Abstract
Modulation of cytosolic (intracellular) Ca2+ concentration (Cai) may be an important host response when airway epithelial cells are exposed to Pseudomonas aeruginosa. We measured Cai in Calu-3 cells exposed from the apical or basolateral surface to cytotoxic and noncytotoxic strains of P. aeruginosa. Apical addition of either noncytotoxic strains or cytotoxic strains failed to affect Cai over a 3-h time period, nor were changes observed after basolateral addition of noncytotoxic strains. In contrast, basolateral addition of cytotoxic strains caused a slow increase in Cai from 100 nM to 200 to 400 nM. This increase began after 20 to 50 min and persisted for an additional 30 to 75 min, at which time the cells became nonviable. P. aeruginosa-induced increases in Cai were blocked by the addition of the Ca channel blocker La3+ to the basolateral but not to the apical chamber. Likewise, replacing the basolateral but not the apical medium with Ca-free solution prevented P. aeruginosa-mediated changes in Cai. With isogenic mutants of PA103, we demonstrated that the type III secretion apparatus, the type III-secreted effector ExoU, and type IV pili were necessary for increased Cai. We propose that translocation of ExoU through the basolateral surface of polarized airway epithelial cells via the type III secretion apparatus leads to release of Ca stored in the endoplasmic reticulum and activation of Ca channels in the basolateral membranes of epithelial cells.
Human airways are constantly exposed to bacteria but maintain sterility by multiple host defense mechanisms. These include the mucociliary escalator, which removes trapped microbes and debris, the secretion of various antimicrobials such as defensins into the airway surface liquid, and the highly impermeable polarized epithelium that lines the airway. In the setting of direct trauma such as mechanical ventilation, the opportunistic pathogen Pseudomonas aeruginosa is able to colonize the airways and establish an infection, leading to inflammation and pneumonia, sepsis, and dissemination to distant organs (38, 54).
Interaction of P. aeruginosa with airway epithelial cells triggers multiple host responses, including upregulation of mucin (41, 42), interleukin-8 (10, 11), defensins (4), and nitric oxide synthase (12). The cellular signaling pathways that lead to these changes are complex and could involve changes in intracellular Ca2+ concentration (Cai). For example, it has been reported that pilin and/or flagellin-mediated bacterial binding to the membrane glycolipid asialo-GM1 leads to increased interleukin-8 secretion through changes in epithelial cell Cai and mitogen-activated protein kinase signaling (8-11, 33, 52, 53, 55, 66, 67).
Bacterial lipopolysaccharide upregulates mucin gene expression through a mitogen-activated protein kinase-NF-κB signaling pathway (42, 65). Activation of Toll-like receptors by various bacterial surface molecules, including lipopolysaccharide and flagellin, may also be important (1, 27, 50, 64, 65). Microarray analysis of rapid changes in gene expression demonstrates that a significant number of human epithelial genes are differentially regulated within a few hours after contact with P. aeruginosa (31).
Many of the published studies have examined P. aeruginosa-host cell signaling in the context of nonpolarized epithelial cells grown in vitro. However, it is becoming increasingly evident that epithelial cell polarity is an important host defense mechanism that adds complexity to these interactions (36). For example, P. aeruginosa appears to bind preferentially to the basolateral surface of polarized airway epithelia (16, 17, 39, 51). Bacterially mediated invasion is greater at less well polarized cells seen at the edges of partially confluent or wounded epithelial monolayers (39). In the well-defined Madin-Darby canine kidney cell line, bacterial invasion is downregulated as the epithelial monolayer polarizes (36).
P. aeruginosa possesses a large armamentarium of cell-associated and secreted virulence factors. Key among these is the type III secretion system, which allows the direct injection of at least four different bacterial effector molecules, ExoS, ExoT, ExoU, and ExoY, into eukaryotic cells. The exoT and exoY genes are present in nearly all isolates, but the production of ExoY protein is variable. Some strains also contain the exoU gene, while others contain the exoS gene (15). ExoT and ExoS possess two distinct functional domains. The N-terminal portion has homology to GTPase-activating proteins and demonstrates GTPase-activating activity towards Rho family GTPases in vitro and in vivo (20, 37). The physiological consequence is disruption of the actin cytoskeleton, cell rounding, inhibition of wound migration and healing, and prevention of bacterial uptake into epithelial cells and macrophages (20, 21, 59).
The C-terminal domain of ExoS has ADP-ribosylase activity directed towards Ras and likely other small GTPases and is cytotoxic to eukaryotic cells (19). Interestingly, the ADP-ribosylase activity of ExoT is only 0.2% of that of ExoS due to mutations in the catalytic site (44). ExoY is an adenylate cyclase that elevates intracellular cyclic AMP concentration in eukaryotic cells and causes rounding of certain cell types (63). The mechanism of action of ExoU remains unknown, but this toxin may be of particular importance in disease (54). ExoU mediates killing of a variety of mammalian cell types in vitro (23, 24). Furthermore, isogenic mutants that do not produce or secrete ExoU are less virulent in a mouse model of pneumonia (26), and an exoU exoT double mutant is defective in causing sepsis in a rabbit model of pneumonia (38). Introduction of the exoU gene into strains that do not naturally harbor it resulted in increased virulence of these strains in a mouse model of acute pneumonia (2).
In this study, we examined the ability of various strains and isogenic mutants of P. aeruginosa to affect Cai in the context of polarized airway epithelia and explicitly address whether bacterially mediated changes in Cai occur preferentially during interactions of P. aeruginosa with the apical or basolateral surface. Cytotoxic and noncytotoxic P. aeruginosa strains were added to either the apical or basolateral surface of Calu-3 cells grown as polarized monolayers on porous filter supports or as islands on cover glasses. After loading with the fluorescent dye fura-2, Cai was measured with digital imaging microscopy. We compared the effects of cytotoxic versus noncytotoxic strains of P. aeruginosa, the role of bacterial contact with the apical and basolateral membranes of the cells, the roles of apical versus basolateral Ca channels in triggering increases in Cai, and the role of pili and type III secretion in modulating cytosolic calcium. Our results support the model that basolateral but not apical addition of cytotoxic strains of P. aeruginosa results in release of Ca stored in the endoplasmic reticulum and activation of Ca channels in the basolateral membranes of epithelial cells.
(Preliminary publication of this work appeared previously [T. Jacob, J. Engel, and T. E. Machen, abstract, Pediatr. Pulmonol. Suppl. 20:274, 2000].)
MATERIALS AND METHODS
Cell culture.
Calu-3 cells are a tracheal serum-like cell line derived from a human pulmonary adenocarcinoma that form highly polarized monolayers with a high transepithelial resistance (RT = 400 to 1,000 Ω/cm2) when grown to confluence on coverslips or on porous filter supports. These cells express high levels of functional, cyclic AMP-stimulated cystic fibrosis transmembrane conductance regulator (CFTR) in their apical membranes (32). Calu-3 cells were grown in Dulbecco's modified Eagle's medium (Sigma, St. Louis, Mo.) supplemented with 10% fetal calf serum in a humidifed 5% CO2-95% air atmosphere. Cells grown to 80% confluence were trypsinized with 0.25% trypsin-0.1% ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) solution for 5 to 15 min. Cells were passaged at a 1:5 dilution, and the remaining cell suspension was seeded directly onto 25-mm-diameter cover glasses or onto permeable filter supports (0.45-, 1.0-, or 3.0-μm pore size; Falcon; Becton Dickinson, Franklin Lakes, N.J.) at a density of 106 cells/cm2.
Growth of the cells as a confluent well-polarized monolayer on a permeable filter allowed independent manipulation of the apical and basolateral bath solutions. Cells were grown until the transepithelial resistance (RT) was greater than 400 Ω/cm2, which typically occurred after 1 to 2 weeks in culture. In some experiments, cells were grown on the “tops” of the filters in the normal configuration, while in others the filter was inverted and the cells were grown on the “bottoms” of the filters. Results from cells grown in these two configurations were indistinguishable.
For some experiments, Calu-3 cells were plated at low densities onto coverslips or onto filters and allowed to grow as small islands; these conditions allowed direct comparison of Cai in the less well polarized cells at the free edge with that in the more polarized cells in the center of the island. In other experiments, the Calu-3 cells were grown as confluent monolayers, mechanically wounded with sterile filter tips or cotton swabs, and then placed back into the incubator for 24 h to allow the wound to heal partly before experimentation. This configuration allowed access of added P. aeruginosa to the apical surfaces of all the cells as well as to the basolateral membranes of the cells located at the free edges of the monolayer where the wound was formed.
Bacterial strains and medium.
PA6206 and PA103 are ExoU- and ExoT-producing cytotoxic strains that lack the exoS gene (Table 1). PAO1 is a noncytotoxic strain that lacks the exoU gene but produces ExoS and ExoT. Several well-characterized isogenic mutants of PA103 were also used. PA103pscJ::Tn5 (PA103pscJ) harbors a transposon insertion in an operon encoding a portion of the type III secretion apparatus and therefore does not secrete any of the known type III-secreted effectors. PA103exsA::Ω (PA103exsA) is defective in the production of the transcription factor ExsA, which is required for transcription of the genes encoding the type III secretion apparatus as well as for transcription of exoU and exoT. PA103ΔU contains an in-frame, nonpolar deletion in the exoU gene but has an otherwise intact type III secretion system and secretes ExoT. PA103pilA carries a gentamicin resistance cassette in the pilin subunit gene pilA and lacks type IV surface pili.
TABLE 1.
P. aeruginosa strains used in this study
| Strain | Relevant characteristic(s) | Sourcea or reference |
|---|---|---|
| PAO1 | P. aeruginosa laboratory strain; known type III-secreted effector proteins are ExoT and ExoS | ATCC |
| PAK | P. aeruginosa laboratory strain; known type III-secreted effector proteins are ExoT and ExoS | J. Mattick |
| PA6206 | Virulent corneal isolate of P. aeruginosa; known type III-secreted effector proteins are ExoT and ExoU | 16 |
| PA103 | Virulent lung isolate of P. aeruginosa; known type III-secreted effector proteins are ExoT and ExoU | 3, 43 |
| PA103exsA | Omega cassette inserted into the exsA gene; defective in type III secretion (PA103exsA::Ω) | 17 |
| PA103pscJ | Tn5-gentamicin cassette inserted into pscJ, defective in type III secretion (PA103pscJ::Tn5) | 35 |
| PA103ΔT | PA103 with an in-frame deletion in exoT | 20 |
| PA103ΔU | PA103 with an in-frame deletion of codons 330 to 571 of exoU | 20 |
| PA103ΔUΔT | PA103 with in-frame deletions in exoU and exoT | 20 |
| PA103pilA | PA103 with a tetracycline cassette inserted into pilA | 6 |
ATCC, American Type Culture Collection.
P. aeruginosa strains were routinely maintained frozen in Trypticase soy broth (TSB) with 10% (vol/vol) glycerol at −70°C. Freshly streaked out bacteria were grown for 24 h at 37°C in Luria-Bertani (LB) or TSB with antibiotics as needed, and then resuspended in sterile normal Ringer's solution to 0.1 U at an optical density at 650 nm (108 CFU/ml) and added to the apical or basolateral medium (total volume of 0.5 to 1.0 ml) at a multiplicity of infection of 50 to 100.
Measurement of cytosolic Cai.
General methods used in this laboratory for measuring Cai in epithelial cells have been described previously (45-47). Cells grown on either filters or coverslips were loaded with the acetoxymethyl (AM) ester of the Ca-sensitive fluorescent dye fura-2 by incubating cells in Ringer's solution containing 10 μM fura-2 for 40 to 60 min at either room temperature or 37°C. Both methods gave roughly equal loading of the dye (data not shown). Once the cells had been loaded with fura-2, the filter or cover glass was mounted in a perfusion chamber on the stage of the imaging microscope and maintained at 37°C in 5% CO2. This experimental setup allowed selective perfusion of either the basolateral or apical surface with different solutions (56) and additions of P. aeruginosa to either side of the epithelium.
Fluorescence ratio imaging measurements of cytosolic Cai were performed with methods that have been reported previously (45-47, 57, 60). Briefly, a Nikon Diaphot inverted microscope was used with either 20× or 40× lenses. A charge-coupled device camera (SenSys; Photometrics, Tucson, Ariz.) collected emission (>510 nm) images during alternate excitation at 380 ± 5 nm and 340 ± 5 nm with a filter wheel (Lambda-10, Sutter Instruments, Novato, Calif.). Axon Imaging Workbench 2.2 (Axon Instruments, Foster City, Calif.) controlled both filters and collection of data. Calibration of fura-2 signals was performed as described previously (45). Each image was corrected for background (region without cells). Dead cells were identified by uptake of the impermeant fluorescent dye propidium iodide as described previously (39).
Solutions.
Epithelial cells were incubated in an HCO3/CO2-buffered Ringer's solution containing 120 mM NaCl, 25 mM NaHCO3, 1.2 mM MgSO4, 2 mM CaCl2, 2.4 mM K2HPO4, 0.6 mM KH2PO4, 10 mM HEPES, and 10 mM glucose (pH 7.4) (chemicals from Sigma Chemical Co., St. Louis, Mo.) or in solutions that were nominally HCO3 and CO2 free: 145 mM NaCl, 1.2 mM MgSO4, 2 mM CaCl2, 2.4 mM K2HPO4, 0.6 mM KH2PO4, 10 mM HEPES, and 10 mM glucose (pH 7.4). Results were similar with the two solutions. Fura-2 (Molecular Probes, Eugene, Oreg.) was prepared as a stock in dimethyl sulfoxide plus the dispersing agent Pluronic (Molecular Probes, Eugene, Oreg.) and added to the cells to a final concentration of 10 μM (<0.1% dimethyl sulfoxide). Propidium iodide was dissolved in distilled water (10 mM) and diluted into the solutions bathing the epithelial cells at 0.1 to 1.0 μM. Thapsigargin was dissolved in dimethyl sulfoxide and added from this stock solution (10 mM) to the Ringer's solutions at a final concentration of 10 μM.
RESULTS
Apical addition of PA6206 to Calu-3 cells increased Cai only in cells at the free edge of a wound.
We first measured Cai under conditions that permitted simultaneous access of apically applied P. aeruginosa to apical membranes of all the epithelial cells as well as to the basolateral membranes of the cells along the free edges of the wound (39). Calu-3 monolayers were grown to confluence on 0.45-μm-pore-size filters, a pore size that prevents bacterial migration across the filter. The monolayer was wounded with a cotton swab and allowed to heal for 24 h, at which time the wound had only partially closed. Changes in Cai were compared between cells at the wound edges and cells that were in confluent regions of the monolayer.
Apical addition of the cytotoxic strain PA6206 (multiplicity of infection of 50) resulted in a steady increase in Cai from control levels of approximately 80 nM (range, 40 to 120 nM) to approximately 300 nM (range, 150 to 400 nM) in cells along the “free edge” after a delay of 20 to 50 min (Fig. 1A, 1B). As shown in Fig. 1B, the elevation of Cai in free edge cells was maintained in the moderately elevated level (150 to 400 nM) for 20 to 50 min. At this time the cells rapidly lost dye, as evidenced by the decrease in fura-2 ratio. This was presumably a consequence of their dying, as control experiments showed that loss of fura-2 coincided with uptake of the impermeant nuclear stain propidium iodide.
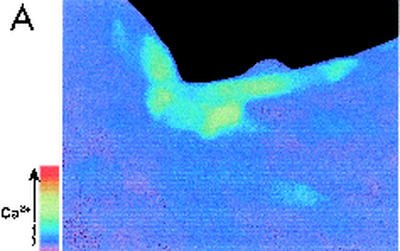
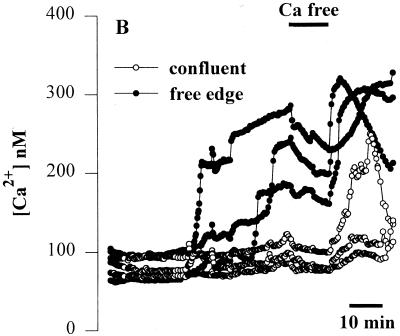
FIG. 1.
Effects of apical addition of P. aeruginosa to wounded Calu-3 cells. The cytotoxic strain PA6206 (multiplicity of infection, 50) was added to the apical chamber of confluent Calu-3 cells grown on 0.45-μm pores that had been mechanically wounded 24 h earlier and loaded with fura-2 prior to addition of bacteria. Ratio imaging microscopy was performed during control conditions and then during addition of P. aeruginosa. (A) Pseudocolor image taken after 60 min of exposure to PA6206 showed that Cai increased in cells adjacent to the free edge of the wound (blue), but not in confluent cells located away from the wound edge. (B) Time course of Cai for three cells along the free edge and three cells in the confluent region of the monolayer following addition of apical PA6206. Cai increased first in the cells along the free edge and only later in cells in the confluent region of the monolayer. Normal perfusion solution was replaced with Ca-free solution at the time shown by the black bar at the top of the figure. This treatment slowed the rate of increase of Cai in cells along the free edge but had only small effects on Cai in those cells that had not responded to P. aeruginosa. Similar results were obtained in 30 free edge cells and >85 confluent cells in a total of three experiments.
When normal perfusion solution was replaced for 10 min with Ca-free solution (Fig. 1B, black bar at the top of the figure), the rate of increase of Cai in cells along the free edge and subsequent addition of normal Ringer's solution caused Cai to increase rapidly. In contrast, incubation in Ca-free solution had only small effects on Cai in those cells that had not responded to the bacteria. These data indicated that the P. aeruginosa-induced rise in Cai may have been at least partially due to Ca entering the cells from the extracellular space.
As cells along the free edge lost viability, the adjacent cells then exhibited increases in Cai followed 20 to 50 min later by loss of fura-2 (data not shown), identical to the pattern of cell killing observed previously (39). In some cases, the depletion of fura-2 in one cell coincided with immediate, though transient, increases in Cai in adjacent cells (data not shown), perhaps due to loss of ATP and ADP from the dying cell and subsequent triggering of purinergic receptors that are present in Calu-3 cells (30).
Basolateral addition of PA6206 increased Cai in all cells of wounded Calu-3 cell monolayers.
We tested whether the susceptibility of the cells at the edges of the wound to P. aeruginosa-mediated changes in Cai was the result of increased bacterial access to the basolateral surface or due to the loss of polarization in the cells along the free edge. Experiments in which bacteria were added to the basolateral surface of similarly wounded Calu-3 cells grown on 1.0- or 3.0-μm-pore-size filters were performed. Under these conditions, basolaterally applied bacteria were able to traverse the filter (39) and specifically access the basolateral surface. Unlike apical addition of PA6206, basolateral application of PA6206 caused increased Cai in cells throughout the monolayer (Fig. 2A), with no preference for the cells along the free edges. Similar to the results obtained when PA6206 was added to the apical side and caused effects first in cells at the free edges, Cai remained elevated for 50 to 60 min. Also, incubation in Ca-free solution (black bar) resulted in a decrease in Cai in all the cells that had responded to P. aeruginosa.
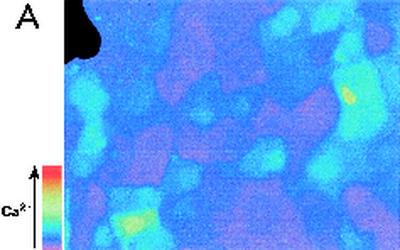
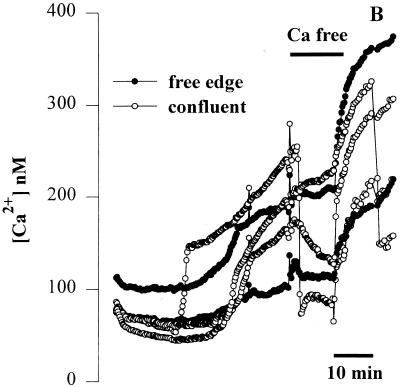
FIG. 2.
Effects of basolateral addition of P. aeruginosa to wounded Calu-3 cells. The cytotoxic strain PA6206 (multiplicity of infection, 50) was added to the basal chamber of confluent Calu-3 cells grown on 3.0-μm pores that had been mechanically wounded 24 h earlier and loaded with fura-2, and ratio imaging microscopy was performed. (A) Pseudocolor image after 40 min of exposure to PA6206 on the basolateral side of the monolayer. As shown by the blue color, basolateral addition of P. aeruginosa caused increased Cai in cells throughout the monolayer. (B) Time course of Cai following basolateral addition of PA6206 was quantified for two cells at the free edge and three cells in the confluent region of the monolayer. Replacement of the normal medium with Ca-free solution (black bar) resulted in a decrease in Cai in all the cells that had responded to P. aeruginosa. Similar results were obtained in >60 cells from free edges and confluent regions in a total of three experiments.
Apical versus basolateral addition of cytotoxic and noncytotoxic P. aeruginosa in confluent monolayers.
We next tested whether the apparent sensitivity of the cells to basolateral addition of P. aeruginosa was related to wounding. Calu-3 cells were grown as fully confluent, polarized monolayers (RT > 400 Ω/cm2) on 3.0-μm-pore-size filters in the absence of wounding. Application of PA6206 to the basolateral surface caused, after a delay of 10 to 50 min, increases in Cai from approximately 90 nM (range, 80 to 100 nM, 50 cells, three experiments) to 350 nM (range, 275 to 425 nM, 50 cells, three experiments) in cells throughout the monolayer (Fig. 3A, top). As with the experiments reported above, elevated Cai was sustained for 20 to 50 min, followed by the rapid loss of fura-2 (and uptake of propidium iodide, not shown), indicating loss of membrane integrity and cell death.
FIG. 3.
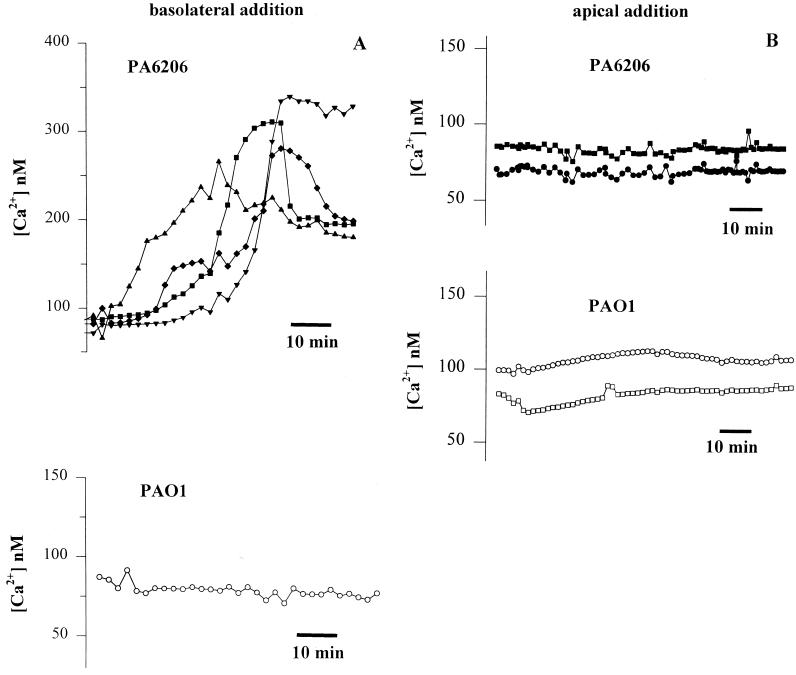
Effects of apical versus basolateral addition of P. aeruginosa on Cai in confluent Calu-3 cell monolayers. Calu-3 cells were grown to confluence on filters with 3.0-μm pores and loaded with fura-2, and either PA6206 or PAO1 (multiplicity of infection of 50) was added to the basolateral (A) or apical (B) side of the monolayer. (A) The upper panel shows representative results from four typical cells following basolateral addition of PA6206. Similar results were obtained in >35 other cells in the field. Basolateral addition of PA6206 caused a slow, sustained increase in Cai from 80 to 100 nM to 200 to 350 nM. Similar results were obtained on >35 cells in each of a total of six experiments. The lower panel shows results from one representative cell during basolateral exposure to PAO1. None of the 75 cells in the field exhibited any increases in Cai during approximately 60 min of exposure to bacteria. Similar results were obtained on >35 cells in each of a total of six experiments. (B) Typical results from two cells upon apical exposure to PA6206 (multiplicity of infection of 50) (upper panel) and from two cells during apical exposure to PAO1 (multiplicity of infection of 50) (lower panel). Neither PAO1 nor PA6206 had any effect on Cai in any of the >100 cells in the fields over the course of >60 min. Similar results were obtained for >35 cells in three experiments each for apical addition of PAO1 and PA6206.
Basolateral addition of the noncytotoxic strain PAO1 had no effect on Cai or cell viability over the course of up to 3 h (Fig. 3A, bottom). Similarly, the noncytotoxic strain PAK also had no effect on Cai when added to the basolateral surface of Calu-3 cells. Basolateral addition of PA6206 (multiplicity of infection of 50) to Calu-3 cells grown as confluent monolayers on 0.45-μm-pore-size filters, a configuration that prevented bacteria from direct contact with the cell layer but allowed access of secreted cytotoxic factors, failed to increase Cai or elicit cytotoxicity (data not shown).
In contrast to the effects of basolateral PA6206, apical addition of either PA6206 or PAO1 to confluent Calu-3 monolayers grown on 1.0- or 3.0-μm-pore-size filters had no effect on Cai over the course of up to 3 h (Fig. 3B). Staining with propidium iodide similarly showed no increase in dead cells for these treatments (data not shown), similar to results presented previously (39).
Together, these experiments demonstrated that in polarized airway epithelia, P. aeruginosa-induced increases in Cai occurred only when cytotoxic strains had direct access to the basolateral surface and could occur in the absence of wounding.
P. aeruginosa-mediated changes in Cai occurred by activation of basolateral Ca channels.
Agonist-induced increases in epithelial cell Cai usually occur in a biphasic manner. First, a rapid spike (often to 1 to 2 μM) is observed due to release of internal Ca stores from the endoplasmic reticulum. This release is followed by a sustained plateau, usually in the range 200 to 500 nM, resulting from Ca entry from the extracellular solution through Ca channels in the plasma membrane that open when the endoplasmic reticulum stores are depleted (store-operated Ca channels). Store-operated Ca channels are present in the basolateral membranes of some epithelial cells (22) and perhaps also the apical membrane (49); they are blocked by La3+ (46, 47). The slow and sustained P. aeruginosa-induced increase in Cai was consistent with entry of Ca from outside the cell through store-operated Ca channels (46).
We tested for the involvement of store-operated Ca channels by treating Calu-3 cells with thapsigargin, a Ca-ATPase pump inhibitor (58). This drug leads to depletion of Ca stores in the endoplasmic reticulum, opening of store-operated Ca channels, and sustained increases in Cai (46). If P. aeruginosa-elicited changes in Cai occurred through the opening of store-operated Ca channels, then thapsigargin and P. aeruginosa should have similar effects on Cai, though the effect of thapsigargin would likely occur faster because it is a lipid-soluble, membrane-permeating inhibitor with high affinity for the Ca pump in the endoplasmic reticulum (58). These experiments were performed on cells grown as islands on cover glasses because this configuration yielded the most informative responses to P. aeruginosa: Cai increased first in cells at the free edges and later in cells in the internal regions of the confluent monolayer.
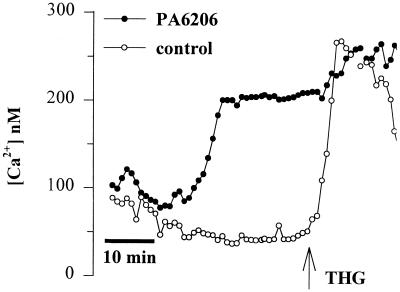
Fura-2-loaded cells were mounted in the perfusion chamber, and then PA6206 (multiplicity of infection of 100) was added. After a delay of 30 to 50 min, Cai increased in some cells along the free edges of the islands (Fig. 4), while there was no effect on cells in the confluent regions of the monolayer, similar to the responses shown in Fig. 1A. Once a P. aeruginosa-induced Cai response had occurred in a free edge cell, thapsigargin (10 μM) was added. As further shown in Fig. 4, thapsigargin caused Cai to increase up to the P. aeruginosa-induced level in the cells in the center of the islands, while there was only a small effect of thapsigargin on the free edge cells in which P. aeruginosa had already elevated Cai. This result was consistent with the idea that P. aeruginosa and thapsigargin had similar effects on Cai in Calu-3 cells. Of note, thapsigargin treatment alone did not elicit cytotoxicity during 2 h of treatment (data not shown).
FIG. 4.
PA6206 and thapsigargin have similar effects on Cai. Calu-3 cells were grown as islands on cover glasses, loaded with fura-2, and then exposed to PA6206 (multiplicity of infection, 100). After Cai in the cell at the periphery had increased to its plateau level, thapsigargin (10 μM; THG, arrow) was added. Thapsigargin caused Cai to increase in the cell that had not responded to P. aeruginosa, while Cai increased in the cell in the center that had not yet responded to P. aeruginosa. Similar results were obtained on 25 cells in three similar experiments.
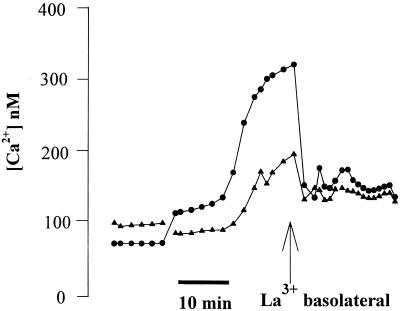
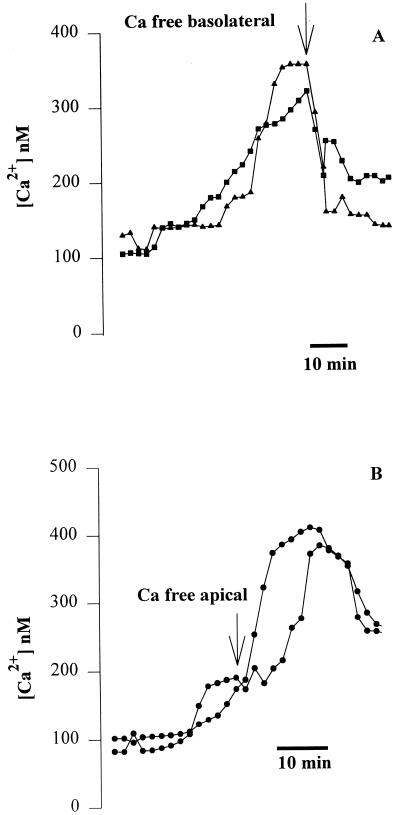
We next tested the role of apical and basolateral store-operated Ca channels in the P. aeruginosa-induced Cai increase. Confluent Calu-3 monolayers were grown on 3.0-μm-pore-size filters, and PA6206 was added to the basolateral side to induce the Cai response. Then the epithelia were treated selectively on the apical or basolateral surface with either La3+, an effective blocker of store-operated Ca channels (46), or Ca-free solutions. Addition of La3+ (1 mM) to only the basolateral surface caused Cai to decrease nearly to baseline, even in cells that had just begun to exhibit an increase in Cai (Fig. 5). Similarly, selective addition of Ca-free solution to the basolateral surface (with 1 mM Ca in the apical solution) caused the P. aeruginosa-induced increase in Cai to return to near the resting level (Fig. 6A). In contrast, Ca-free apical solution (with 1 mM Ca in the basolateral solution) had no significant effect on the P. aeruginosa-induced increase in Cai (Fig. 6B). These results suggested that P. aeruginosa-induced increases in Calu-3 Cai resulted from Ca entry through Ca channels in the basolateral membranes.
FIG. 5.
Basolateral addition of La3+ abolishes basolaterally applied PA6206-induced increases in Cai in Calu-3 cells. Calu-3 cells were grown to confluence on 3.0-μm-pore-size filters, loaded with fura-2, and exposed to PA6206 (multiplicity of infection of 50) from the basolateral surface. After Cai levels had increased, 1 mM La3+ was added to the basolateral surface, resulting in a decrease in Cai back to the baseline. The responses of two typical cells are shown; similar results were obtained with >40 cells in a total of four experiments.
FIG. 6.
Effects of exposure of the basolateral and apical surfaces to Ca-free solution after PA6206-induced increases in Cai in Calu-3 cells. Cells were grown to confluence on 3.0-μm-pore filters, loaded with fura-2, and exposed to PA6206 (multiplicity of infection of 50) on the basolateral surface of the monolayer. (A) After Cai had started to increase in two cells, the basolateral medium was replaced with Ca-free solution. This treatment caused Cai to decrease back to the baseline. Similar results were obtained in >50 cells in a total of four experiments. (B) After Cai had started to increase in two cells, the apical medium was replaced with Ca-free solution. This treatment had no effect on the P. aeruginosa-induced Cai response, which continued to increase. Similar results were obtained from >25 cells in a total of four experiments.
Roles of type III secretion, type IV pili, ExoT, and ExoU in P. aeruginosa-induced changes in Cai.
We investigated the roles of type III secretion, type IV pili, and the type III-secreted effectors ExoT and ExoU in modulating Cai with well-characterized isogenic mutants of the cytotoxic strain PA103. This human lung isolate secretes ExoU and ExoT and lacks the exoS gene. Similar to the results with PA6206, addition of PA103 (multiplicity of infection of 50) to the basolateral side of Calu-3 monolayers grown on 3.0-μm-pore-size filters resulted in a slow, sustained increase in Cai. No changes in Cai were observed upon apical addition of PA103 (data not shown). These results suggested that increases in Cai correlated with cytotoxicity and the secretion of ExoU by the type III secretion system.
We explicitly examined the involvement of type III secretion, type IV pili, and exotoxins T and U on Cai and cytotoxicity. Calu-3 cells were grown as confluent islands on cover glasses to allow easy cellular identification and timed comparisons of the Cai responses of cells on the free edges. We measured three parameters: time from addition of bacteria to the beginning of the increase in Cai, time from the initiation of the Cai response to the time of maximum Cai response, and the maximum Cai reached during the experiment. In separate experiments we confirmed that increases in Cai preceded loss of fura-2 and loss of fura-2 correlated with uptake of propidium iodide and cell death. Typical results with single cells are shown in Fig. 7, and average times and magnitudes of Cai responses for 50 different cells from three different experiments each are presented in Table 2.
FIG. 7.
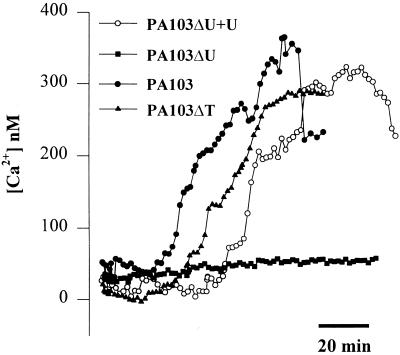
Role of type III-secreted effectors on P. aeruginosa-mediated increases in Cai. Calu-3 cells were grown on cover glasses as islands, then loaded with fura-2, and exposed to PA103, PA103ΔU (lacks ExoU), PA103ΔT (lacks ExoT), or PA103ΔU+U (multiplicity of infection of 50). Responses of typical “free edge” cells from five different Calu-3 islands are shown. The exoU mutant failed to increase Cai, while complementation with exoU expressed from a plasmid (pExoU) restored the ability of PA103ΔU to modulate Cai. Similar results were obtained from >25 cells in three experiments for each bacterial strain.
TABLE 2.
Role of ExoU and ExoT in P. aeruginosa-mediated increases in Caia
| Strain | Avg time from addition to Cai (min) ± SD | Avg time from beginning Cai to maximum Cai (min) ± SD | Maximum Cai (nM) |
|---|---|---|---|
| PA103 | 69 ± 31 | 59 ± 28 | 326 ± 68 |
| PA103ΔU+U | 106 ± 32 | 91 ± 25 | 318 ± 59 |
| PA103ΔT | 87 ± 23 | 88 ± 26 | 289 ± 57 |
PA103 strains were added to the apical surface of Calu-3 cells that had been grown as confluent islands on cover glasses and loaded with Fura-2 prior to bacterial addition. Average time from the addition of the different strains to the time when Cai just began to increase in free edge cells and average time from the beginning of the Cai response (increase) to the time when Cai reached a maximum are shown. Data shown were derived from measuring Cai in 50 cells in three independent experiments. Times from addition of bacteria to first increase in Cai were all statistically significant (P < 0.05) among PA103, PA103ΔU+U, and PA103ΔT. Maximum change in Cai was smaller (P < 0.05) for PA103ΔT compared to PA103 and PA103ΔU+U, which were statistically indistinguishable (P > 0.05).
PA103 caused a slow, sustained increase in Cai in free edge cells, similar to the effects of PA6206 (see Fig. 4). Though the times required between addition of PA103 and the Cai responses were slightly longer than with PA6206—average time was 69 ± 31 min (range, 20 to 80 min) with PA103 versus 47 ± 26 min (range, 10 to 50 min) with PA6206—they failed to reach statistical significance (P > 0.05, Student's two-tailed t test). The time from first increase in Cai to the maximum increase in Cai and the magnitude of the maximum Cai were very similar in PA6206 (52 ± 25 min and 342 ± 84 nM) and PA103 (59 ± 28 min and 326 ± 68 nM) (P ≫ 0.05 in each case). Thus, the two cytotoxic strains PA103 and PA6206 had very similar effects on Cai and cytotoxicity.
Addition of PA103 mutants defective in type III secretion (PA103exsA and PA103pscJ) failed to elicit any significant changes in Cai, even after 3 h of incubation (data not shown). Type IV pili have been implicated in the adhesion of P. aeruginosa to epithelial cells; they are also required for translocation of type III-secreted effectors (35; J. Engel, unpublished data). PA103pilA, which fails to produce the pilin structural subunit and therefore lacks type IV pili, also elicited no increases in Cai (data not shown). These data showed that type III secretion and type IV pili were both necessary to elicit increases in epithelial cell Cai.
To test whether the known PA103 type III-secreted effectors ExoU and ExoT were necessary, PA103ΔU and PA103ΔT were added to the Calu-3 cells. Typical results from single cells are shown in Fig. 7, and average responses from 50 different cells (three different preparations) are summarized in Table 2. While PA103ΔT caused changes in Cai that were similar (but slightly smaller) to those observed with the parental PA103 strain, PA103ΔU, harboring an in-frame deletion in exoU, did not affect Cai (Fig. 7) over the course of 3 h of incubation with the bacteria. Complementation of PA103ΔU with the cloned exoU gene expressed from a low-copy-number plasmid had effects on Cai that were similar to those of wild-type PA103 (Fig. 7 and Table 2).
While the onset of increased Cai induced by PA103ΔU+U was a bit slower than that with the wild type, the maximal levels of Cai were similar (Table 2). All strains that increased Cai were cytotoxic to the cells, as evidenced by loss of fura-2 after 60 min (data not shown). This finding is consistent with previous reports that PA103 and PA103ΔT are cytotoxic to epithelial cells (20). Together, these results suggested that type III-mediated translocation of ExoU into airway epithelial cells was required for the observed increases in Cai, while ExoT was not required.
DISCUSSION
Importance of epithelial polarity in the P. aeruginosa-triggered increase in Cai.
Our studies suggest that ExoU-producing, cytotoxic strains of P. aeruginosa trigger sustained increases in Cai when given access to the basolateral surface of airway epithelial cells. In contrast, apical addition of PA6206 elicited changes in Cai only in cells at the edges of wounded monolayers, not in cells in confluent, highly polarized regions of the monolayer. In these areas, basolateral addition of bacteria was required to trigger the Cai response. The Cai changes seen in cells at the wound edge likely occurred by increased basolateral access or by relocalization of the Cai signaling machinery to the apical surface of the injured cells. We thus conclude that the basolateral surfaces of airway epithelial cells have all the receptors and downstream signaling molecules necessary for ExoU-producing, cytotoxic P. aeruginosa to stimulate increases in Cai. In addition, the capacity of ExoU-producing strains to increase airway epithelial cell Cai required direct host cell contact, as it only occurred in filter-grown monolayers when 3-μm-pore-size filters were used and was not observed with 0.45-μm-pore-size filters.
Noncytotoxic strains PAO1 and PAK and various isogenic, non-ExoU-producing mutants of PA103 had no significant effects on Cai when the bacteria were added from either the apical or basolateral surface of Calu-3 cells. Also, the effects of these different strains of P. aeruginosa were not cell line dependent, as similar results were observed with both Calu-3 and JME cells (cystic fibrosis nasal cell line) (32, 34, 61; G. Lu and T. Machen, unpublished data).
P. aeruginosa, Cai, and control of epithelial cell gene expression.
In contrast to the results presented here, Ratner and colleagues (52) have reported that apical PAO1 caused rapid and transient changes in Cai (increase within 60 to 300 s and then decrease within another 100 s) in an airway epithelial cell line, HBEo− that expresses CFTR. It is unlikely that we missed detecting rapid, transient Cai responses (52) because we observed similar responses in experiments in which images were collected every 2 s or every 20 s (ratio measurements were made every 12 s by Prince and coworkers). In addition, it was extremely unlikely that all the >100 Calu-3 cells in any one field or image would have had rapid PAO1-induced Cai oscillations that were precisely synchronized so that we missed all of them in every experiment.
The different results may have been methodological. Previous work (52) used subconfluent, incompletely polarized HBEo− cells grown on cover glasses, while we used polarized, confluent Calu-3 cells grown on filters or cover glasses. It is also possible that the previous experimenters added bacteria to the chamber more rapidly than we did, and turbulent flows of solution near the epithelial cells could have triggered the release of ATP (30) or other agonists that elicited the changes in Cai. Whatever the explanation for the different Cai responses between this and the previous study (52), it is clear that P. aeruginosa does not universally lead to increases in Cai in polarized airway epithelial cells and also that subsequent activation of host cell gene expression (10, 11, 30, 41) similarly does not require increases in Cai.
P. aeruginosa-induced Cai response requires pili, type III secretion, and ExoU.
As shown by the lack of effect of PA103pilA on Cai, pili were required for the P. aeruginosa-induced changes in Cai. These polar fimbriae act as bacteriophage receptors, are required for twitching motility, and can function as adhesins to asialo-GM1, found on the surface of some cells (24, 40, 53, 55, 67). The lack of effect of pilin-defective strains on Cai may reflect a defect in the type III secretion process such as translocation, and/or it may reflect the lack of binding to a specific receptor.
Our experiments also showed that the type III-secreted protein ExoU was necessary for the P. aeruginosa-mediated increase in Cai. While wild-type PA103 increased Cai, an isogenic mutant of PA103 carrying an in-fame deletion in the exoU gene (PA103ΔU) did not elicit any significant changes in Cai when added to the basolateral surfaces of airway epithelial cells or to the free edges of the cells grown as islands. In addition, complementation of PA103ΔU with the cloned exoU gene restored the ability of the bacterium to increase Cai. Likewise, isogenic PA103 mutants with an intact exoU gene but harboring a transposon mutation in an operon encoding part of the type III secretion machinery (PA103exsA and PA103pscJ) also failed to cause changes in Cai. Interestingly, strains that do not produce or secrete ExoU are also less virulent in an animal model of acute pneumonia (54).
ExoS, ExoT, and ExoY are not required for P. aeruginosa to trigger the Cai response.
PA6206 and PA103, which both exhibit type III secretion of ExoT and ExoU but not ExoS or ExoY, had very similar effects on Cai. Thus, neither ExoS nor ExoY was required for PA6206- or PA103-mediated changes in Cai. PA103ΔT elicited Cai responses similar to those with the wild-type PA103, indicating that ExoT was also not required, though it might play a regulatory role (the effects of PA103ΔT on Cai were somewhat slower and smaller than those of the wild-type PA103). Consistent with these observations, neither PAO1 nor PAK, which both produce ExoS and ExoT but not ExoU, affected Cai.
How does ExoU elicit increases in Cai?
Several observations indicated that the ExoU-triggered increase in Cai did not result from a simple breakdown of the cell membranes with resulting influx of extracellular Ca2+ but instead resulted from a selective process that included opening of Ca channels in the basolateral but not apical membrane. First, P. aeruginosa-induced increases in Cai occurred relatively slowly (over the course of 20 to 50 min) and were small (approximately 300 nM) and sustained (up to 50 min). Second, increases in Cai were reduced by Ca-free basolateral but not apical Ca-free solution and also by the Ca channel blocker La3+. Third, addition of the endoplasmic reticulum Ca pump inhibitor thapsigargin caused Cai to increase to the same level as elicited by P. aeruginosa, while there was no further effect of thapsigargin on Cai in Calu-3 cells in which P. aeruginosa had already elicited its effects on Cai. Fourth and finally, P. aeruginosa-induced increases in Cai occurred 10 to 50 min before cytotoxicity (i.e., loss of fura-2 and uptake of propidium iodide) occurred.
One possible mechanism for the P. aeruginosa-mediated Cai is injection of ExoU into the epithelial cells through the type III secretion apparatus, leading to a release of Ca2+ from the internal stores, perhaps by opening either inositol triphosphate receptors or leak pathways (29). This effect would deplete stored Ca2+ and open store-operated Ca channels in the basolateral membranes of the epithelial cells.
ExoU, Cai, and cytotoxicity.
Although ExoU was sufficient both to elicit increases in Cai and also to cause cytotoxicity, the specific role of increased Cai in ExoU-mediated cytotoxicity remains unclear. It is likely that the increases in Cai alone were not sufficient to elicit cytotoxicity because thapsigargin caused similar increases in Cai without causing cytotoxicity over 3 h of treatment. In addition, neither the cytosolic calcium buffer BAPTA nor the Ca channel blocker La3+ prevented cytotoxicity.
Previous work similarly showed that P. aeruginosa-induced cytotoxicity in corneal epithelial cells was not blocked by BAPTA (13). Thus, ExoU may have other effects on epithelial cells besides the proposed effects to release Ca2+ from the endoplasmic reticulum (23, 59). Further investigations of the effects of ExoU on Cai regulation by the endoplasmic reticulum and other Ca-storing organelles (e.g., mitochondria) may provide clues to how this toxin elicits its cytotoxic and pathophysiological effects.
Acknowledgments
This work was supported by NIH grants RO1DK51799 (T.E.M.) and R01AI42806 (J.N.E.) and by Tobacco Disease-Related Research Program grant 8IT0052 (T.E.M.). J.N.E. is a Career Investigator of the American Lung Association.
Editor: J. T. Barbieri
REFERENCES
- 1.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 2.Allewelt, M., F. T. Coleman, M. Grout, G. P. Priebe, and G. B. Pier. 2000. Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect. Immun. 68:3998-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apodaca, G., M. Bomsel, R. Lindstedt, J. Engel, D. Frank, K. Mostov, and J. Wiener-Kronish. 1995. Characterization of Pseudomonas aeruginosa-induced MDCK cell injury: glycosylation defective host cells are resistant to bacterial killing. Infect. Immun. 63:1541-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bals, R., X. Wang, R. L. Meegalla, S. Wattler, D. J. Weiner, M. C. Nehls, and J. M. Wilson. 1999. Mouse beta-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect. Immun. 67:3542-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comolli, J., A. Hauser, L. Waite, C. Whitchurch, J. Mattick, and J. Engel. 1999. PilU and PilT are required for cytotoxicity and virulence of Pseudomonas aeruginosa. Infect. Immun. 67:3625-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comolli, J., L. Waite, K. Mostov, and J. N. Engel. 1999. The interaction of Pseudomonas aeruginosa pili and asialo-GM1 stimulates epithelial cell cytotoxicity and bacterial internalization. Infect. Immun. 67:3207-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dacheux, D., B. Toussaint, M. Richard, G. Brochier, J. Croize, and I. Attree. 2000. Pseudomonas aeruginosa cystic fibrosis isolates induce rapid, type III secretion-dependent but ExoU-independent oncosis of macrophages and polymorphonuclear neutrophils. Infect. Immun. 68:2916-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bentzmann, S., P. Roger, and E. Puchelle. 1996. Pseudomonas aeruginosa adherence to remodelling respiratory epithelium. Eur. Respir. J. 9:2145-2150. [DOI] [PubMed] [Google Scholar]
- 9.de Bentzmann, S., P. Roger, F. Dupuit, O. Bajolet-Laudinat, C. Fuchey, M. C. Plotkowski, and E. Puchelle. 1996. Asialo GM1 is a receptor for Pseudomonas aeruginosa adherence to regenerating respiratory epithelial cells. Infect. Immun. 64:1582-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiMango, E., A. J. Ratner, R. Bryan, S. Tabibi, and A. Prince. 1998. Activation of NF-κB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J. Clin. Investig. 101:2598-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiMango, E., H. J. Zar, R. Bryan, and A. Prince. 1995. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J. Clin. Investig. 96:2204-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowling, R. B., R. Newton, A. Robichaud, P. J. Cole, P. J. Barnes, and R. Wilson. 1998. Effect of inhibition of nitric oxide synthase on Pseudomonas aeruginosa infection of respiratory mucosa in vitro. Am. J. Resp. Cell. Mol. 19:950-958. [DOI] [PubMed] [Google Scholar]
- 13.Evans, D. J., D. W. Frank, V. Finck-Barbancon, C. Wu, and S. M. Fleiszig. 1998. Pseudomonas aeruginosa invasion and cytotoxicity are independent events, both of which involve protein tyrosine kinase activity. Infect. Immun. 66:1453-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feltman, H., G. Schulert, S. Khan, M. Jain, L. Peterson, and A. R. Hauser. 2001. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 147:2659-2669. [DOI] [PubMed] [Google Scholar]
- 15.Finck-Barbancon, V., J. Goranson, L. Zhu, T. Sawa, J. P. Wiener-Kronish, S. M. Fleiszig, C. Wu, L. Mende-Mueller, and D. W. Frank. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 25:547-557. [DOI] [PubMed] [Google Scholar]
- 16.Fleiszig, S. M., D. J. Evans, N. Do, V. Vallas, S. Shin, and K. E. Mostov. 1997. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect. Immun. 65:2861-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleiszig, S. M., T. S. Zaidi, M. J. Preston, M. Grout, D. J. Evans, and G. B. Pier. 1996. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect. Immun. 64:2288-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank, D., and B. H. Iglewski. 1991. Cloning and sequence analysis of a trans-regulatory locus required for exoenzyme S synthesis in Pseudomonas aeruginosa. J. Bacteriol. 173:6460-6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganesan, A. K., D. W. Frank, R. P. Misra, G. Schmidt, and J. T. Barbieri. 1998. Pseudomonas aeruginosa exoenzyme S ADP-ribosylates Ras at multiple sites. J. Biol. Chem. 273:7332-7337. [DOI] [PubMed] [Google Scholar]
- 20.Garrity-Ryan, L., B. Kazmierczak, R. Kowal, J. Commolli, A. Hauser, and J. Engel. 2000. The arginine finger domain of ExoT is required for actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect. Immun. 68:7100-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geiser, T., B. Kazmierczak, L. Garrity, M. Matthay, and J. Engel. 2001. Pseudomonas aeruginosa ExoT inhibits in vitro lung epithelial wound repair. Cell. Microbiol. 3:223-236. [DOI] [PubMed] [Google Scholar]
- 22.Gordjani, N., R. Nitschke, R. Greger, and J. Leipziger. 1997. Capacitative Ca2+ entry (CCE) induced by luminal and basolateral ATP in polarised MDCK-C7 cells is restricted to the basolateral membrane. Cell Calcium 22:121-128. [DOI] [PubMed] [Google Scholar]
- 23.Hahn, H. P. 1997. The type-4 pilus is the major virulence-associated adhesin of Pseudomonas aeruginosa—a review. Gene 192:99-108. [DOI] [PubMed] [Google Scholar]
- 24.Hauser, A., and J. Engel. 1999. Pseudomonas aeruginosa induces type III secretion-mediated apoptosis in macrophages and epithelial cells. Infect. Immun. 67:5530-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauser, A. R., S. M. Fleiszig, P. J. Kang, K. Mostov, and J. N. Engel. 1998. Defects in type III secretion correlate with internalization of Pseudomonas aeruginosa by epithelial cells. Infect. Immun. 66:1413-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauser, A. R., P. J. Kang, and J. Engel. 1998. PepA, a novel secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol. Microbiol. 27:807-818. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 28.Hirakata, Y., B. B. Finlay, D. A. Simpson, S. Kohno, S. Kamihira, and D. P. Speert. 2000. Penetration of clinical isolates of Pseudomonas aeruginosa through MDCK epithelial cell monolayers. J. Infect. Dis. 181:765-769. [DOI] [PubMed] [Google Scholar]
- 29.Hofer, A. M., S. Curci, T. E. Machen, and L. Schulz. 1996. ATP regulates calcium leak from agonist-sensitive internal calcium stores. FASEB J. 10:302-308. [DOI] [PubMed] [Google Scholar]
- 30.Huang, P., E. R. Lazarowski, R. Tarran, S. L. Milgram, R. C. Boucher, and M. J. Stutts. 2001. Compartmentalized autocrine signaling to the cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc. Natl. Acad. Sci. USA 98:14120-14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ichikawa, J. K., A. Norris, M. G. Bangera, G. K. Geiss, A. B. van't Wout, R. E. Bumgarner, and S. Lory. 2000. Interaction of Pseudomonas aeruginosa with epithelial cells: identification of differentially regulated genes by expression microarray analysis of human cDNAs. Proc. Natl. Acad. Sci. USA 97:9659-9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Illek, B., J. R. Yankaskas, and T. E. Machen. 1997. cAMP and genistein stimulate HCO3− conductance through CFTR in human airway epithelia. Am. J. Physiol. 272:L752-L761. [DOI] [PubMed] [Google Scholar]
- 33.Imundo, L., J. Barasch, A. Prince, and Q. Al-Awqati. 1995. Cystic fibrosis epithelial cells have a receptor for pathogenic bacteria on their apical surface. Proc. Natl. Acad. Sci. USA 92:3019-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jefferson, D. M., J. D. Valentich, F. C. Marini, S. A. Grubman, M. C. Iannuzzi, H. L. Dorkin, M. Li, K. W. Klinger, and M. J. Welsh. 1990. Expression of normal and cystic fibrosis phenotypes by continuous airway epithelial cell lines. Am. J. Physiol. 259:L496-L505. [DOI] [PubMed] [Google Scholar]
- 35.Kang, P. J., A. R. Hauser, G. Apodaca, S. Fleiszig, J. Wiener-Kronish, K. Mostov, and J. N. Engel. 1997. Identification of Pseudomonas aeruginosa genes required for epithelial cell injury. Mol. Microbiol. 24:1249-1262. [DOI] [PubMed] [Google Scholar]
- 36.Kazmierczak, B. I., T. S. Jou, K. Mostov, and J. N. Engel. 2001. Rho GTPase activity modulates Pseudomonas aeruginosa internalization by epithelial cells. Cell. Microbiol. 3:85-98. [DOI] [PubMed] [Google Scholar]
- 37.Krall, R., G. Schmidt, K. Aktories, and J. T. Barbieri. 2000. Pseudomonas aeruginosa ExoT is a Rho GTPase-activating protein. Infect. Immun. 68:6066-6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurahashi, K., O. Kajikawa, T. Sawa, M. Ohara, M. A. Gropper, D. W. Frank, T. R. Martin, and J. P. Wiener-Kronish. 1999. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J. Clin. Investig. 104:743-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee, A., D. Chow, W. Tseng, B. Haus, D. Evans, S. M. Fleiszig, G. Chandy, and T. E. Machen. 1999. Airway epithelial tight junctions and binding and cytotoxicity of Pseudomonas aeruginosa. Am. J. Physiol. 277:L204-L217. [DOI] [PubMed] [Google Scholar]
- 40.Lee, K. K., H. B. Sheth, W. Y. Wong, R. Sherburne, W. Paranchych, R. S. Hodges, C. A. Lingwood, H. Krivan, and R. T. Irvin. 1994. The binding of Pseudomonas aeruginosa pili to glycosphingolipids is a tip-associated event involving the C-terminal region of the structural pilin subunit. Mol. Microbiol. 11:705-713. [DOI] [PubMed] [Google Scholar]
- 41.Li, J. D., A. F. Dohrman, M. Gallup, S. Miyata, J. R. Gum, Y. S. Kim, J. A. Nadel, A. Prince, and C. B. Basbaum. 1997. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc. Natl. Acad. Sci. USA 94:967-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li, J. D., W. Feng, M. Gallup, J. H. Kim, J. Gum, Y. Kim, and C. Basbaum. 1998. Activation of NF-kappaB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells. Proc. Natl. Acad. Sci. USA 95:5718-5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu, P. V. 1966. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis: identity of the lethal toxins produced in vitro and in vivo. J. Infect. Dis. 116:481-489. [DOI] [PubMed] [Google Scholar]
- 44.Liu, S., T. L. Yahr, D. W. Frank, and J. T. Barbieri. 1997. Biochemical relationships between the 53-kilodalton (Exo53) and 49-kilodalton (ExoS) forms of exoenzyme S of Pseudomonas aeruginosa. J. Bacteriol. 179:1609-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Negulescu, P. A., and T. E. Machen. 1990. Intracellular ion activities and membrane transport in parietal cells measured with fluorescent dyes. Methods Enzymol. 192:38-81. [DOI] [PubMed] [Google Scholar]
- 46.Negulescu, P. A., and T. E. Machen. 1993. Ca transport by plasma membrane and intracellular stores of gastric cells. Am. J. Physiol. 264:C843-C851. [DOI] [PubMed] [Google Scholar]
- 47.Negulescu, P. A., W. Reenstra, and T. E. Machen. 1989. Intracellular Ca requirements for stimulus-secretion coupling in parietal cell. Am. J. Physiol. 256:C241-C242. [DOI] [PubMed] [Google Scholar]
- 48.Nicas, T. I., and B. H. Iglewski. 1984. Isolation and characterization of transposon-induced mutants of Pseudomonas aeruginosa deficient in production of exoenzyme S. Infect. Immun. 45:470-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paradiso, A. M., S. J. Mason, E. R. Lazarowski, and R. C. Boucher. 1995. Membrane-restricted regulation of Ca2+ release and influx in polarized epithelia. Nature 377:643-646. [DOI] [PubMed] [Google Scholar]
- 50.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, et al. 1998. Defective lipopolysaccharide signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 51.Ramphal, R., M. T. McNiece, and F. M. Polack. 1981. Adherence of Pseudomonas aeruginosa to the injured cornea: a step in the pathogenesis of corneal infections. Ann. Ophthalmol. 100:1956-1958. [PubMed] [Google Scholar]
- 52.Ratner, A. J., R. Bryan, A. Weber, S. Nguyen, D. Barnes, A. Pitt, S. E. Gelber, A. Cheung, and A. Prince. 2001. Cystic fibrosis pathogens activate Ca2+-dependent mitogen-activated protein kinase signaling pathways in airway epithelial cells. J. Biol. Chem. 276:19267-19275. [DOI] [PubMed] [Google Scholar]
- 53.Saiman, L., and A. Prince. 1993. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J. Clin. Investig. 92:1875-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sawa, T., M. Ohara, K. Kurahashi, S. Twining, D. Frank, D. Doroques, T. Long, M. Gropper, and J. Wiener-Kronish. 1998. In vitro cellular cytotoxicity predicts Pseudomonas aeruginosa virulence in lung infections. Infect. Immun. 66:3242-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheth, H. B., K. K. Lee, W. Y. Wong, G. Srivastava, O. Hindsgaul, R. S. Hodges, W. Paranchych, and R. T. Irvin. 1994. The pili of Pseudomonas aeruginosa strains PAK and PAO bind specifically to the carbohydrate sequence beta GalNAc(1-4)beta Gal found in glycosphingolipids asialo-GM1 and asialo-GM2. Mol. Microbiol. 11:715-723. [DOI] [PubMed] [Google Scholar]
- 56.Sjaastad, M. D., K. S. Zettl, G. Parry, G. L. Firestone, and T. E. Machen. 1993. Hormonal regulation of the polarized function and distribution of Na/H exchange and Na/HCO3 cotransport in cultured mammary epithelial cells. J. Cell Biol. 122:589-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teter, K., G. Chandy, B. Quiñones, K. Pereyra, T. E. Machen, and H.-P. Moore. 1998. Cellubrevin-targeted fluorescence uncovers heterogeneity in the recycling endosomes. J. Biol. Chem. 273:19625-19633. [DOI] [PubMed] [Google Scholar]
- 58.Thastrup, O., A. P. Dawson, O. Scharff, B. Foder, P. J. Cullen, B. K. Drobak, P. J. Bjerrum, S. B. Christensen, and M. R. Hanley. 1994. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions 43:187-193. [DOI] [PubMed] [Google Scholar]
- 59.Vallis, A. J., V. Finck-Barbancon, T. L. Yahr, and D. W. Frank. 1999. Biological effects of Pseudomonas aeruginosa type III-secreted proteins on CHO cells. Infect. Immun. 67:2040-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu, M., J. Llopis, S. Adams, M. McCaffery, T. E. Machen, H.-P. Moore, and R. Y. Tsien. 2000. Organelle pH studies with targeted avidin and fluorescein-biotin. Chem. Biol. 7:197-209. [DOI] [PubMed] [Google Scholar]
- 61.Wunderlich, E., C. Green, G. Chandy, and T. E. Machen. 2000. H and HCO3 transport mechanisms in normal and cystic fibrosis airway epithelia. Pediatr. Pulmonol. Suppl. 20:195. [Google Scholar]
- 62.Yahr, T. L., J. T. Barbieri, and D. W. Frank. 1996. Genetic relationship between the 53- and 49-kilodalton forms of exoenzyme S from Pseudomonas aeruginosa. J. Bacteriol. 178:1412-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yahr, T. L., A. J. Vallis, M. K. Hancock, J. T. Barbieri, and D. W. Frank. 1998. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. USA 95:13899-13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang, R. B., M. R. Mark, A. Gray, A. Huang, M. H. Xie, M. Zhang, A. Goddard, W. I. Wood, A. L. Gurney, and P. J. Godowski. 1998. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 395:284-288. [DOI] [PubMed] [Google Scholar]
- 65.Yang, H., D. W. Young, F. Gusovsky, and J. C. Chow. 2000. Cellular events mediated by lipopolysaccharide-stimulated toll-like receptor 4. MD-2 is required for activation of mitogen-activated protein kinases and Elk-1. J. Biol. Chem. 275:20861-20866. [DOI] [PubMed] [Google Scholar]
- 66.Zar, H., L. Saiman, L. Quittell, and A. Prince. 1995. Binding of Pseudomonas aeruginosa to respiratory epithelial cells from patients with various mutations in the cystic fibrosis transmembrane regulator. J. Pediatr. 126:230-233. [DOI] [PubMed] [Google Scholar]
- 67.Zoutman, D. E., W. C. Hulbert, B. L. Pasloske, A. M. Joffe, K. Volpel, M. K. Trebilcock, and W. Paranchych. 1991. The role of polar pili in the adherence of Pseudomonas aeruginosa to injured canine tracheal cells: a semiquantitative morphologic study. Scanning Microsc. 5:109-126. [PubMed] [Google Scholar]