Abstract
Monocarboxylate transporters (MCT) and sodium-bicarbonate cotransporters (NBC) transport acid/base equivalents and coexist in many epithelial and glial cells. In nervous systems, the electroneutral MCT1 isoform cotransports lactate and other monocarboxylates with H+, and is believed to be involved in the shuttling of energy-rich substrates between astrocytes and neurons. The NBC cotransports bicarbonate with sodium and generates a membrane current. We have expressed these transporter proteins, cloned from rat brain (MCT1) and human kidney (NBC), alone and together, by injecting the cRNA into oocytes of the frog Xenopus laevis, and measured intracellular pH changes and membrane currents under voltage-clamp with intracellular microelectrodes, and radiolabeled lactate uptake into the oocytes. We determined the cytosolic buffer capacity, the H+ and lactate fluxes as induced by 3 and 10 mM lactate in oocytes expressing MCT1 and/or NBC, and in water-injected oocytes, in salines buffered with 5 mM HEPES alone or with 5% CO2/10 mM HCO3− (pH 7.0). In MCT1 + NBC- but not in MCT1- or NBC-expressing oocytes, lactate activated a Na+- and HCO3−-dependent membrane current, indicating that lactate/H+ cotransport via MCT1, due to the induced pH change, stimulates NBC activity. Lactate/H+ cotransport by MCT1 was increased about twofold when MCT1 was expressed together with NBC. Our results suggest that the facilitation of MCT1 transport activity is mainly due to the increase in apparent buffer capacity contributed by the NBC, and thereby suppresses the build-up of intracellular H+ during the influx of lactate/H+, which would reduce MCT1 activity. Hence these membrane transporters functionally cooperate and are able to increase ion/metabolite transport activity.
INTRODUCTION
The transport of energy-rich compounds, such as lactate, pyruvate, and other monocarboxylates, may contribute significantly to the supply of energy in various tissues, and is mediated by different isoforms of the monocarboxylate transporter (MCT) family of proteins, which include at least nine MCT-related sequences in mammalian tissues (Halestrap and Price, 1999; Juel, 2001; Brooks, 2002). The MCT cotransports a proton (H+) with a lactate anion, or with other monocarboxylates, such as pyruvate, hydroxybutyrate, or acetacetate, in an electroneutral mode (Bröer et al., 1998). In the brain, mainly MCT1 and MCT2 are found (Pellerin et al., 1998), differentially expressed in astrocytes and neurons (Bröer et al., 1997). In cultured rat brain cells, MCT1 was expressed primarily in astrocytes, and MCT2 in neurons, and affinity studies indicated a Km of 3–5 mM for MCT1, and near 0.5 mM for MCT2 (Bröer et al., 1997, 1998). Under these conditions, the glial MCT1 would favor extrusion, and the neuronal MCT2 would favor uptake, of lactate or other monocarboxylates (Poitry-Yamate et al., 1995; Schurr et al., 1997; Voutsinos-Porche et al., 2003). It has been suggested that the transfer of lactate from glial cells to neurons via the MCT may be one of the prime trophic functions of glial cells in the brain to meet the high energy requirements of neurons (Dringen et al., 1993; Tsacopulos and Magistretti, 1996; Magistretti et al., 1999). Indeed, lactate can sustain synaptic function in glucose-deprived brain slices (Schurr et al., 1988), and can help to attenuate neuronal damage in a rat model of cerebral ischemia (Schurr et al., 2001).
The sodium-bicarbonate cotransporter (NBC) carries sodium with bicarbonate in an electrogenic mode (Boron and Boulpaep, 1983). The NBC in the brain, first characterized in the giant glial cells of the leech central nervous system (Deitmer and Schlue, 1987, 1989), cotransports one sodium ion with two bicarbonate ions. This transport, thus, generates a membrane current when activated, and its activity and direction are dependent upon the membrane potential (Munsch and Deitmer, 1994; Deitmer and Schneider, 1995). Expression cloning of the NBC from human kidney also suggests a stoichiometry of 1:2 (Heyer et al., 1999). The NBC has been found to operate in epithelial cells, and in the brain in all types of macroglial cells, including astrocytes, oligodendrocytes, Schwann cells, and retinal Müller cells (Romero and Boron, 1999; Deitmer and Rose, 1996). The NBC has been cloned from human kidney and rat brain, and expressed in frog oocytes (Romero et al., 1997; Heyer et al., 1999; Bevensee et al., 2000; Giffard et al., 2000). In situ hybridization revealed mRNA for NBC expression throughout the rat central nervous systems, with particular high levels in the olfactory bulb, the hippocampus dentate gyrus, and the cerebellum (Schmitt et al., 2000). Although mRNA for NBC has been found primarily in astrocytes, it was also evident in neurons (Giffard et al., 2000; Schmitt et al., 2000); transport activity by the NBC has, however, so far only be detected in glial cells, but not in neurons (Deitmer and Schlue, 1987, 1989; Brune et al., 1994; Bevensee et al., 1997).
In this study we have attempted to test the hypothesis that the two transporters, MCT and NBC, interact functionally on the basis that they both transport acid/base equivalents and change the intracellular pH and hence the proton/bicarbonate gradient (Deitmer, 2000, 2002). We have therefore studied the transport activity of MCT1 and NBC expressed in frog oocytes alone and when coexpressed by measuring intracellular pH, the membrane current, and the radiolabeled lactate flux in the nominal absence and in the presence of CO2/HCO3−. Our results indicate that the transport of lactate via MCT1 is increased significantly when MCT1 is coexpressed with NBC, presumably due to the apparent buffer capacity contributed by the NBC activity. The bicarbonate shuttling of the NBC attenuates the intracellular pH changes as induced by inwardly or outwardly cotransported lactate and protons via MCT1, and hence counteracts the dissipation of the proton gradient across the cell membrane, which would reduce MCT1 activity. A preliminary report of part of this study has been presented to the German Physiological Society (Becker et al., 2003).
MATERIAL AND METHODS
Oocytes and injection
For oocyte expression rat MCT1 cloned in the vector pGEMHeJuel, which contains the 5′ and the 3′ untranscribed regions of the Xenopus β-globulin flanking the multiple cloning site, were used (Bröer et al., 1997). The human NBC1 cDNA (hkNBC) cloned in oocyte expression vector pGH19 was kindly provided by Dr. Walter Boron (Choi et al., 1999) and subcloned into the vector pGEMHeJuel. Plasmid DNA was linearized with NotI and transcribed in vitro with T7 RNA-polymerase in the presence of the cap analogon m7G(5′)ppp(5′)G (mMessage mMachine, Ambion, Austin, TX) to produce a capped RNA transcript. The cRNA was purified by phenol/chlorophorm extraction followed by an ammonium acetate/ethanol precipitation and stored at −70°C in DEPC-H2O. Integrity of the cRNA was checked by formaldehyde-gel electrophoresis. X. laevis females were purchased from Horst Köhler (Hamburg, Germany). Oocytes were isolated and singularized by collagenase (Collagenase A, Roche, Mannheim, Germany) treatment in Ca2+-free oocyte Ringer solution at room temperature for 2 h. The singularized oocytes were left overnight in Ca2+-containing oocyte Ringer solution to recover. The oocyte saline (OR2+) had the following composition (in mM): NaCl, 82.5; KCl, 2.5; CaCl2, 1; MgCl2, 1, Na2HPO4; 1, HEPES, 5, titrated with NaOH to pH 7.0. The bicarbonate-containing saline contained (in mM): NaCl, 72.5; KCl, 2.5; CaCl2, 1; MgCl2, 1; Na2HPO4, 1; NaHCO3, 10, gassed with 5% CO2 (pH 7.0) and HEPES, 5, to stabilize the pH. Lactate (0.1, 3, and 10 mM) was added as Na-lactate and exchanged for equimolar amounts of NaCl. In Na+-free saline, NaCl was exchanged by N-methyl-D-glucamine (NMDG) and titrated with HCl to pH 7.0; lactate was added to Na+-free salines as free acid.
Oocytes of the stages V and VI were selected and injected either with 13 ng MCT1-cRNA or with 7 ng MCT1- and 14 ng NBC-cRNA using glass micropipettes and a microinjection device (Nanoliter 2000, World Precision Instruments, Berlin, Germany). Control oocytes were injected with an equivalent volume of DEPC-H2O.
Antibody staining of transporters in oocytes
Frog oocytes, injected with cRNA for the expression of either MCT1, NBC, or both, as well as oocytes injected with DEPC-H2O (control), were fixed in 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS) 3–4 days after cRNA injection. Oocytes were treated with 100% methanol and permeabilized with 0.1% Triton X-100. Unspecific binding sides were blocked with 3% bovine serum albumin (BSA) and 1% normal goat serum (NGS). The cells were incubated in phosphate buffered saline containing the primary antibodies (rabbit anti-Na+/HCO3− cotransporter polyclonal antibody (1:100), chicken anti-monocaboxylate transporter 1 polyclonal antibody (1:300), Chemicon International, Temecula, CA) overnight at 4°C. Oocytes were then incubated with the secondary antibody (Alexa Fluor 546 goat anti-rabbit IgG, Alexa Fluor 488 goat anti-chicken IgG, Molecular Probes, Eugene, OR). The stained oocytes were analyzed with a laser scanning microscope (LSM 510, Carl Zeiss GmbH, Oberkochem, Germany), using either whole oocytes, through which cross sectional optical planes were laid, or bisected oocytes for viewing the cell surface.
Intracellular pH measurements
For measurement of intracellular pH and membrane potential, double-barreled pH-sensitive microelectrodes were used; the manufacture and application have been described in detail previously (Deitmer, 1991). Briefly, two borosilicate glass capillaries of 1.0 and 1.5 mm in diameter were twisted together and pulled to a micropipette. The ion-selective barrel was silanized with a drop of 5% tri-N-butylchlorsilane in 99.9% pure carbon tetrachloride, backfilled into the tip. The micropipette was baked for 4.5 min at 450°C on a hot plate. H+-sensitive cocktail (Fluka 95291, Fluka, Buchs, Switzerland) was backfilled into the tip of the silanized ion-selective barrel and filled up with 0.1 M Na-citrate, pH 6.0. The reference barrel was filled with 3 M KCl. To increase the opening of the electrode tip, it was beveled with a jet stream of aluminium powder suspended in H2O. Calibration of the electrodes was carried out in oocyte salines with a pH of 7.0 and 6.4. The recording arrangement was the same as described previously (Deitmer, 1991; Munsch and Deitmer, 1994). The central and the reference barrel of the electrodes were connected by chlorided silver wires to the headstages of an electrometer amplifier. Electrodes were accepted for use in the experiments when their response exceeded 50 mV per unit change in pH; on average, they responded with 54 mV for unit change in pH. In the experimental chamber they responded faster to a change in saline pH than the fastest reaction expected to occur in the oocyte cytosol.
As described previously (Bröer et al., 1998), optimal pH changes were detected when the electrode was located near the inner surface of the plasma membrane. This was achieved by carefully rotating the oocyte with the impaled electrode. All experiments were carried out at room temperature (22–25°C). Only oocytes with a membrane potential negative to −30 mV were used for experiments.
Buffering power and proton fluxes
The measurements of pHi were stored digitally using homemade PC software (Deitmer and Schneider, 1995), and could be converted into intracellular H+ concentration, [H+]i. This should provide changes in the [H+]i, which take into account the different pH baseline as, e.g., measured in HEPES- and CO2/HCO3−-buffered salines.
Amplitude and rate of change of the measured pHi or of the [H+]i were continuously recorded. The intrinsic buffering power was calculated from the maximal instantaneous pHi changes recorded when changing from HEPES- to 5% CO2/10 mM HCO3−-buffered saline. The CO2-dependent buffering power, βCO2, was calculated from the intracellular bicarbonate concentration in the oocytes (βCO2 = 2.3 × [HCO3−]), and the bicarbonate concentration was obtained from the Henderson-Hasselbalch equation. The total buffer capacity, βt, was defined as the sum of βi and βCO2.
Net acid/base flux JA/B (mM/min), defined as the net transport of acid and/or base equivalents across the cell membrane, was calculated as the product of the rate of pHi change, ΔpHi/t, and the buffering power β. For calculation of the flux rates in HEPES-buffered saline, ΔpHi/t was multiplied with the intrinsic buffer capacity βi of the oocyte. For calculation of the flux rate in CO2/HCO3−-buffered saline, ΔpHi/t was multiplied with the total buffering capacity βt.
Voltage-clamp recording
A borosilicate glass capillary, 1.5 mm in diameter, was pulled to a micropipette and backfilled with 3 M KCl. The resistance of the electrode measured in oocyte saline was around 1 MΩ. For voltage-clamp, both electrodes were connected to the headstages of an Axoclamp 2A or 2B amplifier (Axon Instruments, Union City, CA). The experimental bath was grounded with a chlorided silver wire coated by agar dissolved in oocyte saline.
Radiolabeled flux measurements
For each determination, groups of seven cRNA- or H2O-injected oocytes were washed twice with 4 ml Ca2+-containing oocyte Ringer OR2+ (pH 7.0, see above) or with the bicarbonate-containing saline containing 10 mM NaHCO3 and 5% CO2 (pH 7.0, see above) before incubation at room temperature in a 5 ml polypropylene tube containing 70 μl of the same buffer supplemented with 5 kBq [U-14C]lactate and different amounts of unlabeled substrate. Transport was stopped after different intervals by washing oocytes three times with 4 ml ice-cold OR2+ buffer. Repeated washing steps did not result in leakage of labeled lactate; e.g., when each oocyte contained 29 ± 1 pmol lactate after three washing steps, the same batch of oocytes contained 27.5 ± 1.5 pmol after one additional washing step and 28 ± 4 pmol after a total of six washing steps. Single oocytes were placed into scintillation vials and lysed by addition of 200 μl 10% SDS. After lysis, 3 ml scintillation fluid was added, and the radioactivity determined by liquid scintillation counting. For efflux, equal volumes of [14C]lactate and 200 mM lactate (pH 7.3) were mixed, and subsequently 50 nl of the mixture was injected into oocytes and chilled down to 4°C. Efflux was initiated immediately by washing seven oocytes with buffer equilibrated to room temperature. After the final washing step, 1 ml saline was added, and 100-μl aliqouts were taken for each time point.
Calculation and statistics
For radioactive flux measurements, each individual datapoint represents the difference between the mean ± SE uptake activity of n MCT1 expressing and n noninjected oocytes (usually n = 7). The standard error of the mean (mean ± SE) of the difference was calculated by Gauss' law of error propagation.
For calculation of significance in differences Student's t-test or, if possible, a paired t-test was used. In the figures shown a significance level of p < 0.05 is marked with *, p < 0.01 with **, and p < 0.001 with ***.
RESULTS
To determine the impact of the presence of the NBC on MCT1 activity, we compared the activity of the membrane transporters in Xenopus oocytes, expressing either MCT1 or NBC, MCT1 and NBC together (MCT1 + NBC), or none (H2O-injected). The activity of both transporters was assessed by recording the intracellular pH and the membrane current in voltage-clamp and by [14C]lactate fluxes in the presence of 2–3 different concentrations of L-lactate in saline buffered with HEPES and with CO2/HCO3− (see Methods).
Expression of MCT1 and NBC in oocytes demonstrated by antibody staining
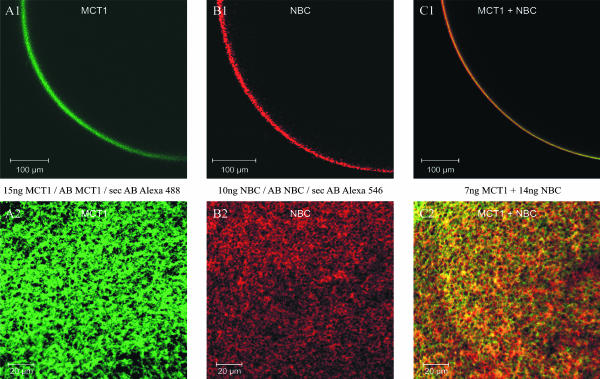
Oocytes injected with the cRNA for the membrane transporters were stained with Alexa dye-linked antibodies against an epitope of the MCT1 and the NBC (see Methods). Confocal images of cross sections (Fig. 1, A1–C1) and of the surface (Fig. 1, A2–C2) of oocytes show staining for both MCT1 (green, Fig. 1 A) and NBC (red, Fig. 1 B) in the plasma membrane of the oocyte, whereas no staining was evident in the cytoplasm. In oocytes, which were injected with cRNA for both MCT1 and NBC (Fig. 1 C), staining for both proteins were identified, and plaques of apparent colocalization of the two proteins could be observed (yellow). Oocytes, which were injected with H2O, or not injected at all, but otherwise treated the same way with the dye-linked antibodies, showed no visible staining (not shown).
FIGURE 1.
Confocal laser scanning images of oocytes injected with cRNA for MCT1 (A1 and A2), for hkNBC (B1 and B2), and with cRNA for both MCT1 + NBC (C1 and C2) after treating oocytes with antibodies, coupled to the dyes Alexa 488 (MCT1) and Alexa 546 (NBC). The images show a cross section (A1–C1) and a surface view (A2–C2) of the oocytes.
Intracellular H+ and HCO3− in oocytes
In HEPES-buffered saline (pH 7.0), the resting, steady-state intracellular pH (pHi) of H2O-injected oocytes was 7.25 ± 0.03 (n = 7), which was similar to the resting pH of oocytes expressing MCT1 that had a pHi of 7.33 ± 0.05 (n = 7). NBC-expressing oocytes had a pH of 7.34 ± 0.05 (n = 8), whereas coexpression of NBC together with MCT1 (MCT1 + NBC) caused a significant increase of the steady-state pHi to 7.46 ± 0.04 (n = 16; p < 0.01). The pHi values correspond to 57 nM (H2O-injected), 49 nM (MCT1), 48 nM (NBC), and 38 nM (MCT1 + NBC) free intracellular H+ concentration ([H+])i, respectively (Table 1).
TABLE 1.
Intracellular pH, [H+], [HCO3−] and Nernst equilibrium potential of H+/HCO3− in oocytes expressing either MCT1, NBC, MCT1+NBC or one (H2O-injected) in salines buffered with 5 mM HEPES or in the presence of added 5% CO2/10 mM HCO3− at pH 7.0
| MCT1 (1) (n = 7) | NBC (2) (n = 8) | MCT1 + NBC (3) (n = 16) | H2O-(4) (n = 7) injected (Control) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oocyte injected with cRNA for | ||||||||||||
| pHi | HEPES | 7.33 ± 0.05 | *** | 2 − | 7.34 ± 0.05 | *** | 3 − | 7.46 ± 0.04 | *** | 4 ** | 7.25 ± 0.03 | *** |
| 3 − | 4 − | |||||||||||
| 4 − | ||||||||||||
| CO2 | 6.91 ± 0.05 | 2 * | 7.07 ± 0.05 | 3 − | 7.09 ± 0.03 | 4 *** | 6.79 ± 0.03 | |||||
| 3 ** | 4 *** | |||||||||||
| 4 − | ||||||||||||
| [H+]i (nM) | HEPES | 49 ± 5 | *** | 2 − | 48 ± 6 | *** | 3 − | 38 ± 4 | *** | 4 * | 57 ± 4 | *** |
| 3 − | 4 − | |||||||||||
| 4 − | ||||||||||||
| 2 * | 3 − | 86 ± 6 | 4 *** | 163 ± 9 | ||||||||
| CO2 | 128 ± 14 | 3 * | 89 ± 11 | 4 *** | ||||||||
| 4 − | ||||||||||||
| [HCO3−]i (mM) | HEPES | 0.14 ± 0.02 | *** | 2 − | *** | 3 − | 0.19 ± 0.02 | *** | 4 *** | 0.11 ± 0.01 | *** | |
| 3 − | 0.14 ± 0.02 | 4 − | ||||||||||
| 4 − | ||||||||||||
| 2 * | 3 − | 12.5 ± 0.8 | 4 *** | 4 *** | 6.2 ± 0.3 | |||||||
| CO2 | 8.3 ± 0.9 | 3 ** | 12.2 ± 1.2 | 4 *** | ||||||||
| 4 − | ||||||||||||
| EH+/HCO3− (mV) | HEPES | 19 ± 3 | *** | 2 − | 20 ± 3 | *** | 3 − | 27 ± 2 | *** | 4 ** | 15 ± 2 | *** |
| 3 − | ||||||||||||
| 4 − | 4 − | |||||||||||
| CO2 | −5 ± 3 | 2 * | 4 ± 3 | 3 − | 5 ± 2 | 4 *** | −12 ± 1 | |||||
| 4 *** | ||||||||||||
Asterisks indicate significance level to column numbered 1–4
In CO2/HCO3−-buffered saline (pH 7.0), the resting pHi of oocytes was lower than in HEPES-buffered saline, due to diffusion of CO2 into the cells. On average, pHi was 6.79 ± 0.03 (n = 7) in H2O-injected oocytes, 6.91 ± 0.05 (n = 7) in oocytes expressing MCT1 alone, 7.07 ± 0.05 (n = 8) in oocytes expressing NBC alone, and 7.09 ± 0.03 (n = 16) in MCT1 + NBC-expressing oocytes. The difference of the steady-state pHi values in MCT1 and H2O-injected on one hand, and in NBC and MCT1 + NBC expressing oocytes on the other hand, was significant (p < 0.05, p < 0.01; Table 1). It was the expression of the NBC, with and without MCT1, that made the difference for the resting pHi, the higher pHi values indicating a larger HCO3− concentration, presumably due to inwardly directed NBC at the holding potential of −40 mV (see below).
The pHi was used to calculate the intracellular HCO3− concentration ([HCO3−]i; Table 1), which was significantly larger in oocytes expressing NBC (p < 0.01) in CO2/HCO3−-buffered saline. The H+/HCO3− Nernst equilibrium potential (EH+/HCO3−) ranged between +27 and +15 mV in HEPES-buffered saline, and between +5 and −12 mV in CO2/HCO3−-buffered saline. The difference in EH+/HCO3− in the two different buffer salines for all oocytes was between 16 and 27 mV, indicating a difference in the H+ gradient across the oocyte membrane by a factor of 2 to 3 in the presence of CO2/HCO3−, as compared to that in HEPES-buffered saline.
Single expression of MCT1 and NBC in oocytes
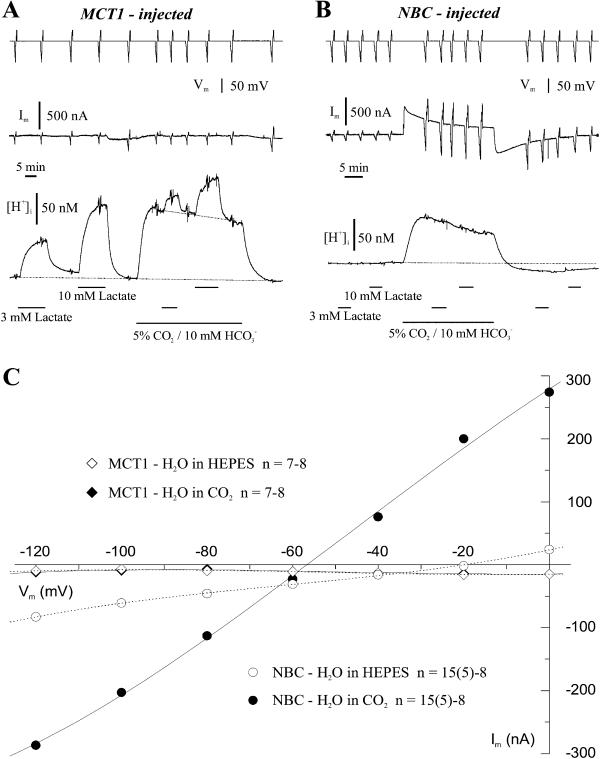
When either MCT1 or NBC was expressed separately in Xenopus oocytes, their ability to transport lactate, as indicated by the intracellular acidification, and their current-voltage relationship were determined in HEPES and CO2/HCO3−-buffered saline (Fig. 2). In voltage-clamped, MCT1-expressing oocytes, lactate (3 and 10 mM) induced a reversible intracellular acidification, which was considerably smaller in the presence of CO2/HCO3−, as compared to that in HEPES-buffered saline, but did not elicit a membrane current (Fig. 2 A). The membrane conductance remained unaltered in lactate, and the current-voltage relationship was linear; both parameters were very similar in HEPES- and in CO2/HCO3−-buffered salines (Fig. 2 C), and only slightly different from those in H2O-injected control oocytes in either buffer (not shown). These results are in line with an electroneutral lactate-H+ cotransport (Bröer et al., 1997, 1998). The smaller acidification evoked by lactate in the presence of CO2/HCO3− was presumably due to the lower pHi caused by CO2/HCO3−, and/or due to the increased buffering power (see below). Introduction of CO2/HCO3− induced a prominent intracellular acidification itself, and removal of CO2/HCO3− initiated a recovery from this acidification in all oocytes. These changes in the [H+]i were not accompanied by any significant changes in membrane current in MCT1-expressing oocytes (Fig. 2 A) or in H2O-injected oocytes (not shown).
FIGURE 2.
Intracellular recordings with pH-selective microelectrodes under two-electrode voltage-clamp (−40 mV, upper trace) in frog oocytes injected with cRNA for MCT1 (A), and with cRNA for hkNBC (B), when 3 and 10 mM lactate was added to the saline buffered with 5 mM HEPES or with 5% CO2/10 mM HCO3− (pH 7.0). Due to the different baselines of pHi in the different buffers, the pHi traces were converted to values of intracellular H+ concentration ([H+]i). The deflections on the voltage and the current traces (middle traces in A and B) are voltage steps to different potentials between −120 and 0 mV, which are used to plot mean current-voltage relationships of the two types of oocytes in the two buffers (C). Oocytes injected with cRNA for NBC showed a prominent conductance in CO2/HCO3−-buffered saline.
In NBC-expressing oocytes, lactate had no effect on either pHi or the membrane current, indicating that the NBC did not mediate lactate/H+ cotransport (Fig. 2 B). When changing to CO2/HCO3−-buffered saline, the [H+]i increased and slowly recovered, and upon removal of CO2/HCO3−, the [H+]i turned into an alkaline undershoot, from which the oocytes slowly recovered back to the initial baseline (Fig. 2 B). These changes in the [H+]i were accompanied by an outward current and an inward current, respectively. The current-voltage relationship shows a large increase in current and in membrane conductance over the membrane potential range between −120 and 0 mV in the presence of CO2/HCO3− with little rectification (Fig. 2 C). These results suggest that the expression of NBC is associated with a prominent increase in membrane conductance in the presence of added HCO3−, the substrate for the NBC. NBC was activated by the diffusion of CO2 into the cell to carry HCO3− into the oocyte, indicated by the outward current, and to extrude HCO3−, indicated by the inward current, when CO2 was removed, respectively.
The reversal potential of the NBC-associated membrane current was near −56 mV, indicating inward transport of Na+ and HCO3− at membrane potentials positive to −56 mV, and outward transport negative to −56 mV. With an intracellular pH (pHi) of 7.07 in NBC-expressing oocytes at an extracellular pH of 7.0 in CO2/HCO3−-buffered saline, a reversal potential of −47 mV is calculated for a stoichiometry of 1 Na+:2 HCO3−, and a reversal potential of −22 mV at a stoichiometry of 1:3 for the NBC. The reversal potential of −56 mV found in this study clearly supports a stoichiometry of 1:2 for the hkNBC expressed in oocytes, as previously reported also for rat kidney NBC (Sciortino and Romero, 1999; Heyer et al., 1999).
Coexpression of MCT1 and NBC in oocytes
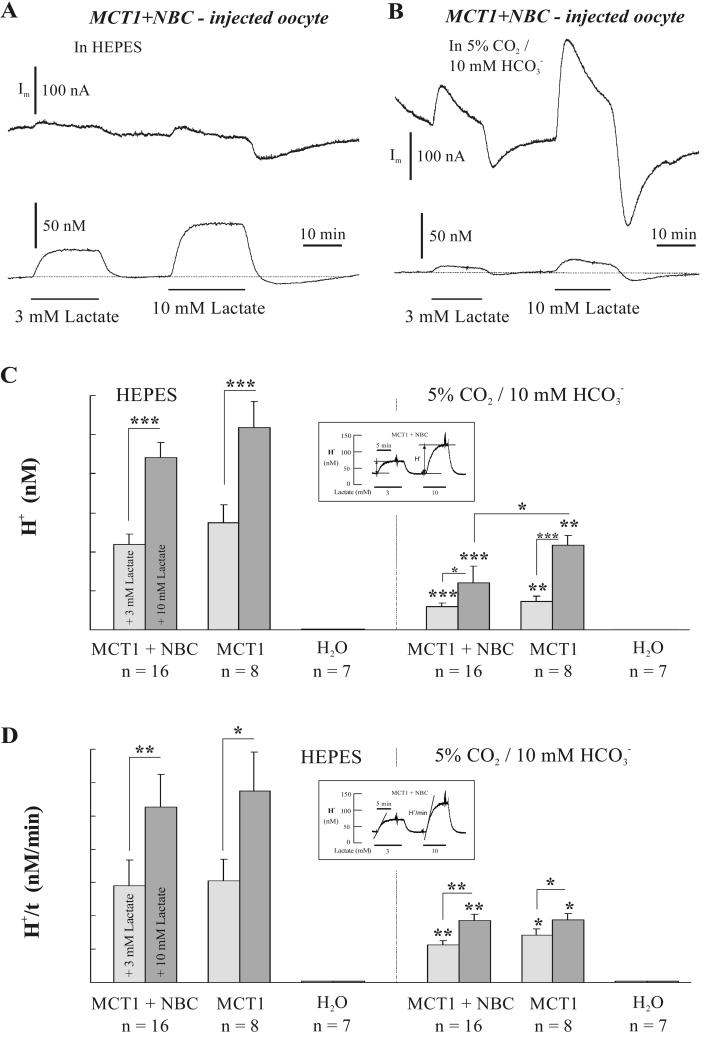
When MCT1 and NBC were coexpressed in oocytes, addition of lactate induced an intracellular acidification and a membrane outward current, which were both dependent on the concentration of the substrate for MCT1 and the buffer present (Fig. 3, A and B). The amplitude of the acidification (Fig. 3 C) and the rate of acidification (Fig. 3 D) in MCT1 + NBC- and in MCT1-expressing oocytes were significantly smaller in the presence of CO2/HCO3−, and there was no change in pHi in H2O-injected oocytes (Fig. 3, C and D). In CO2/HCO3−-buffered saline, the amplitude of the intracellular acidification induced by 10 mM lactate was larger in MCT1-expressing oocytes than in MCT1 + NBC-expressing oocytes, whereas the rate of intracellular acidification was similar in the two types of oocytes for the two lactate concentrations, respectively.
FIGURE 3.
(A and B) Changes of membrane current (Im, upper traces) and intracellular H+ concentration ([H+]I, lower traces) of a MCT1 + NBC expressing oocyte, as induced by 3 and 10 mM lactate added to the saline (pH 7.0) in 5 mM HEPES (A) and in 5% CO2/10 mM HCO3− (B). The recordings in A and B are from the same oocyte. The mean amplitude (ΔH+, C) and the mean rate of change (ΔH+/t, D) of the H+ responses to lactate, as indicated in the insets, were plotted for oocytes injected with cRNA for MCT1 + NBC, with cRNA for MCT1 alone, and with H2O (control), in HEPES- and in CO2/HCO3−-buffered saline. See text for further details.
There were prominent membrane current changes upon the addition and removal of lactate in CO2/HCO3−-buffered saline (Fig. 3 B). These currents were partly transient and showed an overshoot in the alkaline direction upon the removal of lactate. Much smaller, but significant, membrane currents were recorded also in HEPES-buffered saline (Fig. 3 A), indicating that in the nominal absence of CO2/HCO3− there appeared to be still enough HCO3− to maintain some NBC activity; in particular upon removal of lactate, which initiated the pHi recovery from acidosis, a larger inward current signaled a larger activity of NBC in the outward direction, presumably due to endogenously produced HCO3−.
The amount and rate of acidification caused by lactate in CO2/HCO3−-buffered saline was considerably smaller than in HEPES-buffered saline, which would be expected due to the reduced, or reversed, H+ gradient across the cell membrane. We therefore compared only the acid/base fluxes in CO2/HCO3−-buffered saline when evaluating the activity of MCT1 (see below).
Dependence of lactate-induced membrane current on sodium and bicarbonate
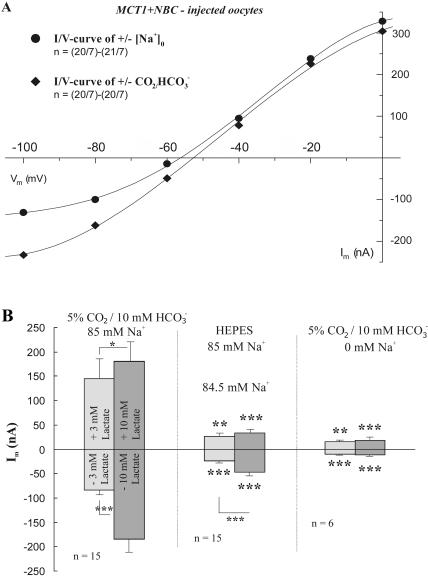
If lactate transport via MCT is affected by NBC in oocytes coexpressing MCT1 + NBC, the current induced by lactate should be indicative for the activation of NBC (since the MCT itself is electroneutral, see above), and therefore be dependent on the presence of both, sodium and bicarbonate, the substrates for NBC. We tested this by measuring the membrane current in MCT1 + NBC-coexpressing oocytes in the presence and absence of either extracellular bicarbonate or sodium (Fig. 4). The current-voltage relationships shown in Fig. 4 A are the differences of the currents measured in the presence and absence of sodium in CO2/HCO3−-buffered saline, and the differences of the currents measured in CO2/HCO3−- and HEPES-buffered, Na+-containing, saline. Both curves show a similar course; they presumably represent the voltage dependence of the NBC current. The difference currents of CO2/HCO3−-HEPES were somewhat larger in the inward direction; NBC inward currents are outward movements of Na+ and HCO3−, and endogeneously produced CO2 HCO3− might contribute to NBC outward flux.
FIGURE 4.
(A) Current-voltage relationships (I/V curves) of oocytes injected with cRNA for MCT1 + NBC, as obtained by subtraction of the I/V curves obtained in the presence and absence of extracellular Na+ in CO2/HCO3−-buffered saline (circles), and as obtained in the presence and absence of CO2/HCO3− (diamonds). (B) Changes in membrane current as induced by addition and removal of 3 and 10 mM lactate in oocytes injected with cRNA for MCT1 + NBC in CO2/HCO3−-buffered saline, in HEPES-buffered saline, and in CO2/HCO3−-buffered, Na+-free, saline. The currents are greatly reduced in the absence of either HCO3− or Na+.
When 3 and 10 mM lactate were added and removed in normal CO2/HCO3−-buffered saline, an outward current and an inward current were generated, respectively, in MCT1 + NBC-coexpressing oocytes. These lactate-induced currents were reduced on average to 18% and 27%, when CO2/HCO3− was removed, and to 10% and 9% in Na+-free saline, for inwardly and outward-directed NBC, as activated by the addition and removal of lactate, respectively (Fig. 4 B). This shows that the current observed in MCT1 + NBC-coexpressing oocytes evoked by lactate is likely to be due to an increased NBC activity.
Assuming that the lactate-induced membrane current remaining in Na+-free saline was not due to NBC activity, it was subtracted from the currents in CO2/HCO3−- and HEPES-buffered saline; the remaining current in HEPES-buffered, nominally CO2/HCO3−-free saline was 9% for the inwardly and 20% for outwardly directed NBC. Outwardly directed NBC is dependent upon intracellular HCO3−, which can still be formed endogenously in CO2/HCO3−-free saline, which could enhance the inward current during removal of lactate. There are potent inhibitors to block NBC, such as DIDS or SITS, for example; these anion transport inhibitors, however, may also affect lactate transport (Eladari et al., 1999). We therefore did not employ these inhibitors, when both anion transporters were expressed in the oocytes, to identify the NBC, but rather relied on the ion substitution experiments as described above.
When the holding potential was changed from −40 to −80 mV, the lactate-induced membrane currents were reduced by nearly 70% in MCT1 + NBC-expressing oocytes in CO2/HCO3−-buffered saline, presumably indicating the voltage dependence of the electrogenic NBC (not shown). As expected, at more negative membrane potential, the inward mode of NBC is impaired due to the larger uphill gradient for HCO3−. The change in HCO3− as evoked by the addition of lactate was also reduced when the oocytes were clamped at −80 mV (not shown).
Buffer capacity
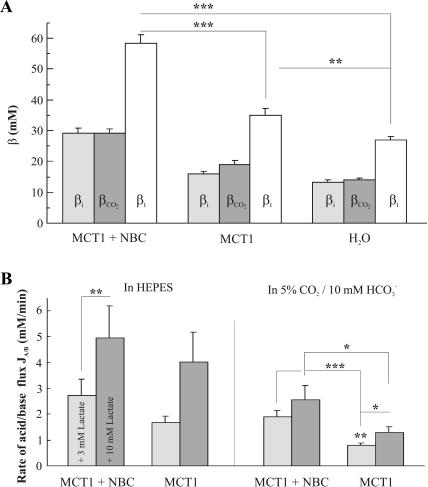
The total buffer capacity of the oocyte (βt) is defined as the sum of intrinsic (βi) and CO2/HCO3−-dependent buffering capacity (βCO2). In HEPES-buffered, nominally CO2/HCO3−-free saline, βi was determined in MCT1 + NBC-expressing oocytes to be 29.1 ± 1.7 mM, and hence 82% larger (p < 0.001; n = 16) than βi of 16 ± 0.9 mM (n = 8) of oocytes expressing MCT1 alone, and 125% larger than βi of 12.9 ± 0.9 mM (n = 7; Fig. 5 A) of H2O-injected oocytes. βi of MCT1-expressing oocytes was 24% larger than of H2O-injected oocytes (p < 0.05; Fig. 5 A). Similarly, βCO2 of MCT1 + NBC-expressing oocytes was 29.1 ± 1.4 mM (n = 16) and hence 53% and 108% larger (p < 0.001) than the βCO2 of 19 ± 1.5 mM (n = 8) in oocytes expressing MCT1 alone, and the βCO2 of 14 ± 0.5 mM in H2O-injected oocytes (n = 7; p < 0.001), respectively. MCT1-expressing and H2O-injected oocytes significantly differed by 36% (p < 0.01).
FIGURE 5.
(A) Buffering capacity β in oocytes expressing MCT1 + NBC, MCT1 alone, and in H2O-injected oocytes. Plotted are the intrinsic buffer capacity (βi), the CO2/HCO3−-dependent buffer capacity (βCO2), and the sum of both, the total buffer capacity (βt). The MCT1 + NBC expressing oocytes have a significantly larger βt than the MCT1 expressing and H2O-injected oocytes. (B) Rate of acid/base fluxes (JA/B) induced by 3 and 10 mM lactate as obtained from pHi recordings and the buffer capacity in MCT1 + NBC expressing and in MCT1 expressing oocytes. The rates of acid/base flux are significantly larger in MCT1 + NBC expressing oocytes than in oocytes expressing MCT1 alone in CO2/HCO3−-buffered saline, but not in HEPES-buffered saline.
The intrinsic and the CO2/HCO3−-dependent buffering capacity add up to a total buffering capacity βt, which was highest in MCT1 + NBC-expressing oocytes, where βt was 58.3 ± 2.9 mM (n = 16). In oocytes expressing MCT1 alone and in H2O-injected oocytes, βt summed up to 35 ± 2.3 mM (n = 8) and 26.9 ± 1.2 mM (n = 7), respectively. βt was significantly different for all three types of oocytes (p < 0.01; Fig. 5 A).
The rate of proton flux
We have previously shown that the intracellular acidification observed during the application of lactate is induced by the transport of H+ cotransported 1:1 with lactate into the oocytes by the MCT1 (Bröer et al., 1998). The rate of H+ uptake should hence be a measure of the lactate uptake and the activity of the MCT1. We determined the rate of acid/base flux JA/B as the product of the maximal rate of pHi change and the buffering power βi or βt in HEPES-buffered and in CO2/HCO3−-buffered saline, respectively. In oocytes coexpressing MCT1 + NBC (n = 16), the acid/base flux rate was 2.7 ± 0.6 mM/min (n = 16) in 3 mM lactate, and 4.9 ± 1.2 mM/min in 10 mM lactate in HEPES-buffered saline. In oocytes expressing MCT1 alone, the acid/base flux rate was 1.7 ± 0.2 mM/min (n = 8) in 3 mM lactate, and 4.0 ± 1.2 mM/min in 10 mM lactate (n = 8; Fig. 5 B). At a lactate concentration of 3 mM, the flux rate was significantly larger in MCT1 + NBC-expressing oocytes than in oocytes expressing MCT1 alone (p < 0.01), whereas no significant difference was determined at a lactate concentration of 10 mM for the two types of oocyte in HEPES-buffered saline.
In MCT1 + NBC-expressing oocytes, the rate of acid/base flux induced by 3 and 10 mM lactate in CO2/HCO3−-buffered saline was 1.9 ± 0.2 mM/min and 2.6 ± 0.6 mM/min, respectively. These flux rates were significantly larger than those in oocytes expressing MCT1 alone, where the values amounted to 0.8 ± 0.1 mM/min (n = 8; p < 0.001) and 1.3 ± 0.2 mM/min (n = 8; p < 0.05), respectively (Fig. 5 B). Thus, oocytes coexpressing MCT1 + NBC, showed a 138% and 100% increase in acid/base flux rate in the presence of 3 and 10 mM lactate, respectively, as compared to MCT1-expressing oocytes in CO2/HCO3−-buffered saline, suggesting that the activity of MCT1 was increased by a factor of 2 when coexpressed with NBC.
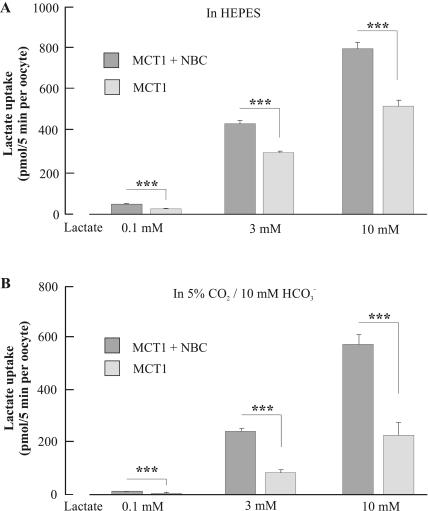
Flux of radiolabeled lactate
To get another, independent, measure of the significance of coexpressing NBC with MCT1, the uptake of radiolabeled [14C]lactate was measured in oocytes expressing MCT1 + NBC and in oocytes expressing MCT1 alone, in HEPES- and in CO2/HCO3−-buffered saline at three different lactate concentrations (Fig. 6). In HEPES-buffered saline and at a lactate concentration of 0.1 mM, MCT1-expressing oocytes took up 38 ± 6 pmol/5min (n = 7), whereas oocytes coexpressing MCT1 + NBC took up 60 ± 5 pmol/5 min (n = 7). A similar picture emerged, when transport activity was determined at higher lactate concentrations. Transporter activities of 314 ± 15 pmol/5 min (n = 7) and 447 ± 29 pmol/5 min per oocyte were measured in HEPES-buffered saline at 3 mM lactate, in MCT1 and MCT1 + NBC expressing oocytes, respectively. At a lactate concentration of 10 mM the presence of NBC increased the transport activity from 534 ± 59 pmol/5 min (n = 7) to 809 ± 60 pmol/5 min (n = 7). The differences between the two groups of oocytes were significant (p < 0.005) at all three lactate concentrations. The stimulatory effect of NBC on MCT1 activity became even more pronounced in the presence of CO2/HCO3−-buffered saline. The transport activities at a lactate concentration of 0.1 mM were 6.4 ± 0.9 pmol/5 min per oocyte (n = 7) and 19 ± 1.4 pmol/5 min per oocyte (n = 7) in MCT1 and MCT + NBC1-expressing oocytes, respectively. At 3 mM lactate, transport rates of 87 ± 10 pmol/5 min per oocyte (n = 7) and 252 ± 10 pmol/5 min per oocyte (n = 7), and at a lactate concentration of 10 mM transport rates of 235 ± 48 pmol/5 min per oocyte (n = 7) and 588 ± 36 pmol/5 min per oocyte (n = 7), were determined for the two groups of oocytes. The difference in flux rates was reflected by the significance levels, which were p = 1.4 × 10−4 (10 mM lactate), p = 4.6 × 10−8 (3 mM lactate), and p = 4.1 × 10−6 (0.1 mM lactate).
FIGURE 6.
Uptake of radiolabeled lactate in the presence of 0.1, 3, and 10 mM lactate in HEPES-buffered saline (A) and in CO2/HCO3−-buffered saline (B) in oocytes expressing MCT1 + NBC and expressing MCT1 alone.
In agreement with the pH-recordings, we found that transport rates were generally lower in CO2/HCO3−-buffered saline than in HEPES-buffered saline. As compared to HEPES-buffered saline, where the resting pHi was 0.37 and 0.42 pH units higher in oocytes expressing MCT1 + NBC and MCT1 alone, respectively, the H+ flux rate was 30–70% smaller in CO2/HCO3−-buffered saline. The radiolabeled lactate flux rate was reduced by 27–73% in the CO2/HCO3−-buffered saline. Thus, similar reductions in the flux rates were obtained for the different buffer systems by both experimental procedures, which were employed to measure MCT1 activity.
Comparison of lactate and acid/base fluxes
The rates of lactate and acid-base fluxes were compared for oocytes expressing MCT1 + NBC and MCT1 alone, during application of 3 mM lactate in HEPES and in CO2/HCO3−-buffered saline. In HEPES-buffered saline, MCT1 + NBC expressing oocytes showed a 1.6-fold larger acid/base flux rate and a 1.4-fold larger lactate flux rate than MCT1-expressing oocytes. In CO2/HCO3−-buffered saline, the acid/base flux rate in MCT1 + NBC expressing oocytes was 2.4-fold, and the radiolabeled lactate flux rate was 2.9-fold larger than in MCT1-expressing cells. These results indicate that the presence of CO2/HCO3− augments lactate transport via MCT1, presumably due to the NBC activity in MCT1 + NBC coexpressing oocytes.
Membrane currents and lactate fluxes
The slope conductance as determined by current-voltage relationships varied between 1.4 and 2.6 μS in HEPES-buffered saline in all oocytes. In the presence of CO2/HCO3−, oocytes expressing MCT1 + NBC had a 6–10-fold larger conductance (11 ± 1.2 μS; n = 3) than oocytes expressing MCT1 alone (1.8 ± 0.1 μS; n = 5) or oocytes injected with H2O (1.1 ± 0.1 μS; n = 6). This suggested that the presence of NBC and its substrate bicarbonate greatly increased the membrane conductance of the oocytes, presumably due to the electrogenic activity of the NBC.
In oocytes expressing MCT1 + NBC, the NBC transports bicarbonate (and sodium) into the oocyte, which slows and attenuates the lactate-induced acidification in MCT1 + NBC-expressing oocytes, and which becomes apparent as an outward current.
Conversely, removal of lactate, which induced a recovery from the acidification in MCT1, and in MCT1 + NBC expressing oocytes (Fig. 1, A–C), generated an inward current in MCT1 + NBC-expressing oocytes (Fig. 4 B). The peak of this inward current was 84 ± 10 nA (n = 15) after the removal of 3 mM lactate, and 185 ± 27 nA (n = 15) after the removal of 10 mM lactate. In HEPES-buffered saline, in both types of oocytes, mean currents of 24–47 nA were measured after the removal of 3 and 10 mM lactate (n = 7–15). Lactate did not elicit a change in membrane current in oocytes expressing MCT1 or NBC alone (see Fig. 2).
Assuming that the reduced H+ concentration changes in MCT1 + NBC-expressing oocytes in CO2/HCO3−-buffered saline, as compared to those in MCT1-expressing oocytes, were due to the activity of the NBC, we tried to relate the reduction in the H+ concentration change induced by 3 and 10 mM lactate in MCT1 + NBC-expressing oocytes with the membrane current generated during the removal of lactate in CO2/HCO3−-buffered saline in oocytes expressing MCT1 + NBC. Although the H+ concentration change increased from 14.7 to 43.3 nM in 3 and 10 mM lactate, respectively, in oocytes expressing MCT1 alone, which is a difference of 28.6 nM, the H+ concentration change rose from 11.9 to 24.3 nM in oocytes expressing MCT1 + NBC, which is a difference of 12.3 nM H+. The augmentation ratio of the difference of these [H+]i changes induced by 3 and 10 mM lactate was 2.3 between MCT1- and MCT1 + NBC-expressing oocytes. The membrane currents in MCT1 + NBC-expressing oocytes as evoked by 3 and 10 mM lactate under these conditions were 84 and 185 nA, respectively, which indicates an increase by a factor of 2.2. This supports the conclusion that the NBC, which generates membrane currents indicative for net bicarbonate fluxes, can account for the reduced H+ concentration change during lactate transport via the MCT1 in oocytes expressing both MCT1 and NBC.
Uptake of 3 and 10 mM radiolabeled lactate showed a similar augmentation between oocytes expressing MCT1 alone and oocytes expressing MCT1 + NBC. In the former, lactate transport increased from 88 to 235 pmol/5 min per oocyte, and in the latter, it increased from 252 to 588 pmol/5 min per oocyte; the differences of 147 and 336 signals an augmentation by a factor of 2.3. Thus, both, measurements of acid/base flux and of radiolabeled lactate flux indicate an enhancement of MCT1 activity by a factor of ∼2 in oocytes, in which MCT1 was coexpressed with NBC.
DISCUSSION
This study compares the lactate-proton cotransport by MCT1 when expressed in Xenopus oocytes alone and when coexpressed with NBC. Our measurements of the total buffering power, the rate of acid/base flux induced by lactate, the flux of radiolabeled lactate, and the membrane current generated by the NBC due to lactate-induced acidosis/alkalosis suggest that lactate transport via MCT1 is doubled when the NBC is coexpressed under the conditions as described here. This indicates a functional cooperation between MCT1 and NBC, which appears to be due to NBC activity partly compensating the H+ fluxes induced by MCT1 activity and hence minimizing the dissipation of the proton gradient, which would reduce MCT1 activity. Cells, which coexpress both proteins in their cell membrane, such as in brain, heart, kidney, and intestine (Abuladze et al., 1998; Burnham et al., 1998; Halestrap and Price, 1999), might have a larger capacity for metabolite/ion transport.
Expression of MCT1 and NBC
Oocytes expressing MCT1 alone responded to lactate with a marked, reversible, intracellular acidification, but not with a change in membrane current (see also Bröer et al., 1997, 1998). The current-voltage relationship was linear, similar to that of H2O-injected control oocytes in either HEPES- or CO2/HCO3−-buffered saline. Oocytes expressing NBC alone did not respond to lactate with either a change in pHi or membrane current, indicating that NBC does not transport lactate. Although the NBC construct used here is derived from the kidney, which is probably not the main NBC1 variant in the brain, it is assumed to have very similar transport properties as the brain NBC (Bevensee et al., 2000). The current-voltage relationship of NBC-expressing oocytes was nearly linear over the voltage-range measured (from −120 to 0 mV), as reported for rat kidney NBC (Heyer et al., 1999; Sciortino and Romero, 1999). It was typical for NBC-expressing oocytes, in contrast to MCT1-expressing oocytes, that the CO2/HCO3−-induced acidification showed a significant recovery, and an undershoot upon removal of CO2/HCO3−. This was accompanied by an outward current during addition, and an inward current during removal, of CO2/HCO3−, indicative for inward and outward going NBC, respectively. At a membrane potential of −40 mV, in contrast to at −80 mV, the NBC acted as an acid remover, given a reversal potential of the NBC of around −56 mV in oocytes expressing NBC either alone or together with MCT1. This is supported by the significantly more alkaline pHi of NBC-expressing oocytes as compared to oocytes expressing MCT1 alone or to H2O-injected oocytes. The reversal potential clearly supports a stoichiometry of the NBC of 1 Na+:2 HCO3−, as was reported for rat kidney NBC (Heyer et al., 1999; Sciortino and Romero, 1999), for leech glial cells (Deitmer and Schneider, 1995), and for cultured rat astrocytes (Bevensee et al., 1997).
The coexpression of MCT1 did not change the reversal potential, suggesting that MCT1 had no effect on the stoichiometry of NBC. Interestingly, the current-voltage relationship of oocytes expressing MCT1 + NBC showed more rectification, in particular at more negative potentials (Fig. 5 A), than oocytes expressing NBC alone. This might be influenced by metabolic processes in oocytes, which can transport monocarboxylates; this issue was not studied any further.
Buffer capacity and proton fluxes
The intrinsic and the CO2/HCO3−-dependent buffer capacities have been determined conventionally using pH shifts in HEPES- and in CO2/HCO3−-buffered saline and intracellular concentrations of bicarbonate for oocytes expressing MCT1 alone or MCT1 + NBC, and for H2O-injected oocytes. It might be expected that the intrinsic buffering capacity βi is the same in the oocytes; however, the βi values were found to be significantly different in all three oocyte types. This suggests that the presence of both MCT1 and of NBC contribute some buffering power to the oocyte. The high affinity of the NBC for bicarbonate would result in significant activity of the NBC even in the nominal absence of CO2/HCO3−, when the HCO3− concentration in the saline (at pH 7.0) from air-CO2 is ∼65 μM and the intracellular HCO3− concentration is ∼180 μM (at pHi 7.46). This is also supported by the more alkaline pHi of 7.46 in MCT1 + NBC-expressing oocytes as compared to the pHi in oocytes expressing MCT1 alone (pHi of 7.33) and the pHi in H2O-injected oocytes (pHi of 7.25). Apparently, bicarbonate ions are accumulated in the oocytes by NBC activity even at these low extracellular HCO3− concentrations. In leech glial cells, a significant NBC activity was indeed detected even in the nominal absence of CO2/HCO3− (Deitmer and Schneider, 1998).
The CO2/HCO3−-dependent buffer capacity was 53% and 108% larger in MCT1 + NBC-expressing oocytes than in oocytes expressing MCT1 alone and in H2O-injected oocytes (p < 0.001), respectively. These salines contain 10 mM bicarbonate, and hence ample substrate for the NBC. Accordingly, the pHi is significantly higher in MCT1 + NBC expressing oocytes (pHi of 7.09) as compared to oocytes expressing MCT1 alone (pHi of 6.91) and H2O-injected oocytes (pHi of 6.79; p < 0.01). These results suggest that the NBC transports considerable amount of bicarbonate into the oocytes under these conditions.
The different buffer capacity in the oocytes has a marked influence on the change in pHi and on the calculation of the acid/base flux rates during lactate-H+ cotransport via MCT1 activity. Although the absolute H+ flux rates as evoked by 3 and 10 mM lactate were smaller in both types of oocytes in CO2/HCO3−-buffered saline, the H+ flux in MCT1 + NBC-expressing oocytes was significantly larger by 62% (in HEPES-buffered saline) and 136% (in CO2/HCO3−-buffered saline) than in oocytes expressing MCT1 alone (p < 0.001 at 3 mM lactate). This suggests an increased activity of the MCT1, when NBC is coexpressed in the oocytes. Possibly, the MCT1 activity is also nearer to saturation at 3 mM, and particularly at 10 mM lactate, when NBC is coexpressed with MCT1. It is conspicuous that at 3 mM lactate, the H+ flux rate is significantly larger in MCT1 + NBC-expressing oocytes even in HEPES-buffered saline, which is likely to be due to the considerable activity of the NBC even in the nominal absence of CO2/HCO3− (see above).
The reduced absolute acid/base flux in CO2/HCO3−- as compared to those in HEPES-buffered saline presumably results from the lower pHi in this saline (De Bruijne et al., 1983). The pHi change induced by lactate was hence affected twofold by CO2/HCO3−, a reduction of the amplitude and rate of acidification due to the lower pHi and due to the increased buffering power in CO2/HCO3−-buffered saline. Therefore, we compared the acid/base fluxes, which includes the buffering capacity, in oocytes expressing MCT1 + NBC with those expressing MCT1 alone only in CO2/HCO3−-buffered saline.
Radiolabeled lactate flux
It might be argued that the enhancement of lactate flux in MCT1 + NBC-expressing oocytes, as compared to oocytes expressing MCT1 alone, is even greatly underestimated in CO2/HCO3−-buffered saline if NBC activity is expected to short-circuit the H+ influx during lactate application by carrying bicarbonate into the oocyte (as pH-selective electrodes record net pH changes). Therefore we performed independent measurements of lactate flux under the same conditions using radiolabeled [14C] lactate. At 3 mM lactate, uptake into oocytes was 43% larger in HEPES-buffered and 188% larger in CO2/HCO3−-buffered saline in MCT1 + NBC-expressing oocytes as compared to oocytes expressing MCT1 alone. These values are 44% smaller for HEPES- and 38% larger for CO2/HCO3−-buffered salines, as obtained for the rates of acid/base flux calculated from the pHi changes and the buffering capacity (see above). Thus, both rates of acid/base flux and radiolabeled lactate flux independently suggest an enhancement of MCT1 activity by a factor of ∼2, when MCT1 is coexpressed with NBC. Again, the lower absolute transport activity of MCT1 at lower pHi values in the presence of CO2/HCO3− is likely to result from the dependence of MCT1 activity on the intracellular pH (De Bruijne et al., 1983).
Membrane currents induced by lactate
Substantial membrane currents were elicited by addition and removal of lactate only in MCT1 + NBC-expressing oocytes and were taken as an indicator for the activity of the electrogenic NBC. This was supported by the dependence of these currents on HCO3− and Na+, the substrates of NBC (see also Sciortino and Romero, 1999; Bevensee et al., 2000). Although these currents were much larger in the presence of CO2/HCO3−, they were significant also in HEPES-buffered saline, supporting the notion that the NBC is activated at micromolar concentrations of bicarbonate as found in the nominal absence of CO2/HCO3− (see above; Deitmer and Schneider, 1998). Addition of lactate evoked an outward current in MCT1 + NBC-expressing oocytes, and removal of lactate an inward current. This is consistent with an electrogenic transport of Na+-HCO3− into the cell, when MCT1 cotransports lactate with H+ into the cell, and with outward transport of Na+-HCO3−, when lactate leaves the cell in cotransport with H+, respectively. The currents were larger during addition and removal of 10 mM lactate as compared to 3 mM lactate, indicating the dependence of NBC activity on the concentration of the MCT1 substrate. It also shows that the NBC activity depends on the MCT1 activity, shuttling bicarbonate ions wherever extra H+ are moved to. This attenuates the resulting pH changes as induced by MCT1 activity, and contributes apparent buffer capacity to the cytosol. Since these transport processes are fast, they cannot be distinguished from the intrinsic or CO2/HCO3−-dependent buffer capacity on the timescale of our pH measurements (Deitmer and Schneider, 1995; Bröer et al., 1998). Thus, transport processes, although classically not considered as part of a cell's buffering system, can significantly contribute to the “H+ muffling” of cells (Thomas et al., 1991).
Functional significance of coexpressed MCT1 and NBC
Since the NBC of epithelial cells (Heyer et al., 1999; Romero and Boron, 1999) and of glial cells (cf. Deitmer, 1991, Deitmer and Rose, 1996) is highly active and fully reversible, NBC activity would also significantly contribute apparent buffering capacity to cells and thereby might increase the efficacy of other acid/base transporters. For glial cells in the nervous system, which express both MCT1 and NBC (Gerhart et al., 1997; Schmitt et al., 2000), our results suggest some interesting functional consequences. Our results on oocytes suggest that the NBC in cells of the central nervous system may help to make the lactate shuttle between astroglial cells and neurons, and thus the glia-neuronal energy transfer, more efficient. In this scenario, intraglial acidification would facilitate lactate export from glial cells, which would then be accompanied by outwardly directed NBC (Deitmer, 2002). Of crucial importance for these processes is the distribution and activity of carbonic anhydrase, both in glial cells and in the extracellular space, which helps to establish a fast equilibrium between carbonic acid and CO2, HCO3−, and H+ (Geers and Gros, 2000; Tong et al., 2000). Interestingly, extracellular carbonic anhydrase has recently been shown to facilitate lactic acid transport in rat skeletal muscle (Wetzel et al., 2001), as well as in neurons and astrocytes in culture (Svichar and Chesler, 2003). By increasing the effective buffering capacity, and hence attenuating the dissipation of the H+ gradient, the NBC enhances the efficacy of MCT1 activity, and even transports Na+ against its steep gradient, when the NBC is outwardly directed. In glial cells, an outwardly directed NBC might also be enhanced by membrane hyperpolarization, as induced by an increase in the membrane K+ conductance (cf. Deitmer and Rose, 1996). In a similar way as described here for MCT1 and NBC, the NBC might also affect the activity of other transporters, which carry acid/base equivalents, such as, e.g., the electrogenic glutamate transporter.
Acknowledgments
We thank Sandra Galic for helping with the lactate flux measurements and Hans-Peter Schneider for excellent technical assistance. We are grateful to Dr. Walter F. Boron, Yale University, New Haven, CT, for providing the human NBC1 cDNA clone.
This study was supported by a stipend by the State of Rheinland-Pfalz to H.B., by a Faculty Research Grant F01049 of the Australian National University to S.B., and by the Deutsche Forschungsgemeinschaft to J.W.D. (De 231/16-1).
References
- Abuladze, N., I. Lee, D. Newman, J. Hwang, K. Boorer, A. Pushkin, and I. Kurtz. 1998. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J. Biol. Chem. 273:17689–17695. [DOI] [PubMed] [Google Scholar]
- Becker, H. M., S. Bröer, and J. W. Deitmer. 2003. Enhancement of lactate transport by the monocarboxylate transporter MCT1 by coexpression with the sodium-bicarbonate cotransporter NBC in Xenopus oocytes. Pflugers Arch. 445:S99. [Google Scholar]
- Bevensee, M. O., B. M. Schmitt, I. Choi, M. F. Romero, and W. F. Boron. 2000. An electrogenic Na+-HCO3− cotransporter (NBC) with a novel COOH-terminus, cloned from rat brain. Am. J. Physiol. Cell Physiol. 278:C1200–C1211. [DOI] [PubMed] [Google Scholar]
- Bevensee, M. O., R. A. Weed, and W. F. Boron. 1997. Intracellular pH regulation in cultured astrocytes from rat hippocampus. I. Role of HCO3−. J. Gen. Physiol. 110:453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron, W. F., and E. L. Boulpaep. 1983. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3 transport. J. Gen. Physiol. 81:53–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröer, S., B. Rahman, G. Pellegri, L. Pellerin, J.-L. Martin, S. Verelysdonk, B. Hamprecht, and P. J. Magistretti. 1997. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylattransporters in astroglial cells and neurons. J. Biol. Chem. 272:3096–3102. [DOI] [PubMed] [Google Scholar]
- Bröer, S., H.-P. Schneider, A. Bröer, B. Rahman, B. Hamprecht, and J. W. Deitmer. 1998. Characterisation of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem. J. 333:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, G. A. 2002. Lactate shuttles in nature. Biochem. Soc. Trans. 30:258–264. [DOI] [PubMed] [Google Scholar]
- Brune, T., S. Fetzer, K. H. Backus, and J. W. Deitmer. 1994. Evidence for electrogenic sodium-bicarbonate cotransport in cultured rat cerebellas astrocytes. Pflugers Arch. 429:64–71. [DOI] [PubMed] [Google Scholar]
- Burnham, C. E., M. Flagella, Z. Wang, H. Amlal, G. E. Shull, and M. Soleimani. 1998. Cloning, renal distribution, and regulation of the rat Na+-HCO3− cotransporter. Am. J. Physiol. 274:F1119–F1126. [DOI] [PubMed] [Google Scholar]
- Choi, I., M. F. Romero, N. Khandoudi, A. Bril, and W. F. Boron. 1999. Cloning and characterization of a human electrogenic Na+-HCO3− cotransporter isoform (hhNBC). Am. J. Physiol. 276:C576–C584. [DOI] [PubMed] [Google Scholar]
- De Bruijne, A. W., H. Vreeburg, and J. Van Steveninck. 1983. Kinetic analysis of L-lactate transport in human erythrocytes via the monocarboxylate-specific carrier system. Biochim. Biophys. Acta. 732:562–568. [DOI] [PubMed] [Google Scholar]
- Deitmer, J. W. 1991. Electrogenic sodium-dependent bicarbonate secretion by glial cells of the leech central nervous system. J. Gen. Physiol. 98:637–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer, J. W. 2000. Glial strategy for metabolic shuttling and neuronal function. Bioessays. 22:747–752. [DOI] [PubMed] [Google Scholar]
- Deitmer, J. W. 2002. A role of CO2 and bicarbonate transporters in metabolic exchanges in the brain. J. Neurochem. 80:721–726. [DOI] [PubMed] [Google Scholar]
- Deitmer, J. W., and C. R. Rose. 1996. pH regulation and proton signaling by glial cells. Prog. Neurobiol. 48:73–103. [DOI] [PubMed] [Google Scholar]
- Deitmer, J. W., and W. R. Schlue. 1987. The regulation of intracellular pH by identified glial cells and neurones in the central nervous system of the leech. J. Physiol. 388:261–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer, J. W., and W. R. Schlue. 1989. An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cells. J. Physiol. 411:179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer, J. W., and H.-P. Schneider. 1995. Voltage-dependent clamp of intracellular pH of identified leech glial cells. J. Physiol. 485:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer, J. W., and H.-P. Schneider. 1998. Acid/base transport across the leech giant glial cell membrane at low external bicarbonate concentration. J. Physiol. 512:459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen, R., H. Wiesinger, and B. Hamprecht. 1993. Uptake of L-lactate by cultured rat brain neurons. Neurosci. Lett. 163:5–7. [DOI] [PubMed] [Google Scholar]
- Eladari, D., R. Chambrey, T. Irinopoulou, F. Leviel, F. Pezy, P. Bruneval, M. Paillard, and R. A. Podevin. 1999. Polarized expression of different monocarboxylate transporters in rat medullary thick limbs of Henle. J. Biol. Chem. 274:28420–28426. [DOI] [PubMed] [Google Scholar]
- Geers, C., and G. Gros. 2000. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol. Rev. 80:681–715. [DOI] [PubMed] [Google Scholar]
- Gerhart, D. Z., B. E. Enerson, O. Y. Zhdankina, R. L. Leino, and L. R. Drewes. 1997. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am. J. Physiol. 273:E207–E213. [DOI] [PubMed] [Google Scholar]
- Giffard, R. G., M. C. Papadopoulos, J. A. van Hooft, L. Xu, R. Giuffrida, and H. Monyer. 2000. The electrogenic sodium bicarbonate cotransporter: developmental expression in rat brain and possible role in acid vulnerability. J. Neurosci. 20:1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap, A. P., and N. T. Price. 1999. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem. J. 343:281–299. [PMC free article] [PubMed] [Google Scholar]
- Heyer, M., S. Müller-Berger, M. F. Romero, W. F. Boron, and E. Frömter. 1999. Stoichiometry of the rat kidney Na+-HCO3− cotransporter expressed in Xenopus laevis oocytes. Pflügers Arch. 438:322–329. [DOI] [PubMed] [Google Scholar]
- Juel, C. 2001. Current aspects of lactate exchange: lactate/H+ transport in human skeletal muscle. Eur. J. Appl. Physiol. 86:12–16. [DOI] [PubMed] [Google Scholar]
- Magistretti, P. J., L. Pellerin, D. L. Rothman, and R. G. Shulman. 1999. Energy on demand. Science. 283:496–497. [DOI] [PubMed] [Google Scholar]
- Munsch, T., and J. W. Deitmer. 1994. Sodium-bicarbonate cotransport current in identified leech glial cells. J. Physiol. 474:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin, L., G. Pellegri, J. L. Martin, and P. J. Magistretti. 1998. Expression of monocarboxylate transporter mRNAs in mouse brain: support for a distinct role of lactate as an energy substrate for the neonatal vs. adult rat brain. Proc. Natl. Acad. Sci. USA. 95:3990–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitry-Yamate, C. L., S. Poitry, and M. Tsacopoulos. 1995. Lactate released by Müller glial cells is metabolized by photoreceptors from mammalia retina. J. Neurosci. 15:5179–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero, M. F., and W. F. Boron. 1999. Electrogenic Na+/HCO3− cotransporters: cloning and physiology. Annu. Rev. Physiol. 61:699–723. [DOI] [PubMed] [Google Scholar]
- Romero, M. F., M. A. Hediger, E. L. Boulpaep, and W. F. Boron. 1997. Expression cloning and characterization of renal electrogenic Na+/HCO3− cotransporter. Nature. 387:409–413. [DOI] [PubMed] [Google Scholar]
- Schmitt, B. M., U. V. Berger, R. M. Douglas, M. O. Bevensee, M. A. Hediger, G. G. Haddad, and W. F. Boron. 2000. Na/HCO3 cotransporters in rat brain: expression in glia, neurons, and choroid plexus. J. Neurosci. 20:6839–6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurr, A., W. Q. Dong, K. H. Reid, C. A. West, and B. M. Rigor. 1988. Lactic acidosis and recovery of neuronal function following cerebral hypoxia in vitro. Brain Res.438:311–314. [DOI] [PubMed] [Google Scholar]
- Schurr, A., R. S. Payne, J. J. Miller, and B. M. Rigor. 1997. Brain lactate is an obligatory aerobic energy substrate for functional recovery after hypoxia: further in vitro validation. J. Neurochem. 69:423–426. [DOI] [PubMed] [Google Scholar]
- Schurr, A., R. S. Payne, J. J. Miller, M. T. Tseng, and B. M. Rigor. 2001. Blockade of lactate transport exacerbated delayed neuronal damage in a rat model of cerebral ischemia. Brain Res. 895:268–272. [DOI] [PubMed] [Google Scholar]
- Sciortino, C. M., and M. F. Romero. 1999. Cation and voltage dependance of rat kidney electrogenic Na+-HCO3− cotransporter, rkNBC, expressed in oocytes. Am. J. Physiol. 277:F611–F623. [DOI] [PubMed] [Google Scholar]
- Svichar, N., and M. Chesler. 2003. Surface carbonic anhydrase activity on astrocytes and neurons facilitates lactate transport. Glia. 41:415–419. [DOI] [PubMed] [Google Scholar]
- Thomas, R. C., J. A. Coles, and J. W. Deitmer. 1991. Homeostatic muffling. Nature. 350:564. [DOI] [PubMed] [Google Scholar]
- Tong, C. K., L. P. Brion, C. Suarez, and M. Chesler. 2000. Interstitial carbonic anhydrase (CA) activity in brain is attributable to membrane-bound CA type IV. J. Neurosci. 20:8247–8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopulos, M., and P. J. Magistretti. 1996. Metabolic coupling between glia and neurons. J. Neurosci. 16:877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutsinos-Porche, B., G. Bonvento, K. Tanaka, P. Steiner, E. Welker, J. Y. Chatton, P. J. Magistretti, and L. Pellerin. 2003. Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron. 37:275–286. [DOI] [PubMed] [Google Scholar]
- Wetzel, P., A. Hasse, S. Papadopoulos, J. Voipio, K. Kaila, and G. Gros. 2001. Extracellular carbonic anhydrase activity facilitates lactic acid transport in rat skeletal muscle fibres. J. Physiol. 531:743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]