Abstract
Different “professional” antigen-presenting cells (APC) have unique characteristics that favor or restrict presentation of microbial antigens to T cells, depending on the organism. Cryptococcus neoformans is a pathogenic yeast that presents unique challenges to APC, including its large size, its rigid cell wall, and its ability to stimulate T cells as a mitogen. T-cell proliferation in response to the C. neoformans mitogen (CnM) requires phagocytosis and processing of the organisms by accessory cells prior to presentation of CnM to T cells. Because of the requirement for uptake of the organism and more limited costimulatory requirements of mitogens, macrophages might be the most likely cellular source for the accessory cell. However, the present study demonstrates that a transiently adherent cell that was CD3−, CD14−, CD19−, CD56−, HLA-DR+, and CD83+ with a dendritic morphology, rather than monocyte-derived or tissue (alveolar) macrophages, was the most efficient APC for presentation of CnM. A large number of these cells bound and internalized the organism, and only a small number of dendritic cells were required for presentation of the mitogen to T cells. Further, the mannose receptor and Fcγ receptor II were required for presentation of C. neoformans, as blocking either of these receptors abrogated both uptake of C. neoformans and lymphocyte proliferation in response to CnM. These studies demonstrate the surprising fact that dendritic cells are the most efficient accessory cells for CnM.
Cryptococcus neoformans is an encapsulated yeast that causes pulmonary, meningeal, and disseminated infections in patients with defective T-cell-mediated immunity, such as those with AIDS, hematological malignancies, and organ transplants (9). There is also substantial experimental evidence that T cells are important in cryptococcosis (10, 31, 48). These T cells respond to cognate cryptococcal antigens (30, 44); however, we have recently shown that C. neoformans also possesses a T-cell mitogen. The evidence for a C. neoformans mitogen (CnM) comes from the ability of CnM to stimulate the proliferation of naive T cells, the ability of allogeneic accessory cells to provide costimulatory activity, and the precursor frequency of responding T cells (49, 52). Similar to some bacterial mitogens (2, 41) and cognate peptide antigens (20), uptake and processing of C. neoformans by antigen-presenting cells (APC) was required prior to presentation of CnM to T cells (74).
B cells, macrophages, and dendritic cells (DC) are all “professional” APC. Each of these APC have unique characteristics that favor or restrict presentation of microbial products to T cells, depending on the organism and the nature of the infection. C. neoformans presents unique challenges to APC that include its large size, its rigid cell wall, and its ability to stimulate T cells as a mitogen. Thus, the APC for C. neoformans would have to have robust phagocytic and processing capabilities but might compromise its costimulatory activity because of the potent immunostimulatory activity of a mitogen. With this in mind, macrophages ought to be the most efficient presenters for C. neoformans, as hypothesized for other microbes (34).
It is clear that many cell types can function as APC. Further, within these cell types, subsets of cells exist. For example, DC can be derived from myeloid or lymphoid precursors (18, 24, 62, 71). Indeed, in umbilical cord blood there is a prevalence of lymphoid DC, which are functionally distinct from myeloid DC (68). Since we had previously demonstrated that cord blood contains the APC required to support T-cell proliferation, this raised the possibility that nonmyeloid subsets of cells could be the responsible APC. Thus, we used peripheral blood, which serves as a source of many of these APC, and attempted to identify the most efficient APC for the T-cell response to C. neoformans.
To determine the APC responsible for presentation of CnM, sequential purification steps were performed on human peripheral blood mononuclear cells (PBMC). First, the cells were characterized by their various abilities to adhere to plastic. The response of transiently adherent cells (TAC) was compared to the response of alveolar macrophages (AM). The phenotype of the APC was determined by sequential immunomagnetic separation and confirmatory flow cytometric analysis. The morphology of APC and the number of APC that had bound and internalized C. neoformans were determined by light and electron microscopy. Finally, the receptor used for uptake of C. neoformans was examined by employing blocking antibodies.
MATERIALS AND METHODS
Preparation of C. neoformans.
C. neoformans strain 67 (ATCC 52817; acapsular mutant) (33) and strain 613 (ATCC 36556; lightly encapsulated; serotype D) (40) were obtained from the American Type Culture Collection (Manassas, Va.). The organisms were maintained as previously described (51) on Sabouraud's slants (Difco, Detroit, Mich.) and passaged to fresh slants monthly. The organisms were killed by autoclaving them at 121°C for 15 min and were stored at 4°C for up to 3 months. To avoid [3H]thymidine ([3H]TdR) incorporation into growing C. neoformans cells, killed organisms, which had previously been shown to elicit responses similar to those elicited by live organisms (50), were used for all studies. Opsonins (other than normal human sera during the proliferation assay) were not used.
Isolation of PBMC and AM.
Human peripheral blood was obtained by venipuncture from healthy adults. The blood was anticoagulated by adding 10 U of heparin (Organon-Teknika-Cappel, Scarborough, Ontario, Canada)/ml. The PBMC were purified by centrifugation (800 × g for 20 min) on a Ficoll-Hypaque density gradient (Lymphoprep; C-six Diagnostics, Woodbridge, Ontario, Canada). The PBMC were harvested and washed three times in Hanks' balanced salt solution (Gibco, Burlington, Ontario, Canada) and then counted and suspended in complete medium containing RPMI 1640 medium (Gibco), 5% heat-inactivated pooled human AB serum (BioWhitaker, Walkersville, Md.), 2 mM l-glutamine (Gibco), 100 U of penicillin/ml, 100 μg of streptomycin/ml, 0.2 μg of amphotericin B/ml (Gibco), 1 mM sodium pyruvate (Gibco), and 0.1 mM nonessential amino acids (Gibco).
AM were obtained as previously described (12). Briefly, patients undergoing bronchoscopy who had normal oxygen saturation and no or minimal pulmonary pathology (i.e., bronchoscopy for mild hemoptysis or a coin lesion) were used as the sources of AM. Following appropriate consent and anesthesia, a bronchofiberscope was introduced and wedged in one of the subsegments of the right middle lobe or lingula that was contralateral to any pathology. Four aliquots of 30 ml of normal saline were introduced and aspirated, and the fluid was immediately placed on ice. The fluid was filtered through a 200-μm-pore-size sterile nylon filter to remove debris. The cells were then washed three times in ice-cold Hanks' balanced salt solution by centrifugation at 400 × g for 10 min at 4°C. The cells were finally resuspended in RPMI medium and counted. By Diff Quik staining, the cells were >90% macrophages. PMBC were obtained from the same patients as a source of T cells and for comparison to blood-derived APC.
T-cell isolation.
T lymphocytes were purified by nonadherence to plastic and rosetting to 2-aminoethylisothiouronium bromide (Sigma, St. Louis, Mo.)-treated sheep red blood cells (Cedarlane, Hornby, Ontario, Canada), followed by nylon wool nonadherence as previously described (65, 75).
APC isolation.
Persistently adherent cells (PAC) were obtained by incubating PBMC on 100-mm-diameter plastic tissue culture plates at 37°C in 5% CO2 in RPMI medium (69). After 2 h, the nonadherent cells were removed and the adherent monolayer was washed twice. The adherent cell population was then removed using a cell scraper (Sarstedt, Montreal, Quebec, Canada), counted, and resuspended in complete medium.
TAC were obtained by incubating PBMC on 100-mm-diameter plastis tissue culture plates for 2 h at 37°C in 5% CO2 in RPMI medium (78). Nonadherent cells were gently harvested, and the adherent cells were resuspended in RPMI medium and 0.1% human serum (BioWhitaker) overnight at 37°C in 5% CO2. Eighteen hours later, the TAC were removed by gentle washing and resuspended in complete medium.
Primary DC were obtained by depletion of cell populations from TAC. TAC were immunolabeled with iron-conjugated antibodies against CD3, CD14, CD19, and CD56 (Dynal, Uppsala, Sweden) according to the manufacturer's instructions. Antibodies and cells were incubated by rocking them at 4°C for 45 min. The cells were separated by exposing them to a magnetic field that separated antibody-bound from unbound cells. The cells were washed three times in this manner in phosphate-buffered saline-1% fetal bovine serum. To determine the efficiency of the depletion, the cells were immunolabeled with fluorescent antibodies or an isotype-matched fluorescent control antibody (Becton Dickinson, San Jose, Calif.), and examined by flow cytometry. In other experiments, DC were isolated by the MACS system (Militnyi Biotec, Gladbach, Germany). Briefly, transiently adherent cells were depleted of CD3, CD14, CD19, and/or CD56 cells according to the manufacturer's instructions using a BS depletion column (Militnyi Biotec). Successful depletion was confirmed by flow cytometry.
Flow cytometry.
Phenotypic analysis was preformed to determine the phenotypes of the isolated populations. The following antibodies were used for immunolabeling: anti-CD3-fluorescein isothiocyanate (FITC), anti-CD14-FITC, anti-CD19-FITC, anti-CD56-FITC, anti-HLA-DR-FITC (all from Becton Dickinson), and anti-CD83-phycoerythrin (Immunotech, Marseille, France). Flow cytometry was performed using a FACScan flow cytometer (Becton Dickinson) with an excitation frequency of 488 nm. At least 10,000 events per sample were analyzed.
Lymphocyte proliferation assays.
To assess lymphocyte proliferation, cells (2 × 105/well) were cultured in 96-well round-bottom tissue culture plates. C. neoformans (2 × 105 organisms/well) or 2.5 μg of concanavalin A (Sigma)/ml was used to stimulate the lymphocytes. All experiments were performed using C. neoformans strain 67 unless otherwise indicated. Cultures were incubated for 5, 7, or 9 days at 37°C in 5% CO2. [3H]TdR incorporation was determined by adding 1 μCi of [3H]TdR (ICN, Montreal, Quebec, Canada) to each well 16 h before the end of incubation. At the end of the incubation period, the cells were harvested on glass filters (Brandel Inc., Gaitherburg, Md.), and counts per minute were determined in a liquid scintillation counter (Beckman, Mississauga, Ontario, Canada).
To block mannose receptors on the surfaces of APC, the cells were incubated in the presence of 10 μg of anti-mannose receptor antibody (Pharmingen, Mississauga, Ontario, Canada)/ml, anti-Fcγ receptor I (FcγRI), anti-FcγRII, anti-C3biR, or isotype-matched antibody (Becton Dickinson).
Phagocytosis assays and light microscopy.
To determine the number of C. neoformans cells taken up by DC, phagocytosis assays were employed as previously described (39) with slight modifications. Briefly, DC were cultured on 24-well plastic tissue culture plates containing plastic 13-mm-diameter coverslips (Nunc, Naperville, Ill.) in complete medium. After 1 h, C. neoformans cells (106/well) were added to the DC. Eighteen hours later, the medium and unbound organisms were removed by washing the plates with phosphate-buffered saline, and the coverslips were air dried and fixed in methanol. The coverslips were then stained with Giemsa stain (ICN) and examined by light microscopy for the number of DC that had bound or internalized C. neoformans, as well as the number of C. neoformans organisms per cell. At least 200 cells were counted per monolayer. Focusing up and down on the cell and the presence of a vacuole surrounding the organisms were used to judge whether the cells were internalized. In preliminary experiments, this technique was as reliable as immunofluorescent labeling and flow cytometric analysis for bound and internalized organisms while having the advantage of being able to determine the morphology of cells.
Electron microscopy.
For scanning electron microscopy, DC were incubated with C. neoformans at an effector-to-target ratio of 1:20 at 37°C on Labtek chamberslides (Nunc). Eighteen hours later, the cells were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer. The fixed cells were then washed in buffer and dehydrated in graded concentrations of ethyl alcohol, dried to the critical point with CO2, and then coated with gold palladium. They were examined with a Hitachi S-450 scanning electron microscope.
For transmission electron microscopy, cells were incubated with C. neoformans in 15-ml conical tubes (Becton Dickinson) for 18 h at 37°C at an effector-to-target ratio of 1:20. The cells were fixed in 2.5% glutaraldehyde-0.1 M cacodylate buffer for 45 min and then washed twice in buffer and postfixed in 1% osmium tetroxide for 30 min. The cells were washed with buffer and then dehydrated through a graded series of ethanol. The pellet was embedded in Spurr resin (JBS, Dorval, Quebec, Canada). Ultrathin sections were cut with a diamond knife and counterstained with uranyl acetate and lead citrate. Sections (90 nm thick) were examined with a transmission electron microscope (Hitachi H7000) at an acceleration voltage of 75 kV.
Statistics.
Data are given as mean ± standard error of the mean (SEM). Each experiment was repeated with different donors on different days. [3H]TdR incorporation is expressed as the mean counts per minute ± SEM of quadruplicate wells. Statistical analysis was performed by one-way analysis of variance. For these tests, a P value of <0.05 was considered significant.
RESULTS
APC for C. neoformans are transiently adherent.
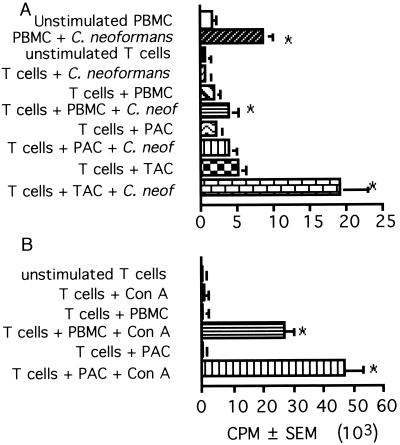
It was previously demonstrated that APC are required for T-cell responses to CnM (75) and that phagocytosis of C. neoformans is required for the APC to present CnM to T cells (73, 74). To establish the type of cell that is the APC, blood mononuclear cells were separated based on their adherence properties, which have been used by other investigators to separate populations of APC (35, 57, 69). In all experiments, APC populations were irradiated so that they would be unable to proliferate but still be capable of processing and presentation. The ability of cells that were persistently adherent to plastic to present CnM to T cells was examined. Since PAC are enriched for monocyte-derived macrophages, we were surprised to find that PAC were a poor source of APC compared to unseparated irradiated PBMC (Fig. 1A). These cells were functional, since they were potent APC for T-cell responses to the mitogenic lectin concanavalin A, and were more potent than irradiated PBMC (Fig. 1B). Cell surface labeling and flow cytometric analysis confirmed that the cells were enriched for CD14+ cells and partially depleted of CD19+ B cells and CD3+ T cells (Table 1), suggesting that CD14+ monocyte-derived macrophages were a poor source of APC for T-cell responses to CnM.
FIG. 1.
PAC are a poor source of APC for C. neoformans, while TAC are a potent source of APC. PBMC, T cells (2 × 105/well), T cells plus irradiated PBMC, T cells plus PAC (105/well), or T cells plus TAC (105/well) were stimulated with killed C. neoformans strain 67 (A), and T cells, T cells plus irradiated PBMC, or T cells plus PAC were stimulated with concanavalin A (Con A) (B). [3H]TdR incorporation was determined 7 days later if the cells were stimulated with C. neoformans and 3 days later if the cells were stimulated with concanavalin A. The experiment was repeated three times with similar results. ∗, P < 0.05 compared to the corresponding unstimulated group.
TABLE 1.
Phenotypes of cells isolated on the basis of adherence to plastic
| Phenotype | % of cells expressing the phenotype (± SEM)
|
||
|---|---|---|---|
| Unseparated PBMC | Persistently adherenta | Transiently adherenta | |
| CD14 | 9.7 ± 1.8 | 45.6 ± 4.9 | 15.9 ± 2.9 |
| CD19 | 10.8 ± 1.3 | 5.7 ± 2.1 | 14.9 ± 5.2 |
| CD3 | 71.2 ± 3.1 | 39.3 ± 5.6 | 18.1 ± 3.3 |
| CD56 | 11.4 ± 1.3 | 17.1 ± 0.6 | 11.4 ± 3.4 |
| HLA-DR | 26.0 ± 2.6 | 32.5 ± 18.4 | 60.0 ± 6.0 |
Mean of six experiments.
Previous studies had demonstrated that cells that were transiently adherent to plastic (adherent after a 2-h incubation but nonadherent after a further overnight incubation) contained a potent source of APC (35, 57, 69). Addition of TAC to T cells induced greater lymphocyte proliferation in response to CnM than either PBMC or PAC (Fig. 1A). This population contained fewer CD14+ cells and a greater percentage of major histocompatibility complex II-positive cells (Table 1) than the persistently adherent population. This observation suggested that another population of cells, other than monocyte-derived macrophages, were the most effective APC for CnM.
TAC are more effective than AM.
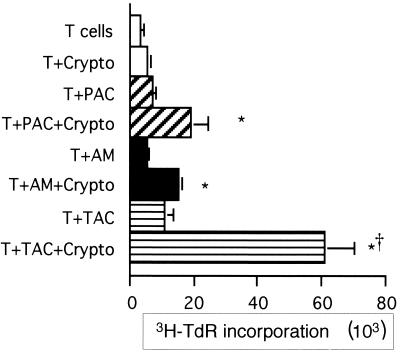
AM are likely to be the first tissue macrophages encountered by inhaled C. neoformans. We therefore considered the possibility that these tissue macrophages might provide a potent source of APC. Indeed, previous studies had suggested that AM were capable of supporting T-cell responses to C. neoformans (79). While AM were capable of providing APC function, the response of T cells to AM was significantly inferior to the response to TAC (Fig. 2) and similar to the response to PAC. Thus, TAC, rather than tissue (alveolar) macrophages, were the more efficient source of APC. We were interested to find that the increased accessory cell function occurred despite the slightly superior ability of AM to phagocytose C. neoformans (data not shown).
FIG. 2.
AM are not as efficient as TAC in presenting C. neoformans to T cells. T cells (2 × 105/well) were incubated alone or in the presence of irradiated PAC (105/well), AM (105/well), or TAC (105/well) in the presence or absence of killed C. neoformans strain 67 (Crypto). Lymphocyte proliferation was determined by [3H]TdR incorporation. The experiment was repeated four times with similar results. ∗, P < 0.05 compared to the corresponding unstimulated group; †, P < 0.05 compared to AM.
Depletion of cytolytic cells.
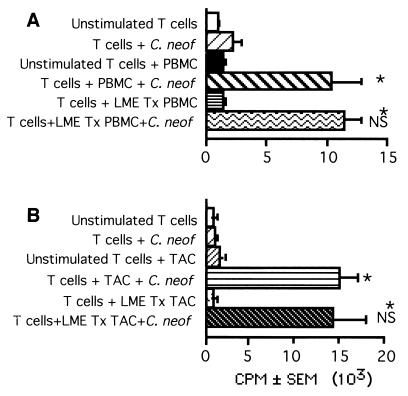
It had been previously demonstrated that the lysosomal compartment and serine proteases were required for processing of C. neoformans (74). This raised the possibility that a leucine methyl ester (LME)-sensitive compartment might be required for processing of C. neoformans. LME concentrates in the lysosome and causes osmotic swelling and eventual rupture of monocytes, macrophages, and natural killer (NK) cells but not DC (61, 76). When PBMC were treated with LME, there was depletion of the monocytes and NK cells such that the resultant population had <1% CD14+ or CD56+ cells. Treating PBMC with LME did not diminish their ability to function as APC for T-cell responses to C. neoformans (Fig. 3A), nor did it abrogate the response when TAC were used as the source of APC (Fig. 3B). Thus, despite the observation that an acidic lysosome is required, and that cathepsins are involved in processing of C. neoformans (74), an LME-sensitive compartment was not required, and the APC was not a monocyte-derived macrophage.
FIG. 3.
Lysis of LME-sensitive cells has no effect on T-cell proliferation in response to C. neoformans. T cells (2 × 105/well) were stimulated by killed C. neoformans strain 67 (C. neof; 2 × 105/well) in the presence or absence of LME-treated (LME Tx) irradiated PBMC (105/well) (A) or LME-treated TAC (105/well) (B). Lymphocyte proliferation was determined by [3H]TdR incorporation. The experiment was repeated twice with similar results. ∗, P < 0.05 compared to the corresponding unstimulated group; NS, not significantly different from cells not treated with LME.
Ability of TAC and PBMC to provide the accessory cell function for encapsulated strains.
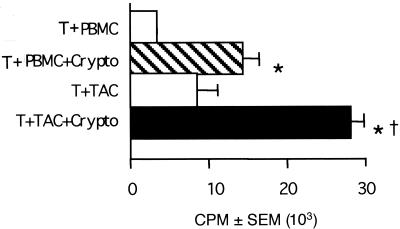
The capsule of C. neoformans is antiphagocytic, and therefore, we considered the possibility that while TAC might be the most effective source of APC for acapsular strains of C. neoformans, the increased demand for phagocytosis that was imposed by encapsulated strains would favor participation by monocyte-derived macrophages. The response of T cells to encapsulated C. neoformans in the presence of TAC was superior to the response in the presence of PBMC (Fig. 4), indicating that the most efficient source of APC for encapsulated C. neoformans was a TAC.
FIG. 4.
PBMC are not as efficient as TAC in presenting encapsulated C. neoformans to T cells. T cells (2 × 105/well) were incubated in the presence of irradiated PBMC (105/well) or TAC (105/well) in the presence or absence of killed C. neoformans strain 36556 (Crypto). Lymphocyte proliferation was assessed by [3H]TdR incorporation. The experiment was repeated with similar results. ∗, P < 0.05 compared to the corresponding unstimulated group; †, P < 0.05 compared to PBMC.
Sequential depletion of the major subsets of PBMC.
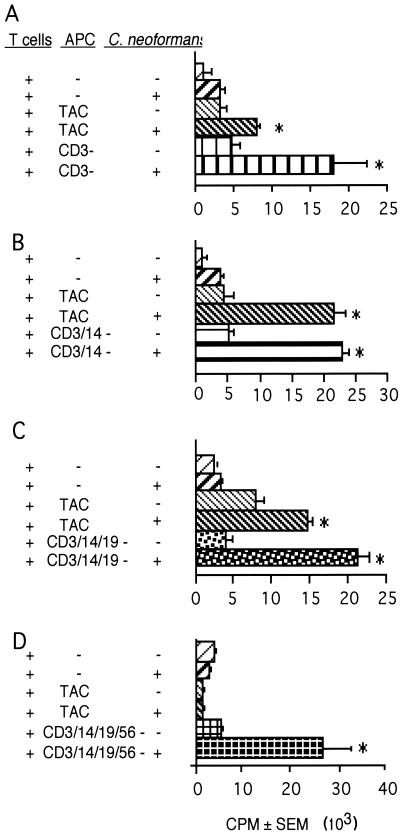
Since TAC possessed large numbers of CD3-, CD14-, CD19-, and CD56-positive cells (Table 1), sequential depletion was performed on this population to enrich or deplete the APC. As each separation was performed, the resultant depleted population was tested for its ability to present CnM to T lymphocytes. Depletion of CD3+ cells from the APC, which were the most prevalent population in TAC, did not diminish the ability of TAC to present C. neoformans to T cells (Fig. 5A).
FIG. 5.
Sequential depletion of TAC resulted in cell populations that could induce T-cell responses to C. neoformans. T cells were placed in culture with various populations of APC with (+) or without (−) killed C. neoformans strain 67. (A) TAC were depleted of CD3 cells (CD3−) (105 cells/well were added to 2 × 105 T cells/well). (B) TAC were depleted of CD3 and CD14 cells (5 × 104 cells/well were added to 2 × 105 T cells/well). (C) TAC were depleted of CD3, CD14, and CD19 cells (5 × 104 cells/well were added to 2 × 105 T cells/well). (D) TAC were depleted of CD3, CD14, CD19, and CD56 cells (2.5 × 104 cells/well were added to 2 × 105 T cells/well). Lymphocyte proliferation was determined by [3H]TdR incorporation. The experiment was repeated three times with similar results. ∗, P < 0.05 compared to the corresponding unstimulated population.
Monocytes are typically CD14+ and are an important APC in many systems (77). When CD14+ and CD3+ cells were depleted, there was no reduction of the cells' ability to support lymphocyte proliferation in response to C. neoformans (Fig. 5B). Additionally, B cells are potent APC for recall responses (11). When CD19+ cells were depleted in addition to depletion of CD14+ and CD3+ cells, it did not reduce lymphocyte proliferation (Fig. 5C).
NK cells were the remaining major phenotype in PBMC. Although NK cells are unlikely to be APC, they do have significant anticryptococcal activity (55). We considered the possibility that they might kill and therefore disrupt C. neoformans, allowing presentation by professional APC. It should be noted that as different cell populations were depleted, it became necessary to reduce the number of APC to reduce the autologous mixed lymphocyte reaction that occurred with the remaining cells. This number of TAC (2.5 × 104) was not sufficient to present C. neoformans to T cells, while the same number of depleted cells (CD3-, CD14-, CD19-, and CD56-depleted TAC) were fully capable of presentation of CnM (Fig. 5D).
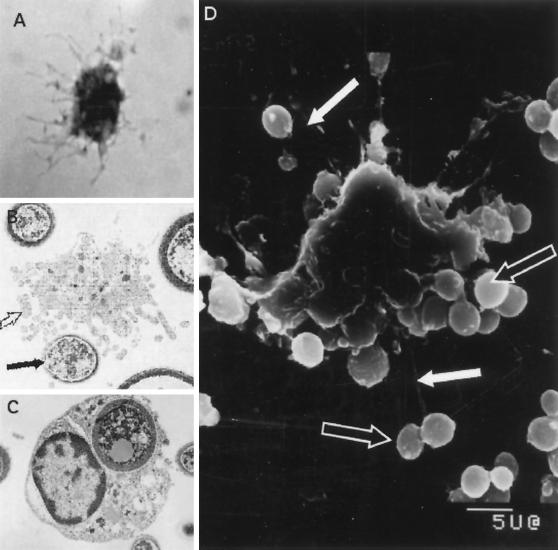
When examined by flow cytometry, the resultant cells were found to be substantially depleted of CD3-positive cells (2.4% ± 1.2%), CD14 cells (1.3% ± 1.8%), CD19 cells (2.0% ± 0.1%), and CD56 cells (2.2% ± 0.9%). Freshly isolated cells expressed HLA-DR (66% ± 5.5% of cells) and CD83 (40% ± 3.5% of cells) (n = 6 experiments). Further, consistent with previous observations (84), when these cells were cultured for 120 h, the percentage of cells that expressed HLA-DR increased to 90% ± 3.5% and the percentage of cells that expressed CD83 increased to 72% ± 13% (n = 4). The depleted cell population was also found to have a characteristic dendritic morphology (Fig. 6A). The cells were agranular and had large irregular or ovoid nuclei, and thin “dendritic” cytoplasmic processes were observed extending from the cell body. Further, these cells were very potent stimulators of a mixed lymphocyte reaction (data not shown). These data suggest that the most effective APC for CnM was a DC rather than a macrophage.
FIG. 6.
CD3-, CD14-, CD19-, and CD56-depleted TAC have morphological features of DC and bind and internalize C. neoformans. (A) Cells were placed in glass tissue culture chambers, and 18 h later the slides were air dried, stained with Diff Quik, and examined by light microscopy. (B to D) Cryptococci are bound and internalized by DC as shown by transmission and scanning electron microscopy. DC were put into culture at a 1:20 ratio with killed C. neoformans strain 67; 18 h later, the cells were fixed in 2.5% glutaraldehyde, processed as described in Materials and Methods, and examined by transmission or scanning electron microscopy. The open arrows indicate dendritic processes; the solid arrows indicate C. neoformans.
Although these studies suggested that a DC was responsible for presentation of C. neoformans, primary DC are felt to be minimally phagocytic (42, 63), and previous studies had indicated that C. neoformans must be phagocytosed to stimulate T cells (73, 74). Studies were therefore performed to examine the interaction between DC and C. neoformans. Scanning electron microscopy demonstrated that DC were capable of interacting with multiple organisms both on the body of the cell and by interactions with the dendrites. The surface contours of the DC suggested that organisms had been internalized (Fig. 6D). To characterize the features of the cells that had taken up C. neoformans, transmission electron microscopy was performed (Fig. 6B and C). This confirmed that the cryptococcal organisms were internalized (Fig. 6C). The cells possessed ovoid or large irregular nuclei, many mitochondria, variable numbers of lysosomes, few cytoplasmic granules, and numerous vesicles, which had previously been described for DC (36, 84). Cells that were binding C. neoformans showed dendritic processes (Fig. 6B). The DC appeared to undergo a morphological change following phagocytosis of C. neoformans involving the loss of dendrites and transformation to a more rounded phenotype. This is consistent with a very rapid shape change that DC undergo upon stimulation (25). The internalized organisms were seen within membrane-bound vesicles consistent with lysosomes (Fig. 6C). Birbeck's granules were not identified.
Number of DC required for T-cell proliferation and number of DC with bound and internalized C. neoformans.
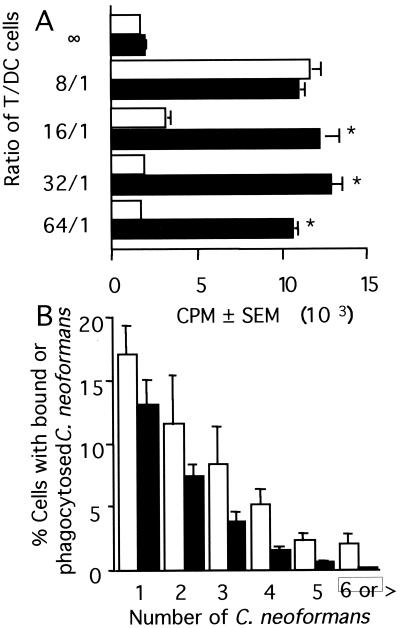
These experiments had suggested that a small number of the depleted TAC were capable of presenting C. neoformans to T cells. In experiments to examine the number of DC necessary to stimulate T cells, <3.1 × 103 DC/well (ratio of DC to T cells, <1/64) were fully capable of presenting C. neoformans to T cells (Fig. 7A).
FIG. 7.
Number of DC required to induce T-cell proliferation in response to C. neoformans and number of C. neoformans organisms that interact with DC. (A) T cells (2 × 105/well) were put into culture with various numbers of DC in the presence (solid bars) or absence (open bars) of killed C. neoformans strain 67 (2 × 105/well). Lymphocyte proliferation was assessed 7 days later by [3H]TdR incorporation. ∗, P < 0.05 compared to the appropriate unstimulated control. The experiment was repeated with similar results. (B) DC (5 × 105) were put into culture with C. neoformans strain 67 (5 × 106) and examined 18 h later by Giemsa staining and light microscopy. At least 200 cells were examined per experiment. Open bars, DC with bound C. neoformans; solid bars, DC with internalized C. neoformans. The experiment was repeated three times with similar results.
The microscopy suggested that a large number of C. neoformans organisms interacted with DC, despite previous observations that DC are poorly phagocytic (70). Thus, the number of organisms that were bound and internalized by DC was determined. DC were put into culture with C. neoformans, and 18 h later, cytocentrifuged slides were prepared, the cells were carefully examined for dendritic morphology, and the numbers of internalized and bound organisms were determined. There was a large percentage of DC that bound C. neoformans (42.3% ± 10.3% of DC had the dendritic process or the cell body in direct apposition with the microbe), and between one and seven organisms were bound to each DC (Fig. 7B). Between 15 and 34% (with an average of 24.9% ± 3.1%) of DC had internalized at least one organism (Fig. 7B). Individual cells were observed taking up as many as six organisms. Thus, DC were capable of taking up large numbers of C. neoformans for processing and presentation of CnM to T cells.
Mechanism of binding of C. neoformans.
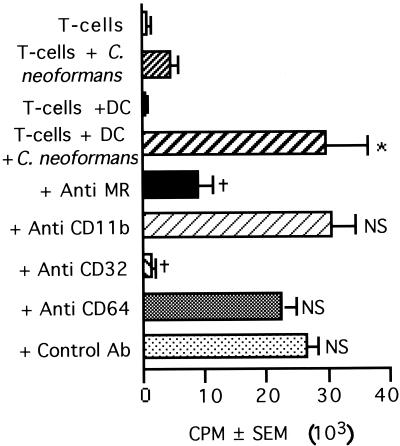
Having determined that a large percentage of DC bind C. neoformans, experiments were performed to identify the receptor on DC that was used for binding the organism. DC express the mannose receptor, β1 integrins (CD11/CD18), FcγRII (CD32), and FcγRIII (CD64) (15, 16, 47). Although the mechanism of interaction with DC had not previously been determined, C. neoformans binds to monocytes and macrophages via CD11b/CD18 and mannose receptors (13, 43). Therefore, experiments were performed to determine whether the mannose receptor, CD11b, or any of the classes of FcγRs might be responsible for the binding of C. neoformans by DC. Antibody directed to the mannose receptor and to CD32 (FcγRII), but not CD11b (CR3) or CD64 (FcγRI), abrogated T-cell responses to C. neoformans (Fig. 8). Antibody directed to the mannose receptor and to CD32 (FcγRII) also reduced the binding and uptake of C. neoformans by DC (Table 2). Thus, both the mannose receptor and CD32 (FcγRII) are involved in the binding and uptake of C. neoformans, which is necessary for presentation to T cells.
FIG. 8.
Blocking the mannose receptor or FcγRII (CD32) inhibits T-lymphocyte proliferation in response to C. neoformans. T cells (2 × 105/well) and DC (5 × 104/well) were stimulated with killed C. neoformans strain 67 (2 × 105/well) for 7 days in the presence of anti-mannose receptor (Anti MR), anti-C3biR (anti CD11b), anti-FcγRII (anti CD32), anti-FcγRI (anti CD64), or control antibody (Ab) at 10 μg/ml. The bars represent the mean counts per minute (± SEM) of quadruplicate cultures. The experiment was repeated three times with similar results. ∗, P < 0.05 compared to T cells plus DC. †, P < 0.05 compared to T cells plus DC plus C. neoformans. NS, nonsignificant compared to T cells plus DC plus C. neoformans.
TABLE 2.
Mannose receptor and FcγRII (CD32) are necessary for binding of C. neoformans by DC
| Expt no. | % of DC that had bound or internalized C. neoformansa
|
||||
|---|---|---|---|---|---|
| IgG control | Anti-MR | Anti-CD64 | Anti-CD32 | Anti-CD16 | |
| 1 | 31 | 23 | 35 | 18 | 47 |
| 2 | 71 | 29 | 63 | 35 | 63 |
5 × 105 DC were incubated with 5 × 106 killed C. neoformans strain 67 cells in 24-well plates with 10 μg of antibody/ml for 18 h and then analyzed by light microscopy after Giemsa staining. Two hundred cells were counted. IgG, immunoglobulin G; MR, mannose receptor.
DISCUSSION
We have made four major observations. (i) The cells responsible for the phagocytosis and processing of C. neoformans that leads to presentation of CnM are transiently adherent, LME resistant, CD3−, CD14−, CD19−, CD56−, HLA-DR+, and CD83+ (with maturation in culture) and have morphological features of DC; (ii) these cells are a better source of APC for CnM than either AM or monocyte-derived macrophages; (iii) both encapsulated and acapsular C. neoformans cells are effectively presented by DC; and (iv) C. neoformans is actively taken up by DC via the mannose receptor and CD32 (FcγRII), and small numbers of DC present CnM to T cells.
The present study demonstrates that DC are potent APC for CnM based on multiple criteria. DC have unique adherence properties whereby they are initially adherent to plastic and then become loosely adherent after overnight incubation (14, 35). DC do not express the surface molecules for monocytes or macrophages (CD14), nor do they express surface molecules typical of B cells (CD19). They express high levels of class II major histocompatibility complex and moderate levels of CD83, and small numbers of cells stimulate a very potent autologous (Fig. 4) and allogeneic (data not shown) mixed lymphocyte reaction. Finally, DC express a typical morphology with large nuclei, an agranular cytoplasm, and thin dendritic cytoplasmic processes extending from the cell body. The cells that were responsible for processing and presentation of CnM exhibited all of these properties, indicating that the cells were DC.
There are a number of properties of DC that might predict that DC would be better APC than macrophages. The initial process of acquiring a microbe requires phagocytosis. Although DC were initially felt to be poorly phagocytic (42) (63), the initial report of phagocytosis of microbes was by Inaba et al. using Mycobacterium bovis BCG (32). Subsequently, it has been shown that DC phagocytose a diverse array of organisms, including Bordetella bronchiseptica (26, 28), Listeria monocytogenes (27), Chlamydia trachomatis (59, 72), Leishmania sp. (4, 22, 38, 80), Borrelia burgdorferi (17), Candida albicans (56), Histoplasma capsulatum (21), and Aspergillus fumigatus (8). Multiple mechanisms are used, including conventional and coiling phagocytosis (7, 8), although we saw no evidence of the latter with C. neoformans. DC are activated by microbes for both expression of potent costimulatory molecules (29, 34, 66, 82) and cytokine production that could provide costimulatory activity (8, 22, 29, 38, 46, 53, 80, 81). The present experiments demonstrate not only that DC are capable of phagocytosis, processing, and presentation of C. neoformans to T cells but that they are better than macrophages.
It has previously been demonstrated that DC participate in cryptococcal immunity. Mice immunized with a protective antigen of C. neoformans accumulate DC in the regional lymphoid compartment, while those immunized with a nonprotective antigen do not (3). Although it is not known whether these cells presented C. neoformans to T cells, accumulation of myeloid DC was associated with protection against C. neoformans while lymphoid DC were not (3). The present studies suggest another role for DC in cryptococcal immunology: a role in the innate response to this organism.
Receptor-mediated uptake of microbes has been demonstrated for B. bronchiseptica, which binds specifically to glycosylated receptors present on the plasma membranes of DC (28). Multiple receptors can be used for the uptake of some microbes. For example, Aspergillus conidia are taken up by mannose receptor, while hyphae are taken up by CR3 and FcγRII and FcγRIII (8). The present study demonstrates that FcγRII and mannose receptors participate in the uptake of C. neoformans.
The present study demonstrates that blocking either mannose receptor or FcγRII resulted in reduced lymphocyte proliferation. It is not clear why blocking either of these receptors would result in such a substantial reduction in proliferation. It is possible that one receptor is required for initial binding and the other for uptake of C. neoformans. Alternatively, it is possible that uptake by one receptor could result in activation of the DC and uptake by the other receptor results in processing that is required for presentation. For example, FcγRs activate cells via immunoreceptor tyrosine-based activation motifs (ITAMs) and activation of tyrosine kinases (1). It has been shown that the functions of cellular activation and receptor-mediated endocytosis are distinct in FcγRs, since an L35A mutation blocks cell signaling without affecting receptor endocytosis (6). On the other hand, while the mannose receptor has been implicated in phagocytosis (5), it has also been implicated in cellular activation. Mannose receptor ligation results in expression of potentially costimulatory cytokines, such as interleukin 1 (IL-1), IL-6, (83), tumor necrosis factor alpha (19), and IL-12 (67). Thus, it is possible that binding to FcγR activates the cells while binding to the mannose receptor is required for internalization or that binding to FcγR is required for internalization while binding to the mannose receptor activates the cells. Additionally, receptor redundancy may be particularly important, since mannose receptors could be used for the initial uptake of acapsular organisms that are acquired from the environment (5, 13), which would potentiate the FcR-mediated uptake of organisms as they rapidly acquire capsule (13, 23).
Different subsets of DC exist (58). DC can be derived from myeloid and lymphoid precursors (18, 24, 62, 71). Freshly isolated lymphoid DC are dependent on IL-3 for survival and use autocrine or exogenous tumor necrosis factor alpha as a maturation signal (37). By contrast, myeloid DC are derived from CD14 cells and differentiate with granulocyte-macrophage colony-stimulating factor and IL-4 (64). Previous studies have demonstrated that lymphoid DC and Langerhans cells lack functional mannose receptors (45, 54) and that lymphoid DC are poorly phagocytic (62). Therefore, the cell responsible for the phagocytosis and processing of C. neoformans is likely to be a myeloid DC. Whether monocytes that have been induced to be myeloid DC in the presence of granulocyte-macrophage colony-stimulating factor and IL-4 are capable of presenting C. neoformans will require further study.
In summary, these studies provide evidence that DC can internalize, and ultimately initiate T-cell responses to, C. neoformans. DC provide a link between innate and adaptive immunity. DC acquire antigens using mechanisms of innate immunity and use these to stimulate components of adaptive immunity (60), but they also provide the mechanism of acquisition of microbial mitogens that stimulate innate T-cell responses.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research, the Canadian Foundation for AIDS research, and the Alberta Lung Association. R.M.S. was supported by a studentship from the National Health Research and Development Program. C.M.M. was supported by a senior scholarship of the Alberta Heritage Foundation for Medical Research.
We thank Laurie Robertson for assistance with flow cytometry.
Editor: T. R. Kozel
REFERENCES
- 1.Amigorena, S., and C. Bonnerot. 1999. Fc receptors for IgG and antigen presentation on MHC class I and class II molecules. Semin. Immunol. 11:385-390. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, A., I. Rutenfranz, and H. Kirchner. 1988. Processing requirements for T cell activation by Mycoplasma arthritidis derived mitogen. Eur. J. Immunol. 18:2109-2112. [DOI] [PubMed] [Google Scholar]
- 3.Bauman, S. K., K. L. Nichols, and J. W. Murphy. 2000. Dendritic cells in the induction of protective and nonprotective anticryptococcal cell-mediated immune responses. J. Immunol. 165:158-167. [DOI] [PubMed] [Google Scholar]
- 4.Blank, C., H. Fuchs, K. Rappersberger, M. Rollinghoff, and H. Moll. 1993. Parasitism of epidermal Langerhans cells in experimental cutaneous leishmaniasis with Leishmania major. J. Infect. Dis. 167:418-425. [DOI] [PubMed] [Google Scholar]
- 5.Bolanos, B., and T. G. Mitchell. 1989. Phagocytosis and killing of Cryptococcus neoformans by rat alveolar macrophages in the absence of serum. J. Leukoc. Biol. 46:521-528. [DOI] [PubMed] [Google Scholar]
- 6.Bonnerot, C., V. Briken, V. Brachet, D. Lankar, S. Cassard, B. Jabri, and S. Amigorena. 1998. Syk protein tyrosine kinase regulates Fc receptor gamma-chain-mediated transport to lysosomes. EMBO J. 17:4606-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouis, D. A., T. G. Popova, A. Takashima, and M. V. Norgard. 2001. Dendritic cells phagocytose and are activated by Treponema pallidum. Infect. Immun. 69:518-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozza, S., R. Gaziano, A. Spreca, A. Bacci, C. Montagnoli, P. di Francesco, and L. Romani. 2002. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J. Immunol. 168:1362-1371. [DOI] [PubMed] [Google Scholar]
- 9.Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans. ASM Press, Washington, D.C.
- 10.Cauley, L. K., and J. W. Murphy. 1979. Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infections. Infect. Immun. 23:644-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chestnut, R. A. W., and H. M. Gray. 1986. Antigen presentation by B cells and its significance in T-B interaction. Adv. Immunol. 39:51-94. [DOI] [PubMed] [Google Scholar]
- 12.Cianciotto, N. P., B. I. Eisenstein, C. H. Mody, G. B. Toews, and N. C. Engleberg. 1989. A Legionella pneumophila gene encoding a species-specific surface protein potentiates initiation of intracellular infection. Infect. Immun. 57:1255-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross, C. E., and G. J. Bancroft. 1995. Ingestion of acapsular Cryptococcus neoformans occurs via mannose and beta-glucan receptors, resulting in cytokine production and increased phagocytosis of the encapsulated form. Infect. Immun. 63:2604-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crow, M. K., and H. G. Kunkel. 1982. Human dendritic cells: major stimulators of the autologous and allogeneic mixed leucocyte reactions. Clin. Exp. Immunol. 49:338-346. [PMC free article] [PubMed] [Google Scholar]
- 15.Ezekowitz, R. A., K. Sastry, P. Bailly, and A. Warner. 1990. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J. Exp. Med. 172:1785-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fanger, N. A., K. Wardwell, L. Shen, T. F. Tedder, and P. M. Guyre. 1996. Type I (CD64) and type II (CD32) Fc gamma receptor-mediated phagocytosis by human blood dendritic cells. J. Immunol. 157:541-548. [PubMed] [Google Scholar]
- 17.Filgueira, L., F. O. Nestle, M. Rittig, H. I. Joller, and P. Groscurth. 1996. Human dendritic cells phagocytose and process Borrelia burgdorferi. J. Immunol. 157:2998-3005. [PubMed] [Google Scholar]
- 18.Galy, A., M. Travis, D. Cen, and B. Chen. 1995. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity 3:459-473. [DOI] [PubMed] [Google Scholar]
- 19.Garner, R. E., K. Rubanowice, R. T. Sawyer, and J. A. Hudson. 1994. Secretion of TNF-alpha by alveolar macrophages in response to Candida albicans mannan. J. Leukoc. Biol. 55:161-168. [DOI] [PubMed] [Google Scholar]
- 20.Germain, R. N. 1986. The ins and outs of antigen processing and presentation. Nature 322:687-689. [DOI] [PubMed] [Google Scholar]
- 21.Gildea, L. A., R. E. Morris, and S. L. Newman. 2001. Histoplasma capsulatum yeasts are phagocytosed via very late antigen-5, killed, and processed for antigen presentation by human dendritic cells. J. Immunol. 166:1049-1056. [DOI] [PubMed] [Google Scholar]
- 22.Gorak, P. M., C. R. Engwerda, and P. M. Kaye. 1998. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur. J. Immunol. 28:687-695. [DOI] [PubMed] [Google Scholar]
- 23.Granger, D. L., J. R. Perfect, and D. T. Durack. 1985. Virulence of Cryptococcus neoformans: regulation of capsule synthesis by carbon dioxide. J. Clin. Investig. 76:508-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grouard, G., M. C. Rissoan, L. Filgueira, I. Durand, J. Banchereau, and Y. J. Liu. 1997. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 185:1101-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunzer, M., P. Friedl, B. Niggemann, E. B. Brocker, E. Kampgen, and K. S. Zanker. 2000. Migration of dendritic cells within 3-D collagen lattices is dependent on tissue origin, state of maturation, and matrix structure and is maintained by proinflammatory cytokines. J. Leukoc. Biol. 67:622-629. [DOI] [PubMed] [Google Scholar]
- 26.Guzman, C. A., M. Rohde, M. Bock, and K. N. Timmis. 1994. Invasion and intracellular survival of Bordetella bronchiseptica in mouse dendritic cells. Infect. Immun. 62:5528-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guzman, C. A., M. Rohde, T. Chakraborty, E. Domann, M. Hudel, J. Wehland, and K. N. Timmis. 1995. Interaction of Listeria monocytogenes with mouse dendritic cells. Infect. Immun. 63:3665-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guzman, C. A., M. Rohde, and K. N. Timmis. 1994. Mechanisms involved in uptake of Bordetella bronchiseptica by mouse dendritic cells. Infect. Immun. 62:5538-5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson, R. A., S. C. Watkins, and J. L. Flynn. 1997. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J. Immunol. 159:635-643. [PubMed] [Google Scholar]
- 30.Huffnagle, G. B., J. L. Yates, and M. F. Lipscomb. 1991. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J. Exp. Med. 173:793-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huffnagle, G. B., J. L. Yates, and M. F. Lipscomb. 1991. T cell-mediated immunity in the lung: a Cryptococcus neoformans pulmonary infection model using SCID and athymic nude mice. Infect. Immun. 59:1423-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inaba, K., M. Inaba, M. Naito, and R. M. Steinman. 1993. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J. Exp. Med. 178:479-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobson, E. S., D. J. Ayers, A. C. Harrell, and C. C. Nicholas. 1982. Genetic and phenotypic characterization of capsule mutants of Cryptococcus neoformans. J. Bacteriol. 150:1292-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiao, X., R. Lo-Man, P. Guermonprez, L. Fiette, E. Deriaud, S. Burgaud, B. Gicquel, N. Winter, and C. Leclerc. 2002. Dendritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. J. Immunol. 168:1294-1301. [DOI] [PubMed] [Google Scholar]
- 35.Knight, S. C., J. Farrant, A. Bryant, A. J. Edwards, S. Burman, A. Lever, J. Clarke, and A. D. Webster. 1986. Non-adherent, low-density cells from human peripheral blood contain dendritic cells and monocytes, both with veiled morphology. Immunology 57:595-603. [PMC free article] [PubMed] [Google Scholar]
- 36.Knight, S. C., A. Stagg, S. Hill, P. Fryer, and S. Griffiths. 1992. Development and function of dendritic cells in health and disease. J. Investig. Dermatol. 99:33S-38S. [DOI] [PubMed] [Google Scholar]
- 37.Kohrgruber, N., N. Halanek, M. Groger, D. Winter, K. Rappersberger, M. Schmitt-Egenolf, G. Stingl, and D. Maurer. 1999. Survival, maturation, and function of CD11c− and CD11c+ peripheral blood dendritic cells are differentially regulated by cytokines. J. Immunol. 163:3250-3259. [PubMed] [Google Scholar]
- 38.Konecny, P., A. J. Stagg, H. Jebbari, N. English, R. N. Davidson, and S. C. Knight. 1999. Murine dendritic cells internalize Leishmania major promastigotes, produce IL-12 p40 and stimulate primary T cell proliferation in vitro. Eur. J. Immunol. 29:1803-1811. [DOI] [PubMed] [Google Scholar]
- 39.Kozel, T. R. 1977. Non-encapsulated variant of Cryptococcus neoformans. II. Surface receptors for cryptococcal polysaccharide and their role in inhibition of phagocytosis by polysaccharide. Infect. Immun. 16:99-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kozel, T. R., and J. Cazin. 1971. Nonencapsulated variant of Cryptococcus neoformans. I. Virulence studies of characterization of soluble polysaccharide. Infect. Immun. 3:287-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Legaard, P. K., R. D. LeGrand, and M. L. Misfeldt. 1992. Lymphoproliferative activity of Pseudomonas exotoxin A is dependent on intracellular processing and is associated with the carboxyl-terminal portion. Infect. Immun. 60:1273-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levine, T. P., and B. M. Chain. 1992. Endocytosis by antigen presenting cells: dendritic cells are as endocytically active as other antigen presenting cells. Proc. Natl. Acad. Sci. USA 89:8342-8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levitz, S. M., A. Tabuni, T. R. Kozel, R. S. MacGill, R. R. Ingalls, and D. T. Golenbock. 1997. Binding of Cryptococcus neoformans to heterologously expressed human complement receptors. Infect. Immun. 65:931-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim, T. S., and J. W. Murphy. 1980. Transfer of immunity to cryptococcosis by T-enriched spleen lymphocytes from Cryptococcus neoformans-sensitized mice. Infect. Immun. 30:5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu, Y. J., H. Kanzler, V. Soumelis, and M. Gilliet. 2001. Dendritic cell lineage, plasticity and cross-regulation. Nat. Immunol. 2:585-589. [DOI] [PubMed] [Google Scholar]
- 46.Marriott, I., T. G. Hammond, E. K. Thomas, and K. L. Bost. 1999. Salmonella efficiently enter and survive within cultured CD11c+ dendritic cells initiating cytokine expression. Eur. J. Immunol. 29:1107-1115. [DOI] [PubMed] [Google Scholar]
- 47.McCarthy, D. A., M. G. Macey, P. A. Bedford, S. C. Knight, D. C. Dumonde, and K. A. Brown. 1997. Adhesion molecules are upregulated on dendritic cells isolated from human blood. Immunology 92:244-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mody, C. H., M. F. Lipscomb, and G. B. Toews. 1990. Depletion of CD4+ (L3T4+) lymphocytes in vivo impairs murine host defense to Cryptococcus neoformans. J. Immunol. 144:1472-1477. [PubMed] [Google Scholar]
- 49.Mody, C. H., K. L. Sims, C. J. Wood, R. M. Syme, J. C. Spurrell, and M. M. Sexton. 1996. Proteins in the cell wall/membrane of Cryptococcus neoformans stimulate both adult and fetal cord blood lymphocytes to proliferate. Infect. Immun. 64:4811-4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mody, C. H., and R. M. Syme. 1993. Effect of polysaccharide capsule and methods of preparation on human lymphocyte proliferation in response to Cryptococcus neoformans. Infect. Immun. 61:464-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mody, C. H., G. B. Toews, and M. F. Lipscomb. 1988. Cyclosporin-A inhibits the growth of Cryptococcus neoformans in a murine model. Infect. Immun. 56:7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mody, C. H., C. J. Wood, R. M. Syme, and J. C. Spurrell. 1999. The cell wall and membrane of Cryptococcus neoformans possess a mitogen for human T lymphocytes. Infect. Immun. 67:936-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohagheghpour, N., A. van Vollenhoven, J. Goodman, and L. E. Bermudez. 2000. Interaction of Mycobacterium avium with human monocyte-derived dendritic cells. Infect. Immun. 68:5824-5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mommaas, A. M., A. A. Mulder, R. Jordens, C. Out, M. C. Tan, P. Cresswell, P. M. Kluin, and F. Koning. 1999. Human epidermal Langerhans cells lack functional mannose receptors and a fully developed endosomal/lysosomal compartment for loading of HLA class II molecules. Eur. J. Immunol. 29:571-580. [DOI] [PubMed] [Google Scholar]
- 55.Murphy, J. W., and D. O. McDaniel. 1982. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J. Immunol. 128:1577-1583. [PubMed] [Google Scholar]
- 56.Newman, S. L., and A. Holly. 2001. Candida albicans is phagocytosed, killed, and processed for antigen presentation by human dendritic cells. Infect. Immun. 69:6813-6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicod, L. P., M. F. Lipscomb, J. C. Weissler, and G. B. Toews. 1989. Mononuclear cells from human lung parenchyma support antigen-induced T lymphocyte proliferation. J. Leukoc. Biol. 45:336-344. [DOI] [PubMed] [Google Scholar]
- 58.O'Doherty, U., M. Peng, S. Gezelter, W. J. Swiggard, M. Betjes, N. Bhardwaj, and R. M. Steinman. 1994. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology 82:487-493. [PMC free article] [PubMed] [Google Scholar]
- 59.Ojcius, D. M., Y. Bravo de Alba, J. M. Kanellopoulos, R. A. Hawkins, K. A. Kelly, R. G. Rank, and A. Dautry-Varsat. 1998. Internalization of Chlamydia by dendritic cells and stimulation of Chlamidia-specific T cells. J. Immunol. 160:1297-1303. [PubMed] [Google Scholar]
- 60.Palucka, K., and J. Banchereau. 1999. Linking innate and adaptive immunity. Nat. Med. 5:868-870. [DOI] [PubMed] [Google Scholar]
- 61.Pechhold, K., and D. Kabelitz. 1993. Human peripheral blood gamma delta T cells are uniformly sensitive to destruction by the lysosomotropic agents leucine methyl ester and leucyl leucine methyl ester. Eur. J. Immunol. 23:562-565. [DOI] [PubMed] [Google Scholar]
- 62.Robinson, S. P., S. Patterson, N. English, D. Davies, S. C. Knight, and C. D. Reid. 1999. Human peripheral blood contains two distinct lineages of dendritic cells. Eur. J. Immunol. 29:2769-2778. [DOI] [PubMed] [Google Scholar]
- 63.Sallusto, F., M. Cella, C. Danieli, and A. Lanzavecchia. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saxon, A., J. Feldhaus, and R. A. Robins. 1976. Single step separation of human T and B cells using AET treated srbc rosettes. J. Immunol. Methods 12:285-288. [DOI] [PubMed] [Google Scholar]
- 66.Seixas, E., C. Cross, S. Quin, and J. Langhorne. 2001. Direct activation of dendritic cells by the malaria parasite Plasmodium chabaudi chabaudi. Eur. J. Immunol. 31:2970-2978. [DOI] [PubMed] [Google Scholar]
- 67.Shibata, Y., W. J. Metzger, and Q. N. Myrvik. 1997. Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: mannose receptor-mediated phagocytosis initiates IL-12 production. J. Immunol. 159:2462-2467. [PubMed] [Google Scholar]
- 68.Sorg, R. V., G. Kogler, and P. Wernet. 1999. Identification of cord blood dendritic cells as an immature CD11c− population. Blood 93:2302-2307. [PubMed] [Google Scholar]
- 69.Steinman, R. M., and Z. A. Cohn. 1974. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J. Exp. Med. 139:380-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steinman, R. M., and J. Swanson. 1995. The endocytic activity of dendritic cells. J. Exp. Med. 182:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Strobl, H., C. Scheinecker, E. Riedl, B. Csmarits, C. Bello-Fernandez, W. F. Pickl, O. Majdic, and W. Knapp. 1998. Identification of CD68+lin− peripheral blood cells with dendritic precursor characteristics. J. Immunol. 161:740-748. [PubMed] [Google Scholar]
- 72.Su, H., R. Messer, W. Whitmire, E. Fischer, J. C. Portis, and H. D. Caldwell. 1998. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable Chlamydiae. J. Exp. Med. 188:809-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Syme, R. M., T. F. Bruno, T. R. Kozel, and C. H. Mody. 1999. The capsule of Cryptococcus neoformans reduces T lymphocyte proliferation by reducing phagocytosis, which can be restored with anticapsular antibody. Infect. Immun. 67:4620-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Syme, R. M., J. C. Spurrell, L. L. Ma, F. H. Green, and C. H. Mody. 2000. Phagocytosis and protein processing are required for presentation of Cryptococcus neoformans mitogen to T lymphocytes. Infect. Immun. 68:6147-6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Syme, R. M., H. Wong, C. J. Wood, and C. H. Mody. 1997. Both CD4+ and CD8+ human lymphocytes are activated and proliferate to Cryptococcus neoformans. Immunology 92:194-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thiele, D. L., and P. E. Lipsky. 1982. The accessory function of phagocytic cells in human T cell and B cell responses. J. Immunol. 129:1033-1039. [PubMed] [Google Scholar]
- 77.Unanue, E. R., and P. M. Allen. 1987. The basis for the immunoregulatory role of macrophages and other accessory cells. Science 236:551-557. [DOI] [PubMed] [Google Scholar]
- 78.Van Voorhis, W. C., L. S. Hair, R. M. Steinman, and G. Kaplan. 1982. Human dendritic cells. Enrichment and characterization from peripheral blood. J. Exp. Med. 155:1172-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vecchiarelli, A., M. Dottorini, D. Pietrella, C. Monari, C. Retini, T. Todisco, and F. Bistoni. 1994. Role of alveolar macrophages as antigen-presenting cells in Cryptococcus neoformans infection. Am. J. Respir. Cell Mol. Biol. 11:130-137. [DOI] [PubMed] [Google Scholar]
- 80.von Stebut, E., Y. Belkaid, T. Jakob, D. L. Sacks, and M. C. Udey. 1998. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J. Exp. Med. 188:1547-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.von Stebut, E., Y. Belkaid, B. V. Nguyen, M. Cushing, D. L. Sacks, and M. C. Udey. 2000. Leishmania major-infected murine Langerhans cell-like dendritic cells from susceptible mice release IL-12 after infection and vaccinate against experimental cutaneous leishmaniasis. Eur. J. Immunol. 30:3498-3506. [DOI] [PubMed] [Google Scholar]
- 82.Wei, S., F. Marches, J. Borvak, W. Zou, J. Channon, M. White, J. Radke, M. F. Cesbron-Delauw, and T. J. Curiel. 2002. Toxoplasma gondii-infected human myeloid dendritic dells induce T-lymphocyte dysfunction and contact-dependent apoptosis. Infect. Immun. 70:1750-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamamoto, Y., T. W. Klein, and H. Friedman. 1997. Involvement of mannose receptor in cytokine interleukin-1β (IL-1β), IL-6, and granulocyte-macrophage colony-stimulating factor responses, but not in chemokine macrophage inflammatory protein 1β (MIP-1β), MIP-2, and KC responses, caused by attachment of Candida albicans to macrophages. Infect. Immun. 65:1077-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou, L. J., and T. F. Tedder. 1995. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J. Immunol. 154:3821-3835. [PubMed] [Google Scholar]