Abstract
We used two strains of Actinobacillus actinomycetemcomitans, one bearing phosphorylcholine (PC) (strain D045D-40) and one devoid of PC antigens (strain DB03A-42), as well as a nonencapsulated strain of Streptococcus pneumoniae (strain 39937), to examine the opsonic properties of physiological concentrations (⩽30 μg/ml) of purified human anti-PC immunoglobulin G (IgG). Anti-PC bound to both A. actinomycetemcomitans DO45D-40 and S. pneumoniae 39937 and induced superoxide anion production by polymorphonuclear neutrophils; induction of the oxidative burst was inhibited by antibodies to either CD16 or CD32. Thus, anti-PC IgG at concentrations present in most human sera promotes the opsonization of PC-expressing strains of A. actinomycetemcomitans in the absence of complement, implying that anti-PC may be a protective antibody against such strains of bacteria.
Phosphorylcholine (PC) is the immunodominant epitope found in pneumococcal C polysaccharide (12, 18) and an antigen present in normal mammalian cells. It has been shown to occur in a variety of gram-negative and gram-positive bacteria (7), as well as in up to one-half of the microorganisms in subgingival and supragingival dental plaque (15). Antibodies to PC are routinely found in human serum in the range of 30 to 300 μg/ml (3, 10, 12, 15). The function of these antibodies is unclear. In mice, the induction of anti-PC antibodies is protective against lethal infections with Streptococcus pneumoniae (2); however, it has been reported that no correlation exists between anti-PC levels and protection against human S. pneumoniae infections in elderly patients (8). Additionally, serum fractions containing anti-PC are not opsonic for S. pneumoniae, and multivalent pneumococcal vaccines containing various amounts of PC fail to induce a significant antibody response against PC (9, 11, 12).
Previous studies indicate that strains of Actinobacillus actinomycetemcomitans, a gram-negative pathogen associated with localized aggressive (juvenile) periodontitis, brain abscesses, and subacute bacterial endocarditis, vary with regard to their levels of expression of PC (5, 13). It has been proposed that PC may be an important virulence factor for A. actinomycetemcomitans, because PC-bearing strains of A. actinomycetemcomitans are able to utilize the platelet-activating factor receptor to enter human vascular endothelial cells and possibly gain access to the circulatory system, where they may seed sites distant from the oral cavity and serve as a source of antigen for the production of anti-PC (13).
It is likely that oral bacteria stimulate a significant portion of the human anti-PC immunoglobulin G (IgG) response, but the function of these antibodies with respect to the control of the oral microflora is unknown. Given the wide range of immunoreactive PC antigens available and the ambiguous role of anti-PC, we chose to examine anti-PC-mediated opsonization and the induction of oxidative bursts in polymorphonuclear neutrophils (PMNs) by using two strains of A. actinomycetemcomitans (D045D-40 and DB03A-42) and a nonencapsulated strain of S. pneumoniae (39937).
Antibodies to PC were isolated from human Cohn Fraction II (Sigma catalog number G-4386) by affinity chromatography on an immobilized p-aminophenyl-PC column (Pierce catalog number 20307) and by stepwise elution with 0.01 M PC-chloride (Sigma catalog number P-0378), and thereafter with 0.01 M p-aminophenyl-PC (Sigma catalog number A-9278). The pH of the concentrated samples was adjusted to 3.0 with 0.4 M HCl, and the IgG and PC-chloride or p-aminophenyl PC were separated on a Sephadex G-25 (Pharmacia) column equilibrated with 0.01 M EDTA-Hanks' balanced salt solution (HBSS). The final product did not contain detectable concentrations of C-reactive protein as determined by enzyme-linked immunosorbent assay. A second antibody preparation was purified by affinity chromatography on lysine-Sepharose for use as a control antibody.
We used A. actinomycetemcomitans strains D045D-40 and DB03A-42 (clinical isolates from patients with aggressive or early-onset periodontitis) and S. pneumoniae 39937 (purchased from the American Type Culture Collection). A. actinomycetemcomitans strains were grown at 37°C in an atmosphere of 5% CO2 and 95% humidity in brain heart infusion broth for 18 h. S. pneumoniae strains were grown in the same medium at 37°C under normal atmospheric conditions. Concentrations of bacteria were determined by measuring the A650 and adjusting the optical density to 1.0, which is nominally equivalent to 5 × 107 bacteria/ml, as estimated in a bacterial counting chamber.
The binding of anti-PC was assessed by diluting the bacteria to 107/ml in HBSS containing 0.1% endotoxin-bovine serum albumin (Sigma catalog number A-2934), adding antibody dilutions, and then incubating the plates at room temperature for 30 min. Bacteria were washed with HBSS-bovine serum albumin, resuspended to their initial volume, and incubated with biotinylated F(ab′)2 goat anti-human IgG (5 μg/ml; Jackson Immunodiagnostics) for 30 min followed by a second incubation with Alexa 488-streptavidin (Molecular Probes catalog number S-11223) for 30 min at room temperature. The cells were examined on a FACScan (Becton Dickinson). Experiments were repeated three times, and similar results were obtained. Results for a typical binding experiment are reported as the mean channel fluorescence (MCF).
PMNs were isolated as described by Scott-Zaki et al. (17). To measure oxidative burst, bacteria were opsonized with anti-PC as described above and resuspended to 108/ml. Fifty microliters of the anti-PC-coated bacteria was added to wells of Dynex Microfluor 2 plates. Fifty microliters of purified PMNs, PMNs plus anti-CD32 (10 μg/ml), or PMNs plus anti-CD16 (30 μg/ml) at a cell concentration of 107/ml in Amplex Red (Molecular Probes), a fluorogenic reagent utilized for the detection of peroxidase, was added to each well. Plates were incubated in a Dynex Microplate fluorometer at 37°C, and readings were taken at 90-s intervals. Phorbol myristate acetate (Sigma) was added to control wells at 50 ng/well. All samples were run in triplicate. PMNs incubated with phorbol myristate acetate were positive controls, while samples containing PMNs plus unopsonized bacteria served as negative controls, and background counts were subtracted at each time point. Results are reported as relative fluorescence units and indicate the increase in Amplex Red fluorescence due to antibody-coated bacteria. For three experiments, the standard error of the mean at each time interval was within 5% of the mean. Purified anti-FcγRII (anti-CD32) and anti-FcγRIII (anti-CD16) were generous gifts from Shaun Ruddy, Virginia Commonwealth University (Richmond).
Affinity-purified anti-PC binds to PC-bearing bacteria.
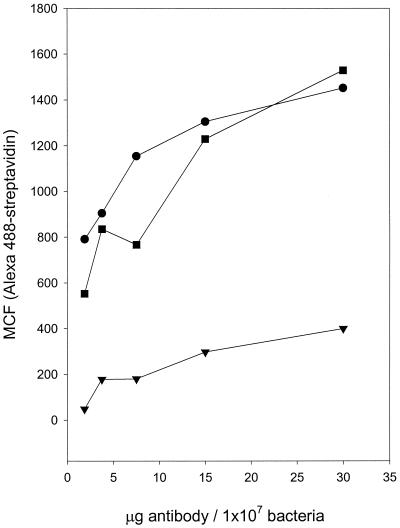
Figure 1 shows that with increasing concentrations of anti-PC, there is a concomitant increase in fluorescence, which begins to plateau at 15 μg/ml for both A. actinomycetemcomitans (D045D-40) and S. pneumoniae (39937). Furthermore, A. actinomycetemcomitans DB03A-42, a strain shown to have a very low incorporation of [3H]choline from culture media and no reactivity with TEPC-15, did not display any more fluorescence than did the human anti-lysine Sepharose (data not shown), which failed to bind to either strain. These data support our previous observations that PC is available as a surface antigen and reactive with human anti-PC.
FIG. 1.
Binding of anti-PC to A. actinomycetemcomitans and S. pneumoniae. Biotinylated affinity-purified human anti-PC was incubated with PC-containing A. actinomycetemcomitans D045D-40 (•) and S. pneumoniae 39937 (▪) in addition to PC-negative A. actinomycetemcomitans DB03A-42 (▾). It was then developed with Alexa 488-streptavidin. Binding, expressed as MCF, was determined by flow cytometry.
Respiratory burst in PMNs is generated by anti-PC-opsonized A. actinomycetemcomitans and S. pneumoniae.
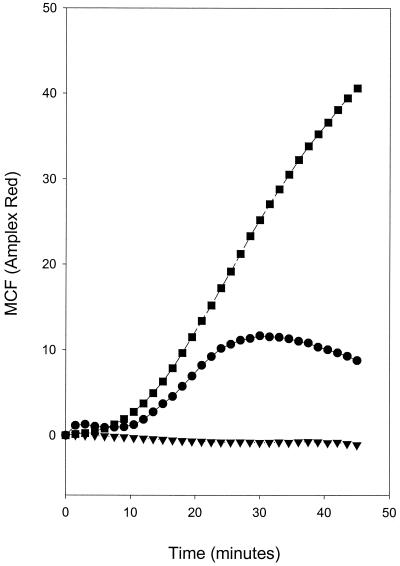
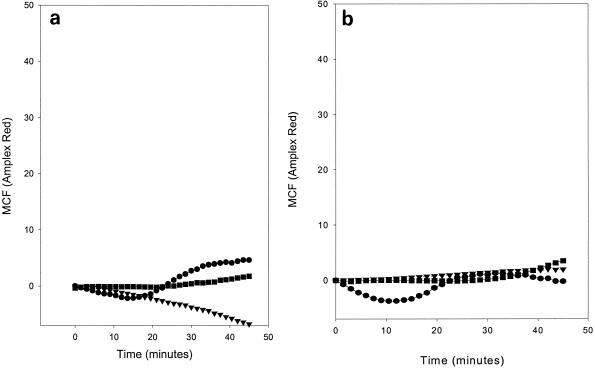
Figure 2 shows that when A. actinomycetemcomitans (D045D-40) and S. pneumoniae (39937) were opsonized with anti-PC and then incubated with PMNs, superoxide was produced. A. actinomycetemcomitans DB03A-42, a strain devoid of PC, generated no increase in superoxide production in either the presence or absence of anti-PC. Similarly, opsonization of the test strains with a control antibody (anti-lysine Sepharose IgG) failed to induce an elevated superoxide response. Figure 3 shows that when PMNs are preincubated with either anti-CD32 or anti-CD16 prior to incubation with opsonized bacteria, the generation of an oxidative burst is virtually eliminated. Thus, human anti-PC bound to A. actinomycetemcomitans (D045D-40) and S. pneumoniae (39937) appears to be capable of cross-linking CD32 on PMNs and inducing superoxide production.
FIG. 2.
Generation of oxidative burst in PMNs incubated with anti-PC-opsonized A. actinomycetemcomitans and S. pneumoniae. PC-containing A. actinomycetemcomitans D045D-40 (•) and S. pneumoniae 39937 (▪) in addition to PC-negative A. actinomycetemcomitans DB03A-42 (▾) were incubated with affinity-purified human anti-PC and then added to PMNs. Readings were made at 90-s intervals over a 45-min time course. The results are expressed as the MCF signal generated by opsonized bacteria minus the signal generated by unopsonized bacteria.
FIG. 3.
Effect of anti-CD16 and anti-CD32 on the generation of an oxidative burst in PMNs incubated with anti-PC-opsonized A. actinomycetemcomitans and S. pneumoniae. PC-containing A. actinomycetemcomitans D045D-40 (•) and S. pneumoniae 39937 (▪) in addition to PC-negative A. actinomycetemcomitans DB03A-42 (▾) were incubated with affinity-purified human anti-PC and then added to PMNs. Readings were made at 90-s intervals over a 45-min time course. The results are expressed as the MCF signal generated by opsonized bacteria minus the signal generated by unopsonized bacteria. (a) PMNs were preincubated with anti-CD16 and then incubated with opsonized and unopsonized bacteria. (b) PMNs were preincubated with anti-CD32 and then incubated with opsonized and unopsonized bacteria.
The data demonstrate that PC-bearing bacterial strains of both A. actinomycetemcomitans and S. pneumoniae can be opsonized by human anti-PC IgG. This opsonization renders them susceptible to binding to and activation of human PMNs through their Fc receptors, resulting in superoxide generation. Baker and Wilson (1) reported that opsonization of A. actinomycetemcomitans strains Y4 and JP2 by IgG from patients with high titers of specific antibody requires complement. The PC content of the strains they utilized is not known, but one strain (designated strain 67) was opsonized with human serum in the absence of high-titer specific antibody, a finding that is consistent with our observations. The prevalence of PC-bearing strains of this organism is not known, though Gmur et al. (5) reported that approximately one-third of their laboratory isolates reacted with PC-specific monoclonal antibodies. Previous data on specific opsonizing antibody that is reactive with surface antigens of periodontal pathogens, such as A. actinomycetemcomitans, indicate that elevated antibody is associated with a decrease in the extent and severity of periodontal diseases (4, 6). Thus, the occurrence of strains bearing PC and anti-PC antibody in patients with early-onset periodontitis may be a measure of the protection afforded by this antibody.
Previous studies in our laboratory have shown that PC-bearing strains of A. actinomycetemcomitans can utilize the platelet-activating factor receptor for entry into endothelial cells (13). This observation, taken in conjunction with data showing increased anti-PC levels in patients with periodontal attachment loss (15), suggests an avenue for an increase in systemic IgG anti-PC. This anti-PC may then opsonize PC-bearing strains of A. actinomycetemcomitans. It is not known whether other oral bacteria bearing PC can use this mechanism to access endothelial cells and the circulation; however, if this is the case, the elevation in anti-PC seen in patients with periodontal attachment loss may be a reflection of invasion.
Anti-PC IgG levels in human serum have been estimated to range from about 5 to over 300 μg/ml. Using affinity-purified anti-PC IgG as a standard, we estimated the concentration in normal serum to be between 30 and 40 μg/ml. Thus, the concentrations to which we exposed the bacterial strains were in the range of those found in most human sera. We have further noted that concentrations of anti-PC in gingival crevicular fluid are elevated in comparison to concentrations in serum (data not shown), indicating not only that anti-PC is accessible to periodontal microorganisms but also that there is local production of these antibodies in the gingiva.
We could block opsonization by applying monoclonal antibodies to either FcγRII or FcγRIII on PMNs, indicating that both receptors are necessary for optimal activation of PMNs by anti-PC-bacterium complexes. There were indications (Fig. 3a) that blocking FcγRIII did not totally eliminate superoxide production, an observation which is consistent with data reported by Scott-Zaki et al. (16, 17), who demonstrated that the cross-linking of FcγRII stimulates superoxide production and chemotaxis in the absence of FcγRIII. We consistently observed a significant reduction in superoxide production in the presence of anti-CD16 and hypothesized that the residual stimulation of superoxide activity may result from the close proximity of PMNs and bacteria in culture, encouraging binding to the less expressed FcγRII.
The likely ability of oral bacteria to induce opsonic anti-PC antibodies may have implications for inflammatory responses external to the oral cavity. The anti-PC produced as a result of exposure to PC-positive A. actinomycetemcomitans (and perhaps other oral microorganisms) may serve as an opsonin for other antigens containing PC, such as oxidized low-density lipoproteins (14). These data, combined with the present data showing PMN activation by binding to the Fc region of bound anti-PC, provide a biological possibility for either a protective immune response in atherosclerosis as a result of exposure to PC-bearing oral bacteria or the enhancement of chronic inflammatory responses found in atherosclerosis.
Acknowledgments
This work was supported in part by Public Health Service grant U19 DE13102 from the National Institute of Dental and Craniofacial Research.
Editor: J. D. Clements
REFERENCES
- 1.Baker, P. J., and M. E. Wilson. 1989. Opsonic IgG antibody against Actinobacillus actinomycetemcomitans in localized juvenile periodontitis. Oral Microbiol. Immunol. 4:98-105. [DOI] [PubMed] [Google Scholar]
- 2.Briles, D. E., C. Forman, and M. Crain. 1992. Mouse antibody to phosphocholine can protect mice from infection with mouse-virulent human isolates of Streptococcus pneumoniae. Infect. Immun. 60:1957-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briles, D. E., G. Scott, B. Gray, M. J. Crain, M. Blaese, M. Nahm, V. Scott, and P. Haber. 1987. Naturally occurring antibodies to phosphocholine as a potential index of antibody responsiveness to polysaccharides. J. Infect. Dis. 155:1307-1314. [DOI] [PubMed] [Google Scholar]
- 4.Califano, J. V., J. C. Gunsolley, K. Nakashima, H. A. Schenkein, M. E. Wilson, and J. G. Tew. 1996. Influence of anti-Actinobacillus actinomycetemcomitans Y4 (serotype b) lipopolysaccharide on severity of generalized early-onset periodontitis. Infect. Immun. 64:3908-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gmur, R., T. Thurnheer, and B. Guggenheim. 1999. Dominant cross-reactive antibodies generated during the response to a variety of oral bacterial species detect phosphorylcholine. J. Dent. Res. 78:77-85. [DOI] [PubMed] [Google Scholar]
- 6.Gunsolley, J. C., J. A. Burmeister, J. G. Tew, A. M. Best, and R. R. Ranney. 1987. Relationship of serum antibody to attachment level patterns in young adults with juvenile periodontitis or generalized severe periodontitis. J. Periodontol. 58:314-320. [DOI] [PubMed] [Google Scholar]
- 7.Harnett, W., and M. M. Harnett. 1999. Phosphorylcholine: friend or foe of the immune system? Immunol. Today 20:125-129. [DOI] [PubMed] [Google Scholar]
- 8.Musher, D. M., A. J. Chapman, A. Goree, S. Jonsson, D. Briles, and R. E. Baughn. 1986. Natural and vaccine-related immunity to Streptococcus pneumoniae. J. Infect. Dis. 154:245-256. [DOI] [PubMed] [Google Scholar]
- 9.Nahm, M. H., J. V. Olander, and M. Magyarlaki. 1997. Identification of cross-reactive antibodies with low opsonophagocytic activity for Streptococcus pneumoniae. J. Infect. Dis. 176:698-703. [DOI] [PubMed] [Google Scholar]
- 10.Nishinarita, S., S. Sawada, and T. Horie. 1990. Phosphorylcholine antibodies in pulmonary infection. Med. Microbiol. Immunol. 179:205-214. [DOI] [PubMed] [Google Scholar]
- 11.Nordenstam, G., B. Andersson, C. Bengtsson, D. Briles, G. Scott, A. Svanborg, and C. Svanborg Edèn. 1989. Age-related change in anti-carbohydrate antibody levels. Am. J. Epidemiol. 129:89-96. [DOI] [PubMed] [Google Scholar]
- 12.Nordenstam, G., B. Andersson, D. Briles, J. W. Brooks, Jr., A. Odèn, A. Svanborg, and C. S. Edèn. 1990. High anti-phosphorylcholine antibody levels and mortality associated with pneumonia. Scand. J. Infect. Dis. 22:187-195. [DOI] [PubMed] [Google Scholar]
- 13.Schenkein, H. A., S. E. Barbour, C. R. Berry, B. Kipps, and J. G. Tew. 2000. Invasion of human vascular endothelial cells by Actinobacillus actinomycetemcomitans via the receptor for platelet-activating factor. Infect. Immun. 68:5416-5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schenkein, H. A., C. R. Berry, D. Purkall, J. A. Burmeister, C. N. Brooks, and J. G. Tew. 2001. Phosphorylcholine-dependent cross-reactivity between dental plaque bacteria and oxidized low-density lipoproteins. Infect. Immun. 69:6612-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schenkein, H. A., J. C. Gunsolley, A. M. Best, M. T. Harrison, C.-L. Hahn, J. Wu, and J. G. Tew. 1999. Antiphosphorylcholine antibody levels are elevated in humans with periodontal diseases. Infect. Immun. 67:4814-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott-Zaki, P., D. Purkall, and S. Ruddy. 2000. Effect of allotype on activation of neutrophils by FcgammaRIIIB cross-linking. Cell. Immunol. 200:8-15. [DOI] [PubMed] [Google Scholar]
- 17.Scott-Zaki, P., D. Purkall, and S. Ruddy. 2000. Neutrophil chemotaxis and superoxide production are induced by cross-linking FcgammaRII receptors. Cell. Immunol. 201:89-93. [DOI] [PubMed] [Google Scholar]
- 18.Tomasz, A. 1967. Choline in the cell wall of a bacterium: novel type of polymer-linked choline in Pneumococcus. Science 157:694-697. [DOI] [PubMed] [Google Scholar]