Abstract
The time-resolved thermodynamics of the flavin mononucleotide (FMN)-binding LOV1 domain of Chlamydomonas reinhardtii phot (phototropin homolog) was studied by means of laser-induced optoacoustic spectroscopy. In the wild-type protein the early red-shifted intermediate LOV715 exhibits a small volume contraction, ΔV715 = −1.50 ml/mol, with respect to the parent state. LOV715 decays within few μs into the covalent FMN-Cys-57 adduct LOV390, that shows a larger contraction, ΔV390 = −8.8 ml/mol, suggesting a loss of entropy and conformational flexibility. The high energy content of LOV390, E390 = 180 kJ/mol, ensures the driving force for the completion of the photocycle and points to a strained photoreceptor conformation. In the LOV-C57S mutated protein the photoadduct is not formed and ΔV390 is undetected. Large effects on the measured ΔVs are observed in the photochemically competent R58K and R58K/D31Q mutated proteins, with ΔV390 = −2.0 and −1.9 ml/mol, respectively, and ΔV715 ≈ 0. The D31Q and D31N substitutions exhibit smaller but well-detectable effects. These results show that the photo-induced volume changes involve the protein region comprising Arg-58, which tightly interacts with the FMN phosphate group.
INTRODUCTION
The phototropin (phot) family comprises membrane-associated kinases (Huala et al., 1997) that undergo self-phosphorylation in response to ultraviolet-A (UV-A) blue light. Phototropins represent the main photoreceptors for phototropism, chloroplast relocation, and stomatal opening in higher plants (Briggs et al., 2001; Kagawa et al., 2001; Jarillo et al., 2001; Kinoshita et al., 2001; Sakai et al., 2001; Briggs and Christie, 2002). The recently characterized Chlamydomonas reinhardtii phot is involved in blue light-mediated gametogenesis (Huang and Beck, 2003). Phot proteins possess two N-terminal photoactive light, oxygen, and voltage (LOV) domains, a subset of the PerArntSim (PAS) superfamily (Zhulin et al., 1997), and a C-terminal serine/threonine kinase domain. Phot-LOV1 and LOV2 bind oxidized flavin mononucleotide (FMN) as chromophore and absorb maximally at ∼450 nm (LOV447) (Christie et al., 1999; Holzer et al., 2002). Blue-light illumination of isolated LOV domains triggers a photocycle involving the formation of a blue-shifted FMN-cysteine C(4a)-thiol adduct (LOV390) (Salomon et al., 2000, 2001) that slowly reverts to LOV447 in the dark (Kasahara et al., 2002). Nonphotochemically formed flavin C(4a)-thiol adducts are catalytic intermediates in flavoenzymes with redox active disulfides (Williams, 1992) and possible intermediates in the biosynthesis of oligomeric enzymes (Eschenbrenner et al., 2001). The photochemical formation of C(4a)-thiol adducts in solution was first described by Knappe and Hemmerich using sulfur compounds as substrates and suggested to proceed via the decay of the excited flavin triplet state (Knappe and Hemmerich, 1972). Accordingly, transient spectroscopic measurements of phot-LOV domains, have revealed that the photocycle comprises a red-shifted transient species, LOV715 (appearing within 20 ns, also referred to as LOV660), spectroscopically similar to the FMN triplet excited state (Swartz et al., 2001; Kottke et al., 2003). Molecular orbital calculations have confirmed the involvement of the FMN triplet state in LOV domains photochemical reactions (Neiss and Saalfrank, 2003). LOV715 decays on the short microsecond timescale into the photoadduct LOV390 (Swartz et al., 2001; Kottke et al., 2003). An analogous photocycle has been described for YtvA, a Bacillus subtilis flavoprotein containing a single LOV domain (Losi et al., 2002), the first characterized member of a growing prokaryotic family (Crosson et al., 2003).
Detailed structural information is now available for the LOV2 domain of phy3 (Crosson and Moffat, 2001, 2002), a phytochrome-phototropin hybrid photoreceptor from the fern Adiantum capillus-veneris (Nozue et al., 1998) and for C. reinhardtii phot-LOV1 (Fedorov et al., 2003), both in the dark (LOV447) and in the photoactivated state (LOV390). The two structures appear very similar. In the latter, the reactive Cys-57 thiol of LOV1 has two conformations, the higher occupied one (70%) is located 4.4 Å from C(4a) whereas in the lower occupied conformation the distance is 3.5 Å (Fedorov et al., 2003).
Despite the fact that much information is now available on the structure, photochemistry, and photophysics of isolated LOV domains, the molecular mechanisms of signal transduction are largely unknown. After light activation, the interaction of the chromophore bearing parts with partner domains and/or effector proteins must change to trigger the subsequent physiological responses. This effect can be basically achieved by light-induced discrete conformational changes (enthalpic effect) or by promoting the formation of conformational substates (entropic effect) (Crosson and Moffat, 2002; Crosson et al., 2003). The first evidence that photoactivation implies conformational changes, both in the chromophore and in the protein secondary structure, was obtained by means of one-dimensional NMR spectroscopy on oat phot1-LOV2 domain (Salomon et al., 2001). Recently, circular dichroism difference spectra have suggested that α-helicity is partially lost upon formation of LOV390 (Corchnoy et al., 2003). However, x-ray crystallography (Crosson and Moffat, 2002; Fedorov et al., 2003) and Fourier transform infrared (FTIR) spectroscopy (Swartz et al., 2002; Ataka et al., 2003) of plant and algal phot-LOV domains, have evidenced that the protein conformational changes are minor and restricted to the vicinity of the chromophore. At variance with these results, temperature-dependent FTIR measurements with a phy3-LOV2 construct (including ∼20 residues upstream and ∼40 downstream of the LOV2 domain itself), suggest progressive alteration of the protein structure that make possible to cryotrap conformational substates of the photoadduct, corresponding to UV-visible spectroscopically silent transitions (Iwata et al., 2003). The relevance of these conformational substates in the room temperature photocycle remain to be clarified, as well as the contribution of the extra residues in the construct and of protein hydration. In the absence of major conformational changes, light activation may affect the dynamics of phot-LOV domains, thus promoting the formation of conformational substates that can interact with partner domains, exemplifying an entropic effect (Crosson and Moffat, 2002; Crosson et al., 2003), in contrast to discrete conformational changes (enthalpic effect). Understanding the thermodynamics of photoadduct formation would thus be of great help in addressing this issue.
In this work we characterized the thermodynamic changes accompanying the formation of LOV715 and LOV390 in C. reinhardtii phot-LOV1 domain, by means of laser-induced optoacoustic spectroscopy (LIOAS). LIOAS is a time-resolved photocalorimetric technique that allows to determine enthalpy (ΔHi) and structural volume changes (ΔVi) of photo-initiated reactions (Braslavsky and Heibel, 1992), in the subnanosecond to microsecond time region. The measured ΔHi allow to estimate the energy content (Ei) of transient species, whereas ΔVi are related to the entropy changes (ΔSi) (Borsarelli and Braslavsky, 1998; Losi et al., 2001).
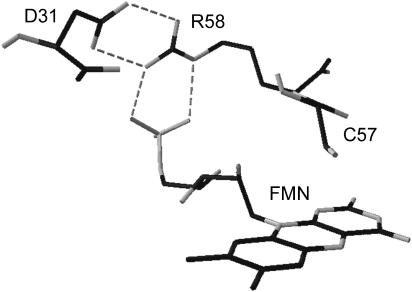
Given that the ΔVi as detected by LIOAS can receive different contributions, (i.e., intrinsic changes in the chromophore bond length, modifications of weak interactions, and solvation effects) and that not necessarily a conformational change implies a volume change of the system, the study of point mutated proteins can give important information on the origin of the measured structural changes. In this work the measurements were carried out with the wild-type LOV1 (LOV1-WT) and with several mutated proteins including LOV1-C57S (where the thiol adduct cannot be formed). Given the suggested involvement of the FMN phosphate group in determining the pH dependence of the dark recovery kinetics in LOV1-WT (Kottke et al., 2003), we also investigated proteins mutated in Arg-58 and Asp-31. Arg-58 is in fact tightly hydrogen bonded to Asp-31 and the FMN-phosphate (Fedorov et al., 2003). The mutated amino acids and their mutual orientation are shown in Fig. 1.
FIGURE 1.
Close-up view of the chromophore pocket obtained from the crystal structure of wild-type LOV1 (PDB entry 1N9L), showing the amino acids that have been mutated in this work. The dashed lines show the hydrogen-bonding interactions involving FMN, R58, and D31. The crystal structure was analyzed using the DeepView Swiss Pdb-Viewer program (Guex and Peitsch, 1997).
Time-resolved data on the decay of LOV715 into LOV390 and the corresponding E390 and ΔV390 provide information on the thermodynamic driving forces that ensure the completion of the photocycle, on the light-induced changes in flexibility and the protein movements. Furthermore, they show how the structural rearrangements involving the hydrogen bond network centered on Arg-58 (Fedorov et al, 2003) largely contribute to the observed light-induced volume changes.
Experimental procedures
Protein expression and purification
The LOV1 (amino acids 16–133) coding gene fragment from the full-length cDNA clone (AV 394090) of the C. reinhardtii phot, was inserted into the Escherichia coli expression vector pET16. The protein, carrying either a maltose binding-protein fusion at the N-terminus or 1 Gly and 10 His, was expressed in E. coli strain BL21. The protein was purified via Amylose Resin (New England Biolabs, Frankfurt, Germany) or Ni-NTA column (Qiagen, Hilden, Germany) according to the supplier's instructions. The C57S, R58K, R58/D31Q, D31Q, D31N, and W98F mutants were generated by site-directed mutagenesis, and expressed and purified in the same way as the wild-type LOV1. More details have been previously given (Kottke et al., 2003; Fedorov et al., 2003). The LOV1 domains were diluted in 10 mM phosphate buffer, pH = 8, 10 mM NaCl.
Instrumentation
Absorption spectra were recorded with a UV-2102PC spectrophotometer (Shimadzu Germany, Duisburg, Germany). Steady-state fluorescence measurements were performed with a Spex Fluorolog spectrofluorometer. FMN (FLUKA, Neu-Ulm, Germany) dissolved in phosphate buffer (FMNfree) was used as a standard (ΦF = 0.26 (van den Berg et al., 2001) to measure the fluorescence quantum yield of the LOV1 proteins.
Time traces of the dark recovery were recorded with a Lambda 9 spectrophotometer (PerkinElmer, Frankfurt, Germany) at 20°C and 475 nm after irradiation for 30 s with a 50-W tungsten lamp (Osram, München, Germany) through a 435-nm cutoff filter (GG435, Schott, Germany).
For the LIOAS experiments, excitation at 355 nm was achieved by the frequency-tripled pulse of a Nd:YAG laser (SL 456G, 6-ns pulse duration, Spectron Laser System, Rugby, Great Britain). Excitation at 450, 466, 425, and 480 nm was achieved by pumping the Nd:YAG laser into a Beta Barium Borate Optical Parametric Oscillator (OPO-C-355, bandwidth 420–515 nm, Laser Technik Vertriebs GmbH, Ertestadt-Friesheim, Germany) as previously described (Losi et al., 2000). The beam was shaped by a 0.5 × 6-mm slit, allowing a time resolution of ∼30 ns by using deconvolution techniques (Rudzki et al., 1985). The experiments were performed in the linear regime of amplitude versus laser fluence, which was up to 35 μJ/pulse. The total incident energy normally used was typically <20 μJ/pulse (<25 μmol/m2). Normally 10 shots were averaged for each waveform. Only a very small fraction of the sample was irradiated by the pulse (<2%) and the sample was gently stirred between each shot. This, together with the slow repetition rate (1 shot per minute) ensures that the concentration of the LOV1447 dark state changes very little during the measurements. This was proven by continuously monitoring the transmitted light during the experiment. New coccine (FLUKA, Neu-Ulm, Germany) was used as a calorimetric reference (Abbruzzetti et al., 1999). The time evolution of the pressure wave was assumed to be a sum of monoexponential functions. The deconvolution analysis yielded the fractional amplitudes (ϕi) and the lifetimes (τi) of the transients (Sound Analysis 3000, Quantum Northwest Inc., Spokane, WA). The time window was between 20 ns and 5 μs. At a given temperature and for each resolved i-th step the fractional amplitude ϕi is the sum of the fraction of absorbed energy released as heat (αi) and the structural volume change per absorbed Einstein (ΔVi), according to Eq. 1 (Braslavsky and Heibel, 1992; Rudzki-Small et al., 1992):
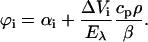 |
(1) |
Eλ is the molar excitation energy, β = (∂V / ∂T)p / V is the volume expansion coefficient, cp is the heat capacity at constant pressure, and ρ is the mass density of the solvent. In this work we used the so-called “two temperature” (TT) method to separate αi from ΔVi (Malkin et al., 1994); the sample waveform was acquired at a temperature for which heat transport is zero, Tβ=0 = 3.2°C and at a slightly higher temperature Tβ>0 (in this work we actually used three different Tβ>0, 6, 7, and 10°C to improve the statistics). At Tβ=0 the LIOAS signal is only due to ΔVi. The reference for deconvolution was recorded at Tβ>0, and Eqs. 2a and 2b were then used to derive αi and ΔVi:
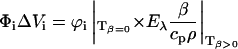 |
(2a) |
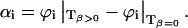 |
(2b) |
RESULTS
Optical spectroscopy and the dark recovery reaction
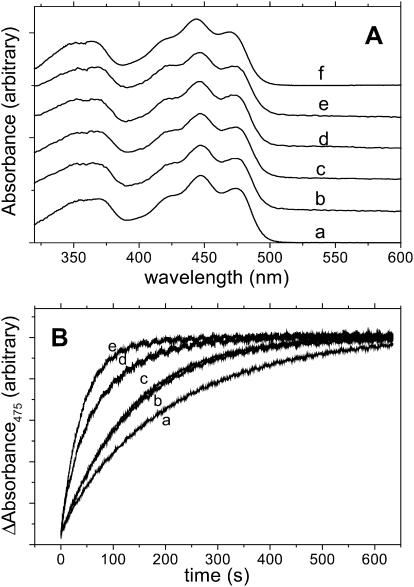
The absorption spectra of the mutated proteins show the same spectral features as wild-type LOV1 (Fig. 2). Only the C57S mutation resulted in a slight blue shift of the spectrum. The fluorescence maxima are the same in both cases, which means that the Stokes shift is larger for the latter protein (Holzer et al., 2002). The fluorescence quantum yield ΦF of LOV1-WT is 0.17 at 25°C and 0.19 at 3°C. LOV1-C57S exhibits a larger ΦF = 0.3 at 25°C independent of temperature and comparable to the free chromophore, FMNfree (ΦF = 0.26 (van den Berg et al., 2001)), in water solutions. The E00 energy (crossing of the absorption and normalized fluorescence spectra) is 246.5 kJ/mol for LOV1-WT and 248.5 kJ/mol for LOV1-C57S whereas for free FMN E00 is 243 kJ/mol (Losi et al., 2002).
FIGURE 2.
(A) Absorption spectra of a, LOV1-WT, and of the mutated: b, D31Q; c, D31N; d, R58K; e, R58K/D31Q; f, C57S. (B) Dark recovery kinetics of the photoadduct to the unphotolyzed parent state, as measured by recording the recovery of the absorbance at 475 nm at 20°C, for LOV1-WT and for the mutated proteins (except C57S).
The recovery of LOV390 to the unphotolyzed dark state LOV447 was followed by recording the absorption change at 475 nm after irradiation, as shown in Fig. 2 (bottom). A monoexponential fitting of the experimental curves gave the lifetime of the intermediate τrec reported in Table 1 for wild-type and mutated samples.
TABLE 1.
Lifetimes for the dark recovery reaction at 20°C
| Protein | τrec (s) |
|---|---|
| LOV1-WT | 204 ± 7 |
| -D31Q | 136 ± 3 |
| -D31N | 131 ± 3 |
| -R58K | 73 ± 2 |
| -R58K/D31Q | 43 ± 2 |
Light-induced energetics and structural changes
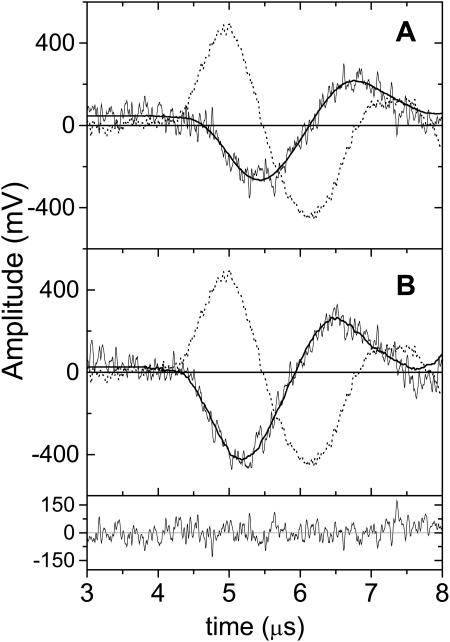
In the LIOAS experiments each signal could be satisfactorily fitted with a two-exponential function (Figs. 3 and 4). The samples were measured at Tβ=0 = 3.2°C and at Tβ>0 = 6, 7, and 10°C. The calorimetric reference signal (new coccine), was measured at Tβ>0. The LIOAS results derived from Eqs. 2a and 2b are reported in Table 2.
FIGURE 3.
A TT LIOAS experiment with LOV1-WT. Signal waveforms recorded after 450-nm excitation, at Tβ>0 = 6°C (A) and at Tβ=0 = 3.2°C. The position and time evolution of the signal with respect to the laser excitation (time = 0), are dictated by the pressure integration time, by the lifetimes of the transient species and by convolution of the signal with the response function of the setup (given by the calorimetric reference, dotted line). Superimposed to the sample signals are the fitting curves, obtained by deconvolution using a sum of two exponentials decay. In this case, at 3.2°C: ϕ1 = −0.3; τ1 < 20 ns; ϕ2 = −1.8, τ2 = 1.32 μs. At 6°C: ϕ1 = 0.1; τ1 < 20 ns; ϕ2 = −1.70; τ2 = 1.28 μs. The residuals distribution is shown (B) for the curve at 3.1°C. The reference compound (new coccine) measured at 6°C, has been used in both cases for deconvolution (the signal for new coccine is zero at 3.2°C).
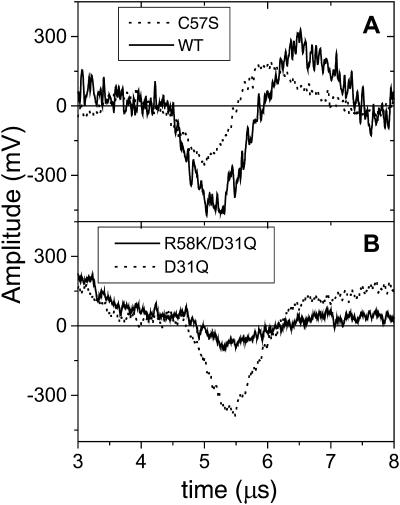
FIGURE 4.
LIOAS signals at Tβ=0 = 3.2°C; at this temperature no heat is deposited and the signal receives contribution only from structural changes. (A) LOV1-C57S (dotted line) and LOV1-WT (solid line). The latter has the largest negative contribution from the large contraction accompanying the formation of LOV390, with τ2 ≈ 1 μs. (B) The effect of the R58K/D31Q double mutation (solid line) is much larger than for the single D31Q substitution (dotted line). A similar effect is observed with the single R58K mutation (see Tables 2 and 3).
TABLE 2.
LIOAS parameters for LOV1 proteins
| LOV1 protein | *α1τ1 < 20 ns | †ΔV1 (ml/mol) | *α2 | †ΔV2 (ml/mol) | §τ2 (μs) |
|---|---|---|---|---|---|
| -WT | 0.36 ± 0.04 | −0.95 ± 0.1 | 0.07 ± 0.07 | −4.4 ± 0.7 | 1 |
| -W98F | 0.33 ± 0.03 | −0.95 ± 0.1 | 0.05 ± 0.04 | −4.8 ± 0.9 | 1 |
| -D31Q | 0.32 ± 0.03 | −0.45 ± 0.1 | 0.1 ± 0.1 | −4.3 ± 0.9 | 1.1 |
| -D31N | 0.31 ± 0.03 | −0.20 ± 0.1 | 0.07 ± 0.07 | −3.2 ± 0.3 | 1.2 |
| -R58K | 0.42 ± 0.03 | ≈−0.03 | 0.11 ± 0.03 | −1.0 ± 0.2 | 1.1 |
| -R58K/D31Q | 0.40 ± 0.03 | ≈−0.03 | 0.06 ± 0.03 | −1.0 ± 0.1 | 1.0 |
| ‡-WTHis | 0.36 ± 0.04 | −0.72 ± 0.1 | 0.09 ± 0.10 | −4.1 ± 0.8 | 1.2 |
| ‡-C57SHis | 0.31 ± 0.03 | −0.95 ± 0.1 | 0.10 ± 0.05 | −0.33 ± 0.1 | 2.0 |
| Sample | |||||
| FMNfree | 0.32 ± 0.03 | −1.1 ± 0.05 | 0.23 ± 0.10 | +0.72 ± 0.1 | 3.2 |
TT measurements: Tβ=0 = 3.2°C; Tβ>0 = 6, 7, and 10°C. The errors come from the average of two independent experiments (three for the WT proteins); in each experiment four waveforms were acquired at Tβ=0 and at Tβ>0.
Fraction of energy released as heat within 20 ns (α1) and in the time-resolved step (α2).
Volume changes per mol of absorbed photon, concomitant with α1 (ΔV1) and α2 (ΔV2).
His = 10 His tag. The tag is the maltose binding protein for the remaining proteins (see Experimental procedures).
The error is within 20%.
The values of α1 and ΔV1 correspond to the fast processes occurring within 20 ns after the laser pulse (not resolved), globally assigned to the formation of LOV715. The time-resolved step, providing the value of α2 and ΔV2 (τ2 ≈ 1 μs), is assigned to the formation of LOV390 upon LOV715 decay. The assignment is based on the lifetimes recovered by optical methods, where two time constants of 800 ns (80%) and 4 μs (20%) have been found (Kottke et al., 2003). We were not able to discriminate the 4-μs lifetime, probably because of the poor time resolution of LIOAS on this time region and the small contribution of this decay.
On the basis of energy balance considerations, α1, the fraction of the absorbed energy that is released within 20 ns (“prompt” heat), is expressed by Eq. 3a, which depicts all the nonradiative decays known to occur on this timescale for phot-LOV domains:
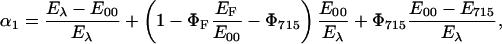 |
(3a) |
with Φ715 = formation quantum yield of LOV715, E715 = energy content of LOV715, EF = average energy of the fluorescence emission (232 kJ/mol for the LOV1 proteins). On the right sight of Eq. 3a, the first term represents the vibrational relaxation to the E00 energy level, the term in parenthesis the internal conversion process and the last term the formation of the red-shifted intermediate LOV715 (triplet state of the FMN chromophore). The different nonradiative pathways occurring within 20 ns are integrated by the piezoelectric transducer and only an overall amplitude, kinetically unresolved, can be retrieved (Losi and Braslavsky, 2003). Eq. 3a also assumes that LOV715 is the only energy storing species formed on this timescale, in agreement with the available transient spectroscopy data (Swartz et al., 2001; Kottke et al., 2003). Eq. 3a can be simplified in Eq. 3b:
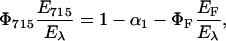 |
(3b) |
from which it is possible to derive the value of Φ715 knowing E715. The latter has been determined for the triplet state of FMN and riboflavin to be between 197 and 209 kJ/mol, by means of energy transfer (Song and Moore, 1968), phosphorescence spectroscopy (Chambers and Kearns, 1969; Lhoste et al., 1966) and LIOAS (Losi et al., 2002). Similarly, by combining the measured heat released and independent quantum yield measurements, we obtained E715 = 203 kJ/mol for YtvA and E715 = 198 kj/mol for the isolated YtvA-LOV domain (Losi et al., 2002, 2003). From this data it appears that the energy content of the FMN chromophore triplet is not appreciably affected by the protein environment in phot-LOV domains and related systems, a conclusion supported by phosphorescence measurements (see Discussion section). We can thus reasonably substitute E715 = 200 kJ/mol in Eq. 3b and calculate Φ715 (Table 3).
TABLE 3.
Molecular thermodynamic changes for LOV1 proteins as compared with B. subtilis YtvA and the isolated YtvA-LOV domain
| LOV1 protein | *Φ715 | †Φ390 | ‡ΔV715 (ml/mol) (τ1 < 20 ns) | §ΔV390 (ml/mol) | ¶E390 (kJ/mol) |
|---|---|---|---|---|---|
| -WT | 0.63 | 0.6 | −1.50 | −8.8 | 180 |
| -W98F | 0.67 | 0.64 | −1.40 | −8.9 | 184 |
| -D31Q | 0.65 | 0.62 | −0.70 | −7.6 | 165 |
| -D31N | 0.7 | 0.66 | −0.30 | −5.1 | 183 |
| -R58K | 0.55 | 0.52 | −0.05 | −2.0 | 155 |
| -R58K/D31Q | 0.58 | 0.55 | −0.05 | −1.9 | 182 |
| -WTHis | 0.63 | 0.6 | −1.15 | −8.0 | 171 |
| -C57SHis | 0.57 | – | −1.70 | – | – |
| Sample | |||||
| ‖**YtvA | 0.62 | 0.49 | −0.71 | −12.5 | 136 |
| **YtvA-LOV | 0.69 | 0.55 | −0.67 | −17.2 | 113 |
For the LOV1 proteins, calculated via Eqs. 3b and 5, taking E715 = 200 kJ/mol and Φ390 = 0.95 × Φ715 (see text). For YtvA and YtvA-LOV see Losi et al. (2002).
ΔV390 = ΔV1/Φ715.
ΔV390 = ΔV1/Φ715 + ΔV2/Φ390.
Energy content of LOV390, Eq. 5.
The fraction of heat released in the microsecond step (time resolved), α2, is related to the enthalpy change during LOV390 formation and can be expressed as follows:
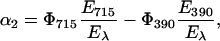 |
(4) |
with E390 = energy level of LOV390 with respect to the parent state. Combining Eqs. 3b and 4 we obtain Eq. 5:
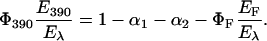 |
(5) |
Given that 95% of LOV715 is converted into LOV390 (Kottke et al., 2003), Φ390 can be readily estimated and Eq. 5 is used to calculate the energy content of the photoadduct, E390 (Table 3).
The structural volume change LOV715 (ΔV715) with respect to the parent state LOV447 coincides with ΔV1/Φ715, whereas we define ΔV390 = ΔV1/Φ715 + ΔV2/Φ390 as the total reaction volume change with respect to the unphotolyzed state upon formation of LOV390. The values of ΔV715 and ΔV390 are reported in Table 3.
In all the samples examined, LOV715 is formed with a small negative ΔV715. This feature is also observed for YtvA, YtvA-LOV, and the formation of the triplet state in FMNfree (Losi et al., 2002, 2003). Only the R58K substitution largely affects the value of ΔV715, which becomes barely detectable.
The formation of LOV390 concides with a more pronounced contraction ΔV390. The value of ΔV390 is strongly reduced upon the R58K substitution (Tables 2 and 3; Fig. 4). Also the D31N mutation has a well-detectable, albeit smaller effect on ΔV390. The W98F mutation has no effect. In C57S, LOV390 cannot be formed and the LOV715 decays biexponentially with 3-μs (relative amplitude = 25%) and 27-μs (75%) lifetimes (Kottke et al., 2003). We detected a very small contraction with ∼2-μs lifetime (Table 2), thus roughly corresponding to the shorter optical decay. Accordingly, from the values of α1, α2 (Table 1), and Φ715 (Table 2), and taking fluorescence into account, it can be calculated that the contraction corresponds to the decay of 24% of the triplet state, whereas 76% is stored as a long-lived triplet.
DISCUSSION
Energy content of the photocycle intermediates
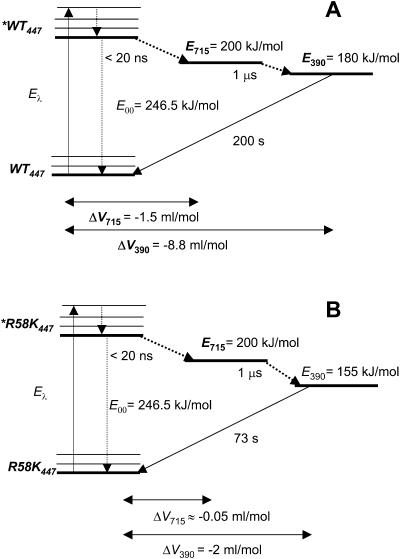
One of the most striking feature of LOV domains and of the related protein YtvA, is the high energy content of the two transient species (Fig. 5).
FIGURE 5.
State diagram for LOV1-WT (A) and the mutated R58K protein (B) with the corresponding light-induced volume changes. E715 is the energy content of the LOV715 transient species. E390 is the energy content of the covalent adduct LOV390. The E00 energy has been estimated to be 246.5 kJ/mol from the crossing of the absorption and fluorescence spectra. The recovery to the parent state, occurring with 200-s lifetime in LOV1-WT and 73 s in R58K, at room temperature (Kottke et al., 2003), falls out of the LIOAS time window.
Previously reported values of E715 for YtvA and YtvA-LOV, that the triplet state energy of FMN is not affected by embedding the chromophore in the protein cavity, namely E715 = ∼200 kJ/mol in all cases (Losi et al., 2002, 2003) (referred to as E660). Accordingly, in isolated phot1-LOV domains, the onset of the phosphorescence is observed between 575 and 600 nm, corresponding to E715 = 206 and 199 kJ/mol, respectively (J. Kennis, unpublished, presented at the International Plant Photobiology Meeting, Marburg, Germany, 2003). Recently we reported a preliminary value of E715 = 170 kJ/mol for LOV1-WT, calculated from the Eq. 3b and Φ715 = 0.75 as determined by means of laser-flash photolysis actinometry (Losi and Braslavsky, 2003). This method may suffer from uncertainties related to the usage of a standard compound as actinometer and to the values of the absorption coefficient of transient species, whereas the value of the triplet energy as determined by phosphorescence measurements is more safe. Nevertheless this discrepancy, albeit not large, must be taken into account. Taken together, the two approaches provide for LOV1-WT a value of Φ715 between 0.63 and 0.75, with E715 between 170 and 200 kJ/mol. The high value of E715 shows that during the formation of the triplet state, the conformational relaxation of the surrounding protein moiety is minor. Low protein motion on the sub-ns timescale is typical also of other photosensors (e.g., rhodopsins, phytochrome, and photoactive yellow protein), in which the primary photochemical event, i.e., photoisomerization leads to the formation of a high-energy intermediate species (Losi and Braslavsky, 2003).
During the decay of LOV715 into the photoadduct LOV390, little energy is released as heat (Table 2, values of α2). As a consequence, the energy of the photoadduct (the putative signaling state), is located well above the unphotolyzed parent state: in LOV1-WT the value of E390 = 180 kJ/mol represents ∼70% of the E00 energy (246.5 kJ/mol) (E390 = 136 kJ/mol, 55% of the the E00 energy if we take Φ390 = 0.75 (Losi and Braslavsky, 2003). This ensures a large driving force for the dark recovery to the unphotolyzed state and points to a strained conformation for LOV390 (Losi et al., 2003). This feature is in sharp contrast with photothermal data obtained for the photoactive yellow protein (PYP), the structural prototype for the PAS domain superfamily among photoreceptors (Taylor and Zhulin, 1999). In PYP photocycle, triggered by chromophore isomerization (Kort et al., 1996), the putative signaling state pB is located only ∼60 kJ/mol above the parent state (∼20% of the E00 energy), suggesting a relaxed protein structure (Takeshita et al., 2002). Accordingly, a large conformational change can be detected at this stage of the photocycle (Xie et al., 2001). In phot-LOV1, on the contrary, light activation results in the formation of a high-energy signaling state, pointing to a strained photoreceptor conformation, in agreement with the minor conformational changes detected with other techniques (Crosson and Moffat, 2002; Fedorov et al., 2003; Ataka et al., 2003). Accordingly, Iwata and co-workers have recently suggested, on the basis of FTIR measurements in the temperature range from 77 to 295 K, that a loosening of the hydrogen bonds in turn and α-helical structures occurs at the lowest temperatures upon formation of the adduct in phy3-LOV2, but this loosening is reverted at higher temperatures with concomitant tightening of the β-structure (Iwata et al., 2003). Similar results, in terms of energy content, have been obtained for YtvA (Losi et al., 2002) and its isolated LOV domain (Losi et al., 2003). This may reflect a basically different mechanism of light activation between phot (and phot-related) receptors and photosensors based on isomerizable chromophores.
The quantum yields Φ715 and Φ390 for LOV1-WT as calculated from the released heat (Eqs. 3b and 5) are 0.63 and 0.60, respectively (assuming that E715 = 200 kJ/mol (Song and Moore, 1968; Chambers and Kearns, 1969; Lhoste et al., 1966; Losi et al., 2002, 2003) and that the efficiency of LOV390 formation from the triplet state is 0.95 (Kottke et al., 2003)). These values are similar to those measured for Ytva (Φ715 = 0.62, Φ390 = 0.49) (Losi et al., 2002) by means of laser-flash photolysis actinometry and for YtvA-LOV (Φ715 = 0.69, Φ390 = 0.55) by employing steady-state illumination and comparison with the full-length protein (Losi et al., 2003). The triplet quantum yield Φ715 = 0.63 as measured here, resembles that for FMN in water solution (Φ715 = 0.60 (Losi et al., 2002)) in agreement with recent findings for plant phot-LOV2 domains (Kennis et al., 2003). For riboflavin values between 0.4 and 0.6 have been reported (Islam et al., 2003; Moore et al., 1977). The value of Φ390 as measured in this work, is necessarily larger than the relative value of 0.3 as measured by Kasahara and co-workers (Kasahara et al., 2002). In fact, under conditions of continuous illumination, due to the fast (few microseconds) formation and long recovery lifetime of the photoadduct, underestimation of Φ390 can be caused by: i), light-induced depopulation of the parent state and consequentely of LOV715; ii), filter effect of LOV1715 at 450 nm; iii), the assumption that no photoproduct is formed while determining the initial slope of the fluorescence signal; and iv), excluding a light-induced back reaction from the photoadduct. However, there is evidence for a photo-induced decay of LOV1390 (Kottke et al., 2003).
The quantum yield for triplet formation in LOV1-WT was recently determined by means of picosecond laser double-pulse excitation and time-resolved fluorescence detection, as Φ715 = 0.255 (Islam et al., 2003), affording Φ390 = 0.240. This is in contrast to the Φ715 and Φ390 determined in this work, albeit we do not know the reason of this discrepancy. With Φ715 = 0.255 and Φ390 = 0.242 we would obtain, from energy balance considerations using Eqs. 3b and 5, E715 = 494 kJ/mol and E390 = 446 kJ/mol, namely the energy level of the transient species would be higher than the excitation energy (Eλ = 265.8 kJ/mol, λex = 450 nm), which is obviously not plausible. The quantum yield of internal conversion reported in that work, ΦIC = 0.575, is also not compatible with the small “prompt” heat (α1 = 0.36) that we measured here. According to Eq. 3a we should in fact detect a value for α1 equal to 0.66. One possible reason for the observed discrepancy could rely on a dependence of Φ715 on the excitation wavelength (λex = 351.3 nm in Islam et al. (2003), λex = 450 nm in this work). Indeed we observed that Φ715 remains constant within blue-light excitation (from 420 to 475 nm), but decreased dramatically by exciting with 355 nm light (Φ715 = 0.37, LIOAS data not shown). This aspect deserves further investigation that goes beyond the scope of this manuscript.
Structural changes and mutagenesis effects
In general a change in volume (ΔV) as measured by means of LIOAS can receive different contributions and does not necessarily imply a protein conformational change in the secondary or tertiary structure. A ΔV may arise from changes in the van der Waals volumes of the protein (which in this case does not change), from solute-solvent effects (i.e., electrostriction, change in hydrogen bonds, and other weak interactions), and from changes in the atom packing within the protein core (Losi and Braslavsky, 2003). In LOV1-WT, the formation of the first transient species LOV715 is accompanied by a small contraction, ΔV715 = −1.50 ml/mol. This feature is similar to the formation of the FMN triplet state in aqueous solution and to the ΔV715 in YtvA and YtvA-LOV (Losi et al., 2002, 2003) (Table 3). The small negative ΔV715 can be related to the larger polarity of the FMN triplet with respect to the parent state (Song, 1968; Neiss and Saalfrank, 2003) inducing strengthening of weak polar interactions with the surrounding environment (Losi et al., 2003).
The decay of LOV715 into LOV390 is accompanied by a larger volume contraction, ΔV2 = −4.4 ml/mol in LOV1-WT. Accordingly, ΔV2 is barely detectable in the C57S mutated protein, for which LOV390 is not formed, confirming that the relatively large ΔV2 corresponds to the establishment of the covalent C(4a)-thiol bond. The small 2-μs contraction as observed in LOV1-C57S, corresponding to the decay of 26% of the triplet state, does not favor a one-step mechanism of quenching via molecular oxygen (which should result in a back expansion), but rather the formation of a species more polar than LOV715, e.g., a radical intermediate that causes electrostriction (volume contraction). This might be in line with the recent observation of a more intense absorption band at 500 nm in the optical transient spectrum of LOV1-C57S, possibly corresponding to a radical species (Kottke et al., 2003). Still, it remains to be clarified why this contribution to the decay is only detectable in the presence of oxygen (Kottke et al., 2003).
In the WT protein, the values of ΔV1 and ΔV2 afford a total ΔV390 = −8.8 mL/mol (−14.7 Å3) with respect to the parent state. A volume contraction is not in contrast with the formation of the covalent bond, which should render the system more rigid and compact, thus decreasing the structural flexibility of the protein (entropy loss). Indeed the magnitude of ΔV390 has been shown to correlate with the conformational flexibility of the parent state in YtvA and YtvA-LOV (Losi et al., 2003).
The large effect of the R58K substitution, (ΔV715 = −0.05 ml/mol, ΔV390 = −2.0 ml/mol) that does not impair the photochemistry, gives important information on the origin of the light-induced volume changes in LOV domains and related systems. The mutation does not alter the absorption and fluorescence spectra, and has minor effects on Φ715 and Φ390, indicating that the microenvironment of the flavin ring is the same as in the WT. The only possible explanation for the large difference in the volume changes can reside on the altered hydrogen bonds and/or other weak interactions that Arg-58 forms in the LOV1-WT. Arg-58 is the center of a hydrogen bond (HB) network in the vicinity of the FMN phosphate (Fedorov et al., 2003). In the dark state the FMN phosphate group is stabilized by HB and/or salt bridges with Arg-58 and Arg-74, involving the oxygen atoms (Arg-58: O1P-Nɛ, 2.8 Å and O2P-NH2, 3.0 Å; Arg-74: O3P-NH1, 2.59 Å). Arg-58 is further hydrogen bonded with Asp-31 (the two residues mutated in this work). Upon formation of LOV390, the lateral chain of Arg-58 moves slightly away from the FMN ribityl chain, causing a length change of the HB with the FMN phosphate (O1P-Nɛ, 2.7 Å and O2P-NH2, 3.2 Å). The HBs with Asp-31 are also slightly rearranged. A larger displacement involves Asn-56, which follows the Cys-57 movement toward FMN and brings it closer to Arg-58 (the distance between the backbone oxygen of Asn-56 and the backbone nitrogen of Arg-58 passes from 3.04 to 2.52 Å) (Fedorov et al., 2003). At the same time, the FMN phosphate group strengthens the weak HB interactions with Arg-74. These movements help stabilizing the photoadduct and must be reversed during the dark recovery reaction. In this scenario, the R58K substitution is not innocent, because Lys has only one terminal NH3+ group, which presumably forms one or two localized HB with the FMN phosphate and hardly any interaction with D31. Concomitantly, the linear hydrocarbon chain of the lysine should stay closer to the FMN ribityl chain, thus lowering its conformational freedom and rendering more difficult the rearrangements in the weak interactions depicted above. The reversibility to the parent state should be, in this context, facilitated with respect to the WT (the photoproduct is less stabilized) and indeed the recovery kinetics is much faster (see Table 1). The dramatic effects of the R58K mutation on the light-induced volume changes show that these very localized modifications are indeed a major source for the LIOAS-measured ΔV390, with little contribution from overall protein conformational changes. Accordingly the peripheral mutation W98F does not affect ΔV390. The double R58K/D31Q mutated protein behaves similar to R58K, confirming the hypothesis that the interaction of K58 with D31 is much weaker than the interaction of R58 with D31 in LOV1-WT. The contribution of R58 to the light-induced structural changes and its influence on the LOV390 lifetimes support the idea that the FMN phosphate group, directly interacting with this residue (Fedorov et al., 2003), is the titratable group responsible for the pH dependency of the recovery kinetics (Kottke et al., 2003). Furthermore, the large effect of the R58K mutation on ΔV715 shows that the above-described movements already start upon formation of the FMN triplet state. The D31N and D31Q substitution also have an effect on ΔV390 and on the recovery kinetics, albeit much smaller, most probably related to the weakening of the HB with R58. The involvement of the FMN ribityl chain and phosphate group in determining the magnitude of ΔV715 and ΔV390, offer an interpretation of formerly reported NMR data on oat phot1-LOV2 (Salomon et al., 2001). In that work, light-induced chemical shift changes of the ribityl carbon atoms and the phosphate moiety were detected for LOV2 reconstituted with isotope-labeled FMN, indicating a conformational change of this chromophore region upon formation of LOV390.
We note that the recovery reaction to the parent state is slower (larger τrec, Table 1) for larger ΔV390 (Table 3) showing that the structural barrier that the system has to overcome to complete the photocycle is a major rate determining factor. This effect is general and has been observed also in YtvA and its isolated YtvA-LOV (Losi et al., 2003).
The fact that the light-induced volume changes originate to a large extent in the vicinity of the chromophore and involve the FMN ribityl chain and phosphate group, does not necessarily imply that this protein region is involved in signal transduction, but solely enlightens peculiar molecular mechanisms that underlie photoactivation. Other regions in LOV domains might to be directly involved in intradomain communication, such as a highly conserved surface-exposed salt bridge between a glutamate and a lysine as recently proposed (Crosson et al., 2003), albeit this hypothesis awaits experimental verification.
CONCLUSIONS
The high energy level of the photoadduct in phot-LOV domains ensures the driving force for the completion of the photocycle and points to a strained protein conformation and little conformational changes with respect to the parent state. This feature appears to be a characteristic of photoreceptors that function according to the phot-LOV domain photoreactivity paradigm. The negative ΔV390 is consistent with the formation of a covalent bond, i.e., loss of conformational flexibility that should be linked to an entropy loss, but receives xlarge contributions from the rearrangements of the hydrogen bonds network centered on Arg-58 and involving the FMN phosphate group. As a whole, the results also stress the importance of weak interaction rearrangements in determining the magnitude of the light-induced volume changes in photosensors (Losi and Braslavsky, 2003).
Acknowledgments
We are grateful to Tina Schiereis and Gudrun Klihm for excellent technical assistance and to Dr. M. Fuhrmann and Professor B. Dick for many helpful suggestions. We thank Professor S.E. Braslavsky for the use of the time-resolved spectroscopic facilities.
The work was supported by the Deutsche Forschungsgemeinschaft (GK640).
References
- Abbruzzetti, S., C. Viappiani, D. H. Murgida, R. Erra-Balsells, and G. M. Bilmes. 1999. Non-toxic, water-soluble photocalorimetric reference compounds for UV and visible excitation. Chem. Phys. Lett. 304:167–172. [Google Scholar]
- Ataka, K., P. Hegemann, and J. Heberle. 2003. Vibrational spectroscopy of an algal phot-LOV1 domain probes the molecular changes associated with blue-light reception. Biophys. J. 84:466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsarelli, C. D., and S. E. Braslavsky. 1998. Volume changes correlate with enthalpy changes during the photoinduced formation of the (MLCT)-M-3 state of ruthenium(II) bipyridine cyano complexes in the presence of salts. A case of the entropy-enthalpy compensation effect\par. J. Phys. Chem. B. 102:6231–6238. [Google Scholar]
- Braslavsky, S. E., and G. E. Heibel. 1992. Time-resolved photothermal and photoacoustic methods applied to photoinduced processes in solution. Chem. Rev. 92:1381–1410. [Google Scholar]
- Briggs, W. R., C. F. Beck, A. R. Cashmore, J. M. Christie, J. Hughes, J. A. Jarillo, T. Kagawa, H. Kanegae, E. Liscum, A. Nagatani, K. Okada, M. Salomon, W. Rudiger, T. Sakai, M. Takano, M. Wada, and J. C. Watson. 2001. The phototropin family of photoreceptors. Plant Cell. 13:993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs, W. R., and J. M. Christie. 2002. Phototropins 1 and 2: versatile plant blue-light receptors. Trends Plant Sci. 7:204–210. [DOI] [PubMed] [Google Scholar]
- Chambers, R. W., and D. R. Kearns. 1969. Triplet states of some common photosensitizing dyes. Photochem. Photobiol. 10:215–219. [DOI] [PubMed] [Google Scholar]
- Christie, J. M., M. Salomon, K. Nozue, M. Wada, and W. R. Briggs. 1999. LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proc. Natl. Acad. Sci. USA. 96:8779–8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corchnoy, S. B., T. E. Swartz, J. W. Lewis, I. Szundi, W. R. Briggs, and R. A. Bogomolni. 2003. Intramolecular proton transfers and structural changes during the photocycle of the LOV2 domain of phototropin 1. J. Biol. Chem. 278:724–731. [DOI] [PubMed] [Google Scholar]
- Crosson, S., and K. Moffat. 2001. Structure of a flavin-binding plant photoreceptor domain: insights into light-mediated signal transduction. Proc. Natl. Acad. Sci. USA. 98:2995–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson, S., and K. Moffat. 2002. Photoexcited structure of a plant photoreceptor domain reveals a light-driven molecular switch. Plant Cell. 14:1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson, S., S. Rajagopal, and K. Moffat. 2003. The LOV domain family: photoresponsive signaling modules coupled to diverse output domains. Biochemistry. 42:2–10. [DOI] [PubMed] [Google Scholar]
- Eschenbrenner, M., L. J. Chlumsky, P. Khanna, F. Strasser, and M. S. Jorns. 2001. Organization of the multiple coenzymes and subunits and role of the covalent flavin link in the complex heterotetrameric sarcosine oxidase. Biochemistry. 40:5352–5367. [DOI] [PubMed] [Google Scholar]
- Fedorov, R., I. Schlichting, E. Hartmann, T. Domratcheva, M. Fuhrmann, and P. Hegemann. 2003. Crystal structures and molecular mechanism of a light-induced signaling switch: the Phot-LOV1 domain from Chlamydomonas reinhardtii. Biophys. J. 84:2492–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex, N., and M. C. Peitsch. 1997. SWISS-MODEL and Swiss-PdBViewer: an environment for comparative protein modeling. Electrophoresis. 18:2714–2723. [DOI] [PubMed] [Google Scholar]
- Holzer, W., A. Penzkofer, M. Fuhrmann, and P. Hegemann. 2002. Spectroscopic characterization of flavin mononucleotide bound to the LOV1 domain of Phot1 from Chlamydomonas reinhardtii. Photochem. Photobiol. 75:479–487. [DOI] [PubMed] [Google Scholar]
- Huala, E., P. W. Oeller, E. Liscum, I. S. Han, E. Larsen, and W. R. Briggs. 1997. Arabidopsis NPH1: a Protein kinase with a putative redox-sensing domain. Science. 278:2120–2123. [DOI] [PubMed] [Google Scholar]
- Huang, K., and C. F. Beck. 2003. Phototropin is the blue-light receptor that controls multiple steps in the sexual life cycle of the green alga Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA. 100:6269–6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, S. D. M., A. Penzkofer, and P. Hegemann. 2003. Quantum yield of triplet formation of riboflavin in aqueous solution and of flavin mononucleotide bound to the LOV1 domain of Phot1 from Chlamydomonas reinhardtii. Chem. Phys. 291:97–114. [Google Scholar]
- Iwata, T., D. Nozaki, S. Tokutomi, T. Kagawa, M. Wada, and H. Kandori. 2003. Light-induced structural changes in the LOV2 domain of Adiantum phytochrome3 studied by low-temperature FTIR and UV-visible spectroscopy. Biochemistry. 42:8183–8191. [DOI] [PubMed] [Google Scholar]
- Jarillo, J. A., H. Gabrys, J. Capel, J. M. Alonso, J. R. Ecker, and A. R. Cashmore. 2001. Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature. 410:952–954. [DOI] [PubMed] [Google Scholar]
- Kagawa, T., T. Sakai, N. Suetsugu, K. Oikawa, S. Ishiguro, T. Kato, S. Tabata, K. Okada, and M. Wada. 2001. Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science. 291:2138–2141. [DOI] [PubMed] [Google Scholar]
- Kasahara, M., T. E. Swartz, M. A. Olney, A. Onodera, N. Mochizuki, H. Fukuzawa, E. Asamizu, S. Tabata, H. Kanegae, M. Takano, J. M. Christie, A. Nagatani, and W. R. Briggs. 2002. Photochemical properties of the flavin mononucleotide-binding domains of the phototropins from Arabidopsis, rice, and Chlamydomonas reinhardtii. Plant Physiol. 129:762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennis, J. T. M., S. Crosson, M. Gauden, I. H. M. van Stokkum, K. Moffat, and R. van Grondelle. 2003. Primary reactions of the LOV2 domain of phototropin, a plant blue-light photoreceptor. Biochemistry. 42:3385–3392. [DOI] [PubMed] [Google Scholar]
- Kinoshita, T., M. Doi, N. Suetsugu, T. Kagawa, M. Wada, and K. Shimazaki. 2001. Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature. 414:656–660. [DOI] [PubMed] [Google Scholar]
- Knappe, W. R., and P. Hemmerich. 1972. Covalent intermediates in flavin-sensitized photodehydrogenation and photodecarboxylation. Z. Naturforsch. 27:1032–1035. [DOI] [PubMed] [Google Scholar]
- Kort, R., H. Vonk, X. Xu, W. D. Hoff, W. Crielaard, and K. J. Hellingwerf. 1996. Evidence for trans-cis isomerization of the p-coumaric acid chromophore as the photochemical basis of the photocycle of photoactive yellow protein. FEBS Lett. 382:73–78. [DOI] [PubMed] [Google Scholar]
- Kottke, T., J. Heberle, D. Hehn, B. Dick, and P. Hegemann. 2003. Phot LOV1: photocycle of a blue-light receptor domain from the green alga Chlamydomonas reinhardtii. Biophys. J. 84:1192–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhoste, J. M., A. Haug, and P. Hemmerich. 1966. Electron paramagnetic resonance studies of the triplet state of flavin and pteridine derivatives. Biochemistry. 5:3290–3300. [Google Scholar]
- Losi, A., and S. E. Braslavsky. 2003. The time-resolved thermodynamics of the chromophore-protein interactions in biological photosensors. Learning from photothermal measurements. Phys. Chem. Chem. Phys. 5:2739–2750. [Google Scholar]
- Losi, A., E. Polverini, B. Quest, and W. Gärtner. 2002. First evidence for phototropin-related blue-light receptors in prokaryotes. Biophys. J. 82:2627–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losi, A., B. Quest, and W. Gärtner. 2003. Listening to the blue: the time-resolved thermodynamics of the bacterial blue-light receptor YtvA and its isolated LOV domain. Photochem. Photobiol. Sci. 2:759–766. [DOI] [PubMed] [Google Scholar]
- Losi, A., A. A. Wegener, M. Engelhard, W. Gärtner, and S. E. Braslavsky. 2000. Aspartate 75 mutation in sensory rhodopsin II from Natronobacterium pharaonis does not influence the production of the K-like intermediate, but strongly affects its relaxation pathway. Biophys. J. 78:2581–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losi, A., A. A. Wegener, M. Engelhardt, and S. E. Braslavsky. 2001. Enthalpy-entropy compensation in a photocycle: the K to L transition in sensory rhodopsin II from Natronobacterium pharaonis. J. Am. Chem. Soc. 123:1766–1767. [DOI] [PubMed] [Google Scholar]
- Malkin, S., M. S. Churio, S. Shochat, and S. E. Braslavsky. 1994. Photochemical energy storage and volume changes in the microsecond time range in bacterial photosynthesis—a laser induced optoacoustic study. J. Photochem. Photobiol. B: Biol. 23:79–85. [Google Scholar]
- Moore, W. M., J. C. McDaniels, and J. A. Hen. 1977. The photochemistry of riboflavin—VI. The photophysical properties of isoalloxazines. Photochem. Photobiol. 25:505–512. [DOI] [PubMed] [Google Scholar]
- Neiss, C., and P. Saalfrank. 2003. Ab initio quantum chemical investigation of the first steps of the photocycle of phototropin: a model study. Photochem. Photobiol. 77:101–109. [DOI] [PubMed] [Google Scholar]
- Nozue, K., T. Kanegae, T. Imaizumi, S. Fukuda, H. Okamoto, K. C. Yeh, J. C. Lagarias, and M. Wada. 1998. A phytochrome from the fern Adiantum with features of the putative photoreceptor NPH1. Proc. Natl. Acad. Sci. USA. 95:15826–15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzki-Small, J., L. J. Libertini, and E. W. Small. 1992. Analysis of photoacoustic waveforms using the nonlinear least square method. Biophys. Chem. 41:29–48. [DOI] [PubMed] [Google Scholar]
- Rudzki, J. E., J. L. Goodman, and K. S. Peters. 1985. Simultaneous determination of photoreaction dynamics and energetics using pulsed, time resolved photoacoustic calorimetry. J. Am. Chem. Soc. 107:7849–7854. [Google Scholar]
- Sakai, T., T. Kagawa, M. Kasahara, T. E. Swartz, J. M. Christie, W. R. Briggs, M. Wada, and K. Okada. 2001. Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc. Natl. Acad. Sci. USA. 98:6969–6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon, M., J. M. Christie, E. Knieb, U. Lempert, and W. R. Briggs. 2000. Photochemical and mutational analysis of the FMN-binding domains of the plant blue light receptor phototropin. Biochemistry. 39:9401–9410. [DOI] [PubMed] [Google Scholar]
- Salomon, M., W. Eisenreich, H. Dürr, E. Scleicher, E. Knieb, V. Massey, W. Rüdiger, F. Müller, A. Bacher, and G. Richter. 2001. An optomechanical transducer in the blue light receptor phototropin from Avena sativa. Proc. Natl. Acad. Sci. USA. 98:12357–12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, P.-S. 1968. On the basicity of the excited state of flavins. Photochem. Photobiol. 7:311–313. [DOI] [PubMed] [Google Scholar]
- Song, P.-S., and T. A. Moore. 1968. Mechanism of the photodephosphorylation of menadiol diphosphate. A model for bioquantum conversion. J. Am. Chem. Soc. 90:6507–6514. [DOI] [PubMed] [Google Scholar]
- Swartz, T. E., S. B. Corchnoy, J. M. Christie, J. W. Lewis, I. Szundi, W. R. Briggs, and R. A. Bogomolni. 2001. The photocycle of a flavin-binding domain of the blue light photoreceptor phototropin. J. Biol. Chem. 276:36493–36500. [DOI] [PubMed] [Google Scholar]
- Swartz, T. E., P. J. Wenzel, S. B. Corchnoy, W. R. Briggs, and R. A. Bogomolni. 2002. Vibration spectroscopy reveals light-induced chromophore and protein structural changes in the LOV2 domain of the plant blue-light receptor phototropin 1. Biochemistry. 41:7183–7189. [DOI] [PubMed] [Google Scholar]
- Takeshita, K., Y. Imamoto, M. Kataoka, F. Tokunaga, and M. Terazima. 2002. Themodynamic and transport properties of intermediate states of the photocyclic reaction of photoactive yellow protein. Biochemistry. 41:3037–3048. [DOI] [PubMed] [Google Scholar]
- Taylor, B. L., and I. B. Zhulin. 1999. PAS domains: internal sensors of oxygen, redox potential and light. Microbiol. Mol. Biol. Rev. 63:479–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg, P. W., J. Widengren, M. A. Hink, R. Rigler, and A. G. Visser. 2001. Fluorescence correlation spectroscopy of flavins and flavoenzymes: photochemical and photophysical aspects. Spectrochim. Acta A: Mol. Biomol. Spectrosc. 57:2135–2144. [DOI] [PubMed] [Google Scholar]
- Williams, C. H., Jr. 1992. In Chemistry and Biochemistry of Flavoenzymes. F. Muller, editor. CRC Press, Boca Raton, FL. 121–211.
- Xie, A., L. Kelemen, J. Hendriks, B. J. White, K. J. Hellingwerf, and W. D. Hoff. 2001. Formation of a new buried charge drives a large-amplitude protein quake in photoreceptor activation. Biochemistry. 40:1510–1517. [DOI] [PubMed] [Google Scholar]
- Zhulin, I. B., B. L. Taylor, and R. Dixon. 1997. PAS domain S-boxes in Archaea, bacteria and sensors for oxygen and redox. Trends Biochem Sci. 22:331–333. [DOI] [PubMed] [Google Scholar]