Abstract
The immunoglobulin A (IgA) protease secreted by pathogenic Neisseria spp. cleaves Lamp1, thereby altering lysosomes in a cell and promoting bacterial intracellular survival. We sought to determine how the IgA protease gains access to cellular Lamp1 in order to better understand the role of this cleavage event in bacterial infection. In a previous report, we demonstrated that the pilus-induced Ca2+ transient triggers lysosome exocytosis in human epithelial cells. This, in turn, increases the level of Lamp1 at the plasma membrane, where it can be cleaved by IgA protease. Here, we show that porin also induces a Ca2+ flux in epithelial cells. This transient is similar in nature to that observed in phagocytes exposed to porin. In contrast to the pilus-induced Ca2+ transient, the porin-induced event does not trigger lysosome exocytosis. Instead, it stimulates exocytosis of early and late endosomes and increases Lamp1 on the cell surface. These results indicate that Neisseria pili and porin perturb Lamp1 trafficking in epithelial cells by triggering separate and distinct Ca2+-dependent exocytic events, bringing Lamp1 to the cell surface, where it can be cleaved by IgA protease.
All pathogenic Neisseria gonorrhoeae strains secrete an immunoglobulin A (IgA) protease (IgAP) that cleaves human IgA1 (hIgA1) at its proline-rich hinge (29). IgA1 is found mainly on mucosal surfaces, and the hydrolysis of hIgA1 by IgAP is thought to promote colonization by disarming bactericidal antibodies at these sites. Lamp1 (lysosome-associated membrane protein 1) is also a substrate for the IgAP (16, 20). Lamp1, an ∼110,000-kDa membrane-spanning glycoprotein, is a major constituent of lysosomes (8). This bilobed polypeptide is spanned by a proline-rich luminal domain which has striking similarity to the hIgA1 hinge (8).
In infected epithelial cells, the secreted IgAP cleaves Lamp1 at its hinge, accelerating its turnover and ultimately reducing its total cellular levels (20). Infected cells also have significantly reduced levels of lysosomal markers Lamp2, CD63, and lysosomal acid phosphatase, even though these polypeptides are not IgAP substrates (3). Cells infected with bacteria with null mutations in iga, the IgAP gene, have normal levels of lysosomal markers, suggesting that IgAP cleavage of Lamp1 modifies lysosomes in an undefined way. The iga mutant also has an intracellular growth defect (20) and transcytoses through an epithelial monolayer at a slower rate (18), suggesting that IgAP cleavage of Lamp1 may be important for bacterial intracellular survival.
To understand how IgAP cleavage of Lamp1 contributes to Neisseria pathogenesis, it is important to determine how this enzyme reaches Lamp1. The majority of newly synthesized Lamp1 is delivered directly from the Golgi apparatus to late endosomes and then lysosomes (7). A small fraction, however, traffics to the plasma membrane before it is endocytosed and delivered to lysosomes (7). Taking these observations into account, there are at least two possible routes by which IgAP may gain access to Lamp1. Protease secreted by adherent bacteria may be delivered to mature lysosomes via the endocytic route. The ability of IgAP to cleave Lamp1 at this site is doubtful, however. IgAP cleavage of Lamp1 is most efficient at neutral pH and much less efficient at pH 5.5 (20), the pH of the lysosome lumen.
A recent report revealed another possible pathway of access. In epithelial cells, the type IV pili of Neisseria cause the release of free Ca2+ from intracellular stores, triggering a transient rise in cytosolic Ca2+ levels in epithelial cells (19). Unlike most Ca2+ transients, which occur rapidly after cellular exposure to stimuli, the pilus-induced flux occurs 12 to 15 min after the addition of pili. This Ca2+ flux triggers lysosome exocytosis, bringing lysosomal Lamp1 to the cell surface and exposing its IgAP cleavage site to the extracellular milieu (2). IgAP secreted by adherent bacteria is then able to cleave plasma membrane Lamp1.
The N. gonorrhoeae porins P1.A and P1.B are relatively conserved membrane-spanning proteins that have been implicated in the pathogenesis of gonococcal infections (35, 36). Like porins of other gram-negative bacteria, the N. gonorrhoeae porins are trimers that form β-pleated barrels within the bacterial membrane (10, 37). Neisserial porins insert into eukaryotic membranes to form ion-gated channels (31, 36). In monocytic cells, the Neisseria porin triggers a transient rise in cytosolic Ca2+ levels. This transient is due to an influx of Ca2+ from the extracellular environment, occurs within 2 min after exposure to porin, and is blocked by ATP (26).
We tested the hypothesis that Neisseria porin would also induce a Ca2+ flux in epithelial cells and that this transient would trigger exocytosis of Lamp1 compartments. We report that porin induces a Ca2+ transient in two human epithelial cell lines. Like the Ca2+ transient in phagocytes, the one observed in epithelial cells occurs within minutes of exposure to porin, is due to an influx of extracellular Ca2+, and can be blocked by ATP. The porin-induced flux also causes a redistribution of vesicular Lamp1 to the plasma membrane, where it is cleaved by IgAP. Interestingly, the redistributed Lamp1 is derived from endosomes, not lysosomes. We conclude that the porin- and pilus-induced Ca2+ transients trigger the exocytosis of separate and distinct Lamp1 compartments in epithelial cells and that these exocytic events serve to increase the amount of Lamp1 that can be accessed by the Neisseria IgAP.
MATERIALS AND METHODS
Cell culture.
The A431 human epidermoid carcinoma cell line was obtained from S. Schmid. Cells were grown in Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco). The T84 human colonic epithelium-like cell line (American Type Culture Collection) was grown in DMEM-F-12-5% FBS.
Bacterial strains.
N. gonorrhoeae strains used in these studies were P1.B producers MS11A (piliated, Opa− [32]) and its derivatives MS11A-307 (MS11A ΔpilE1 ΔpilE2 [22]) and MS11A-500 (MS11A Δiga [17]). Their piliation status was checked by colony morphology; pilin and Opa production and porin type were checked by immunoblotting with the appropriate monoclonal antibodies (MAbs; see below).
Antibodies and chemicals.
MAb 10H5.1.1, against the conserved SM1 epitope (23), was used for immunoblotting to detect pilin production. MAb 3C8, to detect PI.B porin (33), was kindly provided by P. F. Sparling. MAb 4B12 (from M. Blake) was used to detect Opa production. Anti-transferrin receptor (TfR; MAb OKT9) was a kind gift from Caroline Enns. MAb H4A3 (anti-human Lamp1) was obtained from the Developmental Studies Hybridoma Bank and Pharmingen (San Diego, Calif.). Biotinylated MAb H4A3, used to quantitate surface Lamp1, was obtained from Pharmingen and was dialyzed against phosphate-buffered saline (PBS) before use. The streptavidin-horseradish peroxidase (HRP) conjugate was from Roche-Boehringer (Mannheim, Germany). Bovine serum albumin and Probenicid were from Sigma Chemical Co. (St. Louis, Mo.). A goat anti-mouse Alexa 488 antibody, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-PAM, Fura-2/AM, and Lucifer yellow (LY) were from Molecular Probes, Inc. (Eugene, Oreg.). QuantaBlu fluorogenic peroxidase substrate kit, goat anti-mouse IgG-HRP, goat anti-rabbit IgG-HRP, and the Super Signal chemiluminescence kit were from Pierce. HRP-transferrin was from Jackson ImmunoResearch Laboratories, Inc. (Philadelphia, Pa.).
P1.B, purified pili, and crude membrane preparations.
P1.B was purified from a nonpiliated MS11A-PIII− strain (38). P1.B was dialyzed against PBS before experiments were carried out. Purified pili were prepared as described previously (27). Crude membrane preparations from piliated (CMP-P+) or nonpiliated (CMP-P−) derivatives were prepared from MS11A and MS11A-307, as reported previously (2). For appropriate experiments, preparations were supplemented with 2 to 5 mM CaCl2 immediately before use. Contaminating proteins constituted less than 2% of the purified porin and pilus preparations, as judged by Coomassie staining. The absence of pilin in purified porin preparations and the absence of porin in pilus preparations were also confirmed by immunoblotting using appropriate antibodies.
Monitoring fluxes in cytosolic free Ca2+ levels.
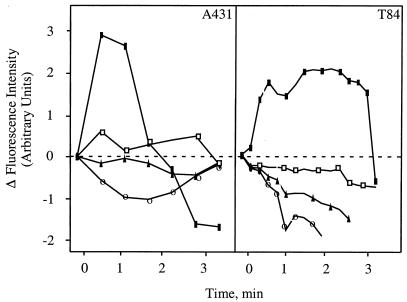
A431 cells were plated in ΔT culture dishes (Bioptechs) at 20% confluence and incubated overnight. Cultures were washed in prewarmed Ca2+-free or Ca2+-replete medium and loaded with 0.1 μM Fura-2/AM (a ratiometric calcium indicator) in Hanks balanced salt solution supplemented with 5 mM MgCl2. Cells were maintained at 34°C throughout the experiment by using the Bioptechs culture dish heating system, including the stage adapter and objective heater. Cells were then stimulated with 7 μg of P1.B/ml, and fluorescence changes in individual cells were captured with a DeltaVision restoration microscopy system (Applied Precision, Inc., Issaquah, Wash.) equipped with a ×60, 1.2-numerical-aperture lens. Fura-2/AM fluorescence was imaged in a single plane through the cells as a time lapse. Successive acquisitions were approximately 15 s apart. Data sets were processed with the DeltaVision SoftworX software package to quantify fluorescence intensity ratios (excitation, 340 or 380 nm; emission, 525 nm) from each frame through a small section of each individual cell. Average ratios (n = 4 cells per sample) were plotted on a graph (see Fig. 1) with Microsoft Excel.
FIG. 1.
Porin P1.B induces an influx of Ca2+ into epithelial cells. A431 and T84 cultures were preloaded with 0.1 μM Fura-2/AM and stimulated for 5 min at 34°C with medium alone (○), with P1.B alone (▪), with P1.B plus ATP (□), or with P1.B plus EGTA (▴). Data were acquired from individual cells with a DeltaVision restoration microscopy system and SoftWorX as described in Materials and Methods.
Quantitation of plasma membrane Lamp1 and TfR levels.
Cultures were incubated with purified protein, live bacteria, or membrane preparations derived from these bacteria for various times and quickly chilled to 4°C by adding ice-cold DMEM and placing the microtiter plates on an ice bath. Surface Lamp1 was quantitated with biotinylated MAb H4A3 for Lamp1 (2), and TfR levels were quantitated with HRP-transferrin (6).
Quantitation of the fluid phase marker LY.
Lysosomes were loaded with 1.5 mg of LY/ml at 37°C in 5% CO2 for 20 h and then chased for 2 h at the same temperature. Endosomes were loaded with 5 mg of LY/ml for 1 h at 16 to 18°C in 5% CO2. After differential loading of lysosomes or endosomes, cells were stimulated with P1.B, CMP-P+ derivatives, CMP-P− derivatives, or pili for different times. Supernatants were treated as previously described (2). LY fluorescence was determined with a Fluostar spectrofluorimeter (BMG Lab Technologies). Relevant calculations for LY release results were obtained after background levels at time zero were subtracted from each sample. Student's paired t test was performed by using MultiStat, version 1.1.
Immunofluorescence of surface TfR.
A431 cells were seeded on sterile coverslips in six-well tissue culture plates. After 24 h, cells were stimulated with 7 μg of purified P1.B/ml for 5 min, chilled to 4°C, and incubated with MAb OKT9 (mouse anti-human TfR) at 1:250 in 10 mM HEPES-DMEM for 1 h at 4°C. Coverslips were then washed in cold PBS and fixed with 4% paraformaldehyde (EM Science) in PBS for 10 min at room temperature. A secondary antibody (goat anti-mouse Alexa 488 [Molecular Probes]) was diluted 1:1,000 in blocking buffer and applied to cells for 1 h at RT. Coverslips were then washed in PBS and mounted on slides with Fluoromount-G (Southern Biotech). The DeltaVision restoration microscopy system was used to acquire and process images. Layout was created with Adobe Photoshop, versions 5.5 and 6.0.
RESULTS
Porin P1.B induces a Ca2+ transient in epithelial cells.
We determined whether Neisseria porin could also induce a Ca2+ flux in human epithelial cells. We chose to stimulate cells with porin at a concentration of 7 μg/ml because previous studies demonstrated that immune cells released Ca2+ when stimulated by porin at this concentration (26). Purified P1.B was added to A431 cells (derived from an epithelioid carcinoma) or T84 colorectal epithelial cells in 5 mM Ca2+ media, and changes in the concentration of cytosolic free Ca2+ were monitored (Fig. 1). Free Ca2+ levels increased at least two- to threefold as reported previously for immune cells (26) within 2 min after the addition of porin. This rise in free-Ca2+ levels was brief, and levels returned to baseline quickly. The Ca2+ response was greatly reduced when cells were exposed to porin in the presence of EGTA (which chelates extracellular Ca2+) and ATP, which inhibits the GTP-binding activity of porin and reduces its influence on Neisseria invasion (35). It has been suggested that extracellular Ca2+ passes directly through the porin channel and that the channel is closed after binding to ATP (26). Finally, Ca2+ levels in cells treated with medium alone were unaffected. These results demonstrate that purified porin induces a brief influx of Ca2+ into the cytosol of two human epithelial cell lines. Similar results were obtained when cells were exposed to crude membrane preparations derived from MS11A-307, a nonpiliated N. gonorrhoeae strain (data not shown).
Porin P1.B triggers Lamp1 exocytosis.
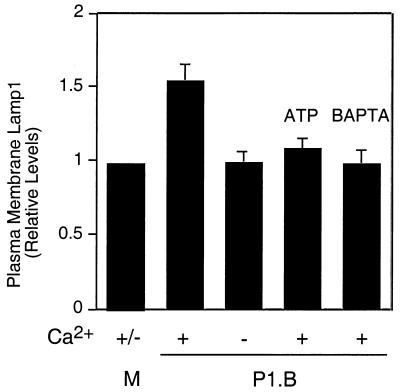
We next determined whether the porin-induced Ca2+ transient results in exocytosis of Lamp1 compartments. A431 cells were incubated with 7 μg of purified P1.B/ml in Ca2+-replete or -depleted medium, and plasma membrane Lamp1 levels were determined (Fig. 2). Membrane Lamp1 levels for cells treated with porin rose, but only when Ca2+ was present in the medium. Increased Lamp1 levels were observed within 5 min after addition of porin. Surface Lamp1 levels for cells exposed to medium alone or to porin preincubated with ATP were unchanged. Plasma membrane Lamp1 levels for cells preloaded with BAPTA-AM (which binds cytosolic free Ca2+) and subsequently exposed to porin were also unchanged. These results indicate that porin triggers Lamp1 exocytosis and that this event is due to the P1.B-induced Ca2+ transient. Similar results were obtained when cells were exposed to crude membrane preparations from P1.B-containing MS11A-307 (data not shown). Thus, Lamp1 exocytosis can be induced by porin.
FIG. 2.
Porin triggers exocytosis of Lamp1 compartments. A431 cultures were incubated for 5 min at 37°C with medium alone (M) or with P1.B in the presence (+) or absence (−) of Ca2+. As indicated, certain cultures were stimulated with P1.B preincubated with ATP. Other cultures were preloaded with BAPTA-AM, a membrane-permeable Ca2+ chelator, before addition of porin. Plasma membrane Lamp1 levels were determined by an antibody-binding assay as described in Materials and Methods (relative surface Lamp1 level for P1.B-infected cultures, 1.59 ± 0.37; P < 0.05; paired Student's t test).
Porin-induced Ca2+ flux does not trigger lysosome exocytosis.
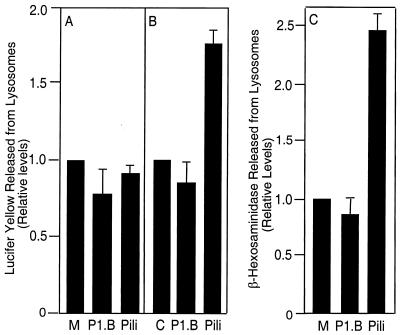
Lamp1 is found in both lysosomes and endosomes. We sought to determine whether the Lamp1 that is newly exposed on the cell surface by the porin-induced Ca2+ transient was lysosomal in origin. Lysosomes in A431 cells were loaded with the fluorescent dye LY for 20 h and chased with LY-free medium for 2 h. Cells were then incubated with 7 μg of porin/ml or 20 μg of pili/ml in Ca2+-replete media for 5 or 15 min, and the amounts of LY released into the medium by these cultures were determined. Porin did not induce lysosome exocytosis, as cultures treated with porin did not release LY into the medium. Indeed, there was a noticeable reduction in LY release by porin-treated cultures compared to that by cultures treated with medium alone (Fig. 3A and B). In agreement with our previous results, pili induced LY release only after 15 min of incubation (2).
FIG. 3.
Effect of porin on lysosome exocytosis. Lysosome exocytosis was determined by the LY (A and B) and β-hexosaminidase (C) release assays. Cultures were incubated with medium alone (M), 7 μg of porin (P1.B)/ml, or 20 μg of purified pili/ml for 5 (A) or 15 min (B and C). Experiments were performed in Ca2+-replete media. Relative LY level in the medium of cultures exposed to pili for 15 min was 1.75 ± 0.11 (P < 0.03; paired Student's t test); relative β-hexosaminidase level in the medium exposed to pili was 2.45 ± 0.2 (P < 0.05; paired Student's t test).
The ability of P1.B to induce lysosome exocytosis was also assessed by monitoring β-hexosaminidase activity in culture supernatants. β-Hexosaminidase is present only in lysosomes; high enzyme activity in the supernatants is indicative of lysosome exocytosis (13). As described above, A431 cultures were incubated for 15 min with 7 μg of purified P1.B/ml or 20 μg of purified pili/ml as a positive control in the presence of extracellular Ca2+. Supernatants were then examined for β-hexosaminidase activity (Fig. 3C). As shown earlier, β-hexosaminidase activity was higher in supernatants from cultures treated with pili (2). In contrast, enzyme activity in supernatants from untreated cultures was similar to that in supernatants from cultures treated with P1.B. Together these data indicate that the porin-induced Ca2+ flux does not trigger lysosome exocytosis.
Porin-induced Ca2+ flux stimulates endosome exocytosis.
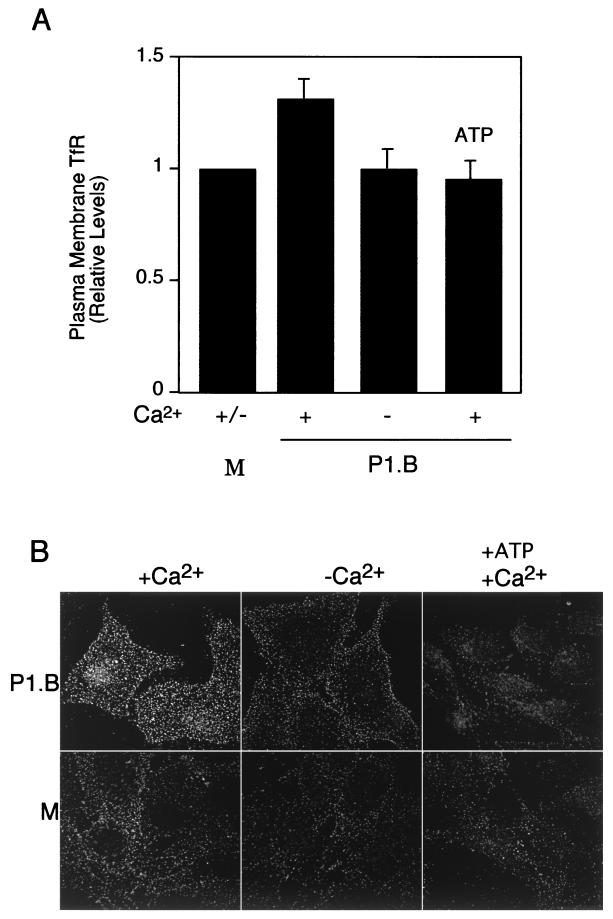
Endosomes destined for lysosomes also contain Lamp1. The early increase in Lamp1 levels on the plasma membrane upon cellular exposure to porin (shown in Fig. 2) may therefore be derived from endosomes. We determined whether porin induced endosome exocytosis. A431 cells were exposed to 7 μg of purified P1.B/ml for 5 min in Ca2+-replete or Ca2+-depleted medium, and the levels of surface TfR were determined (Fig. 4). TfR cycles between early endosomes and the plasma membrane (12); an increase in surface TfR levels upon exposure to porin would be indicative of endosome exocytosis. Under Ca2+-replete conditions, levels of surface TfR in cultures treated with porin were higher than those observed in cultures incubated with medium alone. In contrast, surface TfR levels in cultures incubated with porin plus ATP were similar to those in cultures incubated with medium alone. These results suggest that the porin-induced Ca2+ flux causes a rapid exocytosis of TfR-containing endosomes to the cell surface.
FIG. 4.
Porin-induced Ca2+ influx results in increased levels of plasma membrane TfR. A431 cultures were incubated for 5 min at 37°C with Ca2+-containing medium alone (M) or with P1.B in Ca2+-replete (+) or Ca2+-depleted (−) medium. Certain cultures, as indicated, were exposed to P1.B preincubated with 0.1 mM ATP. (A) Plasma membrane TfR levels were determined by means of an antibody-binding assay as described in Materials and Methods. Plasma membrane TfR levels in porin-treated cultures were normalized to those for cultures exposed to medium alone. Relative TfR level in cultures treated with porin in Ca2+-replete medium, 1.33 ± 0.2 (P < 0.01; paired Student's t test). (B) TfR on the plasma membrane of A431 cells was detected by immunofluorescence microscopy as described in Materials and Methods.
The redistribution of TfR to the plasma membrane was also visualized by immunofluorescence deconvolution microscopy (Fig. 4B). A431 cells were treated with porin for 5 min, and the cultures were quickly chilled to 4°C on ice. Under these conditions, the cells are alive and their membranes are intact but all endocytotic processes are terminated. Cells were subsequently fixed while the temperature was maintained at 4°C. Cells treated with porin in Ca2+-replete medium had significantly higher levels of surface TfR than control cells treated with medium alone. Cells exposed to porin in Ca2+-depleted medium and cells exposed to porin preincubated with ATP had only basal levels of surface TfR. Cells treated with ATP alone, in Ca2+-replete or Ca2+-depleted medium, also displayed only basal levels of surface TfR. Finally, signals were not detected on cells probed solely with either a primary or secondary antibody. These observations lend additional support to our previous findings that porin induces endosome exocytosis.
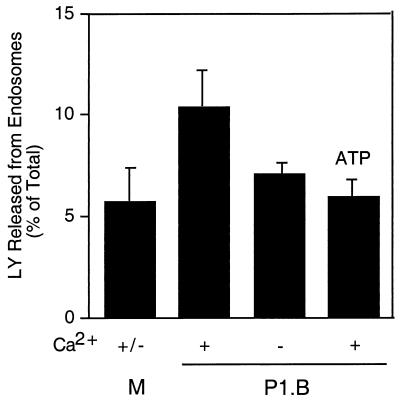
We also examined endosome exocytosis by measuring the release of LY from this compartment. Cells incubated with LY for 60 min at 16 to 18°C accumulate dye in endosomes but not lysosomes (11, 14). Endosomes in A431 cells were loaded with LY and then treated with 7 μg of purified P1.B/ml for 5 min. The amount of LY released into the culture medium was determined (Fig. 5). Cultures treated with porin in Ca2+-replete medium released more LY into the supernatant than cultures treated with medium alone. In contrast, cultures treated with porin in Ca2+-depleted medium released only basal levels of LY, as did cultures treated with porin and ATP. Taken together, results from these experiments indicate that Neisseria porin P1.B induces endosome exocytosis.
FIG. 5.
LY is released from endosomes upon exposure of cells to porin. LY was loaded into the endosomes of A431 cells as described in Materials and Methods. Cultures were stimulated for 5 min at 18°C with Ca2+-replete (+) or Ca2+-depleted (−) medium (M) or with medium containing P1.B. Certain cultures, as indicated, were stimulated with P1.B that had been preincubated with 0.1 mM ATP. LY in the medium was monitored by determining the fluorescence of the samples (excitation wavelength, 485 nm; emission wavelength, 538 nm). The amount of LY released into the medium upon exposure to P1.B was expressed as the percentage of total LY taken up by cells in control wells (LY level in cultures stimulated with P1.B in Ca2+-replete medium, 10.36 ± 1.88; P < 0.01, paired Student's t test).
Lamp1 exposed on the membrane by the porin-induced Ca2+ transient is cleaved by IgAP.
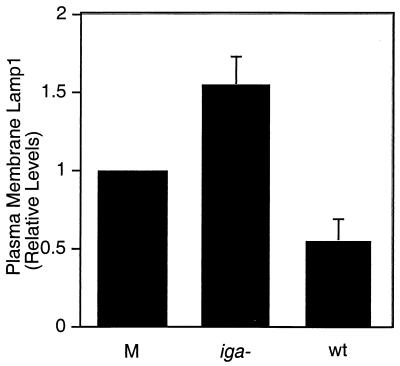
Finally, we determined whether Lamp1 derived from exocytosed endosomes can be cleaved by IgAP. A431 cells were infected with live MS11A, wild type (wt) or iga mutant, at 37°C for 5 min, and plasma membrane Lamp1 levels were determined (Fig. 6). The iga mutant has a null mutation in the gene encoding the IgAP and produces no detectable protease activity (17). Cultures infected with wt MS11A Had lower levels of surface Lamp1 than those incubated with the medium alone. In contrast, cultures infected with the isogenic iga mutant had the highest levels of plasma membrane Lamp1. Similar results were obtained when cells were incubated with the respective CMPs (data not shown). The increase in Lamp1 levels in these cultures reflects the redistribution of Lamp1 to the cell surface by the exocytic events discussed above, as well as the inability of the iga mutant to cleave surface Lamp1. These results demonstrate that plasma membrane Lamp1 can be cleaved by IgAP secreted shortly after infection.
FIG. 6.
Neisseria IgAP cleaves plasma membrane Lamp1 shortly after infection. A431 cultures were infected with live MS11A, iga mutant or wt, or were incubated in medium alone (M) at 37°C for 5 min and then chilled on ice. Plasma membrane Lamp1 levels were determined by an antibody-binding assay as described in Materials and Methods. Numbers are relative to those for controls stimulated with Ca2+-containing medium (relative Lamp1 levels: 1.52 ± 0.15 for the iga mutant and 0.512 ± 0.12 for wt; P < 0.03; paired Student's t test).
DISCUSSION
The Neisseria type IV pilus triggers the release of free Ca2+ from intracellular stores and a transient rise in cytosolic Ca2+ concentration within epithelial cells (2, 19). This, in turn, induces lysosome exocytosis and exposes lysosomal Lamp1 on the plasma membrane for cleavage by IgAP (2). We have tested the hypothesis that neisserial porin would also trigger a Ca2+ flux in epithelial cells and that this transient would induce Lamp1 exocytosis. In this report, we present evidence that the porin P1.B triggers a transient rise in cytosolic Ca2+ levels in two human epithelial cell lines. The porin-induced Ca2+ transient is caused by the influx of extracellular Ca2+, as demonstrated previously for phagocytes (26). Like the pilus-induced Ca2+ transient, the porin-induced flux results in a redistribution of Lamp1 to the cell surface. Unlike the previous event, the Lamp1 newly exposed on the cell surface by the porin-induced Ca2+ transient is derived from endosomes, not lysosomes.
Cells whose lysosomes were loaded with LY did not release the fluorescent dye into the medium when stimulated with porin for 5 min. Indeed, these cells released less LY than control cells treated with medium alone (Fig. 3A). Our results are in agreement with earlier studies showing that Neisseria porin inhibits the degranulation of primary and secondary granules in neutrophils (5, 15) and phagocytes (21).
Cells treated with porin for 5 min did, however, show a transient rise in plasma membrane Lamp1 levels (Fig. 2). If the newly exposed Lamp1 is not derived from lysosomes, then what is its origin? The source is suggested by a previous report showing that a fraction of newly synthesized Lamp1 cycles past the plasma membrane through a series of endocytic compartments en route to lysosomes (1). The newly exposed plasma membrane Lamp1 is likely derived from these endosomes, which had been stimulated to exocytose by the porin-induced Ca2+ flux. Strongly supporting this hypothesis are our data demonstrating that plasma membrane TfR levels in cultures treated by porin are also increased.
So far, porin- and pilus-induced Ca2+ fluxes have been studied mainly with purified protein complexes or membrane preparations containing these complexes. The porin-induced Ca2+ transient occurs within 2 min after exposure to purified P1.B (Fig. 3) (26), and the pilus-induced Ca2+ transient occurs after 10 min of exposure to purified pili (19). The timing of these Ca2+ transients is identical when cells are incubated with membrane preparations containing these complexes (Fig. 1) (2). It will be interesting to determine the nature of the pilus- and porin-induced transients during infection by live bacteria.
The Ca2+ fluxes induced by pili and porin are likely to affect other cellular pathways besides Lamp1 trafficking. Ca2+ is an important second messenger in eukaryotic cells (4, 28), and changes in the cytosolic levels of free Ca2+ regulate numerous cellular processes. In addition, the types of cellular responses differ depending on cytosolic Ca2+ levels, duration of the Ca2+ transient, and origin of Ca2+ (24, 28, 34). The pilus- and porin-induced Ca2+ fluxes may therefore affect overlapping but perhaps not identical sets of cellular signaling pathways.
Influx of Ca2+ into the cytosol of fibroblasts has been demonstrated to accelerate endosome recycling (25). The porin-induced Ca2+ influx may trigger a similar response in human epithelial cells, as endocytosis in porin-treated A431 cells is accelerated (P. Ayala and M. So, unpublished data). The pilus-induced Ca2+ is reported to be derived from the endoplasmic reticulum (ER) (19). Release of Ca2+ from the ER has been shown to trigger lysosome exocytosis (9, 30). The pilus- and porin-induced Ca2+ transients that we observed are consistent with these findings.
The mechanisms by which pili and porin trigger distinct exocytic events are unknown. Many proteins important to endocytic and secretory pathways have been identified, and continued analysis of their activities in targeting, docking, priming, and membrane fusion will yield valuable information in the near future (39).
In summary, the Neisseria pilus and porin complexes are able to modulate Ca2+ levels in human epithelial and monocytic cells (2, 19, 26). In epithelial cells, these Ca2+ transients quickly trigger the exocytosis of both lysosomes and endosomes. These exocytic events cause the redistribution of Lamp1 to the cell surface, where it can be cleaved by IgAP secreted by colonizing bacteria. We have shown previously that Lamp1 cleavage by IgAP leads to lysosome modification and promotion of intracellular survival of the bacteria. It will be interesting to determine whether there are other consequences associated with endosome and lysosome exocytosis triggered by pathogenic Neisseria.
Acknowledgments
We thank Shaun Lee and Dustin Higashi for their creative input, Aurelie Snyder, from the Microscopy Core Facility of the department of Molecular Microbiology and Immunology, for her technical support. We also thank members of the lab for their careful reading of the manuscript.
This work was supported in part by NIH grant RO1 AI32493 awarded to M. So and in part by an Oregon Health Sciences University Tartar Foundation Fellowship awarded to B. Vasquez.
Editor: E. I. Tuomanen
REFERENCES
- 1.Akasaki, K., A. Michihara, K. Mibuka, Y. Fujiwara, and H. Tsuji. 1995. Biosynthetic transport of a major lysosomal membrane glycoprotein, lamp-1: convergence of biosynthetic and endocytic pathways occurs at three distinctive points. Exp. Cell Res. 220:464-473. [DOI] [PubMed] [Google Scholar]
- 2.Ayala, B. P., B. Vasquez, S. Clary, J. A. Tainer, K. Rodland, and M. So. 2001. The pilus-induced Ca2+ flux triggers lysosome exocytosis and increases the amount of Lamp1 accessible to Neisseria IgA1 protease. Cell. Microbiol. 3:265-275. [DOI] [PubMed] [Google Scholar]
- 3.Ayala, P., L. Lin, S. Hopper, M. Fukuda, and M. So. 1998. Infection of epithelial cells by pathogenic neisseriae reduces the levels of multiple lysosomal constituents. Infect. Immun. 66:5001-5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berridge, M., P. Lipp, and M. Bootman. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell. Biol. 1:11-21. [DOI] [PubMed] [Google Scholar]
- 5.Bjerknes, R., H. K. Guttormsen, C. O. Solberg, and L. M. Wetzler. 1995. Neisserial porins inhibit human neutrophil actin polymerization, degranulation, opsonin receptor expression, and phagocytosis but prime the neutrophils to increase their oxidative burst. Infect. Immun. 63:160-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnah, R. A., S. W. Lee, B. Vasquez, C. A. Enns, and M. So. 2000. Alteration of epithelial cell transferrin-homeostasis by Neisseria meningitidis and Neisseria gonorrhoeae. Cell. Microbiol. 2:1-13. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson, S., and M. Fukuda. 1992. The lysosomal membrane glycoprotein lamp-1 is transported to lysosomes by two alternative pathways. Arch. Biochem. Biophys. 296:630-639. [DOI] [PubMed] [Google Scholar]
- 8.Carlsson, S. R., J. Roth, F. Piller, and M. Fukuda. 1988. Isolation and characterization of human lysosomal membrane glycoproteins, h-lamp1 and h-lamp-2. J. Biol. Chem. 263:18911-18919. [PubMed] [Google Scholar]
- 9.Coorssen, R., H. Schmith, and W. Almers. 1996. Ca2+-triggers massive exocytosis in Chinese hamster ovary cells. EMBO J. 15:3787-3791. [PMC free article] [PubMed] [Google Scholar]
- 10.Cowan, S., T. Schirmer, G. Rummel, M. Steiert, R. Ghosh, R. A. Pauptit, J. N. Jansonius, and J. P. Rosenbusch. 1992. Crystal structures explain functional properties of two E. coli porins. Nature 358:727-733. [DOI] [PubMed] [Google Scholar]
- 11.Dunn, W., A. Hubbard, and N. Aronson, Jr. 1980. Low temperature selectively inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused rat liver. J. Biol. Chem. 255:5971-5978. [PubMed] [Google Scholar]
- 12.Goldstein, J. L., M. S. Brown, R. G. W. Anderson, D. W. Russell, and W. J. Schneider. 1985. Receptor-mediated endocytosis: concepts emerging from the LDL receptor. Syst. Annu. Rev. Cell. Dev. Biol. 1:1-39. [DOI] [PubMed] [Google Scholar]
- 13.Green, S., K. Zimmer, G. Griffiths, and I. Mellman. 1987. Kinetics of intracellular transport and sorting of lysosomal membrane and plasma membrane proteins. J. Cell Biol. 105:1227-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffiths, G., B. Hoflack, K. Simons, I. Mellman, and S. Kornfeld. 1988. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell 52:329-341. [DOI] [PubMed] [Google Scholar]
- 15.Haines, K. A., L. Yeh, M. S. Blake, P. Cristello, H. Korchk, and G. Weissmann. 1988. Protein I, a translocatable ion channel from Neisseria gonorrhoeae, selectively inhibits exocytosis from human neutrophils without inhibiting O2− generation. J. Biol. Chem. 263:945-951. [PubMed] [Google Scholar]
- 16.Hauck, C. R., and T. Meyer. 1997. The lysosomal/phagosomal membrane protein h-lamp1 is a target of the IgA1 protease of Neisseria gonorrhoeae. FEBS Lett. 405:86-90. [DOI] [PubMed] [Google Scholar]
- 17.Hopper, S., B. Vasquez, A. Merz, S. Clary, J. S. Wilbur, and M. So. 2000. Effects of the immunoglobulin A1 protease on Neisseria gonorrhoeae trafficking across polarized T84 epithelial monolayers. Infect. Immun. 68:906-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopper, S., J. S. Wilbur, B. L. Vasquez, J. Larson, S. Clary, I. J. Mehr, H. S. Seifert, and M. So. 2000. Isolation of Neisseria gonorrhoeae mutants that show enhanced trafficking across polarized T84 epithelial monolayers. Infect. Immun. 68:896-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Källstrom, H., M. S. Islam, P. O. Berggren, and A. B. Jonsson. 1998. Cell signaling by the type IV pili of pathogenic Neisseria. J. Biol. Chem. 273:21777-21782. [DOI] [PubMed] [Google Scholar]
- 20.Lin, L., P. Ayala, J. Larson, M. Mulks, M. Fukuda, S. R. Carlsson, C. Enns, and M. So. 1997. The Neisseria type 2 IgA1 protease cleaves LAMP1 and promotes survival of bacteria within epithelial cells. Mol. Microbiol. 24:1083-1094. [DOI] [PubMed] [Google Scholar]
- 21.Lorenzen, D., D. Gunther, J. Pandit, T. Rudel, E. Brandt, and T. F. Meyer. 2000. Neisseria gonorrhoeae porin modifies the oxidative burst of human professional phagocytes. Infect. Immun. 68:6215-6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merz, A. J., D. B. Rifenbery, C. G. Arvidson, and M. So. 1996. Traversal of a polarized epithelium by pathogenic neisseriae: facilitation by type IV pili and maintenance of epithelial barrier function. Mol. Med. 2:745-754. [PMC free article] [PubMed] [Google Scholar]
- 23.Merz, A. J., and M. So. 1997. Attachment of piliated, Opa− and Opc− gonococci and meningococci to epithelial cells elicits cortical actin rearrangements and clustering of tyrosine-phosphorylated proteins. Infect. Immun. 65:4341-4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montero, M., M. T. Alonso, E. Carnicero, I. Ibañez-Cuchillo, A. Albillos, A. G. Garcia, J. Garcia-Sancho, and J. Alvarez. 2000. Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat. Cell Biol. 2:57-61. [DOI] [PubMed] [Google Scholar]
- 25.Morimoto, T., S. Popov, K. M. Buckley, and M. M. Poo. 1995. Calcium-dependent transmitter secretion from fibroblasts: modulation by synaptotagmin I. Neuron 15:689-696. [DOI] [PubMed] [Google Scholar]
- 26.Müller, A., D. Gunther, F. Dux, M. Naumann, T. F. Meyer, and T. Rudel. 1999. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. EMBO J. 18:339-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parge, H. E., S. L. Bernstein, C. D. Deal, D. E. McRee, D. Christensen, M. A. Capozza, B. W. Kays, T. M. Fieser, D. Draper, M. So, et al. 1990. Biochemical purification and crystallographic characterization of the fiber-forming protein pilin from Neisseria gonorrhoeae. J. Biol. Chem. 265:2278-2285. [PubMed] [Google Scholar]
- 28.Petersen, O., D. Burdakov, and A. Tepikin. 1999. Polarity in intracellular calcium signaling. Bioessays 21:851-860. [DOI] [PubMed] [Google Scholar]
- 29.Plaut, A. G., J. V. Gilbert, M. S. Artenstein, and J. D. Capra. 1975. Neisseria gonorrhoeae and Neisseria meningitidis: extracellular enzyme cleaves human immunoglobulin A. Science 190:1103-1105. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez, A., P. Webster, J. Ortego, and N. W. Andrews. 1997. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J. Cell Biol. 137:93-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudel, T., A. Schmid, R. Benz, H. A. Kolb, F. Lang, and T. F. Meyer. 1996. Modulation of Neisseria porin (PorB) by cytosolic ATP/GTP of target cells: parallels between pathogen accommodation and mitochondrial endosymbiosis. Cell 85:391-402. [DOI] [PubMed] [Google Scholar]
- 32.Segal, E., P. Hagblom, H. S. Seifert, and M. So. 1986. Antigenic variation of gonococcal pilus involves assembly of separated silent gene segments. Proc. Natl. Acad. Sci. USA 83:2177-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tam, M., T. Buchanan, E. Sandstrom, K. Holmes, J. Knapp, A. Siadak, and R. Nowinski. 1982. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect. Immun. 36:1042-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tse, F., and A. Tse. 1999. Regulation of exocytosis via release of calcium from intracellular stores. Bioessays 21:861-865. [DOI] [PubMed] [Google Scholar]
- 35.van Putten, J. P., T. D. Duensing, and J. Carlson. 1998. Gonococcal invasion of epithelial cells driven by P.IA, a bacterial ion channel with GTP binding properties. J. Exp. Med. 188:941-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weel, J., and J. van Putten. 1991. Fate of the major outer membrane protein P.IA in early and late events of gonococcal infection of epithelial cells. Res. Microbiol. 142:985-993. [DOI] [PubMed] [Google Scholar]
- 37.Weiss, M. S., and G. E. Schulz. 1993. Porin conformation in the absence of calcium. Refined structure at 2.5 Å resolution. J. Mol. Biol. 231:817-824. [DOI] [PubMed] [Google Scholar]
- 38.Wetzler, L. M., M. S. Blake, K. Barry, and E. C. Gotschlich. 1992. Gonococcal porin vaccine evaluation: comparison of Por proteosomes, liposomes, and blebs isolated from rmp deletion mutants. J. Infect. Dis. 166:551-555. [DOI] [PubMed] [Google Scholar]
- 39.Zimmerberg, J., P. S. Blank, I. Kolosova, M. S. Cho, M. Tahara, and J. R. Coorssen. 2000. A stage-specific preparation to study the Ca2+-triggered fusion steps of exocytosis: rationale and perspectives. Biochimie 82:303-314. [DOI] [PubMed] [Google Scholar]