand. 1997. The influence of cholesterol on phospholipid membrane curvature and bending elasticity. Biophys. J. 73:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z., and R. P. Rand. 1998. Comparative study of the effects of several n-alkanes on phospholipid hexagonal phases. Biophys. J. 74:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik, L., M. M. Kozlov, and J. Zimmerberg. 1995. Topical review: lipids in biological membrane fusion. J. Membr. Biol. 146:1–14. [DOI] [PubMed] [Google Scholar]
- Chupin, V., J. A. Killian, and B. de Kruijff. 1987. 2H-nuclear magnetic resonance investigations on phospholipid acyl chain order and dynamics in the gramicidin-induced hexagonal HII phase. Biophys. J. 51:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis, P. R., and B. de Kruijff. 1979. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim. Biophys. Acta. 559:399–420. [DOI] [PubMed] [Google Scholar]
- Dubos, R. J. 1939. Studies on a bactericidal agent extracted from a soil bacillus. J. Exp. Med. 70:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epand, R. M., N. L. Fuller, and R. P. Rand. 1996. Role of the position of unsaturation on the phase behaviour and intrinsic curvature of phosphatidylethanolamines. Biophys. J. 71:1806–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller, N. L., and R. P. Rand. 2001. The influence of lysolipids on the spontaneous curvature and bending elasticity of phospholipid membranes. Biophys. J. 81:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawrisch, K., V. A. Parsegian, D. A. Hajduk, M. W. Tate, and S. M. Gruner. 1992. Energetics of a hexagonal-lamellar-hexagonal-phase transition sequence in dioleoylphosphatidylethanolamine membranes. Biochemistry. 31:2856–2864. [DOI] [PubMed] [Google Scholar]
- Gruner, S. M. 1985. Intrinsic curvature hypothesis for biomembrane lipid composition: A role for non-bilayer lipids. Proc. Natl. Acad. Sci. USA. 82:3665–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner, S. M., V. A. Parsegian, and R. P. Rand. 1986. Directly measured deformation energy of phospholipid HII hexagonal phases. Faraday Discuss. Chem. Soc. 81:29–37. [DOI] [PubMed] [Google Scholar]
- Helfrich, W. 1973. Elastic properties of lipid bilayers: theory and possible experiments. Z. Naturforsch. 28C:693–703. [DOI] [PubMed] [Google Scholar]
- Helfrich, P., and E. Jakobsson. 1990. Calculation of deformation energies and conformations in lipid membranes containing gramicidin channels. Biophys. J. 57:1075–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, S. L., S. M. Bezrukov, S. M. Gruner, M. W. Tate, I. Vodyanoy, and V. A. Parsegian. 1993. Probability of alamethicin conductance states varies with nonlamellar tendency of bilayer phospholipids. Biophys. J. 65:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, S. L., S. M. Gruner, and K. Gawrisch. 1996. Small concentrations of alamethicin induce a cubic phase in bulk phosphatidylethanolamine mixtures. Biochim. Biophys. Acta. 1278:241–246. [DOI] [PubMed] [Google Scholar]
- Killian, J. A. 1992. Gramicidin and gramicidin-lipid interactions. Biochim. Biophys. Acta. 1113:391–425. [DOI] [PubMed] [Google Scholar]
- Killian, J. A., K. N. J. Burger, and B. de Kruijff. 1987. Phase separation and hexagonal HII phase formation by gramicidins A, B and C in dioleoylphosphatidylcholine model membranes. A study on the role of the tryptophan residues. Biochim. Biophys. Acta. 897:269–284. [DOI] [PubMed] [Google Scholar]
- Killian, J. A., and B. de Kruijff. 1985a. Importance of hydration for gramicidin-induced hexagonal HII phase formation in dioleoylphosphatidylcholine model membranes. Biochemistry. 24:7890–7898. [DOI] [PubMed] [Google Scholar]
- Killian, J. A., and B. de Kruijff. 1985b. Thermodynamic, motional, and structural aspects of gramicidin-induced hexagonal HII phase formation in phosphatidylethanolamine. Biochemistry. 24:7881–7890. [DOI] [PubMed] [Google Scholar]
- Killian, J. A., and B. de Kruijff. 1986. The influence of proteins and peptides on the phase properties of lipids. Chem. Phys. Lipids. 40:259–284. [DOI] [PubMed] [Google Scholar]
- Killian, J. A., K. U. Prasad, D. W. Urry, and B. de Kruijff. 1989. A mismatch between the length of gramicidin and the lipid acyl chains is a prerequisite for HII phase formation in phosphatidylcholine model membranes. Biochim. Biophys. Acta. 978:341–345. [DOI] [PubMed] [Google Scholar]
- Killian, J. A., C. W. Van den Berg, H. Tournois, S. Keur, A. J. Slotboom, G. J. M. Van Scharrenburg, and B. de Kruijff. 1986. Gramicidin-induced hexagonal HII phase formation in negatively charged phospholipids and the effect of N- and C- terminal modification of gramicidin on its interaction with zwitterionic phospholipids. Biochim. Biophys. Acta. 857:13–27. [DOI] [PubMed] [Google Scholar]
- Kirk, G. L., S. M. Gruner, and D. L. Stein. 1984. A thermodynamic model of the lamellar to inverse hexagonal phase transition of lipid membrane-water systems. Biochemistry. 23:1093–1102. [Google Scholar]
- Koeppe, R. E., and O. S. Andersen. 1996. Engineering the gramicidin channel. Annu. Rev. Biophys. Biomol. Struct. 25:231–258. [DOI] [PubMed] [Google Scholar]
- Kozlov, M. M., S. Leikin, and R. P. Rand. 1994. Bending, hydration and interstitial energies quantitatively account for the hexagonal-lamellar-hexagonal reentrant phase transition in dioleoylphosphatidylethanolamine. Biophys. J. 67:1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikin, S., M. M. Kozlov, N. L. Fuller, and R. P. Rand. 1996. Measured effects of diacylglycerol on structural and elastic properties of phospholipid membranes. Biophys. J. 71:2623–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom, G., M. Sjolund, and L. Rilgors. 1988. Effect of normal-alkanes and peptides on the phase-equilibria in phosphatidylcholine water-systems. Liquid Crystals. 3:783–790. [Google Scholar]
- Luzzati, V., and F. Husson. 1962. The structure of the liquid-crystalline phases of lipid-water systems. J. Cell Biol. 12:207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, C., M. Goulian, and O. S. Andersen. 1998. Energetics of inclusion-induced bilayer deformations. Biophys. J. 74:1966–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian, V. A., R. P. Rand, N. L. Fuller, and D. C. Rau. 1986. Osmotic stress for the direct measurement of intermolecular forces. Methods Enzymol. 127:400–416. [DOI] [PubMed] [Google Scholar]
- Ramachandran, L. K. 1975. The gramicidins. Biochem. Rev. XLVI:1–17. [Google Scholar]
- Rand, R. P., N. L. Fuller, S. M. Gruner, and V. A. Parsegian. 1990. Membrane curvature, lipid segregation, and structural transitions for phospholipids under dual-solvent stress. Biochemistry. 29:76–87. [DOI] [PubMed] [Google Scholar]
- Rand, R. P., and W. A. Pangborn. 1973. A structural transition in egg lecithin-cholesterol bilayers at 12°C. Biochim. Biophys. Acta. 318:299–305. [Google Scholar]
- Scarlata, S., and S. M. Gruner. 1997. Role of phosphatidylethanolamine lipids in the stabilization of protein-lipid contacts. Biophys. Chem. 67:269–279. [DOI] [PubMed] [Google Scholar]
- Sjolund, M., L. Rilfors, and D. Lindblom. 1989. Reversed hexagonal phase formation in lecithin alkane water-systems with different acyl chain unsaturation and alkane length. Biochemistry. 28:1323–1329. [DOI] [PubMed] [Google Scholar]
- Szule, J. A., N. L. Fuller, and R. P. Rand. 2002. The effects of acyl chain length and saturation of diacylglycerols and phosphatidylcholines on membrane monolayer curvature. Biophys. J. 83:977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournois, H., J. Leunissen-Bijvelt, C. W. M. Haest, J. de Gier, and B. de Kruijff. 1987. Gramicidin-induced hexagonal HII phase formation in erythrocyte membranes. Biochemistry. 26:6613–6621. [DOI] [PubMed] [Google Scholar]
- Van Echteld, C. J. A., B. de Kruijff, A. J. Verkleij, J. Leunissen-Bijvelt, and J. de Gier. 1982. Gramicidin induces the formation of non-bilayer structures in phosphatidylcholine dispersions in a fatty acid chain length dependent way. Biochim. Biophys. Acta. 692:126–138. [Google Scholar]
- Van Echteld, C. J. A., R. Van Stigt, B. de Kruijff, J. Leunissen-Bijvelt, A. J. Verkleij, and J. de Gier. 1981. Gramicidin promotes formation of the hexagonal HII phase in aqueous dispersions of phosphatidylethanolamine and phosphatidylcholine. Biochim. Biophys. Acta. 648:287–291. [Google Scholar]
- Wallace, B. A. 1986. Structure of gramicidin A. Biophys. J. 49:295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]