Abstract
Formamide-induced detubulation of rat ventricular myocytes was used to investigate the functional distribution of the Na/Ca exchanger (NCX) and Na/K-ATPase between the t-tubules and external sarcolemma. Detubulation resulted in a 32% decrease in cell capacitance, whereas cell volume was unchanged. Thus, the surface-to-volume ratio was used to assess the success of detubulation. NCX current (INCX) and Na/K pump current (Ipump) were recorded using whole-cell patch clamp, as Cd-sensitive and K-activated currents, respectively. Both inward and outward INCX density was significantly reduced by ∼40% in detubulated cells. INCX density at 0 mV decreased from 0.19 ± 0.03 to 0.10 ± 0.03 pA/pF upon detubulation. Ipump density was also lower in detubulated myocytes over the range of voltages (−50 to +100 mV) and internal [Na] ([Na]i) investigated (7–22 mM). At [Na]i = 10 mM and −20 mV, Ipump density was reduced by 39% in detubulated myocytes (0.28 ± 0.02 vs. 0.17 ± 0.03 pA/pF), but the apparent Km for [Na]i was unchanged (16.9 ± 0.4 vs. 17.0 ± 0.3 mM). These results indicate that although thet-tubules represent only ∼32% of the total sarcolemma, they contribute ∼60% to the total INCX and Ipump. Thus, the functional density of NCX and Na/K pump in the t-tubules is 3–3.5-fold higher than in the external sarcolemma.
INTRODUCTION
The sarcolemma of mammalian cardiac ventricular myocytes has invaginations (transverse (t-) tubules) that occur periodically at the level of the Z-line, and comprise 21–64% of the total sarcolemmal membrane area (Page et al., 1971; Page and Surdyk-Droske, 1979; Soeller and Cannell, 1999; Bers, 2001). Immunocytochemical studies have shown that L-type calcium channels are concentrated in the t-tubules, in close apposition to the ryanodine receptor (Carl et al., 1995; Scriven et al., 2000) at the dyadic junction between the sarcolemma and the sarcoplasmic reticulum (SR) membrane. This allows the fast inward spread of excitation-contraction coupling so that SR Ca release is spatially and temporally synchronized throughout the cell (Yang et al., 2002; Brette et al., 2002; Bers, 2001). In contrast, in atrial and neonatal ventricular myocytes, and Purkinje cells, which lack appreciable t-tubules, [Ca]i rises first at the edge of the cell and then propagates into the cell interior (Huser et al., 1996; Haddock et al., 1999; Cordeiro et al., 2001).
While there is general agreement that L-type Ca channels are concentrated in the t-tubules, the distribution of the Na/Ca exchanger (NCX), and Na/K-ATPase, the major routes for Ca and Na extrusion in cardiac cells, is less clear. Immunocytochemical studies show either a rather even distribution of NCX between surface sarcolemma and t-tubules (Kieval et al., 1992) or a concentration in the t-tubules (Frank et al., 1992). The Na/K ATPase is observed in both t-tubules and surface sarcolemma (McDonough et al., 1996), but the relative distribution is unclear. Moreover, these immunohistochemical methods do not provide functional data, nor are they particularly quantitative with respect to transporter localization or function.
A novel method has recently been developed to detubulate rat ventricular myocytes and make direct functional measurements of ion channel and transporter function in surface vs. t-tubule membranes (Kawai et al., 1999; Brette et al., 2002; Yang et al., 2002). Upon withdrawal of 1.5 M formamide, the t-tubules seal off and only currents carried by surface sarcolemmal channels and transporters are accessible. Notably, this procedure has no effect on ionic currents measured in atrial myocytes, which lack endogenous t-tubules, indicating that formamide exposure and withdrawal does not by itself alter ion channel function (Brette et al., 2002). Using this technique, it has been shown that ∼87% of the L-type calcium current (ICa) occurs within the t-tubules (Kawai et al., 1999), in agreement with immunolabeling data. Furthermore, it was found that in detubulated ventricular myocytes the spatial distribution of [Ca]i after electrical stimulation is similar to that in atrial cells, i.e., [Ca]i initially increases close to the surface membrane and then propagates into the cell (Yang et al., 2002).
Yang et al. (2002) showed that while detubulation has no effect on Na current density (suggesting equal t-tubule vs. surface density), it nearly abolishes NCX current (INCX) and the transient increase of extracellular [Ca] upon caffeine application. They concluded that NCX activity is located almost entirely in the t-tubules. However, in that study, INCX density was very small, even in control cells, making quantitative analysis difficult. Therefore, the first aim of the present study was to quantitatively reinvestigate the functional distribution of NCX between external sarcolemma and t-tubules. The ability of NCX to extrude Ca is controlled by intracellular [Na] ([Na]i), which depends on Na/K-ATPase function. During the cardiac cycle, NCX accounts for >60% of the total Na influx, vs. ∼20% via Na channels (Bers et al., 2003). Thus, our second aim was to test the hypothesis that Na/K pump and NCX function are both concentrated in the t-tubules vs. the external sarcolemma.
MATERIALS AND METHODS
Myocytes isolation and detubulation procedure
The procedure for isolation of ventricular myocytes has been described previously (Despa et al., 2002) and approved by the Loyola University Chicago animal welfare committee. Briefly, rats were anesthetized by I.P. injection of Nembutal (∼0.1 mg/g). Hearts were excised quickly and placed on a Langendorff perfusion apparatus. Hearts were perfused for 5–7 min with nominally Ca free Tyrode's solution, then perfusion proceeded with added collagenase (1 mg/ml) and albumin (0.05%). When the heart became flaccid, the left ventricular tissue was cut into small pieces for further incubation (5 or 10 min) with 0.4 mg/ml collagenase. The tissue was then filtered and Ca concentration in the cell suspension was progressively increased to 1 mM. The standard Tyrode's solution used in these experiments contained (in mM): 140 NaCl, 4 KCl, 1 MgCl2, 10 glucose, 5 HEPES, and 1 CaCl2 (pH = 7.4). All experiments were done at room temperature (23–25°C).
Detubulation was induced by osmotic shock as described previously (Kawai et al., 1999). Briefly, 1.5 M formamide was added to the cell suspension for 15–20 min, then the cells were returned to the standard solution. Detubulation occurs because of the osmotic shock produced by formamide withdrawal. For some experiments, 1 mM ouabain was added during the last 2 min of formamide treatment and the first 2 min of formamide removal. Ouabain was then removed from the bathing solution ∼1 h before the experiments were started, which is sufficient to fully reverse Ipump blockade (not shown).
Cell volume was determined using the formula v = (πlwd)/4 (Boyett et al., 1991), where l and w are the measured cell length and width, respectively. The cell depth (d) was calculated by assuming the cell to be an elliptical cylinder with a minor-to-major axis ratio of 1:3 (Boyett et al., 1991).
Electrophysiological recording
Isolated ventricular myocytes, plated on laminin-coated glass coverslips, were whole-cell voltage clamped using patch electrodes made from borosilicate glass capillaries. When filled with the standard pipette solution, electrode resistance was 1.5–2.5 MΩ. Current signals were recorded using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA). Membrane capacitance (Cm) and series resistance were calculated from a 5-mV voltage step and were then compensated up to 70% for measurement of membrane current. The standard pipette solution contained (in mM): 10 NaCl, 20 CsCl, 80 CsOH, 80 glutamic acid, 20 TEA-Cl, 10 HEPES, 5 Tris-ATP, 5.7 MgCl2 (≈1 mM free Mg), 5 BAPTA, 1 di-Br-BAPTA, 1.8 CaCl2 (100 nM free Ca) or 2.69 CaCl2 (200 nM free Ca), at pH = 7.2.
ICa, Na/Ca exchange, and Na/K pump current measurements
For these experiments, myocytes were treated for 10 min with 1 μM thapsigargin to empty the SR of Ca. ICa was measured shortly after reaching the whole-cell configuration with a depolarization step from −40 mV (where the cell was held for 50 ms to inactivate Na channels) to 0 mV. The myocytes were then superfused with a solution containing (in mM): 140 NaCl, 1 CaCl2, 1 MgCl2, 2 BaCl2, 10 HEPES, 10 glucose, 0.01 nifedipine, and 0.01 niflumic acid (pH = 7.4 with Tris). Myocytes were held for a few minutes at the calculated reversal potential of NCX (Erev: −33 mV for [Ca]i = 100 nM, −15 mV for [Ca]i = 200 nM) to minimize fluxes through NCX and thus allow [Ca]i and [Na]i to equilibrate with the pipette concentrations. INCX-voltage curves were obtained using a voltage ramp protocol. From the holding potential at −33 mV, the cell membrane was first depolarized to +100 mV for 40 ms, then hyperpolarized to −100 mV over 400 ms. This was followed by a 400-ms ascending ramp to +100 mV. This ramp clamp was repeated every 12 s. The protocol was repeated in the presence of 1 mM Cd and the difference in current was taken as INCX. Next, 4 mM K was added to the external solution (in the presence of 1 mM Cd) to activate the Na/K-ATPase, at Em = −20 mV, and the outward shift in the membrane current taken as Ipump. The ramp protocol was repeated in the presence of external K and the difference current (between the presence and the absence of K) was used to determine the Ipump-voltage relationship.
Simultaneous Ipump and [Na]i measurements
In a different set of experiments, Ipump and [Na]i were measured simultaneously as described previously (Despa and Bers, 2003). The approach was to use relatively high resistance electrodes (3–5 MΩ when filled with the internal solution) so that [Na]i is mainly controlled by the membrane transporters and not by the pipette. To derive the [Na]i -dependence of Ipump, myocytes were Na loaded by inhibiting the Na/K pump for 10–12 min in K free external solution (Em = −20 mV). Then the pump was abruptly re-activated by adding back K (4 mM) and both membrane current and [Na]i were measured. The internal and external solutions were similar to those used for INCX measurements, except for omitting Ca, nifedipine, and niflumic acid from the external solution and blocking NCX with 1 mM Cd. In the same experiments, we also measured the rate of change of [Na]i (d[Na]i/dt) upon Na/K-pump activation, using methods previously described to account for [Na] in the pipette and passive entry of Na into the cell from the bath (Despa and Bers, 2003).
[Na]i was measured using dual excitation ratiometric measurements (at 340 and 380 nm) with the fluorescent indicator SBFI (Molecular Probes, Eugene, OR). For this, 1 mM SBFI was added to the internal solution. For each cell, the background signal at both wavelengths was measured on seal formation and subtracted from the signals after dye loading. In situ calibration of SBFI was done at the end of each experiment by exposing the cell to divalent-free solutions with 0, 10, and 20 mM extracellular [Na], in the presence of 10 μM gramicidin and 100 μM strophanthidin.
[Ca]i measurements
Confocal Ca imaging was performed using a laser-scanning unit (Microradiance 2000, Bio-Rad, Hercules, CA) attached to a Nikon Diaphot inverted microscope (Nikon, Melville, NY). Control and detubulated (with ouabain washed off from the external sarcolemma in the group treated with ouabain during detubulation) myocytes were loaded with 10 μM Fluo-3 AM (Molecular Probes) for 40 min, then myocytes were perfused with a standard Tyrode's solution with 2 mM CaCl2. The dye was excited with the 488-nm line of an argon ion laser and fluorescence measured at >500 nm. Transversal line scan images were acquired at 2-ms intervals.
Statistical analysis
Data are expressed as mean ± SE. Statistical discriminations were performed using unpaired t-tests. Values of P < 0.05 were considered significant.
RESULTS
Effect of formamide treatment on membrane capacitance, surface-to-volume ratio, and ICa
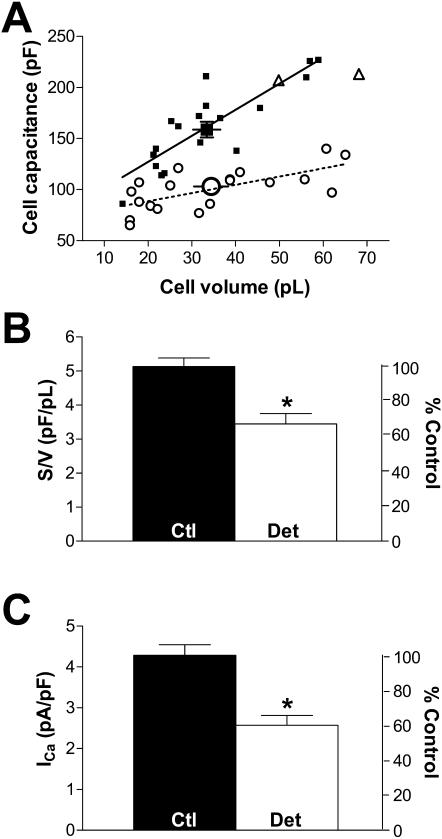
Previous experiments showed that >80% of myocytes are detubulated after formamide treatment (Kawai et al., 1999; Brette et al., 2002). Because membrane capacitance decreases significantly upon formamide treatment, but cell volume is unchanged (Kawai et al., 1999), the surface-to-volume ratio could be used to assess detubulation of the myocytes used for patch clamp measurements. Fig. 1 A shows the relation between Cm and volume for control and formamide-treated myocytes. The surface-to-volume ratio was calculated from the linear regression of these data points. This plot allowed us to exclude two “detubulated” cells (out of 21) from the analysis (triangles in Fig. 1 A) that do not appear to be detubulated based upon this regression.
FIGURE 1.
Effect of detubulation on cell surface, volume, and calcium current. (A) Cell capacitance vs. volume for all control (▪) and detubulated (○) cells studied. Mean volume and capacitance values are shown by the bigger symbol. A linear regression was calculated for control (solid line) and detubulated (dotted line) cells. Note the presence of two outlier “detubulated” cells (▵) near the control regression line. These cells were not considered for currents analysis. (B) Mean data for surface-to-volume ratio (S:V) in control (n = 18, black bar) and detubulated cells (n = 19, open bar). (C) Mean ICa in control (n = 22, black bar) and detubulated cells (n = 21, open bar). The right axis represents value normalized to control data. All bars are means ± SE; asterisk indicates P < 0.05.
We confirmed that the cell volume does not change significantly with detubulation (33.4 ± 3.1 pl in 18 control cells vs. 34.4 ± 3.8 pl in 19 detubulated cells, P > 0.05), whereas Cm decreases significantly, by 32%, from 156 ± 7 pF (n = 24) to 106 ± 5 pF (n = 19, P < 0.05). Fig. 1 B shows that the surface-to-volume ratio is significantly lower in detubulated myocytes (5.1 ± 0.2 pF/pL in control vs. 3.4 ± 0.3 pF/pL in detubulated cells, P < 0.05). We also routinely measured ICa in each cell shortly after patch rupture, and confirmed that ICa density is significantly lower in detubulated vs. control myocytes (2.6 ± 0.2 vs. 4.3 ± 0.3 pA/pF, respectively, P < 0.05, Fig. 1 C).
Effect of detubulation on INCX
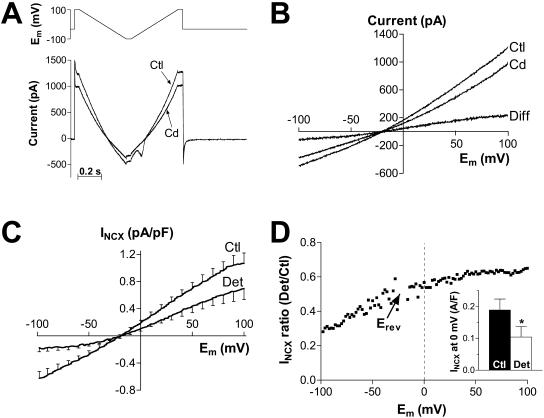
INCX was measured using a voltage ramp protocol (Fig. 2 A, top) as the current sensitive to 1 mM Cd. Fig. 2 A shows original current records in a control myocyte in the absence and presence of Cd. A small contamination of the ascending ramp by Cd-sensitive cardiac Na current is apparent. The voltage dependence of the membrane current recorded without and with Cd and the difference current, i.e., INCX, are shown in Fig. 2 B. The data are from the descending ramp, although there were no differences in INCX between the ascending and the descending ramp (apart from the INa contamination). This indicates that subsarcolemmal [Ca]i and [Na]i are not appreciably affected by the NCX fluxes during the ramp protocol. Fig. 2 C shows mean INCX density-voltage relationship for control and detubulated myocytes at 100 nM free [Ca]i. In both cases, the current reversed at −20 mV, which is slightly positive to the calculated NCX reversal potential (−33 mV). Both inward and outward INCX density is reduced in detubulated cells (Fig. 2 D), indicating that INCX has a higher density in the t-tubules vs. external sarcolemma. This is emphasized in the inset of Fig. 2 D, which shows the average INCX at 0 mV is reduced by 45% (0.19 ± 0.03 pA/pF in control vs. 0.10 ± 0.03 pA/pF in detubulated myocytes). It is unclear why the relative amount of INCX in detubulated cells is smaller at negative potentials (Fig. 2 D); at positive potentials the ratio was relatively constant. By using the value at 0 mV we obtain a conservative (i.e., minimum) estimate of the percentage of INCX located in the t-tubules. Similar results were obtained for [Ca]i = 200 nM (n = 10; not shown).
FIGURE 2.
INCX is concentrated in the t-tubules. (A) Representative example of the membrane current recorded in a control cell during the voltage ramp protocol (top panel) in the absence (Ctl) and in the presence (Cd) of 1 mM Cd. Free [Ca]i in the pipette was 100 nM. (B) Current-voltage relationship for the currents shown in A during the descending ramp and the difference current (Diff). (C) Mean current density-voltage relationship of INCX, i.e., the Cd-sensitive current, recorded in 12 control (Ctl) and 11 detubulated (Det) myocytes. (D) Voltage dependence of the ratio between INCX in detubulated and control cells. (Inset) Mean INCX density at 0 mV in control and detubulated cells.
Sealed-off t-tubules still transport Ca
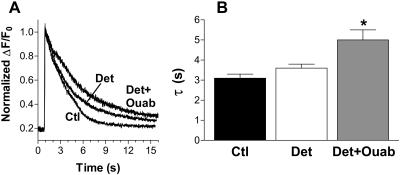
It has been shown previously (Brette et al., 2002) that the t-tubules seal off inside the cell after detubulation. Thus, the NCX molecules localized in the t-tubules might still be functionally active in removing Ca from the cytoplasm. During caffeine-induced Ca transients (Fig. 3) [Ca]i decline is primarily mediated by NCX (Bers, 2001). So, if 50% of NCX molecules were in the t-tubules and were nonfunctional, then the rate of [Ca]i decline during caffeine-induced Ca transients should be slowed by ∼50%. Fig. 3 shows that [Ca]i decline is slowed only by 14% in detubulated myocytes ([Ca]i decay time constant increased from 3.1 ± 0.2 to 3.6 ± 0.2 s). This suggests that NCX in the sealed-off t-tubules is still largely functional, and therefore that the sealed-off t-tubules are able to generate high intratubular [Na] via Na/K-ATPase function (to drive Ca extrusion via NCX). To block Na/K-ATPase in sealed-off t-tubules 1 mM ouabain was entrapped there during detubulation (and washed away from surface pumps after detubulation occurred). Without functional Na/K-ATPase in the sealed-off t-tubules there should be no [Na] gradient to fuel NCX. In this case the rate of [Ca]i decline was slowed 38% vs. control (τ = 5.0 ± 0.5 s). This is consistent with a high fraction of NCX molecules in the t-tubules and the t-tubules still able to transport Na and Ca after formamide treatment. All myocytes in Fig. 3 were identically conditioned by electrical stimulation at 0.5 Hz before caffeine application. The amplitude of the caffeine-induced Ca transient was not significantly different between groups.
FIGURE 3.
NCX in the sealed-off t-tubules membrane might still be functionally active. (A) Normalized traces of caffeine-induced Ca transient in a control (Ctl), detubulated (Det), and detubulated with 1 mM ouabain entrapped in the sealed-off t-tubules (Det+ouab) myocytes. All myocytes were stimulated at steady state (at 0.5 Hz) before the application of 10 mM caffeine. (B) Mean data for the decay time of caffeine-induced Ca transient in 18 control cells, 14 detubulated myocytes, and 11 detubulated cells with 1 mM ouabain.
Effect of detubulation on Ipump
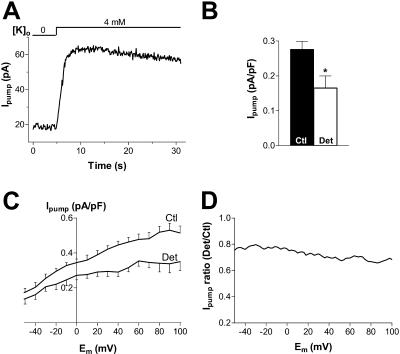
To measure Ipump, myocytes were held at −20 mV and the Na/K pump activated by adding 4 mM K to the external solution (Fig. 4 A). No change in the membrane current was observed when 10 mM ouabain was present in the external solution (not shown), indicating that under our experimental conditions, the outward shift induced by external K is entirely Ipump. Mean data from these experiments (Fig. 4 B) indicate that Ipump density in detubulated myocytes was 60% of that in control myocytes (0.17 ± 0.03 vs. 0.28 ± 0.02 pA/pF).
FIGURE 4.
Ipump is concentrated in the t-tubules. (A) Representative example of Ipump measured at −20 mV as the outward shift induced by 4 mM K in a control cell. (B) Mean data for Ipump in 16 control and 14 detubulated myocytes. (C) Mean Ipump-voltage relationship recorded in 8 control and 8 detubulated myocytes. (D) Voltage dependence of the ratio between Ipump in detubulated and control cells.
The voltage-dependence of Ipump was also assessed using the ramp protocol described above. The protocol was applied in the absence and in the presence of external K. Fig. 4 C shows the mean Ipump density-voltage relationship in control and detubulated cells. Ipump density was reduced in detubulated cells over the whole voltage range investigated (Fig. 4 D). The mean detubulated/control ratio had a slight Em-dependence and was slightly higher than the value in Fig. 4 B (where a larger number of cells were studied).
The [Na]i-dependence of Ipump was studied in a different set of experiments: Ipump and [Na]i were measured simultaneously during rapid reactivation of the pump by re-addition of 4 mM K to the bathing solution under conditions designed to allow control of [Na]i to be dominated by transsarcolemmal Na fluxes (see Methods). Fig. 5, A and B, and C and D, show representative examples in a control and a detubulated myocyte, respectively. Pump re-activation resulted in a rapid outward shift in the membrane current, followed by a decay accompanied by a decline in [Na]i. It has recently been shown that the decay of Ipump occurs in two phases, with a first, rapid decline at practically constant [Na]i and a second, slower phase where Ipump and [Na]i decline in parallel (Despa and Bers, 2003). The initial Ipump sag was attributed to local subsarcolemmal [Na]i depletion due to the rapid Na extrusion via the pump itself (at 37°C). The initial Ipump sag was less obvious here, although still present in 5 out of 7 control cells and 3 out of 8 detubulated myocytes. This is probably because Ipump density is 3–4-fold lower at 23°C, especially in detubulated myocytes, and the apparent depletion depends strongly on Ipump magnitude.
FIGURE 5.
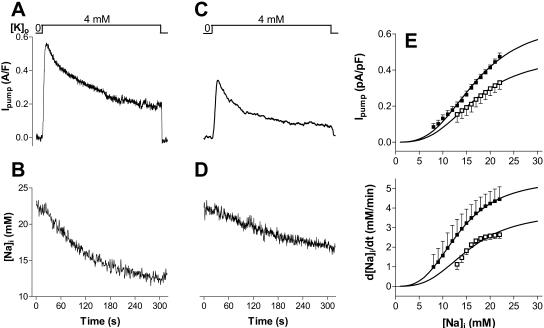
[Na]i-dependence of Ipump in control and detubulated myocytes. Changes in Ipump (A and C) and [Na]i (B and D) upon abrupt Na/K pump re-activation after 10–12 min of pump blockade in the absence of external K, in a control (A and B) and a detubulated (C and D) myocyte. (E) Mean Ipump and d[Na]i/dt at various [Na]i in seven control and eight detubulated myocytes. Data are fit with a Hill equation (Vmax/(1+(Km/[Na]i)3).
Fig. 5 E shows the [Na]i-dependence of Ipump and the rate of [Na]i decline (−d[Na]i/dt) in control and detubulated myocytes. To derive the maximum transport rate (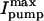 and d[Na]i/dtmax) and the [Na]i for half-maximal activation of the pump (Km), mean data were fitted with a Hill expression.
and d[Na]i/dtmax) and the [Na]i for half-maximal activation of the pump (Km), mean data were fitted with a Hill expression. 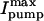 in detubulated cells was 71% of that in control (0.48 ± 0.01 vs. 0.67 ± 0.02 pA/pF) whereas the Km was unchanged (16.9 ± 0.4 vs. 17.0 ± 0.3 mM). Similarly, the d[Na]i/dtmax in detubulated cells was 69% of that in control (3.8 ± 0.3 vs. 5.5 ± 0.1 mM/min) with comparable Km (15.6 ± 1.1 and 13.4 ± 0.1). The lower
in detubulated cells was 71% of that in control (0.48 ± 0.01 vs. 0.67 ± 0.02 pA/pF) whereas the Km was unchanged (16.9 ± 0.4 vs. 17.0 ± 0.3 mM). Similarly, the d[Na]i/dtmax in detubulated cells was 69% of that in control (3.8 ± 0.3 vs. 5.5 ± 0.1 mM/min) with comparable Km (15.6 ± 1.1 and 13.4 ± 0.1). The lower 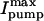 and d[Na]i/dtmax in detubulated cells also suggests that the Na/K pumps are concentrated in the t-tubules in rat ventricular myocytes.
and d[Na]i/dtmax in detubulated cells also suggests that the Na/K pumps are concentrated in the t-tubules in rat ventricular myocytes.
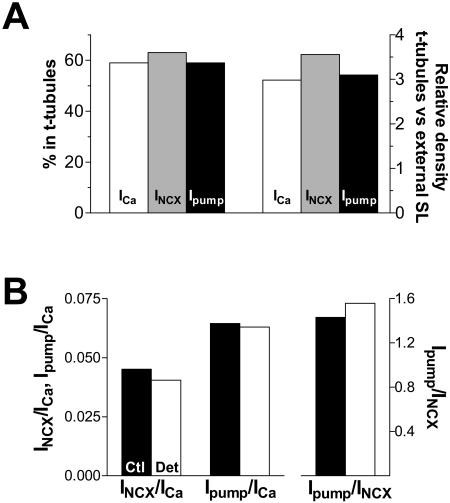
The central findings of this study are summarized in Table 1 and Fig. 6. While the t-tubules represent only ∼32% of the total sarcolemma (based on the decrease in membrane capacitance), they contribute ∼60% to the total INCX and Ipump. This indicates that the functional density of NCX and Na/K pump in the t-tubules is 3–3.5-fold higher than in the external sarcolemma (Fig. 6 A). The ratio of Ipump and INCX is similar in control and detubulated myocytes (Fig. 6 B), suggesting that the relative concentration of NCX and Na/K-ATPase in t-tubules is the same (see also Table 1 and Discussion). However, NCX could be more concentrated in the t-tubule than the Na/K-ATPase, since there was Em-dependence of the INCX ratio (Fig. 2 D).
TABLE 1.
Distribution of mean (±SE) surface area, ICa, INCX, and Ipump in the external sarcolemma and t-tubules
| Surface area (pF) | ICa* (pA/pF) | INCX† (pA/pF) | Ipump | |
|---|---|---|---|---|
| Total SL (control cells) | 156 ± 7 | 4.3 ± 0.3 | 0.19 ± 0.03 | 0.28 ± 0.02 |
| External SL (detubulated cells) | 106 ± 5 | 2.6 ± 0.2 | 0.10 ± 0.03 | 0.17 ± 0.03 |
| t-tubules (calculated) | 50 | 7.8 | 0.38 | 0.51 |
| % in t-tubules | 32 | 59 | 63 | 59 |
| Density t-tub/ext SL | 3.0 | 3.6 | 3.1 |
May be an underestimate; see Discussion.
The values are for [Ca]i = 100 nM at 0 mV.
FIGURE 6.
The relative distribution of Ca channels, NCX, and Na/K pumps between t-tubules and external sarcolemma. (A) Percentage of the whole-cell ICa, INCX, and Ipump originating in the t-tubules and the ratio between the current density in the t-tubules and external sarcolemma. The currents in t-tubules were calculated as the difference in the whole-cell current between control and detubulated myocytes. These currents were divided by the difference in the membrane capacitance in control versus detubulated cells to derive the current density in t-tubules. (B) The mean ratio between ICa, INCX, and Ipump in control and detubulated cells. The values at 0 mV and 100 nM [Ca]i were used for INCX.
DISCUSSION
It is increasingly clear that the subcellular localization of channels and transporters is crucial to their function. A classical example in cardiac myocytes is the close co-localization of L-type Ca channels and SR Ca release channels at the junctions between the surface and SR membranes. This forms the structural basis of the local control theory of excitation-contraction coupling (ECC).
NCX is a main player in ECC in cardiac cells because it extrudes the Ca ions that enter via ICa. Here we showed that, like L-type Ca channels, NCX activity is found predominantly in the t-tubules of rat ventricular myocytes. Because NCX brings in three Na ions for each Ca ion extruded, it also represents an important route for Na entry. Indeed, >60% percent of Na influx during the cardiac cycle enters the cell via NCX (Bers et al., 2003). To maintain [Na]i at steady state, a similar amount of Na has to be extruded via the Na/K pump. Therefore, the location of Na/K pumps with respect to NCX might be important in [Na]i regulation and, ultimately, in ECC. The present data show that, functionally, Na/K pumps are also located preferentially in the t-tubules and that the percentage of NCX and Na/K pump activities in the t-tubules are comparable. Notably, Na channels are at equal density in surface and t-tubular membranes (Yang et al., 2002).
Relation to previous studies
A previous study using the same technique (acute detubulation and INCX measurements) showed NCX activity apparently located almost exclusively in the t-tubules (Yang et al., 2002): no significant decrease in current could be detected in detubulated cells when Ni was applied to block INCX (although the INCX traces shown agree with the quantitative conclusions here). However, the INCX density measured by Yang et al. (2002) even in control cells was ∼20× lower than that reported here, and this may explain their failure to detect significant INCX in detubulated myocytes. It is not obvious why INCX density was so low in the previous study, but the lack of ATP in the pipette solution may have greatly reduced INCX availability (Hilgemann, 1990). Yang et al. (2002) also assessed Ca extrusion via NCX by measuring a transient rise in local [Ca]o upon abrupt application of caffeine (with the sarcolemmal Ca-ATPase blocked by carboxyeosin). In detubulated myocytes they could not detect a rise in [Ca]o with this approach. However, it is possible that this technique is insufficiently sensitive to detect low levels of Ca efflux. The present results confirm that the density of INCX is much higher in t-tubules than the surface sarcolemma (Frank et al., 1992; Yang et al., 2002), and provides more quantitative analysis than previous immunofluorescence or electrophysiological results. We conclude that at least 63% of INCX occurs in t-tubules, where it is 3.6-fold more concentrated than in surface sarcolemma (see Table 1). Our data also suggest that NCX remains functionally active in the sealed-off t-tubular membrane after detubulation.
The Na/K-ATPase is present in both t-tubule and surface sarcolemma (based on immunofluorescence detection, McDonough et al., 1996), but there are no previous quantitative determinations of relative distribution. Our data show that 50–60% of Ipump activity occurs in t-tubules and is ∼3-fold more concentrated there than in surface sarcolemma. Interestingly, there was no difference in the Km of the pump for [Na]i in control and detubulated myocytes, although McDonough et al. (1996) showed that the α1 isoform of the pump is located predominantly in the t-tubules of rat ventricular myocytes whereas the α2 isoform is more homogeneously distributed, and the different isoforms have different [Na]i sensitivities (Blanco and Mercer, 1998).
It should be noted that if detubulation of myocytes were incomplete, we would be underestimating both the percent of INCX and Ipump in the t-tubules (∼60%) and the relative density in t-tubule vs. surface sarcolemma (∼3-fold). We think this effect is likely to be small for two reasons. First, previous work has shown virtual abolition of t-tubules in 80% of rat ventricular myocytes using this approach (Kawai et al., 1999). Although we used the surface-to-volume ratio to reject nondetubulated myocytes, we cannot exclude the possibility that some incompletely detubulated myocytes are included in the analysis. Second, the 32% reduction in capacitance measured here corresponds almost exactly to direct electron microscopic measurements of the percent of total sarcolemma in t-tubules in rat ventricular myocytes (33%; Page, 1978; Page and Surdyk-Droske, 1979). On the other hand, earlier stereology values were somewhat lower (21%; Page et al., 1971) and recent estimates from optical fluorescence microscopy are higher (64%; Soeller and Cannell, 1999).
During whole-cell voltage clamp it is possible that t-tubular current and capacitance could be underestimated (Kim and Vergara, 1998). If this is the case, the observed 32% decrease in cell capacitance may underestimate the percentage of the cell membrane and ionic current within the t-tubules. However, if the capacitance and membrane currents are underestimated to a similar degree, the concentration of these currents in the t-tubules as determined here will not be affected.
In the present study, we found ICa to be concentrated only threefold in the t-tubules vs. the cell surface sarcolemma. This contrasts with a previous report using the same methods (Kawai et al., 1999) which showed an ∼9-fold concentration of ICa in the t-tubules. However, our experimental conditions were optimized to record NCX and Na/K currents. The ICa measurement was taken just after patch rupture and may not represent the true steady-state value after dialysis of the cell interior. Thus, for ICa we are inclined to place more confidence in the value determined by Kawai et al. (1999), than that which we report here. Therefore, despite the apparent similarity in inferred distribution of ICa, INCX, and Ipump in Fig. 6, we believe that ICa is likely to be more concentrated in t-tubules than either INCX or Ipump.
Functional consequences of the observed NCX and Na/K ATPase distributions
The present data suggest that NCX and Na/K ATPase function are both at least ∼3× more concentrated in the t-tubules than in the surface membrane. While this may suggest a functional coupling of these transporters, our results do not allow any inferences regarding the possible co-localization of these proteins. NCX and Na/K ATPase are, however, co-localized in smooth muscle (Moore et al., 1993) and have been shown to interact functionally via a “fuzzy” subsarcolemmal space with restricted Na and Ca diffusion in cardiac guinea pig (Fujioka et al., 1998) and rabbit (Terracciano, 2001) cells. Although L-type Ca channels and NCX are not co-localized (Scriven et al., 2000), the concentration of NCX at the t-tubules means that the activity of the exchanger may be preferentially modulated by Ca released from the SR, and this is supported by functional measurements (Trafford et al., 1995; Weber et al., 2002). Thus, this Ca plays an important role in determining NCX function and hence Ca efflux. The Na/K ATPase present at the t-tubules might then rapidly extrude Na entering in exchange for Ca, thus preventing a local increase in [Na]i near NCX and the effect this would have on NCX activity. A previous study (Yang et al., 2002) indicated that Na channels are uniformly distributed in the t-tubules and external sarcolemma. The fact that Na/K ATPase follows the spatial distribution of NCX rather than Na channels supports the idea that Na/K ATPase localization is dictated by functional requirements, since NCX brings in ∼3× more Na than Na channels during the cardiac cycle (Bers et al., 2003).
Acknowledgments
We thank Brian French for technical support.
This work was funded by the Wellcome Trust, National Institutes of Health (grants HL-64098 and HL-64724), and the American Heart Association (fellowship #0225554Z).
S. Despa and F. Brette contributed equally to this study.
References
- Bers, D. M. 2001. Excitation-contraction coupling and cardiac contractile force. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- Bers, D. M., W. H. Barry, and S. Despa. 2003. Intracellular Na+ regulation in cardiac myocytes. Cardiovasc. Res. 57:897–912. [DOI] [PubMed] [Google Scholar]
- Blanco, G., and R. W. Mercer. 1998. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am. J. Physiol. 275:F633–F650. [DOI] [PubMed] [Google Scholar]
- Boyett, M., J. E. Frampton, and M. S. Kirby. 1991. The length, width and volume of isolated rat and ferret ventricular myocytes during twitch contractions and change in osmotic strength. Exp. Physiol. 76:259–270. [DOI] [PubMed] [Google Scholar]
- Brette F., K. Komukai, and C. H. Orchard. 2002. Validation of formamide as a detubulation agent in isolated rat cardiac cells. Am. J. Physiol. 283:H1720–H1728. [DOI] [PubMed] [Google Scholar]
- Carl, S. L., K. Felix, A. H. Caswell, N. R. Brandt, W. J. Ball, Jr., P. L. Vaghy, G. Meissner, and D. G. Ferguson. 1995. Immunolocalization of sarcolemmal dihydropyridine receptor and sarcoplasmic reticular triadin and ryanodine receptor in rabbit ventricle and atrium. J. Cell Biol. 129:672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro, J. M., K. W. Spitzer, W. R. Giles, P. E. Ershler, M. B. Cannell, and J. H. Bridge. 2001. Location of the initiation site of calcium transients and sparks in rabbit heart Purkinje cells. J. Physiol. 531:301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despa, S., M. A. Islam, S. M. Pogwizd, and D. M. Bers. 2002. Intracellular [Na+] and Na+ pump rate in rat and rabbit ventricular myocytes. J. Physiol. 539:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despa, S., and D. M. Bers. 2003. Na/K pump current and [Na]i in rabbit ventricular myocytes; local [Na]i depletion and Na buffering. Biophys. J. 84:4157–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, J. S., G. Mottino, D. Reid, R. S. Molday, and K. D. Philipson. 1992. Distribution of the Na+-Ca2+ exchange protein in mammalian cardiac myocytes: an immunofluorescence and immunocolloidal gold-labeling study. J. Cell Biol. 117:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka, Y., S. Matsuoka, T. Ban, and A. Noma. 1998. Interaction of the Na+-K+ pump and Na+-Ca2+ exchange via [Na+]i in a restricted space of guinea-pig ventricular cells. J. Physiol. 509:457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock, P. S., W. A. Coetzee, E. Cho, L. Porter, H. Katoh, D. M. Bers, M. S. Jafri, and M. Artman. 1999. Subcellular [Ca2+]i gradients during excitation-contraction coupling in newborn rabbit ventricular myocytes. Circ. Res. 85:415–427. [DOI] [PubMed] [Google Scholar]
- Hilgemann, D. W. 1990. Regulation and deregulation of cardiac Na+-Ca2+ exchange in giant excised sarcolemmal membrane patches. Nature. 344:242–245. [DOI] [PubMed] [Google Scholar]
- Huser, J., S. L. Lipsius, and L. A. Blatter. 1996. Calcium gradients during excitation-contraction coupling in cat atrial myocytes. J. Physiol. 494:641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai, M., M. Hussain, and C. H. Orchard. 1999. Excitation-contraction coupling in rat ventricular myocytes after formamide-induced detubulation. Am. J. Physiol. 277:H603–H609. [DOI] [PubMed] [Google Scholar]
- Kieval, R. S., R. J. Bloch, G. E. Lindenmayer, A. Ambesi, and W. J. Lederer. 1992. Immunofluorescence localization of the Na-Ca exchanger in heart cells. Am. J. Physiol. 263:C545–C550. [DOI] [PubMed] [Google Scholar]
- Kim, A. M., and J. L. Vergara. 1998. Supercharging accelerates t-tubule membrane potential changes in voltage clamped frog skeletal muscle fibers. Biophys. J. 75:2098–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough, A. A., Y. Zhang, V. Shin, and J. S. Frank. 1996. Subcellular distribution of Na pump isoform subunits in mammalian cardiac myocytes. Am. J. Physiol. 270:C1221–C1227. [DOI] [PubMed] [Google Scholar]
- Moore, E. D., E. F. Etter, K. D. Philipson, W. A. Carrington, K. E. Fogarty, L. M. Lifshitz, and F. S. Fay. 1993. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature. 365:657–660. [DOI] [PubMed] [Google Scholar]
- Page, E. 1978. Quantitative ultrastructural analysis in cardiac membrane physiology. Am. J. Physiol. 235:C147–C158. [DOI] [PubMed] [Google Scholar]
- Page, E., L. P. McCallister, and B. Power. 1971. Stereological measurements of cardiac ultrastructures implicated in excitation-contraction coupling. Proc. Natl. Acad. Sci. USA. 68:1465–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, E., and M. Surdyk-Droske. 1979. Distribution, surface density, and membrane area of diadic junctional contacts between plasma membrane and terminal cisterns in mammalian ventricle. Circ. Res. 45:260–267. [DOI] [PubMed] [Google Scholar]
- Scriven, D. R., P. Dan, and E. D. Moore. 2000. Distribution of proteins implicated in excitation-contraction coupling in rat ventricular myocytes. Biophys. J. 79:2682–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeller, C., and M. B. Cannell. 1999. Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circ. Res. 84:266–275. [DOI] [PubMed] [Google Scholar]
- Terracciano, C. M. N. 2001. Rapid inhibition of the Na+-K+ pump affects Na+-Ca2+ exchanger-mediated relaxation in rabbit ventricular myocytes. J. Physiol. 533:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafford, A. W., M. E. Diaz, S. C. O'Neill, and D. A. Eisner. 1995. Comparison of subsarcolemmal and bulk calcium concentration during spontaneous calcium release in rat ventricular myocytes. J. Physiol. 488:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, C. R., V. Piacentino III, K. S. Ginsburg, S. R Houser, and D. M. Bers. 2002. Na/Ca exchange current and submembrane [Ca] during the cardiac action potential. Circ. Res. 90:182–189. [DOI] [PubMed] [Google Scholar]
- Yang, Z., C. Pascarel, D. S. Steele, K. Komukai, F. Brette, and C. H. Orchard. 2002. Na+-Ca2+ exchange activity is localized in the t-tubules of rat ventricular myocytes. Circ. Res. 91:315–322. [DOI] [PubMed] [Google Scholar]