Abstract
Fluorescence quenching methods have been used to study interactions of anionic phospholipids with the potassium channel KcsA from Streptomyces lividans. Quenching of the Trp fluorescence of KcsA reconstituted into mixtures of dioleoylphosphatidylcholine (DOPC) and an anionic phospholipid with dibromostearoyl chains is more marked at low mole fractions of the brominated anionic phospholipid than is quenching in mixtures of dibromostearoylphosphatidylcholine and nonbrominated anionic lipid. The quenching data are consistent with two classes of binding site for lipid on KcsA, one set corresponding to annular binding sites around KcsA to which DOPC and two-chain anionic phospholipids bind with similar affinities, the other set (non-annular sites) corresponding to sites at which anionic phospholipids can bind but from which DOPC is either excluded or binds with very low affinity. The binding constant for tetraoleoylcardiolipin at the annular sites is significantly less than that for DOPC, being comparable to that for dioleoylphosphatidylethanolamine. Tetraoleoylcardiolipin binds with highest affinity to the non-annular sites, the affinity for dioleoylphosphatidylglycerol being the lowest. The affinity for dioleoylphosphatidylserine decreases at high ionic strength, suggesting that electrostatic interactions between the anionic phospholipid headgroup and positively charged residues on KcsA are important for binding at the non-annular site. The effect of ionic strength on the binding of phosphatidic acid is less marked than on phosphatidylserine. The value of the binding constant for the non-annular site depends on the extent of Trp fluorescence quenching following from binding at the non-annular site. It is suggested that the non-annular site to which binding is detected in the fluorescence quenching experiments corresponds to the binding site for phosphatidylglycerol detected at monomer-monomer interfaces in x-ray diffraction studies.
INTRODUCTION
The structure of the K+ channel KcsA of the bacterium Streptomyces lividans has been determined at high resolution (Zhou et al., 2001). KcsA is a homotetramer, each monomer containing two transmembrane α-helices separated by a conserved region, the P-loop that forms part of the ion conduction pathway. The pore structure of KcsA is believed to be very similar to those of the mammalian Kv and Kir family of K+ channels, and to those of the voltage-gated Na+ and Ca2+ channels (Sansom et al., 2002, Jiang et al., 2003). The presence of anionic phospholipid is essential for the function of KcsA, the channel remaining closed in the absence of anionic lipid (Heginbotham et al., 1998; Valiyaveetil et al., 2002). KcsA purified using dodecylmaltoside as detergent contains ∼0.7 molecules of the anionic lipid phosphatidylglycerol per KcsA monomer and the crystal structure of KcsA shows one lipid molecule per monomer bound in a deep cleft at each monomer-monomer interface in the tetrameric structure (Fig. 1). The headgroup of this bound lipid molecule is not resolved in the crystal structure, and the lipid has therefore been modeled as a diacylglycerol with one C14 and one C9 chain (Zhou et al., 2001), but it is presumed to be phosphatidylglycerol (Valiyaveetil et al., 2002). Anionic lipid is not required for the formation of the KcsA tetramer since tetramers are formed when KcsA is reconstituted into bilayers of zwitterionic phospholipid in the absence of anionic lipid (Valiyaveetil et al., 2002; Williamson et al., 2002). Instead, it has been suggested that the presence of the anionic lipid “co-factor” bound between the transmembrane α-helices is important in gating of the channel because opening and closing the gate must involve movement of the transmembrane α-helices, which will be affected by the presence of the lipid molecule bound between the transmembrane α-helices (Valiyaveetil et al., 2002).
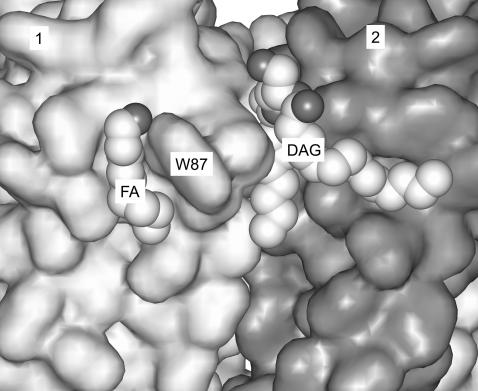
FIGURE 1.
The non-annular binding site for anionic phospholipid on KcsA. The lipid molecule, modeled as diacylglycerol (DAG) in the crystal structure of KcsA, is bound at the interface between monomers 1 and 2, whose surfaces are shaded light and dark gray, respectively. The partial lipid molecule modeled as nonan-1-ol (FA) is also shown (PDF file 1K4C).
Although only a few crystal structures of membrane proteins show resolved lipid molecules, many of these lipid molecules are bound to deep clefts between transmembrane helices as in KcsA; examples include cardiolipin bound to the bacterial photosynthetic reaction center and cardiolipin and other lipid molecules bound to cytochrome c oxidase and cytochrome bc1 (Iwata et al., 1995; Harrenga and Michel, 1999; McAuley et al., 1999; Lange et al., 2001; Camara-Artigas et al., 2002). These lipid molecules are often essential for activity, with cardiolipin, for example, being required for activity of many of the proteins important in bioenergetics (Jiang et al., 2000; Schlame et al., 2000; Heimpel et al., 2001). Binding sites for hydrophobic molecules located between transmembrane α-helices have been referred to as non-annular sites, to distinguish them from the boundary or annular sites that make up the lipid-exposed external surface of the protein (Simmonds et al., 1982). The idea of non-annular binding sites developed from fluorescence quenching studies with the Ca2+-ATPase of sarcoplasmic reticulum, that showed the existence of sites on Ca2+-ATPase to which hydrophobic molecules could bind, but from which normal phospholipids were excluded (Simmonds et al., 1982; de Foresta et al., 1989; Lee, 2002).
Binding constants for phospholipids at the annular sites around a variety of membrane proteins have been determined (Marsh and Horvath, 1998; Lee, 2003) but there have as yet been no measurements for binding constants of phospholipids at non-annular sites. It is not known to what extent binding at non-annular sites is structurally specific, and it is not known if binding of anionic phospholipids to these sites is driven by nonspecific charge effects or by specific interactions such as hydrogen bonding. Further, the lack of knowledge about the binding constants at these sites means that it is not known which lipids will occupy these sites in the native membrane and whether or not these sites will be fully occupied in the native membrane.
In previous studies we have shown that lipid binding constants for the annular sites around KcsA can be obtained using fluorescence quenching methods in which the degree of occupancy of the lipid binding sites around KcsA by bromine-containing phospholipids is determined from the level of quenching of the fluorescence of the Trp residues in the protein (Williamson et al., 2002). Phospholipids containing brominated fatty acyl chains are prepared by addition of bromine across the double bond in the corresponding phospholipid containing cis-unsaturated fatty acyl chains. Phospholipids containing brominated fatty acyl chains behave much like conventional phospholipids with unsaturated fatty acyl chains, because the bulky bromine atoms have effects on lipid packing that are similar to those of a cis double bond (East and Lee, 1982). KcsA contains five Trp residues of which Trp-26 and Trp-113, at the cytoplasmic ends of transmembrane α-helices M1 and M2 respectively, and Trp-87 at the extracellular end of M2, are exposed to the lipid bilayer. Binding of brominated phospholipids to the annular sites around KcsA would be expected to lead to quenching of the fluorescence of these lipid-exposed Trp residues. In contrast, Trp-67 and Trp-68 are located away from the lipid-protein interface as part of the short pore helix that points into the intracellular cavity. However, both Trp-67 and Trp-68 are close to the sn-1 chain of the lipid bound at the non-annular site, which is inserted between the pore helix of one monomer and a transmembrane α-helix of the adjacent monomer (Fig. 1). Thus binding of brominated anionic phospholipids to the non-annular sites would also be expected to result in quenching of Trp fluorescence. Importantly, KcsA still forms tetramers in the absence of phosphatidylglycerol (Williamson et al., 2002; Valiyaveetil et al., 2002) so that binding to the non-annular binding sites can be treated as a simple equilibrium binding reaction. Here it is shown that binding constants at the non-annular sites can indeed be obtained from analysis of fluorescence quenching data.
EXPERIMENTAL PROCEDURES
Dioleoylphosphatidylcholine (DOPC), dioleoylphosphatidylethanolamine (DOPE), dioleoylphosphatidic acid (DOPA), dioleoylphosphatidylserine (DOPS), dioleoylphosphatidylglycerol (DOPG), and tetraoleoylcardiolipin (TOCL) were obtained from Avanti Polar Lipids (Alabaster, AL). Phospholipids were brominated as described in East and Lee (1982) to give dibromostearoylphosphatidylcholine (BrPC), dibromostearoylphosphatidylethanolamine (BrPE), dibromostearoylphosphatidic acid (BrPA), dibromostearoylphosphatidylserine (BrPS), dibromostearoylphosphatidylglycerol (BrPG), and dibromostearoylcardiolipin (BrCL), respectively.
Purification of KcsA and reconstitution
A plasmid containing the kcsA gene (Schrempf et al., 1995) with a poly-His epitope at the N-terminus was the generous gift of Professor Schrempf. KcsA was purified using Mega-9 (Calbiochem, Nottingham, UK) as detergent, as described in Williamson et al. (2002). Purified KcsA was reconstituted into lipid bilayers by mixing lipid and KcsA in cholate, followed by dilution into buffer to decrease the concentration of cholate below its critical micelle concentration, as described (Williamson et al., 2002). For reconstitution into bilayers of a single phospholipid, the required phospholipid (0.6 μmol) was dried from a chloroform solution onto the walls of a thin glass vial. Buffer (300 μl; 20 mM HEPES and 1 mM EGTA at pH 7.2) containing 20 mM cholate was added and the sample was sonicated to clarity in a bath sonicator (Ultrawave, Cardiff, UK). KcsA (100 μg) was then added and the suspension left at room temperature for 15 min, followed by incubation on ice until use. For reconstitution into bilayers containing a mixture of two lipids, stock solutions of the two lipids (2 mM lipid) were prepared in cholate-containing buffer as described above. Aliquots of the two stock solutions were then mixed in the appropriate proportions to give 200-μl samples of the mixed lipids. The mixtures were incubated at 60°C for 15 min and then left at room temperature for 1 h and then mixed with KcsA, as described above. For reconstitutions with phosphatidylethanolamine, this procedure sometimes gave inconsistent results; and in these cases lipid mixtures were first prepared by mixing the lipids in chloroform solution, which were then dried down and dissolved in cholate, followed by reconstitution as described above.
Fluorescence measurements
For fluorescence measurements 50 μl of the sample were diluted into 3 ml buffer (20 mM HEPES and 1 mM EGTA, at pH 7.2) and the fluorescence recorded on an SLM (Urbana, IL) 8000C fluorimeter with excitation at 290 nm, at 25°C. Unless otherwise stated, the concentration of KcsA was 0.24 μM and the molar ratio of lipid to KcsA was 100:1. Wavelengths of maximum fluorescence emission intensity were obtained by fitting fluorescence emission spectra to skewed Gaussian curves, as described in Williamson et al. (2002).
Analysis of fluorescence results
Quenching of Trp fluorescence by bromine-containing molecules requires the Trp residue and the bromine atom to be close in space. Quenching of the fluorescence of a particular Trp residue in KcsA by a brominated phospholipid molecule will therefore depend on the level of occupancy of the lipid binding sites immediately adjacent to that Trp residue by brominated phospholipid. Quenching of Trp fluorescence due to binding of brominated phospholipids to the annular sites around KcsA has been fitted to a lattice model for quenching (London and Feigenson, 1981; Caffrey and Feigenson, 1981; Mall et al., 1998; O'Keeffe et al., 2000) using the equation
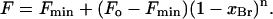 |
(1) |
Here Fo and Fmin are the fluorescence intensities for KcsA in nonbrominated and in brominated lipid, respectively; F is the fluorescence intensity in the phospholipid mixture when the mole fraction of brominated lipid is xBr; and n represents the number of lipid sites from which the fluorescence of an average Trp residue can be quenched. Quenching of fluorescence for KcsA in mixtures of two different species of lipid that may have different affinities for annular sites on KcsA was fitted to the equation,
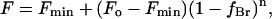 |
(2) |
where fBr , the fraction of sites occupied by brominated lipid, is related to xBr by
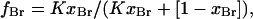 |
(3) |
where K is the binding constant of the brominated lipid relative to that of the nonbrominated lipid.
The experimental data were also fitted to a model involving separate annular and non-annular binding sites, as in the previous analysis of binding at non-annular sites on the Ca2+-ATPase (Simmonds et al., 1982). It is assumed that, although both anionic and zwitterionic lipids can bind to the annular sites, possibly with different affinities, only anionic phospholipids can bind to the non-annular sites. The degree of occupation of the non-annular sites, 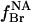 , by brominated anionic phospholipid, is given by
, by brominated anionic phospholipid, is given by
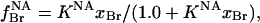 |
(4) |
where KNA is the association constant for binding to the non-annular sites and xBr is the mole fraction of brominated anionic phospholipid in the membrane. If binding of brominated phospholipid to the non-annular site results in quenching of the fluorescence of all five Trp residues in KcsA, then the fluorescence intensity is given by (Simmonds et al., 1982)
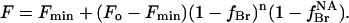 |
(5) |
If, however, as seems more likely, it is assumed that binding at the non-annular sites only results in quenching of the three Trp residues on the same side of the membrane as the non-annular binding site (the extracellular side), then assuming that the fluorescence intensity of all Trp residues are equal in the absence of quenching, the total fluorescence intensity will be given by
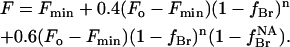 |
(6) |
The experimental data were fitted to the above equations using the nonlinear least-squares routine in the SigmaPlot (SPSS, Chicago, IL) package.
RESULTS
Fluorescence emission in reconstituted membranes
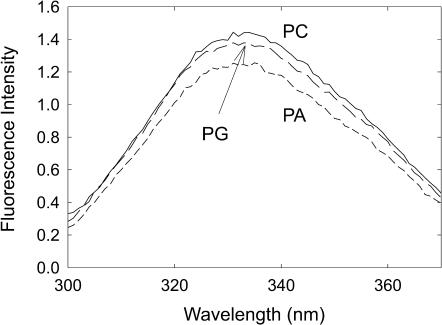
KcsA was reconstituted into bilayers of defined composition by mixing KcsA with phospholipid in cholate solution, followed by dilution into buffer to drop the concentration of cholate below its critical micelle concentration and reform membranes (Williamson et al., 2002). The fluorescence emission maxima for KcsA reconstituted into bilayers of anionic lipid are identical to that in DOPC (Fig. 2; Table 1). Spectra were uncorrected and differ slightly from those reported in Williamson et al. (2002), which were recorded on a different instrument. The fluorescence intensity in DOPA is ∼10% lower than that in DOPC (Fig. 2). In mixtures of DOPC and DOPA or DOPC and DOPS fluorescence intensities decrease with increasing mole fraction of anionic lipid, the maximum fluorescence quenching observed in DOPS being the same as that in DOPA (Figs. 3 and 4). The presence of DOPG (Fig. 2) or TOCL resulted in no significant decrease in fluorescence intensity. Fluorescence intensities for KcsA reconstituted into DOPE are more variable between reconstitutions than those with other phospholipids, possibly connected with the tendency of DOPE to form nonlamellar phases, but the fluorescence emission maximum was very similar to that in the other phospholipids (Table 1), and the fluorescence intensity in a 9:1 mixture of DOPE:DOPC, in which intensities were reproducible, were the same as in DOPC.
FIGURE 2.
Fluorescence emission spectra for KcsA. Spectra are shown for KcsA reconstituted into bilayers of DOPC (solid line), DOPG (long dash), and DOPA (short dash). Wavelengths of maximum emission are listed in Table 1. The concentration of KcsA was 0.24 μM and the molar ratio of lipid to KcsA was 100:1. The buffer was 20 mM HEPES and 1 mM EGTA at pH 7.2.
TABLE 1.
Fluorescence properties of reconstituted KcsA
| Phospholipid | Emission max (nm) | n number | Quenching by brominated lipid (F/F)o |
|---|---|---|---|
| DOPC | 332.6 ± 0.2 | 1.69 | 0.61 ± 0.01 |
| DOPA | 331.8 ± 0.2 | 2.49 ± 0.25 | 0.48 ± 0.01 |
| DOPE | 331.9 ± 0.1 | 1.77 ± 0.27 | 0.62 ± 0.01 |
| DOPG | 332.1 ± 0.2 | 1.53 ± 0.12 | 0.58 ± 0.01 |
| DOPS | 331.2 ± 0.3 | 1.77 ± 0.12 | 0.49 ± 0.01 |
| TOCL | 332.5 ± 0.4 | 0.90 ± 0.15 | 0.63 ± 0.04 |
Wavelengths of maximum emission were determined by fitting the fluorescence emission spectra to a skewed Gaussian peak as described under Experimental Procedures. n is the number of lattice sites close enough to a Trp residue to cause quenching when occupied by a brominated lipid and was obtained by fitting quenching data to Eq. 1. The value of n for DOPC is the average value reported previously for quenching in brominated phosphatidylcholines (Williamson et al., 2002). Levels of quenching in brominated lipid were determined as F/Fo where Fo is the fluorescence intensity in DOPC and F is the fluorescence intensity in the corresponding brominated phospholipid. For DOPE the value of F/Fo corresponds to that recorded in a 9:1 mixture of BrPE:DOPC. For all measurements the buffer was 20 mM HEPES and 1 mM EGTA at pH 7.2.
Fluorescence quenching by brominated phospholipids
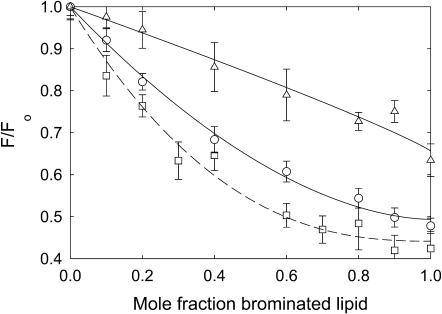
Reconstitution of KcsA into a mixture of a phospholipid with two oleoyl chains and the corresponding phospholipid with two dibrominated fatty acyl chains leads to fluorescence quenching, the level of quenching increasing with increasing mole fraction of brominated lipid (Fig. 5). For phosphatidylserine, the data fit to Eq. 1 with a value for n, the number of sites from which the fluorescence of a Trp residue can be quenched, of 1.77 ± 0.12, in agreement with the value of 1.69 determined previously for quenching by brominated phosphatidylcholines (Williamson et al., 2002). Quenching in brominated phosphatidylethanolamine and phosphatidylglycerol fits to an n number the same as for phosphatidylcholine (Table 1). Quenching by brominated cardiolipin fits to a value for n of 0.90 ± 0.15 (Fig. 5), a value close to half that for the other phospholipids, consistent with the four-chain structure of cardiolipin compared to the two-chain structure of the other phospholipids. Quenching in brominated phosphatidic acid fits to a significantly larger n number of 2.49 ± 0.25 (Fig. 5), possibly because of the small size of the phosphatidic acid headgroup. For the subsequent analyses, an n-value of 1.69 was used for all two-chain phospholipids except for phosphatidic acid, where an n-value of 2.49 was used. In studies of quenching in mixtures of cardiolipin and phosphatidylcholine, the mole fraction of cardiolipin was calculated based on the number of moles of fatty acyl chains to account for the fact that TOCL contains four chains and DOPC contains two chains; an n-value of 1.69 was used to describe quenching.
FIGURE 5.
Quenching of KcsA fluorescence by brominated phospholipids. KcsA was reconstituted into bilayers containing mixtures of nonbrominated lipid and the corresponding brominated lipid at pH 7.2. Fluorescence intensities are expressed as a fraction of the fluorescence for KcsA reconstituted in the nonbrominated lipid. Phospholipids were as follows: (○), phosphatidylserine; (□), phosphatidic acid; and (▵), cardiolipin. The lines show fits to Eq. 1 giving the values for n listed in Table 1. The buffer was 20 mM HEPES and 1 mM EGTA at pH 7.2.
The ratios of the fluorescence intensities for KcsA reconstituted into bilayers of brominated phospholipids compared to the fluorescence intensity in DOPC are listed in Table 1. Levels of fluorescence quenching in BrPE, BrPG, and BrCL are comparable to those in BrPC; higher levels of fluorescence quenching are observed in BrPA and BrPS (Table 1), attributable to the additional quenching of ∼10% caused by the lipid headgroups of phosphatidic acid and phosphatidylserine. The level of quenching observed with BrPS decreases with increasing ionic strength; in 500 mM KCl, the level of quenching (0.60 ± 0.01; Fig. 6) is comparable to that in BrPC.
FIGURE 6.
Quenching of KcsA fluorescence in mixtures with phosphatidylserine at high ionic strength. The buffer was 20 mM HEPES, 1 mM EGTA at pH 7.2, and 500 mM KCl. KcsA was reconstituted into bilayers containing mixtures of BrPC and DOPS (□), and BrPS and DOPC (○). Fluorescence intensities are expressed as F/Fo where Fo is the fluorescence intensity in the nonbrominated lipid. In A, the solid lines show fits to Eq. 2 giving the values for relative binding constants listed in Table 2. In B, the solid line for the BrPC/DOPS experiment shows a fit to Eq. 2 giving the annular binding constant and the lines for the BrPS/DOPC experiments show fits to the annular/non-annular binding site model, assuming (solid line) that all the fluorescence can be quenched from the non-annular sites (Eq. 5) and (broken line) that only 60% of the fluorescence can be quenched from the non-annular sites (Eq. 6). Values for the binding constants are listed in Table 3.
Fluorescence quenching in mixtures of phospholipids of two different classes
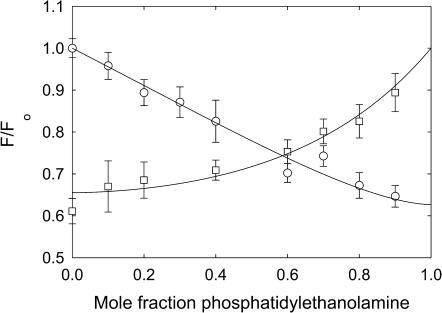
Fig. 7 shows fluorescence quenching for KcsA in BrPC/DOPE and BrPE/DOPC mixtures. In BrPC/DOPE mixtures displacement of BrPC from around KcsA by DOPE leads to an increase in fluorescence intensity whereas in BrPE/DOPC mixtures displacement of DOPC from around KcsA by BrPE leads to a decrease in fluorescence intensity, as described by Eq. 2. In mixtures of BrPC and DOPE, fluorescence intensities fit to Eq. 2 with a value for the binding constant for DOPE relative to DOPC of 0.57 ± 0.09 (Table 2). In mixtures of BrPE and DOPC, fluorescence intensities fit to Eq. 2 with a value for the binding constant for DOPE relative to DOPC of 0.69 ± 0.10, equal within experimental error to that obtained from analysis of the BrPC/DOPE mixtures, as expected (Table 2). These results confirm that DOPE and DOPC show simple competitive binding for KcsA and that the binding is unaffected by whether the phospholipid contains cis-unsaturated fatty acyl chains or the corresponding dibrominated fatty acyl chains.
FIGURE 7.
Quenching of KcsA fluorescence in mixtures with phosphatidylethanolamine. KcsA was reconstituted into bilayers containing mixtures of BrPC and DOPE (□) and BrPE and DOPC (○). Fluorescence intensities are expressed as F/Fo where Fo is the fluorescence intensity in the nonbrominated lipid. The solid lines show fits to Eq. 2 giving the values for relative binding constants listed in Table 2.
TABLE 2.
Relative lipid binding constants for KcsA determined from fluorescence quenching data
| Binding constant K relative to DOPC
|
|||
|---|---|---|---|
| Lipid X | K+ concentration (mM) | X/BrPC | BrX/DOPC |
| Phosphatidylethanolamine | 0 | 0.57 ± 0.09 | 0.69 ± 0.10 |
| Phosphatidylserine | 0 | 0.83 ± 0.16 | 2.16 ± 0.17 |
| 500 | 1.27 ± 0.22 | 1.43 ± 0.16 | |
| Phosphatidic acid | 0 | 1.38 ± 0.26 | 1.95 ± 0.22 |
| 500 | 1.47 ± 0.36 | 1.67 ± 0.14 | |
| Phosphatidylglycerol | 0 | 1.26 ± 0.06 | 1.66 ± 0.20 |
| Cardiolipin | 0 | 0.41 ± 0.09 | 1.61 ± 0.23 |
Lipid binding constants relative to DOPC were determined by fitting quenching data for KcsA in mixtures of lipid X with BrPC and brominated lipid X in mixtures with DOPC to Eq. 2. The mole fraction of cardiolipin was calculated on a chain basis, to account for the fact that cardiolipin contains four fatty acyl chains compared to two chains in phosphatidylcholine.
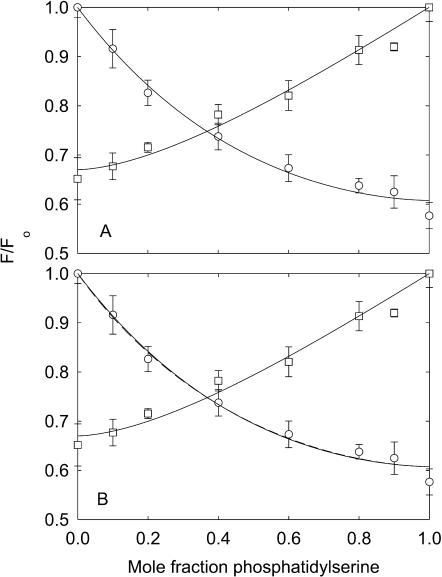
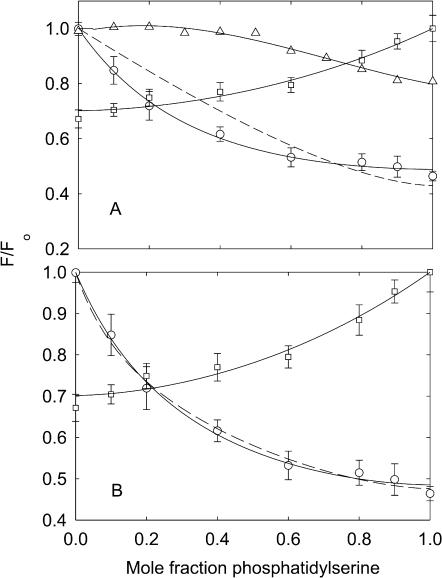
Results obtained with phosphatidylserine are very different from those obtained with phosphatidylethanolamine (Fig. 3 A). Fluorescence quenching in mixtures of BrPC and DOPS fits to Eq. 2 with a value for the binding constant for DOPS relative to DOPC of 0.83 ± 0.16 (Table 2), indicating little discrimination in binding between DOPS and DOPC. However, in mixtures of BrPS and DOPC, quenching is more extensive at low mole fractions of brominated lipid than in mixtures of BrPC and DOPS, and the data fit to a value for the binding constant for DOPS relative to DOPC of 2.16 ± 0.17 (Fig. 3 A, Table 2), indicating a preference for phosphatidylserine over phosphatidylcholine. Fig. 3 A also shows (dotted line) the best fit of the data for the BrPS/DOPC experiment to Eq. 2 fixing the relative binding constant at the value (0.83) obtained from the BrPC/DOPS experiment; the very poor fit to the data shows that the same binding constant cannot be used to describe quenching in both the BrPS/DOPC and the BrPC/DOPS experiments.
FIGURE 3.
Quenching of KcsA fluorescence in mixtures with phosphatidylserine. KcsA was reconstituted into bilayers containing mixtures of BrPC and DOPS (□), BrPS and DOPC (○), and DOPC and DOPS (▵). Fluorescence intensities are expressed as F/Fo where Fo is the fluorescence intensity in the nonbrominated lipid. In A, the solid lines for the BrPC/DOPS and BrPS/DOPC experiments show fits to Eq. 2 giving the values for relative binding constants listed in Table 2. The broken line shows an attempt to fit the data for the BrPS/DOPC experiment to the binding constant determined from the BrPC/DOPS experiment, as described in the text. In B, the solid line for the BrPC/DOPS experiment shows a fit to Eq. 2 giving the annular binding constant and the lines for the BrPS/DOPC experiments show fits to the annular/non-annular binding site model, assuming (solid line) that all the fluorescence can be quenched from the non-annular sites (Eq. 5), and (broken line) that only 60% of the fluorescence can be quenched from the non-annular sites (Eq. 6). Values for the binding constants are listed in Table 3. The buffer was 20 mM HEPES and 1 mM EGTA at pH 7.2.
Clearly, if there were a single class of binding site for phospholipids on KcsA, the binding constant determined from the BrPC/DOPS experiment would be the same as that determined from the BrPS/DOPC experiment, as observed for mixtures of phosphatidylcholine and phosphatidylethanolamine (Fig. 7). The fact that the binding constants are not equal is consistent with the presence of two classes of binding site for lipid on KcsA, one class corresponding to a large number of sites showing little selectivity for anionic lipid over phosphatidylcholine, explaining the relatively small effect of DOPS in the BrPC/DOPS mixtures, and the other class of site corresponding to a smaller number of sites showing high specificity for phosphatidylserine, explaining the relatively large effect of BrPS in BrPS/DOPC mixtures. Similar observations have been made previously in studies of the effects of cholesterol and brominated cholesterol on the fluorescence of Ca2+-ATPase in BrPC and DOPC, respectively, and interpreted in terms of a large number of annular sites to which both cholesterol and phosphatidylcholine can bind, and a small number of non-annular sites to which only cholesterol can bind (Simmonds et al., 1982). Adapting this same model to KcsA, fluorescence quenching for KcsA can be described by Eq. 5 where both phosphatidylserine and phosphatidylcholine can bind to the annular sites but only anionic lipid can bind to the non-annular sites. The model further assumes that binding to both the annular and the non-annular sites can quench the fluorescence of all the Trp residues; as with the previous experiments with Ca2+-ATPase it is assumed that the level of quenching following from binding to the non-annular sites is directly proportional to the occupancy of the sites by BrPS. As shown in Fig. 3 B, the data for quenching in BrPS/DOPC mixtures fits well to Eq. 5, with a relative binding constant of 0.83 for BrPS relative to DOPC at the annular sites and a binding constant of 2.53 ± 0.32 mole fraction−1 for binding at the non-annular sites (Table 3).
TABLE 3.
Annular and non-annular binding constants for KcsA
| Binding constant
|
||||
|---|---|---|---|---|
| Non-annular binding constant (mole fraction−1)
|
||||
| Lipid | KCl (mM) | Annular binding constant (relative to DOPC) | Model 1 | Model 2 |
| Phosphatidylserine | 0 | 0.83 ± 0.16 | 2.53 ± 0.32 | 5.71 ± 1.11 |
| 500 | 1.27 ± 0.22 | 0.26 ± 0.25 | 0.49 ± 0.48 | |
| Phosphatidic acid | 0 | 1.38 ± 0.26 | 1.51 ± 0.62 | 3.39 ± 1.41 |
| 500 | 1.47 ± 0.36 | 0.48 ± 0.33 | 0.94 ± 0.64 | |
| Phosphatidylglycerol | 0 | 1.26 ± 0.06 | 0.66 ± 0.35 | 1.46 ± 0.73 |
| Cardiolipin | 0 | 0.41 ± 0.09 | 2.77 ± 0.67 | 7.65 ± 3.96 |
Annular lipid binding constants relative to DOPC were determined by fitting quenching data for KcsA in mixtures of BrPC and anionic lipid to Eq. 2. Non-annular binding constants were determined by fitting quenching data for KcsA in mixtures of DOPC and brominated anionic lipid to Eq. 5 (Model 1) or Eq. 6 (Model 2). The mole fraction of cardiolipin was calculated on a chain basis, to account for the fact that cardiolipin contains four fatty acyl chains compared to two chains in phosphatidylcholine.
Quenching of Trp fluorescence by brominated phospholipids requires the brominated lipid and Trp to be close, quenching fitting to a Förster-type equation with a value of Ro—the distance at which quenching is 50% efficient—of ∼8–9 Å (Bolen and Holloway, 1990; Mall et al., 2001). Three of the Trp residues in KcsA (Trp-87, Trp-67, and Trp-68) are on the same side of the membrane as the lipid molecule bound to the non-annular site, and close to it; the other two Trp residues (Trp-26 and Trp-113) are more distant, on the opposite side of the membrane (Valiyaveetil et al., 2002). The data were also therefore fitted to a model (Eq. 6) in which only 60% of the Trp fluorescence could be quenched from the non-annular sites. This also gave a good fit to the data, with an increased value for the non-annular binding constant of 5.71 ± 0.32 mole fraction−1 (Fig. 3 B, Table 3).
Fits of fluorescence quenching data for BrPC/DOPS and BrPS/DOPC mixtures in the presence of 500 mM KCl to Eq. 2 gave binding constants much closer than those obtained at low salt, although the binding constant DOPS relative to DOPC derived from the BrPS/DOPC experiments was still slightly greater than that derived from the BrPC/DOPS experiments (Fig. 6, Table 2). This suggests that the non-annular binding constant of BrPS is reduced at high ionic strength. Thus fits of the data to the two-site, annular/non-annular binding site models (Eqs. 5 and 6) give much reduced binding constants for the non-annular site compared to those obtained at low salt (Fig. 6, Table 3).
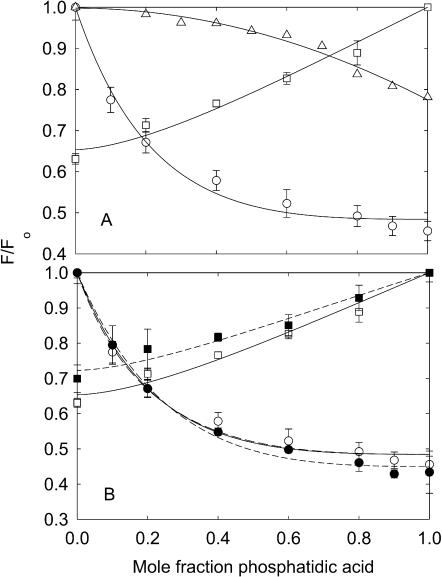
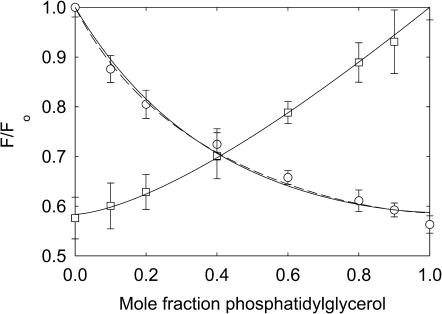
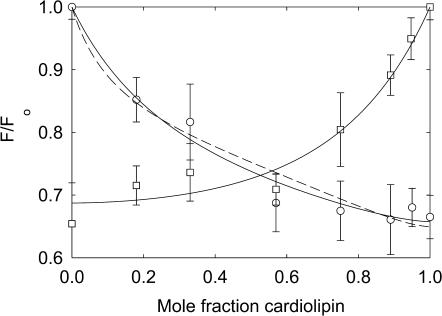
Fluorescence quenching in mixtures with phosphatidic acid (Fig. 4) and phosphatidylglycerol (Fig. 8) are very similar to those in mixtures of phosphatidylserine. Quenching is again more marked at low mole fractions of brominated lipid when the brominated lipid is the anionic lipid than when it is BrPC. The data fit to Eq. 2 with the parameters listed in Table 2 and to Eqs. 5 and 6 with the parameters listed in Table 3. Effects of 500 mM KCl on quenching in mixtures with phosphatidic acid are less marked than those on quenching in mixtures with phosphatidylserine (Fig. 4). Fluorescence quenching in mixtures with cardiolipin (Fig. 9) are consistent with relatively weak binding of cardiolipin at the annular sites with strong binding at the non-annular sites (Tables 2 and 3).
FIGURE 4.
Quenching of KcsA fluorescence in mixtures with phosphatidic acid. KcsA was reconstituted into bilayers containing mixtures of BrPC and DOPA (□,▪), BrPA and DOPC (○,•), and DOPC and DOPA (▵), in 20 mM HEPES, and 1 mM EGTA, at pH 7.2, in the absence of K+ (unfilled symbols) or in the presence of 500 mM KCl (solid symbols). Fluorescence intensities are expressed as F/Fo where Fo is the fluorescence intensity in the nonbrominated lipid. In A, the solid lines for the BrPC/DOPA and BrPA/DOPC experiments in the absence of K+ show fits to Eq. 2 giving the values for relative binding constants listed in Table 2. In B, the solid line for the BrPC/DOPA experiment in the absence of K+ shows a fit to Eq. 2 giving the annular binding constant and the lines for the BrPA/DOPC experiments in the absence of K+ show fits to the annular/non-annular binding site model, assuming (solid line) that all the fluorescence can be quenched from the non-annular sites (Eq. 5) and (broken line) that only 60% of the fluorescence can be quenched from the non-annular sites (Eq. 6). Fits of the data in 500 mM KCl to Eqs. 2 and 6 are shown by the dotted lines. Values for the binding constants are listed in Table 3.
FIGURE 8.
Quenching of KcsA fluorescence in mixtures with phosphatidylglycerol. KcsA was reconstituted into bilayers containing mixtures of BrPC and DOPG (□) and BrPG and DOPC (○). Fluorescence intensities are expressed as F/Fo where Fo is the fluorescence intensity in the nonbrominated lipid. The solid line for the BrPC/DOPG experiment shows a fit to Eq. 2 giving the annular binding constant and the lines for the BrPG/DOPC experiments show fits to the annular/non-annular binding site model, assuming (solid line) that all the fluorescence can be quenched from the non-annular sites (Eq. 5) and (broken line) that only 60% of the fluorescence can be quenched from the non-annular sites (Eq. 6). Values for the binding constants are listed in Table 3. The buffer was 20 mM HEPES and 1 mM EGTA at pH 7.2.
FIGURE 9.
Quenching of KcsA fluorescence in mixtures with cardiolipin. KcsA was reconstituted into bilayers containing mixtures of BrPC and TOCL (□) and BrCL and DOPC (○). Fluorescence intensities are expressed as F/Fo where Fo is the fluorescence intensity in the nonbrominated lipid. The solid line for the BrPC/TOCL experiment shows a fit to Eq. 2 giving the annular binding constant and the lines for the BrCL/DOPC experiments show fits to the annular/non-annular binding site model, assuming (solid line) that all the fluorescence can be quenched from the non-annular sites (Eq. 5) and (broken line) that only 60% of the fluorescence can be quenched from the non-annular sites (Eq. 6). Values for the binding constants are listed in Table 3. The buffer was 20 mM HEPES and 1 mM EGTA at pH 7.2. The mole fraction of cardiolipin was calculated on a chain basis to account for the fact that cardiolipin contains four fatty acyl chains and phosphatidylcholine, two.
DISCUSSION
The presence of anionic phospholipids is required for the potassium channel KcsA to open, the exact structure of the anionic lipid headgroup not being important since the channel is activated by phosphatidylglycerol, phosphatidylserine, and cardiolipin (Heginbotham et al., 1998; Valiyaveetil et al., 2002). Anionic lipid is not required for formation of the KcsA tetramer, since tetramers are formed when KcsA denatured in SDS is reconstituted into bilayers of phosphatidylcholine (Valiyaveetil et al., 2002). Rather, it was suggested that the anionic phospholipid bound between the transmembrane α-helices of the channel affected channel opening because channel opening involves movement of the transmembrane α-helices (Valiyaveetil et al., 2002). KcsA purified from E. coli membranes using dodecylmaltoside as detergent contains 0.7 mole fraction of phosphatidylglycerol per mole of KcsA monomer, suggesting the presence of a single site per monomer with relatively high affinity for phosphatidylglycerol; it is assumed that it is binding to this site that affects channel opening (Valiyaveetil et al., 2002). The crystal structure of KcsA shows a single lipid molecule per monomer, modeled as a diacylglycerol because the headgroup structure was not resolvable, but assumed to be a phosphatidylglycerol molecule, bound in a deep cleft (Zhou et al., 2001; Valiyaveetil et al., 2002), in a type of site referred to previously as a non-annular binding site (Simmonds et al., 1982; Lee, 2003). The crystal structure also shows a single chain modeled as nonan-1-ol, probably corresponding to part of the chain of a detergent molecule. The chain is located in a shallow groove on the surface of the protein, illustrating how the annular phospholipids might bind to the hydrophobic surface of the protein (Fig. 1).
The Trp residues in KcsA are located in two bands, one on each side of the membrane close to the expected interface between the hydrocarbon core of the bilayer and the lipid headgroup region (Williamson et al., 2002). The observation that reconstitution into bilayers of anionic phospholipid results in no significant change in the position of the fluorescence emission maximum (Table 1) suggests that no large conformation change for KcsA occurs on reconstitution into anionic phospholipid. Fluorescence intensities in DOPS and DOPA are ∼10% lower than in DOPC (Table 1). Trp fluorescence is quenched in solution by acidic groups, by a mechanism involving electron or proton transfer requiring close contact between the Trp and the acidic group; oleic acid, for example, has been shown to quench the Trp fluorescence of Ca2+-ATPase and of a simple hydrophobic analogue of Trp incorporated into lipid bilayers (Froud et al., 1986). Quenching of the Trp fluorescence of KcsA by DOPS and DOPA is consistent with a location for the lipid headgroups close to some or all of the Trp residues on KcsA.
Reconstitution of KcsA into bilayers of lipids containing 9,10-dibromostearyl chains leads to extensive quenching of fluorescence (Table 1). Levels of fluorescence quenching in bilayers of the brominated anionic phospholipids, when corrected for the effect of the anionic lipid headgroup, are very similar to that observed in BrPC (Table 1). Since the level of fluorescence quenching of KcsA observed in bilayers of brominated phospholipid depends on the distance of separation between the bromine groups and the Trp residues (Williamson et al., 2002) this observation again argues against any large change in conformation for KcsA on reconstitution into bilayers of anionic phospholipid.
Lipid binding constants
Relative lipid binding constants for the annular sites around membrane proteins have been estimated from electron spin resonance (ESR) and fluorescence studies (Marsh and Horvath, 1998; Williamson et al., 2002; Lee, 2003). However, there have, as yet, been no reports of binding constants for phospholipids at non-annular sites. Here we have shown that such information can be obtained for KcsA from fluorescence quenching studies with brominated phospholipids. Fluorescence quenching in mixtures of BrPC with anionic phospholipids are consistent with simple competition between anionic phospholipids and BrPC for binding at the annular sites, with binding constants for the anionic phospholipids relative to DOPC close to 1 except for cardiolipin (Table 3), suggesting that charge interactions are relatively unimportant for binding to annular sites. Studies of binding of anionic phospholipids to a range of membrane proteins using ESR methods also showed little selectivity in binding, binding of the anionic phospholipids being no more than a factor of two stronger than phosphatidylcholine (Marsh and Horvath, 1998). Binding of phosphatidylethanolamine to the annular sites on KcsA is slightly weaker than binding of phosphatidylcholine (Table 2), despite the fact that phosphatidylcholine is absent from the cytoplasmic membrane of Streptomyces lividans whose major zwitterionic lipid is phosphatidylethanolamine (Hoischen et al., 1997). Phosphatidylethanolamine was also found to bind less strongly than phosphatidylcholine to the Ca2+-ATPase of skeletal muscle sarcoplasmic reticulum, a membrane in which phosphatidylcholine is the major zwitterionic lipid (East and Lee, 1982). The binding constant for phosphatidylethanolamine is similar to that for cardiolipin (Table 3) and it is possible that this is related to the fact that both these phospholipids have a tendency to form curved, hexagonal HII phases as well as the normal planar bilayer phase.
Quenching in mixtures of brominated anionic phospholipid with DOPC was more marked at low mole fractions of brominated phospholipid than would have been expected if binding at the annular sites showed little specificity for anionic phospholipids compared to DOPC (Figs. 3, 4 and 8). This is consistent with binding of the brominated anionic phospholipids to the non-annular binding sites for anionic phospholipids observed in the crystallographic studies of KcsA (Fig. 1), sites at which zwitterionic phospholipids can either not bind, or to which they bind with very low affinity. Quenching by brominated anionic phospholipids will then arise both from binding to the annular sites and from binding to the non-annular sites. A model describing quenching of this type was proposed previously in studies of the binding of brominated cholesterol to Ca2+-ATPase (Simmonds et al., 1982), and is used here. An unknown in the fitting procedure is the fraction of the Trp fluorescence that can be quenched from the non-annular sites. Inasmuch as quenching of Trp fluorescence by bromine requires the bromine to be located close to the Trp residue (Bolen and Holloway, 1990; Mall et al., 2001), and inasmuch as three of the five Trp residues in KcsA are on the same side of the membrane as the non-annular site, it is probable that the fluorescence of only 60% of the Trp residues will be quenched from the non-annular sites (Eq. 6) and all the data reported here fit to this model (Table 3). However, all the data also fit to a model in which binding at the non-annular sites quenches the fluorescence of all the Trp residues (Eq. 5). It is not possible to distinguish between these two possibilities from the data presented here, but the experiments do provide information about the likely order of magnitude of the binding constants and about their dependence on lipid headgroup structure.
Non-annular binding constants for phosphatidylserine and phosphatidic acid are equal within experimental error, the non-annular binding constant for phosphatidylglycerol being significantly lower and that for cardiolipin being significantly higher (Table 3). The binding constant for phosphatidylserine decreases very markedly in the presence of 500 mM KCl (Table 3), suggesting that binding at the non-annular binding sites is strongly dependent on electrostatic interactions. The effect of 500 mM KCl on the non-annular binding constant for phosphatidic acid is less marked than its effect for phosphatidylserine (Fig. 4; Table 3) suggesting that charge interactions make less of a contribution to binding of phosphatidic acid than phosphatidylserine. Weaker binding of phosphatidylglycerol than of the other anionic phospholipids (Table 3) could be a result of the presence of the relatively bulky and hydrophobic glycerol moiety in the lipid headgroup. The anionic phospholipid headgroups probably interact with Arg-64 and Arg-89 located in the girdle of charged residues above Trp-87, the interaction being a relatively nonspecific one since the anionic lipid headgroup is not resolved in the crystal structure (Fig. 1).
Binding of anionic lipids to the non-annular binding site in the native membrane
The plasma membrane of the gram-negative Escherichia coli in which KcsA is expressed contains ∼20% anionic phospholipid, predominantly phosphatidylglycerol; it is not known if the distribution of phosphatidylglycerol between the two leaflets of the bilayer is uniform or asymmetric (Huijbregts et al., 2000). If the binding constant for phosphatidylglycerol is 1.46 mole fraction−1 (Table 3) then the non-annular binding site would be 25% occupied at a mole fraction of phosphatidylglycerol of 0.2 and it would be 40% occupied at a mole fraction of phosphatidylglycerol of 0.4, corresponding to the situation where all the phosphatidylglycerol was in the outer leaflet of the plasma membrane, the side on which the non-annular binding site of KcsA is located. Fractional occupation of the non-annular binding site by anionic phospholipid in the native membrane would be surprising, inasmuch as the site needs to be occupied for KcsA to be functional (Valiyaveetil et al., 2002). However, the lipid composition of the plasma membrane of the Gram-positive S. lividans is different from that of KcsA. The lipid composition of S. lividans appears not to have been determined, but in S. ambofaciens ∼40% of the lipid is cardiolipin with small amounts of phosphatidylinositol as the only other anionic lipid (Schauner et al., 1999) and in S. hygroscopicus ∼20% of the lipid is cardiolipin with ∼5% phosphatidic acid and 10% modified phosphatidylinositols (Hoischen et al., 1997). The phosphatidylglycerol content of the Streptomyces family appears to vary widely between strains (Lechevalier et al., 1981). Given the strong binding of cardiolipin to the non-annular binding site in KcsA (Table 3) it is likely that the site will be occupied by cardiolipin in the native membrane. With a binding constant of 7.65 mole fraction−1 for cardiolipin expressed on a chain basis, the non-annular site would be ∼75% occupied in a membrane containing 20% cardiolipin and ∼90% occupied in a membrane containing 40% cardiolipin.
Acknowledgments
We thank Professor Schrempf for the generous gift of the KcsA construct, and we thank the Biotechnology and Biological Sciences Research Council for financial support and for a studentship to S.J.A.
References
- Bolen, E. J., and P. W. Holloway. 1990. Quenching of tryptophan fluorescence by brominated phospholipid. Biochemistry. 29:9638–9643. [DOI] [PubMed] [Google Scholar]
- Caffrey, M., and G. W. Feigenson. 1981. Fluorescence quenching in model membranes. 3. Relationship between calcium adenosinetriphosphatase enzyme activity and the affinity of the protein for phosphatidylcholines with different acyl chain characteristics. Biochemistry. 20:1949–1961. [DOI] [PubMed] [Google Scholar]
- Camara-Artigas, A., D. Brune, and J. P. Allen. 2002. Interactions between lipids and bacterial reaction centers determined by protein crystallography. Proc. Natl. Acad. Sci. USA. 99:11055–11060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Foresta, B., M. le Maire, S. Orlowski, P. Champeil, S. Lund, J. V. Moller, F. Michelangeli, and A. G. Lee. 1989. Membrane solubilization by detergent: use of brominated phospholipids to evaluate the detergent-induced changes in Ca2+-ATPase/lipid interaction. Biochemistry. 28:2558–2567. [DOI] [PubMed] [Google Scholar]
- East, J. M., and A. G. Lee. 1982. Lipid selectivity of the calcium and magnesium ion dependent adenosinetriphosphatase, studied with fluorescence quenching by a brominated phospholipid. Biochemistry. 21:4144–4151. [DOI] [PubMed] [Google Scholar]
- Froud, R. J., J. M. East, E. K. Rooney, and A. G. Lee. 1986. Binding of long-chain alkyl derivatives to lipid bilayers and to Ca2+-Mg2+-ATPase. Biochemistry. 25:7535–7544. [DOI] [PubMed] [Google Scholar]
- Harrenga, A., and M. Michel. 1999. The cytochrome-c oxidase from Paracoccus denitrificans does not change the metal center ligation upon reduction. J. Biol. Chem. 274:33296–33299. [DOI] [PubMed] [Google Scholar]
- Heginbotham, L., L. Kolmakova-Partensky, and C. Miller. 1998. Functional reconstitution of a prokaryotic K+ channel. J. Gen. Physiol. 111:741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimpel, S., G. Basset, S. Odoy, and M. Klingenberg. 2001. Expression of the mitochondrial ADP/ATP carrier in Escherichia coli—renaturation, reconstitution, and the effect of mutations on 10 positive residues. J. Biol. Chem. 276:11499–11506. [DOI] [PubMed] [Google Scholar]
- Hoischen, C., K. Gura, C. Luge, and J. Gumpert. 1997. Lipid and fatty acid composition of cytoplasmic membranes from Streptomyces hygroscopicus and its stable protoplast-type L form. J. Bacteriol. 179:3430–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts, R. P. H., A. I. P. M. de Kroon, and B. de Kruijff. 2000. Topology and transport of membrane lipids in bacteria. Biochim. Biophys. Acta. 1469:43–61. [DOI] [PubMed] [Google Scholar]
- Iwata, S., C. Ostermeier, B. Ludwig, and H. Michel. 1995. Structure at 2.8 Å resolution of cytochrome-c oxidase from Paracoccus denitrificans. Nature. 376:660–669. [DOI] [PubMed] [Google Scholar]
- Jiang, F., M. T. Ryan, M. Schlame, M. Zhao, Z. M. Gu, M. Klingenberg, N. Pfanner, and M. L. Greenberg. 2000. Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function. J. Biol. Chem. 275:22387–22394. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, V. Uta, M. Cadene, B. T. Chait, and R. MacKinnon. 2003. X-ray structure of a voltage-dependent K+ channel. Nature. 423:33–41. [DOI] [PubMed] [Google Scholar]
- Lange, C., J. H. Nett, B. L. Trumpower, and C. Hunte. 2001. Specific roles of protein-phospholipid interactions in the yeast cytochrome bc1 complex structure. EMBO J. 20:6591–6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechevalier, M. P., A. E. Stern, and H. A. Lechevalier. 1981. Phospholipids in the taxonomy of actinomycetes. In Actinomycetes. K. P. Schaal and G. Pulverer, editors. Gustav Fischer Verlag, Stuttgart, Germany. 111–116.
- Lee, A. G. 2002. Ca2+-ATPase structure in the E1 and E2 conformations: mechanism, helix-helix and helix-lipid interactions. Biochim. Biophys. Acta. 1565:246–266. [DOI] [PubMed] [Google Scholar]
- Lee, A. G. 2003. Lipid-protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta. 1612:1–40. [DOI] [PubMed] [Google Scholar]
- London, E., and G. W. Feigenson. 1981. Fluorescence quenching in model membranes. 2. Determination of local lipid environment of the calcium adenosinetriphosphatase from sarcoplasmic reticulum. Biochemistry. 20:1939–1948. [DOI] [PubMed] [Google Scholar]
- Mall, S., R. P. Sharma, J. M. East, and A. G. Lee. 1998. Lipid-protein interactions in the membrane: studies with model peptides. Faraday Discuss. 111:127–136. [DOI] [PubMed] [Google Scholar]
- Mall, S., R. Broadbridge, R. P. Sharma, J. M. East, and A. G. Lee. 2001. Self-association of model transmembrane α-helices is modulated by lipid structure. Biochemistry. 40:12379–12386. [DOI] [PubMed] [Google Scholar]
- Marsh, D., and L. I. Horvath. 1998. Structure, dynamics and composition of the lipid-protein interface. Perspectives from spin-labelling. Biochim. Biophys. Acta. 1376:267–296. [DOI] [PubMed] [Google Scholar]
- McAuley, K. E., P. K. Fyfe, J. P. Ridge, N. W. Isaacs, R. J. Cogdell, and M. R. Jones. 1999. Structural details of an interaction between cardiolipin and an integral membrane protein. Proc. Natl. Acad. Sci. USA. 96:14706–14711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keeffe, A. H., J. M. East, and A. G. Lee. 2000. Selectivity in lipid binding to the bacterial outer membrane protein OmpF. Biophys. J. 79:2066–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom, M. S. P., I. H. Shrivastava, J. N. Bright, J. Tate, C. E. Capener, and P. C. Biggin. 2002. Potassium channels: structures, models, simulations. Biochim. Biophys. Acta. 1565:294–307. [DOI] [PubMed] [Google Scholar]
- Schauner, C., A. Dary, A. Lebrihi, P. Leblond, B. Decaris, and P. Germain. 1999. Modulation of lipid metabolism and spiramycin biosynthesis in Streptomyces ambofaciens unstable mutants. Appl. Microbiol. Biotechnol. 65:2730–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlame, M., D. Rua, and M. L. Greenberg. 2000. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 39:257–288. [DOI] [PubMed] [Google Scholar]
- Schrempf, H., O. Schmidt, R. Kummerlen, S. Hinnah, D. Muller, M. Betzler, T. Steinkamp, and R. Wagner. 1995. A prokaryotic potassium ion channel with two predicted transmembrane segments from Streptomyces lividans. EMBO J. 14:5170–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds, A. C., J. M. East, O. T. Jones, E. K. Rooney, J. McWhirter, and A. G. Lee. 1982. Annular and non-annular binding sites on the (Ca2+ + Mg2+)-ATPase. Biochim. Biophys. Acta. 693:398–406. [DOI] [PubMed] [Google Scholar]
- Valiyaveetil, F. I., Y. Zhou, and R. Mackinnon. 2002. Lipids in the structure, folding and function of the KcsA K+ channel. Biochemistry. 41:10771–10777. [DOI] [PubMed] [Google Scholar]
- Williamson, I. M., S. J. Alvis, J. M. East, and A. G. Lee. 2002. Interactions of phospholipids with the potassium channel KcsA. Biophys. J. 83:2026–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y., J. H. Morals-Cabral, A. Kaufman, and R. Mackinnon. 2001. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature. 414:43–48. [DOI] [PubMed] [Google Scholar]