Abstract
Unlike all plant inward-rectifying potassium channels, the carrot channel KDC1 has two histidine pairs (H161,H162) in the S3–S4 and (H224,H225) in the S5–S6 linkers. When coinjected with KAT1 in Xenopus oocytes, KDC1 participates in the formation of heteromultimeric KDC1:KAT1 channels and the ionic current is potentiated by extracellular Zn2+. To investigate the potential interactions between KDC1 and zinc, a KDC1-KAT1 dimer was constructed. The dimeric and heteromeric channels displayed similar characteristics and the same sensitivity to zinc and other metals; this result suggests that zinc binding is mediated by residues in a single channel subunit. The KDC1:KAT1 currents were also potentiated by external Pb2+ and Cd2+ and inhibited by Ni2+. To investigate further the role of KDC1-histidines, these amino acids were mutated into alanines. The single mutations H225A, H161A, and H162A did not affect the response of the heteromeric channels to zinc. Conversely, the single mutant H224A and the double mutants (H224A,H225A) and (H161A,H162A) abolished zinc potentiation, but not that induced by Pb2+ or Cd2+. These results suggest that Zn2+ potentiation cannot be ascribed to simple electrostatic interactions between zinc and channel residues and that histidine 224 is crucial for zinc but not for lead potentiation of the current.
INTRODUCTION
Metal ions have a significant impact on nutrient assumption, growth rate, and biomass production of plants growing in highly mineralized, acidic, or metal-contaminated soils. In soils, the concentrations of heavy metals, such as zinc or lead, may range in the order of hundreds ppm (Brady, 1984), but in contaminated soils the concentrations of the same metals are significantly higher. In these conditions, for example, tolerant plants may accumulate millimolar concentrations of bound zinc in the cytoplasm and several mmoles of soluble zinc in the vacuoles (Marschner, 1995). Metal assumption and redistribution in the different tissues of harvestable and edible plants may have important consequences on human health (Patterson et al., 1998; Xintaras, 1998). For example, many metals, like cadmium, lead, and mercury are toxic to humans and are mutagenic and carcinogenic. They may affect different organs such as the kidney, liver, and the hematopoietic system. The intellectual development of children can be highly compromised by the presence of high concentrations of metals such as lead (Johnson, 1998).
On the other hand, the improvement of plant performances by modification of specific molecular transport mechanisms may be of primary importance for the success of phytoremediation, an emerging environmentally friendly technique which uses metal-tolerant plants as solar-driven pumps to decontaminate soils and waters polluted by heavy metals. The relief of metal inhibition of ionic channels involved in mineral nutrition might be part of a winning strategy to select plants that are tolerant to heavy metals.
Heavy metals can also be used as tools to investigate the biophysical properties of ion channels. Indeed, interactions of metals with specific amino acids, their interactions with ions permeating within the pore, as well as metal competition with physiological divalent ions (such as Ca2+ and Mg2+) may provide useful information regarding the structure of the pore as well as on channel modulation by chemicophysical parameters and physiological compounds.
These reasons suggest it is useful to investigate the basic biophysical mechanisms affecting nutrient absorption and redistribution in plants, verifying whether metals affect the properties of classical permeation pathways.
We previously characterized (Paganetto et al., 2001) the biophysical properties of KDCl, a potassium channel cloned from carrot roots (K+ Daucus carota 1) (Downey et al., 2000). This clone does not express functional channels when injected alone in Xenopus laevis oocytes but expresses functional channels when coinjected in oocytes (Paganetto et al., 2001) together with KAT1 (potassium Arabidopsis thaliana 1) (Schachtman et al., 1992). KDC1 participates in the formation of channels that show peculiar properties in response to heavy metals: the current mediated by the KDC1:KAT1 channel was not inhibited (as occurs in other plant channels investigated so far) (Hoth and Hedrich, 1999; Paganetto et al., 2001) but, on the contrary, it was increased with the addition of Zn2+ to the bath solution.
On the basis of these considerations, we decided to investigate further the molecular mechanisms responsible for KDC1 modulation by zinc and other heavy metals. As potential binding sites responsible for KDCl response to metals, here we investigate in detail the role played by the two-histidines pairs (Fig. 1 A), located in the S3–S4 and S5–S6 linkers of the proteins.
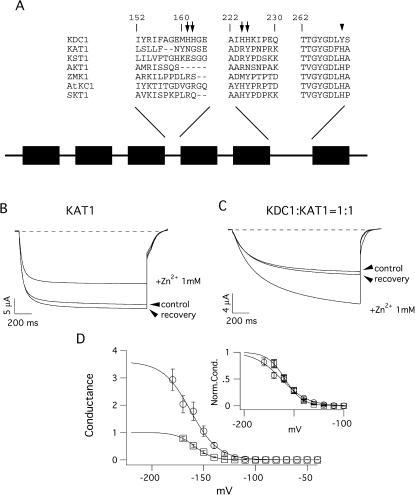
FIGURE 1.
KDC1 and KAT1 schematic structure and effects of zinc on the heteromeric KDC1:KAT1 channel. (A) Segment sequences comprising KDC1 histidines compared with the correspondent segments in other plant inward-rectifying potassium channels. All channels belong to the Shaker family and therefore comprise six hydrophobic transmembrane segments. The S4 segment is the voltage sensor motif and the putative pore region is located between the S5 and S6 segments. The locations of the KDC1 histidines are indicated by arrows in the segments comprised between S3 and S4 and S5 and S6. Instead KAT1 has only one histidine residue in the pore region (H267), whereas in the correspondent position KDC1 has a tyrosine (Y269), indicated by the arrowhead. (B) The addition of 1 mM extracellular Zn2+ typically inhibited up to 30–40% of the KAT1 current. (C) Conversely, with the addition of 1 mM Zn2+, the heteromeric KDC1:KAT1 channels displayed a marked increase in the current. Applied transmembrane potentials to −150 mV. (D) Increase of the macroscopic conductance in the KDC1:KAT1 channel after addition of 5 mM zinc (unfilled circles) compared with the conductance of the same channel in the absence of zinc (unfilled squares, control). (Inset) Comparison of the normalized conductances obtained in the presence and absence of zinc. The symbols have the same meaning as in the panel. Each data point was obtained from three to eight experiments. Standard ionic solution, pH 5.6.
MATERIALS AND METHODS
Molecular biology
Kdc1 was amplified from carrot root cDNA using an RT-PCR strategy (Downey et al., 2000). The coding sequence was cloned as a blunt-ended (sense orientation) fragment into the SmaI site of vector pGEMHE (Liman et al., 1992). To provide a linear template for in vitro transcription pGEMHE-KDC1 was digested with SphI which cuts after the 3′ UTR of the Xenopus β-globin gene. Linearized DNA was purified and dissolved in RNAase free water. The mCAP mRNA Capping Kit (Stratagene, La Jolla, CA) was used to synthesize sense-strand RNA. The RNA was quantified by optical density at 260 nm and visualized on an agarose gel. Mutant KDC1 and KAT1 were obtained using the Quikchange Site-Directed Mutagenesis Kit (Stratagene). All mutants were sequenced to confirm the mutation. In vitro transcription of wild-type and mutant constructs was performed using the mCAP-RNA Capping Kit. A dimeric cDNA was constructed by eliminating the stop codon of KDC1 and linking the 3′ end of the coding region of KDC1 with the 5′ end of the coding region of KAT1; in this way the carboxyterminus of KDC1 is linked to the aminoterminus of KAT1; only two extra amino acids, Ser and Lys, are left between the two channels. The dimeric construct was subcloned in pGemHe vector.
Injection of RNA in oocytes
Xenopus laevis oocytes were isolated (Hedrich et al., 1995) and injected with KDC1 and KAT1 mRNA (0.5 μg/μl) using a Drummond Nanoject microinjector (25–50-nl/oocyte; Drummond Scientific, Broomall, PA). RNA concentration was quantified spectroscopically. All the experiments shown were performed using a single batch of the two RNAs (KDC1 and KAT1); however, similar results were also obtained using other KDC1 samples. Current recordings were made 2–6 days after injection. KDC1 and KAT1 (or KAT1 mutants) were simultaneously injected in the weight ratio of 1:1. We verified that oocytes, injected with KAT1 and a volume of distilled water identical to that used to coinject KDC1, did not show changes of the KAT1 channel properties (Véry et al., 1994).
Voltage-clamp recordings
Whole cell K+ currents were measured with a two-microelectrode homemade voltage-clamp amplifier (designed by F. Conti), using 0.2−0.4 Mohm electrodes filled with 3 M KCl. The following bath standard solution was used (in mM): 100 KCl, 2 MgCl2, 1 CaCl2, 10 MES/TRIS, pH 5.6, or HEPES for pH 7.6 for the experiments on different pH. Unless otherwise indicated all the experiments were performed at pH 5.6. Various concentrations of different metals were added as metal-chloride salts. Currents were typically filtered with a cutoff frequency of 1 kHz before acquisition. Unless otherwise indicated each data point represents the mean ± SE obtained from at least three different experiments. The relative open probability was obtained dividing the steady-state currents by (V–Vrev), normalized to the saturation value of the calculated Boltzmann distribution.
RESULTS
KDC1 coinjected with KAT1 in Xenopus oocytes forms heteromultimeric KDC1:KAT1 inward-rectifying channels which activate at more negative potentials and display a much slower kinetic of activation with respect to KAT1 (Paganetto et al., 2001). We have already demonstrated that, compared to KAT1, coexpressed channels also display a peculiar tolerance to zinc, as illustrated in Fig. 1, B and C, where the effects induced by 1 mM zinc on KAT1 and KDC1:KAT1 currents are shown. It can be observed that whereas the KAT1 currents are reduced by 30–40% on adding zinc to the bath solution (Fig. 1 B), the KDC1:KAT1 currents are markedly potentiated by zinc (Fig. 1 C) (Paganetto et al., 2001). Fig. l D exemplifies the substantial increase induced by 5 mM Zn2+ in the conductance of the KDC1:KAT1 channel (unfilled circles), compared to the same channel in control conditions (i.e., in the absence of zinc, unfilled squares). The inset shows a comparison of the two conductances normalized to their maximum values. We have already proposed that this peculiar property, ascribed to the presence of KDC1 in the KDC1:KAT1 complexes (Paganetto et al., 2001), might depend on the four histidine residues present in each KDC1 subunit (Downey et al., 2000). Indeed, KDC1 has two pairs of histidines in the linkers connecting α-helices S3–S4 (H161,H162) and S5–S6 (H224,H225) (Fig. 1 A). Moreover, in the KDC1 pore, a tyrosine (Y269) residue substitutes the histidine characterizing all other plant inward-rectifying potassium channels (for a review, see Czempinski et al., 1999).
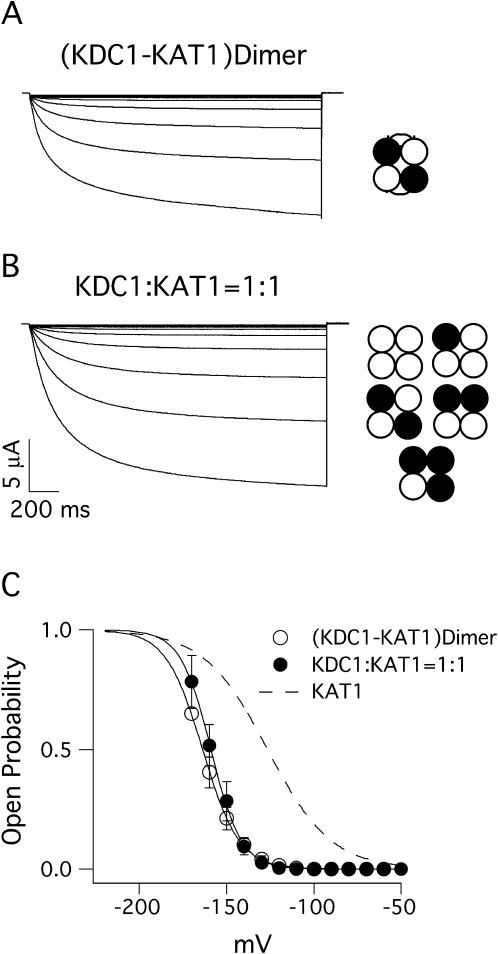
Before investigating the role of these histidines in zinc potentiation, we verified the participation of KDC1 to heteromeric channels. The coinjection of mRNA expressing different potassium channels may result in the formation of heteromeric channels comprising a combination of a variable number of subunits which are presumably arranged according to different structural combinations and symmetries (Naranjo, 1997). To evaluate both the effects of this variability and the interactions of KDC1-histidines belonging to different KDC1 subunits, we constructed a mRNA tandem that expresses a KDC1 subunit covalently linked to a KAT1 subunit. When injected in oocytes this construct expresses a functional channel which in the following is indicated with the term (KDC1-KAT1) heterodimer or simply dimer. It is reasonable to assume that this construct leads to the expression of a single, uniform population of channels, each one containing two KDC1 subunits alternating with two KAT1 subunits, arranged as schematized in Fig. 2 A (right) (for detail and description on dimer arrangement see Experimental Procedures in Heginbotham and MacKinnon, 1992). The currents mediated by the dimeric channel can be compared with those recorded when oocytes were injected with KDC1 and KAT1 at a 1:1 weight ratio (Fig. 2 B). It can be observed that the two channels display very similar Boltzmann characteristics (Fig. 2 C). This confirms that when KDC1 and KAT1 are coinjected at a 1:1 weight ratio the majority of heteromers presumably comprise two KDC1 and two KAT1 subunits. Besides showing the same characteristics in control conditions, the dimeric channels also displayed a sensitivity to Zn2+ comparable to that exhibited by the coinjected channels. Fig. 3 A shows the typical current recorded in oocytes expressing the (KDC1-KAT1) dimer with the addition of two extracellular concentrations of zinc (0.1 mM and 1 mM) in response to a hyperpolarizing pulse to −150 mV from a holding potential of 0 mV: a reversible zinc potentiation comparable to that already recorded on KDC1:KAT1 channels can be observed. The dose-response curves for both channels are shown in Fig. 3 B. The ratio between the steady state of the current in the presence and in the absence of Zn2+ (IZn/IControl) and the open probabilities of the two types of channels in the presence of 1 mM Zn2+ did not show appreciable differences (Fig. 3 C). As the heteromeric and dimeric channels display similar properties in all the experiments performed, for the sake of simplicity in the following we refer to coexpressed channels, comparing occasionally these results with those performed on the dimer adopted as a control system which guarantees the presence of two KDC1 subunits per channel.
FIGURE 2.
Functional expression of KDC1-KAT1 heterodimers and KDC1:KAT1 coexpressed channels. Ionic currents elicited by voltage steps from −50 to −170 mV in −10-mV increments from an holding potential of 0 mV in oocytes injected with (A) KDC1-KAT1 dimers and (B) coinjected with KDC1 and KAT1 at 1:1 weight ratio. In the model representation, each subunit is represented by a circle and the linker domain is represented by an arc. Dark subunits represent KDC1; white subunits represent KAT1. In B, the structure comprising four KDC1 subunits is omitted as this channel either is not expressed or does not carry potassium currents. We assume that in both cases (heteromeric or dimeric complexes) the channel comprises four subunits. (C) Typical open-probability characteristics of the dimer and coinjected channels. Data represent mean ± SE of the normalized steady state obtained from families of ionic currents like those in A and B plotted as a function of the applied potential. Continuous curves are the best fit of the open probability. They gave the following values for the half-activation potentials, V1/2 and the apparent gating valence, z (n = number of experiments): (KDC1-KAT1) dimer (V1/2 = −163 mV, z = 2.4, n = 3), KDC1:KAT1 channel (V1/2 = −159 mV, z = 2.9, n = 5). The dashed line represents the open probability of KAT1 channel. Standard ionic solution, pH 5.6.
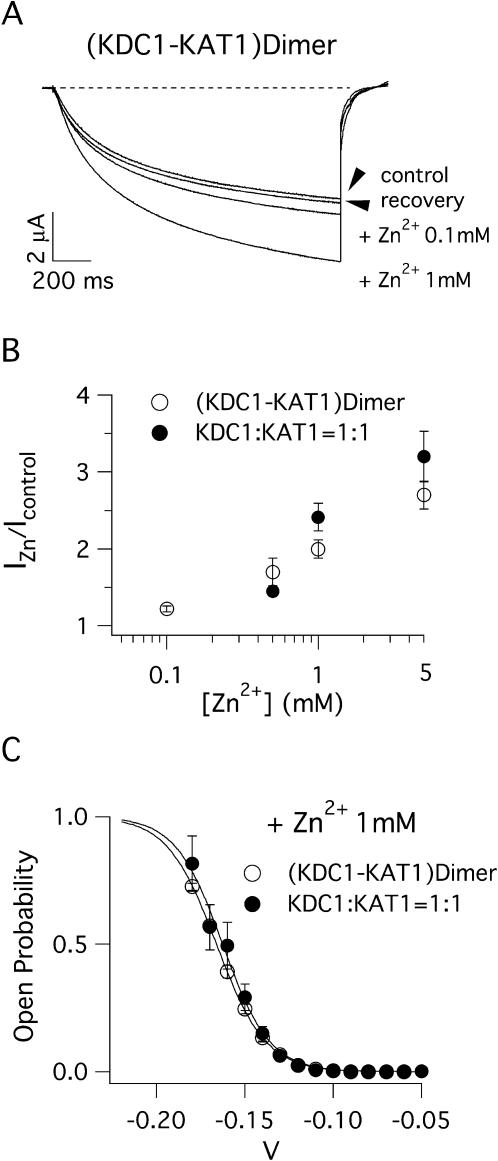
FIGURE 3.
KDC1-KAT1 heterodimeric channels display the same zinc sensitivity as KDC1:KAT1 heteromultimeric channel. (A) Typical ionic currents recorded in oocytes before (control) and after (recovery) the application of ZnCl2 to the bath solution. Like in KDC1:KAT1 channels current was enhanced (∼1.6-fold) in the presence of 1 mM of Zn2+. Applied transmembrane potentials to −150 mV. (B) Zn2+ dose-response curves for the dimeric (KDC1-KAT1) and heteromeric (KDC1:KAT1) channels. Data were obtained from at least three different experiments. IZn/Icontrol represents the steady state of the ionic current recorded in the presence of Zn2+ with respect to the control (i.e., Zn2+ = 0). (C) Comparison of the open probability in presence of 1 mM Zn2+. Continuous lines are the best fit of the open probability and gave the following values: KDC1-KAT1 (V1/2 = −166 mV, z = 1.8, n = 4) and KDC1:KAT1 (V1/2 = −163 mV, z = 1.9, n = 4). Standard ionic solution, pH 5.6.
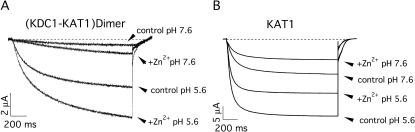
As histidines play a central role in the pH-sensing mechanisms of plant potassium channels (Hoth et al., 1997; Hoth and Hedrich, 1999), taking into account the peculiar histidine composition of KDC1, we also investigated the pH dependence of KDC1:KAT1 channels. Fig. 4 shows the dimeric (Fig. 4 A) and KAT1 (Fig. 4 B) currents recorded at V = −150 mV at pH 5.6 and 7.6. The dimeric channel was still potentiated by acidic pH, and KDC1 increases fourfold the pH-dependent potentiation with respect to KAT1 (Fig. 4 and Table 1). Moreover Zn2+ effect on current is independent of the pH for the KAT1 but not for the dimer.
FIGURE 4.
The pH and zinc modulation of the KDC1-KAT1 current. (A) Acidic pHs determine a potentiation of the current of the KDC1-KAT1 channels. Compare this pH potentiation of the current with that induced on KAT1 by the same pH variation (B). At neutral pH, zinc potentiation of the dimeric current is even greater with respect to pH 5.6. On the contrary, the inhibition of the KAT1 current in the presence of zinc is not affected by the pH (see Table 1). Standard ionic solution.
TABLE 1.
Current ratios describing the potentiation or inhibition of the channel at different pHs (5.6 and 7.6) and in the presence/absence of Zn2+ at the two different pHs in oocytes injected with either the KDC1-KAT1 tandem construct or with KAT1
| Current ratio | (KDC1-KAT1) Dimer (N = 4) | KAT1 (N = 4) |
|---|---|---|
| IpH5.6/IpH7.6 | 10.5 ± 0.6 | 2.7 ± 0.4 |
| (IZn/Icontrol) pH 5.6 | 1.7 ± 0.4 | 0.74 ± 0.08 |
| (IZn/Icontrol) pH 7.6 | 3.6 ± 1.3 | 0.74 ± 0.14 |
Histidines are prominent in the zinc-binding domains of many enzymes (Choi and Lipton, 1999; Christianson, 1991; Vallee and Falchuck, 1993), so to verify whether the KDC1 histidines comprised in the S3–S4 and the S5–S6 linkers play a major role in Zn2+-current potentiation, we mutated each of them into an alanine and injected in oocytes the mutated dimer or coinjected the mutated KDC1 cRNA together with KAT1. Substitutions of single and double histidines gave rise to both heterodimeric and heteromultimeric functionally mutated channels. Conversely the simultaneous quadruple mutation of all the histidines (H161, H162, H224, and H225) did not produce functional channels.
Fig. 5 shows the macroscopic currents measured in the KDC1:KAT1 channels when the histidines in the S5–S6 linker, facing the extraplasmatic side of KDC1 channel, were mutated into alanines. Both the single mutations H224A (Fig. 5 A) or H225A (Fig. 5 B) as well as the double mutation (H224A,H225A) (Fig. 5 C) gave rise to the formation of functional channels that displayed the typical basic properties conferred to heteromeric channels by KDC1: i.e., like the wild-type KDC1:KAT1, the mutated channels also displayed a threshold of activation shifted toward more negative membrane potentials (for example, Fig. 5 G illustrates the shift determined by H224A) and slow activation times which appeared to be dependent on the mutation (Figs. 5 and 6). Interestingly, the activation time constants of mutated channels were always slower than the times of homomeric KAT1 channels in similar conditions and in one case (H225A:KAT1) even slower than the wild-type (compare the kinetics of traces in Fig. 5 B with those in Fig. 1, B and C). These differences provide further support to the hypothesis that these currents are not due to a loss of function of the channel comprising one or more mutant subunits but to functional channels comprising mutated KDC1.
FIGURE 5.
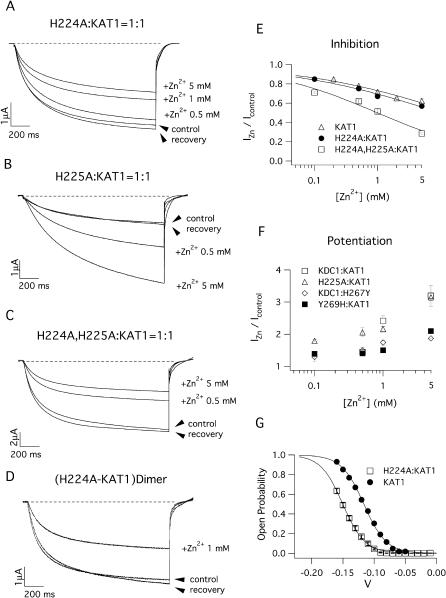
Sensitivity to external Zn2+ of heteromeric channels comprising KDC1 mutated in the S5–S6 histidines. Ionic currents recorded in the presence of Zn2+ in the bath solution from oocytes injected with mRNA mixtures containing (A) H224A:KAT1, (B) H225A:KAT1, and (C) (H224A,H225A):KAT1. Holding, step, and tail potentials were 0 mV, −150 mV, and −70 mV, respectively. (D) 1 mM Zn2+ reversibly inhibits the current mediated by the dimeric mutated channel H224A-KAT1. Compare these effects with those induced on the heteromultimeric channel shown in A. (E) Dose-response inhibition for KAT1 and mutated KDC1 (H224A and H224A,H225A): KAT1 heteromeric channels. Continuous lines are the best fit of the equation IZn/Icont = Kin/(Kin + xn) where Ki is the zinc concentration that inhibits one-half of the maximal current and n is the Hill coefficient. We found Ki = 15 mM for KAT1 (n = 0.4), Ki = 9.7 mM for H224A:KAT1 (n = 0.4), and Ki = 0.98 mM for H224A,H225A:KAT1 (n = 0.5). (F) Dose-response curves of current potentiation for KDC1 and mutated KDC1 (H225A and Y269H) as well as mutated KAT1 (H267Y). Data represent mean ± SE from at least three experiments. Applied transmembrane potentials to −150 mV. (G) Comparison of the normalized conductances of KAT1 and H224A:KAT1 heteromultimeric channels; the significant shift of the opening probability induced by KDC1 on KAT1 characteristics can be observed. Standard ionic solution, pH 5.6.
FIGURE 6.
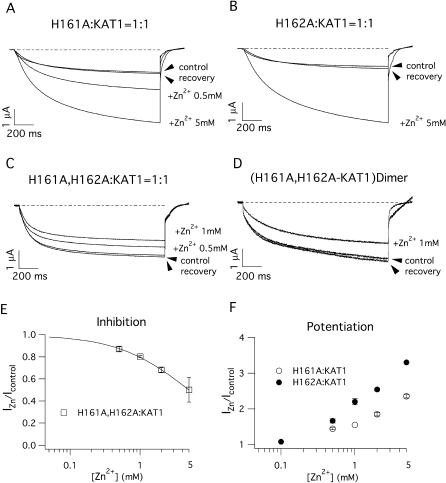
Sensitivity to external Zn2+ of heteromeric channels comprising KDC1 mutated in the S3–S4 histidines. Typical ionic currents recorded in oocytes coinjected with (A) H161A and KAT1 and (B) H162A and KAT1. In both cases, the addition of Zn2+ resulted in an increase in the ionic current. (C) On the contrary, the current evoked by the KDC1 double mutant H161A,H162A coinjected with KAT1 is slightly blocked by the addition of Zn2+. (D) Inhibition of the current induced by Zn2+ on the heterodimeric double mutant H161A,H162A-KAT1. (E) Magnitude of the inhibition and (F) potentiation as a function of Zn2+ concentration. Steady-state current in the presence of Zn2+ (IZn) was normalized with respect to the control (Icontrol). In E the continuous line represents the best fit of the Hill equation with Ki = 4.98 mM and n = 0.8. Data represent mean ± SE from at least three experiments. Other conditions as in Fig. 5. Standard ionic solution, pH 5.6.
The mutant channels responded in different ways to Zn2+: the current potentiation, found in the wild-type KDC1:KAT1 channel, was abolished in the single H224A:KAT1 mutant (Fig. 5 A) and in the double mutant (H224A,H225A):KAT1 (Fig. 5 C). In both cases the current markedly decreased upon addition of Zn2+, like in KAT1 channels, as summarized in Figs. 5 E and 7 B. Indeed, the inhibition of the current in presence of 5 mM of Zn2+ (which was 62% of the control for KAT1 and 57% for H224A:KAT1 channels) increased to 28% of the control in the double mutant (H224A,H225A):KAT1. Notably the effects induced by Zn2+ on the H224A-KAT1 dimer (Fig. 5 D) are fully comparable to those induced by this metal on the correspondent coexpressed channel (Fig. 5 A).
FIGURE 7.
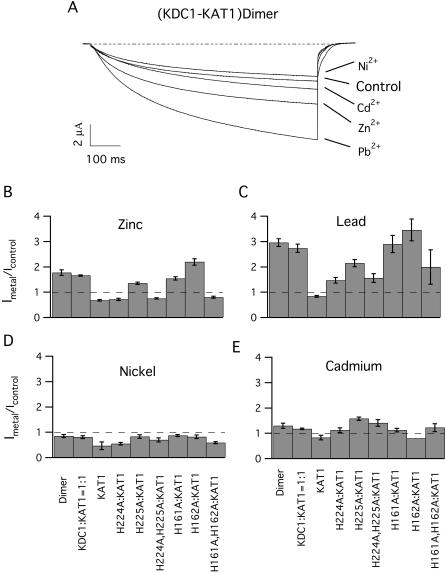
Sensitivity of heteromultimeric and heterodimeric channels to different metal ions. Current traces obtained in control conditions and in the presence of various metals (at 1-mM concentration) in oocytes injected with the (KDC1-KAT1) dimer (A). Currents were elicited by voltage steps to −150 mV; other conditions as in Fig. 3. It can be observed that all metal ions, with the exception of Ni2+, determined a differentiated potentiation of the current. External Pb2+ typically increases the current up to 3×. (B–E) Normalized current potentiation or inhibition, (Imetal/Icontrol), elicited by 1 mM Zn2+, Pb2+, Ni2+, and Cd2+, respectively. Oocytes were injected with KAT1, the dimeric channel, coinjected with KAT1, and either wild-type or mutated KDC1 (at 1:1 weight ratio). A comparison of the effects induced by different metals shows that not only zinc but also lead (C) gives rise to a consistent potentiation of the current. Interestingly, the single mutation H224A abolished the potentiation of the current elicited by Zn2+ (B), but determined only a partial decrease of Pb2+-potentiation (C). On the other side, mutation H225A had little effect on zinc potentiation (B) or Ni2+-inhibition (D). Similarly, the two single mutations H161A and H162A had only slight effects on current potentiation mediated by Zn2+ (B), Pb2+ (C), and Cd2+ (E) and on current inhibition induced by Ni2+ (D). The double mutations (H161A,H162A) and (H224A,H225A) completely abolished the potentiation mediated by zinc although a residual current could still be measured with the addition of Pb2+ (C) and Cd2+ (E) in the double mutant H224A,H225A. Error bars represent the standard deviations. Data were obtained from 3 to 18 experiments. Standard ionic solution, pH 5.6.
Conversely, the single mutation H225A still displayed a reversible Zn2+-dependent current increase (Fig. 5 B) similar to that found for the wild-type KDC1:KAT1 channels (Fig. 5 F). This figure also shows that mutations introduced in the pore of either KAT1 (H267Y, i.e., histidine 267 was mutated into a tyrosine) or KDC1 (Y269H, i.e., tyrosine 269 was mutated into a histidine) do not significantly affect the current potentiation. Therefore the KAT1 histidine located in the pore does not play any role in the Zn2+-mediated current increase.
Mutations in the histidines in the S3–S4 linker were less effective than those in the S5–S6 linker. In fact, mutation H161A did not induce significant modifications in zinc modulation (Fig. 6 A). Like H161A, the mutation H162A also did not abolish the Zn2+-potentiation of the current (Fig. 6 B). Current potentiation in this case was similar to that observed in the wild-type channel (compare the potentiations in Figs. 5 F and 6 F) and larger than that observed in the H161A mutant (Fig. 6 F). It was necessary to mutate both the histidines in the S3–S4 linker (H161A,H162A) to abolish current potentiation, and to obtain a current inhibition (Fig. 6 C); this inhibition reached almost 50% of the control current when Zn2+ was raised to 5 mM (Fig. 6 E). Note that the kinetics of current activation as well as the zinc effects are entirely comparable in the double mutant dimeric and heteromultimeric channels (Fig. 6, C and D).
Finally, we investigated the effects induced by other heavy metals on both wild-type and mutated channels. Fig. 7 A shows the effects induced on (KDC1-KAT1) dimeric currents by 1 mM Zn2+, Pb2+, Cd2+, and Ni2+. Like for Zn2+ (Fig. 5, E and F, and Fig. 6, E and F), the other metals also determine effects which are dependent on metal concentrations (not shown).
It is important to note that, also in this case, the heteromeric and the dimeric channels displayed similar behavior with the addition of all metals (Fig. 7, B–E): namely Zn2+ (Fig. 7 B) and Pb2+ (Fig. 7 C) induced a significant potentiation of the current, whereas Cd2+ (Fig. 7 E) only induced a small increase. On the other hand, Ni2+ (Fig. 7 D) determined a slight decrease of the current which was smaller than the significant inhibition induced by this metal on the homomeric KAT1 channel.
It can be observed that although the single mutation H224A completely abolishes the Zn2+-potentiation of the current, it does not completely remove Pb2+- and Cd2+-potentiation (Fig. 7, C and E), and the Ni2+-inhibition of the current mediated by this mutant is similar to that observed in homomeric KAT1 channels. Also, the double mutations (H161A,H162A) and (H224A,H225A) that abolished the potentiation mediated by Zn2+ did not completely abolish the current potentiation elicited by Pb2+. Instead, these two double mutations completely eliminated the relief of current inhibition introduced by KDC1 in the presence of Ni2+.
As in the case of Zn2+, mutation H225A had minor consequences both on Pb2+- and Cd2+-potentiation and on the inhibition of the current after the addition of Ni2+. Similarly, the two single mutations H161A and H162A had only small consequences on current potentiation mediated by Pb2+ as well as on the current inhibition induced by Ni2+. Surprisingly, H162A, but not H161A, significantly reduced the slight potentiation induced by Cd2+.
As it is well known that DEPC (diethylpyrocarbonate), a histidine-specific reagent, irreversibly binds to imidazole rings of histidines (Chakrabarti, 1990; De Biasi et al., 1993) preventing zinc-binding and protonation, we verified (not shown) that perfusing Xenopus oocytes with 1 mM DEPC determined a rapid and irreversible decrease of the heteromultimeric current that decreased in a few seconds to almost 20% of its initial value. However, DEPC also determined a slower and irreversible decrease of the homomeric KAT1 currents. Interestingly, whereas the residual KAT1 current was further inhibited by the addition of 1 mM zinc, the residual KDC1:KAT1 current was unaffected by zinc. This suggests that DEPC irreversibly binds to residues, like external histidines, responsible for Zn2+-potentiation, therefore preventing the subsequent binding of the metal.
DISCUSSION
The coinjection of KDC1 and KAT1 in Xenopus oocytes gives rise to the formation of heteromeric potassium channels, comprising KDC1 and KAT1 subunits. Compared to KAT1, KDC1:KAT1 channel displays a slower kinetic of current activation, a threshold of activation shifted toward more negative membrane potentials, and a peculiar potentiation of the current after the addition of zinc to the extracellular solution.
In this article we try to answer two important questions: how KDC1 combines with KAT1 to form a tetrameric structure, and whether the different histidine composition of KDC1 with respect to other plant inward-rectifying channels plays an important role in conferring zinc tolerance to the coexpressed channel. Indeed, although KDC1 has two couples of histidines in the S3–S4 and S5–S6 linkers, it lacks the well-conserved histidine (replaced by a tyrosine, Y269) present in the permeation pore of all other plant inward-rectifying potassium channels.
Dimeric construct
The formation of heteromeric channels and the important role played by these two histidine pairs was investigated and supported by the robust current expression observed in oocytes injected with the dimeric KDC1-KAT1 tandem construct which displayed characteristics almost identical to those of the coinjected KDC1:KAT1 channels (Fig. 2 C) as well as the same sensitivity to metal ions (Fig. 7). It is reasonable to assume that potassium channels formed by the KDC1-KAT1 tandem are based on alternating KDC1 and KAT1 subunits (Heginbotham and MacKinnon, 1992). Under this hypothesis, in these channels each KDC1 subunit interacts with two KAT1 subunits; this suggests that the interaction of histidines belonging to two different KDC1 subunits is not needed to mediate the interaction with zinc and the subsequent current potentiation.
pH dependence
In accordance with the pH dependence of monomeric KAT1 and KDC1 expressed in cultured cells (Downey et al., 2000), we found that lowering the extracellular pH from 7.6 to 5.6 induced a 10-fold increase in the current. Interestingly, at neutral pH, zinc potentiation of the current was even larger than at acidic pH (threefold with respect to the 1.8-fold at pH 5.6, see Table 1). The fact that the pKa of histidine in proteins ranges from 5.0 to 8.0 (with respect to a pKa = 6.0 of free histidines) (Clyne et al., 2002) supports the histidines as obvious candidates for proton-binding sites involved in the pH-dependent potentiation of the current (Hoth et al., 1997, Hoth and Hedrich, 1999). Moreover, the lower Zn-potentiation of the dimeric current observed at acidic pH suggests that protons and zinc possibly act at the same binding site. The different potentiation of the current with acidification (∼threefold for KAT1 channel instead of the 10-fold for dimeric channel) could possibly be ascribed not only to the different composition of external histidines in the two channels but also to some differences in the pore region, which were shown to be crucial for H+ binding (Hoth et al., 2001). Future experiments on mutant dimeric channels will help to identify the amino acids important in this process. Similar to what was observed in KAT1 and KST1, the current of heteromeric channels that contain KDC1 increases with the external pH, suggesting that parts of the pore segments crucial for pH modulation are conserved in these three channels (Hoth et al., 2001).
Zn2+-potentiation
It has been demonstrated that histidine residues are also responsible for Zn2+-modulation of the current amplitude (either inhibition or potentiation) in several ion channels (Choi and Lipton, 1999; Clyne et al., 2002; Hoth et al., 1997; Wang et al., 1995). Specifically, the inhibition of the current induced by zinc in other plant inward-rectifying channels has already been ascribed to zinc-binding to histidine present in the permeation pore, like H271 in KST1 (Hoth and Hedrich, 1999) or H267 in KAT1 (Paganetto et al., 2001).
Therefore, we hypothesize that the zinc potentiation of our KDC1:KAT1 channel might depend on two concurrent mechanisms: namely, 1), a relief of current inhibition owing to the absence of the histidine in the pore region of KDC1, and 2), an increase of the current due to zinc-binding to the histidine couples accessible at the extracellular side at the S3–S4 and the S5–S6 linkers. Indeed, it has been demonstrated that bidentate binding sites, comprising from two to six residues, represent the major protein binding sites for Zn2+. These binding sites frequently involve histidine and/or other residues like cysteine, aspartate, or glutamate (Choi and Lipton, 1999; Vallee and Falchuck, 1993). Interestingly, two glutamates (E159 and E164) symmetrically flank histidines H161–H162 in the S3–S4 linker and another glutamate, E229, is present near histidines H224–H225 at the S5–S6 linker.
Investigating the role played by the two pairs of histidines in zinc potentiation, we found that histidine 224 plays a crucial role in the interaction between zinc and the channel as the substitution of H224 with an alanine was sufficient to abolish the potentiation of the current elicited by Zn2+. Interestingly, the single mutation H225A only slightly affected zinc potentiation of the current. The simultaneous mutation of the two histidines H224 and H225 reinforced the effects induced by the single H224A mutation as the double mutant displayed a more pronounced inhibition of the current with the addition of Zn2+. The deduced Hill coefficients did not change appreciably in the two Zn2+ inhibition curves, being respectively 0.4 and 0.5 for the single H224A and double (H224A,H225A) mutant. This suggests that both in the single and double mutants the binding of only one Zn2+ ion is sufficient to inhibit the current. This binding site, responsible for current inhibition, could be identified with the histidine conferred to the inner pore by each KAT1 subunit participating in channel formation. Accordingly, when the histidine residues were changed in the pore region of the KDC1:KAT1 channels, replacing the KDC1 tyrosine at position 269 with a histidine (Y269H), the current was still enhanced by zinc (Fig. 5 F); this indicates that the KAT1-histidine in the pore region is not involved in the current potentiation induced by zinc. On the contrary, as already proposed (Hoth and Hedrich, 1999; Paganetto et al., 2001), our results are compatible with the hypothesis that the pore histidines are responsible for the inhibition of the current mediated by zinc when the groups responsible for current potentiation are mutated into alanines and potentiation is abolished.
In the histidine pair at the S3–S4 linker, whereas the single mutations H161A and H162A did not significantly affect Zn2+-potentiation of the current, the simultaneous mutation of both histidines (H161A, H162A) determined effects comparable to those induced by H224A, i.e., this double mutation also completely abolished the Zn2+-potentiation of the current. Zinc-binding to H161 and H162 is also compatible with the structure recently proposed for potassium voltage-dependent channels (Jiang et al., 2003), as, according to this model, in the open state the S3–S4 linker is exposed to the external solution and therefore can interact with molecules present in the extracellular solution. However, to verify a state-dependence of zinc-binding is beyond the objectives of this work.
Zinc “tolerance” conferred by KDC1 to the heteromeric channel cannot be simply emulated by introducing similar mutations in KAT1, as the introduction of two histidines in the KAT1 S5–S6 linker, at positions corresponding to H224 and H225 in KDC1, did not give rise to the expression of functional channels in Xenopus oocytes (not shown). Indeed, the different effects induced by the mutations introduced in KDC1 suggest that Zn2+-potentiation of the current is a finely tuned mechanism where zinc interacts with both histidine pairs and which presumably depends on the structure and organization of the entire subunit or channel. We can also speculate that possibly in the H161–H162 a double mutation is needed to abolish Zn2+-potentiation as the two glutamates possibly support the interaction of the histidine cleft. The second pair being supported by only one glutamate is more sensitive to single histidine mutations like H224A.
Other metals
To investigate whether current potentiation is a property that can be observed also with the addition of other heavy metals, we compared the effects induced by Ni2+, Pb2+, and Cd2+ with those induced by Zn2+ on wild-type and mutated channels. All these metals decreased the current mediated by the KAT1 channel and affected at different levels the currents in oocytes coinjected with the wild-type KDC1 and KAT1.
Surprisingly, whereas Cd2+ induced only a small potentiation of the KDC1:KAT1 current, lead induced a current potentiation which resulted to be even greater than that observed with the addition of Zn2+. Contrary to Zn2+, Pb2+-potentiation of the current was only partially abolished by the mutations introduced in the two histidine pairs, whereas Cd2+ induced a modest current potentiation, which was not particularly sensitive to the mutations introduced in the four histidines, suggesting the importance of other amino acids as binding sites of these two metals.
The behavior of channels in the presence of Ni2+ is particularly interesting. Ni2+, which also react strongly with histidines (Gordon and Zagotta, 1995; Maroney, 1999), decreased the current of both wild-type and mutant KDC1:KAT1 channels, but shows a profile (in Fig. 7 D) very similar to that of Zn2+. The presence of KDC1 resulted in a decrease of the current inhibition (Ni2+-relief) which was abolished by the same mutations which removed zinc potentiation. Indeed, in the presence of nickel, the inhibition of the current was maximum in KAT1 and in heteromeric channels comprising H224A, (H224A,H225A), and (H161A,H162A) mutants. This suggests that, in the case of Ni2+, between the two mechanisms already hypothesized to explain zinc effects, the machinery responsible for the current increase (ascribed to external histidines) gives a minor contribution, if any, with respect to that responsible for the relief of current inhibition (ascribed to the absence of the histidine in the pore).
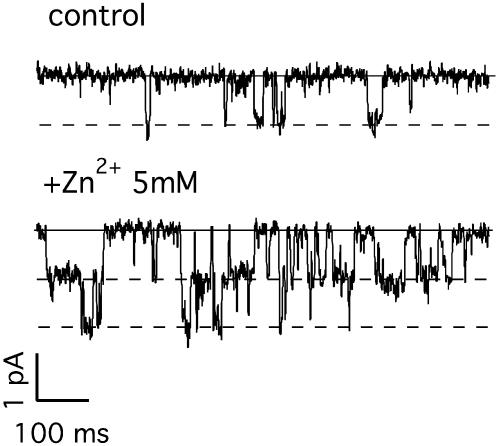
Finally, the comparison of measurements performed on different metals also confirms that current potentiation cannot be ascribed to nonspecific electrostatic interactions of zinc and other charged divalent ions with the lipid and/or protein negative charges. Actually Zn2+-potentiation of the current might be ascribed to different mechanisms such as 1), a shift of the open probability of the channel which affects either the gating charges or the channel activation threshold; 2), an increase of the single channel conductance; and 3), a recruitment of silent channels from a nonconducting pool. One can exclude the first possibility, as zinc only slightly affects the normalized G(V), as shown in the inset in Fig. 1 D. Instead, zinc determines an increase of the absolute conductance (Fig. 1 D) which depends on Zn2+ concentration. The significant increase of the macroscopic conductance (e.g., fourfold in Fig. 1 D) cannot be ascribed to a correspondent increase of the single channel conductance as demonstrated by preliminary single channel experiments performed on the KDC1-KAT1 dimer showing that the single channel conductance does not change appreciably; on the contrary the time spent by the channel(s) in the open state visibly increases in the presence of Zn2+ (Fig. 8). Therefore, the hypothesis of a recruitment of silent channels from a pool of nonconducting channels seems to be the more realistic possibility. A confirmation will possibly come from detailed single channel analysis to be performed on single channel experiments like those shown in Fig. 8.
FIGURE 8.
Effects of Zn2+ on the dimer single channel conductance. Unitary events measured at −160 mV in an outside-out patch in oocytes injected with the (KDC1-KAT1) dimer, before control (upper trace), and after the application of Zn2+ (lower trace) to the bath solution. The data were filtered at 0.5 kHz and sampled at 10 kHz. Internal solution was 100 mM KCl, 2 mM MgCl2, 11 mM EGTA, 10 mM HEPES, and 0.5 mM ATP, at pH 7.2. External standard ionic solution, pH 5.6.
CONCLUSION
In summary, our results show that KDC1 participates in the formation of heteromultimeric channels which present a peculiar tolerance to different heavy metals. We also identify two histidine pairs (H161 and H162) and (H224 and H225) that react well with zinc ions and are needed for potentiation of the current mediated by zinc present in the bath solution. Histidine H224 is the most sensitive, whereas the other three histidines appear to be less crucial, since only the simultaneous mutation of two of them modifies the interaction properties of the channel with heavy metals. As single and double mutations in both histidine pairs are able to completely abolish zinc potentiation, we argue that in some way all these histidines participate and interact in a coordinated manner (within each KDC1 subunit) in mediating zinc-binding. On the other hand, interactions between histidines belonging to distinct KDC1 subunits presumably are not important.
We have recently demonstrated that KDC1 also expresses functional channels when coinjected in oocytes with other carrot channels (Formentin et al., 2003). Preliminary results, showing that KDC1 confers on these heteromeric channels properties that are very similar to those conferred on KAT1, support the hypothesis of a functional modulatory role of KDC1 in potassium transport in vivo. Indeed, KDC1 is homologous to AtKC1, which is expressed in Arabidopsis thaliana root hairs as an integral functional K+ selective channel (Pilot et al., 2003; Ivashikina et al., 2001; Reintanz, 2002). Moreover, AtKC1 seems to participate in the formation of heteromeric channels as an α-subunit which alters some critical functions of K+ potassium channels in root hair cells (Reintanz, 2002). As it has been suggested that heteromultimeric assembly of α-subunits might provide the molecular basis for the functional diversity of potassium channels observed in vivo, one can argue that in carrots, KDC1 might play a role similar to that of AtKC1 in A. thaliana. Indeed, besides conferring its peculiar sensitivity to metal ions, KDC1 shifts the activation threshold of heteromeric inward potassium channels toward more negative membrane potentials, whereas selectivity to potassium ions remains practically unchanged (Paganetto et al., 2001). One can observe that this shift of the activation threshold is consistent with the positive shift of the threshold observed in Atkc1 knockout plants and might possibly contribute to modulating the influx of potassium ions at the root level and in other tissues where KDC1 is expressed.
It is tempting to speculate that in the future it could be possible to identify channel genes homologous to KDC1 in plants tolerant to heavy metals. In the roots of these plants potassium assumption, instead of being depleted, could possibly be increased by large concentrations of metals like zinc and nickel. Moreover, as Arabidopsis adaptation to K+ starvation and salt stress seems to induce mainly an increase in the expression of AtKC1 in leaf peripheral tissues, one could investigate whether KDC1 participation in tetrameric potassium channels contributes to raising resistance to salt stress through an increased or unaltered assumption of potassium in salty soils (Pilot et al., 2003).
Acknowledgments
We thank Alessio Accardi for his extremely helpful critical revision of the manuscript.
This work was supported by the Ministero Dell'Istruzione Universitá e Ricerca, fondi per gli Investimenti Della Ricerca di Base, Project N-RBAUO183A9.
Patrick Downey's present address is Schering-Plough Research Institute, San Raffaele Biomedical Science Park, Milan, Italy.
References
- Brady, N. C. 1984. The Nature and Properties of Soil. Ninth Ed. MacMillan Publishing Company, Collier MacMillan Publishers, New York, London.
- Chakrabarti, P. 1990. Geometry of interaction of metal ions with histidines residues in protein. Prot. Eng. 4:57–63. [DOI] [PubMed] [Google Scholar]
- Choi, Y. B., and S. A. Lipton. 1999. Identification and mechanism of action of two histidine residues underlying high-affinity Zn2+ inhibition of the NMDA receptor. Neuron. 23:171–180. [DOI] [PubMed] [Google Scholar]
- Christianson, D. W. 1991. Structural biology of zinc. Adv. Protein Chem. 42:281–355. [DOI] [PubMed] [Google Scholar]
- Clyne, J. D., L. D. LaPointe, and R. I. Hume. 2002. The role of histidine residues in modulation of the rat P2X2 purinoreceptor by zinc and pH. J. Physiol. 539:347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czempinski, K., N. Gaedeke, S. Zimmermann, and B. Müller-Röber. 1999. Molecular mechanisms and regulation of plant ion channels. J. Exp. Bot. 50:955–966. [Google Scholar]
- De Biasi, M., J. A. Drewe, G. E. Kircsh, and A. M. Brown. 1993. Histidine substitution identifies a surface position and confers Cs+ selectivity on a K+ pore. Biophys. J. 65:1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey, P., I. Szabò, N. Ivashikina, A. Negro, F. Guzzo, P. Ache, R. Hedrich, Terzi, M., and F. Lo Schiavo. 2000. Kdc1, a novel carrot root hair K+ channel: cloning, characterisation and expression in mammalian cells. J. Biol. Chem. 275:39420–39426. [DOI] [PubMed] [Google Scholar]
- Formentin, E., A. Costa, M. Bregante, P. Gavazzo, A. Naso, C. Picco, F. Gambale, F. Lo Schiavo, and M. Terzi. 2003. KDC2: a novel carrot AKT1-like potassium channel. Proc. Intl. Congr. Plant Mol. Biol. June 2003, Barcelona. S24–S14.
- Gordon, S. E., and W. N. Zagotta. 1995. Subunit interactions in coordination of Ni2+ in cyclic nucleotide-gated channels. Proc. Natl. Acad. Sci. USA. 92:10222–10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R., O. Moran, E. Conti, H. Bush, D. Becker, F. Gambale, I. Dreyer, A. Kuch, K. Neuwinger, and K. Palme. 1995. Inward rectifier potassium channels in plants differ from their animal counterparts in response to voltage and channel modulators. Eur. Biophys. J. 24:107–115. [DOI] [PubMed] [Google Scholar]
- Heginbotham, L., and R. MacKinnon. 1992. The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron. 8:483–491. [DOI] [PubMed] [Google Scholar]
- Hoth, S., I. Dreyer, P. Dietrich, D. Becker, B. Müller-Röber, and R. Hedrich. 1997. Molecular basis of plant-specific acid activation of K+ uptake channels. Proc. Natl. Acad. Sci. USA. 94:4806–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth, S., and R. Hedrich. 1999. Susceptibility of the guard cell K+ uptake channel KST1 towards Zn2+ requires histidine residues in the S3–S4 linker and in the channel pore. Planta. 209:543–546. [DOI] [PubMed] [Google Scholar]
- Hoth, S., D. Geiger, D. Becker, and R. Hedrich. 2001. The pore of plant K+ channel is involved in voltage and pH sensing: domain-swapping between different K+ channel a-subunits. Plant Cell. 13:943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashikina, N., D. Becker, P. Ache, O. Meyerhoff, H. H. Felle, and R. Hedrich. 2001. K+ channel profile and electrical properties of Arabidopsis root hairs. FEBS Lett. 508:463–469. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, V. Ruta, M. Cadene, B. T. Chait, and R. MacKinnon. 2003. X-ray structure of a voltage-dependent K+ channel. Nature. 423:33–41. [DOI] [PubMed] [Google Scholar]
- Johnson, F. M. 1998. The genetic effects of environmental lead. Mutat. Res. 410:123–140. [DOI] [PubMed] [Google Scholar]
- Liman, E. R., J. Tytgat, and P. Hess. 1992. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 9:861–871. [DOI] [PubMed] [Google Scholar]
- Maroney, M. J. 1999. Structure/function relationships in nickel metallobiochemistry. Curr. Opin. Chem. Biol. 3:188–199. [DOI] [PubMed] [Google Scholar]
- Marschner, H. 1995. Mineral Nutrition of Higher Plants. Academic Press, London.
- Naranjo, D. 1997. Assembly of Shaker K-channels from random mixture of subunits carrying different mutations. In From Ion Channel to Cell-to-Cell Conversation. R. Latorre and J. C. Sáez, editors. Plenum Press, New York and London. 35–65.
- Paganetto, A., M. Bregante, P. Downey, F. Lo Schiavo, S. Hoth, R. Hedrich, and F. Gambale. 2001. A novel K+ channel expressed in carrot roots with a low susceptibility toward metal ions. J. Bioen. Biomemb. 33:63–71. [DOI] [PubMed] [Google Scholar]
- Patterson, C. C., H. Shirahata, and J. E. Ericson. 1998. Lead in ancient human bones and its relevance to historical developments of social problems with lead. Sci. Tot. Environ. 61:167–200. [DOI] [PubMed] [Google Scholar]
- Pilot, G., F. Gaymard, K. Mouline, I. Cherèl, and H. Sentenac. 2003. Regulated expression of Arabidopsis Shaker K+ channel genes involved in K+ uptake and distribution in the plant. Plant Mol. Biol. 51:773–787. [DOI] [PubMed] [Google Scholar]
- Reintanz. 2002. AtKC1, a silent Arabidopsis potassium channel a-subunit modulates root hair K+ influx. Proc. Natl. Acad. Sci. USA. 99:4079–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman, D. P., J. I. Schroeder, W. J. Lucas, J. A. Anderson, and R. F. Gaber. 1992. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 258:1654–1658. [DOI] [PubMed] [Google Scholar]
- Vallee, B. L., and K. H. Falchuck. 1993. The biochemical basis of zinc physiology. Physiol. Rev. 73:79–118. [DOI] [PubMed] [Google Scholar]
- Véry, A. A., C. Bosseux, F. Gaymard, H. Sentenac, and J. B. Thibaud. 1994. Level of expression in Xenopus oocytes affects some characteristics of a plant inward-rectifying voltage-gated K+ channel. Pflugers Arch. 428:422–424. [DOI] [PubMed] [Google Scholar]
- Wang, T. L., A. Hackam, W. B. Guggino, and G. R. Cutting. 1995. A single histidine residue is essential for zinc inhibition of GABAr1 receptor. J. Neurosc. 15:7684–7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xintaras, C. 1998. Impact of Lead-Contaminated Soil in Public Health. U.S. Department of Health and Human Services, Atlanta, Georgia.