Abstract
Using single molecule force spectroscopy we examine the response of heparin chains to mechanical stretching. We find that at forces below 200 pN heparin behaves as a simple entropic spring. At ∼200 pN heparin displays a large enthalpic elasticity, which is evident as a pronounced plateau in the force-extension relationship. We determine that this enthalpic elasticity is produced by sugar rings of heparin flipping to more energetic and more extended conformations. We estimate that in vivo, the forces which stretch heparin are comparable to the forces that trigger conformational transitions in our single molecule atomic force microscopy measurements. We hypothesize that these conformational transitions have biological significance in that they provide a mechanism to finely regulate the affinity of various ligands toward heparin, for example, in secretory granules undergoing exocytosis and during the mechanical interactions between cells and the extracellular matrix.
INTRODUCTION
Glycosaminoglycans (GAG) are heteropolysaccharides made of disaccharide repeats. Typically, one of the constituent monosaccharides is uronic acid, and the other is either a glucosamine or a galactosamine sugar linked by α(1→4) or β(1→3) glycosidic bonds. GAG molecules are negatively charged due to a high density of sulfate and carboxylate groups. GAG can be found either in soluble form, as in the case of heparin and hyaluronic acid, or covalently linked to the serine residues of a protein backbone, forming proteoglycans. GAG play major roles in the extracellular matrix where they serve as ligands controlling cell migration and cell adhesion. Of particular interest to us is the GAG heparin, a soluble molecule that has a major role in defining the physical and chemical properties of the extracellular matrix and that binds fibronectin with high affinity (Sharma et al., 1999). Heparin is also found in the condensed proteoglycan gel in the lumen of secretory granules of the mast cell (Parpura and Fernandez, 1996). Heparin is known to have a plethora of binding proteins and vast roles in animal physiology (Jackson and McLean, 1991) and is commonly used as a blood anticoagulant.
Most of the studies examining the properties of heparin have been done with in vitro biochemistry in the absence of a mechanical tension. However, the results of Harold Erickson and collaborators demonstrated that the extracellular matrix is normally under a stretching force (Ohashi et al., 1999). Furthermore, the recent data demonstrating the presence of cryptic sites in the fibronectin molecule show that this matrix protein functions as a force transducer. Since heparin polysaccharides bind to the fibronectin matrix in vivo, it is likely that heparin also is placed under mechanical stress and that there are force-driven conformations in heparin that are physiologically significant.
Another important source of heparin is found in the secretory granules of mast cells. These granules contain a polymeric matrix composed of heparin sulfate proteoglycan, which stores a variety of secretory products such as amines, enzymes, and chemotactic factors (Fernandez et al., 1991; Humphries et al., 1999; Metcalfe et al., 1981). Because of the high density of fixed negative charges associated with the sulfate groups of heparin, the matrix acts like a cationic ion exchanger that regulates the release of histamine during exocytosis (Fernandez et al., 1991; Metcalfe et al., 1981; Parpura and Fernandez, 1996; Uvnas and Aborg, 1983). During exocytosis, the influx of sodium counterions, from the extracellular medium into the matrix, is accompanied by a large influx of water causing the matrix to swell (Curran and Brodwick, 1991; Fernandez et al., 1991; Monck et al., 1991; Parpura and Fernandez, 1996). The swelling pressure of an expanding granule matrix that stretches the proteoglycan molecules was determined to be as high as 12 bar (Nanavati and Fernandez, 1993). It is then likely that during exocytosis the heparin molecules that form the granule matrix are placed under a stretching force.
These considerations together show that heparin molecules are likely to function in a physiological environment that normally includes a mechanical stretching force. Yet nothing is known about the properties of heparin under a mechanical load.
A commonly held view proposes that the pyranose ring, the main building block of polysaccharides, is locked into a chair conformation and serves as a somewhat rigid platform for ligand binding. The structural features of polysaccharide molecules such as heparin are thought to result mainly from their secondary structure (Venkataraman et al., 1994). Pyranose-based sugars have two distinct chair conformations: 4C1 and 1C4 separated by a large energy barrier. In addition to the chair conformers, pyranoses also have an intermediate “boat” conformer. In the absence of an applied force, the most stable conformation of a pyranose is that of the 4C1 chair. Recent studies using atomic force microscopy (AFM) have shown that application of a force to amylose and pectin polysaccharides drives a conformational change in the pyranose ring which is evident as a sudden elongation of the molecule (Marszalek et al., 1998, 1999). This elongation was shown to result from an increase in the distance between glycosidic oxygen atoms caused by a force-induced transition between the chair and boat conformations of the pyranose ring (Marszalek et al., 1998, 1999). Here we use single molecule force spectroscopy to examine the mechanical properties of heparin molecules derived either from intact secretory granules or from soluble heparin.
MATERIALS AND METHODS
Single molecule force spectroscopy on single molecules
Our custom made AFM apparatus was described in detail elsewhere (Oberhauser et al., 1998); it is capable of measuring the extensibility of individual molecules. The spring constant of each individual AFM cantilever (Si3N4 tip, TwinTips, Digital Instruments, Santa Barbara, CA; or microlevers from Thermomicroscopes, Sunnyvale, CA) was calibrated in solution, using the equipartition theorem. This method gives values for the spring constant of the cantilever which are within 20% of the values obtained by other methods.
Heparin
We used two types of heparin: a commercial product (heparin sodium salt from bovine intestinal mucosa, product No. 51536; Sigma, St. Louis, MO) and an isolate from secretory granules of mast cells. Mast cell secretory granules were prepared from beige mice (bgj/bgj strain; Jackson Laboratories, Bar Harbor, ME) following the procedure described by Oberhauser and Fernandez (Oberhauser and Fernandez, 1993) and in Marszalek et al. (1997a). Granules were lysed in pure water, centrifuged, and resuspended in (phosphate-buffered saline (PBS)) buffer whose pH was increased to 11 by the addition of NaOH. This procedure dissociates heparin chains from the protein core of the heparin sulfate proteoglycan. Such dissociated heparin molecules were used, without further purification in AFM pulling experiments. A layer of heparin molecules was created by drying a drop of solution containing either the granular material or commercial heparin (1 mg/ml in PBS) onto glass coverslips followed by extensive rinsing. This procedure leaves for measurements only these molecules that are strongly adsorbed to the glass surface (Li et al., 1998). AFM stretching measurements werfe carried out in PBS mixed with ethanol (final concentration of ethanol 30%).
Molecular modeling of heparin
The pyranose structures of heparin residues were generated and all calculations were carried out with the program PC Spartan Pro for Windows (v.1.07; Wavefunction, Inc., Irvine, CA) on a PC. First, the molecules were conformationally relaxed with the molecular mechanics method MMFF and then further optimized ab initio (HF/6-31G*). To simulate AFM stretching of pyranose rings with Spartan, the distance between the glycosidic oxygen atoms O1 and O4 was constrained and allowed to increase in steps of ∼0.05 Å while the geometry of the ring, at each step, was optimized with the semiempirical quantum mechanical method MNDO.
RESULTS AND DISCUSSION
Extensibility of single heparin molecules
We dissociated heparin molecules from the protein core of the proteoglycan matrix by an alkaline treatment of secretory granules (see Materials and methods; Fig. 1 A) and measured their extensibility in an atomic force microscope. Fig. 1 B shows a family of force-extension recordings obtained from individual molecules from the material prepared by dissociating the matrices obtained from many mast cell secretory granules (see Materials and Methods). We measured a family of force-extension curves for molecules with contour lengths that varied between ∼30 nm and ∼350 nm (Fig. 1 B). The varying contour lengths result either from molecules with different sizes or, most likely, from the random attachment of the AFM tip to the heparin molecule. Regardless of their contour length, all the recordings show a pronounced plateau in the force-extension curve, marking an enthalpic transition at forces above ∼200 pN. This plateau can be interpreted as an increased extensibility of the molecules. Such an elastic behavior was already observed for several different polysaccharides studied with AFM such as amylose, dextran, and pectin (Li et al., 1999; Marszalek et al., 2001, 2002, 1998, 1999; Rief et al., 1997). The enthalpic elasticity of these polymers was linked to their structural features characterized by a significant number of glycosidic and/or aglycone bonds in the axial orientation. Under a stretching force these axial linkages promote conformational transitions of the pyranose rings from the chair to the boat or the inverted chair conformation (Marszalek et al., 2001, 2002, 1998, 1999). This observation supports our hypothesis that the recordings shown in Fig. 1 B were obtained on polysaccharide molecules with a significant percentage of α anomeric residues with axial bonds, which is consistent with the structure of heparin (see Fig. 1A). It is important to note that the force at which heparin molecules display their enthalpic elasticity (F = 201 ± 63 pN; n = 12) coincides with the force at which α-D-glucopyranose residues of amylose flip from their chair to a boatlike conformation (275 ± 45 pN)(Li et al., 1999; Marszalek et al., 1998). The plateau force of ∼200 pN is characteristic and indicative of 1→4 linkages between the pyranose monomers, because other linkages are known to produce plateaus at higher forces. For example, the transition in dextran, whose backbone is primarily formed by 1→6 linkages, occurs at ∼800 pN (Rief et al., 1997; Marszalek et al., 1998). Taken together, these observations suggest that the plateau observed in the force-extension curve of heparin is generated by α anomeric residues which are jointed by 1→4 linkages. In Fig. 1 C we plotted the recordings from Fig. 1 B after normalizing the molecules' extension by the molecule end-to-end distance determined at a force of 100 pN. Here, we make use of this property of the freely jointed chain model that guarantees that the chain extension at any given force is proportional to the contour length of the chain (Flory, 1953). This normalizing procedure allows us to compare recordings obtained on segments of various lengths and to examine whether the features observed in force-extension recordings scale with the molecule length, and also allows us to verify if the pulling experiments were carried out on single molecules. Indeed, after normalization, all the recordings superimpose reasonably well, which confirms that they were obtained on single molecules whose enthalpic extensibility scales with the length of the molecule. This last observation suggests that similar to other polysacharides like amylose or dextran, the enthalpic elasticity of heparin reflects the mechanical properties of its building blocks: the sugar rings and not the secondary structure of the polysaccharide chain (Li et al., 1998).
FIGURE 1.

Elasticity of single native heparin molecules isolated from mast cell secretory granules. (A) Simplified diagram of heparin structure. Heparin consists of repeats of two dimers: a 1→4 linked α-L-iduronic acid (IdoA) and α-D-glucosamine (GlcN) (∼80%) and a 1→4 linked β-D-glucuronic acid (GlcA) and α-D-GlcN (∼20%) with the average number of dimers in a heparin chain ranging from 50 to 150. (B) Force-extension curves of single native heparin molecules obtained from the heparin matrix of mast cell secretory granules. Heparin was dissociated from the protein backbone at alkaline pH (Fernandez et al., 1991). (C) Normalized force-extension curves of native heparin superimpose on each other, proving that the recordings were made on single molecules. The relative increase in the contour length of heparin molecules due to its enthalpic elasticity (a hump in the curves) was determined to be 11.6 ± 3.1% (n = 12). In all cases, the extension x, was normalized by the length of the molecule measured at 100 pN.
We also examined the extensibility of individual heparin molecules from a commercial heparin sample that was extracted from bovine intestinal mucosa and then further purified (see Materials and Methods). In Fig. 2 A, we show a family of AFM force-extension recordings obtained from these molecules. The force extension curves for mucosal heparin molecules show features similar to those of the granule heparin shown in Fig. 1. They have a wide range of contour lengths, varying between ∼30 nm and up to ∼400 nm. These recordings also display a pronounced enthalpic elasticity marked as a plateau in the force-extension curve that occurs at around 230 pN (228 ± 53 pN; n = 19). The force-extension curves shown in Fig. 2 A superimpose after normalizing their extensions (Fig. 2 B), confirming that they were obtained from single molecules whose elastic properties scaled linearly with the contour length (Rief et al., 1997). In some experiments, a single heparin molecule remained attached to the AFM cantilever long enough to repeat several extension and relaxation cycles. In these recordings we observed that the extension and relaxation traces were superimposable. The lack of hysteresis demonstrates that the enthalpic changes in heparin structure that occur upon stretching are fully reversible and in equilibrium. These observations suggest that similar to other α-linked glucans, the enthalpic elasticity of heparin originates in sugar rings which undergo force-induced conformational transitions that lengthen the molecule when placed under a stretching force.
FIGURE 2.
Mechanical properties of commercial heparin. (A) Force-extension curves obtained from heparin derived from bovine intestinal mucosa. (B) Normalized force-extension curves for commercial heparin. The relative increase in contour length due to the enthalpic elasticity was determined to be 14.4 ± 3% (n = 19). The extension x was normalized by the length of the molecule measured at 100 pN. The conformational transitions underlying the enthalpic elasticity of heparin are reversible. Three sets of recordings performed on the same molecules during stretching and subsequent relaxation did not show any hysteresis and were separated only to reveal the forward and backward traces.
Force-induced conformational transitions in heparin residues
The enthalpic elasticity of single molecules of native and commercial heparin measured with AFM (Figs. 1 B and 2 A) is suggestive of the ability of heparin residues to undergo reversible force-induced conformational transitions that can increase the length of the molecule in a stepwise manner. We examined whether this observation is consistent with the atomic structure of heparin. As shown in the inset of Fig. 2 A, heparin is a linear heteropolysaccharide composed of repeats of two types of dimers. Dimer A consists of α-L-iduronic acid (IdoA) and α-D-glucosamine (GlcN) residues jointed by the 1→4 linkage. Dimer B consists of 1→4-linked β-D-glucuronic acid (GlcA) and α-D-GlcN residues (Rao, 1998). Typically, in mature heparin there is ∼80% of dimer A and ∼20% of dimer B. In addition, heparin has a complex pattern of sulfation (not shown in Fig. 2 A) that typically includes ∼3 sulfate groups per disaccharide (Faham et al., 1996). Typical sulfation sites in GlcN units are N2 and O6 (occasionally also at O3) and in IdoA/GlcA units are O2 sites (Faham et al., 1996; Rao et al., 1998) with the amount of sulfation exceeding 70% (Roden et al., 1992).
Conformational studies on heparin indicate that α-D-GlcN and β-D-GlcA residues are in the regular 4C1 chair conformation (Rao et al., 1998). In this conformation, GlcN units have the glycosidic bond in the axial orientation (perpendicular to the plane of the pyranose ring) and the aglycone bond in the equatorial orientation (in the plane of the ring). In β-D-GlcA units, both the glycosidic and the aglycone bonds are in the equatorial orientation. We depicted the α-l-IdoA residue in the 1C4 inverted chair conformation. However, reliable information on the ground-state conformation of this residue, in a free (not bound) heparin molecule, is not available. There is an ongoing controversy surrounding the predominant conformation of IdoA residues in solution (Faham et al., 1996; and Rao et al., 1995; Ernst et al., 1998; and Ferro et al., 1986). If IdoA residues are in the 1C4 conformation, as we assume here, then both the glycosidic and the aglycone bonds on this residue are in the axial orientation.
Our earlier force spectroscopy studies on several α-D-glucans and α-D-galactans showed that forced conformational transitions in those polysaccharides are induced by axial linkages that work as atomic levers to generate torque and flip the pyranose rings from the chair to a boatlike or inverted chair conformation (Marszalek et al., 1999). These conformational changes increase the separation of the glycosidic oxygen atoms and therefore are favored in polysaccharide chains subjected to mechanical stretching (Marszalek et al., 2001, 2002, 1998, 1999). We also showed that polysaccharides with equatorial linkages, such as cellulose, do not undergo force-induced conformational transitions upon stretching because these linkages generate minimal torque on the pyranose ring and also provide a maximal separation of the glycosidic oxygen atoms in the ground energy conformation (typically 4C1). Force-extension curves obtained from polysaccharide molecules such as cellulose, that do not change conformation under a stretching force, are well described by classical models such as the freely jointed chain model of polymer elasticity (FJC). For an FJC polysaccharide, the main mechanism of extension involves aligning the sugar rings with the direction of the force (reducing its entropy) with a minimal enthalpic component resulting from the deformation of covalent bonds. By contrast, the force-extension curves of polysaccharide molecules such as dextran or amylose deviate substantially from the FJC model due to the force-driven conformational changes of their pyranose rings (e.g., Marszalek et al., 1998, 1999).
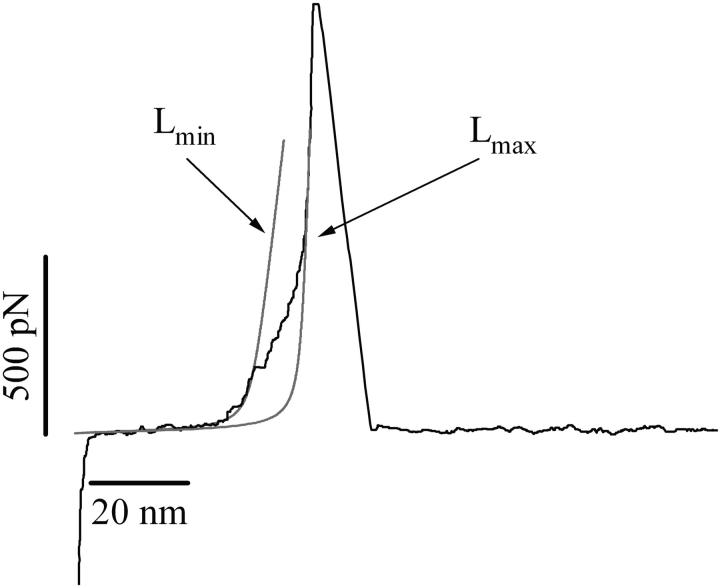
The analysis of heparin structure carried out above suggests that the enthalpic elasticity captured in the force-extension recordings of heparin originates from conformational transitions within α-D-GlcN and α-L-IdoA units because these residues provide axial linkages in their ground energy conformations. In Fig. 3 we examine in detail conformations of heparin residues before and after subjecting them to stretching forces using the molecular mechanics approach. Fig. 3, A–D, shows ab initio minimized (HF/6-31G* level) conformers of IdoA, GlcN, and GlcA residues. These conformers were subjected to a stretching procedure where the distance between the glycosidic oxygen atoms was constrained and allowed to increase in small steps (<0.05 Å) while the geometry of the molecule was optimized at each step with a semiempirical method, MNDO. Fig. 3 B shows that an IdoA residue subjected to the stretching along the line connecting the glycosidic oxygen atoms O1 and O4 flips from the inverted chair conformation 1C4 to the 4C1 chair conformation. This conformational transition increases the O1O4 distance from 4.682 Å to 5.428 Å, which amounts to ∼16% of the initial separation. Fig. 3 C shows that a GlcN residue subjected to a similar procedure flips from the 4C1 chair conformation to a skew-boat conformation, and this transition increases the O1O4 distance from 4.659 Å to 5.566 Å or by ∼19%. Fig. 3 D shows that a GlcA residue subjected to stretching forces will remain in the 4C1 conformation and the O1O4 distance will increase by a negligible amount. Assuming that a hypothetical heparin chain is composed of 80% of dimer A and 20% of dimer B, we estimate the total increase in the length of the chain, as a result of conformational transitions in GlcN and IdoA residues, to be 15.8%. In Fig. 4 we show an example of the force-extension recording of a heparin molecule from the commercial sample, together with the fits of the FJC model to the data before and after the enthalpic extension. This allows us to measure the increase in the contour length of the molecule due to the conformational transitions in the IdoA and GlcN residues. We find that this increase amounts to 14.4 ± 3% (n = 19). We conclude that the experimental value agrees, within the limit of experimental uncertainty, with the theoretical estimate. Similarly, we measured the increase in the contour length of native heparin molecules upon stretching to be 11.6 ± 3.1% (n = 12). This value is lower than the theoretical estimate, and it is possible that this difference may reflect a higher percentage of GlcA in native heparin as compared to commercial heparin and to the idealized structure shown in Fig. 2.
FIGURE 3.

Force-induced conformational transitions in heparin residues. (A) Atomic structure of heparin showing the relative proportion of IdoA and GlcA. (B) IdoA residues are assumed to be in the 1C4 conformation with the glycosidic and aglycone bonds in the axial orientation. The application of a stretching force to the glycosidic oxygen atoms flips the ring to the inverted chair conformation (4C1). This transition increases the spacing between the oxygen atoms from 4.682 Å to 5.428 Å. (C) GlcN are initially in the relaxed 4C1 chair conformation with the glycosidic bond in the axial orientation. Upon application of a stretching force, the ring flips to a boatlike conformation and the distance between the glycosidic oxygens atoms increases from 4.659 Å to 5.566 Å. (D) GlcA are initially in the 4C1 chair conformation with the aglycone and glycosidic bonds in the equatorial orientation. The applied force does not induce any significant conformational transition but only slightly deforms the ring, and the distance between the glycosidic oxygen atoms practically remains constant. To simulate the application of a stretching force to the pyranose ring, the distance between the glycosidic oxygen atoms was forced to increase in steps of ∼0.05 Å. At each step, geometry of the ring was optimized with the molecular mechanics quantum mechanical method, MNDO. The formula for calculation of the fractional extension of the molecule depicted in A, caused by a stretching force is shown at the bottom.
FIGURE 4.
Characteristic extensional transition of a heparin molecule examined with the freely jointed chain (FJC) model of polymer elasticity; force-extension relationship of a heparin molecule from a commercial source. The thin red lines are the fits of the FJC model before and after the enthalpic extension of the heparin chain. These fits are used to measure the increase in the molecule contour length (from Lmin to Lmax) due to the conformational transitions of the heparin ring structures.
Are there in vivo forces capable of stretching heparin into its force-driven conformers?
So far we determined that the mechanical stretching of single heparin molecules can force the sugar rings of IdoA and GlcN residues into extended conformations (e.g., a boatlike or a 4C1 chair). Can similar conformational transitions occur in vivo, for example, during exocytotic swelling of secretory granules or in a stretched fibronectin matrix? To answer these questions we need to estimate the stretching force that a single heparin molecule experiences in these settings.
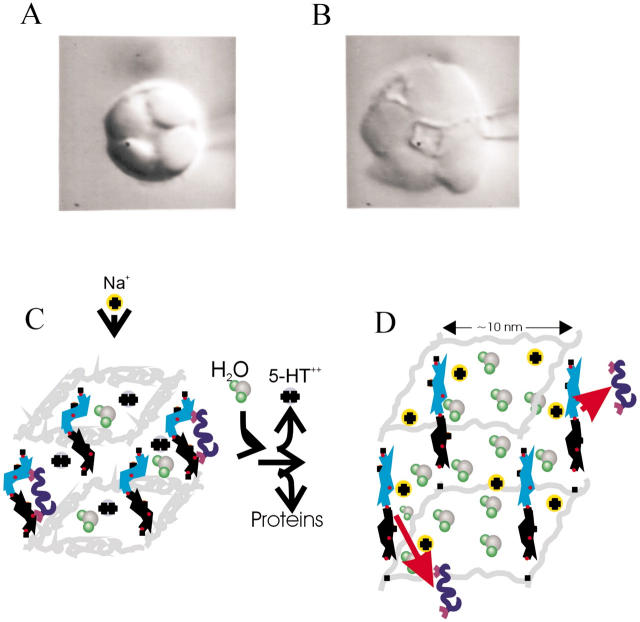
First, we examine the forces generated upon swelling of the proteoglycan matrix (Fig. 5, A and B). We note that, when examined in vitro, the exocytosed matrices remain condensed for weeks and do not dissolve in the extracellular medium. We also note that to dissolve a proteoglycan matrix, the linkage between heparin chains and the core protein must be cut. These observations suggest that the proteoglycan gel is a cross-linked molecular network (Fig. 5, C). It is not known if this cross-linking between heparin chains and proteins is chemical in nature or if it involves physical entanglements of individual heparin polymers. Regardless of the nature of this cross-linking, it must be strong enough to enable the matrix to withstand the large stretching forces that occur upon swelling. A force that a swelling matrix can exert was directly measured, and the associated swelling pressure was determined to be ∼12 bar (Nanavati and Fernandez, 1993). On the other hand, we know that the matrix is only weakly cross-linked because large molecules such as concanavalin A (∼5 nm in length) can easily penetrate it (Oberhauser and Fernandez, unpublished observation). In addition, AFM measurements of the elastic modulus of the matrix confirmed that the matrix behaves as a weakly cross-linked ion exchanger (Parpura and Fernandez, 1996). We therefore assume that in the swollen matrix there is a single heparin molecule per ∼100 nm2 of the granule surface and that such a molecule is attached to the matrix at two points at least, and therefore is subjected to tensile forces upon swelling of the matrix (see Fig. 5 D). Then, a pressure of 12 bar corresponds to a stretching force of 120 pN per single heparin molecule. From this rough estimate we clearly see that the forces within the swollen matrix are of such a magnitude that they can affect the conformation of pyranose rings in heparin. Hence, it is likely that during exocytosis the sugar residues of heparin undergo force-induced conformational transitions. It is therefore possible that these conformational transitions have biological significance in regulating release of many molecules that are bound to heparin. In Fig. 4 we describe the mechanism of release that, in addition to ion exchange (Marszalek et al., 1996, 1997a,b), now also includes conformational transitions as the driving force for release of uncharged ligands. In contrast to charged amines which are small molecules that interact with localized charges on heparin, proteins are large, and their binding to heparin may be coordinated by hydroxyls or other functional groups on several sugar rings in a conformation dependent manner. In Fig. 5, C and D, we illustrate this situation by assuming that before exocytosis, the binding of a protein ligand is coordinated by a dimer of IdoA and GlcN residues. In the relaxed state of heparin these sugars are in their ground energy conformations (1C4 and 4C1, respectively) which assure a high affinity for the binding of the ligand molecule. Upon exocytotic swelling of the matrix, the stretching forces flip the IdoA residue to the 4C1 conformation and the GlcN residue to a boatlike conformation. In this extended state, the distance between the putative binding sites on the dimer increases significantly, the binding affinity decreases dramatically, and the ligand molecule is allowed to diffuse out of the matrix (Fig. 5 D).
FIGURE 5.
Stretching of the heparin proteoglycan matrix controls release of secretory products from mast cells secretory granules. In Fig. 5, A and B, we show two images of a mast cell from a beige mouse mutant before and after exocytosis (Monck et al., 1991). The image on the left shows the granules before inducing exocytosis, and the image on the right shows the enlarged granules which swelled after the opening of the exocytotic fusion pore and the discharge of secretory products into the extracellular medium. In exocytosis, the secretory granule matrix must expand against the rigid cytoskeleton network and direct measurements of the force exerted by a swollen matrix. Nanavati and Fernandez (1993) indicated that the components of the matrix experience large internal stretching forces. (C and D) Schematic showing components of the polymeric matrix of a secretory granule from the mast cell and their role in binding and release of secretory products upon swelling of the matrix. Protein components of the proteoglycan matrix are represented by coiled springs (gray traces), and heparin molecules are represented by its sugar dimers. The sugar chains are much longer than the protein core, and the remaining parts of the chains are omitted. Iduronic acid residues are represented by pyranose rings in blue, and glucosamine residues are represented by pyranose rings in black. Critical oxygen atoms are shown as red filled circles. Sulfate groups and other details of heparin structure are omitted for simplicity. Orange circles represent negative charges fixed on the matrix whereas divalent cations (blue circles) represent charged amines (serotonin, histamine) stored in the matrix. The blue coiled structures with the handles represent other secretory products (e.g., proteins) that interact with heparin. When the matrix is exposed to the extracellular medium, sodium ions replace positively charged secretory products and bring water molecules, which generate a swelling pressure on the matrix. Heparin chains are stretched and release uncharged secretory products, e.g., proteins. We model the heparin-protein interaction as a bivalent contact where a forced conformational transition of the receptor (e.g., heparin dimer) changes the affinity of the interaction. On the left (C), the iduronic acid and glucosamine residues are in their relaxed 1C4 and 4C1 conformations, respectively, which coordinate binding of a ligand with high affinity. Upon stretching (D) the IdoA ring flips to the extended 4C1 conformation, and the glucosamine ring flips to the extended boatlike conformation. The distance between the binding sites on the sugar rings increases significantly, and the bonds with the ligand are broken.
The case for the mechanical stretching of heparin is even more compelling in the case of the extracellular matrix. Using a novel micromachined substrate containing several thousand tiny cantilevers, Galbraith and Sheetz (1997) demonstrated that cells generate traction forces of up to several nN at their adhesive contacts. Furthermore, cell migration has been shown to depend on the elasticity of the substrate that they are migrating on. Pelham (Pelham and Wang, 1997) constructed a series of polyacrylamide-based substrates that were coated with type I collagen, another component of the extracellular matrix. These artificial matrices could be designed to have an elasticity that varied more than 10-fold between 0.05 and 0.7 N/m (Pelham and Wang, 1997). They found that the cells readily sensed the elasticity of the substrate with cells becoming less motile and more spread with increasing rigidity of the substrate. The observation that cells can detect the flexibility of the substrate is a crucial cue that led the authors to state that “communications through physical signals are as important as communications through chemical messengers.” These experiments together show that the traction forces of cell adhesion are transmitted to the extracellular matrix and that the mechanical properties of this underlying matrix determines the behavior of the cells. Indeed, studies of cell adhesion at the single molecule level have definitively shown that receptor-ligand bonds are challenged by forces in the range between 100 and 200 pN during rolling cell adhesions (Alon et al., 1997, 1995). Furthermore, the mechanical compliance of the connection was shown to be a determinant of the adhesive interaction (Chen et al., 1997). In all of these mechanical reactions the cell membrane receptors exert a force on their matrix ligands suggesting that the matrix, due to its intimate interaction with cells, must itself be under mechanical tension. Harold Erickson and collaborators recently proved this point by constructing a recombinant fibronectin protein that contained a GFP module spliced in between the 3F3 and 4F3 modules. Using this fluorescent fibronectin, they monitored the location and density of a fibronectin matrix assembled by cultured Chinese hamster ovary (CHO) cells (Ohashi et al., 1999). The striking finding was that when the fibronectin fibrils were cut or separated from cells they contracted to one fourth of the length they had in the intact matrix (Ohashi et al., 1999). This result demonstrated that fibronectin proteins are highly extended and under tension in an intact extracellular matrix. The implication of these experiments was that some of the FNIII modules of fibronectin had been mechanically unfolded. We now know that the FNIII modules will unfold when exposed to stretching forces ranging between 85 pN and up to ∼250 pN (Oberhauser et al., 2002). Given that heparin is intertwined with the FN molecules, it is likely that the same stretching forces that trigger the unfolding of some of the FNIII modules will also trigger conformational changes in the heparin molecule as well. Our observations on the extensibility of heparin contrasts with the widespread view that the pyranose ring serves as a rigid scaffold for ligand binding in the extracellular matrix. In heparin, functional groups attached to the pyranose ring coordinate the binding to fibronectin modules (12F3, 13F3, and 14F3) and other extracellular matrix proteins (Sharma et al., 1999). For example, Chen and Hansma (2000), using atomic force microscopy, imaged and mapped the contact points between the heparan sulfate and collagen IV and laminin-1 in the basement membrane. These binding sites may be affected by a stretching force. For example, our ab initio calculations of the pyranose conformers of pectin predict that a chair inversion reaction will change the spacing and the orientation of the hydroxyl groups in positions 2 and 3 of the ring. A forced chair inversion reaction would increase the O2-O3 distance by 0.7 Å and also change the orientation of the functional groups from equatorial to axial (Marszalek et al., 1999). Similar changes in the iduronic and/or the glucosamino sugars of heparin will have a large effect in the binding affinity to fibronectin modules. Hence, heparin and other GAGs present in the extracellular matrix may serve as detectors that send biological signals in a narrow force range.
A specific heparin pentasaccharide sequence is known to bind to 13F3 with very high affinity (10−8 M). We hypothesize that the high binding affinity is dependent on the orientation of the sulfate and carboxylate groups, which hydrogen bond to the charged amino acids in the 13F3 module (Hricovini et al., 1999). If a force-driven conformational change were to occur in heparin, the spatial arrangement of the sulfate and carboxylate groups will change dramatically, altering the binding affinity of heparin for 13F3.
Conclusions
Using single molecule force spectroscopy techniques, we showed that the elasticity of heparin molecules have a large enthalpic component that occurs at a stretching force of ∼230 pN. We determined that this enthalpic elasticity represents force-induced conformational transitions in GlcN and IdoA sugar residues. We estimated that forces stretching the proteoglycan matrix of secretory granules or the extracellular matrix are capable of inducing similar conformational transitions in heparin molecules in vivo. We hypothesize that these conformational transitions in heparin and similar conformational transitions in other glycosaminoglycans control ligand binding affinity during exocytotic release and during interactions between migrating cells and the extracellular matrix.
Acknowledgments
This work was supported by grants from the National Science Foundation and the National Institutes of Health.
References
- Alon, R., S. Chen, K. D. Puri, E. B. Finger, and T. A. Springer. 1997. The kinetics of L-selectin tethers and the mechanics of selectin-mediated rolling. J. Cell Biol. 138:1169–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon, R., D. A. Hammer, and T. A. Springer. 1995. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 374:539–542. [DOI] [PubMed] [Google Scholar]
- Chen, S., R. Alon, R. C. Fuhlbrigge, and T. A. Springer. 1997. Rolling and transient tethering of leukocytes on antibodies reveal specializations of selectins. Proc. Natl. Acad. Sci. USA. 94:3172–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. H., and H. G. Hansma. 2000. Basement membrane macromolecules: insights from atomic force microscopy. J. Struct. Biol. 131:44–55. [DOI] [PubMed] [Google Scholar]
- Curran, M. J., and M. S. Brodwick. 1991. Ionic control of the size of the vesicle matrix of beige mouse mast cells. J. Gen. Physiol. 98:771–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst, S., G. Venkataraman, V. Sasisekharan, R. Langer, C. L. Cooney, and R. Sasisekharan. 1998. Pyranose ring flexibility. Mapping of physical data for iduronate in continuous conformational space. J. Am. Chem. Soc. 120:2099–2107. [Google Scholar]
- Faham, S., R. E. Hileman, J. R. Fromm, R. J. Linhardt, and D. C. Rees. 1996. Heparin structure and interactions with basic fibroblast growth factor. Science. 271:1116–1120. [DOI] [PubMed] [Google Scholar]
- Fernandez, J. M., M. Villalon, and P. Verdugo. 1991. Reversible condensation of mast cell secretory products in vitro. Biophys. J. 59:1022–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro, D. R., A. Provasoli, M. Ragazzi, G. Torri, B. Casu, G. Gatti, J. C. Jacquinet, P. Sinaÿ, M. Petitou, and J. Choay. 1986. Evidence for conformational equilibrium of the sulfated L-iduronate residue in heparin and in synthetic heparin mono- and oligo-saccharides: NMR and force-field studies. J. Am. Chem. Soc. 108:6773–6778. [Google Scholar]
- Flory, P. J. 1953. Principles of Polymer Chemistry. Cornell University Press, Ithaca, NY.
- Galbraith, C. G., and M. P. Sheetz. 1997. A micromachined device provides a new bend on fibroblast traction forces. Proc. Natl. Acad. Sci. USA. 94:9114–9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hricovini, M., M. Guerrini, and A. Bisio. 1999. Structure of heparin-derived tetrasaccharide complexed to the plasma protein antithrombin derived from NOEs, J-couplings and chemical shifts. Eur. J. Biochem. 261:789–801. [DOI] [PubMed] [Google Scholar]
- Humphries, D. E., G. W. Wong, D. S. Friend, M. F. Gurish, W. T. Qiu, C. Huang, A. H. Sharpe, and R. L. Stevens. 1999. Heparin is essential for the storage of specific granule proteases in mast cells. Nature. 400:769–772. [DOI] [PubMed] [Google Scholar]
- Jackson, R. L., and L. R. McLean. 1991. Human postheparin plasma lipoprotein lipase and hepatic triglyceride lipase. Methods Enzymol. 197:339–345. [DOI] [PubMed] [Google Scholar]
- Li, H. B., M. Rief, F. Oesterhelt, and H. E. Gaub. 1998. Single molecule force spectroscopy on xanthan. Advanced Materials. 10:316–319. [Google Scholar]
- Li, H. B., M. Rief, F. Oesterhelt, X. Zhang, J. C. Shen, and H. E. Gaub. 1999. Single molecule force spectroscopy on polysaccharides by AFM-nanomechanical fingerprint of alpha-(1,4)-linked polysaccharides. Chem. Phys. Lett. 305:197–201. [Google Scholar]
- Marszalek, P., B. Farrell, and J. M. Fernandez. 1996. Ion-exchange gel regulates neurotransmitter release through the exocytotic fusion pore. Soc. Gen. Physiol. Ser. 51:211–222. [PubMed] [Google Scholar]
- Marszalek, P. E., B. Farrell, P. Verdugo, and J. M. Fernandez. 1997a. Kinetics of release of serotonin from isolated secretory granules. I. Amperometric detection of serotonin from electroporated granules. Biophys. J. 73:1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek, P. E., B. Farrell, P. Verdugo, and J. M. Fernandez. 1997b. Kinetics of release of serotonin from isolated secretory granules. II. Ion exchange determines the diffusivity of serotonin. Biophys. J. 73:1169–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek, P. E., H. Li, and J. M. Fernandez. 2001. Fingerprinting polysaccharides with single-molecule atomic force microscopy. Nat. Biotechnol. 19:258–262. [DOI] [PubMed] [Google Scholar]
- Marszalek, P. E., H. Li, A. F. Oberhauser, and J. M. Fernandez. 2002. Chair-boat transitions in single polysaccharide molecules observed with force-ramp AFM. Proc. Natl. Acad. Sci. USA. 99:4278–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek, P. E., A. F. Oberhauser, Y. P. Pang, and J. M. Fernandez. 1998. Polysaccharide elasticity governed by chair-boat transitions of the glucopyranose ring. Nature. 396:661–664. [DOI] [PubMed] [Google Scholar]
- Marszalek, P. E., Y. P. Pang, H. Li, J. El Yazal, A. F. Oberhauser, and J. M. Fernandez. 1999. Atomic levers control pyranose ring conformations. Proc. Natl. Acad. Sci. USA. 96:7894–7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe, D. D., M. Kaliner, and M. A. Donlon. 1981. The mast cell. Crit. Rev. Immunol. 3:23–74. [PubMed] [Google Scholar]
- Monck, J. R., A. F. Oberhauser, G. Alvarez de Toledo, and J. M. Fernandez. 1991. Is swelling of the secretory granule matrix the force that dilates the exocytotic fusion pore? Biophys. J. 59:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanavati, C., and J. M. Fernandez. 1993. The secretory granule matrix: a fast-acting smart polymer. Science. 259:963–965. [DOI] [PubMed] [Google Scholar]
- Oberhauser, A. F., C. Badilla-Fernandez, M. Carrion-Vazquez, and J. M. Fernandez. 2002. The mechanical hierarchies of fibronectin observed with single-molecule AFM. J. Mol. Biol. 319:433–447. [DOI] [PubMed] [Google Scholar]
- Oberhauser, A. F., and J. M. Fernandez. 1993. Patch clamp studies of single intact secretory granules. Biophys. J. 65:1844–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser, A. F., P. E. Marszalek, H. P. Erickson, and J. M. Fernandez. 1998. The molecular elasticity of the extracellular matrix protein tenascin. Nature. 393:181–185. [DOI] [PubMed] [Google Scholar]
- Ohashi, T., D. P. Kiehart, and H. P. Erickson. 1999. Dynamics and elasticity of the fibronectin matrix in living cell culture visualized by fibronectin-green fluorescent protein. Proc. Natl. Acad. Sci. USA. 96:2153–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura, V., and J. M. Fernandez. 1996. Atomic force microscopy study of the secretory granule lumen. Biophys. J. 71:2356–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham, R. J., Jr., and Y. Wang. 1997. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA. 94:13661–13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, V. S., P. V. Balaji, and P. K. Qasba. 1995. Controversial iduronate ring conformation in dermatan sulphate. Glycobiology. 5:273–279. [DOI] [PubMed] [Google Scholar]
- Rao, V. S. R., P. K. Qasba, P. V. Balaji, and R. Chandrasekaran. 1998. Conformation of Carbohydrates. Harwood Academic Publishers, Amsterdam.
- Rief, M., F. Oesterhelt, B. Heymann, and H. E. Gaub. 1997. Single molecule force spectroscopy on polysaccharides by atomic force microscopy. Science. 275:1295–1297. [DOI] [PubMed] [Google Scholar]
- Roden, L., S. Ananth, P. Campbell, T. Curenton, G. Ekborg, S. Manzella, D. Pillion, and E. Meezan. 1992. Heparin: an introduction. Adv. Exp. Med. Biol. 313:1–20. [DOI] [PubMed] [Google Scholar]
- Sharma, A., J. A. Askari, M. J. Humphries, E. Y. Jones, and D. I. Stuart. 1999. Crystal structure of a heparin- and integrin-binding segment of human fibronectin. EMBO J. 18:1468–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnas, B., and C. H. Aborg. 1983. Cation exchange—a common mechanism in the storage and release of biogenic amines stored in granules (vesicles)? I. Comparative studies on the uptake of sodium and biogenic amines by the weak cation (carboxyl) exchangers Amberlite IRC-50 and Sephadex C-50 and by biogenic (granule-enriched) materials in vitro. Acta Physiol. Scand. 119:225–234. [DOI] [PubMed] [Google Scholar]
- Venkataraman, G., V. Sasisekharan, C. L. Cooney, R. Langer, and R. Sasisekharan. 1994. A stereochemical approach to pyranose ring flexibility: its implications for the conformation of dermatan sulfate. Proc. Natl. Acad. Sci. USA. 91:6171–6175. [DOI] [PMC free article] [PubMed] [Google Scholar]