Abstract
Corticotropin-releasing hormone (CRH) exerts an anti-inflammatory effect indirectly, via cortisole production, and a proinflammatory effect directly on immune cells. The aim of the present work was to examine the effect of CRH on macrophage-derived cytokines both in vitro and in vivo. For the in vitro experiments we used two types of macrophages: (i) the RAW264.7 monocyte/macrophage cell line and (ii) thioglycolate-elicited peritoneal macrophages from BALB/c mice. We have found that CRH enhanced lipopolysaccharide (LPS)-induced tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6 production. For the in vivo experiments we have used the LPS-induced endotoxin shock model in BALB/c mice, an established model for systemic inflammation in which macrophages are the major source of the proinflammatory cytokines responsible for the development of the shock. Administration of antalarmin, a synthetic CRH receptor 1 (CRHR1) antagonist, prior to LPS prolonged survival in a statistically significant manner. The effect was more evident at the early stages of endotoxin shock. CRHR1 blockade suppressed LPS-induced elevation of the macrophage-derived cytokines TNF-α, IL-1β, and IL-6, confirming the role of CRH signals in cytokine expression. In conclusion, our data suggest that CRH signals play an early and crucial role in augmenting LPS-induced proinflammatory cytokine production by macrophages. Our data suggest that the diffuse neuroendocrine system via CRH directly affects the immune system at the level of macrophage activation and cytokine production.
Corticotropin-releasing hormone (CRH) affects the immune system. This effect can be indirect, via the end products of the two major axes of the adaptive response to stress, regulated by CRH, i.e., cortisole of the hypothalamus-pituitary-adrenals (HPA) axis and catecholamines of the sympathetic system (13). CRH also affects the immune system in a direct manner. Indeed, CRH is released at the site of inflammation by nerve terminals and epithelial cells, affecting resident immune cells (13, 33). It should be noted that while the indirect effect of CRH is anti-inflammatory, its direct paracrine effect is definitely proinflammatory. Thus, blockade of its paracrine effect by specific anti-CRH serum attenuates the inflammatory response in several models of inflammation in vivo (15, 33). A major immune target of CRH is the mast cell (13, 25). However, in addition to mast cells a growing list of immune cells exhibits specific CRH binding sites. Thus, mouse splenocytes (34), human peripheral blood monocytes and lymphocytes (24), and monocytes/macrophages and Th cells (2) have CRH receptors. CRH receptors are also present in inflamed synovium (9) and inflamed subcutaneous tissues (19). CRHR1 expression is upregulated by lipopolysaccharide (LPS) stimulation (22). The aim of the present work was to study the effect of CRH on macrophages, the main source of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6, which initiate the inflammatory response. Since macrophages express CRH receptors, we hypothesized that CRH may exert a direct effect on the mechanisms that underline their activation. Thus, the first part of the present work consists of experiments examining the effect of CRH on proinflammatory cytokine production from macrophages in vitro. For this purpose we have employed the RAW264.7 cell line, which derives from a mouse myeloma and produces all proinflammatory cytokines in response to LPS, and thioglycolate-elicited peritoneal macrophages from BALB/c mice.
The biological effects of CRH are mediated by at least two different receptors, CRHR1 and CRHR2 that belong to the G-protein-coupled receptor superfamily (21). CRH exhibits an affinity towards CRHR1 10 times higher than that toward CRHR2 (21). In the immune system, CRHR1 receptors have been identified in spleen and thymus (3, 22).
The recent synthesis of non-peptide receptor antagonists for the CRHR1 receptor has provided a useful tool for a more accurate evaluation of the functional significance of CRH at the tissue level. Antalarmin, a pyrrolopyrimidine compound, is a specific CRHR1 receptor antagonist (32). Administration of antalarmin inhibits CRH-induced local inflammation (32) when used at a concentration of 20 mg/kg of body weight. Treatment of animals with antalarmin at this concentration for up to 1 week does not appear to influence significantly basal or stress-induced plasma adrenocorticotropin (ACTH) and corticosterone levels (10, 35).
The second part of the work consists of experiments examining the effect of CRH on the inflammatory response in vivo. In vivo studies on the direct effect of CRH on the inflammatory response have been focused on models of local inflammation restricted to confined areas of the body. We hypothesized that peripheral CRH may play a proinflammatory role by targeting macrophages in vivo. In addition, CRH may contribute in the development of systemic and potentially lethal inflammatory reactions, which depend on secretion of proinflammatory cytokines by macrophages. The LPS-induced endotoxin shock in mice is a widely used in vivo model for the study of acute systemic inflammatory reaction and macrophage activation.
We examined the effect of CRH on macrophages in vivo using the LPS-induced systemic endotoxin shock in mice. Specifically, to determine the role of locally secreted CRH in the development of endotoxin shock we treated mice with the specific CRHR1 inhibitor antalarmin in a dose and time frame that does not affect significantly the HPA axis (6, 35). Corticosterone was monitored both in antalarmin-treated and control animals. Blockade of CRHR1 did not affect the LPS-induced HPA axis response. Both our in vitro and in vivo data suggest that CRH augments the response of macrophages to inflammatory agents.
MATERIALS AND METHODS
Cell culture.
RAW264.7 cells were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, 10 mM l-glutamine, penicillin (100 U/ml), and streptomycin (0.1 mg/ml) (all purchased from Gibco) at 5% CO2 and 37°C. Cells were plated in 25-cm2 flasks at a concentration of 4 × 105/ml 1 day prior to stimulation. Cells were then stimulated with Escherichia coli-derived LPS (10 μg/ml; serotype 0111:B4 [catalog no, L2630]; Sigma) and synthetic CRH (Sigma) at a concentration of 10−8 M.
Isolation and stimulation of thioglycolate-elicited macrophages.
A 4% thioglycolate solution was prepared and autoclaved 2 days prior to administration. A 1.5-ml aliquot of the thioglycolate solution was injected intraperitoneally (i.p.) in BALB/c mice, and peritoneal macrophages were isolated by lavage of the peritoneal cavity with DMEM. Cells were then cultured in DMEM supplemented with 10% fetal calf serum, 10 mM l-glutamine, penicillin (100 U/ml), and streptomycin (0.1 mg/ml) (all from Gibco). Cells were moved to plates at a concentration of 5 × 105/ml and maintained in culture 24 h prior to stimulation.
Isolation of total RNA and RT-PCR.
Primers for actin were sense, 5′-TCA GAA GAA CTC CTA TGT GG-3′, and antisense, 5′-TCT CTT TGA TGT CAC GCA CG-3′, giving a 499-bp product; those for TNF-α were 5′-CAC GCT CTT CTG TCT ACT GAA CTT CG-3′ and 5′-GGC TGG GTA GAG AAT GGA TGA ACA CC-3′, giving a 590-bp product; those for IL-1β were 5′-GGA TGA GGA CAT GAG CAC CT-3′ and 5′-TCC ATT GAG GTG GAG AGC TT-3′, resulting in a 196-bp product; those for IL-6 were 5′-TGA AGT TCC TCT CTG CAA GAG ACT-3′ and 5′-TGA GGA AGG CCG TGG TTG T-3′, giving a 200-bp product. Total cellular RNA was isolated using Trizol reagent (Gibco). Following reverse transcription (RT) (Thermoscript RT; Invitrogen), 1 μl of the cDNA product was amplified by PCR (Platinum Taq polymerase; Invitrogen), at 33 cycles, annealing to a temperature of 55°C. It should be noted that at 33 cycles all mRNA amplifications were at the exponential phase of amplification as indicated by a standard curve performed for each pair of primers (data not shown). The amplified products (10 μl) were separated on a 3% agarose gel and visualized by ethidium bromide staining using the Bio-Rad molecular analyst system. The quantitation was performed using TINAscan software. Each experiment was repeated four times.
Animals and reagents.
Male 20- to 25-g BALB/c mice, 8 to 10 weeks old, were used. They were kept in our animal facility for at least 1 week prior to each experiment to allow adjustment and confirmation of their health. All experiments were approved by the animal care committee. Each animal received rodent laboratory chow and water ad libitum. Antalarmin was kindly provided by G. P. Chrousos (Pediatric and Reproductive Endocrinology Branch, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md.). Antalarmin was initially dissolved into 100% ethanol at a concentration of 200 mg/ml and then diluted in a 1:1 ratio with Cremaphor EL (Sigma) and finally brought to a working stock of 2-mg/ml antalarmin in 10% ethanol and 10% Cremaphor EL in sterile water. E. coli LPS (serotype 0111:B4) and Salmonella enterica serovar Enteritidis LPS (catalog no. L6011) were purchased from Sigma. The antibodies and the reagents for the TNF-α, IL-1β and IL-6 determination were purchased from R&D.
LPS-induced endotoxin shock.
For the determination of the 50% lethal dose (LD50), groups composed of five mice were received i.p injections of either 200, 400, 600, 700, or 1,000 μg of Salmonella-derived LPS (Sigma) per mouse dissolved in phosphate-buffered saline at a concentration of 10 mg/ml. Survival of animals was monitored for a period of 7 days. The same protocol was used for E. coli-derived LPS (0111:B4). To determine the effect of antalarmin on the survival of mice injected with LPS, 40 mice were divided in four different groups; the first group received antalarmin at a concentration of 20 mg/kg of body weight; the second received antalarmin at 20 mg/kg of body weight and LPS at a concentration of 0.7 mg per 25 g of body weight; the third group received LPS and the antalarmin diluent; and the fourth group received the antalarmin diluent alone. Mice were pretreated with antalarmin or the diluent 1.5 h prior to LPS injection, according to published protocols (28, 32) and in order not to alter significantly the HPA axis response (10, 35). Antalarmin alone had no effect in the survival of animals, and injection of antalarmin alone was not repeated in the course of the experiments.
ELISA.
Serum from trunk blood was collected as follows: (i) for the determination of TNF-α 1 h after LPS administration and (ii) at 4 h for the determination of IL-1β or IL-6 levels. Each time point and treatment group was composed of five animals per experiment. Sera were collected and frozen until used for cytokine determination by enzyme-linked immunosorbent assay (ELISA) according to the instructions of the manufacturer (R&D). Similarly, cell culture supernatants were collected 24 h following stimulation and stored at −70°C until analyzed.
Radioimmunoassay.
Corticosterone was measured by radioimmunoassay in serum collected 1 h following LPS administration. Five animals per treatment were used. Sera were frozen at −70°C and analyzed as recommended by the manufacturer (ICN).
Statistical analysis.
Statistical analysis of the LPS-induced endotoxin shock survival was performed using the Kaplan-Meyer method and the Mantel-Haenszel log rank test to determine the P values for the differences in the survival. For the statistical evaluation of the ELISA data, we used analysis of variance and post hoc comparison of means followed by two multiple comparison tests: Fisher's least-significant-difference test and the Newman-Keuls test.
RESULTS
In vitro studies on the effect of CRH on macrophages. (i) CRH enhances LPS-induced cytokine production from RAW264.7 cells.
To determine the effect of CRH on macrophages, RAW264.7 cells were cultured in media containing serum and stimulated with E. coli-derived LPS in the presence or absence of CRH at a concentration of 10−8 M. The concentration used is within the physiological range for CRH in peripheral tissues since in the placenta it is found at a concentration of 10−6 M and in the adrenals it can vary between 10−6 to 10−9 M (29, 36). Treatment of cells for 24 h in the presence of LPS stimulated the secretion of TNF-α, IL-1β, and IL-6. In the presence of CRH the levels of all three cytokines were significantly higher, indicating that CRH augments the LPS signal (Fig. 1). However, there was only a minimal effect on cytokine secretion when cells were treated with CRH alone. Specifically, CRH significantly augmented LPS-induced TNF-α secretion (P = 0.04), IL-1β secretion (P = 0.01), and IL-6 secretion (P = 0.04).
FIG. 1.
CRH augments LPS-induced proinflammatory cytokine secretion from RAW264.7 macrophages. (A) TNF-α levels in culture medium of cells treated with CRH, LPS, and CRH plus LPS. TNF-α levels are significantly higher when cells are treated with CRH and LPS than when they are treated with LPS alone (*, P = 0.04). (B) CRH potentiates LPS-induced IL-1β secretion in a significant manner (**, P = 0.01). (C) CRH potentiates LPS-induced IL-6 secretion from RAW264.7 cells (*, P = 0.04). Unstimulated cells were unable to secrete detectable amounts of any of the above cytokines.
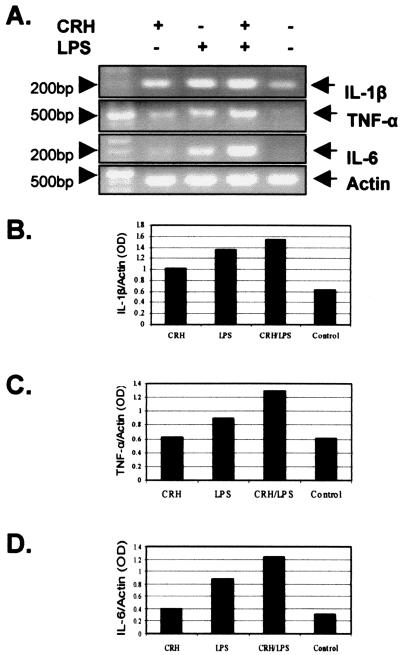
To determine whether CRH has an effect on cytokine transcription, RNA was isolated from cells treated with LPS in the presence or absence of CRH and the levels of TNF-α, IL-1β, and IL-6 mRNA were estimated using a semiquantitative RT-PCR approach. The PCRs were carried out at 33 cycles where the amplification was at the exponential phase, as determined by the curve of the amplification of each product (data not shown). As indicated in Fig. 2A CRH has a minor enhancing effect at the basal mRNA levels of all three cytokines and a stronger augmenting effect on the LPS-induced levels. Densitometric analysis of the RT-PCR products following normalization versus actin revealed that CRH alone induced minimal transcription of IL-1β (Fig. 2B), TNF-α (Fig. 2C), or IL-6 (Fig. 2D), but it strongly augmented the LPS-induced cytokine transcription. The increase that occurred at the transcriptional level was lower than the increase indicated at the protein level, suggesting that there may be an additional effect of CRH at the posttranscriptional level. Alternatively, this may be the result of the lower sensitivity of the semiquantitative approach of RT-PCR. The same experiment was repeated four times with similar results.
FIG. 2.
(A) CRH augments proinflammatory cytokines at the transcriptional level. IL-1β (upper panel), TNF-α (second panel), and IL-6 (third panel) mRNA levels were determined by a semiquantitative RT-PCR approach. CRH induces expression of all three cytokines and further potentiates the LPS-induced transcriptional activation. (B, C, and D) Densitometric analysis of the RT-PCR products of IL-1β (B), TNF-α (C), and IL-6 (D).
(ii) CRH enhances LPS-induced cytokine production in thioglycolate-elicited peritoneal macrophages.
To determine whether CRH exerts the same effect in primary macrophages, we treated thioglycolate-induced peritoneal macrophages with CRH and CRH plus LPS. Thioglycolate-induced macrophages are primed inflammatory macrophages and using this approach one could study inflammatory macrophages without having to accelerate them with LPS. CRH was unable to induce TNF-α, IL-1β, or IL-6 transcription but significantly augmented the LPS-induced proinflammatory cytokine expression (Fig. 3). The densitometric data were analyzed and showed differences similar to the ones observed in RAW264.7 cells (Fig. 3, lower panels). Thus, CRH has a potent effect in both activated RAW264.7 cells and activated primary macrophages, and it cannot elicit cytokine expression in the absence of a potent costimulus such as LPS.
FIG. 3.
CRH augments LPS-induced proinflammatory cytokine expression in thioglycolate-induced peritoneal macrophages from BALB/c mice. IL-1β (A) (upper panel), TNF-α (B) (upper panel), and IL-6 (C) (upper panel) mRNA expression was estimated by RT-PCR. The RT-PCR products were quantitated by densitometry as the ratio of cytokine RNA to the actin levels (lower panels).
In vivo experiments. (i) Determination of LPS LD50 and LD100.
The LD50 and LD100 of LPS in our BALB/c mice was determined as follows. LPS was administered i.p. at concentrations of 0.2, 0.4, 0.6, 0.7, and 1 mg per 25 g of body weight. One hundred percent of the animals treated with LPS at 0.2 mg/25 g survived, compared to 80% of the animals treated at 0.4 mg/25 g and 40% at 0.6 mg/kg, and none of the animals survived at 0.7 or 1 mg/25 g. The LD50 was estimated at 0.5 mg per 25 g of body weight, and the LD100 was estimated at 0.7 mg per 25 g of body weight and above. For the purpose of our experiment we wanted to use a higher dose than the LD50 to determine the possible protective effect of CRHR1 blockade. Thus, mice were injected with 0.7 mg of LPS/25 g, an LD100 dose but not too high to mask a possible protective effect of CRHR1 blockade.
(ii) Antalarmin had no effect on peripheral corticosterone triggered by LPS-induced endotoxin shock.
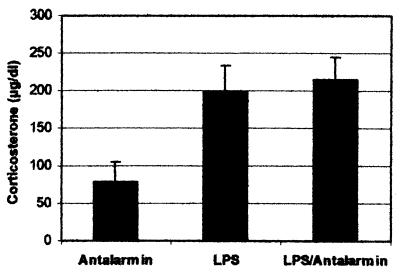
Antalarmin is a potent CRHR1 antagonist and may, therefore, affect the HPA axis and its final product corticosterone. Corticosterone is known to suppress macrophage activation and cytokine production both in vivo and in vitro. Alteration of the HPA axis response to LPS in antalarmin-treated animals would conceivably affect survival. To exclude this hypothesis, corticosterone was measured in all animals, i.e., those treated with LPS and those with LPS plus antalarmin. No difference in corticosterone levels was observed (Fig. 4), suggesting that antalarmin did not affect the HPA axis response to LPS in a significant manner.
FIG. 4.
Corticosterone levels in LPS- and LPS-antalarmin-treated BALB/c mice. Sera were collected 1.5 h following LPS treatment.
(iii) Antalarmin prolonged survival of mice subjected to LPS-induced septic shock.
To determine the role of CRHR1 signals in the cascade of events that take place during septic shock, mice were subjected to a lethal dose of LPS with or without i.p. administration of antalarmin 1.5 h prior to the administration of LPS, to ensure absorbance according to previous reports (28, 32). Two different types of LPS were used to confirm that the results where not specific to a particular type of LPS. i.p. injection of LPS at a dose of 0.7 mg per 25 g of bodyweight induced lethality within 12 to 31 h after injection. Specifically, in mice treated with Salmonella serovar Enteritidis-derived LPS alone, lethality was observed between 14 to 31 h. At 18 h 60% of the animals had died, compared to only 20% of the mice pretreated with antalarmin (Fig. 5A). Overall, survival was significantly prolonged in the mice pretreated with antalarmin (P = 0.022) (Fig. 5A). Similarly, 72% of the injected with E. coli-derived LPS mice and pretreated with antalarmin were still alive at 18 h, while all the animals treated with E. coli-LPS alone had died (Fig. 5B). Mice that were treated with LPS plus antalarmin and survived the endotoxin shock were observed over a period of 7 days and were still alive at the end of this period, indicating that treatment with antalarmin not only prolonged but also improved the survival. All animals treated with antalarmin alone survived. The overall survival was significantly improved in the presence of antalarmin (P = 0.002) (Fig. 5B). The experiment was repeated three times for each LPS subtype using 10 animals per group.
FIG. 5.
(A) Antalarmin prolonged survival of animals treated with Salmonella serovar Enteritidis-derived LPS. Antalarmin or the antalarmin diluent was administered 1.5 h prior to LPS. Survival was observed over a period of 7 days. Control mice received the antalarmin diluent alone. Antalarmin significantly prolonged survival (P = 0.022). (B) Antalarmin prolonged survival of animals treated with E. coli-derived LPS. Antalarmin or the antalarmin diluent was administered 1.5 h prior to LPS. Survival was observed over a period of 7 days. Antalarmin significantly prolonged survival (P = 0.002).
(iv) Antalarmin suppressed endotoxin-induced proinflammatory cytokines.
LPS administration resulted in an acute elevation of TNF-α in plasma, peaking at 1 h. TNF-α was significantly reduced in mice pretreated with antalarmin compared to LPS alone (Fig. 6A) (n = 5 animals per group; P = 0.001). Similarly, IL-1β and IL-6 in plasma reach a peak 3 to 4 h following LPS treatment and remain elevated throughout septic shock. Both IL-1β and IL-6 increased at 4 h following LPS administration but were significantly lower in mice that were pretreated with antalarmin (Fig. 6B and C) (n = 5 animals per group [P = 0.013] for IL-1β; n = 5 animals per group [P < 0.0001] for IL-6).
FIG. 6.
(A) TNF-α levels in mice subjected to LPS-induced endotoxin shock. Trunk blood was collected 1 or 2 h following treatment with E. coli-derived LPS. Treatment with antalarmin significantly reduced TNF-α (n = 5 animals in each group; ***, P = 0.001). (B) IL-1β levels in mice subjected to LPS-induced endotoxin shock. Trunk blood was collected 4 or 6 h following treatment with E. coli-derived LPS. Antalarmin significantly reduced IL-1β (n = 5 animals in each group; *, P < 0.05). (C) IL-6 levels in mice subjected to LPS-induced endotoxin shock. Trunk blood was collected 4 h following treatment with E. coli-derived LPS. Antalarmin significantly reduced IL-6 (n = 5 animals in each group; ***, P < 0.0001; **, P < 0.01). (A to C) C, control; A, antalarmin.
To determine whether the difference in cytokine levels in the presence of antalarmin is a result of a change in kinetics, we measured TNF-α at 2 h following LPS injection and found that the levels of TNF-α in the mice that were pretreated with antalarmin remained significantly lower than in the animals treated with LPS alone (P < 0.001). Similar differences were observed when measuring IL-1β and IL-6 6 h following LPS injection (Fig. 6B and C). Thus, LPS-treated animals had significantly higher levels of IL-1β (P < 0.01) and IL-6 (P < 0.001) than mice treated with LPS plus antalarmin at 6 h. We could, therefore, conclude that antalarmin prolonged survival during LPS-induced septic shock by lowering proinflammatory cytokine levels rather than altering their kinetics.
DISCUSSION
Peripheral CRH is present at the site of inflammation and plays an important proinflammatory role. Indeed, CRH has been detected in subcutaneous inflammation induced by carrageenin (15), in the synovia of patients with rheumatoid arthritis (8), and in the colonic mucosa of patients with ulcerative colitis (16). Blockade of CRH by specific antibodies inhibits carrageenin-induced subcutaneous local inflammation in rats (15). The role of CRH has been associated mainly with mast cells since its administration results in mast cell degranulation, an effect inhibited by the CRHR1 antagonist antalarmin (25).
Macrophages are among the initiator cells during an inflammatory response and are the main source of a series of proinflammatory cytokines. Activation of macrophages occurs through antigenic signals such as bacterial LPS, which binds on Toll-like receptor 4 and activates cytokine transcription and secretion (7, 27). During both local and systemic inflammation, macrophages are the predominant source of proinflammatory cytokines. The aim of this work was to investigate the role of CRH on LPS-induced cytokine secretion from macrophages both in vitro and in vivo. We have found that CRH had a stimulatory effect on cytokine production in both types of macrophages used. Specifically, treatment of RAW264.7 cells with LPS in the presence of CRH further induced secretion of TNF-α, IL-1β, and IL-6. The most-profound effect was on IL-1β secretion, in which CRH induced 5.7-fold more IL-1β. CRH had a similar proinflammatory effect on primary inflammatory macrophages in culture such as thioglycolate-elicited peritoneal macrophages. It should be noted that in pituitary cells CRH induces phosphorylation and activation of CREB (31), a transcription factor that plays a central role in IL-1β transcription. Thus, CRH may enhance the LPS-induced IL-1β secretion through activation of cyclic AMP and CREB. Furthermore, we have previously shown that CRH activates the ERK1/ERK2 mitogen-activated protein kinase pathway (11), which is required for TNF-α expression (26) and the nuclear export of its mRNA (12), suggesting a potential mechanism through which CRH potentiates LPS-induced TNF-α levels. CRH also activates the AP-1 transcription complex (4), which is known to participate in TNF-α and IL-6 transcription, suggesting that the CRH-driven effect on TNF-α levels involves activation of TNF-α transcription. Indeed, CRH activated transcription of both IL-1β and TNF-α as shown in Fig. 2. The activation observed at the transcriptional level is not as potent as the one observed at the protein level indicating that the effect of CRH may occur at both levels.
Induction of proinflammatory cytokines is minimal when cells are exposed to CRH alone but is more profound when cells are primed with LPS. It seems that either CRH requires primed macrophages to exert its effect or LPS induces CRH receptors and, thus, amplifies the effect of CRH. Using an alternative approach, we treated with CRH thioglycolate-induced peritoneal macrophages from BALB/c mice. These cells are primed and are ready to produce cytokines but are not activated by the potent signal of LPS. CRH was unable to induced cytokine expression alone but in those cells without costimulus but presence of CRH further enhanced the LPS effect. It could therefore be concluded that CRH has a profound stimulatory effect on activated macrophages, possibly due to higher levels of its receptors or due to effects of LPS in intracellular signaling cascades. In addition it confirms that the effect of CRH on macrophages is not confined on the myeloma cell line RAW264.7 but it also applies on primary macrophages.
Since CRH exerts an effect on macrophage activation in vitro, we hypothesized was that peripheral CRH deriving from the diffuse neuroendocrine system may play an important role in systemic inflammation in vivo, including activation of the cascade of events following exposure to LPS and leading to the endotoxin shock syndrome. LPS, a group of bacterial endotoxins, stimulates monocytes/macrophages, to produce the proinflammatory cytokines TNF-α, IL-1β, and IL-6, which in turn are the principal initiators of the endotoxin shock syndrome. Based on the preceding observations, we hypothesized that in systemic inflammatory responses CRH exerts an early effect most probably augmenting the response of monocytes/macrophages. To test our hypothesis we have used antalarmin, a synthetic CRH-R1 antagonist in doses and timing shown not to affect the HPA axis, in mice exposed to LPS. More specifically, following the development of the experimental protocol and the titration of LPS, a marginally lethal dose of LPS was injected i.p. in mice, and their survival rate and systemic cytokine response (TNF-α, IL-1β, and IL-6) were measured and compared to those observed for similarly treated animals which had been pretreated with antalarmin. Blockade of the CRHR1 receptor prolonged survival of LPS-treated mice in a statistically significant manner and ameliorated the elevation of plasma levels of TNF-α, IL-1β, and IL-6. This suppression of peripheral cytokines was observed at different time points during the endotoxin shock suggesting that antalarmin did not change the kinetics of cytokine expression.
Antalarmin did not affect the HPA axis when administered on a short-term basis. Indeed, it does not affect basal or stress-induced ACTH or corticosterone levels (10, 32, 35). However, even if our dose of antalarmin had an inhibitory effect on ACTH and subsequently decreased plasma corticosterone levels, this hypocortisolism would have had a completely opposite effect in the survival of mice and the prevention of macrophage-derived cytokines since glucocorticoids exert a potent immunosuppressive and anti-inflammatory effect. To confirm our hypothesis, we measured plasma corticosterone levels and found no differences between the mice treated with LPS alone and those treated with LPS plus antalarmin. Thus, the effect of antalarmin in our experimental model is most likely restricted to the periphery without affecting the HPA axis.
Interestingly, IL-1β had the strongest response, which, in agreement with the strong potentiation of IL-1β expression in RAW264.7 cells, indicates that this cytokine is a major mediator of the proinflammatory properties of CRH. Thus, our data suggest that CRHR1 signals are involved in the early stages during the development of LPS-induced endotoxin shock. It appears that CRH may play a permissive or facilitating role in the activation of monocyte/macrophages since blockade of the CRH-R1 receptor significantly attenuated proinflammatory cytokine secretion mainly produced by activated monocyte/macrophages.
Blockade of CRHR1 by antalarmin in LPS-induced endotoxin shock dramatically reduced the levels of TNF-α, a cytokine that plays a central role in the promotion of inflammation and the initiation of septic shock. The importance of TNF-α in septic shock has been demonstrated repeatedly. TNF-α is rapidly secreted by macrophages following immunogen recognition. In experimental models of LPS-induced endotoxin shock in knockout animals lacking TNF-α expression or molecules that contribute to TNF-α expression results in attenuation of septic shock (12, 20). Furthermore, anti-TNF-α neutralizing antibodies exert a protective effect in experimental sepsis or patients in septic shock (1). This is the first report implicating a neuropeptide receptor in the regulation of TNF-α secretion during septic shock.
A cross talk between the immune system and the diffuse neuroendocrine system was first reported when it was shown that IL-1β affects CRH production in the hypothalamus (5, 17, 23). Recently, a similar cross talk has been demonstrated in endometrial cells, where IL-6 induces CRH secretion by activating the CRH promoter (18), and in the adrenals, where CRH regulates IL-6 expression (30). In general, TNF-α, IL-1β, and IL-6 promote ACTH and glucocorticoid secretion, activating the stress system (14). In the present report we suggest a possible involvement of CRH/CRHR1 in the secretion of such proinflammatory cytokines, highlighting a bidirectional cross talk between the major proinflammatory cytokines and the CRH/CRHR1 system. High expression levels of CRH, urocortin, and their receptors CRHR1 and CRHR2 have been detected in the spleen, the thymus, and activated macrophages (3, 22), suggesting that the peripheral CRH system is present in lymphoid organs, where it may exert paracrine/autocrine effect(s). The role of CRH signaling in cells of the immune system is still unclear. Several models have been proposed in which CRH induces mast cell degranulation, promoting the proinflammatory process (14, 25). Furthermore, involvement of CRH/CRHR1 signaling appears to be necessary for the development of local inflammation induced by carrageenin or turpentine (15, 28). Monocytes/macrophages play a central role both in local and systemic inflammation, being responsible for the secretion of proinflammatory cytokines. The fact that antalarmin affects all major cytokines involved in this model indicates that CRHR1 signaling may play a role in augmenting the initial LPS signal in macrophages.
In conclusion, our data support the hypothesis that the proinflammatory effect of CRH involves macrophage activation via the CRHR1 receptor.
Acknowledgments
S.A. and C.T. contributed equally to this work.
We thank M. Venihaki and K. Karalis for their helpful suggestions and G. Samonis for stimulating discussions during the course of this work.
This work was partially supported by a grant from Medicon Hellas Co (ELKE 296,2001) and from the Hellenic Ministry of Health (KESY 335/6235/1998).
Editor: J. D. Clements
REFERENCES
- 1.Abraham, E., R. Wunderink, H. Silverman, T. M. Perl, S. Nasraway, H. Levy, R. Bone, R. P. Wenzel, R. Balk, R. Allred, et al. 1995. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. JAMA 273:934-941. [PubMed] [Google Scholar]
- 2.Audhya, T., R. Jain, and C. S. Hollander. 1991. Receptor-mediated immunomodulation by corticotropin-releasing factor. Cell Immunol. 134:77-84. [DOI] [PubMed] [Google Scholar]
- 3.Baigent, S. M., and P. J. Lowry. 2000. mRNA expression profiles for corticotrophin-releasing factor (CRF), urocortin, CRF receptors and CRF-binding protein in peripheral rat tissues. J. Mol. Endocrinol. 25:43-52. [DOI] [PubMed] [Google Scholar]
- 4.Becquet, D., F. Guillaumond, O. Bosler, and A. M. Francois-Bellan. 2001. Long-term variations of AP-1 composition after CRH stimulation: consequence on POMC gene regulation. Mol. Cell Endocrinol. 175:93-100. [DOI] [PubMed] [Google Scholar]
- 5.Berkenbosch, F., J. van Oers, A. del Rey, F. Tilders, and H. Besedovsky. 1987. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science 238:524-526. [DOI] [PubMed] [Google Scholar]
- 6.Bornstein, S. R., E. L. Webster, D. J. Torpy, S. J. Richman, N. Mitsiades, M. Igel, D. B. Lewis, K. C. Rice, H. G. Joost, M. Tsokos, and G. P. Chrousos. 1998. Chronic effects of a nonpeptide corticotropin-releasing hormone type I receptor antagonist on pituitary-adrenal function, body weight, and metabolic regulation. Endocrinology 139:1546-1555. [DOI] [PubMed] [Google Scholar]
- 7.Cario, E., I. M. Rosenberg, S. L. Brandwein, P. L. Beck, H. C. Reinecker, and D. K. Podolsky. 2000. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 164:966-972. [DOI] [PubMed] [Google Scholar]
- 8.Crofford, L. J., H. Sano, K. Karalis, T. C. Friedman, H. R. Epps, E. F. Remmers, P. Mathern, G. P. Chrousos, and R. L. Wilder. 1993. Corticotropin-releasing hormone in synovial fluids and tissues of patients with rheumatoid arthritis and osteoarthritis. J. Immunol. 151:1587-1596. [PubMed] [Google Scholar]
- 9.Crofford, L. J., H. Sano, K. Karalis, E. L. Webster, E. A. Goldmuntz, G. P. Chrousos, and R. L. Wilder. 1992. Local secretion of corticotropin-releasing hormone in the joints of Lewis rats with inflammatory arthritis. J. Clin. Investig. 90:2555-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deak, T., K. T. Nguyen, A. L. Ehrlich, L. R. Watkins, R. L. Spencer, S. F. Maier, J. Licinio, M. L. Wong, G. P. Chrousos, E. Webster, and P. W. Gold. 1999. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology 140:79-86. [DOI] [PubMed] [Google Scholar]
- 11.Dermitzaki, E., C. Tsatsanis, A. Gravanis, and A. N. Margioris. 2002. Corticotropin-releasing hormone induces Fas ligand production and apoptosis in PC12 cells via activation of p38 mitogen-activated protein kinase. J. Biol. Chem. 277:12280-12287. [DOI] [PubMed] [Google Scholar]
- 12.Dumitru, C. D., J. D. Ceci, C. Tsatsanis, D. Kontoyiannis, K. Stamatakis, J. H. Lin, C. Patriotis, N. A. Jenkins, N. G. Copeland, G. Kollias, and P. N. Tsichlis. 2000. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 103:1071-1083. [DOI] [PubMed] [Google Scholar]
- 13.Elenkov, I. J., and G. P. Chrousos. 1999. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol. Metab. 10:359-368. [DOI] [PubMed] [Google Scholar]
- 14.Elenkov, I. J., E. L. Webster, D. J. Torpy, and G. P. Chrousos. 1999. Stress, corticotropin-releasing hormone, glucocorticoids, and the immune/inflammatory response: acute and chronic effects. Ann. N. Y. Acad. Sci. 876:1-13. [DOI] [PubMed] [Google Scholar]
- 15.Karalis, K., H. Sano, J. Redwine, S. Listwak, R. L. Wilder, and G. P. Chrousos. 1991. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science 254:421-423. [DOI] [PubMed] [Google Scholar]
- 16.Kawahito, Y., H. Sano, S. Mukai, K. Asai, S. Kimura, Y. Yamamura, H. Kato, G. P. Chrousos, R. L. Wilder, and M. Kondo. 1995. Corticotropin releasing hormone in colonic mucosa in patients with ulcerative colitis. Gut 37:544-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lumpkin, M. D. 1987. The regulation of ACTH secretion by IL-1. Science 238:452-454. [DOI] [PubMed] [Google Scholar]
- 18.Makrigiannakis, A., A. N. Margioris, E. Zoumakis, C. Stournaras, and A. Gravanis. 1999. The transcription of corticotropin-releasing hormone in human endometrial cells is regulated by cytokines. Neuroendocrinology 70:451-459. [DOI] [PubMed] [Google Scholar]
- 19.Mousa, S. A., M. Schafer, W. M. Mitchell, A. H. Hassan, and C. Stein. 1996. Local upregulation of corticotropin-releasing hormone and interleukin-1 receptors in rats with painful hindlimb inflammation. Eur. J. Pharmacol. 311:221-231. [DOI] [PubMed] [Google Scholar]
- 20.Pasparakis, M., L. Alexopoulou, V. Episkopou, and G. Kollias. 1996. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184:1397-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrin, M. H., and W. W. Vale. 1999. Corticotropin releasing factor receptors and their ligand family. Ann. N. Y. Acad. Sci. 885:312-328. [DOI] [PubMed] [Google Scholar]
- 22.Radulovic, M., F. M. Dautzenberg, S. Sydow, J. Radulovic, and J. Spiess. 1999. Corticotropin-releasing factor receptor 1 in mouse spleen: expression after immune stimulation and identification of receptor-bearing cells. J. Immunol. 162:3013-3021. [PubMed] [Google Scholar]
- 23.Sapolsky, R., C. Rivier, G. Yamamoto, P. Plotsky, and W. Vale. 1987. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science 238:522-524. [DOI] [PubMed] [Google Scholar]
- 24.Singh, V. K., and H. H. Fudenberg. 1988. Binding of [125I]corticotropin releasing factor to blood immunocytes and its reduction in Alzheimer's disease. Immunol. Lett. 18:5-8. [DOI] [PubMed] [Google Scholar]
- 25.Theoharides, T. C., L. K. Singh, W. Boucher, X. Pang, R. Letourneau, E. Webster, and G. Chrousos. 1998. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology 139:403-413. [DOI] [PubMed] [Google Scholar]
- 26.van der Bruggen, T., S. Nijenhuis, E. van Raaij, J. Verhoef, and B. S. van Asbeck. 1999. Lipopolysaccharide-induced tumor necrosis factor alpha production by human monocytes involves the raf-1/MEK1-MEK2/ERK1-ERK2 pathway. Infect. Immun. 67:3824-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasselon, T., and P. A. Detmers. 2002. Toll receptors: a central element in innate immune responses. Infect. Immun. 70:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venihaki, M., P. Dikkes, A. Carrigan, and K. P. Karalis. 2001. Corticotropin-releasing hormone regulates IL-6 expression during inflammation. J. Clin. Investig. 108:1159-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venihaki, M., A. Gravanis, and A. N. Margioris. 1997. Comparative study between normal rat chromaffin and PC12 rat pheochromocytoma cells: production and effects of corticotropin-releasing hormone. Endocrinology 138:698-704. [DOI] [PubMed] [Google Scholar]
- 30.Venihaki, M., A. Gravanis, and A. N. Margioris. 1998. KAT45 human pheochromocytoma cell line. A new model for the in vitro study of neuro-immuno-hormonal interactions. Ann. N. Y. Acad. Sci. 840:425-433. [DOI] [PubMed] [Google Scholar]
- 31.Waeber, G., N. Thompson, T. Chautard, M. Steinmann, P. Nicod, F. P. Pralong, T. Calandra, and R. C. Gaillard. 1998. Transcriptional activation of the macrophage migration-inhibitory factor gene by the corticotropin-releasing factor is mediated by the cyclic adenosine 3′, 5′-monophosphate responsive element-binding protein CREB in pituitary cells. Mol. Endocrinol. 12:698-705. [DOI] [PubMed] [Google Scholar]
- 32.Webster, E. L., D. B. Lewis, D. J. Torpy, E. K. Zachman, K. C. Rice, and G. P. Chrousos. 1996. In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: suppression of pituitary ACTH release and peripheral inflammation. Endocrinology 137:5747-5750. [DOI] [PubMed] [Google Scholar]
- 33.Webster, E. L., D. J. Torpy, I. J. Elenkov, and G. P. Chrousos. 1998. Corticotropin-releasing hormone and inflammation. Ann. N. Y. Acad. Sci. 840:21-32. [DOI] [PubMed] [Google Scholar]
- 34.Webster, E. L., D. E. Tracey, M. A. Jutila, S. A. Wolfe, Jr., and E. B. De Souza. 1990. Corticotropin-releasing factor receptors in mouse spleen: identification of receptor-bearing cells as resident macrophages. Endocrinology 127:440-452. [DOI] [PubMed] [Google Scholar]
- 35.Wong, M. L., E. L. Webster, H. Spokes, P. Phu, M. Ehrhart-Bornstein, S. Bornstein, C. S. Park, K. C. Rice, G. P. Chrousos, J. Licinio, and P. W. Gold. 1999. Chronic administration of the non-peptide CRH type 1 receptor antagonist antalarmin does not blunt hypothalamic-pituitary-adrenal axis responses to acute immobilization stress. Life Sci. 65:L53-L58. [DOI] [PubMed] [Google Scholar]
- 36.Zoumakis, E., E. Chatzaki, I. Charalampopoulos, A. N. Margioris, E. Angelakis, E. Koumantakis, and A. Gravanis. 2001. Cycle and age-related changes in corticotropin-releasing hormone levels in human endometrium and ovaries. Gynecol. Endocrinol. 15:98-102. [PubMed] [Google Scholar]