Abstract
Many aquatic microorganisms use gas vesicles to regulate their depth in the water column. The molecular basis for the novel physical properties of these floatation organelles remains mysterious due to the inapplicability of either solution or single crystal structural methods. In the present study, some folding constraints for the ∼7-kDa GvpA building blocks of the vesicles are established via matrix-assisted laser desorption ionization time-of-flight mass spectrometry studies of intact and proteolyzed vesicles from the cyanobacterium Anabaena flos-aquae and the archaea Halobacterium salinarum. The spectra of undigested vesicles show no evidence of posttranslational modification of the GvpA. The extent of carboxypeptidase digestion shows that the alanine rich C-terminal pentapeptide of GvpA is exposed to the surface in both organisms. The bonds that are cleaved by Trypsin and GluC are exclusively in the extended N-terminus of the Anabaena flos-aquae protein and in the extended C-terminus of the Halobacterium salinarum protein. All the potentially cleavable peptide bonds in the central, highly conserved portion of the protein appear to be shielded from protease attack in spite of the fact that some of the corresponding side chains are almost certainly exposed to the aqueous medium.
INTRODUCTION
Gas vesicles are gas-filled intracellular structures that provide buoyancy in many unicellular aquatic organisms. They have been found in over 150 species of procaryotes, in at least five phyla of bacteria, and in two of the phyla of archaea (Walsby, 1994). Gas vesicles generally take the shape of a cylindrical shell with hollow conical end-caps, although less regular shapes are found in some species. The most important difference among species is in the width of the vesicles, which relates to the ecological niche (Walsby, 1994; Walsby et al., 1995; Bright and Walsby, 1999)—narrower vesicles, with a larger shell/volume ratio, can withstand higher pressures and are found in organisms living in deeper aquatic environments. In this study, we focus on the gas vesicles of two organisms: the cyanobacterium Anabaena flos-aquae and the archaeon Halobacterium salinarum. H. salinarum gas vesicles are wider than those of A. flos-aquae (Fig. 1) and, with their relatively low shell/volume ratio, they provide efficient buoyancy in shallow salt flats.
FIGURE 1.
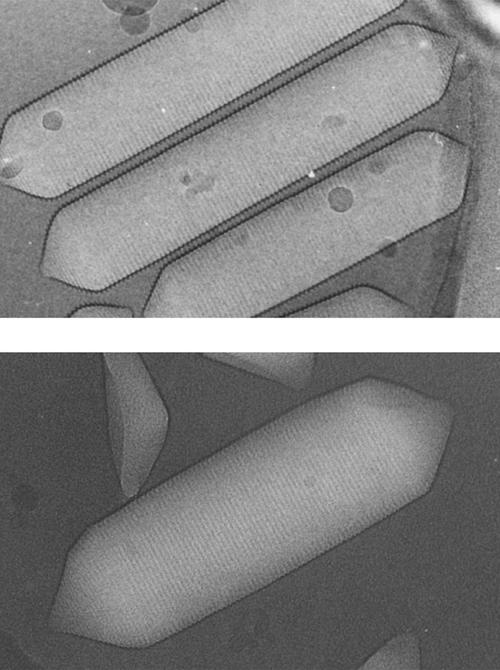
Electron micrographs (∼150,000×) of gas vesicles from A. flos-aquae (top, courtesy of N. Grigorieff and A. E. Walsby) and H. salinarum (bottom, obtained in collaboration with L. A. Melanson and D. DeRosier).
The gas vesicle shell has a composition more like that of a virus capsid than that of a vesicle membrane: it contains no lipids and is built almost exclusively of repeating units of the 7- to 8-kDa gas vesicle protein A (GvpA) (Hayes et al., 1986; Walker et al., 1984). Adhering to this basic shell, in a reinforcing fashion, are varying amounts of the larger, ∼21-kDa gas vesicle protein C (GvpC) (Hayes et al., 1992). The shell is freely permeable to small gas molecules, including water vapor (Walsby, 1977). The fact that water does not condense inside has been attributed to a highly hydrophobic and highly concave interior surface, both of which make the nucleation of water droplets very difficult (Walsby, 1972).
The GvpA sequences for A. flos-aquae (Hayes et al., 1986; Tandeau de Marsac et al., 1985) and H. salinarum (Jones et al., 1991) (Fig. 2) show that they differ mostly at the two ends. The central sequences (S8–E58 in A. flos-aquae and S5–E55 in H. salinarum) are highly homologous with conserved residues at 36 positions and conservative substitutions in the other 15. This region is strongly hydrophobic, but carries a net negative charge with 8 acidic residues balanced by only 5 basic residues. In A. flos-aquae, both ends of the protein are electrically neutral, the 7-residue N-terminal sequence carrying an EK pair and the 11-residue C-terminal sequence having no ionizable residues at all. In contrast, in H. salinarum, both ends of the protein are negatively charged, the 4-residue N-terminal sequence having one acidic residue and no basic residues and the 20-residue C-terminal sequence carrying 5 acidic residues and only one basic residue. The latter highly-charged segment is expected to be exposed to the exterior and may be responsible for the relatively large diameter of the vesicles formed in H. salinarum.
FIGURE 2.
Aligned amino acid sequences of GvpA from A. flos-aquae and H. salinarum (from Protein Data Bank). (a) Comparison of the GvpA sequence from A. flos-aquae (top) and the p-GvpA sequence from H. salinarum (bottom) with conserved residues shaded. This alignment differs from those of others (Jones et al., 1991; Walsby, 1994) in identifying the limited commonality at the C-terminus, as well as the extensive commonality in the central part of the sequence. (b) Comparison of different GvpA sequences from H. salinarum. From top to bottom: p-GvpA, c-GvpA and “low abundance” GvpA.
Since GvpA is one of the most hydrophobic proteins known (Walsby, 1994; Walsby and Hayes, 1989), hydrophobic interactions are expected to be important in forming the contacts between the GvpA units of the gas vesicle shell. However, the shells are not soluble in detergent. In normal sodium dodecyl sulfate-polyacrylamide gel electrophresis, most of the protein remains in the loading well and only a small portion moves through the gel as a family of GvpA oligomers (Walsby and Hayes, 1988). Gas vesicles can be dissolved in highly protic acids, such as 80% formic acid, but solution NMR shows that the protein is unfolded under these conditions (unpublished results in collaboration with P. Rossi and C. J. Turner) and dialysis to remove the formic acid causes the protein to precipitate rather than reassemble into vesicles (Walsby, 1994; Walker and Walsby, 1983). In contrast, gas vesicles in vivo are assembled and disassembled according to the organism's needs and the GvpA monomers are recycled in the process (Hayes and Walsby, 1984).
Although gas vesicles have been studied for almost 40 years (Bowen and Jensen, 1965; Walsby, 1977), their structure remains mysterious. Because the vesicles dissolve only under denaturing conditions, solution structure techniques cannot be applied. Electron micrographs of gas vesicles (e.g., in Fig. 1) show the presence of ribs running perpendicular to the vesicle axis. The spacing between the ribs is uniform throughout, including both the cylindrical and conical sections of the vesicle. Infrared spectra (Jones and Jost, 1971; Wober, 1974), x-ray diffraction data (Blaurock and Walsby, 1976), and atomic force microscopy (McMaster et al., 1996) indicate that gas vesicles have considerable β-sheet structure. Since the combination of extensive β-structure and insolubility in all but highly protic solvents is reminiscent of amyloids, comparison of gas vesicle structure and amyloid structure should prove interesting when both become available.
In recent years, matrix assisted laser desorption ionization time-of-flight mass spectrometery (MALDI-TOF MS) has been widely used for analysis of proteins directly from organelles or even whole cells (Rubakhin et al., 2000; Arnold et al., 1999). In this study we apply MALDI-TOF MS to suspensions of gas vesicles to determine 1), whether any posttranslational modification of GvpA is involved in gas vesicle assembly; and 2), which peptide bonds of GvpA are exposed to enzymatic cleavage in intact vesicles. This information can assist us in understanding GvpA folding.
MATERIALS AND METHODS
Isolation of gas vesicles
A. flos-aquae from the Cambridge Collection of Algae and Protozoa (CCAP, strain 1403/13f, Cambridge, UK) was grown in Jaworski's medium (CCAP) in static cultures at ∼500 Lux light intensity in Erlenmeyer flasks. About one-quarter of the flask volume was filled with medium and flasks were loosely covered by foil so gas exchange was not blocked. Floating cells were collected from the surface by pipette and lysed in 0.7 M sucrose by osmotic shrinkage of the protoplasts (Walsby, 1972).
H. salinarum strain II-7 (vacuole+, ruberin−, purple membrane+, in the lineage of Pfeifer et al., 1981) were provided by Mary Betlach. Colonies, selected for high gas vesicle production according to milky appearance, were grown in synthetic medium (Gochanauer and Kushner, 1969). Gas vesicle-rich cells were harvested by flotation in static cultures and lysed as described previously (Oesterhelt and Stoeckenius, 1974).
Gas vesicles were generally isolated and washed by six to seven cycles of flotation in water or buffer. In some cases, adhering GvpC was removed by washing with 6 M urea, 0.1 M Tris (Hayes et al., 1992). In the case of A. flos-aquae gas vesicles, flotation could be accelerated by centrifugation and washing could be accelerated by filtration on Millipore (Bedford, MA) membrane filters (Walsby, 1974). However, the more fragile H. salinarum gas vesicles couldn't withstand the pressure applied during centrifugation and filtration, and were isolated solely by repeated static flotation. On the other hand, these wider vesicles float more readily due to their greater buoyancy.
Enzymatic digestion
Intact gas vesicles were digested by mixing a gas vesicle suspension with protease in an appropriate buffer. The initial concentration of gas vesicles was 1–14 mg/ml, as determined by correlating the optical density (OD) of gas vesicle suspensions at 550 nm with the mass of a lyophilized aliquot of the suspension. The protease/Gvp mass ratio in most cases was 1:50. C-terminus digestion with carboxypeptidase-Y (CPY) (Sequazyme C-Peptide Sequencing Kit from Applied Biosystems, Foster City, CA) was carried out in ammonium citrate buffer, pH 6.1, at room temperature for up to 3 days. GluC (Sigma, St. Louis, MO) digestion was done in 0.1 M ammonium phosphate buffer at 37°C (Tomasselli et all., 1986) for up to 24 h. Trypsin (Sigma) digestion was conducted in 0.2 M NH4HCO3 at 37°C (Gorelic et al., 1977) for up to 28 h. In this case, the sample volume was large enough to monitor turbidity (OD550) during digestion.
MALDI MS analysis
All mass spectra were acquired on a PerSeptive Biosystems (Framingham, MA) Voyager DE-RP mass spectrometer with a nitrogen laser operating at 337 nm. We used the linear delayed extraction method in positive-ion mode. Different instrument settings were used for samples of undigested and digested vesicles, as described in Table 1.
TABLE 1.
Instrument settings
| Undigested vesicles | Digested vesicles | |
|---|---|---|
| Accelerating voltage | 25,000 volts | 20,000 volts |
| Grid voltage | 90% | 91.5% |
| Guide wire voltage | 0.05% | 0.05% |
| Extraction delay time | 150 ns | 125 ns |
| Mass range observed | 50,000 channels | 50,000 channels |
| Dwell time | 2 ns | 2 ns |
| Low mass gate | 1500 amu | 600 amu |
| Mass range displayed | 3000–46,000 amu | 600 to 8900 amu |
| Digitizer input bandwidth | 20 MHz | 500 MHz |
| Laser power | 2500–3600 | 2400–3300 |
Initial MALDI experiments were conducted using several different preparation procedures. The intact gas vesicles were dispersed in water, or 80% formic acid, or 0.01% trifluoroacetic acid (TFA) in 30:70 acetonitrile (ACN)/water. We tried both sinapinic acid and 2,5-dihydroxybenzoic acid (DHB) matrices. We used the sinapinic acid matrix in its usual solvent system (30% ACN in 0.3% TFA), but we tried the DHB matrix at 10 mg/ml in several different solvent systems: 100% water, 100% methanol, 100% ACN, 100% acetone, various mixtures of these four solvents, and 0.3% TFA in various ACN/water mixtures.
The most uniform crystals were obtained from DHB matrix in ACN/water mixtures, the uniformity increasing as the proportion of ACN increased. But even when the matrix crystals themselves were very uniform, the matrix/sample mixtures were not homogeneous in appearance—the protein seemed to form a globular mass not at all integrated with the needle-like DHB crystals—and we found it difficult to get consistent signals from one place to another in any given sample well. In addition, the strength of the signal from any given spot decreased rapidly as the laser impinged upon it, so reusing or returning to a spot that had given a good signal before did not necessarily give a good signal again.
We improved our results dramatically by sonicating the matrix/sample mixtures instead of vortexing them, and by then spotting them onto a sample plate which had been preheated to 45–50°C. For the first time we were able to get 10 successive spectra from the same sample—equal parts of GvpA from A. flos-aquae (suspended in 0.01%TFA in 30/70 ACN/water) and DHB (10 mg/ml in 0.3% TFA in 90/10 ACN/water). These 10 successive spectra gave an average mass ion value of 7415 amu (range 7426.56–7396.16 amu) using PerSeptive Biosystems Cal Mix 3 in standard diluent (30% ACN in 0.1% TFA) as an external standard to create a two-point calibration file based on bovine insulin [(M + H)+ average mass 5735 amu] and thioredoxin [(M + H)+ average mass 11674.5 amu].
Once we established a technique for getting reproducible signals from the gas vesicle samples, we used Cal Mix 3 as an internal rather than an external standard. Mixing 10 parts DHB (10 mg/ml in 90/10 ACN/water with no TFA), 10 parts GvpA (suspended in 0.01% TFA in 30/70 ACN/water), and 1 part Cal Mix 3, 10 successive spectra gave an average mass for GvpA of A. flos-aquae of 7395 (20–25 amu lower than the values obtained when Cal Mix 3 was used as an external standard).
Later we found that we could use aqueous suspensions of GvpA, and from that point our sample preparation remained as follows: 10 parts of DHB at 10 mg/ml in 90/10 ACN/water, 10 parts of gas vesicles suspended in water or proteolysis buffer, and 1 part of either internal standard or 0.01% TFA. The internal standard was either Cal Mix 3, or 10 μM bovine insulin (Sigma), in the standard diluent. The mixtures were sonicated for 10 min. Then 1 μl was deposited on a warm (45°C) gold or stainless steel target and the droplet was air dried.
RESULTS
Intact gas vesicles
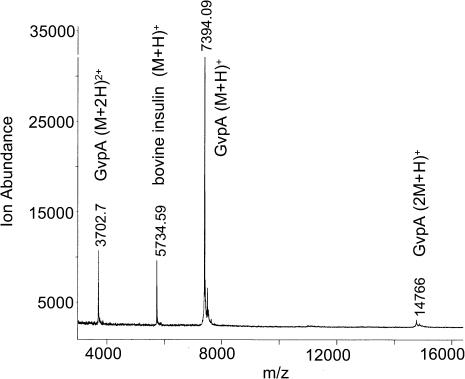
Fig. 3 shows a MALDI-TOF mass spectrum of undigested gas vesicles of A. flos-aquae. The major peak corresponds to singly charged GvpA monomers (m/z ratio = 7394), and smaller peaks correspond to singly charged dimers (m/z = 14,766) and doubly charged monomers (m/z = 3703). The mass of A. flos-aquae GvpA, calculated as an average from 25 spectra, equals 7397 Da. This matches the mass calculated from the amino acid sequence and indicates an absence of posttranslational modification. Note that GvpC (peak at mass 22,010) could only be seen in freshly prepared samples that were not washed with urea (spectra not shown), This is consistent with reports that GvpC is lost from the surface of isolated vesicles over time (Hayes et al., 1992).
FIGURE 3.
Mass spectrum of undigested gas vesicles of A. flos-aquae.
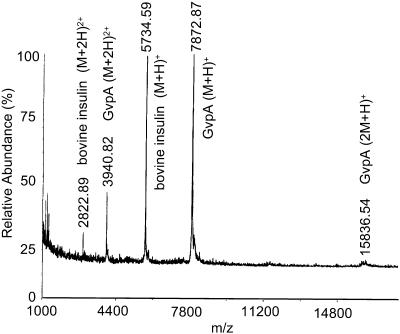
Fig. 4 shows a mass spectrum of undigested gas vesicles of H. salinarum. The average GvpA mass of 7873 equals the monomer mass calculated from the amino acid sequence for p-GvpA with no posttranslational modification. The absence of c-GvpA is consistent with reports that c-GvpA is most commonly expressed in vac− revertants (Pfeifer et al., 1997). The absence of “low abundance” GvpA (DasSarma et al., 1987), also referred to as GvpB (Surek et al., 1988), is consistent with our selection of colonies with high gas vesicle production.
FIGURE 4.
Mass spectrum of undigested gas vesicles of H. salinarum.
Trypsin cleavage of R-X and K-X bonds
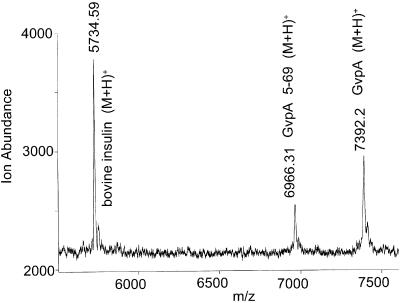
There are three lysines and three arginines in GvpA of A. flos-aquae. After digestion with trypsin for 24 h most gas vesicles of A. flos-aquae remain inflated (OD550 reduction over 24 h doesn't exceed 10%). The mass spectrum (Fig. 5) shows that some of the GvpA remains undigested. The only digestion product observed (m/z = 6966) was a GvpA monomer missing the four amino acids from the N-terminus (GvpA 5-69). This demonstrates that the K4-T5 bond is exposed to the exterior surface in many of the subunits. The other five K-X and R-X bonds, all in the central portion of the protein that is closely homologous to that of H. salinarum, appear to be inaccessible from the exterior solution in all subunits.
FIGURE 5.
Mass spectrum of A. flos-aquae gas vesicles digested with trypsin for 24 h.
H. salinarum GvpA has two lysines and three arginines (the remaining basic residue being a histidine). These gas vesicles collapsed (as indicated by the loss of turbidity) after 24 h of digestion with trypsin. The mass spectrum (Fig. 6) shows that some of the GvpA remains undigested. The peak at m/z = 6318 corresponds to digestion product GvpA 1-59, indicating that the K59-I60 bond is exposed to the exterior in some of the subunits. Again, K-X and R-X bonds in the central portion of the protein that is closely homologous to that of A. flos-aquae appear to be inaccessible from the exterior solution in all subunits. The peak at m/z = 6997 cannot be explained as a result of digestion with trypsin. We attribute this cleavage of the L65-T66 bond to chymotrypsin, a contaminant in most trypsin preparations.
FIGURE 6.
Mass spectrum of H. salinarum gas vesicles digested with trypsin for 24 h.
Carboxypetidase-Y digestion of the C-terminus
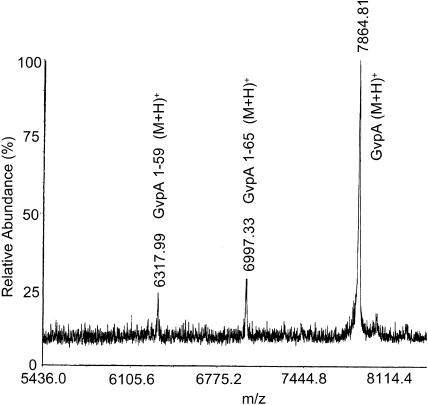
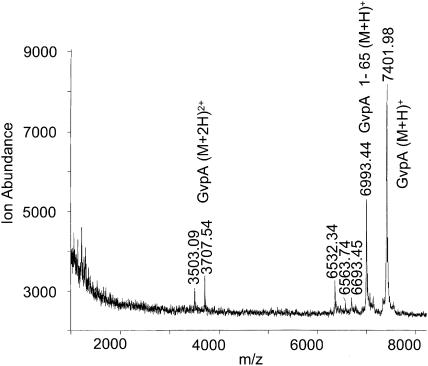
The main CPY digestion product for the gas vesicles of A. flos-aquae (Fig. 7) is GvpA 1-65 (m/z = 6993). Smaller digestion peaks were not reproducible and some of the GvpA remains undigested. The results indicate that the last five peptide bonds in the alanine-rich C-terminus are exposed to the surface in a significant fraction of the subunits.
FIGURE 7.
Mass spectrum of A. flos-aquae gas vesicles digested with CPY for 17 h. Further digestion did not produce additional products.
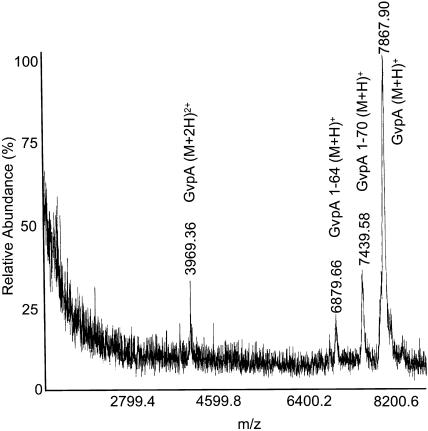
The main CPY digestion product for H. salinarum (Fig. 8) corresponds to GvpA 1-70 (m/z = 7440). However, some 1-64 GvpA (m/z = 6880) and undigested GvpA can be seen as well. The results indicate that, as in the A. flos-aquae protein, the last five peptide bonds in the alanine-rich C-terminus are exposed to the surface in some of the subunits. Furthermore, in a fraction of these subunits, another six peptide bonds are exposed, leading to a cleavage that has no analog in the A. flos-aquae protein.
FIGURE 8.
Mass spectrum of H. salinarum gas vesicles digested with CPY for 28 h. Further digestion did not produce additional products.
Endoproteinase GluC cleavage of E-X and D-X bonds
There are three aspartate residues and six glutamate residues in GvpA of A. flos-aquae. However, treatment of A. flos-aquae gas vesicles with GluC shows no sign of digestion. Thus none of the nine D-X or E-X bonds appear to be accessible from solution in any subunits.
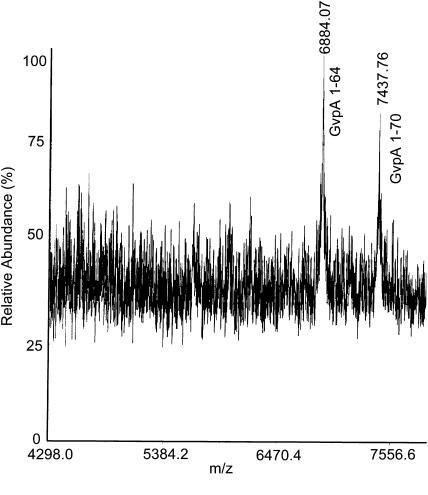
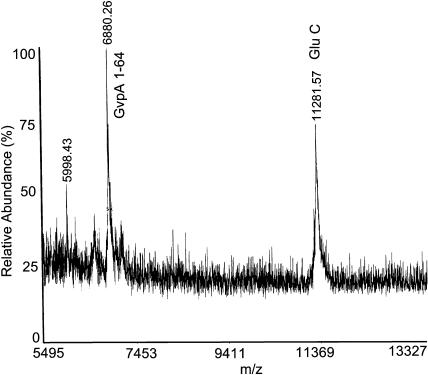
H. salinarum GvpA has five aspartate residues and nine glutamate residues. After digestion of H. salinarum gas vesicles with GluC for 4 h (Fig. 9), no undigested GvpA remains. Some GvpA 1-70 (m/z = 7438) was produced, but the main product was GvpA 1-64 (m/z = 6884). After digestion for 24 h (Fig. 10) only GvpA 1-64 was observed. However, consistent with the absence of digestion in the A. flos-aquae protein, none of the D-X or E-X bonds in the central portions of the protein appear to be accessible from the exterior solution in any subunits.
FIGURE 9.
Mass spectrum of H salinarum gas vesicles digested with GluC for 4 h.
FIGURE 10.
Mass spectrum of H salinarum gas vesicles digested with GluC for 24 h.
DISCUSSION
The gas vesicle shell constitutes an unusual interface between an aqueous phase and a gas phase. Both its rigidity and its insolubility in detergents suggest possible stabilization by covalent cross-links. However, MALDI-TOF MS results show that the mass of GvpA from gas vesicles of both A. flos-aquae and H. salinarum is that expected from the amino acid sequence. Thus, if there are any posttranslational modifications at all, they must be ones that do not survive the conditions of MALDI sample preparation.
Proteases provide probes of the exposure of peptide bonds to the surface of the vesicles. The results obtained here are summarized in Fig. 11. Of particular interest is where enzymatic cleavage fails to occur. In the central highly conserved region of the protein (S8–E58 in A. flos-aquae and S5–E55 in H. salinarum), none of the numerous R-X, K-X, E-X, or D-X bonds appear to be accessible to protease in the aqueous suspension. In addition, the flanking bonds D4-S5 and E56-I57 in H. salinarum appear to be inaccessible to GluC. Thus the peptide bonds of most of the central region of the protein are shielded from protease whether the ionizable side chains reach to the surface or not (about which, more below). On the other hand, the C-terminal pentamer of GvpA is digested by CPY in both species.
FIGURE 11.
Summary of the proteolysis of intact vesicles. GvpA sequences are shown for A. flos-aquae and H. salinarum as in Fig. 2. Potentially cleavable bonds (shaded) and actually cleaved bonds (arrows) are shown for trypsin (upper panel) and GluC (lower panel). In addition, the extent of C-terminal digestion by CPY is indicated by the angle brackets in the upper panel.
The action of trypsin shows exposure of the K4-T5 bond in the extended N-terminal segment of A. flos-aquae and exposure of the K59-I60 bond in the extended C-terminal segment of H. salinarum. Interestingly, the integrity of the vesicle structure is not compromised by loss of some of the N-termini in A. flos-aquae vesicles. On the other hand, loss of some of the C-termini in H. salinarum vesicles is sufficient to cause collapse of the vesicles. The radical change in structure corresponds to a radical change in the composition of the digested monomers. Whereas the intact protein contains 14 acidic residues and 6 basic residues, the loss of the E-rich 60–75 segment leaves only 10 acidic residues and 6 basic residues. Thus, the excess of acidic residues, and likely negative charge of the protein, has been halved in the affected monomers. This suggests that uniform electrostatic repulsions may be important in keeping the relatively wide vesicles of this species inflated.
The charged moieties of GvpA can contribute more specifically to the stability of the protein fold by forming internal salt bridges or interacting with water to define the outer surface of the structure. Given the excess of acidic residues over basic residues (by 3 in A. flos-aquae and 8 in H. salinarum), it is possible that all of the basic residues are involved in salt bridges. However, trypsin digestion shows that K4 in A. flos-aquae and K59 in H. salinarum are exposed to the exterior surface of the gas vesicles in at least some of the subunits. Thus, at most 5 basic residues are buried in these subunits. This leaves at least 4 of the 9 acidic residues in A. flos-aquae and at least 9 of the 14 acidic residues in H. salinarum without salt bridge partners. Since it is thermodynamically expensive to bury unpaired charges, these acidic residues are expected to be at the aqueous surface. However, no D-X or E-X bonds are accessible to GluC in A. flos-aquae and at most 3 are accessible to GluC in H. salinarum. This leaves at least 4 in A. flos-aquae and at least 6 in H. salinarum that are inaccessible to GluC. If these acidic residues are not buried, then their peptide bonds must be shielded from protease by well-packed side chains.
The enzymatic digestion observed in this study is not generally uniform. The exceptional case is GluC which leaves all the GvpA of A. flos-aquae intact and completely cleaves all the GvpA of H. salinarum at E64-L65. In contrast, trypsin and CPY cleave a fraction of the vesicle subunits. The most interesting explanation for this would be a unit cell of the vesicle lattice that contains more than one GvpA monomer per unit cell. Other possibilities are kinetics that are slower than the self-digestion of the enzyme and some nonspecific protection (e.g., through aggregation) before or during proteolysis. At this time there is no way to distinguish between these possibilities.
CONCLUSIONS
From the foregoing studies of intact and proteolyzed gas vesicles, we conclude that:
MALDI-TOF MS can be used to analyze gas vesicle proteins from suspensions of intact gas vesicles.
MALDI TOF MS shows no evidence of posttranslational modification of GvpA in either A. flos-aquae or H. salinarum. Thus, if any posttranslational modification is present in the gas vesicles, it must be labile under the conditions of the MALDI preparation.
Enzyme digestion shows that the K4-T5 bond is exposed to the exterior in at least some subunits of A. flos-aquae GvpA in aqueous suspensions of gas vesicles. There is no corresponding bond in H. salinarium GvpA.
Enzyme digestion also shows that the K59-I60 and E64-L65 bonds are exposed to the exterior in at least some subunits of H. salinarium GvpA in aqueous suspensions of gas vesicles. There are no corresponding bonds in A. flos-aquae GvpA.
In vesicles of both species, the C-terminus of GvpA is exposed to the exterior. Five C-terminal residues were removed by CPY in many subunits and a much smaller fraction of subunits were digested further.
In the central, highly conserved part of GvpA, none of the R-X, K-X, E-X, or D-X bonds are accessible to protease. This is despite the fact that some of the acidic groups are almost certainly located at the aqueous interface. These side chains must be tightly packed to protect their peptide bonds.
Acknowledgments
We thank Nikolaus Grigorieff and Anthony Walsby for their electron micrographs of Anabaena flos-aquae gas vesicles, Linda A. Melanson and David DeRosier for obtaining electron micrographs of Halobacterium salinarum gas vesicles, and Paolo Rossi and Christopher J. Turner for obtaining a 15N HSQC NMR spectrum of dissolved gas vesicles.
This work was supported by National Institutes of Health grant R01 GM-36810 and benefited from use of the electron microscopy facilities of the Keck Institute for Cellular Visualization at Brandeis University and the solution NMR facilities of the Harvard-MIT Center for Magnetic Resonance (supported by National Center for Research Resources grant RR-00995).
References
- Arnold, A. J., J. N. Karty, A. D. Ellington, and J. P. Reilly. 1999. Monitoring the growth of a bacteria culture by MALDI-MS of whole cells. Anal. Chem. 71:1990–1996. [DOI] [PubMed] [Google Scholar]
- Blaurock, A. E., and A. E. Walsby. 1976. Crystalline structure of the gas vesicle wall from Anabaena flos-aquae. J. Mol. Biol. 105:183–199. [DOI] [PubMed] [Google Scholar]
- Bowen, C. C., and T. E. Jensen. 1965. Blue-green algae — fine structure of gas vacuoles. Science. 147:1460–1462. [DOI] [PubMed] [Google Scholar]
- Bright, D. I., and A. E. Walsby. 1999. The relationship between critical pressure and width of gas vesicles in isolates of Planktothrix rubescens from Lake Zurich. Microbiology. 145:2769–2775. [DOI] [PubMed] [Google Scholar]
- DasSarma, S., T. Damerval, J. G. Jones, and N. Tandeau de Marsac. 1987. A plasmid-encoded gas vesicle protein gene in halophilic archaebacterium. Mol. Microbiol. 1:365–370. [DOI] [PubMed] [Google Scholar]
- Gochanauer, M. B., and D. J. Kushner. 1969. Growth and nutrition of extremely halophilic bacteria. Can. J. Microbiol. 15:1157–1165. [DOI] [PubMed] [Google Scholar]
- Gorelic, P. D., M. Greenwald, B. Frangione, M. Pras, and E. C. Franklin. 1977. The amino acid sequence of duck amyloid A (AA) protein. J. Immunol. 118:1113–1118. [PubMed] [Google Scholar]
- Hayes, P. K., B. Buchholz, and A. E. Walsby. 1992. Gas vesicles are strengthened by the outer-surface protein, GvpC. Arch. Microbiol. 157:229–234. [DOI] [PubMed] [Google Scholar]
- Hayes, P. K., and A. E. Walsby. 1984. An investigation into the recycling of gas vesicle protein derived from collapsed gas vesicles. J. Gen. Microbiol. 130:1591–1596. [Google Scholar]
- Hayes, P. K., A. E. Walsby, and J. E. Walker. 1986. Complete amino acid sequence of cyanobacterial gas-vesicle protein indicated 70-residue molecule that corresponds in size to the crystallographic unit cell. Biochem. J. 236:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D. P., and M. Jost. 1971. Characterization of the protein from gas vacuole membranes of the blue-green algae, Microcystis aeruginosa. Planta. 100:277–287. [DOI] [PubMed] [Google Scholar]
- Jones, J. G., D. C. Young, and S. DasSarma. 1991. Structure and organization of the gas vesicle gene cluster on the Halobacterium halobium plasmid pNRC100. Gene. 102:117–122. [DOI] [PubMed] [Google Scholar]
- McMaster, T. J., M. J. Miles, and A. E. Walsby. 1996. Direct observation of protein secondary structure in gas vesicles by atomic force microscopy. Biophys. J. 70:2432–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt, D., and W. Stoeckenius. 1974. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red in purple membrane. Methods Enzymol. 31:667–678. [DOI] [PubMed] [Google Scholar]
- Pfeifer, F., K. Krüger, R. Röder, A. Mayr, S. Ziesche, and S. Offner. 1997. Gas vesicle formation in halophilic Archaea. Arch. Microbiol. 167:259–268. [DOI] [PubMed] [Google Scholar]
- Pfeifer, F., G. Weidinger, and W. Goebel. 1981. Genetic variability in Halobacterium halobium. J. Bacteriol. 145:375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubakhin, S. S., R. W. Garden, R. R. Fuller, and J. V. Sweedler. 2000. Measuring the peptides in individual organelles with mass spectrometry. Nat. Biotechnol. 18:172–175. [DOI] [PubMed] [Google Scholar]
- Surek, B., B. Pillay, U. Rdest, K. Beyreuther, and W. Goebel. 1988. Evidence for two different gas vesicle proteins and genes in Halobacterium halobium. J. Bacteriol. 70:1746–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandeau de Marsac, N., D. Mazel, D. A. Bryant, and J. Houmard. 1985. Molecular cloning and nucleotide sequence of a developmentally regulated gene from the cyanobacterium calothrix PCC 7601: a gas vesicle protein gene. Nucleic Acids Res. 13:7223–7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasselli, A. B., R. Frank, and E. Schiltz. 1986. The complete primary structure of GTP: AMP phosphotransferase from beef heart mitochondria. FEBS Lett. 202:303–307. [DOI] [PubMed] [Google Scholar]
- Walker, J. E., P. K. Hayes, and A. E. Walsby. 1984. Homology of gas vesicle protein in cyanobacteria and halobacteria. J. Gen. Microbiol. 130:2709–2715. [Google Scholar]
- Walker, J. E., and A. E. Walsby. 1983. Molecular weight of gas vesicle protein from the planktonic cyanobacterium Anabaena flos-aquae and implications for structure of the vesicle. Biochem. J. 209:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby, A. E. 1972. Structure and function of gas vacuoles. Bacteriol. Rev. 36:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby, A. E. 1974. The isolation of gas vesicles from blue-green algae. Methods Enzymol. 31A:678–686. [DOI] [PubMed] [Google Scholar]
- Walsby, A. E. 1977. The gas vacuoles of blue-green algae. Sci. Am. 237:90–97. [Google Scholar]
- Walsby, A. E. 1994. Gas vesicles. Microbiol. Rev. 58:94–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby, A. E., and P. K. Hayes. 1988. The minor cyanobacterial gas vesicle protein gvpC is attached to the outer surface of the gas vesicle. J. Gen. Microbiol. 134:2647–2657. [Google Scholar]
- Walsby, A. E., and P. K. Hayes. 1989. Gas vesicle proteins. Biochem. J. 264:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby, A. E., P. K. Hayes, and R. Boje. 1995. The gas vesicles, buoyancy and vertical distribution of cyanobacteria in the Baltic Sea. Eur. J. Phycol. 30:87–94. [Google Scholar]
- Wober, W. 1974. Gasvakuol-membranen aus Halobacterium halobium. Fortschr. Med. 92:676. [Google Scholar]