Abstract
Haemophilus ducreyi is the etiologic agent of the sexually transmitted genital ulcer disease chancroid. H. ducreyi serum resistance protein A (DsrA) is a member of a family of multifunctional outer membrane proteins that are involved in resistance to killing by human serum complement. The members of this family include YadA of Yersinia species, the UspA proteins of Moraxella catarrhalis, and the Eib proteins of Escherichia coli. The role of YadA, UspA1, and UspA2H as eukaryotic cell adhesins and the function of UspA2 as a vitronectin binder led to our investigation of the cell adhesion and vitronectin binding properties of DsrA. We found that DsrA was a keratinocyte-specific adhesin as it was necessary and sufficient for attachment to HaCaT cells, a keratinocyte cell line, but was not required for attachment to HS27 cells, a fibroblast cell line. We also found that DsrA was specifically responsible for the ability of H. ducreyi to bind vitronectin. We then theorized that DsrA might use vitronectin as a bridge to bind to human cells, but this hypothesis proved to be untrue as eliminating HaCaT cell binding of vitronectin with a monoclonal antibody specific to integrin αvβ5 did not affect the attachment of H. ducreyi to HaCaT cells. Finally, we wanted to examine the importance of keratinocyte adhesion in chancroid pathogenesis so we tested the wild-type and dsrA mutant strains of H. ducreyi in our swine models of chancroid pathogenesis. The dsrA mutant was less virulent than the wild type in both the normal and immune cell-depleted swine models of chancroid infection.
Chancroid is a sexually transmitted genital ulcer disease caused by the gram-negative bacterium Haemophilus ducreyi. Two to seven days after contact, a small tender papule surrounded by erythema develops at the site of inoculation. This papule progresses to a pustule and then to a painful soft necrotic ulcer with ragged edges. The floor of the ulcer is composed of necrotic tissue and a mixture of inflammatory cells (28). If left untreated, the ulcer can persist for weeks or even months, but it may eventually heal. H. ducreyi infection has never been shown to become systemic (22).
Chancroid is particularly common in Africa, Asia, and Latin America and is considered uncommon in the United States (41). There were 4,986 reported cases of chancroid in the United States in 1987; by 1999, however, the number had fallen to 143 (42). While the official number of United States chancroid cases has declined, it is suspected that chancroid is more prevalent than reported as chancroid-positive patients are often misdiagnosed (29). One reason for this is that chancroid is difficult to diagnose without specific laboratory tests that are unavailable to most health care providers (6).
The ability of certain strains of H. ducreyi to cause chancroid infection appears to be correlated with their ability to attach to human cells. When eight H. ducreyi strains were tested in an adherence assay, the six strains that adhered to over 90% of the human foreskin epithelial cells were virulent in the temperature-dependent rabbit model of H. ducreyi infection, while the two nonadherent strains were not virulent (40). The level of attachment of virulent strain 35000 to human foreskin fibroblasts is significantly greater than the level of attachment of avirulent strain A77 at 25 and 35°C (2). While previous work has described various eukaryotic cells to which H. ducreyi binds (2, 9, 16, 24, 25, 33, 40, 44), little is known about the bacterial proteins mediating the adhesive phenotype of H. ducreyi.
Recently, we described the H. ducreyi serum resistance protein A (DsrA). This H. ducreyi outer membrane protein is essential for resistance to killing by normal human serum (13). DsrA is a member of a family of surface-exposed outer membrane proteins that impart both resistance to killing by complement and the tendency to form stable multimers (21, 35) (Pfam database accession number PF03895). Other members of this family include YadA of the pathogenic Yersinia species, the UspA proteins of Moraxella catarrhalis, and the Eib proteins of Escherichia coli. Interestingly, YadA and the UspA proteins also function as eukaryotic cell adhesins. YadA mediates the adherence of Yersinia pseudotuberculosis and Yersinia enterocolitica to intestinal tissue, to brush border membranes and polystyrene surfaces (32), to epithelial cells (19), and to fibronectin-coated coverslips (39). Expression of UspA1 and UspA2H is responsible for adherence of M. catarrhalis to human epithelial cell lines (1, 23, 27). The homology between DsrA and these proteins suggested that DsrA might function as a possible H. ducreyi adhesin.
Herein, we describe the specific role of DsrA in the attachment of H. ducreyi to keratinocytes by demonstrating elimination of attachment in a mutant and restoration of this defect via complementation and by conferring attachment ability to Haemophilus influenzae through recombinant (rDsrA) expression. We also demonstrated that DsrA is specifically responsible for binding of vitronectin and that the attachment of H. ducreyi to keratinocytes occurs independent of vitronectin association.
MATERIALS AND METHODS
Cell culture.
The HaCaT keratinocyte cell line (8) was a gift from Bernard Weissman (Lineburger Comprehensive Cancer Center, University of North Carolina at Chapel Hill). HaCaT cells were cultured in T-75 flasks (Nuclon Δ Surface Nunc) by using RPMI 1640 (Gibco BRL catalog no. 11875-093) supplemented with 10 μM HEPES (pH 7.5) and 10% fetal bovine serum (FBS) (Gibco BRL catalog no. 26140-079) without antibiotics. The human foreskin fibroblast cell line utilized was HS27 (ATCC 1634-CRL; American Type Culture Collection, Manassas, Va.). HS27 cells were cultured in T-75 flasks by using Dulbecco's modified Eagle medium (Gibco BRL catalog no. 11995-065) supplemented with 10 μM HEPES (pH 7.5) and 10% FBS (Gibco BRL catalog no. 26140-079) without antibiotics. All cells were maintained in a 35°C humidified atmosphere with 5% CO2. Four-well dishes (Nuclon Δ Surface Nunc) were seeded with 105 cells. Attachment assays were performed as soon as the cells appeared to be confluent. Cells were grown on plastic and therefore did not differentiate.
Bacterial culture and inoculum preparation.
For the attachment assays H. ducreyi and H. influenzae strains were cultured on chocolate agar plates. The chocolate agar plates consisted of 2.5% brain heart infusion, 1.5% agar, 1% hemoglobin, and 1% IsoVitaleX (Becton Dickinson Microbiology Systems catalog no. 211876). The chocolate agar plates used for H. ducreyi also contained 10% FBS (Gibco BRL catalog no. 26140-079), but the plates used for H. influenzae did not. Plasmid-containing strains were cultured on selective media that included antibiotics (0.1 mg of chloramphenicol per 100 ml). The liquid medium used contained 3.79% brain heart infusion, 1% IsoVitaleX (Becton Dickinson Microbiology Systems catalog no. 211876), and 50 μg of hemin per ml.
For the attachment assay, bacteria were recovered from freezer stocks on chocolate agar plates and incubated at 35°C in the presence of 5% CO2. After 16 to 18 h of growth, bacteria were swabbed into 2 ml of liquid medium. The suspension was vortexed for 5 s and allowed to settle for 5 min. After 5 min, the top 1 ml was removed, added to a flask containing 9 ml of liquid medium, and incubated for approximately 4 h at 35°C at 100 rpm. Turbidity was measured with a Klett meter, and the suspension was adjusted with liquid medium to obtain a final concentration of approximately 1 × 108 CFU/ml. Bacteria were pelleted and then resuspended in tissue culture medium at a concentration of approximately 1 × 108 CFU/ml and diluted into a tissue culture medium solution so that the final concentration was approximately 106 CFU/ml. Tissue culture bacterial solutions were serially diluted and plated to determine the precise number of input CFU.
For the swine experiments, H. ducreyi 35000 and the isogenic dsrA mutant strain FX517 (Table 1) were prepared as previously described (20, 36, 37). Briefly, bacteria were grown from frozen stocks on chocolate agar plates containing 10% FBS and were passaged once. Bacteria were harvested from the plates with swabs and resuspended in phosphate-buffered saline (PBS). The bacteria were passed through a 30-gauge needle in order to reduce clumping. Serial dilutions were cultured in duplicate to determine bacterial cell concentrations.
TABLE 1.
Strains and plamids
| Strain or plasmid | Relevant genotype and/or phenotype | Source or reference |
|---|---|---|
| Strains | ||
| H. ducreyi | ||
| 35000 | Wild type, parent | Stanley Spinola, Indiana University |
| FX517 | 35000 dsrA mutant, Cmr | 13 |
| H. influenzae Rd | Nontypeable | American Type Culture Collection |
| Plasmids | ||
| pLSKS | Shuttle vector without insert of dsrA, Cmr Kanr Strr Sulr | 43 |
| pUNCH1260 | dsrA expression vector, Cmr Strr | 13 |
Attachment assays.
We added 500 μl of a 106-CFU/ml suspension of bacteria in tissue culture medium to confluent monolayers of 105 cells in four-well tissue culture dishes (multiplicity of infection, 5 to 10) in medium supplemented with 10% FBS unless otherwise indicated. After 2 h of coincubation at 35°C in the presence of 5% CO2, the wells were randomly designated either adherent or total-count wells. Total bacterial counts were determined by removing bacteria and eukaryotic cells from the plastic with a sterile wooden stick. Suspensions were vortexed, serially 10-fold diluted, and plated in duplicate. Adherent bacteria were defined as viable bacteria that remained adherent to cells after five washes with 500 μl of PBS. After washing, cells were scraped from the plastic with a sterile wooden stick. The suspensions were then vortexed. Adherent cells counts were determined by serial dilution and plating in duplicate. The percentage of adherence was determined as follows: (number of adherent CFU after 2 h of incubation/total number of CFU) × 100.
To ensure that plasmids were maintained throughout the assay, bacterial strains were plated onto both antibiotic-containing and non-antibiotic-containing chocolate agar plates. There were no differences between the numbers of CFU.
H. influenzae expressing DsrA.
We introduced plasmid pUNCH1260 into H. influenzae strain Rd by electroporation. pUNCH1260 contains the complete H. ducreyi dsrA gene in broad-host-range plasmid pLSKS.
rDsrA antiserum production.
We amplified the dsrA gene using 5′ primer dsrA19 (ATT AAT GCA GCA GCC GCC AAA GTT TGC TGG) and 3′ primer dsrA20 (GCG GCC GCG AAT TCA TAC CCA ACA GAA CCA CC). The 5′ and 3′ primers contained engineered AseI and NotI sites, respectively. PCR was performed by using Ready To Go tubes (Pharmacia), and strain 35000 chromosomal DNA was used as the template. The conditions for PCR were as follows: one initial hot start cycle of 5 min at 95°C, followed by 30 cycles of 1 min at 95°C, annealing for 1 min at 65°C, and extension for 1 min at 72°C. A single PCR product was obtained, ligated into the TA cloning vector, and transformed into DH5α. White colonies were screened for size (4.6 kb), and four clones were designated pUNCH1250A to pUNCH1250D. These clones were digested with AseI and NotI, and the insert isolated was ligated into NdeI/NotI-digested pET-30. Ligation mixtures were transformed into BL2(DE3)/pLysS. Transformants were induced with isopropyl-β-d-thiogalactopyranoside (IPTG) and screened for expression of rDsrA by using anti-native DsrA (13). The fusion protein did not have a signal sequence but did have a C-terminal hexahistidine tag and was directed to inclusion bodies. rDsrA was purified as previously described (14).
Rabbits were immunized four times with 200 μg of rDsrA per dose. The first immunization mixture contained Freund's complete adjuvant, and the remaining immunization mixtures contained incomplete Freund's adjuvant. Preimmune sera and sera present after four immunizations were obtained and screened by Western blotting.
Vitronectin binding assay.
We used a previously described assay for detection of serum components bound by H. ducreyi, modified as follows. H. ducreyi (approximately 2 × 108 CFU) was suspended in 1 ml of gonococcal medium base broth (GCB) with 5% heat-inactivated normal human serum (NHS). After binding for 30 min at 35°C, the bacteria were extensively washed (four times) with GCB and then transferred to a new tube and washed once with PBS. Bacterial pellets with bound components of NHS (approximately 2 × 107 CFU) were subjected to Western blotting by using a sheep anti-human vitronectin (Affinity Biologicals) at a 1:5,000 dilution. The secondary antibody used was anti-sheep alkaline phosphatase (Sigma) at a 1:5,000 dilution. 5-Bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium were used as the substrates.
Biotinylation of vitronectin.
We used the previously described method (11) for biotinylation. Briefly, 100 μg of vitronectin (kindly supplied by Jos van Putten and Tom Duensing) in PBS (pH 8.0) was mixed with 100 μg of NHS-biotin (Pierce) in a 500-μl (total volume) mixture for 2 h at room temperature. Excess aldehyde groups were blocked by addition of 20 μl of 1 M Tris (pH 8.0), followed by incubation at room temperature for an additional 30 min. Proteins were dialyzed overnight at 4°C to remove unreacted biotin.
Whole-cell radioimmunoprecipitation with biotinylated ligands.
The assay used for whole-cell radioimmunoprecipitation with biotinylated ligands was a modification of a previously described assay used for identifying antibodies reacting with surface-exposed epitopes of gonococci (12, 38). H. ducreyi was surface iodinated, washed, and suspended in 0.5 ml of GCB (approximately 1 × 108 CFU per tube; 1 × 106 cpm per tube). Biotinylated vitronectin (5 μg) was added and allowed to bind at 37°C for 30 min. Control tubes lacking vitronectin were used to determine nonspecific binding. The bacteria were washed twice in PBS, and cell pellets were lysed with 1 ml of 2% Zwittergent in TEN (Tris-buffered saline with 5 mM EDTA; pH 8.0) at 37°C with mixing for 1 h. Insoluble debris was removed by centrifugation (15,000 × g, 10 min), and soluble material was mixed with 40 μl of a 50% slurry of Neutravidin-agarose (Pierce). After the preparation was rocked at 4°C for 2 h, Neutravidin-agarose with bound proteins was washed five times with 2% Zwittergent in TEN (changing tubes once) and finally with TEN without detergent. Laemmli sample buffer was added, and samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography performed with an intensifying screen at −70°C.
Inhibition of vitronectin binding.
Monoclonal antibody inhibition of HaCaT cell binding of vitronectin has been described previously (34). Briefly, the vitronectin binding assay was performed by allowing 100 μl of a 10-μg/ml solution of vitronectin (Sigma catalog no. V9881) in 50 mM sodium carbonate buffer (pH 9.6) to coat wells of a 96-well plate (Nunc-Immuno MaxiSorp plate) overnight at 4°C. Equal numbers of HaCaT cells were placed on the plates and allowed to attach to the vitronectin-coated wells in the presence of various concentrations of the anti-αvβ5 integrin antibody (mouse anti-human integrin αvβ5 preservative-free monoclonal antibody; CHEMICON International, Inc. catalog no. MAB1961Z), the mouse immunoglobulin G1 isotype control antibody (Pharmingen catalog no. 03171D), or no antibody for 1 h at 35°C. After incubation, the wells were washed five times with PBS. The cells were then fixed with a 2.5% glutaraldehyde solution. The fixed cells were washed twice with borate buffer. After the preparations were stained with methylene blue, cell numbers were determined by reading the absorbance at 595 nm. The percentage of attachment was calculated by dividing the absorbance of a well that received antibodies by the average absorbance of the wells that were coated with vitronectin but did not receive any antibody. Wells that received HaCaT cells but were not coated with vitronectin served as the background wells.
Animal experiments.
Crossbred (Yorkshire, Landrace, and Hampshire cross) 6- to 12-week-old female pigs were used to examine the virulence of a dsrA mutant of H. ducreyi. The methods used, including the immune cell depletion treatment, have been described previously (20, 36, 37). Briefly, after juvenile swine ears were cleaned with a mixture of water and 95% ethanol, they were inoculated dorsally with 10-μl portions of 106- to 107-CFU/ml suspensions of strains 35000 and FX517 by using Multi-test multiple-skin-test applicators (Lincoln Diagnostics catalog no. MH-6).
The biopsy, recovery, and histology methods used have been described previously (20, 36, 37). Briefly, biopsies were taken 2 and 7 days after inoculation with a 6-mm punch (Acu-Punch USA; Acuderm Inc. catalog no. CE0413) and were bisected with a scalpel (Acu-Scalpel; Acuderm Inc. catalog no. S100.) One half of each biopsy was minced and plated on appropriate chocolate agar plates for recovery, and the other half was fixed for histological analysis. The sample halves used for histological observation were fixed in 4% paraformaldehyde in PBS at 4°C, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (Histopathology Reference Laboratory, Richmond, Calif.). Stained biopsies from normal pigs were blindly scored for histology by using our previously developed scoring system (37). We checked that proper antibiotic resistance and susceptibility had been maintained when viable colonies were recovered by patching both 35000 and FX517 onto both plain chocolate agar plates and plates containing the correct antibiotics.
Statistical analysis.
Statistical analysis was performed by using Sigma Stat, version 2.0 (Jandel Scientific, San Rafael, Calif.). The specific methods utilized are indicated below.
RESULTS
DsrA expression is necessary for efficient attachment of H. ducreyi to HaCaT cells but not to HS27 cells.
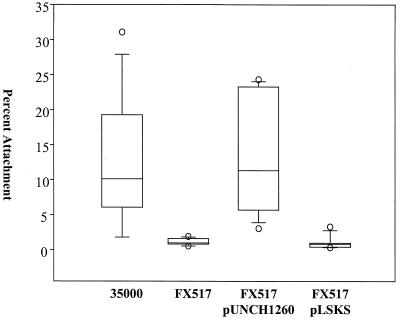
We compared the attachment of the wild-type parent strain (35000) and the attachment of the isogenic dsrA mutant strain (FX517) to HaCaT cells in parallel. HaCaT cells are a spontaneously transformed human keratinocyte epithelial cell line that maintains its full epidermal differentiation capacity (8). Approximately 10-fold less FX517 than 35000 adhered to HaCaT cells (Fig. 1) (P ≤ 0.001, as determined by the Mann-Whitney rank sum test). A non-dsrA cryptic mutation was not responsible for FX517's attachment defect as FX517 complemented with pUNCH1260, a plasmid containing dsrA (13), attached to HaCaT cells at wild-type levels (Fig 1). DsrA expression was responsible for the restoration of attachment, as the pLSKS vector did not enhance attachment (Fig. 1).
FIG. 1.
Effect of DsrA expression on H. ducreyi attachment to HaCaT cells. In each assay, approximately 106 CFU of each strain was incubated with 105 HaCaT cells for 2 h. The percentage adherence was determined as follows: (number of adherent CFU after 2 h of incubation/total number of CFU after 2 h of incubation) × 100. The data are presented in a box plot due to unequal variance. Each box indicates the median, 25th, and 75th percentiles, and the error bars indicate the 10th and 90th percentiles.
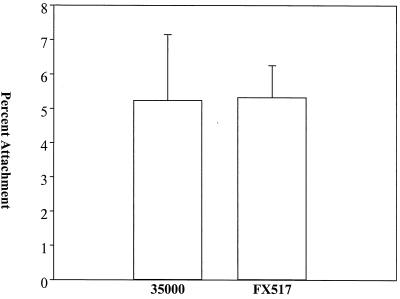
To investigate the role of DsrA in the attachment of H. ducreyi to fibroblasts, we repeated the attachment assays using HS27, a human foreskin fibroblast cell line. FX517 and 35000 adhered equally well to this fibroblast cell line (Fig. 2). This result indicated that DsrA was not required for attachment of H. ducreyi to fibroblasts.
FIG. 2.
Effect of DsrA expression on H. ducreyi attachment to HS27 cells. In each assay, approximately 106 CFU of each strain was incubated with 105 HS27 cells for 2 h. The percentage of adherence was determined as follows: (number of adherent CFU after 2 h of incubation/total number of CFU after 2 h of incubation) × 100. The data are means and standard deviations.
DsrA is sufficient for attachment to HaCaT cells.
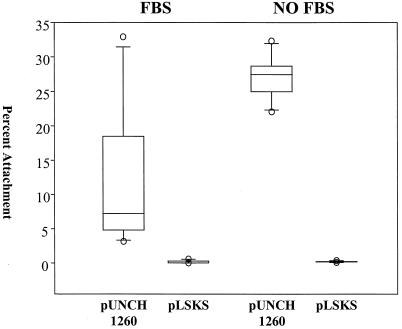
The related bacterial strain H. influenzae Rd does not express DsrA and does not readily attach to HaCaT cells (40). We transformed H. influenzae with either pUNCH1260 or pLSKS to examine the effect of DsrA expression on adherence. H. influenzae transformed with pUNCH1260 expressed a protein whose size was consistent with the size of DsrA, and the identity of the protein was confirmed by Western blotting (data not shown). rDsrA clearly fractionated into the Sarkosyl-insoluble outer membrane preparations, suggesting that it was correctly localized to the outer membrane of H. influenzae (data not shown). Transformation with pUNCH1260 significantly enhanced the ability of H. influenzae to adhere to HaCaT cells compared to the results obtained after transformation with the vector, pLSKS, alone (P ≤ 0.001, as determined by the Mann-Whitney rank sum test) (Fig 3). In fact, rDsrA-expressing H. influenzae attached to HaCaT cells at levels similar to the levels of attachment of H. ducreyi 35000.
FIG. 3.
rDsrA enhanced the attachment of H. influenzae to HaCaT cells. rDsrA confers an attachment phenotype to H. influenzae in the presence and in the absence of FBS. The attachment data are presented in a box plot due to unequal variance. Each box indicates the median, 25th, and 75th percentiles, and the error bars indicate the 10th and 90th percentiles.
DsrA directly binds vitronectin.
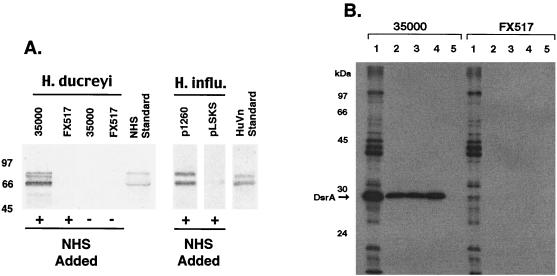
Since DsrA exhibits homology to UspA2 and UspA2 mediates M. catarrhalis binding of vitronectin (27), we investigated the possibility that DsrA binds vitronectin. We attempted to pull vitronectin out of NHS with various H. ducreyi strains. Only 35000, a strain that expressed DsrA, bound vitronectin (Fig 4A). As wild-type H. ducreyi could bind vitronectin from NHS, we used vitronectin to affinity purify its receptor from H. ducreyi. Biotinylated human vitronectin, recombinant human vitronectin, and bovine vitronectin purified only one radiolabeled outer membrane protein from 35000 and no radiolabeled outer membrane protein from FX517 (Fig. 4B). The protein from 35000 was the approximately the same size as DsrA. Since it was possible that a nonradiolabeled protein was the vitronectin receptor and DsrA was just in a complex with this protein, we expressed rDsrA in H. influenzae Rd, a strain that normally does not bind vitronectin. Cloned dsrA, but not the plasmid vector alone, conferred upon H. influenzae the ability to bind vitronectin in solution (Fig. 4A). As only bacteria expressing DsrA bound vitronectin and vitronectin affinity purified an outer membrane protein receptor only from DsrA-expressing H. ducreyi, we concluded that DsrA was solely responsible for the binding of vitronectin by H. ducreyi.
FIG. 4.
DsrA is responsible for the binding of vitronectin by H. ducreyi. (A) Western blot of DsrA-expressing and non-DsrA-expressing strains that were exposed to NHS, solublized, transferred, and probed with anti-human vitronectin antibody. (B) Autoradiograph of affinity-purified vitronectin binding proteins from iodinated H. ducreyi strains 35000 and FX517. Lanes 1, iodinated H. ducreyi whole cells; lanes 2 to 5, affinity purification using human vitronectin, recombinant human vitronectin, bovine vitronectin, and no vitronectin, respectively. The position of DsrA is indicated by the arrow. HuVn, human vitronectin.
DsrA-mediated attachment to HaCaT cells does not require vitronectin.
DsrA is required for both H. ducreyi adherence to keratinocytes and vitronectin binding. Accordingly, we considered the possibility that H. ducreyi might use the vitronectin present in FBS (18) as a bridge to attach to HaCaT cells. Since H. ducreyi died in tissue culture media lacking FBS (data not shown), we performed side-by-side H. influenzae attachment experiments with tissue culture media that contained and lacked FBS. A lack of FBS had no impact on the ability of H. influenzae Rd transformed with pUNCH1260 to attach to HaCaT cells (Fig. 3). Similarly, a lack of FBS did not enhance the ability of H. influenzae Rd transformed with pLSKS to adhere. When the attachment percentages obtained in the non-FBS-containing H. influenzae experiments were compared, there was once again a statistically significant difference between the attachment of the DsrA-expressing bacteria and the attachment of the non-DsrA-expressing bacteria (P ≤ 0.001, as determined by the Mann-Whitney rank sum test). This result suggested that vitronectin was not required for attachment mediated by DsrA.
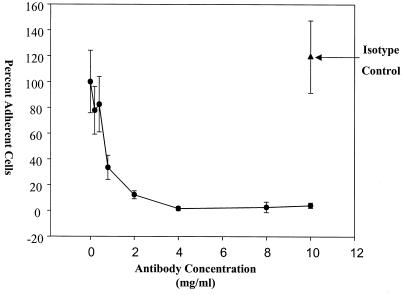
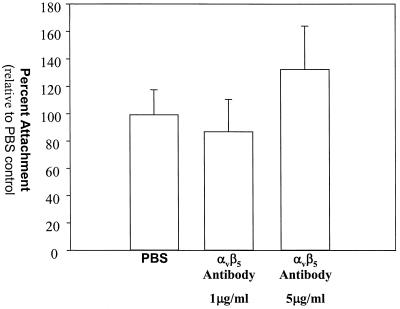
Additional evidence that vitronectin does not play a role in the attachment of H. ducreyi to HaCaT cells was obtained from experiments performed with a function-blocking monoclonal antibody to integrin αvβ5. Integrin αvβ5 is the only vitronectin receptor in HaCaT cells (34); accordingly, a monoclonal antibody to integrin αvβ5 completely blocked HaCaT cell binding to immobilized vitronectin in a concentration-dependent fashion (Fig. 5). H. ducreyi effectively adhered to HaCaT cells in 5- and 1-μg/ml solutions of this monoclonal antibody (Fig. 6). These antibody concentrations inhibited HaCaT cell binding to vitronectin by over 65 and 99%, respectively (percentages based on two-parameter exponential decay regression analysis of the data). Since the monoclonal antibody to αvβ5 blocked the binding of HaCaT cells to vitronectin but did not affect the attachment of H. ducreyi to HaCaT cells, we concluded that H. ducreyi does not use vitronectin as a bridge to attach to HaCaT cells and in fact binds to HaCaT cells independent of vitronectin.
FIG. 5.
Effect of a blocking αvβ5 monoclonal antibody on HaCaT cells binding to vitronectin. Symbols: •, percentage of HaCaT cell attachment in the presence of various concentrations of the αvβ5 antibody; ▴, percentage of HaCaT cell attachment in the presence of the isotype control. The data are normal and are means and standard deviations.
FIG. 6.
Effect of a blocking αvβ5 monoclonal antibody on H. ducreyi attachment to HaCaT cells. The concentrations of the αvβ5 antibody are indicated. The data are normal and are means and standard deviations.
Expression of DsrA is essential for complete virulence in the swine model of chancroid infection in both normal and immune cell-depleted animals.
We multiply inoculated the ears of three normal and three cyclophosphamide (CPA)-treated immune cell-depleted animals with strains 35000 and FX517. Lesion biopsies were collected 2 and 7 days after inoculation. Lesion biopsies, not animals, were considered independent units. After bisection, one half of each biopsy was analyzed for organism recovery, and the other half was fixed, sectioned, and stained with hematoxylin and eosin. We scored the stained samples by using a previously described histology scoring system (37). Briefly, a score of 1 indicates normal skin, a score of 2 indicates the presence of dermal perivascular and interstitial mononuclear cell infiltrate, a score of 3 indicates the presence of intraepidermal pustules, a score of 4 indicates the presence of epidermal pustules plus keratinocyte cytopathology and acanthosis, and a score of 5 indicates ulceration or epidermal necrosis with dermal erosion and a confluence of immune cells. The histology slides were coded and scored blindly to prevent introduction of bias. Only lesions from normal animals were scored with our five-point system as it is not possible to score histology with our scale in immune cell-depleted animals since it is the immune cells themselves which contribute almost entirely to lesion severity (36).
Normal pigs.
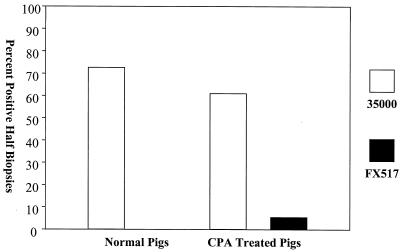
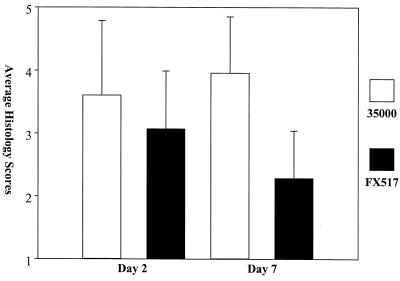
FX517 was impaired in its ability to infect normal pigs, as measured by its decreased recovery and histology scores compared to those of 35000. The average number of CFU recovered from FX517-infected lesion biopsies was 10-fold lower than the average number of CFU recovered from 35000-infected lesion biopsies on day 2, and there were no viable bacteria in any of the FX517-inoculated lesions on day 7 (Fig. 7). The differences in recovery between 35000- and FX517-inoculated lesions were statistically significant on day 2 (P = 0.014, as determined by the Mann-Whitney rank sum test) and day 7 (P = 0.014, as determined by the Mann-Whitney rank sum test). These results suggested that the dsrA mutant was cleared faster from the normal pigs as there were fewer FX517 culture-positive biopsies and the few positive biopsies contained far fewer live organisms. The histology scores for the FX517-inoculated lesions were significantly lower on day 7 than the histology scores for the 35000-infected lesions (P ≤ 0.001, as determined by a t test) (Fig. 8). The mean lesion histology score for 35000 on day 7 was 3.96, while the mean histology score for FX517 was 2.28 (Fig. 8). The decreased recovery on day 2, the lack of recovery of viable organisms on day 7, and the significantly reduced histology scores for strain FX517 relative to the scores for 35000 on day 7 suggested that by day 7 the FX517 infection had experienced much greater clearance than the 35000 infection. These results are in agreement with the findings obtained with the human challenge model (7), as Bong et al. concluded that the dsrA mutant was attenuated in virulence in the human challenge model.
FIG. 7.
Percentages of day 7 lesion biopsies from normal and CPA-treated, immune cell-depleted pigs that were culture positive. The percentages were determined as follows: (number of culture-positive lesion biopsies/total number of lesion biopsies) × 100.
FIG. 8.
Average histology scores from day 2 and 7 lesion biopsies from normal pigs. The data are normal and are means and standard deviations.
CPA-treated pigs.
The results obtained with the CPA-treated, immune cell-depleted animals were similar to the results obtained with the normal pigs. The percentage of FX517 culture-positive biopsies was greatly reduced compared to the percentage of 35000 culture-positive biopsies on both days 2 and 7 (Fig. 7). While there was not a statistically significant difference in the recovery of viable organisms between the 35000- and FX517-infected lesions on day 2, there was a statistically significant difference on day 7 (P = 0.012, as determined by a t test). Furthermore, in the CPA-treated, immune cell-depleted animals only one of the FX517 lesions contained viable H. ducreyi on day 7, while more than one-half of the 35000-inoculated lesions contained viable bacteria. This is a significant finding as FX517 died even when immune cell-mediated clearance was greatly impaired, as the numbers of neutrophils, monocytes, and lymphocytes were severely reduced after CPA treatment (Table 2).
TABLE 2.
Effect of CPA treatment on pigs
| Day | % Reduction in cell no. after CPA treatmenta
|
||
|---|---|---|---|
| Neutrophils | Monocytes | Lymphocytes | |
| 0 | 97.707 ± 4.157 | 97.955 ± 3.542 | 83.469 ± 11.921 |
| 2 | 99.710 ± 0.502 | 97.754 ± 3.889 | 87.220 ± 5.555 |
| 7 | 93.253 ± 3.789 | 83.535 ± 7.447 | 84.660 ± 2.328 |
The values are the mean percent reductions in cell number ± standard deviations. The percentages were based on data obtained from blood drawn prior to the start of treatment. Treatment was begun 4 days prior to inoculation.
DISCUSSION
Without adhesion to host cells, initiation of natural infection is highly unlikely (5, 31), yet little is known about how H. ducreyi adheres to human cells. Similarity to other bacterial adhesins inspired us to investigate DsrA as a possible H. ducreyi adhesin. We measured the adherence of wild-type H. ducreyi strain 35000 and the isogenic dsrA mutant FX517 to HaCaT cells and HS27 cells. While there was no difference in the percentages of attachment of FX517 and 35000 to HS27 cells, there was consistently a 10-fold difference in the percentages of attachment to HaCaT cells. Before we concluded that DsrA was both necessary and sufficient for attachment of H. ducreyi to HaCaT cells, we had to rule out two competing possibilities. The first possibility was that there was an undetected mutation elsewhere in the genome and that this mutation was responsible for the reduced attachment of FX517. The second possibility was that the loss of DsrA, a major outer membrane protein, induced the component actually responsible for mediating adherence to be aberrantly localized or expressed. To test the first hypothesis, we measured the attachment ability of FX517 transformed with a plasmid containing the dsrA gene. To test the second hypothesis, we measured the attachment phenotype conferred by rDsrA expression in the closely related strain H. influenzae Rd.
DsrA expression successfully restored attachment of the mutant to wild-type levels. From these experiments, we concluded that DsrA was necessary for attachment and that an undetected mutation was not responsible for the attachment defect. Expression of rDsrA in H. influenzae Rd dramatically increased attachment to HaCaT cells, while transformation with the control plasmid had no discernible effect. The ability to transfer the attachment phenotype along with protein expression demonstrated that DsrA was sufficient for attachment to HaCaT cells.
Recent findings obtained with the human challenge model call into question the significance of keratinocyte binding in chancroid infection as H. ducreyi is not often found in association with keratinocytes (3, 4). However, attachment to keratinocytes may be an important part of chancroid pathogenesis as both the human challenge model and the swine model use Multi-test multiple-skin-test applicators. These devices deliver the majority of organisms through the epidermis to the dermis (unpublished results) (3). Even in human challenge samples examined immediately after inoculation, the majority of the bacteria are located in the dermis (3). As this inoculation method appears to bypass the epithelial layer, it is possible that this procedure does not accurately reflect the natural mode of transmission or inoculation. Therefore, the lack of association between H. ducreyi and keratinocytes in the human challenge model does not eliminate the possibility that bacterial interactions with keratinocytes are a significant part of H. ducreyi pathogenesis.
After we determined that DsrA was required for H. ducreyi to attach to HaCaT cells, our next goal was to determine the mechanism of attachment. As DsrA binds vitronectin, we thought that DsrA might use vitronectin as a bridge to connect to the HaCaT cell surface. Support for this idea came from the fact that other bacteria, such as Neisseria gonorrhoeae (10, 11, 17) and Pneumocystis carinii (26), effectively use vitronectin as a bridging molecule to attach to and invade human cells. Vitronectin is a major cell attachment-promoting protein present in FBS (18), so to test the role of vitronectin in the attachment of H. ducreyi, we first attempted to perform the attachment assays in the absence of FBS. Unfortunately, these attachment assays were not successful as H. ducreyi did not survive in tissue culture media lacking FBS (data not shown). Fortunately, H. influenzae persisted without a problem in tissue culture media lacking FBS. Therefore, we decided that parallel to performing the H. influenzae Rd experiments in the presence of FBS, we would also perform experiments in the absence of FBS. Expression of DsrA, regardless of the presence of FBS in the tissue culture media, significantly enhanced the attachment of H. influenzae to HaCaT cells.
These data did not support our vitronectin bridging model, but we did not discard our model yet. We concluded that we needed to block vitronectin association with HaCaT cells and then observe its effect on the adherence of H. ducreyi to HaCaT cells. As integrin αvβ5 is solely responsible for the binding of vitronectin by HaCaT cells (34), we used an anti-human integrin αvβ5 monoclonal antibody to inhibit HaCaT cell binding to vitronectin in a concentration-dependent fashion. This antibody had no discernible effect on the adherence of H. ducreyi to HaCaT cells. As the αvβ5 monoclonal antibody blocked the binding of HaCaT cells to vitronectin but did not affect the attachment of H. ducreyi to HaCaT cells, we concluded that H. ducreyi binds HaCaT cells independent of an association with vitronectin.
The dsrA mutant is avirulent in the human challenge model of chancroid infection (7). Nevertheless, we decided to test the importance of DsrA expression in H. ducreyi pathogenesis with the swine model of chancroid infection. These experiments were done both as a validation of the swine model and because of the unique opportunities for experimentation that exist with the swine model. With pigs, we can observe the progression of pustules to ulcers and perform experiments on immunosuppressed animals. These latter experiments allow examination of the bacterial contribution to infection independent of the host response (37).
FX517 was impaired in virulence in both the normal and immunosuppressed swine models of chancroid infection. Decreased virulence was demonstrated in the CPA-treated pigs by the decreased recovery of FX517 relative to the recovery of 35000 and by the lower number of lesions that contained any viable FX517 cells on day 7. The loss of DsrA left H. ducreyi sufficiently attenuated in virulence that even in the absence of immune cell-mediated clearance, FX517 was not as virulent as 35000.
In normal pigs, the average recovery of FX517 from lesion biopsies and the percentage of FX517 culture-positive biopsies were significantly reduced compared to the data obtained with 35000 on both day 2 and day 7. In fact, no viable FX517 cells were found in any lesions biopsied 7 days after inoculation. These data and the reduced average histology scores from the day 7 biopsies suggested that normal animals cleared the dsrA mutant faster than they cleared the wild type. As DsrA is also required for serum resistance in H. ducreyi (13), the decreased survival of the bacteria in the animals could be due to either the decreased attachment ability or the increased serum susceptibility or both. Determination of the specific effects of the two phenotypes awaits mapping of the individual functions to distinct areas of the protein and generation of specific mutants in which the two functions attributed to DsrA are separated.
While we have identified a means of attachment, we do not suggest that this is the only means of host association. It is likely that H. ducreyi employs multiple means of attachment. In fact, there are at least two other bacterial factors that have demonstrated roles in H. ducreyi attachment. One is the H. ducreyi homolog of GroEL, a 58.5-kDa heat shock protein (Hsp), which partially influences the adherence of H. ducreyi to HEp-2 cells (15). The other ligand involves the flp operon. Products of the flp operon mediate the attachment of H. ducreyi to certain nonbiological surfaces and human foreskin fibroblasts (30). Interestingly, while products of the flp operon mediate attachment to human foreskin fibroblasts, they are not involved in the attachment of H. ducreyi to HaCaT cells (30). DsrA exhibited opposite specificities as it was necessary and sufficient for attachment to HaCaT cells but was not required for the attachment of H. ducreyi to HS27 fibroblasts. The fact that these two adhesins have complementary specificities suggests that they might function in different stages of infection. Bauer et al. have shown in the human challenge model that at the pustular stage of the disease the majority of the bacteria are found in the dermis (3, 4). Perhaps DsrA is required during an early stage of infection, while the products of the flp operon are essential later during the pustular and ulcerative stages. In conclusion, we think that DsrA is not only an important adhesin for H. ducreyi but also an important virulence factor and possible vaccine candidate.
Acknowledgments
This study was supported by NIH NIAID grants AI42824 (to T.H.K.) and AI31496 (to C.E.). Additional support came from an NSF predoctoral fellowship (to L.E.C.).
We are extremely grateful to Bonnie Olsen and Annice Roundtree for their expert technical assistance. We are grateful to Thomas Duensing and Jos van Putten for their advice on the biotinylation of vitronectin and their generous gifts of bovine vitronectin. We thank David Sane of Wake Forest University for his gift of human vitronectins. Finally, we are thankful for the insightful comments and suggestions regarding the manuscript given by Janne Cannon, Robert Fulcher, and Marcia Hobbs.
Editor: D. L. Burns
REFERENCES
- 1.Aebi, C., E. R. Lafontaine, L. D. Cope, J. L. Latimer, S. L. Lumbley, G. H. McCracken, Jr., and E. J. Hansen. 1998. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis 035E. Infect. Immun. 66:3113-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfa, M. J., P. Degagne, and T. Hollyer. 1993. Haemophilus ducreyi adheres to but does not invade cultured human foreskin cells. Infect. Immun. 61:1735-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer, M. E., M. P. Goheen, C. A. Townsend, and S. M. Spinola. 2001. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infect. Immun. 69:2549-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer, M. E., and S. M. Spinola. 2000. Localization of Haemophilus ducreyi at the pustular stage of disease in the human model of infection. Infect. Immun. 68:2309-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beachey, E. H. (ed.). 1980. Bacterial adherence, series B, vol. 6. Chapman and Hall, London, United Kingdom.
- 6.Beck-Sague, C. M., J. R. Cordts, K. Brown, S. A. Larsen, C. M. Black, J. S. Knapp, J. C. Ridderhof, F. G. Barnes, and S. A. Morse. 1996. Laboratory diagnosis of sexually transmitted diseases in facilities within the United States. Results of a national survey. Sex. Transm. Dis. 23:342-349. [DOI] [PubMed] [Google Scholar]
- 7.Bong, C. T., R. E. Throm, K. R. Fortney, B. P. Katz, A. F. Hood, C. Elkins, and S. M. Spinola. 2001. DsrA-deficient mutant of Haemophilus ducreyi is impaired in its ability to infect human volunteers. Infect. Immun 69:1488-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boukamp, P., R. T. Petrussevska, D. Breitkreutz, J. Hornung, A. Markham, and N. E. Fusenig. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106:761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brentjens, R. J., S. M. Spinola, and A. A. Campagnari. 1994. Haemophilus ducreyi adheres to human keratinocytes. Microb. Pathog. 16:243-247. [DOI] [PubMed] [Google Scholar]
- 10.Dehio, M., O. G. Gomez-Duarte, C. Dehio, and T. F. Meyer. 1998. Vitronectin-dependent invasion of epithelial cells by Neisseria gonorrhoeae involves alpha(v) integrin receptors. FEBS Lett. 424:84-88. [DOI] [PubMed] [Google Scholar]
- 11.Duensing, T. D., and J. P. van Putten. 1997. Vitronectin mediates internalization of Neisseria gonorrhoeae by Chinese hamster ovary cells. Infect. Immun. 65:964-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkins, C., K. B. Barkley, N. H. Carbonetti, A. J. Coimbre, and P. F. Sparling. 1994. Immunobiology of purified recombinant outer membrane porin protein I of Neisseria gonorrhoeae. Mol. Microbiol. 14:1059-1075. [DOI] [PubMed] [Google Scholar]
- 13.Elkins, C., K. J. Morrow, Jr., and B. Olsen. 2000. Serum resistance in Haemophilus ducreyi requires outer membrane protein DsrA. Infect. Immun. 68:1608-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkins, C., K. Yi, B. Olsen, C. Thomas, K. Thomas, and S. Morse. 2000. Development of a serological test for Haemophilus ducreyi for seroprevalence studies. J. Clin. Microbiol. 38:1520-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frisk, A., C. A. Ison, and T. Lagergard. 1998. GroEL heat shock protein of Haemophilus ducreyi: association with cell surface and capacity to bind to eukaryotic cells. Infect. Immun. 66:1252-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frisk, A., and T. Lagergard. 1998. Characterization of mechanisms involved in adherence of Haemophilus ducreyi to eukaryotic cells. APMIS 106:539-546. [PubMed] [Google Scholar]
- 17.Gomez-Duarte, O. G., M. Dehio, C. A. Guzman, G. S. Chhatwal, C. Dehio, and T. F. Meyer. 1997. Binding of vitronectin to opa-expressing Neisseria gonorrhoeae mediates invasion of HeLa cells. Infect. Immun. 65:3857-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayman, E. G., M. D. Pierschbacher, S. Suzuki, and E. Ruoslahti. 1985. Vitronectin—a major cell attachment-promoting protein in fetal bovine serum. Exp. Cell Res. 160:245-258. [DOI] [PubMed] [Google Scholar]
- 19.Heesemann, J. A. L. G. 1987. Genetic evidence that the outer-membrane protein YOP1 of Yersinia enterocolitica mediates adherence and phagocytosis resistance to human epithelial cells. FEMS Microbiol. Lett. 40:37-41. [Google Scholar]
- 20.Hobbs, M. M., L. R. San Mateo, P. E. Orndorff, G. Almond, and T. H. Kawula. 1995. Swine model of Haemophilus ducreyi infection. Infect. Immun. 63:3094-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoiczyk, E., A. Roggenkamp, M. Reichenbecher, A. Lupas, and J. Heesemann. 2000. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 19:5989-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonasson, J. A. 1993. Haemophilus ducreyi. Int. J. STD AIDS 4:317-321. [DOI] [PubMed] [Google Scholar]
- 23.Lafontaine, E. R., L. D. Cope, C. Aebi, J. L. Latimer, G. H. McCracken, Jr., and E. J. Hansen. 2000. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J. Bacteriol. 182:1364-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagergard, T., M. Purven, and A. Frisk. 1993. Evidence of Haemophilus ducreyi adherence to and cytotoxin destruction of human epithelial cells. Microb. Pathog. 14:417-431. [DOI] [PubMed] [Google Scholar]
- 25.Lammel, C. J., N. P. Dekker, J. Palefsky, and G. F. Brooks. 1993. In vitro model of Haemophilus ducreyi adherence to and entry into eukaryotic cells of genital origin. J. Infect. Dis. 167:642-650. [DOI] [PubMed] [Google Scholar]
- 26.Limper, A. H., J. E. Standing, O. A. Hoffman, M. Castro, and L. W. Neese. 1993. Vitronectin binds to Pneumocystis carinii and mediates organism attachment to cultured lung epithelial cells. Infect. Immun. 61:4302-4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMichael, J. C., M. J. Fiske, R. A. Fredenburg, D. N. Chakravarti, K. R. VanDerMeid, V. Barniak, J. Caplan, E. Bortell, S. Baker, R. Arumugham, and D. Chen. 1998. Isolation and characterization of two proteins from Moraxella catarrhalis that bear a common epitope. Infect. Immun. 66:4374-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehregan, A. H., K. Hashimoto, and H. Pinkus. Pinkus' guide to dermatohistopathology, 5th ed. Appleton & Lange, Norwalk, Conn.
- 29.Mertz, K. J., D. Trees, W. C. Levine, J. S. Lewis, B. Litchfield, K. S. Pettus, S. A. Morse, M. E. St. Louis, J. B. Weiss, J. Schwebke, J. Dickes, R. Kee, J. Reynolds, D. Hutcheson, D. Green, I. Dyer, G. A. Richwald, J. Novotny, I. Weisfuse, M. Goldberg, J. A. O'Donnell, and R. Knaup. 1998. Etiology of genital ulcers and prevalence of human immunodeficiency virus coinfection in 10 US cities. The Genital Ulcer Disease Surveillance Group. J. Infect. Dis. 178:1795-1798. [DOI] [PubMed] [Google Scholar]
- 30.Nika, J., J. L. Latimer, C. K. Ward, R. Blick, N. J. Wagner, L. D. Cope, G. Mahairas, R. S. Munson, Jr., and E. J. Hansen. 2002. Haemophilus ducreyi requires the flp gene cluster for microcolony formation in vitro. Infect. Immun. 70:2965-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ofek, I., and E. H. Beachey. 1980. Bacterial adherence. Adv. Intern. Med. 25:503-532. [PubMed] [Google Scholar]
- 32.Paerregaard, A., F. Espersen, J. Hannover Larsen, and N. Hoiby. 1990. Adhesion of Yersinia enterocolitica to human epithelial cell lines and to rabbit and human small intestinal tissue. APMIS 98:53-60. [PubMed] [Google Scholar]
- 33.Parsons, L. M., R. J. Limberger, and M. Shayegani. 1997. Alterations in levels of DnaK and GroEL result in diminished survival and adherence of stressed Haemophilus ducreyi. Infect. Immun. 65:2413-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinartz, J., B. Schafer, R. Batrla, C. E. Klein, and M. D. Kramer. 1995. Plasmin abrogates alpha v beta 5-mediated adhesion of a human keratinocyte cell line (HaCaT) to vitronectin. Exp. Cell Res. 220:274-282. [DOI] [PubMed] [Google Scholar]
- 35.Sandt, C. H., and C. W. Hill. 2001. Nonimmune binding of human immunoglobulin A (IgA) and IgG Fc by distinct sequence segments of the EibF cell surface protein of Escherichia coli. Infect. Immun. 69:7293-7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.San Mateo, L. R., K. L. Toffer, P. E. Orndorff, and T. H. Kawula. 1999. Immune cells are required for cutaneous ulceration in a swine model of chancroid. Infect. Immun. 67:4963-4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.San Mateo, L. R., K. L. Toffer, P. E. Orndorff, and T. H. Kawula. 1999. Neutropenia restores virulence to an attenuated Cu,Zn superoxide dismutase-deficient Haemophilus ducreyi strain in the swine model of chancroid. Infect. Immun. 67:5345-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swanson, J., L. W. Mayer, and M. R. Tam. 1982. Antigenicity of Neisseria gonorrhoeae outer membrane protein(s) III detected by immunoprecipitation and Western blot transfer with a monoclonal antibody. Infect. Immun. 38:668-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tertti, R., M. Skurnik, T. Vartio, and P. Kuusela. 1992. Adhesion protein YadA of Yersinia species mediates binding of bacteria to fibronectin. Infect. Immun. 60:3021-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Totten, P. A., J. C. Lara, D. V. Norn, and W. E. Stamm. 1994. Haemophilus ducreyi attaches to and invades human epithelial cells in vitro. Infect. Immun. 62:5632-5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trees, D. L., and S. A. Morse. 1995. Chancroid and Haemophilus ducreyi: an update. Clin. Microbiol. Rev. 8:357-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.United States Department of Health and Human Services. 2000. Tracking the hidden epidemics: trends in STDs in the United States, 2000. Centers for Disease Control and Prevention, Atlanta, Ga.
- 43.Wood, G. E., S. M. Dutro, and P. A. Totten. 1999. Target cell range of Haemophilus ducreyi hemolysin and its involvement in invasion of human epithelial cells. Infect. Immun. 67:3740-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaretzky, F. R., and T. H. Kawula. 1999. Examination of early interactions between Haemophilus ducreyi and host cells by using cocultured HaCaT keratinocytes and foreskin fibroblasts. Infect. Immun. 67:5352-5360. [DOI] [PMC free article] [PubMed] [Google Scholar]