Abstract
Mice incapable of generating an efficient Th2 response because of functional deletion of the genes for signal transducer and activation of transcription 6 (Stat6), interleukin-4 receptor alpha chain (IL-4Rα), or IL-4 plus IL-13 (IL-4/IL-13) were no more resistant than wild-type (WT) mice to airborne infection with virulent Mycobacterium tuberculosis. WT mice were able to control infection and hold it at a stationary level following 20 days of log linear M. tuberculosis growth. Likewise, infection was kept under control and was held at the same stationary level in IL-4/IL-13−/− mice but progressed to a slightly higher level in Stat6−/− and IL-4Rα−/− mice. The onset of stationary-level infection in WT mice was associated with the expression of Th1-mediated immunity, as evidenced by an approximately 100- to 1,000-fold increase in the lungs in the synthesis of mRNA for IL-12, gamma interferon (IFN-γ), and inducible nitric oxide synthase (NOS2) that was sustained for at least 100 days. IL-12 is essential for the induction of Th1 immunity, IFN-γ is a key Th1 cytokine involved in mediation of immunity, and NOS2 is an inducible enzyme of macrophages and is needed by these cells to express immunity. In response to infection, the lungs of Stat6−/− mice showed increases in synthesis of mRNA for IL-12, IFN-γ, and NOS2 similar to that seen in WT mice. In IL-4/IL-13−/− mice, however, synthesis of mRNA for IFN-γ and NOS2 reached higher levels than in WT mice. These results argue against the notion that a Th2 response is partly or wholly responsible for the inability of Th1-mediated immunity to resolve infection with a virulent strain of M. tuberculosis.
The rational design of an antituberculosis vaccine capable of protecting susceptible humans will be based on a detailed knowledge of the type of immunity that provides most humans with the ability to resist the disease. Most of our recent knowledge about immunity to tuberculosis comes from studies of tuberculosis in mice that have been rendered incapable of generating one or more lymphocyte subpopulations by targeted gene deletion. According to these studies, immunity to Mycobacterium tuberculosis infection is mediated predominately by M. tuberculosis-specific CD4 and CD8 αβ T cells (5, 8, 13, 35). The responsible CD4 T cells are Th1 cells, as evidenced by the demonstration that protective immunity is not generated in mice subjected to targeted gene deletion and thereby rendered incapable of making interleukin-12 (IL-12) (7) or gamma interferon (IFN-γ) (6, 14). It is generally agreed that the function of IFN-γ secreted by Th1 cells is to activate the antimycobacterial functions of the macrophages in which M. tuberculosis bacilli reside at sites of infection. In order for macrophages to express immunity, however, they must generate the inducible isoform of nitric oxide synthase (NOS2) that catalyzes high-output generation of NO from l-arginine. Mice rendered incapable of synthesizing NOS2 are greatly deficient in an ability to express anti-M. tuberculosis immunity, as evidenced by progressive M. tuberculosis growth in major organs and early death (24, 38). However, Th1-mediated immunity, as expressed by activated macrophages, is not successful at resolving infection but functions, instead, to control M. tuberculosis growth and hold infection at a stationary level from about day 20 of infection on (for a discussion, see reference 27). This is of limited usefulness to the host, however, because stationary-level lung infection induces progressive lung pathology that is eventually lethal (10, 11). Presumably, therefore, in order for mice to successfully defend against tuberculosis, they would need to be capable of completely resolving lung infection or of reducing it to a level incapable of inducing pathology. It is generally assumed that this would require the generation of higher levels of Th1-mediated immunity.
A possible reason for the inability of Th1-mediated immunity to resolve M. tuberculosis infections is that conditions arise during the course of infection that favor the production of M. tuberculosis-specific Th2 cells over Th1 cells (17, 34). This would be in keeping with the Th1/Th2 paradigm that has been invoked by some to explain the failure of Th1-mediated immunity to protect mice against infection with Leishmania major (23) in particular and against intracellular microbial infections in general (18, 22, 39). In keeping with this explanation are publications (17, 31) showing that an early Th1 response to M. tuberculosis infection in mice is joined by a Th2 response at about the time that infection enters a chronic phase. A similar Th1-to-Th2 response sequence has been shown to occur in mice infected with Mycobacterium bovis BCG (36) and Mycobacterium avium (1). The presence of T cells secreting Th2 cytokines in humans with active tuberculosis has likewise been taken as evidence that a Th2 response is responsible in part for progression of disease (2, 45).
One way to test whether a Th2 response is responsible for the inadequacy of Th1-mediated immunity would be to determine whether mice rendered deficient in the ability to generate a Th2 response are capable of generating higher levels of Th1-mediated immunity to M. tuberculosis and of causing the infection to resolve. It is known (21, 42), in this regard, that the efficient generation of a Th2 response requires the synthesis and secretion of the Th2-polarizing cytokines IL-4 and IL-13, which, on binding to their respective receptors on T cells, activate the Stat6 pathway for transcriptional activation of Th2 cytokine genes. Therefore, if a Th2 response is responsible for the inability of Th1-mediated immunity to resolve M. tuberculosis infection, mice in which the genes encoding IL-4 plus IL-13 or Stat6 have been functionally deleted should generate a higher level of immunity and show more resistance to M. tuberculosis infection than wild-type (WT) mice. The same should apply to mice in which the gene for the IL-4 receptor alpha chain (IL-4Rα) has been deleted, since IL-4Rα is a common component of IL-4R and IL-13R. The purpose of this paper is to show that mice in which the aforementioned genes have been deleted are no more capable than WT mice at defending against M. tuberculosis infection.
MATERIALS AND METHODS
Mice.
WT, IL-4Rα−/−, and IL-4/IL-13−/− mice from a C57BL/6 background were purchased from the Trudeau Institute animal breeding facility. WT and Stat6−/− mice from a BALB/c background were obtained from the same source. It is known (26) that BALB/c and C57BL/6 are resistant mouse strains. Mice of both strains were used in experiments at 10 weeks of age.
Bacteria and infection.
M. tuberculosis strain H37Rv (TMC 102), originally obtained from the Trudeau Mycobacterial Culture (TMC) collection, was grown as a suspension culture in Proskauer and Beck medium containing 0.01% Tween 80 and harvested while in log-phase growth, as described previously (10). The culture was subjected to two 5-s bursts of ultrasound to break up clumps and was then diluted in phosphate-buffered saline containing 0.01% Tween 80 for use in infection via the respiratory route. This was done with an aerosol infection apparatus (Tri Instruments, Jamaica, N.Y.) and involved exposing mice to an aerosol generated by nebulizing 10 ml of a suspension of 106 CFU per ml for 30 min. At the times indicated, the mice were sacrificed, and their lungs, livers, and spleens were removed and homogenized on ice with motorized Teflon pestles in tight-fitting glass tubes containing phosphate-buffered saline-Tween 80. The homogenates were subjected to 10-fold serial dilution, and the dilutions were plated on enriched Middlebrook 7H11 agar. Colonies were counted after 3 weeks of incubation at 37°C. The significance of differences between means was determined with Student's t test by using Prism version 3 for Windows (GraphPad Software, San Diego, Calif.).
Quantitation of host gene expression in infected lungs by real-time RT-PCR.
Lungs were harvested at the times indicated in Results and snap-frozen in liquid nitrogen. Total RNA was extracted from lung tissue homogenized in Trizol (Life Sciences, Carlsbad, Calif.) used in accordance with the manufacturer's instructions. The RNA pellet was dissolved in diethyl pyrocarbonate-treated distilled water. To remove contaminated genomic DNA, RNA samples were treated with RNase-free DNase I (Ambion, Austin, Tex.) for 1 h at 37°C. Aliquots of RNA samples were then passed through RNeasy minicolumns (Qiagen, Valencia, Calif.), treated with a DNA-free kit (Ambion), and stored at −70°C. A RiboGreen quantitation kit (Molecular Probes, Eugene, Oreg.) was used to quantify RNA for real-time reverse transcription-PCR (RT-PCR) analysis. The assay was repeated three times with different dilutions of the samples.
Primers and probes were designed with Primer Express software (PE Biosystems, Foster City, Calif.), whereas those for IL-12p40 and IL-4 were designed according to a published procedure (32) and purchased from Integrated DNA Technologies (Coralville, Iowa). Probes contained a fluorescent dye (6-carboxy-fluorescein) and a quencher (Black Hole Quencher 1). The melting temperature of the hybridized probe (approximately 70°C) was always 10°C higher than that of the PCR primers (57 to 60°C).
To make RNA standards, each amplicon of IL-12p35, IL-12p40, IFN-γ, NOS2, and IL-4 was generated by PCR from WT mouse lung mRNA by using the same primers that were used for real-time RT-PCR. The amplicons were cloned behind the T7 RNA polymerase promoter in the pGEM-T Easy vector system (Promega, Madison, Wis.). The sequence of each cloned amplicon was determined by thermocycler sequencing. After the linearization of plasmid DNA, amplicons were transcribed with T7 RNA polymerase (Promega). Template DNA was removed by digestion with DNase I, and RNA was purified by using RNeasy minicolumns and quantified with the RiboGreen assay. It was determined that 1 μg of an average 1,000-bp mRNA contained 1.8 × 1012 molecules. To obtain a standard curve, serial dilution of each transcript was performed in triplicate to give dilutions ranging from 109 to 101 molecules. The dilutions were then subjected to real-time RT-PCR analysis as described below.
For real-time RT-PCR, 2 to 5 μg of RNA was transcribed by using a random hexamer, an oligonucleotide deoxyribosylthymine primer, and a TaqMan Gold RT-PCR kit (PE Biosystems) in accordance with the manufacturer's instructions. Real-time PCR to enumerate IL-12p35, IL-12p40, IFN-γ, NOS2, and IL-4 amplicons was performed with the ABI-Prism 7700 sequence detector. Reaction conditions were programmed on a dedicated Power Macintosh 7200 computer. PCR amplification was performed with a total of 25 μl containing 10 μl of cDNA sample, 2.5 μl of 10× Taqman buffer A, 3 to 9 mM MgCl2, 200 μM (each) dATP, dCTP, and dGTP, 400 μM dUTP, 0.1 to 0.3 μM (each) primer, 0.625 U of AmpliTaq Gold, and 0.25 U of AmpErase uracil N-glycosylase (PE Biosystems). The reaction also contained 0.1 to 0.2 μM detection probe. Amplification was performed with triplicate wells under the following conditions: 2 min at 50°C and 10 min at 94°C followed by a total of 40 two-temperature cycles (15 s at 94°C and 1 min at 60°C). The copy number in each sample was calculated by use of the formula N = (Ct − b)/m, where N is the copy number, Ct is the threshold cycle, b is the y intercept, and m is the slope of the standard curve line.
Histology and immunocytochemistry.
Lung tissue was fixed in 10% neutral buffered formalin for 18 h, washed in distilled water, dehydrated in ethanol, and embedded in wax, in accordance with standard procedures. Paraffin sections were cut on a rotary microtome, stained for acid-fast bacteria by a modified basic fuchsin stain, and counterstained with methylene blue or azure A and eosin, as described in a previous publication (27). Immunocytochemistry to detect NOS2 in lung sections involved reacting deparaffinized lung sections with 0.1 μg of affinity-purified monospecific rabbit immunoglobulin (Ig) anti-mouse NOS2 (Transduction Laboratories, Lexington, Ky.) per ml as the primary antibody. After being washed, the sections were reacted with biotinylated goat Ig anti-rabbit Ig as the second reagent, and after being washed, they were incubated with avidin-coupled biotinylated horseradish peroxidase with diaminobenzidine as the substrate (Vectastain ABC kit; Novocastra Laboratories Ltd., Burlingame, Calif.) to produce the reaction product, in accordance with the supplier's instructions. The sections were then subjected to acid-fast staining and counterstaining as described above. Photomicrographs were taken with a Nikon Microphot-Fx microscope.
RESULTS
Growth of M. tuberculosis in WT, Stat6−/−, IL-4Rα−/−, and IL-4/IL-13−/− mice.
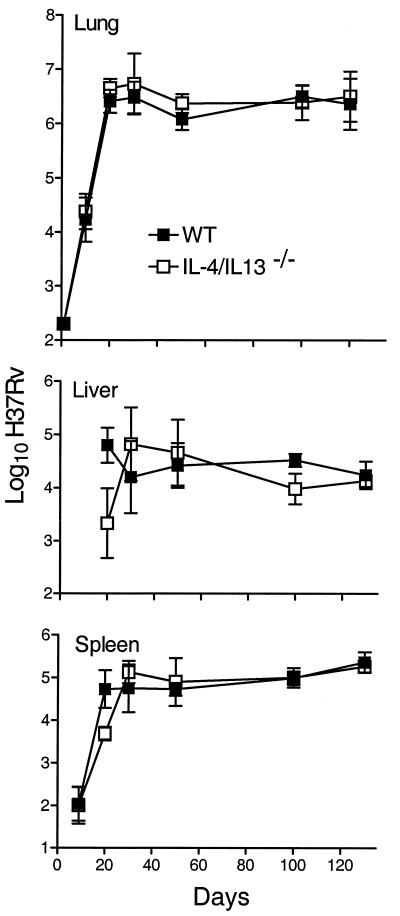
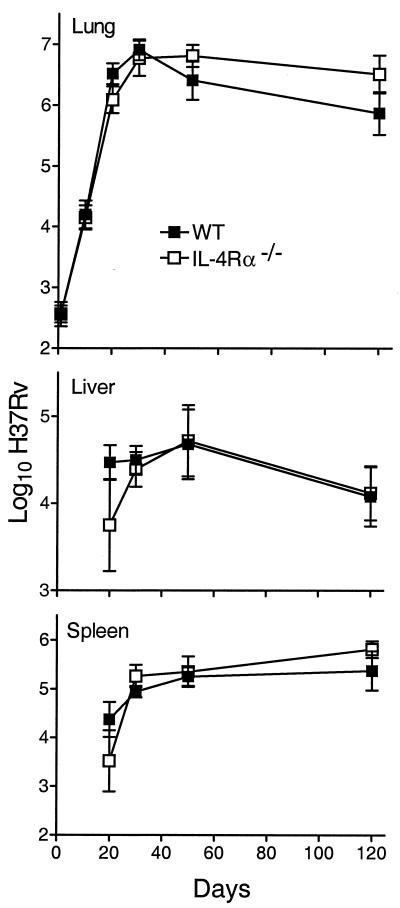
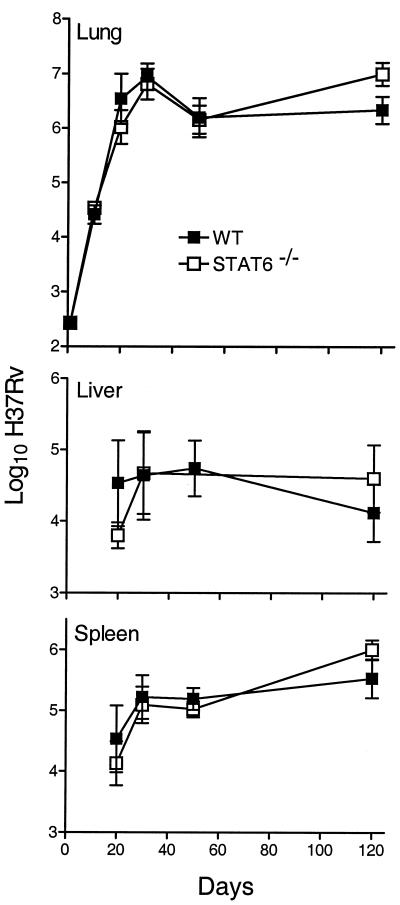
The growth of M. tuberculosis in the lungs, livers, and spleens of WT, Stat6−/−, IL-4Rα−/−, and IL-4/IL-13−/− mice infected via the respiratory route is shown in Fig. 1 to 3. There was little or no difference between WT and mutant mice in their ability to deal with M. tuberculosis growth for at least 50 days of infection. In all cases, M. tuberculosis grew progressively for about 20 days to reach a level of about 6.5 logs, after which infection was held at an approximately stationary level until day 50. However, while the level of infection remained approximately stationary from day 50 until day 120 in WT and IL-4/IL-13−/− mice, infection in Stat6−/− and IL-4Rα−/− mice was almost 1 log higher (P = 0.0068 and 0.0338, respectively) at day 120. The slightly higher level of infection in the spleens of Stat6−/− and IL-4Rα−/− mice at day 120 is in keeping with the higher level of infection in the lungs. It will be noted that infection in the livers and spleens was not evident in WT or mutant mice until day 20 and that there were fewer CFU in these organs in mutant mice on that day. This could indicate that the dissemination of M. tuberculosis from the lungs was delayed in mutant mice, although the differences in the CFU numbers were not statistically significant.
FIG. 1.
Growth of 2 × 102 CFU of M. tuberculosis strain H37Rv, administered by aerosol, in the lungs, livers, and spleens of WT and IL-4/IL-13−/− mice. In the lungs of both types of mice, H37Rv grew progressively for approximately 20 days, after which infection was controlled and held at a stationary level of approximately 6.5 logs until day 120, when the experiment was terminated. There was no significant difference in the growth of H37Rv in the livers and spleens of WT and IL-4/IL-13−/− mice. Data are means ± standard deviations of results from five mice per group per time point.
FIG. 3.
Growth of M. tuberculosis strain H37Rv in the lungs, livers, and spleens of WT and IL-4Rα−/− mice. There was no significant difference between WT and Stat6−/− mice in the kinetics of growth of H37Rv in their organs up to day 50 of infection. However, at day 120, the lungs of mutant mice contained 0.75 log more CFU of H37Rv (P = 0.0338) than the lungs of WT mice. Data are means ± standard deviations of results from five mice per group per time point.
IL-12, IFN-γ, IL-4, and NOS2 gene expression in the lungs of WT and targeted mutant mice.
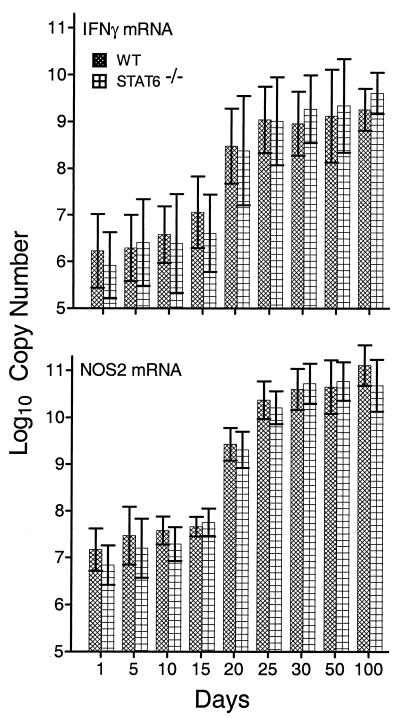
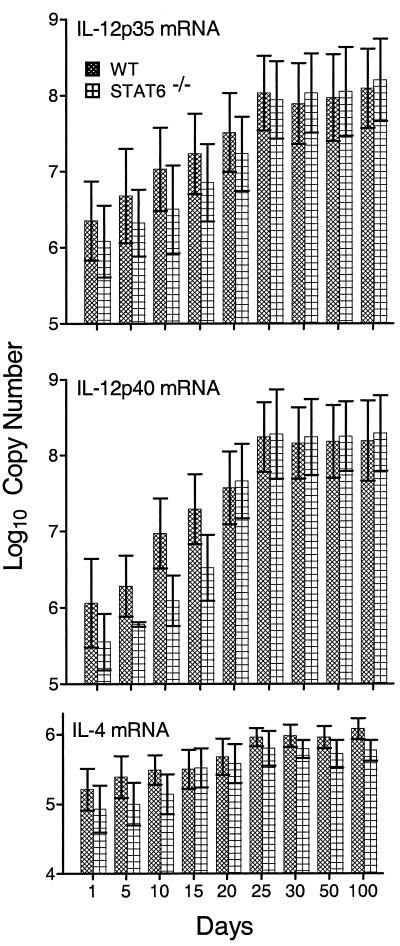
It is generally believed that the expression of anti-M. tuberculosis immunity at sites of infection in the lungs depends on the accumulation at these sites of M. tuberculosis-specific Th1 cells that secrete IFN-γ and other Th1 cytokines that function to activate the antibacterial function of M. tuberculosis-infected macrophages at infectious foci. Therefore, mediation of immunity in the lungs by Th1 cells would be evidenced by an increase in the synthesis of mRNA for IFN-γ, whereas increased anti-M. tuberculosis function of macrophages would be evidenced by an increase in the synthesis of mRNA for NOS2. On the other hand, an increase in the synthesis of IL-4 mRNA would point to the possible presence in the lungs of functioning Th2 cells. Figures 4 and 5 show the results of a real-time RT-PCR analysis of changes in the levels of synthesis of mRNA for IFN-γ, NOS2, IL-12p35, IL-12p40, and IL-4 in the lungs of WT versus Stat6−/− mice over time of infection. Levels of mRNA are expressed as copy numbers per total lung RNA. It can be seen (Fig. 4) that in both WT and Stat6−/− mice, the level of IFN-γ mRNA per total lung RNA increased approximately 1,000-fold between days 10 and 25 of infection and remained elevated from day 20 until day 100. Again, in the lungs of both types of mice, mRNA copy numbers for NOS2 increased more than 1,000-fold between days 15 and 25 and remained elevated through day 100. The copy numbers of mRNA for both subunits of IL-12 (Fig. 5) also increased substantially (100- to 500-fold) by day 25, and these increased levels of expression were maintained in the lungs of both WT and mutant mice until at least day 100 of infection. Therefore, according to the expression of genes for two key Th1 cytokines involved in Th1 immunity and for a macrophage-inducible enzyme indicative of enhanced macrophage mycobacteriostatic function, maintenance of persistent M. tuberculosis infection at a stationary level was associated with continuous expression of Th1-mediated immunity. The absence of Stat6 did not result in increased levels of Th1-mediated immunity in response to M. tuberculosis infection.
FIG. 4.
Changes in the copy number (per total lung RNA) of mRNA for IFN-γ and NOS2 over time of infection with 2 × 102 CFU of M. tuberculosis strain H37Rv administered via the respiratory route. The copy numbers of mRNA for both proteins increased approximately 1,000-fold between days 15 and 25 of infection, and this increase was sustained until day 100. Data are means ± standard deviations of results from three individual experiments.
FIG. 5.
Changes in copy number of mRNA (per total lung RNA) for IL-12p35, IL-12p40, and IL-4 during the course of M. tuberculosis strain H37Rv infection. The copy number for IL-12p35 increased approximately 100-fold in the lungs of both types of mice over the first 25 days of infection and remained at this elevated level until day 100. The copy number of IL-12p40 mRNA increased about 500-fold in the lungs of WT mice and about 1,000-fold in the lungs of Stat6−/− mice during the first 25 days of infection. In both cases, the copy number of IL-12p40 remained elevated until day 100. The copy number of IL-4 mRNA increased less than 10-fold in the lungs of Stat6−/− mice and WT mice over the first 25 days of infection but remained elevated through day 100. Data are means ± standard deviations of results of three individual experiments. Similar results were obtained with repeat experiments.
Infection in both Stat6−/− and WT mice caused an increase in the synthesis of mRNA for IL-4 between days 15 and 21 (Fig. 5), and increased synthesis of IL-4 mRNA was sustained throughout the course of infection. Thus, in the absence of Stat6, the increased transcription of IL-4 was equivalent in magnitude to that observed in WT mice. Moreover, the kinetics of increased IL-4 mRNA synthesis were the same in both types of mice and were similar to those of increased IFN-γ and IL-12 mRNA synthesis. However, the increase in the level of IL-4 gene expression was small (approximately 10-fold) relative to the increases in copy numbers of mRNA for IL-12 and IFN-γ (approximately 1,000-fold).
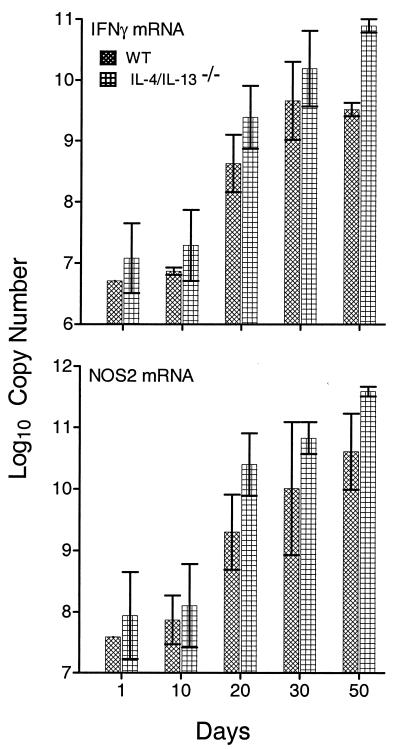
A similar result was obtained when the levels of IFN-γ and NOS2 gene expression were compared in WT and IL-4/IL-13−/− mice (Fig. 6). In this case, however, mutant mice showed about a 1-log-larger increase in both IFN-γ and NOS2 mRNA copy numbers than WT mice did at day 50 of infection (P < 0.0001 and P = 0.0202, respectively), indicating that the absence of both IL-4 and IL-13 resulted in increased levels of Th1-mediated immunity. The experiment was not continued beyond day 50 because of a shortage of double mutant mice.
FIG. 6.
Changes in copy number (per total lung RNA) of mRNA for IFN-γ and NOS2 in the lungs of WT and IL-4/IL-13−/− mice over 50 days of M. tuberculosis strain H37Rv infection. The copy number of IFN-γ mRNA increased approximately 1,000-fold in WT mice and double mutant mice during the first 30 days of infection and increased even further at day 50 in the lungs of mutant mice. Likewise, the NOS2 mRNA copy number increased approximately 1,000-fold in the lungs of WT and mutant mice during the first 30 days of infection and continued to increase until day 50. Data are means ± standard deviations of results from three mice per time point. Additional experiments gave similar results.
Because activation of Stat6 requires binding of IL-4Rα to IL-4 or IL-13, mice deficient in this receptor can be considered functionally equivalent to Stat6−/− mice. Therefore, IFN-γ, IL-12, and NOS2 gene expression levels were not measured in the lungs of IL-4Rα−/− mice.
Immunocytochemical demonstration of NOS2 protein in infected macrophages in the lungs of WT and mutant mice.
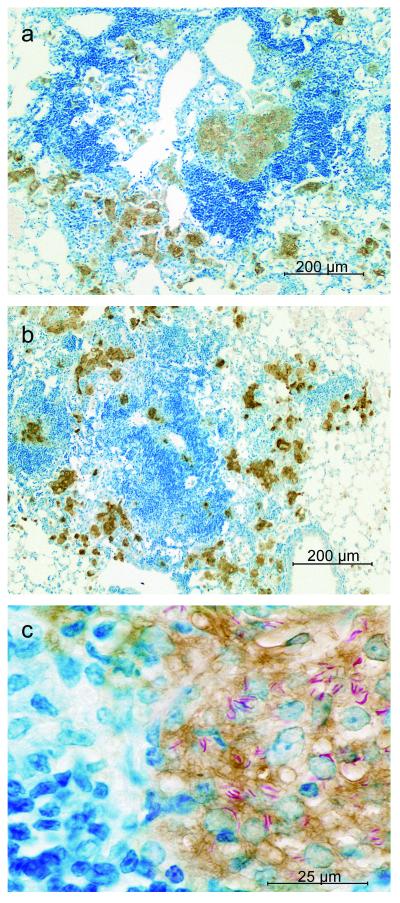
In view of the results described above, it was considered important to determine whether increased NOS2 mRNA synthesis in the lungs of WT, Stat6−/−, and IL-4/IL-13−/− mice was associated with the synthesis of NOS2 protein by macrophages at sites of infection. This would indicate that macrophages had acquired antimicrobial function, which would indicate in turn that IFN-γ was synthesized and secreted at sites of infection. An examination of lung sections stained for NOS2 protein by immunocytochemistry showed that the focal lesions in WT and Stat6−/− mice (Fig. 7) were similar in cellular composition. In both types of mice, sites of lung infection at day 50 were characterized by numerous collections of macrophages in close proximity to large aggregates of lymphoid cells. In both WT and Stat6−/− mice, most of the macrophages stained positively for NOS2, and at higher magnification, the cytoplasm of many of the NOS2-positive macrophages showed the presence of acid-fast bacilli. Because WT lesions looked the same as those in Stat6−/− mice, only a Stat6−/− mouse lesion is shown at higher magnification. Again, the lung lesions of IL-4/IL-13−/− mice are not shown because they had the same cellular composition as did WT and Stat6−/− mice up to day 100.
FIG. 7.
Photomicrographs of sections of lungs of WT and Stat6−/− mice at day 50 of infection stained for NOS2 by immunocytochemistry. The low-power micrographs show that infection-induced lesions in WT (a) and Stat6−/− (b) lungs were similar, being composed of accumulations of macrophages that stained positively for NOS2 (brown) in close proximity to large aggregates of lymphoid cells (dark blue). At higher magnification (c), the cells that stained brown for NOS2 in Stat6−/− lesions each showed a large pale nucleus typical of epithelioid macrophages, whereas the nuclei of nearby lymphocytes were compact and densely stained. Many of the macrophages can be seen to contain acid-fast bacilli. The lesions of WT mice displayed similar characteristics under high-power magnification.
DISCUSSION
In agreement with a recently published study (27), our results show that implantation of M. tuberculosis in the lungs of mice is followed by a 3-week period of progressive M. tuberculosis growth after which bacterial growth is controlled and infection is held at an approximately stationary level. It is known (10, 11, 27) that stationary-level lung infection gives rise to progressive lung pathology that is eventually lethal. Presumably, therefore, in order to prevent lung disease from progressing, infection would need to be completely resolved or its level would need to be greatly reduced, and this presumably would require that the host generate a higher level of Th1 immunity. It has been suggested (17, 34), in keeping with the Th1/Th2 paradigm, that the failure of Th1-mediated immunity to resolve M. tuberculosis infection is the result of the generation of a Th2 response that exerts a negative influence on the development of Th1-mediated immunity. Evidence that this might be the case in M. tuberculosis-infected mice is that a Th1 response to M. tuberculosis infection is joined by a Th2 response at about the time that infection enters a chronic stationary phase (17, 31). This evidence is based on studies of in vitro cytokine production by cells from M. tuberculosis-infected mice. The results presented here argue against such a role for Th2 cells by showing that mice depleted of the genes for IL-4 and IL-13, and therefore incapable of producing the only two Th2 cytokines deemed capable of causing unpolarized or already polarized M. tuberculosis-specific T cells to acquire a Th2 phenotype, were no more capable than WT mice at controlling M. tuberculosis growth in lungs and other organs. In support of this conclusion are additional results showing that IL-4Rα−/− mice whose T cells are devoid of an ability to be signaled by IL-4 or IL-13 to initiate Th2 gene transcription via the Stat6 pathway were somewhat less resistant to M. tuberculosis than WT mice, rather than being more resistant. Likewise, Stat6−/− mice were less resistant, rather than more resistant, than WT mice. These results are in agreement with results of a previous study (30) showing that the IL-4−/− mice were no better than WT mice at defending against airborne M. tuberculosis infection. In that particular study, however, the possibility that IL-13 compensated for the absence of IL-4 could not be excluded.
The finding that the mutant mice used in this study were no more capable than WT mice at controlling M. tuberculosis infection in lungs and other organs does not in itself represent evidence that the former mice generated the same level of immunity as the latter. To make this conclusion, it was necessary to show that the levels of immunity expressed in the lungs of mutant and WT mice were similar. This was investigated in this study by measuring the changes in IL-12, IFN-γ, and NOS2 mRNA synthesis in lungs over the time of infection. It is known that IL-12 is essential in the induction of Th1-mediated anti-M. tuberculosis immunity (7), whereas IFN-γ is essential (6, 14) for the ability of M. tuberculosis-specific Th1 cells to mediate immunity via the activation of macrophages, the cells that need to express immunity at the sites of infection. Activation of the function of macrophages is evidenced by synthesis by these cells of NOS2, an inducible enzyme needed for the high-output generation of NO that is essential for control of M. tuberculosis growth (24, 38). It is shown here that the levels of mRNA for IL-12p35 and IL-12p40, IFN-γ, and NOS2 in the lungs of WT and Stat6−/− mice increased substantially (approximately 3 logs) between days 10 and 20 of infection, immediately preceding the control of M. tuberculosis growth. It is also shown that the increased levels of mRNA for these proteins were sustained until at least day 100 of infection and that increased NOS2 mRNA synthesis was associated with the synthesis of NOS2 protein by infected macrophages in lung lesions. According to these indicators of Th1-mediated immunity, there was no difference between WT and Stat6−/− mice in the level of immunity generated. These results indicate, therefore, that stationary-phase infection was maintained in both types of mice by the continuous mediation and expression of Th1-mediated immunity. In keeping with this interpretation are published findings showing that interference with the expression of Th1-mediated immunity by treatment of mice combating stationary-level infection with anti-CD4 monoclonal antibody (37) results in resumption of M. tuberculosis growth.
An additional key finding presented here is that in the lungs of WT mice, increased IL-4 gene expression in response to M. tuberculosis infection was relatively minor (less than 10-fold) compared to increased transcription of IL-12 and IFN-γ (100- to 1,000-fold). The additional finding that IL-4 mRNA synthesis in the lungs of WT mice reached essentially the same level as that in Stat6−/− mice is in keeping with the knowledge (9, 20, 29) that there is a Stat6-independent pathway for IL-4 production based on an intrinsic capacity of T cells to make this and other cytokines in response to ligation of their T-cell receptors (3, 16). This alternate pathway is inefficiently expressed, however, except in cases where appropriate hosts are infected with pathogens that favor a Th2 response (12, 28). It is becoming increasingly apparent, in this connection, that it is the properties of the particular pathogen and its interaction with antigen-presenting cells that determine whether the T-cell response becomes biased towards the generation of Th1 or Th2 cells (15, 19). Thus, certain pathogens favor the induction of a Th2 response while others favor induction of a Th1 response (19). It seems reasonable to assume on the basis of the results presented here that M. tuberculosis is an example of an infectious agent that invokes a dominant Th1 response. Indeed, the demonstration here that IL-4 gene expression was subservient to IL-12, IFN-γ, and NOS2 gene expression and was no higher in Stat6−/− BALB/c mice than in WT BALB/c mice might mean that in both types of mice, transcription of the IL-4 gene was activated via the Stat6-independent pathway. This is a different situation than that seen in BALB/c mice infected with certain pathogens, such as Leishmania mexicana (40), Trypanosoma cruzi (44), or ectromelia virus (25), where the absence of Stat6 results in a larger and more protective Th1 response. On the other hand, according to the present study, IL-4/IL-13−/− mice did generate significantly higher levels of Th1-mediated immunity than did WT mice on day 50 of infection, as measured by the levels of IFN-γ and NOS2 gene expression. This would indicate that either or both of these Th2 cytokines had a negative influence on the generation of Th1-mediated anti-M. tuberculosis immunity in WT mice. This finding is being further investigated in this laboratory. Regardless, the higher levels of Th1-mediated immunity generated in the absence of IL-4 and IL-13 did not provide the double mutant mice with a superior ability to deal with infection. This suggests that failure of WT mice to resolve M. tuberculosis infection is likely not the result of an insufficient level of Th1-mediated immunity.
It was not anticipated in this study that the sustained expression of Th1-mediated immunity in lungs over an 80-day period of infection, as evidenced by a sustained increased synthesis of mRNA for IL-12, IFN-γ, and NOS2, would be associated with an elevated and sustained synthesis of mRNA for IL-12p35 and IL-12p40, given that it is generally believed that IL-12 functions in the initiation rather than in the expression of Th1-mediated immunity. It is becoming increasingly evident (33, 41, 46), however, that continuous synthesis of IL-12 is needed for the maintenance and preservation of already ongoing protective Th1 responses. It is possible that continuous synthesis of IL-12 serves to ensure that antigen-committed Th1 cells maintain their Th1 phenotype. It has been demonstrated in the case of human tuberculosis that IL-12 is present in the bronchoalveolar lavage fluid (43) and pleural fluid (47) of patients with ongoing active pulmonary disease, indicating that active disease is associated with the continuous expression of Th1 immunity.
On the basis of the evidence presented here, it would seem reasonable to conclude that the inability of anti-M. tuberculosis Th1-mediated immunity to resolve infection is not due to a negative influence of Th2 cells on Th1 immunity. It remains possible, however, that immunity is inadequate because of a negative influence of cytokines, such as IL-10, that can be produced by cells other than Th2 cells. It is possible, for example, that some type of balance between the macrophage-activating action of IFN-γ and the macrophage-deactivating action of IL-10 (4) is created, such that the level of activation that can be reached by macrophages is high enough to be mycobacteriostatic but not mycobacteriocidal. It is unlikely, however, that IL-10 functions this way during M. tuberculosis infection, because it has been convincingly shown (30) that targeted mutant mice devoid of an ability to make IL-10 are identical to WT mice in terms of their ability to control M. tuberculosis lung infection and hold it at a stationary level for over 120 days of infection.
FIG. 2.
Growth of M. tuberculosis strain H37Rv in the lungs, livers, and spleens of WT and Stat6−/− mice. There was no significant difference between WT and Stat6−/− mice in the kinetics of growth of the pathogen in their organs up to day 50 of infection. At day 120, however, the level of infection in the lungs of Stat6−/− mice was 0.75 log higher (P = 0.0068) than that in the lungs of WT mice. The level of infection was also significantly higher in the spleens of Stat6−/− mice on day 120 (P = 0.0392), although not in the livers. Data are means ± standard deviations of results from five mice per group per time point.
Acknowledgments
This work was supported by grant AI-37844 from the National Institute of Allergy and Infectious Diseases and grant HL-64565 from the National Heart, Lung, and Blood Institute.
Editor: B. B. Finlay
REFERENCES
- 1.Azouaou, N., M. Petrofsky, L. S. Young, and L. E. Bermudez. 1997. Mycobacterium avium infection in mice is associated with time-related expression of Th1 and Th2 CD4 T-lymphocyte response. Immunology 91:414-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baliko, Z., L. Szereday, and J. Szekeres-Bartho. 1998. Th2 biased immune response in cases with active Mycobacterium tuberculosis infection and tuberculin anergy. FEMS Immunol. Med. Microbiol. 22:199-204. [DOI] [PubMed] [Google Scholar]
- 3.Bix, M., Z. E. Wang, B. Thiel, N. J. Schork, and R. M. Locksley. 1998. Genetic regulation of commitment to interleukin 4 production by CD4+ T cell-intrinsic mechanisms. J. Exp. Med. 188:2289-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogdan, C., Y. Vodovitz, and C. Nathan. 1991. Macrophage deactivation by IL-10. J. Exp. Med. 174:1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boom, W. H. 1996. The role of T cell subsets in Mycobacterium tuberculosis infection. Infect. Agents Dis. 5:73-81. [PubMed] [Google Scholar]
- 6.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in IFN-γ gene disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper, A. M., J. Magram, J. Ferrante, and I. M. Orme. 1997. Interleukin 12 (IL-12) is crucial for the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J. Exp. Med. 186:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper, A. M., B. M. Saunders, C. D. D'Souza, A. A. Frank, and I. M. Orme. 1997. Mycobacterium tuberculosis-driven processes in gene-disrupted mice. Bull. Inst. Pasteur 95:85-95. [Google Scholar]
- 9.Dent, A. L., J. Hu-Li, W. E. Paul, and L. M. Staudt. 1998. T helper type 2 inflammatory disease in the absence of interleukin 4 and transcription factor STAT6. Proc. Natl. Acad. Sci. USA 95:13823-13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn, P. L., and R. J. North. 1995. Virulence ranking of some Mycobacterium tuberculosis and Mycobacterium bovis strains according to their ability to multiply in the lungs, induce lung pathology, and cause mortality in mice. Infect. Immun. 63:3428-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn, P. L., and R. J. North. 1996. Persistent infection with virulent but not avirulent Mycobacterium tuberculosis in the lungs of mice causes progressive pathology. J. Med. Microbiol. 45:103-109. [DOI] [PubMed] [Google Scholar]
- 12.Finkelman, F. D., S. C. Morris, T. Orekhova, M. Masaaki, D. Donaldson, S. L. Reiner, N. L. Reilly, L. Schopf, and J. F. Urban. 2000. Stat6 regulation of in vivo IL-4 responses. J. Immunol. 164:2303-2310. [DOI] [PubMed] [Google Scholar]
- 13.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 14.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giacomini, E., E. Iona, L. Ferroni, M. Miettinen, L. Fattorini, G. Orefici, I. Julkunen, and E. M. Coccia. 2001. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a different cytokine gene expression that modulates T cell response. J. Immunol. 166:7034-7041. [DOI] [PubMed] [Google Scholar]
- 16.Grogan, J. L., M. Mohrs, B. Harmon, D. A. Lacy, J. W. Sedat, and R. M. Locksley. 2001. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity 14:205-215. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Pando, R., H. Orozcoe, A. Sampieri, L. Pavon, C. Velasquillo, J. Larriva-Sahd, J. M. Alcocer, and M. V. Madrid. 1996. Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology 89:26-33. [PMC free article] [PubMed] [Google Scholar]
- 18.Jankovic, D., Z. Lui, and W. C. Gause. 2001. Th1- and Th2-cell commitment during infectious disease: asymmetry in divergent pathways. Trends Immunol. 22:450-457. [DOI] [PubMed] [Google Scholar]
- 19.Jankovic, D., A. Sher, and G. Yap. 2001. Th1/Th2 effector choice in parasitic infection: decision making by committee. Curr. Opin. Immunol. 13:403-409. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan, M. H., A. L. Wurster, S. T. Smiley, and M. J. Grusby. 1999. Stat6-dependent and -independent pathways for IL-4 production. J. Immunol. 163:6536-6540. [PubMed] [Google Scholar]
- 21.Kaplan, M. H., and M. J. Grusby. 1998. Regulation of T helper cell differentiation by STAT molecules. J. Leukoc. Biol. 64:2-5. [DOI] [PubMed] [Google Scholar]
- 22.Liew, F. Y. 2002. Th1 and Th2 cells: a historical perspective. Nat. Rev. Immunol. 2:55-60. [DOI] [PubMed] [Google Scholar]
- 23.Locksley, R. M., and S. L. Reiner. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 24.MacMicking, J. D., R. J. North, R. LaCourse, J. S. Mudgett, S. K. Shah, and C. F. Nathan. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94:5243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahalingam, S., G. Karupiah, K. Takeda, S. Akira, K. I. Matttaei, and P. S. Foster. 2001. Enhanced resistance in Stat6-deficient mice to infection with ectromelia virus. Proc. Natl. Acad. Sci. USA 98:6812-6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medina, E., and R. J. North. 1998. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology 93:270-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mogues, T., M. E. Goodrich, L. Ryan, R. LaCourse, and R. J. North. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohrs, M., C. Holscher, and F. Brombacher. 2000. Interleukin-4 receptor alpha-deficient BALB/c mice show an unimpaired T helper 2 polarization in response to Leishmania major infection. Infect. Immun. 68:1773-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noben-Trauth, N., L. D. Shultz, F. Brombacher, J. F. J. Urban, H. Gu, and W. E. Paul. 1997. An interleukin 4 (IL-4)-independent pathway for CD4 T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc. Natl. Acad. Sci. USA 94:10838-10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.North, R. J. 1998. Mice incapable of making IL-4 or IL-10 display normal resistance to infection with Mycobacterium tuberculosis. Clin. Exp. Immunol. 113:55-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orme, I. M., A. D. Roberts, J. P. Griffin, and J. S. Abrams. 1993. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J. Immunol. 141:518-525. [PubMed] [Google Scholar]
- 32.Overbergh, L., D. Valckx, M. Waer, and C. Mathieu. 1999. Quantitation of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine 11:305-312. [DOI] [PubMed] [Google Scholar]
- 33.Park, A. Y., B. D. Hondowicz, and P. Scott. 2000. IL-12 is required to maintain a Th1 response during Leishmania major infection. J. Immunol. 165:896-902. [DOI] [PubMed] [Google Scholar]
- 34.Power, C. A., G. Wei, and P. A. Bretscher. 1998. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by intravenous, subcutaneous, or intradermal route. Infect. Immun. 66:5743-5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raupach, B., and S. H. Kaufmann. 2001. Immune responses to intracellular bacteria. Curr. Opin. Immunol. 13:417-428. [DOI] [PubMed] [Google Scholar]
- 36.Sander, B., U. Skansen-Saphir, O. Damm, L. Hakansson, and J. Andersson. 1995. Sequential production of Th1 and Th2 cytokines in response to live bacillus Calmette-Guerin. Immunology 86:512-518. [PMC free article] [PubMed] [Google Scholar]
- 37.Scanga, C. A., V. P. Mohan, K. Yu, H. Joseoh, J. Chan, and J. L. Flynn. 2000. Depletion of CD4 T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase. J. Exp. Med. 192:347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scanga, C. A., V. P. Mohan, K. Tanaka, D. Alland, J. L. Flynn, and J. Chan. 2001. The inducible nitric oxide synthase locus confers protection against aerogenic challenge of both clinical and laboratory strains of Mycobacterium tuberculosis in mice. Infect. Immun. 69:7711-7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spellberg, B., and J. E. Edwards, Jr. 2001. Type 1/type 2 immunity in infectious diseases. Clin. Infect. Dis. 32:76-102. [DOI] [PubMed] [Google Scholar]
- 40.Stamm, L. M., A. Raisanen-Sokolowski, M. Okano, M. Russell, and J. R. David. 1998. Mice with STAT6-targeted gene disruption develop a Th1 response and control cutaneous leishmaniasis. J. Immunol. 161:6180-6188. [PubMed] [Google Scholar]
- 41.Stobie, L., S. Gurunathan, C. Prussin, D. L. Sacks, N. Glaichenhaus, C. Y. Wu, and R. A. Seder. 2000. The role of antigen and IL-12 in sustaining Th1 memory cells in vivo: IL-12 is required to maintain memory/effector Th1 cells sufficient to mediate protection to an infectious parasitic challenge. Proc. Natl. Acad. Sci. USA 97:8427-8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szabo, S. J., L. H. Glimcher, and I. C. Ho. 1997. Genes that regulate interleukin expression in T cells. Curr. Opin. Immunol. 9:776-781. [DOI] [PubMed] [Google Scholar]
- 43.Taha, R. A., T. C. Kotsimbos, Y. L. Song, D. Menzies, and Q. Hamid. 1997. IFN-gamma and IL-12 are increased in active compared with inactive tuberculosis. Am. J. Respir. Crit. Care Med. 155:1135-1139. [DOI] [PubMed] [Google Scholar]
- 44.Tarleton, R. L., M. J. Grusby, and L. Zhang. 2000. Increased susceptibility of Stat4-deficient and enhanced resistance in Stat6-deficient mice to infection with Trypanosoma cruzi. J. Immunol. 165:1520-1525. [DOI] [PubMed] [Google Scholar]
- 45.van Krevel, R., E. Karyadi, F. Preyers, M. Leenders, B. J. Kullberg, R. H. Nelwan, and J. W. van der Meer. 2000. Increased production of interleukin 4 by CD4 and CD8 T cells from patients with tuberculosis is related to the presence of pulmonary cavities. J. Infect. Dis. 181:1194-1197. [DOI] [PubMed] [Google Scholar]
- 46.Yap, G., M. Persin, and A. Sher. 2000. IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J. Immunol. 165:628-631. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, M., M. K. Gately, E. Wang, J. Gong. S. F. Wolf, S. Lu, R. L. Modlin, and P. F. Barnes. 1994. Interleukin 12 at sites of disease in tuberculosis. J. Clin. Investig. 93:1733-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]