Abstract
We investigate the issue of end versus side branching of actin filaments by Arp2/3 complex, using a combination of analytic theory, polymerization assays, and quantitative modeling. The analytic theory shows that the effect of capping protein on the initial stages of actin polymerization in the presence of Arp2/3 complex depends strongly on whether new Arp2/3 complex-induced branches grow from the sides or ends of existing filaments. Motivated by these results, we measure and quantitatively model the kinetics of actin polymerization in the presence of activated Arp2/3 complex, for a range of concentrations of capping protein. Our model includes the most important types of events involving actin and actin-binding proteins, and can be adjusted to include end branching, side branching, or both. The side-branching model gives a better fit to the experimental data than the end-branching model. An end-plus-side model including both types of branching gives a moderate improvement in the quality of the fit. Another side-branching model, based on aging of subunits' capacity for branch formation, gives a significantly better fit than the end-plus-side model. We discuss implications for actin polymerization in cells.
INTRODUCTION
Actin polymerization, which plays a crucial role in many forms of cell motility, often involves the formation of networks of actin filaments. The networks include branches between the side of one actin filament and the pointed end of another filament. Arp2/3 complex is present at the branching points, and is required for branch formation. Branched networks are seen in both ultrastructure studies of cell cytoplasm (Svitkina et al., 1997; Svitkina and Borisy, 1999) and purified proteins in vitro (Mullins et al., 1998). The role of Arp2/3 complex in stimulating actin polymerization is discussed in two recent reviews (Pollard et al., 2000; Higgs and Pollard, 2001). The branching process leads to autocatalytic polymerization (Higgs et al., 1999; Machesky et al., 1999; Pantaloni et al., 2000) in which the rate of creation of new filaments increases with the filament concentration. However, the details of the process by which new “daughter” filaments are generated are not well understood. Theories based on branching on filament sides and at filament barbed ends have been proposed. Structural studies of the Arp2/3 complex alone and at filament branch points (Volkmann et al., 2001; Robinson et al., 2001) support the side-branching model. In addition, fluorescence microscopy movies of filament branch formation have shown that side branching does occur, with a bias toward barbed ends in some studies (Amann and Pollard, 2001; Ichetovkin et al., 2002) or pointed ends in other studies (Fujiwara et al., 2002). On the other hand, arguments based on electron microscopy data for mother/daughter filament lengths, and the polymerization kinetics of actin in the presence of activated Arp2/3 complex, have been used to support the end-branching model (Pantaloni et al., 2000). A refinement of the side-branching model, in which the capacity of filament subunits to form new side branches ages over time, has also been proposed on the basis of fluorescence-microscopy movies (Ichetovkin et al., 2002).
Here, we discriminate among these models with experimental data from the polymerization kinetics of filament assembly in solution. The autocatalytic behavior of the Arp2/3 complex-induced branching results in a steep climb of the polymerized-actin density up to its final value. The steepness of the climb is reduced by the presence of capping protein (CP), which binds to barbed ends and thus slows polymerization. We reasoned that the strength of the CP effects should be different in end- versus side-branching models. In end-branching models, capping a filament makes it unavailable for branching, but in side-branching models the capped filament remains available, and the CP serves only to slow the growth of filaments. These differences are demonstrated in a simplified analytic model that is described below. In end-branching models, the autocatalytic growth rate goes to zero abruptly at a certain CP concentration; in side-branching models, it decreases with increasing CP concentration but never goes to zero. We have thus measured the time course of actin polymerization in the presence of Arp2/3 complex activated by GST-VCA and a range of CP concentrations, and modeled the results using both end- and side-branching models.
Simplified model for short times
At short times, the rates for growth, branching, and capping are determined by the initial concentrations of actin, Arp2/3 complex, and CP. We thus treat the polymerization kinetics via effective first-order rate constants. We also ignore pointed-end growth and capping for simplicity. Pointed ends grow slowly compared to barbed ends, and filaments nucleated by Arp2/3 complex have capped pointed ends. We thus obtain the following equations for side branching:
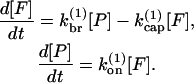 |
(1) |
Here [F] is the concentration of uncapped filaments, [P] is the polymerized-actin concentration, 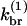 is the branching rate per subunit along the side of a filament,
is the branching rate per subunit along the side of a filament, 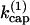 is the capping rate, and
is the capping rate, and 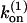 is the net barbed-end monomer addition rate; all of these are assigned constant values determined by the initial protein concentrations. By transforming the expressions in Eq. 1 into a second-order equation for [F], one readily shows that [F(t)] = [F(0)] exp(κt), where
is the net barbed-end monomer addition rate; all of these are assigned constant values determined by the initial protein concentrations. By transforming the expressions in Eq. 1 into a second-order equation for [F], one readily shows that [F(t)] = [F(0)] exp(κt), where
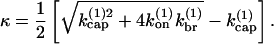 |
(2) |
For end branching, we assume in general that filament capping excludes branching, which leads to the rate equation
 |
(3) |
For this model, 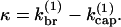 However, for completeness we also include the case in which capping allows branching,
However, for completeness we also include the case in which capping allows branching,
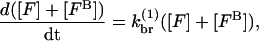 |
(4) |
where [FB] is the density of filaments capped at the barbed end. Then 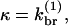 so that κ is independent of
so that κ is independent of 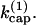
Fig. 1 shows that the behavior of κ differs strongly between side- and end-branching models. Both end-branching models give straight lines, whereas the side-branching model has a gradually decaying form. These differences are sufficiently large that they should persist in more realistic models, and in measurements of polymerization kinetics. This motivates the more detailed studies described below.
FIGURE 1.
Calculated short-time autocatalytic growth rates κ for side-branching model (solid line), end-branching model (dashed line), and modified end-branching model in which capped filaments can branch (dotted line). 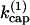 is the barbed-end capping rate. κ(0) is the value of κ at
is the barbed-end capping rate. κ(0) is the value of κ at 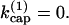
EXPERIMENTAL METHODS
CP purification
The plasmid for the expression of mouse CP α1 and β2 subunits (cDNA accession numbers U16740 and U10407, α1 and β2, respectively) was constructed by Dorothy Schafer in a pET-3d vector using the strategy described (Soeno et al., 1998) for chicken α1β1 CP. Mouse α1β2 CP was expressed and purified from BL21 Star (DE3) Escherichia coli as described (Palmgren et al., 2001). Purified CP was stored at −70°C in 10 mM TrisCl at pH 8.0, 40 mM KCl, 0.5 mM DTT, and 50% glycerol. Concentration of CP was determined using the extinction coefficient 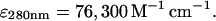
Arp2/3 complex purification
Arp2/3 complex was purified from bovine calf thymus as described (Higgs et al., 1999) with minor modifications. Purified complex was stored at −70°C in 20 mM Tris at pH 7.5, 100 mM KCl, 1 mM EGTA, 2 mM MgCl2, 0.1 mM ATP, 0.5 mM DTT, 1 mM NaN3, and 50% glycerol. Concentration of Arp2/3 complex was determined using the extinction coefficient 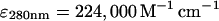 (Egile et al., 1999).
(Egile et al., 1999).
GST-VCA purification
The plasmid for the expression of the VCA domains from human N-WASp as a GST-fusion in the pGEX-4T1 vector, constructed as described (Egile et al., 1999), was a kind gift from Marie-France Carlier. GST-VCA was expressed in BL21 Star E. coli and purified using glutathione-agarose and standard protocols. GST-VCA was stored at −70°C in 50 mM Tris at pH 8.0, 150 mM NaCl, 0.5 mM EDTA, 0.5 mM DTT, 1 mM NaN3, and 50% glycerol. Concentration of GST-VCA was determined using the extinction coefficient 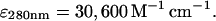
Actin polymerization assays
Actin was purified from chicken-breast skeletal muscle as described (Spudich and Watt, 1971). Actin monomers were purified by gel-filtration on a Sephacryl S-300 column (Vt ∼ 530 ml; 2.6 × 100 cm) equilibrated in ATP-G-buffer (10 mM Tris at pH 7.5, 0.2 mM ATP, 0.5 mM DTT, and 0.2 mM CaCl2). Actin was labeled with pyrenyliodoacetamide (Molecular Probes, Eugene, OR) as described (Cooper et al., 1983). Actin polymerization was followed by monitoring the fluorescence of pyrene-actin for 1000 s, at 25°C (with a reading taken every s) on a PTI Quantmaster spectrofluorimeter (Photon Technology International, Santa Clara, CA), with excitation at 368 nm and emission at 386 nm. Actin was used at a final concentration of 2.0 μM (5% pyrene labeled), with final buffer conditions of 10 mM Tris at pH 7.5, 100 mM KCl, 2 mM MgCl2, 0.2 mM ATP, 0.5 mM DTT, 0.2 mM CaCl2, and 1 mM EGTA. The G-actin was primed before the initiation of polymerization. To an aliquot of Ca2+-G-actin in ATP-G-buffer a 1/10th volume of 10 mM EGTA; 1 mM MgCl2 was added and the mixture preincubated for 90 s, at 25°C, to allow exchange of the Ca2+ for Mg2+ on the actin. A 1/20th volume of 200 mM Tris at pH 7.5, 2M KCl, 40 mM MgCl2, and 20 mM EGTA was then added to initiate polymerization. Arp2/3 complex, GST-VCA and CP were in 10 mM Tris at pH 7.5, 100 mM KCl, 2 mM MgCl2, 0.2 mM ATP, 0.5 mM DTT, 0.2 mM CaCl2, and 1 mM EGTA.
Spectrin-actin seeded (SAS) polymerization assays
Spectrin-actin seeds (SAS) were prepared from human erythrocytes as described (DiNubile et al., 1995). Capping protein was added to the actin mixture immediately after the priming incubation followed by the addition of the polymerization salt and finally by addition of 10 μl SAS. The solution was mixed by pipetting up and down twice. The reaction mixture was then transferred to the cuvette, and the pyrene fluorescence intensity measured.
Capping protein nucleation assays
Capping protein was added to the actin mixture immediately after the priming incubation and treated as before.
Activated Arp2/3 complex and CP polymerization assays
Arp2/3 complex (at 14.3 nM), GST-VCA (at 25 nM), and CP were added together to the actin mixture immediately after the priming incubation and treated as before. The delay between the addition of the all the reactants, mixing, and the commencement of fluorescence measurements was 12–15 s. The critical concentration of actin in the absence and presence of CP was measured as described (Cooper and Pollard, 1985).
Quantitative modeling methods
Our kinetic model treats filament growth, Arp2/3 complex-induced branching, barbed-end capping by CP, pointed-end capping by Arp2/3 complex, spontaneous nucleation of filaments, nucleation of new filaments by CP, and nucleation of new filaments by Arp2/3 complex. It can treat side branching, end branching, or both, by appropriate choice of parameters. We use parts of it to evaluate the input parameters for our branching simulations, and the whole model to perform the branching simulations. The parameters in the model are adjusted to minimize the mean-squared error in the fit to the data, using the Berkeley Madonna package, Ver. 8.01 (http://www.BerkeleyMadonna.com).
The rate equations are as follows:
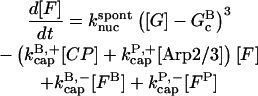 |
(5) |
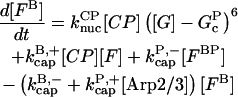 |
(6) |
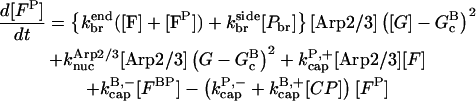 |
(7) |
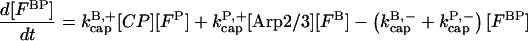 |
(8) |
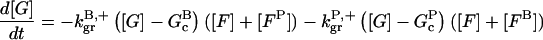 |
(9) |
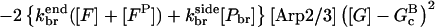 |
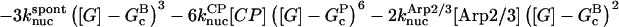 |
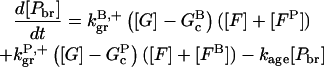 |
(10) |
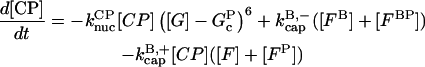 |
(11) |
 |
(12) |
In these equations, B and P denote barbed and pointed ends, respectively, and plus (+) and minus (−) denote on and off rates. [F], [FB], [FP], and [FBP] are the concentrations of filaments that are uncapped, or capped at the barbed and/or pointed ends, [G] is the free-monomer concentration, and 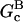 and
and 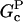 are the critical concentrations. Pbr is the concentration of filament subunits that are capable of forming side branches (which is equal to the polymerized-actin concentration in simple side-branching models). In the rate constants, the subscript gr denotes growth, cap denotes capping, nuc denotes nucleation, br denotes branching, and age denotes aging. The rates
are the critical concentrations. Pbr is the concentration of filament subunits that are capable of forming side branches (which is equal to the polymerized-actin concentration in simple side-branching models). In the rate constants, the subscript gr denotes growth, cap denotes capping, nuc denotes nucleation, br denotes branching, and age denotes aging. The rates 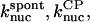 and
and 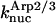 are for spontaneous, CP-induced (pointed-end growth), and Arp2/3 complex-induced (barbed-end growth) nucleation, respectively. The branching rate constants,
are for spontaneous, CP-induced (pointed-end growth), and Arp2/3 complex-induced (barbed-end growth) nucleation, respectively. The branching rate constants, 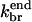 and
and  are given per filament and per subunit along a filament, respectively. Aging refers to the decrease of the ability of subunits to form branches on the side of a filament over time (Ichetovkin et al., 2002). The justification for our choice of powers of concentration in the branching and nucleation terms is given below.
are given per filament and per subunit along a filament, respectively. Aging refers to the decrease of the ability of subunits to form branches on the side of a filament over time (Ichetovkin et al., 2002). The justification for our choice of powers of concentration in the branching and nucleation terms is given below.
Evaluation of input parameters for branching models
The parameter values were obtained by fits to the polymerization data, except for three values taken from the literature. The values are summarized in Table 1. For the growth-rate parameters, we use the literature values (Higgs et al., 1999; Pollard, 1986) 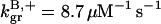 (for pH 7.5) and
(for pH 7.5) and 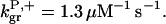 There is no published value of the pointed-end uncapping rate
There is no published value of the pointed-end uncapping rate 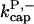 corresponding to Arp2/3 complex detachment. However, pointed-end uncapping should lead to branch detachment, so the branch detachment rate can provide an upper bound to the uncapping rate. Recent experiments (Weaver et al., 2001; Blanchoin et al., 2000) have measured the time course of branch detachment. We have fit the data of Weaver et al. (2001) for the number of branched filaments as a function of time with an exponential function. This yields a detachment rate constant of 0.0018 s−1, which is also consistent with the data of Blanchoin et al. (2000). We use this value of
corresponding to Arp2/3 complex detachment. However, pointed-end uncapping should lead to branch detachment, so the branch detachment rate can provide an upper bound to the uncapping rate. Recent experiments (Weaver et al., 2001; Blanchoin et al., 2000) have measured the time course of branch detachment. We have fit the data of Weaver et al. (2001) for the number of branched filaments as a function of time with an exponential function. This yields a detachment rate constant of 0.0018 s−1, which is also consistent with the data of Blanchoin et al. (2000). We use this value of 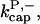 and also perform a parallel series of fits with
and also perform a parallel series of fits with 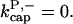 As described under Methods, we measured the critical concentrations
As described under Methods, we measured the critical concentrations 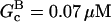 for the barbed end and
for the barbed end and 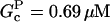 for the pointed end.
for the pointed end.
TABLE 1.
Parameter values
| Parameter | 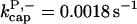 |
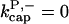 |
Source |
|---|---|---|---|
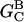 |
0.07 μM | 0.07 μM | Present |
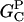 |
0.69 μM | 0.69 μM | Present |
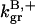 |
8.7 μM−1 s−1 | 8.7 μM−1 s−1 | (Higgs et al., 1999) |
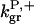 |
1.3 μM−1 s−1 | 1.3 μM−1 s−1 | (Pollard, 1986) |
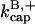 |
8.0 μM−1 s−1 | 8.0 μM−1 s−1 | Model I |
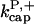 |
0.71 μM−1 s−1 | 0.18 μM−1 s−1 | Model IV - end |
| 1.07 μM−1 s−1 | 0.36 μM−1 s−1 | Model IV - side | |
| 0.98 μM−1 s−1 | 0.27 μM−1 s−1 | Model IV - end-plus-side | |
| 0.80 μM−1 s−1 | 0.33 μM−1 s−1 | Model V | |
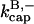 |
4.2 × 10−4 s−1 | 4.2 × 10−4 s−1 | Model I |
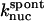 |
1.05 × 10−9μM−2 s−1 | 1.05 × 10−9μM−2 s−1 | Model II |
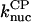 |
2.9 × 10−5μM−6 s−1 | 2.9 × 10−5μM−6 s−1 | Model III |
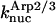 |
8.7 × 10−5μM−2 s−1 | 1.19 × 10−4μM−2 s−1 | Model IV - end |
| 1.1 × 10−5μM−2 s−1 | 6.8 × 10−6μM−2 s−1 | Model IV - side | |
| 8.7 × 10−6μM−2 s−1 | 0 | Model IV - end-plus-side | |
| 0 | 0 | Model V | |
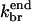 |
0.29 μM−3 s−1 | 0.158 μM−3 s−1 | Model IV - end |
| 0.082 μM−3 s−1 | 0.43 μM−3 s−1 | Model IV - end-plus-side | |
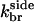 |
5.4 × 10−4μM−3 s−1 | 6.0 × 10−4μM−3 s−1 | Model IV - side |
| 5.3 × 10−4μM−3 s−1 | 4.9 × 10−4μM−3 s−1 | Model IV - end-plus-side | |
| 1.44 × 10−3μM−3 s−1 | 1.22 × 10−3μM−3 s−1 | Model V | |
| kage | 0.0087 s−1 | 0.0059 s−1 | Model V |
In rate constants, subscript gr denotes growth, cap denotes capping, nuc denotes nucleation, br denotes branching, and age denotes aging; superscripts P and B denote pointed and barbed ends, respectively. Pointed-end capping is by Arp2/3 complex and barbed-end capping is by CP. Columns headed 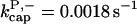 and
and 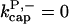 denote fits obtained with these values of
denote fits obtained with these values of 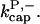
To obtain the remaining input parameters, we used several models corresponding to subsets of Eqs. 5–12.
Model I includes Eqs. 5–9, and 11, neglecting the kbr, 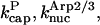 and
and 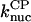 terms. It was used to evaluate
terms. It was used to evaluate 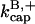 and
and 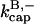 from the polymerization kinetics (progress curves) of a solution of spectrin-actin seeds (SAS) in 2 μM actin and varying concentrations of CP. The
from the polymerization kinetics (progress curves) of a solution of spectrin-actin seeds (SAS) in 2 μM actin and varying concentrations of CP. The 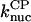 terms are ignored because of the very low concentrations of CP used in these experiments. The initial density of SAS ([FP] at t = 0) was used as a fitting parameter, which gave a value of 0.11 nM. Fig. 2 shows that Model I gives a good fit to the data. The best-fit value of
terms are ignored because of the very low concentrations of CP used in these experiments. The initial density of SAS ([FP] at t = 0) was used as a fitting parameter, which gave a value of 0.11 nM. Fig. 2 shows that Model I gives a good fit to the data. The best-fit value of  is 7.99 μM−1 s−1, consistent with the range of values observed previously (Schafer et al., 1996). We note that we used bacterially expressed CP, and previous studies used CP purified from tissue. In both cases, the β1 and β2 isoforms gave similar results. The value of
is 7.99 μM−1 s−1, consistent with the range of values observed previously (Schafer et al., 1996). We note that we used bacterially expressed CP, and previous studies used CP purified from tissue. In both cases, the β1 and β2 isoforms gave similar results. The value of 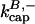 is 4.16 × 10−4 s−1, also in line with the previous estimates.
is 4.16 × 10−4 s−1, also in line with the previous estimates.
FIGURE 2.
Effect of CP on polymerization of spectrin-actin seeds. [CP] increases from top to bottom: 0 nM, 0.25 nM, 0.5 nM, and 0.75 nM. Smooth curves, Model I.
Model II includes the 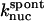 term of Eq. 5, and the kgr and
term of Eq. 5, and the kgr and 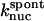 terms of Eq. 9. It was used to obtain
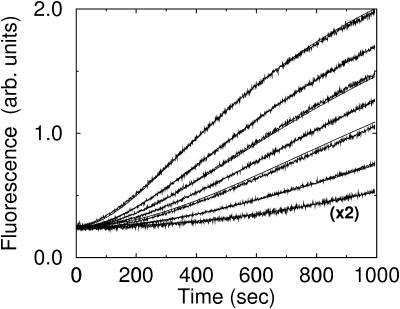
terms of Eq. 9. It was used to obtain 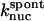 from the measured polymerization kinetics of a 2 μM actin solution. Fig. 3 (lowest curve, multiplied by a factor of 2 for visibility) shows that the fit, with
from the measured polymerization kinetics of a 2 μM actin solution. Fig. 3 (lowest curve, multiplied by a factor of 2 for visibility) shows that the fit, with 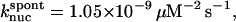 is quite accurate. This model is simpler than the multistep models (Frieden, 1983; Buzan and Frieden, 1996) generally used to study filament nucleation, but the quality of the fit indicates that it is sufficiently accurate to describe the limited range of times, and the single initial concentration, employed here.
is quite accurate. This model is simpler than the multistep models (Frieden, 1983; Buzan and Frieden, 1996) generally used to study filament nucleation, but the quality of the fit indicates that it is sufficiently accurate to describe the limited range of times, and the single initial concentration, employed here.
FIGURE 3.
Spontaneous nucleation of actin filaments, and nucleation by CP. CP concentration increases from bottom to top: 0 nM, 2 nM, 5 nM, 7 nM, 10 nM, 15 nM, and 25 nM. Smooth curves, Model II (bottom curve, multiplied by a factor of 2 for visibility) and Model III (remaining curves).
Model III treats CP-induced filament nucleation using Eqs. 5, 6, 9, and 11, neglecting all kbr terms and terms involving [Arp2/3]. We obtained 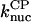 using this model, by measuring the polymerization kinetics of a solution of 2 μM actin in the presence of concentrations of CP varying from 0 to 25 nM. Fig. 3 shows that the polymerization is strongly accelerated by CP, indicating that at these concentrations, CP-induced nucleation dominates spontaneous nucleation. The fit, with
using this model, by measuring the polymerization kinetics of a solution of 2 μM actin in the presence of concentrations of CP varying from 0 to 25 nM. Fig. 3 shows that the polymerization is strongly accelerated by CP, indicating that at these concentrations, CP-induced nucleation dominates spontaneous nucleation. The fit, with 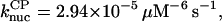 is excellent. Because the correct choice of power of concentration to use in Model III is not clear, we have tried other powers as well. Using a rate proportional to
is excellent. Because the correct choice of power of concentration to use in Model III is not clear, we have tried other powers as well. Using a rate proportional to 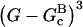 instead of
instead of  gives a fit that is somewhat worse but still quite acceptable. The sixth power may give a better fit by giving a combined description of multiple steps treated in more complete models (Cooper and Pollard, 1985).
gives a fit that is somewhat worse but still quite acceptable. The sixth power may give a better fit by giving a combined description of multiple steps treated in more complete models (Cooper and Pollard, 1985).
Results for branching models
In our comparisons between different branching models, we use the parameters determined above where possible. However, because we cannot ascertain all of the parameters independently, we include parameters that are allowed to vary to optimize the fit. Figs. 4–7 show the polymerization kinetics of a solution of 2 μM actin, 14.3 nM Arp2/3 complex, 25nM GST-VCA, and CP concentrations ranging up to 25 nM. The CP slows the polymerization, changing the characteristic time over which the actin goes from mainly monomeric to mainly polymerized from approximately one hundred to several hundred seconds. The fitting errors (in arbitrary units, but consistent from model to model) obtained for several branching models are given in Table 2. In the curve fits given in Figs. 4–7, we use 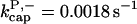 ; the error values for
; the error values for 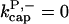 are given in Table 2.
are given in Table 2.
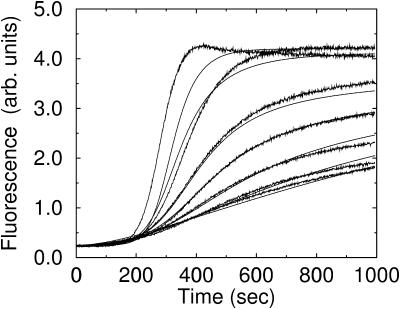
FIGURE 4.
Effect of CP on Arp2/3 complex-induced polymerization of actin. Capping protein concentration increases from top to bottom: 0 nM, 2 nM, 5 nM, 7 nM, 10 nM, 15 nM, and 25 nM. Smooth curves, Model IV with 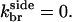
FIGURE 7.
Aging model for Arp2/3 complex-induced polymerization of actin. Capping protein concentration increases from top to bottom: 0 nM, 2 nM, 5 nM, 7 nM, 10 nM, 15 nM, and 25 nM. Smooth curves, Model V.
TABLE 2.
Quality of fit
| Model | 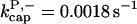 |
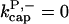 |
With [CP] = 0 | Npar |
|---|---|---|---|---|
| IV | ||||
| End branching | 22.8 | 24.6 | 33.8 | 3 |
| (CP allow) | 19.3 | 19.1 | 3 | |
| (Combined) | 19.2 | 19.0 | 4 | |
| Side branching | 9.8 | 8.2 | 12.7 | 3 |
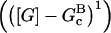
|
10.7 | 9.8 | 3 | |
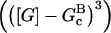
|
9.7 | 8.4 | 3 | |
| End-plus-side | 9.8 | 6.4 | 10.0 | 4 |
| V | ||||
| Side branching | 4.2 | 4.4 | 6.4 | 4 |
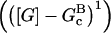
|
4.8 | 4.8 | 4 | |
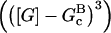
|
10.6 | 7.4 | 4 |
Errors given in arbitrary units, same in all models. Runs, in general, leave the [CP] = 0 curve out of the fitting procedure. The column labeled With [CP] = 0 indicates the error obtained by minimizing with this curve, Npar is the number of fitting parameters.
We use two models in our branching simulations:
Model IV treats branching polymerization in the absence of subunit aging effects. The rate equations include all of Eqs. 5–12, with kage = 0. End branching is obtained by taking 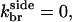 and side branching by taking
and side branching by taking 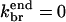 ; an end-plus-side model is obtained by allowing both of these rates to be nonzero. The rate of formation of new branches should be proportional to the concentration of free, activated Arp2/3 complex, which we take to be proportional to the total concentration of free Arp2/3 complex. Provided that the timescale of the activation of Arp2/3 complex by GST-VCA is less than the characteristic time over which the actin polymerizes, this will be an accurate approximation. A value of 0.8 μM−1 s−1 has been measured (Marchand et al., 2001) for the rate of WASp-VCA attachment to Arp2/3 complex, which would give a rate of 0.02 s−1 with our value of 25 nM for the GST-VCA concentration if we assume the same rate constant; in addition, an activation step with a rate constant of 0.034 s−1 has been found (Zalevsky et al., 2001). These rates are much faster than the characteristic rates for our Arp2/3 complex-induced polymerization curves shown below, with the exception of the [CP] = 0 curve. The power of 2 in the
; an end-plus-side model is obtained by allowing both of these rates to be nonzero. The rate of formation of new branches should be proportional to the concentration of free, activated Arp2/3 complex, which we take to be proportional to the total concentration of free Arp2/3 complex. Provided that the timescale of the activation of Arp2/3 complex by GST-VCA is less than the characteristic time over which the actin polymerizes, this will be an accurate approximation. A value of 0.8 μM−1 s−1 has been measured (Marchand et al., 2001) for the rate of WASp-VCA attachment to Arp2/3 complex, which would give a rate of 0.02 s−1 with our value of 25 nM for the GST-VCA concentration if we assume the same rate constant; in addition, an activation step with a rate constant of 0.034 s−1 has been found (Zalevsky et al., 2001). These rates are much faster than the characteristic rates for our Arp2/3 complex-induced polymerization curves shown below, with the exception of the [CP] = 0 curve. The power of 2 in the 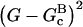 factor multiplying the kbr terms gives the best overall fit to the data, as discussed below.
factor multiplying the kbr terms gives the best overall fit to the data, as discussed below.
In Fig. 4, the data are fit to the end-branching model. There are three fitting parameters: 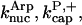 and
and 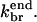 In the [CP] = 0 curve the data shows a pronounced overshoot in the polymerized fraction, which cannot be modeled within the constraints of the models used here. It is absent in the remaining curves, which have lower polymerization rates. For this reason, and because of the lag time for Arp2/3 complex activation discussed above, we give the [CP] = 0 curve zero fitting weight in most of our runs. However, we perform a few additional runs to establish whether this choice affects the relative quality of fit of the models. The end-branching model provides a poor description of the variation of the polymerization kinetics with [CP]. The general tendency of this model is to cut off polymerization too abruptly at large times. To make up for this tendency, the fit procedure adjusts
In the [CP] = 0 curve the data shows a pronounced overshoot in the polymerized fraction, which cannot be modeled within the constraints of the models used here. It is absent in the remaining curves, which have lower polymerization rates. For this reason, and because of the lag time for Arp2/3 complex activation discussed above, we give the [CP] = 0 curve zero fitting weight in most of our runs. However, we perform a few additional runs to establish whether this choice affects the relative quality of fit of the models. The end-branching model provides a poor description of the variation of the polymerization kinetics with [CP]. The general tendency of this model is to cut off polymerization too abruptly at large times. To make up for this tendency, the fit procedure adjusts 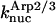 to a very high value, and this results in much too rapid polymerization at early times. When the value of
to a very high value, and this results in much too rapid polymerization at early times. When the value of 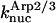 is limited to smaller values, the end-branching model gives much too low a polymerized fraction at large times.
is limited to smaller values, the end-branching model gives much too low a polymerized fraction at large times.
We have also considered a variant of this model in which CP at filament ends does not prevent branch formation (see the CP allow results in Table 2). This gives a somewhat better fit than the conventional end-branching model, but much better fits are obtained with the side-branching models described below. Allowing for the possibility that capped filaments can branch at a rate less than uncapped ones results in a “combined” four-parameter model which, however, fits only marginally better than the CP allow model (see Table 2.)
The side-branching model gives a better description, as is seen in Fig. 5. The overall shape of the experimental data is well reproduced. The main deficiency of the model is that the curves for low [CP] have too small a slope, whereas those at high [CP] have too high a slope, and thus the effect of increasing [CP] is somewhat underestimated. The error (Table 2) of this model, for both values of 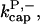 is less than half that of the end-branching model. To evaluate the reliability of the fit, we have performed several other runs. Keeping the [CP] = 0 curve in the fitting procedure gives results essentially identical to the previous ones. We have also varied the exponent m in the proportionality of the branching rate to
is less than half that of the end-branching model. To evaluate the reliability of the fit, we have performed several other runs. Keeping the [CP] = 0 curve in the fitting procedure gives results essentially identical to the previous ones. We have also varied the exponent m in the proportionality of the branching rate to 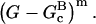 As seen in Table 2, varying m in the range 1–3 results in variations of ∼10 to 20% in the error. Although the m = 3 error for
As seen in Table 2, varying m in the range 1–3 results in variations of ∼10 to 20% in the error. Although the m = 3 error for 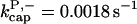 is slightly less than that for m = 2, we use m = 2 in most of calculations. This allows us to compare the models on a roughly equal footing without including one more fitting parameter, since m = 2 gives close to the minimum error for all of the models with errors of 10 or below. Fig. 6 shows the fit for the end-plus-side model, with four fitting parameters. The error for
is slightly less than that for m = 2, we use m = 2 in most of calculations. This allows us to compare the models on a roughly equal footing without including one more fitting parameter, since m = 2 gives close to the minimum error for all of the models with errors of 10 or below. Fig. 6 shows the fit for the end-plus-side model, with four fitting parameters. The error for 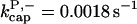 is only marginally reduced, but that for
is only marginally reduced, but that for  is reduced by ∼25%.
is reduced by ∼25%.
FIGURE 5.
Side-branching model for Arp2/3 complex-induced polymerization of actin. Capping protein concentration increases from top to bottom: 0 nM, 2 nM, 5 nM, 7 nM, 10 nM, 15 nM, and 25 nM. Smooth curves, Model IV, with 
FIGURE 6.
End-plus-side model for Arp2/3 complex-induced polymerization of actin. Capping protein concentration increases from top to bottom: 0 nM, 2 nM, 5 nM, 7 nM, 10 nM, 15 nM, and 25 nM. Smooth curves, Model IV.
Model V treats the aging model, in which branches form along filament sides, and the ability of subunits to form new branches ages at a rate kage. It uses Eqs. 5–12, with  As Fig. 7 shows, it gives the best fit of any of the models treated. In Table 2, the errors are considerably smaller than those for the end-plus-side model. Since the aging model has the same number of adjustable parameters as the end-plus-side model (four), it appears to be a much better choice. In fact, the discrepancies between this model's predictions and the experimental data are smaller than the differences between this model and any of the other models. It is not clear what the aging process is. The value of kage, 0.0059 s−1–0.0087 s−1, is much smaller than the ATP hydrolysis rate constant, 0.3 s−1 (Blanchoin and Pollard, 2002). It is closer to the phosphate release rate, which is in the range 0.002–0.003 s−1 (Melki et al., 1996; Blanchoin and Pollard, 1999). However, kage exceeds these values by a factor of 2 to 4, suggesting that phosphate release is not the only factor governing aging. One possibility is that a partial reduction in subunits' ability to generate new branches occurs upon rapid hydrolysis, with a greater reduction coming with subsequent phosphate release. Disentangling these two contributions is probably beyond the resolution of the types of experiments described here. Examination of Table 1 shows that inclusion of aging effects increases the rate constant for side branching by a factor of 2 to 3. This is expected, since the number of branching-competent monomers is reduced by the aging effects, and the increased rate constant per monomer compensates for this reduction.
As Fig. 7 shows, it gives the best fit of any of the models treated. In Table 2, the errors are considerably smaller than those for the end-plus-side model. Since the aging model has the same number of adjustable parameters as the end-plus-side model (four), it appears to be a much better choice. In fact, the discrepancies between this model's predictions and the experimental data are smaller than the differences between this model and any of the other models. It is not clear what the aging process is. The value of kage, 0.0059 s−1–0.0087 s−1, is much smaller than the ATP hydrolysis rate constant, 0.3 s−1 (Blanchoin and Pollard, 2002). It is closer to the phosphate release rate, which is in the range 0.002–0.003 s−1 (Melki et al., 1996; Blanchoin and Pollard, 1999). However, kage exceeds these values by a factor of 2 to 4, suggesting that phosphate release is not the only factor governing aging. One possibility is that a partial reduction in subunits' ability to generate new branches occurs upon rapid hydrolysis, with a greater reduction coming with subsequent phosphate release. Disentangling these two contributions is probably beyond the resolution of the types of experiments described here. Examination of Table 1 shows that inclusion of aging effects increases the rate constant for side branching by a factor of 2 to 3. This is expected, since the number of branching-competent monomers is reduced by the aging effects, and the increased rate constant per monomer compensates for this reduction.
Examination of the With [CP] = 0 column in Table 2 shows that our conclusions are robust to inclusion of this curve in the fitting procedure. Side branching fits better than end branching, and Model V gives the best fit.
However, none of the models accurately describes the [CP] = 0 curve. As mentioned above, this curve has an overshoot in the polymerized fraction that is not obtained by any of our model variants. A similar overshoot was seen when light scattering was used in a control experiment without CP (data not shown), so the overshoot is not an artifact of the pyrene label. No actin polymerization theories to date have predicted this feature correctly, which may require inclusion of ATP hydrolysis, phosphate release, or annealing/severing of filaments. These processes can have a substantial effect on the critical concentration (Pantaloni et al., 1984).
We have not performed experiments for other actin or Arp2/3 complex concentrations. However, we have run simulations for some of the concentrations used by Pantaloni et al. (2000). The qualitative behavior of the simulations is consistent with their results, with the characteristic rate for polymerization increasing with the Arp2/3 complex concentration. Obtaining a quantitative fit to this data would involve revising several of the simulation parameters because of the different experimental conditions.
DISCUSSION
The analysis presented above has two main conclusions. First, if branching is restricted to a single type of model, side-branching models fit the experimental polymerization data better than end-branching models. Second, a model incorporating aging effects, in which the branching ability of subunits decays over time, gives significant improvements over end-plus-side models. Thus, most of the branching occurs along the sides of filaments, and newly formed portions of the filaments support a higher rate of branching.
These findings can help discriminate between several branching models that have been proposed previously. Support for end-branching models has been drawn (Pantaloni et al., 2000) from both electron-microscopy images of branched filaments and the polymerization kinetics in the presence of activated Arp2/3 complex. The electron-microscopy images showed a strong correlation between the lengths of mother and daughter filaments, suggesting dominance of end branching. The kinetic arguments favoring end branching were based on three types of experimental protocols. We have used our rate-equation methodology to model these results. Model V explains all three data sets, whereas Model IV explains the results for only two. The experiments were as follows:
Actin polymerization after addition of Arp2/3 complex at a delay time t0 after the initiation of polymerization. Measurements of the time Δt1/2 after t0 required to reach 50% polymerization suggested that Δt1/2 decreases with t0 but stabilizes at a value of t0 at which the polymerized actin concentration [P] is still increasing. Since the branch generation rate in side-branching models is proportional to [P], this would appear to refute side-branching models. However, because the polymerization is autocatalytic, it has an exponential time dependence. Therefore the time required to reach a certain value of [P] should depend only logarithmically on the initial value of [P]. Over a finite observation interval, it is difficult to distinguish a logarithmic variation from an approach to a limit. Our simulations for Model IV confirm the slow variation of Δt1/2 with t0. In the simulations for Model V, Δt1/2 approaches a finite limit with increasing t0, giving a somewhat better description of the experimental data than Model IV.
Actin polymerization in the presence of varying numbers of seed filaments, with the initial value of [P] fixed. The polymerization rate increased with the number of seed filaments. In side-branching models, the filament generation rate should be mainly determined by [P], leading to little variation with the number of seed filaments. Our simulations confirm this expectation, so that these experimental results cannot be explained by Model IV. However, in Model V, increasing the number of seed filaments increases the density of freshly polymerized actin, which enhances the branching rate. In our simulations, the polymerization thus is accelerated by the larger number of seed filaments. The effect is, however, significantly smaller than the experimental one.
Actin polymerization in the presence of a constant number of seed filaments, with lengths of 0.04 μm, 1 μm, or 2 μm. The polymerization rate was affected only slightly by the filament lengths. In side-branching models, the filament generation rate should be proportional to the filament lengths. However, under the experimental conditions that were used, already 50 s into the experiment (a time much shorter than the time courses used) a filament would elongate by ∼3 μm, masking the initial differences between the filament lengths. Our simulations of Models IV and V yield very small effects from the filament-length differences, consistent with the experimental data. We also note that the functional antagonism between Arp2/3 complex and capping proteins, derived by Pantaloni et al. (2000) from the dependence of the critical concentration on the Arp2/3 and capping-protein concentrations, is consistent with our side-branching models. In both Models IV and V the critical concentration increases from the barbed-end value toward the pointed-end value as [CP] increases. The value of [CP] required to substantially change the critical concentration increases with [Arp2/3].
Subsequently, several fluorescence-microscopy studies have provided real-time images of the branch formation process. In the study of Amann and Pollard (2001) filaments of rhodamine-labeled actin were attached to a substrate, and their growth and branching monitored. Branches clearly formed from sides in many cases. New branches were formed on the average 1.56 ± 1.42 μm from the barbed end of the mother filaments, and no preference was found for branch formation right at either end of the mother filament. However, a modest preference for branching in the barbed-end half of the mother filament was found. In a similar study (Ichetovkin et al., 2002), phalloidin-labeled filaments were used as seeds for the growth of rhodamine-actin filaments, and side branching was again found to dominate. An observed correlation between mother and daughter branch lengths, and an increased branching frequency along newly formed filaments, suggested a preference for branching near, but not at, the barbed end. Another study (Fujiwara et al., 2002) used TMR-5-MA labeled actin, with filament motion reduced by use of methylcellulose in the solution rather than attachment to a substrate. Side branching dominated, but 10% of the new branches were formed near the barbed end. Unlike the other two fluorescence studies, new branches formed preferentially near pointed ends. Our results here support the dominance of side branching, found in all of the real-time fluorescence studies. They are also consistent with the small enhancement of branching near the filaments' barbed ends, noted in Amann and Pollard (2001) and Ichetovkin et al. (2002).
Relevance of the present results for actin polymerization in cells
In cells, new branches are formed in the vicinity of the obstacle (plasma membrane or intracellular pathogen) against which the growing actin network exerts its force. If the maximum distance at which new branches form is d, and both end and side branching are present, then the ratio Nside/Nend of the number of filaments formed by side branching to the number formed by end branching is 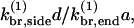 where the k-values are effective first-order rate constants containing appropriate powers of concentration, and a = 2.8 nm is the step size per subunit along a filament (Holmes et al., 1990). If we assume the same concentration factors for side and end branching, then
where the k-values are effective first-order rate constants containing appropriate powers of concentration, and a = 2.8 nm is the step size per subunit along a filament (Holmes et al., 1990). If we assume the same concentration factors for side and end branching, then 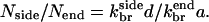 The value of d is not precisely known. However, an approximate upper bound for the width of the branching region is the spacing lbr between branches. If new branches formed much farther than lbr from the obstacle, then an increase in the network density going away from the obstacle would be observed in electron micrographs, and such an increase is not seen (Svitkina et al., 1997; Svitkina and Borisy, 1999). We therefore assume that d ≤ lbr; lbr is roughly 15 subunit spacings (Svitkina and Borisy, 1999). Then,
The value of d is not precisely known. However, an approximate upper bound for the width of the branching region is the spacing lbr between branches. If new branches formed much farther than lbr from the obstacle, then an increase in the network density going away from the obstacle would be observed in electron micrographs, and such an increase is not seen (Svitkina et al., 1997; Svitkina and Borisy, 1999). We therefore assume that d ≤ lbr; lbr is roughly 15 subunit spacings (Svitkina and Borisy, 1999). Then, 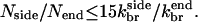 In the end-plus-side model (Table 1, using the set with smaller
In the end-plus-side model (Table 1, using the set with smaller 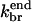 ),
), 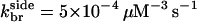 and
and 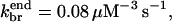 so Nside/Nend ≤ 0.09; if
so Nside/Nend ≤ 0.09; if 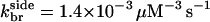 is taken from the aging model, we obtain Nside/Nend ≤ 0.3. Thus, even though most of the branches in the in vitro studies are formed along filament sides, the same rate parameters could lead to most filaments being formed at filament ends in the cellular environment. For side branching to be the dominant mechanism in the cellular environment, the ratio
is taken from the aging model, we obtain Nside/Nend ≤ 0.3. Thus, even though most of the branches in the in vitro studies are formed along filament sides, the same rate parameters could lead to most filaments being formed at filament ends in the cellular environment. For side branching to be the dominant mechanism in the cellular environment, the ratio 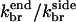 would need to be very low.
would need to be very low.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org.
Acknowledgments
We are grateful to Marie-France Carlier for supplying plasmids used in our GST-VCA purification protocol.
This research was supported by the National Institutes of Health under grant GM38542.
References
- Amann, K. J., and T. D. Pollard. 2001. Direct real-time observation of actin filament branching mediated by Arp2/3 complex using total internal reflection fluorescence microscopy. Proc. Natl. Acad. Sci. USA. 98:15009–15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchoin, L., K. J. Amann, H. N. Higgs, J.-P. Marchand, D. A. Kaiser, and T. D. Pollard. 2000. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature. 404:1007–1011. [DOI] [PubMed] [Google Scholar]
- Blanchoin, L., and T. D. Pollard. 1999. Mechanism of interaction of Acanthamoeba actophorin (ADF/cofilin) with actin filaments. J. Biol. Chem. 274:15538–15546. [DOI] [PubMed] [Google Scholar]
- Blanchoin, L., and T. D. Pollard. 2002. Hydrolysis of ATP by polymerized actin depends on the bound divalent cation but not profilin. Biochemistry. 41:597–602. [DOI] [PubMed] [Google Scholar]
- Buzan, J. M., and C. Frieden. 1996. Yeast actin: polymerization kinetic studies of wild type and a poorly polymerizing mutant. Proc. Natl. Acad. Sci. USA. 93:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, J. A., and T. D. Pollard. 1985. Effect of capping protein on the kinetics of actin polymerization. Biochemistry. 24:793–799. [DOI] [PubMed] [Google Scholar]
- Cooper, J. A., S. B. Walker, and T. D. Pollard. 1983. Pyrene actin: documentation of the validity of a sensitive assay for actin polymerization. J. Muscle Res. Cell Motil. 4:253–262. [DOI] [PubMed] [Google Scholar]
- DiNubile, M. J., L. Cassimeris, M. Joyce, and S. H. Zigmond. 1995. Actin filament barbed-end capping activity in neutrophil lysates—the role of capping protein-β(2). Mol. Biol. Cell. 6:1659–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egile, C., T. P. Loisel, V. Laurent, R. Li, D. Pantaloni, P. J. Sansonetti, and M.-F. Carlier. 1999. Activation of the Cdc42 effector N-WASp by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 146:1319–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden, C. 1983. Polymerization of actin: mechanism of the Mg2+-induced process at pH 8 and 20°C. Proc. Natl. Acad. Sci. USA. 80:6513–6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara, I., S. Suetsugu, S. Uemura, T. Takenawa, and S. Ishiwata. 2002. Visualization and force measurement of branching by Arp2/3 complex and N-WASP in actin filament. Biochem. Biophys. Res. Commun. 293:1550–1555. [DOI] [PubMed] [Google Scholar]
- Higgs, H. N., L. Blanchoin, and T. D. Pollard. 1999. Influence of the C terminus of Wiskott-Aldrich syndrome protein WASp and the Arp2/3 complex on actin polymerization. Biochemistry. 38:15212–15222. [DOI] [PubMed] [Google Scholar]
- Higgs, H. N., and T. D. Pollard. 2001. Regulation of actin filament formation through Arp2/3 complex. Annu. Rev. Biochem. 70:649–676. [DOI] [PubMed] [Google Scholar]
- Holmes, K. C., D. Popp, W. Gebhard, and W. Kabsch. 1990. Atomic model of the actin filament. Nature. 347:44–49. [DOI] [PubMed] [Google Scholar]
- Ichetovkin, I., W. Grant, and J. Condeelis. 2002. Cofilin produces newly polymerized actin filaments that are preferred for dendritic nucleation by the Arp2/3 complex. Curr. Biol. 12:79–84. [DOI] [PubMed] [Google Scholar]
- Marchand, J.-B., D. A. Kaiser, T. D. Pollard, and H. N. Higgs. 2001. Interaction of WASp/Scar proteins with actin and vertebrate Arp2/3 complex. Nat. Cell Biol. 3:76–82. [DOI] [PubMed] [Google Scholar]
- Machesky, L. M., D. M. Mullins, H. N. Higgs, D. A. Kaiser, L. Blanchoin, R. C. May, M. E. Hall, and T. D. Pollard. 1999. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc. Natl. Acad. Sci. USA. 96:3739–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki, R., S. Fievez, and M.-F. Carlier. 1996. Continuous monitoring of Pi release following nucleotide hydrolysis in actin or tubulin assembly using 2-amine-6-mercapto-7-methylpurine ribonucleoside and purine-nucleoside phosphorylase as an enzyme-linked assay. Biochemistry. 35:12038–12045. [DOI] [PubMed] [Google Scholar]
- Mullins, R. D., J. A. Heuser, and T. D. Pollard. 1998. The interaction of Arp2/3 complex with actin: nucleation, high-affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl. Acad. Sci. USA. 95:6181–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren, S., P. J. Ojala, M. A. Wear, J. A. Cooper, and P. Lappalainen. 2001. Interactions with PIP2, ADP-actin monomers, and capping protein regulate the activity and localization of yeast twinfilin. J. Cell Biol. 155:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaloni, D., M.-F. Carlier, M. Coué, A. A. Lal, S. L. Brenner, and E. D. Korn. 1984. The critical concentration of actin in the presence of ATP increases with the number concentration of filaments and approaches the critical concentration of actin·ADP. J. Biol. Chem. 259:6274–6283. [PubMed] [Google Scholar]
- Pantaloni, D., R. Boujemaa, D. Didry, P. Gounon, and M.-F. Carlier. 2000. The Arp2/3 complex branches filament barbed ends: functional antagonism with capping proteins. Nat. Cell Biol. 2:385–391. [DOI] [PubMed] [Google Scholar]
- Pollard, T. 1986. Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J. Cell Biol. 103:2747–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard, T. D., L. Blanchoin, and R. D. Mullins. 2000. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 29:545–576. [DOI] [PubMed] [Google Scholar]
- Robinson, R. C., K. Turbedsky, D. A. Kaiser, J.-B. Marchand, H. N. Higgs, S. Choe, and T. D. Pollard. 2001. Crystal structure of Arp2/3 complex. Science. 294:1679–1684. [DOI] [PubMed] [Google Scholar]
- Schafer, D. A., P. B. Jennings, and J. A. Cooper. 1996. Dynamics of capping protein and actin assembly in vitro: uncapping barbed ends by polyphosphoinositides. J. Cell Biol. 135:169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeno, Y., H. Abe, S. Kimura, K. Maruyama, and T. Obinata. 1998. Generation of functional β-actinin (CapZ) in an E. coli expression system. J. Muscle Res. Cell Motil. 19:639–646. [DOI] [PubMed] [Google Scholar]
- Spudich, J. A., and S. Watt. 1971. The regulation of rabbit skeletal muscle contraction. J. Biol. Chem. 246:4866–4871. [PubMed] [Google Scholar]
- Svitkina, T. M., and G. G. Borisy. 1999. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 145:1009–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina, T. M., A. B. Verkhovsky, K. M. McQuade, and G. G. Borisy. 1997. Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J. Cell Biol. 139:397–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann, N., K. J. Amann, C. E. Stoilova-McPhie, D. C. Winter, L. Hazelwood, J. E. Heuser, R. Li, T. D. Pollard, and D. Hanein. 2001. Structure of Arp2/3 complex in its activated state and in actin filament branch junctions. Science. 293:2456–2459. [DOI] [PubMed] [Google Scholar]
- Weaver, A. M., A. V. Karginov, A. W. Kinley, S. A. Weed, Y. Li, J. T. Parsons, and J. A. Cooper. 2001. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 11:370–374. [DOI] [PubMed] [Google Scholar]
- Zalevsky, J., L. Lempert, H. Kranitz, and R. D. Mullins. 2001. Different WASP family proteins stimulate different Arp2/3 complex-dependent actin-nucleating activities. Curr. Biol. 11:1903–1913. [DOI] [PubMed] [Google Scholar]