Abstract
Juvenile hormone (JH) regulates insect development. JH present in the hemolymph is bound to a specific glycoprotein, juvenile hormone binding protein (JHBP), which serves as a carrier to deploy the hormone to target tissues. In this report structural changes of JHBP from Galleria mellonella induced by guanidine hydrochloride have been investigated by a combination of size-exclusion chromatography, protein activity measurements, and spectroscopic methods. Molecules of JHBP change their conformation from a native state via two unstable intermediates to a denatured state. The first intermediate appears in a compact state, because it slightly changes its molecular size and preserves most of the JHBP secondary structure of the native state. Although the second intermediate also preserves a substantial part of the secondary structure, it undergoes a change into a noncompact state changing its Stokes radius from ∼30 to 39 Å. Refolding experiments showed that JHBP molecules recover their full protein structure, as judged from the CD spectrum, fluorescence experiments, and JH binding activity measurements. The free energy of unfolding in the absence of the denaturant, ΔGD–N, is calculated to be 4.1 kcal mol−1.
INTRODUCTION
Juvenile hormone (JH) exhibits a stimulating effect on the reproductive maturation and regulation of insect development. It inhibits metamorphosis, arrests insects in their premetamorphotic stage, induces insect diapause, and affects the migratory behavior of insects (Gilbert et al., 1976). JH is transported to the target cells via JH-binding protein, JHBP (Trowell, 1992; Kochman and Wieczorek, 1995; Gilbert et al., 2000). JHBP from Galleria mellonella belongs to class I of monomeric, hemolymph JH carrier proteins of low molecular weight and high affinity of binding (for review, see Goodman and Chang, 1985; Kochman and Wieczorek, 1995). Nearly all the hemolymph JH molecules are bound to JHBP and protected from the action of nonspecific hydrolases (Kochman and Wieczorek, 1995; Hidayat and Goodman, 1994; Sanburg et al., 1975; Kramer and Childs, 1977).
JHBP from G. mellonella is a glycoprotein of molecular weight 25,880 Da (Duk et al., 1996) and known primary structure (Rodriguez Parkitna et al., 2002). In solution, JHBP is a single polypeptide chain protein which contains two disulphide bridges (Kołodziejczyk et al., 2001). The amino acid composition of mature JHBP showed no methionyl or tryptophanyl residues (Ożyhar and Kochman, 1987). Analysis of JHBP molecules' susceptibility to a proteolytic degradation has shown that digestion with carboxypeptidase A or Y, trypsin, and chymotrypsin does not change JHBP binding activity, whereas digestion with subtilisin proceeds extremely slowly (Wieczorek and Kochman, 1991). This suggests that JHBP molecules or their portion(s) may exhibit great stability. On the other hand, JHBP molecules from G. mellonella are prone to irreversible aggregation and loss of stability in low-salt concentrations as observed in our laboratory. Recently JHBP from G. mellonella has been crystallized, and a space group and unit-cell parameters of the crystals were described (Kołodziejczyk et al., 2003). Results of the secondary structure modeling of G. mellonella JHBP indicate that its molecule probably contains a β-barrel motif flanked by α-helices and thus could be evolutionarily related to the family of calicins (Rodriguez Parkitna et al., 2002). This group of proteins includes avidins, fatty acid binding proteins, and lipocalins. JHBP shows considerable homology to the secondary structure of human serum retinol binding protein (RBP), a member of the lipocalin superfamily (Rodriguez Parkitna et al., 2002). It has been previously found that RBP and other proteins belonging to the same superfamily exhibit low values of ΔGD–N and a similar reversible progression of unfolding (Greene et al., 2001; Burns et al., 1998). The mechanisms of folding and unfolding, and thus the stability of small, reversibly folding proteins with a β-structure have been extensively investigated in recent years (Hamada and Goto, 1997; Schonbrunner et al., 1997; Forge et al., 2000; Roumestand et al., 2001; Chanez-Cardenas et al., 2002; Srimathi et al., 2002). Analysis of thermodynamic data shows that the free energy change of unfolding protein molecules, composed mostly of a β-structure, is in the lower range of the values described for globular proteins (Privalov, 1979).
To shed some light on the structural stability of JHBP we felt that it might be of interest to investigate the effect of a perturbant (GdmCl) on JHBP molecules. To our knowledge, no studies on the structural stability, unfolding, and refolding mechanisms of JHBP(s) from any species have yet been reported.
MATERIALS AND METHODS
Chemicals
JHBP was isolated from the hemolymph of the wax moth larvae G. mellonella by immunoaffinity chromatography (Wieczorek et al., 1996). Juvenile hormone III (JH III) was purchased from Sigma Chemical (St. Louis, MO). 10-[3H]-labeled JH III was purchased from NEN Chemicals (Boston, MA).
GdmCl stock solutions of ∼8.0 M were prepared, and the concentration was checked by refractive index measurements using Eq. 1 according to Nozaki (1972):
 |
(1) |
where ΔN is the difference in the refractive index between the denaturant solution and the buffer at the sodium D line.
All the solute samples were prepared in a 100 mM Na2HPO4/NaH2PO4 buffer, pH 7.2. All measurements were done at 25°C.
ANS (Sigma) was purified as described previously (York et al., 1978). All other chemicals used in this work were of the highest purity commercially available.
The following absorption coefficients were used for the spectrophotometric determination of concentrations: JHBP, 0.46 mg−1 cm−1 mL at 280 nm (Krzyżanowska et al., 1998); ANS, 6.24 mM−1 cm−1 at 351 nm (Ferguson and Cahnmann, 1975); and JH III, 13,830 M−1 cm−1 at 220 nm (Trautmann et al., 1974).
UV difference spectrum of JHBP in the presence of GdmCl
GdmCl-induced changes in the absorption spectrum of JHBP in the presence of 4.0 M denaturant concentration were monitored in the range of 240–350 nm with a Cary 3E spectrophotometer (Varian, Cary, NC). Two tandem cells (Hellma-238-QS) with a total optical path length of 1 cm were used for measuring the difference spectra. The baseline was recorded for the protein and GdmCl solutions placed separately in two compartments of each tandem cell. After mixing the contents of the sample cell, the difference spectrum was recorded against the nonmixed solutions present in the reference cell. The contents of the reference cell were mixed after the difference spectrum was recorded, and the baseline was checked again. Measurements were made at final concentrations: 2.6 × 10−5 M JHBP and 4.0 M GdmCl for 2 h.
Activity measurements
Juvenile-hormone-binding activity was determined by a charcoal assay (Goodman et al., 1976) except that 0.1% gelatin was added to the JHBP solution as previously described (Ożyhar and Kochman, 1987). Briefly, 1 μl of 2 × 10−5 M 10-[3H]-labeled JH III (∼12,000 dpm) in hexane was added to a glass tube, and the solvent was allowed to evaporate. Then, in each tube of hormone a 10-μl sample of JHBP (3.8 μM) was mixed with 190 μl 0.1% gelatin in 10 mM MOPS and 100 mM NaCl, at pH 7.2, 25°C. This solution was incubated for 30 min at 4°C and mixed with 25 μl of freshly prepared charcoal suspension. After 10 min of incubation at 4°C, the contents of the tube were centrifuged at 12,000 × g for 2 min at 4°C. The amount of hormone bound to JHBP was determined from the measurements of radioactivity in 150 μl of the supernatant. Glassware that could come into contact with aqueous solutions of JH was coated with polyethylene glycol.
Effect of GdmCl on the JH binding activity of JHBP
The defined volumes of GdmCl solution were added to JHBP samples in a phosphate buffer (final JHBP concentration 0.1 mg mL−1) to obtain an increasing denaturant concentration in the range from 0 to 4.0 M. After 1 h of incubation at 4°C the protein samples were withdrawn from the incubation mixture and used for the JHBP activity assay. The activity test described above was modified so that each tube of hormone was mixed with 0.1% gelatin in 10 mM MOPS and 100 mM NaCl at pH 7.2 supplemented with GdmCl of the same concentration as in the JHBP sample. A blank sample titration was performed in the same manner as described above, except that the buffer replaced the protein solution.
Fluorescence analysis of the GdmCl effect on a microenvironment of JHBP tyrosine residues
The influence of GdmCl on the fluorescence of JHBP tyrosine residues was monitored (λex = 275 nm) using the Fluorolog-3 instrument (SPEX, Jobin-Yvon, Horiba, ISA Katowice, Poland). The defined volumes of GdmCl solution were added to JHBP samples in a phosphate buffer (final JHBP concentration 0.1 mg mL−1) to obtain an increasing denaturant concentration range from 0 to 4.0 M. After 1 h of incubation at 4°C the protein samples were withdrawn from the incubation mixture, and the fluorescence emission spectra were recorded in a range of 290 to 360 nm. Analogous control experiments were done to investigate the effect of GdmCl on 30.9 μM L-tyrosine amide (corresponding to the concentration of tyrosine residues in 0.1 mg mL−1 JHBP).
Analysis of the GdmCl effect on JHBP secondary structure with CD spectroscopy
CD spectra of JHBP (0.1 mg mL−1 in the phosphate buffer) were obtained after mixing JHBP with GdmCl in a final concentration range between 0 and 4.5 M, using a JASCO (Vienna, Austria) spectropolarimeter J-600 in the thermostated cell with an optical path of 1 mm at 25°C. Because equilibration of the CD signal was significantly prolonged for GdmCl concentrations in the unfolding transition region, i.e., 1.5–2.5 M of GdmCl (25–100 min), the final ellipticity was determined as an extrapolated value from the equation y = A(1-exp(−kt))+off, which represents the ellipticity dependence on the time of a sample incubation with the denaturant.
Analysis of GdmCl-induced conformational transition of JHBP molecules
Two-state approximation
Pre- and posttransition baselines were linearly extrapolated to a general forms, yN = ax + b and yD = cx + d, where x is the GdmCl concentration, and yN and yD are the ellipticity at 222 nm (Θ222) of the native (N) and denatured (D) molecules, respectively. Ellipticities of intermediate states can be obtained for any given denaturant concentration by extrapolation of the baselines before and after the transition. The percentage of molecules in D form was calculated for each GdmCl concentration using Eq. 2,
 |
(2) |
where yex represents the experimental value of Θ222. The conformational transition constant (KD) was calculated from Eq. 3,
 |
(3) |
The free energy change (ΔGD) of the transition was calculated from Eq. 4, using a two-state model based on the equilibrium assumptions (Ghélis and Yon, 1982; Pace, 1886),
 |
(4) |
To estimate the conformational stability expressed as ΔGD–N, the linear part of the plot ΔGD versus GdmCl concentration was extrapolated to a zero GdmCl concentration using experimental points from the transition region according to Eq. 5,
 |
(5) |
where m is a denaturant susceptibility parameter (m = −δΔGD/δ[GdmCl]).
Number of conformational states
An analytical approach to analyze the number of conformational protein states for protein unfolding induced by GdmCl has been described in detail by Uversky and Ptitsyn (1994). It was shown that the changes of physical parameters upon denaturation can be presented as superpositions of the contributions of native (N) and denatured molecules (D),
 |
(6) |
where X is the measured parameter, XN and XD are its values for the N state and the D state, respectively, and fN and fD are respective fractions of molecules. In the case of multistate transition through intermediates, In, one may write
 |
(7) |
where fI is the fraction of molecules in the intermediate state(s), n is the number of intermediate states, and XI is the value of parameter X in the nth state. The relative populations of two additional substates (fI1 and fI2) in the case of a four-state transition can be expressed by Eq. 8,
 |
(8) |
All the experimental data (JHBP binding activity, Tyr and ANS fluorescence changes, and CD signal changes) used to conclude the number of conformational states were obtained for the experiments made at the same JHBP concentration (0.1 mg mL−1) and the same time (1 h) of JHBP incubation with GdmCl.
Analysis of the GdmCl effect on JHBP-ANS complex fluorescence
Defined volumes of GdmCl solution were added to JHBP samples in the phosphate buffer (final JHBP concentration 0.1 mg mL−1; i.e., 3.9 μM) to obtain increasing denaturant concentrations in the range from 0 to 4.0 M. After 1 h of incubation at 25°C the protein samples were supplemented with ANS (final concentration of ANS 77.3 μM and JHBP 3.5 μM). ANS solutions contained the same GdmCl concentration as the JHBP samples before mixing. Changes in emission maximum wavelength and fluorescence intensity were monitored in the 400–540 nm range after 2 min of incubation with ANS (λex = 374 nm). Analogous control experiments were done to investigate the influence of GdmCl on the fluorescence of 30.9 μM L-tyrosine amide in the presence of ANS.
Analysis of JHBP refolding from JHBP binding activity, CD spectra, and JHBP-ANS fluorescence spectra
Recovery of JHBP binding activity was measured as follows. The defined volume of GdmCl stock solution was added to the JHBP solution (0.49 mg mL−1) in a phosphate buffer to obtain a 3.5 M denaturant concentration. After 1 h of incubation the reaction mixture was diluted twofold with a phosphate buffer and then immediately diluted 20-fold with the 10 mM MOPS and 100 mM NaCl buffer of pH 7.2 supplemented with 0.1% gelatin. Final JHBP and GdmCl concentrations were 0.01 mg mL−1 of protein and 0.09 M of denaturant. After another hour of incubation the JHBP binding activity was measured. The binding parameters of native and refolded JHBP were determined based on Scatchard analysis (Scatchard, 1949). Increasing amounts of JH III (1.6 × 10−8 M−2 × 10−6 M) dissolved in hexane were added to glass tubes. After the solvent evaporated, 0.47 μM JHBP in 10 mM MOPS, 100 mM NaCl, and 0.1% gelatin at pH 7.2 was added. Then after 5 h of incubation at 4°C, the bound and unbound hormones were separated using the method described above. The data points were fitted to the Scatchard equation B/F = −B/Kd + nBT/Kd, where B is the concentration of the bound JH, F is the concentration of unbound JH, BT is the total JHBP concentration, Kd is the dissociation constant, and n is the number of JH binding sites per one JHBP molecule.
The refolding experiments analyzed with CD measurements were done as follows. JHBP (0.7 mg mL−1) was denatured in 3.5 M GdmCl for 1 h. Then this solution was diluted to a 0.02-mg mL−1 protein concentration and a 0.09-M GdmCl concentration, and the far-UV CD spectra were recorded.
The ANS binding ability by refolded JHBP was monitored by measuring the fluorescence spectra. The position of the fluorescence emission maximum and fluorescence intensity measured at 481 nm were determined after ANS was added to the incubation mixture. JHBP of a 0.8 mg mL−1 concentration was incubated for 1 h with 2.5 M GdmCl. Then the sample was progressively diluted to a final protein concentration of 0.1 mg mL−1 and the different GdmCl concentrations. The fluorescence spectra were recorded 1 h after the initialization of refolding and 2 min after ANS was added (13.7-fold molar excess of ANS over JHBP concentration) (λEX = 374 nm).
Size-exclusion HPLC analysis
Hydrodynamic dimensions (Stokes radius, Rs) of JHBP molecules in different conformational states were measured using the gel filtration method. Measurements were carried out with a Superdex 75 HR 10/30 column (V0 = 8 mL) using a Pharmacia HPLC instrument (Warsaw, Poland). A set of globular proteins with known Rs values was used for column calibration both in native conditions and in the presence of 6.0 M GdmCl. A set of standards included horse myoglobin (MW = 17.8 kDa) (Andrews, 1970);  Å (Laurent and Killander, 1964);
Å (Laurent and Killander, 1964);  Å (Uversky, 1993); ovalbumin (MW = 45.0 kDa) (Andrews, 1970);
Å (Uversky, 1993); ovalbumin (MW = 45.0 kDa) (Andrews, 1970);  Å (Laurent and Killander, 1964);
Å (Laurent and Killander, 1964);  Å (Uversky, 1993); BSA (MW = 66.3 kDa) (Uversky, 1993);
Å (Uversky, 1993); BSA (MW = 66.3 kDa) (Uversky, 1993);  Å (Uversky, 1993);
Å (Uversky, 1993);  Å (Uversky, 1993); RNase A (MW = 13 700 kDa) (Uversky, 1993);
Å (Uversky, 1993); RNase A (MW = 13 700 kDa) (Uversky, 1993);  Å (Uversky, 1993); and
Å (Uversky, 1993); and  Å, where
Å, where  and
and  are the Stokes radii of native (N) and completely unfolded (U) proteins, respectively. Hydrodynamic dimensions of native and completely unfolded globular proteins were determined experimentally or calculated from empirical Eqs. 9 and 10 as described by Uversky (1993),
are the Stokes radii of native (N) and completely unfolded (U) proteins, respectively. Hydrodynamic dimensions of native and completely unfolded globular proteins were determined experimentally or calculated from empirical Eqs. 9 and 10 as described by Uversky (1993),
 |
(9) |
 |
(10) |
JHBP samples (0.1 mg mL−1) were incubated with various concentrations of GdmCl (0–3.0 M) for 30 min at 25°C and then they were centrifugated at 17,000 × g at 4°C for 30 min. Finally, 200-μl samples containing 20 μg protein were injected into the column which was previously equilibrated with the appropriate concentration of denaturant. Elution volumes were used to calculate the Kav values for each sample,
 |
(11) |
where Ve is the elution volume of the sample, V0 is the void volume, and Vt is the total volume of the gel bed. The analytical approach used by Uversky and Ptitsyn (1994) for β-lactamase unfolding studies was applied to analyze the stages of JHBP unfolding from a native N state to a fully denatured D state.
RESULTS
GdmCl-induced far-UV difference spectra of JHBP molecule
The JHBP UV difference spectrum between native JHBP and denatured JHBP in 4.0 M GdmCl shows two minima at 279 and 287 nm (Fig. 1). The locations of these minima correspond to the difference spectra of the perturbed tyrosine residues as similarly observed for unfolded pancreatic RNase from red deer; the protein is like G. mellonella JHBP, which contains tyrosine residues and no tryptophan (Schmid, 1989). Time-dependent changes of the difference signal at 287 nm (Dɛ287) showed that the main conformational change induced by GdmCl occurs in the first 2 min of the interaction and then continues slowly for at least 2 h more (Fig. 1, inset).
FIGURE 1.
UV difference spectrum of JHBP perturbed by GdmCl. JHBP with a final concentration 2.63 × 10−5 M in 100 mM phosphate buffer, pH 7.2 at 25°C was incubated with 4.0 M GdmCl. Difference spectra were monitored after 2, 5, 13, 30, 90, and 120 min of mixing of the initial solutions in the tandem sample cell. (Inset) Time-dependent changes of the difference signal monitored at 287 nm. Data points were taken from the difference spectra of JHBP monitored at 4.0 GdmCl in the conditions described above. Baselines recorded before mixing the contents of the sample, and reference tandem cells and after mixing the contents of the reference cell at the end of experiment, are indicated; for details, see Materials and Methods.
Effect of GdmCl on JHBP binding activity
The pattern of JHBP activity changes after 1 h of incubation with GdmCl is presented in Fig. 2. Increasing GdmCl concentrations from 0.0 to 0.6 M result in slight fluctuations of JHBP activity. In the GdmCl concentration range of 0.6–1.4 M, a sharp decrease of JHBP activity was observed. In a control experiment GdmCl had a small effect on the ability of JH III adsorption by charcoal (Fig. 2, inset).
FIGURE 2.
Effect of GdmCl on JHBP binding activity. JHBP (0.1 mg mL−1) in the phosphate buffer was incubated for 1 h with different concentrations of GdmCl. JHBP activity was measured as described in the Materials and Methods section. Solid lines were obtained by fitting the experimental points with the spline function of SigmaPlot 3.0 program for better visualization. Each bar represents mean ± SD for five determinations. (Inset) Effect of GdmCl on the activity assay. The measurement was performed in the same manner as described above, except the phosphate buffer replaced the protein solution. Each bar represents mean ± SD for three determinations.
Detection of the exposure of hydrophobic side-chain residues to the solvent during GdmCl-induced JHBP unfolding
Previous studies have shown that ANS binding to the hydrophobic part of proteins results in a blue shift of the ANS fluorescence emission maximum and a pronounced increase in fluorescence intensity (Lakowicz, 1999; Semisotnov et al., 1991). For example, ANS binding to the aldolase active-site environment resulted in a shift of the ANS fluorescence maximum from 520 to 487 nm and an ∼90-fold increase in fluorescence intensity (Kasprzak and Kochman, 1980). Intrinsic JHBP fluorophores fluorescence in this region of the spectrum is negligible (not shown). It appeared that GdmCl caused a decrease in fluorescence intensity and a red shift of the ANS emission maximum (Fig. 3 A, inset). In a control experiment it was found that GdmCl had little effect on ANS fluorescence in the presence of L-tyrosine amide (Fig. 3 B, inset). Between 0 and 0.8 M denaturant concentrations the fluorescence intensity of the JHBP-ANS complex is rather constant (Fig. 3 A). In the range of 0.8–1.5 M GdmCl concentration fluorescence intensity decreases dramatically and reaches a constant value at 1.8 M GdmCl. Fig. 3 B shows the dependence of a position of the ANS emission maximum on a GdmCl concentration. One may observe a small shift of the ANS emission maximum from ∼481 nm to 485 nm between 0 and 0.2 M GdmCl, respectively. Between 1.0 and 1.4 M denaturant concentrations the maximum shifts from 485 to 520 nm, and it reaches a plateau at 1.8 M GdmCl, corresponding to the value observed for unbound ANS in the control experiment.
FIGURE 3.
Fluorescence analysis of the effect of GdmCl on JHBP-ANS complex. (A) Changes in fluorescence intensity monitored at 481 nm for the JHBP (3.51 μM)-ANS (77.3 μM) mixture titrated with GdmCl (λEX = 374 nm). Data points were taken from the fluorescence spectra (examples are presented in the inset) recorded at indicated GdmCl concentrations and presented as the difference between ANS fluorescence intensity in the presence of JHBP and the absence of JHBP. Each bar represents mean ± SD for three determinations. (B) Shift of the fluorescence emission wavelength maximum of ANS and JHBP-ANS complex due to GdmCl addition (λEX = 374 nm of JHBP-ANS complex; ANS 77.3 μM, JHBP 3.51 μM in the phosphate buffer). Each bar represents mean ± SD for three determinations. (A, inset) Examples of the fluorescence emission spectra (λEX = 374 nm) of ANS (77.3 μM) and JHBP (3.51 μM) in the presence of 0-M, 1.2-M, and 2.5-M concentrations of GdmCl. (B, inset) Effect of GdmCl on the fluorescence intensity changes of ANS (77.3 μM) in the presence of L-tyrosine-amide (30.9 μM).
JHBP conformational changes affect the microenvironment of tyrosine residues
The fluorescence emission spectrum of native JHBP excited at 275 nm displays a maximum of 303 nm which corresponds to the excitation of tyrosine residues (Fig. 4, inset). In the presence of 0–0.1 M GdmCl an increase in JHBP fluorescence intensity was observed; then, a decrease in intensity was observed in the range of 0.4–1.2 M GdmCl (Fig. 4). Above a 1.2 M denaturant concentration, changes in fluorescence intensity are small (in the range of experimental error), indicating that tyrosine residues are randomly exposed to solvent.
FIGURE 4.
Effect of GdmCl on the fluorescence intensity of JHBP tyrosine residues. JHBP unfolding was monitored by fluorescence intensity at λEM = 303 nm (λEX = 275 nm). Each bar represents mean ± SD for three determinations. (Inset) Representative fluorescence spectra of JHBP (0.1 mg mL−1) in the phosphate buffer after incubation without GdmCl (solid line) and with 0.3-M (dotted line) and 2.5-M (dashed line) concentrations of GdmCl (λEX = 275 nm).
Changes in the secondary structure of JHBP induced by GdmCl revealed a two-state denaturation transition
The CD spectrum of native JHBP protein consists of two distinct bands: a broad negative maximum between 217 and 221 nm with a shoulder of ∼208 nm and a positive maximum at 195–196 nm (Fig. 5, inset). This spectrum is the same as previously reported (Krzyżanowska et al., 1998). Due to the high absorption of the GdmCl solution far-UV CD measurements were reliable only above 210 nm; nonetheless, conformational changes associated with the secondary structure transition can be observed. Between 0 and 0.8 M GdmCl concentrations JHBP exhibits a CD spectrum corresponding to a folded nativelike structure (Fig. 5, inset) composed of ∼8% α-helix and 62% β-structures. The values obtained were recalculated data from previously presented CD spectra (Krzyżanowska et al., 1998). Above 2.5 M GdmCl, JHBP loses the regular secondary structure, as seen from the JHBP CD spectra (Fig. 5, inset). Analysis of the JHBP unfolding transition monitored by the variations in ellipticity at 222 nm shows that the transition midpoint c1/2 value equals 1.67 M (Fig. 5). The ΔGD–N = 4.1 kcal mol−1 value, obtained from Eq. 5, reflects the equilibrium constant between native and unfolded populations of JHBP molecules. The m-value, which is the measure of the dependence of ΔGD on the denaturant concentration, is 2.5 kcal mol−1 M−1. The complete unfolding of JHBP molecules was observed above 2.5 M GdmCl (Fig. 5).
FIGURE 5.
Effect of GdmCl on CD spectra of JHBP. Dependence of molar ellipticity measured at 222 nm on various GdmCl concentrations. Data points were taken from the spectra of JHBP monitored in the 0–4.5 M GdmCl range, after 1 h of incubation with denaturant and corrected for the changes of JHBP concentration and GdmCl concentrations. The final values of ellipticities from the unfolding transition region were determined as extrapolated values; see Materials and Methods. (Inset) Examples of far-UV CD spectra of JHBP (0.1 mg mL−1 in the phosphate buffer) after 1 h of incubation without GdmCl (solid line) and with 0.8-M (dotted line) and 2.8-M (dashed line) concentrations of GdmCl.
JHBP unfolding monitored by gel filtration
Estimation of JHBP molecules Stokes radius in the native and unfolded state
Fig. 6 presents the elution profiles of JHBP preincubated with GdmCl in the 0–3.0 M concentration range. A bimodal distribution of JHBP molecules in the transition region was observed. This result suggests that JHBP undergoes an “all-or-none” transition between “compact” and “noncompact” states, slowly exchanging with each other. The Stokes radii determined for standard proteins in the presence and absence of denaturant are in agreement with the Rs values calculated with Eqs. 9 and 10 (not shown). This indicates that the Superdex 75 column can be used to determine JHBP molecular dimensions in the native and completely unfolded states. Based on the elution profiles of native JHBP and the protein denatured in 3.0 M GdmCl, the Stokes radius of the native molecule is 23.0 Å and the relative molecular mass is 27.4 kDa. This value is close to 25.88 kDa which was measured with mass spectrometry (ESI-MS; Duk et al., 1996). Calculated with the empirical Eq. 9 the value of Rs equals 23.9 Å. The Rs value for JHBP, found from size-exclusion chromatography after 1 h of incubation in 3.0 M GdmCl, is 39.0 Å (Fig. 6). This value is smaller in comparison with the 47.0 Å value calculated for an unfolded molecule. A distinct, nearly 40% increase in the JHBP Stokes radius (30–39 Å) between 0.7 and 3.0 M GdmCl indicates the swelling of a noncompact molecule contrary to the small increase of ∼2 Å of Rs of compact-state molecules (22–24 Å) observed between 0 and 1.2 M GdmCl (Fig. 6). Six hours of JHBP incubation with GdmCl showed similar elution patterns as for a 1-h incubation period, except that in the 0–0.6 M GdmCl range a poorly visible peak of high molecular weight material of Rs 64.6 Å at 0.2 M GdmCl was detected (data not shown). The gel filtration data were analyzed also as relative areas of the two peaks corresponding to the compact and noncompact JHBP molecules (data not shown). The appearance of the noncompact form is accompanied with the simultaneous decline of the protein in a soluble form. This is due to the aggregation of JHBP molecules. Aggregation of JHBP molecules was confirmed by light-scattering experiments (data not shown).
FIGURE 6.
JHBP unfolding induced by GdmCl monitored by gel filtration chromatography. Native JHBP with a final concentration of 3.9 μM in the phosphate buffer was incubated for 1 h at 25°C with indicated concentrations of GdmCl. After 30 min of centrifugation at 14,000 rpm at 4°C, 21 μg of the protein was applied on the Superdex HR 75 column and equilibrated at 25°C with indicated concentrations of GdmCl. The elution profile was monitored at 280 nm. Stokes radii (RS) of JHBP molecules eluted from the column are indicated above each peak. The values of RS, expressed in Å, were calculated with the HPLC instrument software from the positions of protein peaks.
Refolding of JHBP monitored by activity, CD, and fluorescence measurements
Refolding experiments were performed to answer the question of whether or not JHBP incubated with high GdmCl concentration solutions is able to return to a native protein structure. It was found that 2 h after the dilution of the unfolded protein sample incubated with 3.5 M GdmCl, JHBP molecules recovered 100% of the control sample activity. The JHBP control sample was treated in the same manner as refolded JHBP except that GdmCl was omitted. The binding parameters obtained from a linear regression fit of the data presented in Fig. 7 to the Scatchard equation are Kd = 0.5 μM, n = 0.95, and r2 = 0.95 for native JHBP and Kd = 0.64 μM, n = 1.06, and r2 = 0.93 for refolded protein (Fig. 7, inset), where r2 is the goodness-of-fit parameter. The Kd value obtained for native JHBP is in agreement with the data published by Ożyhar and Kochman (1987).
FIGURE 7.
Comparison of the binding affinities of JH III to native and refolded JHBP. 0.47 μM JHBP in 10 mM MOPS, 100 mM NaCl, and 0.1% gelatin, at pH 7.2, incubated with various concentrations of JH III at 4°C for 5 h. Separation of free and bound hormones was performed by the charcoal method (see Materials and Methods) (•). The defined volume of GdmCl stock solution was added to the JHBP solution (0.49 mg mL−1) in the phosphate buffer to obtain a 3.5 M denaturant concentration. After 1 h of incubation the reaction mixture was diluted twofold with the phosphate buffer and then 20-fold with 10 mM MOPS, 100 mM NaCl, and 0.1% gelatin, at pH 7.2. After another hour of incubation JHBP binding activity was measured (○). (Inset) Scatchard plots for the binding of JH III by native (•) and refolded (○) JHBP. Solid lines are obtained using the values of parameters (see text) obtained from linear least-squares fits described by the Scatchard equation.
The reversibility of JHBP unfolding was also analyzed using CD spectrum changes and the ANS binding ability of JHBP molecules. The spectra of the native JHBP (solid line), denatured with 3.5 M GdmCl (dotted line) and refolded (dashed line) molecules transferred from 3.5 M GdmCl to 0.09 M denaturant concentration are presented in Fig. 8. One can observe that a decrease of the denaturant concentration caused nearly full regeneration of the native protein CD spectrum (Fig. 8).
FIGURE 8.
The CD spectra of native JHBP (solid line), denatured JHBP (dotted line), and refolded JHBP (dashed line, normalized for protein concentration). JHBP (0.7 mg mL−1) was incubated for 1 h with 3.5 M GdmCl. Then the reaction mixture was diluted with the phosphate buffer to 0.02 mg mL−1 and 0.09 M concentrations of the protein and GdmCl, respectively, and the far-UV CD spectra were recorded.
As shown above (Fig. 3) the interactions between ANS and JHBP are strongly perturbed by GdmCl. The progress curve of JHBP refolding (dotted line), monitored by the inspection of ANS fluorescence, exhibits a hysteresis in comparison with the curve obtained during unfolding (Fig. 9, A and B, solid line). Dilution of denatured JHBP to a final GdmCl concentration of <0.5 M results in the protein which binds ANS as the native protein in the control experiment.
FIGURE 9.
Comparison of JHBP unfolding (•) and refolding (○) pathways monitored by the changes in the fluorescence of JHBP-ANS complex. JHBP (0.1 mg mL−1) was incubated for 1 h with GdmCl. The position of fluorescence emission maximum (λEX = 374 nm; results in A) and fluorescence intensity at 481 nm (B) was determined after ANS was added (13.7-fold molar excess over JHBP) to the incubation mixture (•). JHBP of the initial concentration 0.8 mg mL−1 was incubated for 1 h with 2.5 M GdmCl. Then the sample was diluted to the final protein concentration of 0.1 mg mL−1 and the indicated GdmCl concentration. After 1 h of incubation, ANS (13.7-fold molar excess over JHBP) was added, and the fluorescence spectra were recorded (λEX = 374 nm) (○).
The results of JHBP activity analysis and the CD and fluorescence measurements indicate that the GdmCl-induced perturbation of JHBP's structure is reversible. Therefore, a thermodynamic analysis of denaturation free energy change can be used.
DISCUSSION
Four-state GdmCl-induced unfolding of JHBP
This is the first detailed study on the stability of JHBP molecules. Fig. 6 shows that the protein molecules gradually increase their size when exposed to a denaturant. Initially, all the conformational changes seem to be associated with a kind of compact structure. However, the loss of integrity of a JH binding site was already observed at low perturbant concentrations (Fig. 2) which transform JHBP molecules to a noncompact state. This means that protein unfolding involves several transitions. The first transition is associated with protein deactivation, the second transition leads to exposure of hydrophobic fragments as seen from ANS fluorescence analysis, and the third transition is represented by the loss of the JHBP secondary structure as it appears from CD analysis.
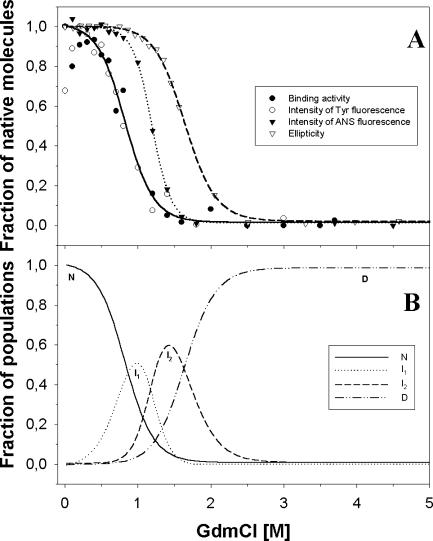
Fig. 10 A presents the collected results of the effects of GdmCl on JHBP binding activity, JHBP intrinsic fluorophores fluorescence intensity, ANS interaction with JHBP, and the JHBP secondary structure. It appears that the curves representing the perturbant effect on JHBP binding activity and on the tyrosine residues microenvironment are almost superimposed. The results nicely correlate with the data published by Krzyżanowska et al. (1998), which have shown that the JHBP conformational change induced by JH binding results in changes in the protein tyrosine residues microenvironment. The change in Tyr fluorescence and protein activity starts to appear at a 0.1 M GdmCl concentration. These changes in the region of denaturant concentration from 0 to 0.6 M slightly deviate from the data curves. Thus, it is difficult to ascribe them to the stable transition state. This lack of continuity suggests that, up to a 0.6 M GdmCl concentration, unstable intermediates susceptible to aggregation appear. This suggestion is supported by the detection of oligomeric forms of JHBP in light-scattering experiments (data not shown). Above 0.6 M GdmCl a dramatic destruction of the JHBP active site was observed, as well as noncompact state JHBP molecules, in size-exclusion chromatography (Fig. 6). The second stage of JHBP conformational changes induced by GdmCl was detected with an ANS probe. The changes in fluorescence intensity and the shift of the ANS wavelength maximum indicate that an ANS binding site(s), probably a hydrophobic pocket, is destroyed above 1.2 M GdmCl. Moreover, most of the JHBP molecules exist in a noncompact state in this range of perturbant concentration. As one may expect, the JHBP secondary structure is the most stable. The curve representing CD-signal changes shows a shift of ∼0.5 M GdmCl concentration from the curve of ANS fluorescence intensity changes. This shift shows that, above a 1.5-M denaturant concentration, JHBP molecules still have ∼60% of their secondary structure (Fig. 10 A). At 3.0 M GdmCl the protein appears in a denatured state. It seems that the two disulfide bridges present in JHBP from G. mellonella help protein molecules to maintain their secondary structure at higher denaturant concentrations. Their presence is reflected in the smaller Stokes radius of the denatured protein than what is calculated for the unfolded JHBP molecules. The data presented in Fig. 10 A allowed us to estimate the number of conformational states and the population of intermediates induced by GdmCl by means of Eqs. 6–8 (Fig. 10 B).
FIGURE 10.
Comparison of the results obtained for GdmCl-induced unfolding of JHBP with CD, ANS fluorescence intensity, JHBP tyrosine residues fluorescence intensity, and the binding activity stages of conformational changes of JHBP molecules by GdmCl. (A) The fraction of native molecules. Fractions of unfolded species in the presence of increased GdmCl concentrations under the same experimental conditions were obtained based on the data shown in Figs. 2, 3 A, 4, and 5. Calculations were made with the equation fD = (Y–YN)/(YD–YN), where YN and YD are the values of the measured parameter for the native and denatured states. Y refers to the value of this parameter at given conditions. (B) Populations of conformational states of JHBP molecules at different GdmCl concentrations. Populations of native molecules and intermediates were expressed as fractions of all JHBP molecules. The fraction of native molecules was calculated from JHBP binding activity data from Eq. 7, assuming fN = 1.0 at 0 M GdmCl and fN = 0 at 2.0 M GdmCl. The fraction of molecules in a D state (fD) was obtained from CD measurements by Eq. 7, with fD = 0.0 for 0.0 M GdmCl and fD = 1.0 for 3.5 M GdmCl. Fractions of intermediates I (fI1) and II (fI2) were calculated with Eq. 8.
The four conformational states of JHBP molecules can be characterized as follows:
N. A monomeric, native state population of JHBP molecules of Stokes radius 23.0 Å; molecules maintain a compact structure up to 1.5 M GdmCl.
I1. An unstable intermediate that appears in the 0.1–1.5-M GdmCl range and consists of no more than 50% of the population of whole JHBP molecules; JHBP molecules lose the ability to bind JH with increasing GdmCl concentrations but are able to bind ANS. They reveal a tendency for slow aggregation.
I2. A population of unstable molecules in the 0.6–3.0 M GdmCl range, unable to bind ANS above 1.4 M GdmCl. They are inactive; up to a 2.5 M denaturant concentration they preserve part of their secondary structure.
D. A denatured state of JHBP molecules, which appears above 1.0 M GdmCl. Above 3.0 M GdmCl all JHBP populations exist in a D state. The Stokes radius of JHBP molecules equals 39.0 Å. Molecules are able to refold to a nativelike structure.
Assuming that JHBP molecules may occur in four different states (Fig. 10 B), one may attempt to calculate the population of molecules in a particular state assuming that all the molecules in the absence of GdmCl are in the N state. The fraction of molecules in the D state (fD) was calculated from the CD signal at 222 nm by Eq. 6, assuming fD = 0 at 0 M GdmCl and fD = 1 at 2.4 M GdmCl. Fractions fI1 and fI2 of intermediates I1 and I2, respectively, were calculated from Eq. 8. As shown in Fig. 10 B, the population of native molecules decreases immediately with the concomitant increase of two unstable intermediates (I1 and I2), reaching their maxima at ∼1.0 M and 1.4 M GdmCl. Above 3.0 M GdmCl all JHBP molecules are transformed into a D state.
Refolding of JHBP molecules
In a wide range of GdmCl concentrations JHBP is able to refold almost 100% as seen from activity, CD measurements, and ANS fluorescence spectroscopy. Scatchard analysis of JH binding to JHBP molecules shows that JHBP is able to reactivate after incubation with 3.5 M GdmCl. The binding affinity of a hormone to the protein molecule is in the same range of experimental error for native JHBP molecules as for refolded molecules. ANS fluorescence analysis shows that denaturant also induces reversible changes in JHBP-ANS binding. The CD spectra of the native and refolded proteins are very similar. Therefore, analysis of the N-D transition fulfills the condition of reversibility and allows the calculation of a ΔGD–N value from Fig. 5.
Implications for folding of JHBP as β-structure protein
JHBP shows considerable homology to the secondary structure of RBP protein, which consists of 9% α-helix and 45% β-sheet, despite having as little as 10% sequence similarity (Rodriguez Parkitna et al., 2002). Both β-structure proteins have disulfide bridges (RBP: three) and comparable molecular weight (RBP: 21,000 Da) (Greene et al., 2001). Interestingly, both proteins have similar stability measured by chemical denaturation characterized, in the case of RBP, by ΔGD–N = 4.76 kcal mol−1, m = 2.67 kcal mol−1 M−1, and c1/2 = 1.79 M (Greene et al., 2001). Moreover, three other mostly β-sheet proteins (CRABP I, cellular retinoic acid binding protein I; CRBP II, cellular retinol binding protein II; and IFABP, intestinal fatty acid binding protein) belonging to the same superfamily have somewhat higher values of ΔGD–N (5.5–8.3 kcal mol−1, obtained after urea denaturation) and a similar reversible progress of unfolding (Burns et al., 1998). For each of these proteins no significant population of folding intermediates was observed at equilibrium, but intermediates were detected during folding and unfolding of all of the investigated proteins (Burns et al., 1998). We also found examples of proteins functionally unrelated to JHBP, i.e., MTCP1 oncogene product P13 (ΔGD–N = 5.7 kcal mol−1; Roumestand et al., 2001) and SH3 domain of spectrin (ΔGD–N = 3.7 kcal mol−1; Viguera et al., 1994) whose structure is comprised of a β-barrel motif. Thermodynamic and kinetic analysis showed that the free energy change of JHBP unfolding is similar to the value reported in the literature for small β-structure proteins and even smaller than for small globular proteins (ΔGD–N = 5–9 kcal mol−1; Privalov, 1979). This result confirms the theoretical prediction which locates JHBP among β-barrel proteins.
Acknowledgments
The authors acknowledge Dr. Marek Lisowski from the University of Wrocław for assistance with CD spectroscopy and Prof. Jacek Otlewski from the University of Wrocław for discussions and comments.
This work was supported by a grant from the State Committee for Scientific Research (3-P04A-003-23), a grant from the Ministry of Scientific Research and Information Technology (3-P04A-040-25), and in part by the Wrocław University of Technology.
Abbreviations used: ANS, 1-anilinonaphthalene-8-sulfonic acid; GdmCl, guanidine hydrochloride; JHBP, juvenile hormone binding protein; JH, juvenile hormone.
References
- Andrews, P. 1970. Estimation of molecular size (Stokes' radius) by column methods. In Methods of Biochemical Analysis. D. Glick, editor. J. Wiley & Sons, New York. 20–26.
- Burns, L. L., P. M. Dalessio, and I. J. Ropson. 1998. Folding mechanism of three structurally similar β-sheet proteins. Prot. Struct. Funct. Genet. 33:107–118. [DOI] [PubMed] [Google Scholar]
- Chanez-Cardenas, M. E., D. A. Fernandez-Velasco, E. Vazquez-Contreras, R. Coria, G. Saab-Rincon, and R. Perez-Montfort. 2002. Unfolding of triosephosphate isomerase from Trypanosoma brucei: identification of intermediates and insight into the denaturation pathway using tryptophan mutants. Arch. Biochem. Biophys. 399:117–129. [DOI] [PubMed] [Google Scholar]
- Duk, M., H. Krotkiewski, E. Forest, J. M. Rodriguez Parkitna, M. Kochman, and E. Lisowska. 1996. Evidence for glycosylation of the juvenile-hormone-binding protein from Galleria mellonella hemolymph. Eur. J. Biochem. 242:741–746. [DOI] [PubMed] [Google Scholar]
- Ferguson, R. N., and H. J. Cahnmann. 1975. Preparation of tritium-labeled 8-anilino-1-naphthalenesulfonic acid. Biochemistry. 14:287–289. [DOI] [PubMed] [Google Scholar]
- Forge, V., M. Hosino, K. Kuwata, M. Arai, K. Kuwajima, C. A. Batt, and Y. Goto. 2000. Is folding of β-lactoglobulin non-hierarchic? Intermediate with native-like β-sheet and non-native α-helix. J. Mol. Biol. 296:1039–1051. [DOI] [PubMed] [Google Scholar]
- Ghélis, C., and J. Yon. 1982. Physicochemical studies of the unfolding-folding equilibrium. In Molecular Biology: Protein Folding. B. Horecker, N. O. Kaplan, J. Marmur, and H. A. Scheraga, editors. Academic Press, New York. 297–338.
- Gilbert, L. I., W. Goodman, and J. Nowock. 1976. The possible roles of binding proteins in juvenile hormone metabolism and action. Actualites sur les hormones d'invertebres. Colloq. Int. CNRS. 251:413–434. [Google Scholar]
- Gilbert, L. I., N. A. Granger, and R. M. Roe. 2000. The juvenile hormones: historical facts and speculations on future research directions. Insect Biochem. Mol. Biol. 30:617–644. [DOI] [PubMed] [Google Scholar]
- Goodman, W., W. E. Bollenbacher, H. L. Zvenko, and L. I. Gilbert. 1976. A competitive binding protein assay for juvenile hormone. In The Juvenile Hormones. L. I. Gilbert, editor. Plenum Press, New York. 75–95.
- Goodman, W. G., and E. S. Chang. 1985. Juvenile hormone cellular and hemolymph binding proteins. In Comprehensive Insect Physiology, Biochemistry and Pharmacology. G. Kerkut and L. I. Gilbert, editors. Pergamon Press, Oxford, UK. 491–510.
- Greene, L. H., E. D. Chrysina, L. I. Irons, A. C. Papageorgiou, K. R. Acharya, and K. Brew. 2001. Role of conserved residues in structure and stability: tryptophans of human serum retinol-binding protein, a model for the lipocalin superfamily. Protein Sci. 10:2301–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada, D., and Y. Goto. 1997. The equilibrium intermediate of β-lactoglobulin with non-native α-helical structure. J. Mol. Biol. 269:479–487. [DOI] [PubMed] [Google Scholar]
- Hidayat, P., and W. G. Goodman. 1994. Juvenile hormone and hemolymph juvenile hormone binding protein titers and their interaction in the hemolymph of fourth stadium Manduca sexta. Insect Biochem. Mol. Biol. 24:709–715. [Google Scholar]
- Kasprzak, A. A., and M. Kochman. 1980. Interaction of fructose-1,6-bisphosphate aldolase with adenine nucleotides. Binding of 5′-mononucleotides and phosphates to rabbit muscle aldolase. Eur. J. Biochem. 104:443–450. [DOI] [PubMed] [Google Scholar]
- Kochman, M., and E. Wieczorek. 1995. Proteins involved in juvenile hormone signal transmission. In Insects: Chemical, Physiological and Environmental Aspects. D. Konopińska, G. Goldsworthy, R. J. Nachman, J. Nawrot, I. Orchard, G. Rosiński, and W. Sobótka, editors. University of Wrocław Press, Wrocław, Poland. 92–118.
- Kołodziejczyk, R., P. Dobryszycki, A. Ożyhar, and M. Kochman. 2001. Two disulphide bridges are present in juvenile hormone binding protein from Galleria mellonella. Acta Biochim. Polon. 48:917–920. [PubMed] [Google Scholar]
- Kołodziejczyk, R., M. Kochman, G. Bujacz, P. Dobryszycki, A. Ożyhar, and M. Jaskolski. 2003. Crystallization and preliminary crystallographic studies of juvenile hormone-binding protein from Galleria mellonella haemolymph. Acta Crystallogr. D59:519–521. [DOI] [PubMed] [Google Scholar]
- Kramer, K. J., and C. N. Childs. 1977. Interaction of juvenile hormone with carrier proteins and hydrolases from insect haemolymph. Insect Biochem. 7:397–403. [Google Scholar]
- Krzyżanowska, D., M. Lisowski, and M. Kochman. 1998. UV-difference and CD spectroscopy studies on juvenile hormone binding to its carrier protein. J. Pept. Res. 51:96–102. [DOI] [PubMed] [Google Scholar]
- Lakowicz, J. R. 1999. Principles of Fluorescence Spectroscopy. Kluwer Academic/Plenum Publishers, New York.
- Laurent, T. C., and J. Killander. 1964. A theory of gel filtration and its experimental verification. J. Chromatogr. 14:317–330. [Google Scholar]
- Nozaki, Y. 1972. The preparation of guanidine hydrochloride. Methods Enzymol. 26:43–51. [DOI] [PubMed] [Google Scholar]
- Ożyhar, A., and M. Kochman. 1987. Juvenile-hormone-binding protein from the hemolymph of Galleria mellonella (L). Isolation and characterization. Eur. J. Biochem. 162:675–682. [DOI] [PubMed] [Google Scholar]
- Pace, C. N. 1986. Determination and analysis of urea and guanidine hydrochloride denaturation curves. Methods Enzymol. 131:266–280. [DOI] [PubMed] [Google Scholar]
- Privalov, P. L. 1979. Stability of proteins: small globular proteins. Adv. Protein Chem. 33:167–241. [DOI] [PubMed] [Google Scholar]
- Rodriguez Parkitna, J. M., A. Ożyhar, J. R. Wiśniewski, and M. Kochman. 2002. Cloning and sequence analysis of Galleria mellonella juvenile hormone binding protein—a search for ancestors and relatives. Biol. Chem. 383:1343–1355. [DOI] [PubMed] [Google Scholar]
- Roumestand, C., M. Boyer, L. Guignard, P. Barthe, and C. A. Royer. 2001. Characterization of the folding and unfolding reactions of a small β-barrel protein of novel topology, the MTCP1 oncogene product P13. J. Mol. Biol. 312:247–259. [DOI] [PubMed] [Google Scholar]
- Sanburg, L. L., K. J. Kramer, F. J. Kezdy, and J. H. Oberlander. 1975. Role of juvenile hormone esterases and carrier proteins in insect development. Nature. 253:266–267. [DOI] [PubMed] [Google Scholar]
- Scatchard, G. 1949. The attraction of proteins for small molecules and ions. Ann. N. Y. Acad. Sci. 51:660–672. [Google Scholar]
- Schmid, F. X. 1989. Spectral methods of characterizing protein conformation and conformational changes. In Protein Structure: A Practical Approach. T. E. Creighton, editor. IRL Press at Oxford University Press, Oxford, England. 251–285.
- Schonbrunner, N., K.-P. Koller, and T. Kiefhaber. 1997. Folding of the disulfide-bonded β-sheet protein tendamistat: rapid two-state folding without hydrophobic collapse. J. Mol. Biol. 268:526–538. [DOI] [PubMed] [Google Scholar]
- Semisotnov, G. V., N. A. Rodionova, O. I. Razgulyaev, V. N. Uversky, A. F. Gripas, and R. I. Gilmanshin. 1991. Study of the “molten globule” intermediate state in protein folding by a hydrophobic fluorescent probe. Biopolymers. 31:119–128. [DOI] [PubMed] [Google Scholar]
- Srimathi, T., T. K. S. Kumar, Y. Chi, I.-M. Chiu, and C. Yu. 2002. Characterization of the structure and dynamics of the a near-native equilibrium intermediate in the unfolding pathway of an all β-barrel protein. J. Biol. Chem. 277:47507–47516. [DOI] [PubMed] [Google Scholar]
- Trautmann, K. H., A. Schuler, M. Suchy, and H. K. Wipf. 1974. A method for the qualitative and quantitative determination of three natural insect juvenile hormones. Evidence of methyl 10,11-epoxy-3,7,11-trimethyl-2-trans-6-trans-dodecadienoate in Melolontha melolontha. Z. Naturforsch. [C]. 29:161–168. [PubMed] [Google Scholar]
- Trowell, S. C. 1992. High affinity juvenile hormone carrier proteins in the haemolymph of insects. Comp. Biochem. Physiol. 103B:795–807. [Google Scholar]
- Uversky, V. N. 1993. Use of fast protein size-exclusion liquid chromatography to study the unfolding of proteins which denature through the molten globule. Biochemistry. 32:13288–13298. [DOI] [PubMed] [Google Scholar]
- Uversky, V. N., and O. B. Ptitsyn. 1994. “Partly folded” state, a new equilibrium state of protein molecules: four-state guanidinium chloride-induced unfolding of β-lactamase at low temperature. Biochemistry. 33:2782–2791. [DOI] [PubMed] [Google Scholar]
- Viguera, A. R., J. C. Martinez, V. V. Filimonov, P. L. Mateo, and L. Serrano. 1994. Thermodynamic and kinetic analysis of the SH 3 domain of spectrin shows a two-state folding transition. Biochemistry. 33:2142–2150. [DOI] [PubMed] [Google Scholar]
- Wieczorek, E., and M. Kochman. 1991. Conformational change of the haemolymph juvenile-hormone-binding protein from Galleria mellonella (L). Eur. J. Biochem. 201:347–353. [DOI] [PubMed] [Google Scholar]
- Wieczorek, E., J. M. Rodriguez Parkitna, J. Szkudlarek, A. Ożyhar, and M. Kochman. 1996. Immunoaffinity purification of juvenile hormone-binding protein from Galleria mellonella hemolymph. Acta Biochim. Pol. 43:603–610. [PubMed] [Google Scholar]
- York, S. S., R. C. Lawson, Jr., and D. M. Worah. 1978. Binding of recrystallized and chromatographically purified 8-anilino-1-naphthalenesulfonate to Escherichia coli lac repressor. Biochemistry. 17:4480–4486. [DOI] [PubMed] [Google Scholar]