Abstract
A molecular understanding of volatile anesthetic mechanisms of action will require structural descriptions of anesthetic-protein complexes. Previous work has demonstrated that the halogenated alkane volatile anesthetics halothane and chloroform bind to the hydrophobic core of the four-α-helix bundle (Aα2-L38M)2 (Johansson et al., 2000, 2003). This study shows that the halogenated ether anesthetics isoflurane, sevoflurane, and enflurane are also bound to the hydrophobic core of the four-α-helix bundle, using isothermal titration calorimetry. Isoflurane and sevoflurane both bound to the four-α-helix bundle with Kd values of 140 ± 10 μM, whereas enflurane bound with a Kd value of 240 ± 10 μM. The ΔH° values associated with isoflurane, sevoflurane, and enflurane binding were –7.7 ± 0.1 kcal/mol, −8.2 ± 0.2 kcal/mol, and –7.2 ± 0.1 kcal/mol, respectively. The ΔS° values accompanying isoflurane, sevoflurane, and enflurane binding were −8.5 cal/mol K, −10.4 cal/mol K, and −8.0 cal/mol K, respectively. The results indicate that the hydrophobic core of (Aα2-L38M)2 is able to accommodate three modern ether anesthetics with Kd values that approximate their clinical EC50 values. The ΔH° values point to the importance of polar interactions for volatile general anesthetic binding, and suggest that hydrogen bonding to the ether oxygens may be operative.
INTRODUCTION
A molecular understanding of volatile general anesthetic mechanisms of action will require structural descriptions of anesthetic-protein complexes. Because the in vivo sites of action remain to be determined, the structural features of anesthetic binding sites on proteins are being explored using well defined model systems, such as the serum albumins and four-α-helix bundle proteins (Eckenhoff and Johansson, 1997). Studies with these model systems have suggested that volatile general anesthetics preferentially bind to preexisting appropriately sized packing defects, or cavities, within the protein matrix. In addition, favorable polar interactions with hydrophobic core side chains can enhance anesthetic binding affinity (Johansson et al., 2000; Manderson and Johansson, 2002).
Previous work has demonstrated that the halogenated alkane volatile anesthetics halothane and chloroform bind to the designed packing defect in the hydrophobic core of the four-α-helix bundle (Aα2-L38M)2 with dissociation constants that are comparable to their clinical EC50 values (Johansson et al., 2000, 2003). Because halogenated ether anesthetics are currently used primarily in the United States and Europe it is of interest to determine whether these molecules can also associate with the current four-α-helix bundle design. In contrast to halothane and chloroform, these halogenated ether anesthetics are not efficient quenchers of tryptophan fluorescence, precluding the use of this spectroscopic approach (Johansson et al., 1995) to monitor anesthetic binding. The current study therefore uses isothermal titration calorimetry to examine the binding of three halogenated ether anesthetics to the four-α-helix bundle (Aα2-L38M)2. In addition to providing values for dissociation constants, isothermal titration calorimetry has the distinct advantage of allowing both the enthalpic and entropic contributions to binding to be resolved (Leavitt and Friere, 2001).
To calibrate the results against earlier work using tryptophan fluorescence quenching, isothermal titration calorimetry was used initially to determine the energetics of halothane binding to the four-α-helix bundle. The binding of the ether anesthetics isoflurane, sevoflurane, and enflurane to the four-α-helix bundle (Aα2-L38M)2 was then characterized thermodynamically, after having shown that isothermal titration calorimetry and tryptophan fluorescence quenching gave comparable quantitative results with regard to halothane binding.
MATERIALS AND METHODS
9-Fluorenylmethoxycarbonyl (Fmoc) amino acids and Fmoc 2,4-dimethoxybenzhydrylamide resin were purchased from Perkin Elmer (Foster City, CA). Halothane (2-bromo-2-chloro-1,1,1-trifluoroethane) was obtained from Halocarbon Laboratories (Hackensack, NJ). The thymol preservative present in the commercial halothane was removed with an aluminum oxide column (Johansson et al., 1995). Enflurane (2-chloro-1,1,2-trifluoroethyl difluoromethyl ether) was obtained from Anaquest (Madison, WI). Isoflurane (1-chloro-2,2,2-trifluoroethyl difluoromethyl ether) was purchased from Ohmeda PPD, Inc. (Liberty Corner, NJ), and sevoflurane (fluoromethyl 2,2,2-trifluoro-1-(trifluoromethyl) ethyl ether from Abbott Laboratories (North Chicago, IL). All other chemicals were of reagent grade.
Peptide synthesis and preparation
The peptide Aα2-L38M (Johansson et al., 2000) was assembled as a C-terminus carboxyamide on a 0.25-mM scale using Fmoc amino acids and Fmoc 2,4-dimethoxybenzhydrylamide resin on an Applied Biosystems model 433A solid-phase peptide synthesizer (Perkin Elmer, Foster City, CA). Crude peptides were purified to homogeneity using reversed-phase C18 HPLC with aqueous-acetonitrile gradients containing 0.1% (v/v) 2,2,2-trifluoroacetic acid. Laser desorption mass spectrometry confirmed the peptide identity.
Isothermal titration calorimetry
Isothermal titration calorimetry was performed using a MicroCal VP-ITC titration microcalorimeter (Northampton, MA) at 20°C. The four-α-helix bundle (Aα2-L38M)2 at a concentration of 123 μM in 130 mM NaCl, pH 7.0, was placed in the 1.4-ml calorimeter cell, and anesthetic (5 mM in 130 mM NaCl, pH 7.0) was added sequentially in 10 μl aliquots (for a total of 29 injections) at 5-min intervals. The heat of reaction per injection (microcalories per second) was determined by integration of the peak areas using the Origin Version 5.0 software (1998). This software provides the best-fit values of the heat of binding (ΔH°), the stoichiometry of binding (n), and the dissociation constant (Kd) from plots of the heat evolved per mol of anesthetic injected versus the anesthetic/(Aα2-L38M)2 molar ratio (Wiseman et al., 1989). The heats of dilution were determined in parallel control experiments by injecting either 130 mM NaCl, pH 7.0, into a 123 μM four-α-helix bundle (Aα2-L38M)2 solution or 5 mM anesthetic (in 130 mM NaCl, pH 7.0) into the 130-mM NaCl, pH 7.0, solution. These heats of dilution are subtracted from the corresponding four-α-helix bundle-anesthetic binding experiments before curve-fitting.
The overall shape of the titration curve depends upon the c value ([(Aα2-L38M)2]/Kd) (Wiseman et al., 1989) and is rectangular for high c values (>500) and flat for low c values (<0.1). Earlier work using tryptophan fluorescence quenching indicates that halothane binds to the four-α-helix bundle (Aα2-L38M)2 with a Kd of 200 ± 10 μM (Johansson et al., 2000). To achieve a c value in the ideal range for isothermal titration calorimetry (5–50) would therefore require prohibitively high concentrations of protein (on the order of 1–10 mM). The four-α-helix bundle concentration used was 123 μM (c = 0.6), resulting in a shallow titration curve for halothane. Because the hydrophobic core of the four-α-helix bundle (Aα2-L38M)2 contains two identical binding sites for the anesthetics (Fig. 1), n was set as 1.0 so that deconvolution of the resulting isotherms only required the Kd and ΔH° values to be minimized. Allowing all three variables to float simultaneously may be associated with more variable results because of the potential for multiple minima (Wiseman et al., 1989).
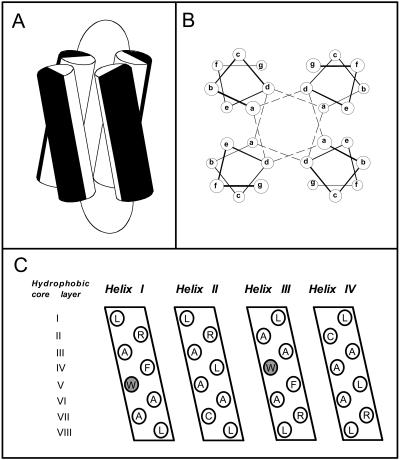
FIGURE 1.
(A) Modeled structure of the synthetic four-α-helix bundle. The cylinders represent the two 27-residue amphiphilic α-helical portions of each 62-residue di-α-helical peptide, joined by an eight-residue glycine linker. Black and white halves of each cylinder represent hydrophilic and hydrophobic residues, respectively. (B) End-on view of anti four-α-helix bundle, showing the interaction of the hydrophobic core residues at the heptad a and d positions. The dashed lines indicate how successive hydrophobic core layers are composed of two a and two d residues. (C) An opened-out and flattened representation of the (Aα2-L38M)2 bundle illustrating the amino acids present at the hydrophobic heptad a and d positions. There are a total of eight hydrophobic core layers, each composed of two a and two d position residues. The heptad a position W15 residues are shaded. Equivalent binding sites for anesthetic molecules reside in hydrophobic core layers III and VI where larger leucines were replaced with smaller alanines (Johansson et al., 1998).
RESULTS
Binding of the volatile anesthetic halothane to the hydrophobic core of the four-α-helix bundle (Aα2-L38M)2
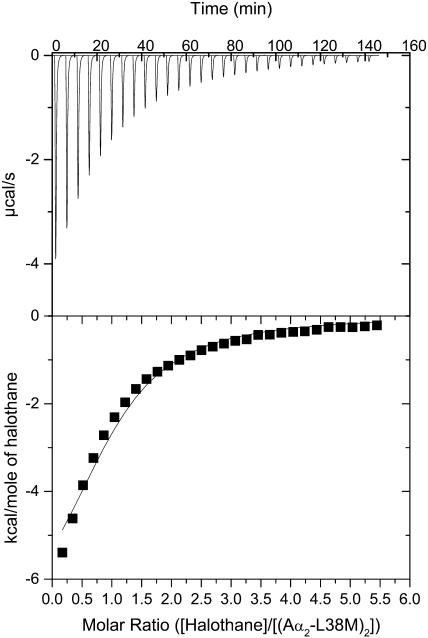
A representative calorimetric titration at pH 7.0 of the four-α-helix bundle (Aα2-L38M)2 with halothane is shown in Fig. 2. Each peak in the binding isotherm (Fig. 2, upper panel) represents a single injection of halothane. The negative deflections from the baseline on addition of halothane indicate that heat was evolved (an exothermic process). The enthalpy change associated with each injection of halothane was plotted versus the halothane/(Aα2-L38M)2 molar ratio (Fig. 2, lower panel), and the ΔH°, Kd, the free energy change associated with binding (ΔG°), and the change in entropy associated with binding (ΔS°) were determined from the plots. The Kd value of 60 ± 5 μM is quite comparable to the value of 200 ± 10 μM obtained using tryptophan fluorescence quenching (Johansson et al., 2000), supporting the validity of the results. The other thermodynamic parameters underlying halothane binding to the four-α-helix bundle (Aα2-L38M)2 are given in Table 1.
FIGURE 2.
Titration of the four-α-helix bundle (Aα2-L38M)2 with halothane, showing the calorimetric response as successive injections of ligand are added to the reaction cell. Panel B depicts the binding isotherm of the calorimetric titration shown in panel A. The continuous line represents the least-squares fit of the data to a single-site binding model.
TABLE 1.
Dissociation constants and thermodynamic data for binding of volatile general anesthetics to the four-α-helix bundle (Aα2-L38M)2
| Anesthetic | Kd (μM) | ΔG° (kcal/mol) | ΔH° (kcal/mol) | ΔS° (eu)* |
|---|---|---|---|---|
| Halothane | 60 ± 5 | −5.7 ± 0.1 | −7.7 ± 0.2 | −6.9 |
| Isoflurane | 140 ± 10 | −5.2 ± 0.1 | −7.7 ± 0.1 | −8.5 |
| Sevoflurane | 140 ± 10 | −5.2 ± 0.1 | −8.2 ± 0.2 | −10.4 |
| Enflurane | 240 ± 10 | −4.9 ± 0.1 | −7.2 ± 0.1 | −8.0 |
The entropy unit (eu) is cal/mol K.
Binding of the volatile anesthetics isoflurane, sevoflurane, and enflurane to the hydrophobic core of the four-α-helix bundle (Aα2-L38M)2
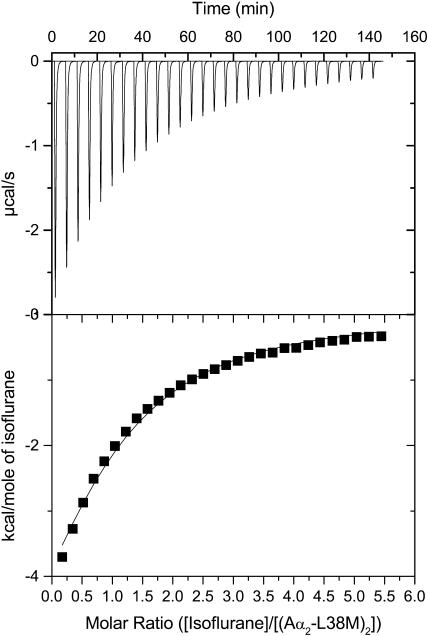
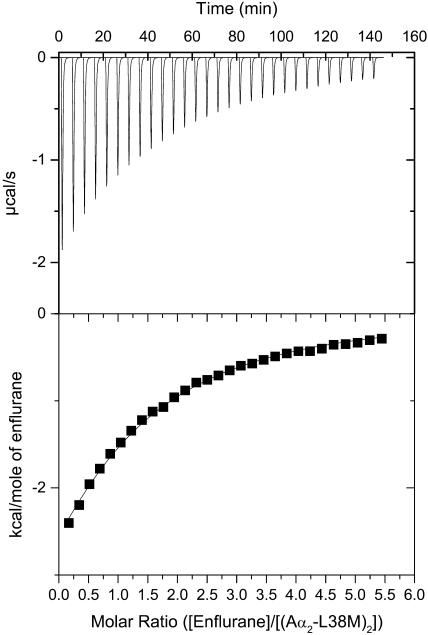
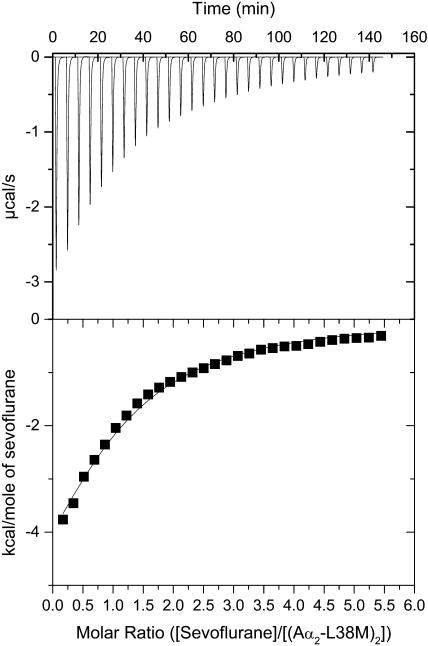
Representative calorimetric titrations at pH 7.0 of the four-α-helix bundle (Aα2-L38M)2 with isoflurane, sevoflurane, and enflurane are shown in Figs. 3–5. Each peak in the binding isotherms (Figs. 3–5, upper panels) represents a single injection of anesthetic. The negative deflections from the baseline on addition of anesthetic in each case indicate that heat was evolved. The enthalpy change associated with each injection of anesthetic was plotted versus the anesthetic/(Aα2-L38M)2 molar ratio (Figs. 3–5, lower panels), and the ΔH°, Kd, ΔG°, and ΔS° were determined from the plots. The Kd values for isoflurane, sevoflurane, and enflurane binding to the four-α-helix bundle (Aα2-L38M)2 were 140 ± 10 μM, 140 ± 10 μM, and 240 ± 10 μM, respectively. The other thermodynamic parameters underlying the binding of isoflurane, sevoflurane, and enflurane to the four-α-helix bundle (Aα2-L38M)2 are given in Table 1.
FIGURE 3.
Titration of the four-α-helix bundle (Aα2-L38M)2 with isoflurane, showing the calorimetric response as successive injections of ligand are added to the reaction cell. Panel B depicts the binding isotherm of the calorimetric titration shown in panel A. The continuous line represents the least-squares fit of the data to a single-site binding model.
FIGURE 5.
Titration of the four-α-helix bundle (Aα2-L38M)2 with enflurane, showing the calorimetric response as successive injections of ligand are added to the reaction cell. Panel B depicts the binding isotherm of the calorimetric titration shown in panel A. The continuous line represents the least-squares fit of the data to a single-site binding model.
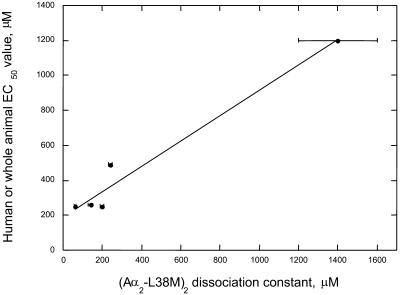
The four-α-helix bundle (Aα2-L38M)2 follows the Meyer-Overton rule
The Meyer-Overton rule has played a central role in the field of general anesthetic mechanisms research (Miller, 1985). This relationship shows that the olive oil/gas partition coefficients of anesthetic molecules correlate with their potency in animals. The ability of model proteins to obey the Meyer-Overton rule is used as an indication of how well they mimic the in vivo sites of anesthetic action (Miller, 1985). Fig. 6 shows a plot of the experimentally determined dissociation constants for halothane, chloroform, isoflurane, sevoflurane, and enflurane binding to the hydrophobic core of the four-α-helix bundle (Aα2-L38M)2 versus human or whole animal potency (EC50) data. Although limited to six data points, the binding site in the hydrophobic core of the four-α-helix bundle (Aα2-L38M)2 behaves in accord with the Meyer-Overton rule, suggesting that it represents a reasonable model of the actual central nervous system targets.
FIGURE 6.
Meyer-Overton plot of the human or whole animal potency data versus the experimental dissociation constant for binding to the four-α-helix bundle (Aα2-L38M)2. The halothane clinical EC50 value in man is 250 μM (Franks and Lieb, 1994), the chloroform EC50 value in dogs is 1.2 ± 0.2 mM (Eger et al., 1969), and the EC50 values in humans for isoflurane, sevoflurane, and enflurane are 260 μM, 260 μM, and 490 μM, respectively (Krasowski and Harrison, 1999). Two data points are for halothane, using the current Kd value and that of 200 ± 10 μM determined using fluorescence spectroscopy as reported earlier (Johansson et al., 2000). Note that the isoflurane and sevoflurane data points superimpose. The least-squares linear regression correlation coefficient is 0.982.
DISCUSSION
Although the in vivo sites of action of the volatile general anesthetics remain to be determined, it is currently argued that membrane proteins in the central nervous system represent likely targets (Franks and Lieb, 1994; Krasowski and Harrison, 1999). Based upon the few x-ray crystal structures that are available it is evident that the transmembrane domains of membrane proteins typically consist of bundles of α-helices (Doyle et al., 1998; Toyoshima et al., 2000; Bass et al., 2002; Dutzler et al., 2003). In addition, there is evidence that volatile general anesthetics interact directly with the transmembrane segments of ligand-gated ion channels (Eckenhoff, 1996; Mascia et al., 2000; Ishizawa et al., 2002). The ability of four-α-helix bundles to model the transmembrane domains is therefore being examined to provide detailed structural descriptions of anesthetic-protein interactions with the ultimate goal of understanding the mechanisms of anesthetic action.
Techniques that allow the direct measurement of volatile anesthetic binding to protein targets have been introduced during the past decade. These methods rely on 19F-NMR spectroscopy (Dubois and Evers, 1992; Dubois et al., 1993), photoaffinity labeling with halothane (Eckenhoff and Shuman, 1993), and fluorescence spectroscopy (Johansson et al., 1995; Manderson and Johansson, 2002). Isothermal titration calorimetry was first introduced a little over a decade ago (Wiseman et al., 1989) and is increasingly being used to study the thermodynamics of a broad range of molecular interactions. One prior study (Ueda and Yamanaka, 1997) has reported the use of isothermal titration calorimetry to characterize the binding of chloroform to bovine serum albumin. A Kd of 0.47 mM was determined for chloroform binding to bovine serum albumin and the molar heat of binding was −2.5 ± 0.1 kcal/mol.
The current study was designed to determine whether the hydrophobic core of the four-α-helix bundle (Aα2-L38M)2 is able to bind the halogenated ether anesthetics isoflurane, sevoflurane, and enflurane. Earlier studies have shown that this four-α-helix bundle design is able to bind both halothane and chloroform with dissociation constants that approximate their respective clinical EC50 values (Johansson et al., 2000, 2003). These studies relied on the ability of both halothane and chloroform to effectively quench the fluorescence of W15 located in the hydrophobic core of the four-α-helix bundle. In the current study, isothermal titration calorimetry was used to define the binding energetics associated with halogenated ether anesthetic binding. The results indicate that the hydrophobic core of (Aα2-L38M)2 is able to accommodate three modern ether anesthetics with Kd values that approximate their clinical EC50 values of 260–490 μM (Krasowski and Harrison, 1999). The results therefore suggest that the same site on a protein can bind structurally diverse volatile general anesthetics with affinities comparable to their clinical EC50 values. The ΔH° values point to the importance of polar interactions for anesthetic binding, and suggest that hydrogen bonding to the ether oxygen may be operative.
Isoflurane has been shown to bind to bovine serum albumin with Kd values of 1.4 ± 0.2 and 1.3 ± 0.2 mM using 19F-NMR spectroscopy (Dubois and Evers, 1992; Xu et al., 2000). Using a competitive photoaffinity labeling approach, Eckenhoff and Shuman (1993) reported a Kd value of 1.5 ± 0.2 mM for isoflurane binding to bovine serum albumin. Similarly, a tryptophan fluorescence anisotropy study determined that isoflurane bound to bovine serum albumin with a Kd of 1.6 ± 0.4 mM (Johansson et al., 1999). In addition, isoflurane has been shown to bind to nicotinic acetylcholine receptors from Torpedo nobiliana with an average Kd value of 0.36 ± 0.03 mM using 19F-NMR spectroscopy and gas chromatography (Xu et al., 2000). Sevoflurane binds to bovine serum albumin with a Kd value of 4.5 ± 0.6 mM as determined using 19F-NMR spectroscopy (Dubois et al., 1993). No report that directly addresses enflurane binding to proteins is currently available in the literature. The four-α-helix bundle (Aα2-L38M)2 therefore binds the halogenated ethers ∼10-fold more tightly than serum albumin. Of interest is that this relatively simple protein is able to bind the structural isomers enflurane and isoflurane with the relative affinities that mirror their respective in vivo potencies.
For the binding of all four volatile general anesthetics to the four-α-helix bundle (Aα2-L38M)2, ΔH° was somewhat more negative than ΔG° (Table 1). Because ΔG° = ΔH° - TΔS°, it follows that the entropy term will also be negative under these conditions. The binding of all four volatile general anesthetics to the four-α-helix bundle is therefore driven by enthalpy, whereas the entropy term is counteracting complex formation. This may be explained by the fact that the dissociation constants reflect relatively tight binding of anesthetic molecules to the four-α-helix bundle, compared to other proteins where direct binding has been demonstrated (Dubois and Evers, 1992; Dubois et al., 1993; Eckenhoff and Shuman, 1993, Johansson et al., 1995). Under these conditions, complex formation leads to a reduction in the degrees of freedom of both the anesthetic and the side chains at the binding site on the four-α-helix bundle, yielding a net entropic loss (Smithrud et al., 1991). As shown in Table 1, the binding of the anesthetic ethers isoflurane, sevoflurane, and enflurane to the four-α-helix bundle was associated with a greater entropic loss compared to halothane binding. Because the anesthetic ethers are predicted to be able to accept hydrogen bonding partners in the binding site on the four-α-helix bundle, this should impose additional geometric constraints on the complex that may be absent in the case of halothane, where more traditional hydrogen bonding interactions should be lacking. Furthermore, the dehydration of halothane that accompanies binding to the four-α-helix bundle may be associated with a greater net entropy increase compared to that associated with anesthetic ether dehydration.
Isothermal titration calorimetry may prove to be of considerable value as a method for examining the interactions of the volatile general anesthetics with a variety of proteins, including potential membrane targets. An advantage over 19F-NMR is that anesthetic molecules that are not fluorinated, such as nitrous oxide and cyclopropane, can be studied. Photoaffinity labeling has the disadvantage of being limited to studies on halothane, although the binding of other anesthetics to proteins can be examined using competitive approaches (Eckenhoff and Shuman, 1993). Compared to the fluorescence quenching technique (Johansson et al., 1995), isothermal titration calorimetry has the advantage of allowing nonquenching, or poorly quenching, anesthetics to be studied, as in the current manuscript. The main limitation of isothermal titration calorimetry is that high protein concentrations are required, because of the inherent relatively weak energetics of the interactions of volatile general anesthetics with macromolecules.
Recent efforts to crystallize the four-α-helix bundle (Aα2-L38M)2 have met with success and heavy atom derivatives are currently being investigated. The current results suggest that this four-α-helix bundle represents an attractive system for atomic-level structural studies in the presence of bound anesthetic. Such studies will provide much needed insight into how volatile anesthetics interact with biological macromolecules, and will provide guidelines regarding the general architecture of binding sites on central nervous system proteins.
FIGURE 4.
Titration of the four-α-helix bundle (Aα2-L38M)2 with sevoflurane, showing the calorimetric response as successive injections of ligand are added to the reaction cell. Panel B depicts the binding isotherm of the calorimetric titration shown in panel A. The continuous line represents the least-squares fit of the data to a single-site binding model.
Acknowledgments
Mass spectrometry was performed at the Protein Chemistry Laboratory, University of Pennsylvania, Philadelphia, PA.
This work was supported by National Institutes of Health grant GM55876.
References
- Bass, R. B., P. Strop, M. Barclay, and D. C. Rees. 2002. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 298:1582–1587. [DOI] [PubMed] [Google Scholar]
- Doyle, D. A., J. Morais-Cabral, R. A. Pfuetzner, A. Kuo, J. M. Gulbis, S. L. Cohen, B. T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- Dubois, B. W., and A. S. Evers. 1992. 19F-NMR spin-spin relaxation (T2) method for characterizing anesthetic binding to proteins: analysis of isoflurane binding to albumin. Biochemistry. 31:7069–7076. [DOI] [PubMed] [Google Scholar]
- Dubois, B. W., S. F. Cherian, and A. S. Evers. 1993. Volatile anesthetics compete for common binding sites on bovine serum albumin: A 19F-NMR study. Proc. Natl. Acad. Sci. USA. 90:6478–6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzler, R., E. B. Campbell, and R. MacKinnon. 2003. Gating the selectivity filter in ClC chloride channels. Science. 300:108–112. [DOI] [PubMed] [Google Scholar]
- Eckenhoff, R. G., and H. Shuman. 1993. Halothane binding to soluble proteins determined by photoaffinity labeling. Anesthesiology. 79:96–106. [DOI] [PubMed] [Google Scholar]
- Eckenhoff, R. G. 1996. An inhalational anesthetic binding domain in the nicotinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA. 93:2807–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenhoff, R. G., and J. S. Johansson. 1997. Molecular interactions between inhaled anesthetics and proteins. Pharmacol. Rev. 49:343–367. [PubMed] [Google Scholar]
- Eger, E. I., C. Lundgren, S. L. Miller, and W. C. Stevens. 1969. Anesthetic potencies of sulfur hexafluoride, carbon tetrafluoride, chloroform and Ethrane in dogs: Correlation with the hydrate and lipid theories of anesthetic action. Anesthesiology. 30:129–135. [PubMed] [Google Scholar]
- Franks, N. P., and W. R. Lieb. 1994. Molecular and cellular mechanisms of general anaesthesia. Nature. 367:607–614. [DOI] [PubMed] [Google Scholar]
- Ishizawa, Y., R. Pidikiti, P. A. Liebman, and R. G. Eckenhoff. 2002. G protein-coupled receptors as direct targets of inhaled anesthetics. Mol. Pharmacol. 61:945–952. [DOI] [PubMed] [Google Scholar]
- Johansson, J. S., R. G. Eckenhoff, and P. L. Dutton. 1995. Binding of halothane to serum albumin demonstrated using tryptophan fluorescence. Anesthesiology. 83:316–324. [DOI] [PubMed] [Google Scholar]
- Johansson, J. S., B. R. Gibney, F. Rabanal, K. S. Reddy, and P. L. Dutton. 1998. A designed cavity in the hydrophobic core of a four-α-helix bundle improves volatile anesthetic binding affinity. Biochemistry. 37:1421–1429. [DOI] [PubMed] [Google Scholar]
- Johansson, J. S., H. Zou, and J. W. Tanner. 1999. Bound volatile general anesthetics alter both local protein dynamics and global protein stability. Anesthesiology. 90:235–245. [DOI] [PubMed] [Google Scholar]
- Johansson, J. S., D. Scharf, L. A. Davies, K. S. Reddy, and R. G. Eckenhoff. 2000. A designed four-α-helix bundle that binds the volatile general anesthetic halothane with high affinity. Biophys. J. 78:982–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson, J. S., K. Solt, and K. S. Reddy. 2003. Binding of the general anesthetics chloroform and 2,2,2-trichloroethanol to the hydrophobic core of a four-α-helix bundle protein. Photochem. Photobiol. 77:89–95. [DOI] [PubMed] [Google Scholar]
- Krasowski, M. D., and N. L. Harrison. 1999. General anaesthetic actions on ligand-gated ion channels. Cell. Mol. Life Sci. 55:1278–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt, S., and E. Friere. 2001. Direct measurement of protein binding energetics by isothermal titration calorimetry. Curr. Opin. Struct. Biol. 11:560–566. [DOI] [PubMed] [Google Scholar]
- Manderson, G. A., and J. S. Johansson. 2002. The role of aromatic side chains in the binding of volatile general anesthetics to a four-α-helix bundle. Biochemistry. 41:4080–4087. [DOI] [PubMed] [Google Scholar]
- Mascia, M. P., J. R. Trudell, and R. A. Harris. 2000. Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc. Natl. Acad. Sci. USA. 97:9305–9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, K. W. 1985. The nature of the site of general anesthesia. Int. Rev. Neurobiol. 27:1–61. [DOI] [PubMed] [Google Scholar]
- Smithrud, D. B., T. B. Wyman, and F. Diederich. 1991. Enthalpically driven cyclophane-arene inclusion complexation: Solvent-dependent calorimetric studies. J. Am. Chem. Soc. 113:5420–5426. [Google Scholar]
- Toyoshima, C., M. Nakasako, H. Nomura, and H. Ogawa. 2000. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature. 405:647–655. [DOI] [PubMed] [Google Scholar]
- Ueda, I., and M. Yamanaka. 1997. Titration calorimetry of anesthetic-protein interaction: negative enthalpy of binding and anesthetic potency. Biophys. J. 72:1812–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman, T., S. Williston, J. F. Brandts, and L.-N. Lin. 1989. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal. Biochem. 179:131–137. [DOI] [PubMed] [Google Scholar]
- Xu, Y., T. Seto, P. Tang, and L. Firestone. 2000. NMR study of volatile anesthetic binding to nicotinic acetylcholine receptors. Biophys. J. 78:746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]