Abstract
To help understand the molecular mechanisms of Pasteurella multocida toxin (PMT) action, we searched for a cellular protein interacting with PMT. The ligand overlay assay revealed a 60-kDa cellular protein that binds to a region from the 840th to 985th amino acids of the toxin. This protein was identified as vimentin by peptide mass fingerprinting. The N-terminal head domain of vimentin was further found to be responsible for the binding to the toxin.
Pasteurella multocida, a causative agent of progressive atrophic rhinitis in pigs, produces a protein toxin (P. multocida toxin [PMT]) with a molecular mass of 146 kDa (2, 17, 23, 27). Several lines of evidence have shown that PMT is one of the major virulence factors that cause turbinate atrophy in atrophic rhinitis (3-5, 8, 13, 34). PMT is also known to be a potent mitogen in various types of cells (10, 25, 29, 31, 38). Many research groups have pointed out that the cellular effects of PMT are mediated by at least two different types of GTPases. PMT-treated cells have shown increases in inositol 1,4,5-trisphosphate and diacylglycerol levels, Ca2+ mobilization, and activation of protein kinase C (10, 21, 29, 32, 33), suggesting the involvement of phospholipase C (PLC) in the PMT actions. PLC comprises the β, γ, and δ isozymes, which are considered to be regulated by heterotrimeric GTPases of the Gq/11 family, several tyrosine kinases, and little-known pathways, respectively. A PMT-induced Ca2+-dependent Cl− current in Xenopus oocytes could be inhibited by antibodies against PLCβ1 and -α subunits of heterotrimeric GTPase Gq and G11 (36). These results clearly indicate that PMT activates the signal pathway from the GTPases of the Gq/11 family to PLCβ1 to elicit the toxic effects. Moreover, it was found that Gq but not G11 likely plays an important role in the PMT-induced activation of the PLC, because G11-deficient fibroblasts retained their ability to produce inositol phosphates in response to PMT, whereas Gq-deficient fibroblasts did not. On the other hand, PMT has also been known to cause formations of stress fibers and focal adhesions and tyrosine phosphorylations of focal adhesion kinase and paxillin, both of which are localized at the focal adhesions (6, 16, 38). These effects of the toxin could be blocked by C3 exoenzyme, an inhibitor of Rho function, indicating that Rho is involved in these toxic actions. The Rho-mediated PMT actions are likely independent of the Gq signaling pathway, because Rho was activated and stress fiber formations were induced by PMT in Gq/11 double-deficient fibroblasts (38). Thus, it is now believed that PMT independently stimulates the two different signaling pathways through the heterotrimeric GTPase Gq and the small GTPase Rho. However, the real target molecules for the toxin and nature of its molecular action remain unknown.
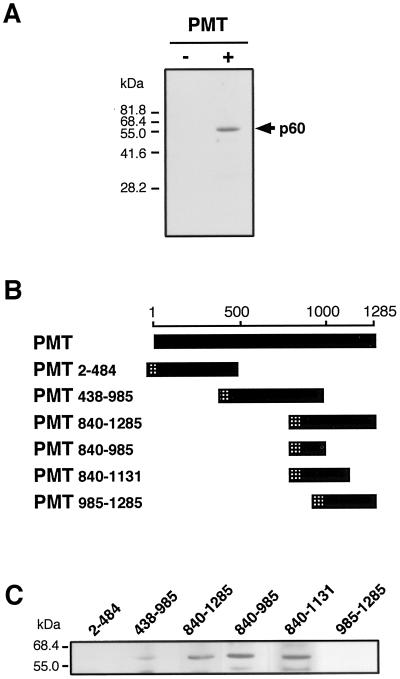
In the present study, to help elucidate the actions of PMT, we used the ligand overlay assay in an attempt to search for a cellular substance that has the ability to associate with the toxin. Swiss 3T3 cells, which are highly sensitive to PMT (25, 29, 33), were homogenized by sonication, and the supernatant after centrifugation at 10,000 × g for 20 min was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotting onto polyvinylidene difluoride membranes. Recombinant PMT was purified from extracts of Escherichia coli harboring pSN131, a PMT expression vector, provided by S. Nagai, Nippon Institute for Biological Science, Tokyo, Japan, by the method reported by Nakai et al. (23) and was overlaid at 0.4 μM on the membrane. Substances interacting with PMT were visualized by an enhanced chemiluminescence system (Amersham) after incubation of the membrane with rabbit anti-PMT antibody and horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG) antibody (Cappel). As shown in Fig. 1A, a 60-kDa cellular protein (p60) appeared from the cell lysate. To define the region responsible for the binding to p60, we subjected various deletion mutants of PMT to the assay. DNAs encoding the deletion mutants with the N-terminal FLAG tags were generated by PCRs with the suitable nucleotide primers and pSN131 as a template and subcloned into pET21d expression vectors (Novagen). The mutant proteins were purified by affinity chromatography with anti-FLAG M2 antibody beads (Sigma) from extracts of E. coli harboring the vectors. They were designated by amino acid numbers covering each mutant (Fig. 1B). As shown in Fig. 1C, PMT438-985, PMT840-1285, PMT840-985, and PMT840-1131, but not PMT2-484 and PMT985-1285bound to p60, indicating that PMT specifically binds to p60 via the region ranging from amino acid positions 840 to 985. To identify p60, we then separated the cellular proteins of the cell lysate by SDS-PAGE and excised gel pieces at the position corresponding to that of p60. The proteins were digested in gel with trypsin, and the resulting peptides, which were extracted with 0.1% trifluoroacetic acid in 50% acetonitrile from the gel, were subjected to peptide mass fingerprinting by matrix-assisted laser desorption ionization-time of flight mass spectrometry. As a result, 19 tryptic peptides matched with 57.4% of the whole sequence of mouse vimentin, an intermediate filament component (Table 1). The ligand overlay assay revealed that PMT actually bound to mouse vimentin, provided by M. Inagaki, Aichi Cancer Center Research Institute (Fig. 2A). Furthermore, the binding of PMT to vimentin was confirmed by a pull-down assay as described below. Purified glutathione S-transferase (GST)-tagged PMT840-985 or GST coupled with glutathione Sepharose 4B (Amersham) was mixed with vimentin and allowed to react at 4°C for 3 h. The Sepharose beads were washed, and the proteins that precipitated with the beads were analyzed by Western blot analysis with goat anti-vimentin antiserum (V4630; Sigma) and HRP-conjugated anti-goat IgG antibody (Chemicon). Vimentin was precipitated with GST-PMT840-985, but not with GST, indicating that PMT is associated with vimentin in solution (Fig. 2B). Vimentin is composed of three functional domains, designated head, rod, and tail, from the N terminus to the C terminus. Each domain that was expressed as GST-tagged protein in E. coli was subjected to the ligand overlay assay. PMT840-1285 was found to bind to the GST-tagged head domain that migrated to the position of the predicted molecular size, whereas there were no positive signals in the GST-tagged rod and tail domains, except nonspecifically reactive bands (Fig. 3).
FIG. 1.
Ligand overlay assay with PMT and its deletion mutants. The lysates of Swiss 3T3 cells were subjected to SDS-PAGE, transferred to a membrane, and incubated with full-length PMT (A) or the deletion mutants of the toxin (C) for 1 h. The deletion mutants of PMT used in this experiment are schematically represented in panel B. The numbers with the mutant names indicate the positions of the N- and C-terminal amino acids. Crosshatched bars indicate FLAG peptides. After stringent washing, the toxin that remained on the membrane was detected by Western blot analysis with anti-PMT polyclonal antibody or anti-FLAG M2 antibody. The positions of the molecular mass standards are shown on the left.
TABLE 1.
Summary of mass values observed for tryptic peptides of p60 and corresponding sequence of vimentin
| Sequence position of amino acida | Mass (Da)
|
|
|---|---|---|
| Observed | Theoretical | |
| 217-221 | 645.8 | 645.36b |
| 71-77 | 701.8 | 701.39b |
| 64-70 | 788.8 | 788.47b |
| 28-35 | 914.6 | 914.46b |
| 69-77 | 970.7 | 970.58b |
| 4-12 | 1028.5 | 1028.51b |
| 440-449 | 1173.5 | 1173.73b |
| 50-63 | 1444.3 | 1444.71b |
| 36-49 | 1495.4 | 1495.79b |
| 13-27 | 1527.4 | 1527.70b |
| 410-423 | 1557.5 | 1557.91b |
| 424-439 | 1838.5 | 1838.97b |
| 78-99 | 2498.5 | 2498.75 |
| 71-99 | 3181.4 | 3181.53 |
| 378-409 | 3847.5 | 3848.43 |
| 100-142 | 5105.2 | 5105.80 |
| 222-269 | 5554.5 | 5555.26 |
| 222-272 | 5925.6 | 5925.67 |
| 217-269 | 6180.0 | 6181.95 |
Sequence data were obtained from the Swiss-Prot database under accession no. P20152.
Monoisotopic mass.
FIG. 2.
Association of PMT with vimentin. (A) Purified mouse vimentin (1.5 μg) was subjected to the ligand overlay assay with PMT. (B) Pull-down assay with GST-PMT840-985. Vimentin (2 μg) was mixed with GST-PMT840-985- or GST-coupled glutathione Sepharose 4B and incubated at 4°C for 3 h. Vimentin associated with the beads was extracted by boiling in SDS-PAGE buffer and subjected to Western blot analysis with anti-vimentin antibody as described in the text. The positions of the molecular mass standards are shown on the left.
FIG. 3.
Association of the head domain of vimentin with PMT. The lysates of E. coli expressing the head (H; amino acids 1 to 94), rod (R; amino acids 95 to 406), and tail (T; amino acids 407 to 465) domains of vimentin in the GST-tagged forms were subjected to the ligand overlay assay with PMT840-1285. Arrows indicate the predicted position of each domain. (A) The expression of the fusion proteins was estimated by Western blot analysis with anti-GST polyclonal antibody. The amount of each fragment was estimated from the intensity of the blotted protein band. (B) Ligand overlay assay. An equal amount of each fragment was applied to each lane. Note that the head domain at the predicted position was reacted with PMT840-1285, whereas the rod and the tail domains were not. The bands that appeared at the position below 25 kDa should be nonspecifically reactive proteins, because they were observed even in the lysate from naive E. coli. The positions of the molecular mass standards are shown on the left.
PMT is a single-chain polypeptide consisting of 1,285 amino acid residues. Its N-terminal region shows 24 and 27% homologies with E. coli cytotoxic necrotizing factors 1 and 2 (CNF1 and -2, respectively) (7, 26). In addition, it was reported that PMT possesses biological activities apparently similar to those of Bordetella dermonecrotic toxin (DNT) (22), which has homology in the C-terminal region with CNF1 and -2 (11, 18, 35). Therefore, it has been proposed that CNFs, DNT, and PMT could be classified into the same toxin family (18). CNFs deamidate and DNT deamidates or polyaminates the Rho GTPases. These modifications make the GTPases constitutively active, which probably underlies their toxicities on target tissues (9, 12, 19, 30). In contrast to CNFs and DNT, however, PMT did not show similar enzymatic activities (25). Thus, clarification of the target molecules and mode of action of PMT is now an important issue in this area. In the present study, we demonstrated that vimentin shows affinity to PMT. To our knowledge, this is the first report to identify cellular substances that associate with the toxin. Recently, it was shown that the C-terminal fragment of PMT induces inositol phosphate production and rearrangement of the actin cytoskeleton, and the N-terminal fragment competitively inhibits the action of the toxin (1, 28). These results imply that receptor binding and intracellularly active domains reside on the N- and C-terminal regions on PMT, respectively. According to these reports, the C-terminal fragments of PMT encompassing amino acids 581 through 1285 or 681 through 1285 were active when introduced into cells by electroporation and microinjection (1, 28). Because these fragments include the region (PMT840-985) responsible for binding to vimentin, it is possible that the binding of PMT to vimentin may play a role in its intracellular action. Vimentin is a major component of type III intermediate filament (IF) and is predominantly expressed in mesenchymal cells and many types of tumor cells. Although the roles of IF and vimentin in cellular function remain to be elucidated, some reports have shown that IF contributes to the maintenance of cell shape, cell motility, and cytokinesis, some aspects of which have been shown to be regulated by phosphorylations of distinct sites of the head domain (14, 15, 20, 24, 37). Furthermore, it has been reported that vimentin associates with a variety of cellular substances through the head domain and is thereby involved in various aspects of cellular events. In the present study, PMT was also found to bind to the head domain of vimentin. These facts imply that vimentin might be one of the accessory components for PMT actions, although at present it is difficult to predict a correlation between vimentin or IF and the activation of Gq-PLCβ or Rho. To clarify this issue, we are now attempting to examine the effects of PMT on vimentin-deficient cells and intracellular localization of vimentin, IF, and PMT in the intoxicated target cells.
Acknowledgments
We thank S. Nagai for pSN131 and M. Inagaki for mouse vimentin.
This work was supported in part by Grant-in Aid for Scientific Research (B) no. 13470059 from the Japan Society for the Promotion of Science.
Editor: J. T. Barbieri
REFERENCES
- 1.Busch, C., J. Orth, N. Djouder, and K. Aktories. 2001. Biological activity of a C-terminal fragment of Pasteurella multocida toxin. Infect. Immun. 69:3628-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buys, W. E. C. M., H. E. Smith, A. M. I. E. Kamps, E. M. Kamp, and M. A. Smits. 1990. Sequence of the dermonecrotic toxin of Pasteurella multocida ssp. multocida. Nucleic Acids Res. 18:2815-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominick, M. A., and R. B. Rimler. 1986. Turbinate atrophy in gnotobiotic pigs intranasally inoculated with protein toxin isolated from type D Pasteurella multocida. Am. J. Vet. Res. 47:1532-1536. [PubMed] [Google Scholar]
- 4.Dominick, M. A., and R. B. Rimler. 1988. Turbinate osteoporosis in pigs following intranasal inoculation of purified Pasteurella toxin: histomorphometric and ultrastructural studies. Vet. Pathol. 25:17-27. [DOI] [PubMed] [Google Scholar]
- 5.Elias, B. G., G. Boros, M. Albert, S. Tuboly, P. Gergely, L. Papp, I. B. Vetro, P. Rafai, and E. Molnar. 1990. Clinical and pathological effects of the dermonecrotic toxin of Bordetella bronchiseptica and Pasteurella multocida specific-pathogen-free piglets. Jpn. J. Vet. Sci. 52:677-688. [DOI] [PubMed] [Google Scholar]
- 6.Essler, M., K. Hermann, M. Amano, K. Kaibuchi, J. Heesemann, P. C. Weber, and M. Aepfelbacher. 1998. Pasteurella multocida toxin increases endothelial permeability via Rho kinase and myosin light chain phosphatase. J. Immunol. 161:5640-5646. [PubMed] [Google Scholar]
- 7.Falbo, V., T. Pace, L. Picci, E. Pizzi, and A. Caprioli. 1993. Isolation and nucleotide sequence of the gene encoding cytotoxic necrotizing factor 1 of Escherichia coli. Infect. Immun. 61:4909-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felix, R., H. Fleisch, and P. L. Frandsen. 1992. Effect of Pasteurella multocida toxin on bone resorption in vitro. Infect. Immun. 60:4984-4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flatau, G., E. Lemichez, M. Gauthler, P. Chardin, S. Paris, C. Fiorentini, and P. Boquet. 1997. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature 387:729-733. [DOI] [PubMed] [Google Scholar]
- 10.Higgins, T. E., A. C. Murphy, J. M. Staddon, A. J. Lax, and E. Rozengurt. 1992. Pasteurella multocida toxin is a potent inducer of anchorage-independent cell growth. Proc. Natl. Acad. Sci. USA 89:4240-4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horiguchi, Y. 2001. Escherichia coli cytotoxic necrotizing factors and Bordetella dermonecrotic toxin: the dermonecrosis-inducing toxins activating Rho small GTPases. Toxicon 39:1619-1627. [DOI] [PubMed] [Google Scholar]
- 12.Horiguchi, Y., N. Inoue, M. Masuda, T. Kashimoto, J. Katahira, N. Sugimoto, and M. Matsuda. 1997. Bordetella bronchiseptica dermonecrotizing toxin induces reorganization of actin stress fibers through deamidation of Gln-63 of the GTP-binding protein Rho. Proc. Natl. Acad. Sci. USA 94:11623-11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimman, T. G., C. W. G. M. Löwik, L. J. A. van de Wee-Pals, C. W. Thesingh, P. Defize, E. M. Kamp, and O. L. M. Bijvoet. 1987. Stimulation of bone resorption by inflamed nasal mucosa dermonecrotic toxin-containing conditioned medium from Pasteurella multocida, and purified dermonecrotic toxin from P. multocida. Infect. Immun. 55:2110-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosako, H., M. Amano, M. Yanagida, K. Tanabe, Y. Nishi, K. Kaibuchi, and M. Inagaki. 1997. Phosphorylation of glial fibrillary acidic protein at the same sites by cleavage furrow kinase and Rho-associated kinase. J. Biol. Chem. 272:10333-10336. [DOI] [PubMed] [Google Scholar]
- 15.Kosako, H., H. Goto, M. Yanagida, K. Matsuzawa, M. Fujita, Y. Tomono, T. Okigaki, H. Odai, K. Kaibuchi, and M. Inagaki. 1999. Specific accumulation of Rho-associated kinase at the cleavage furrow during cytokinesis: cleavage furrow-specific phosphorylation of intermediate filaments. Oncogene 18:2783-2788. [DOI] [PubMed] [Google Scholar]
- 16.Lacerda, H. M., A. J. Lax, and E. Rozengurt. 1996. Pasteurella multocida toxin, a potent intracellularly acting mitogen, induces p125FAK and paxillin tyrosine phosphorylation, actin stress fiber formation, and focal contact assembly in Swiss 3T3 cells. J. Biol. Chem. 271:439-445. [DOI] [PubMed] [Google Scholar]
- 17.Lax, A. J., N. Chanter, G. D. Pullinger, T. Higgins, J. M. Staddon, and E. Rozengurt. 1990. Sequence analysis of the potent mitogenic toxin of Pasteurella multocida. FEBS Lett. 277:59-64. [DOI] [PubMed] [Google Scholar]
- 18.Lemichez, E., G. Flatau, M. Bruzzone, P. Boquet, and M. Gauthier. 1997. Molecular localization of the Escherichia coli cytotoxic necrotizing factor CNF1 cell-binding and catalytic domains. Mol. Microbiol. 24:1061-1070. [DOI] [PubMed] [Google Scholar]
- 19.Masuda, M., L. Betancourt, T. Matsuzawa, T. Kashimoto, T. Takao, Y. Shimonishi, and Y. Horiguchi. 2000. Activation of Rho through a cross-link with polyamines catalyzed by Bordetella dermonecrotizing toxin. EMBO J. 19:521-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuzawa, K., H. Kosako, N. Inagaki, H. Shibata, H. Mukai, Y. Ono, M. Amano, K. Kaibuchi, Y. Matsuura, I. Azuma, and M. Inagaki. 1997. Domain-specific phosphorylation of vimentin and glial fibrillary acidic protein by PKN. Biochem. Biophys. Res. Commun. 234:621-625. [DOI] [PubMed] [Google Scholar]
- 21.Murphy, A. C., and E. Rozengurt. 1992. Pasteurella muoltocida toxin selectively facilitates phosphatidylinositol 4,5-bisphosphate hydrolysis by bombesin, vasopressin, and endothelin. J. Biol. Chem. 267:25296-25303. [PubMed] [Google Scholar]
- 22.Nakai, T., A. Sawata, M. Tsuji, and K. Kume. 1984. Characterization of dermonecrotic toxin produced by serotype D strains of Pasteurella multocida. Am. J. Vet. Res. 45:2410-2413. [PubMed] [Google Scholar]
- 23.Nakai, T., A. Sawata, M. Tsuji, Y. Samejima, and K. Kume. 1984. Purification of dermonecrotic toxin from a sonic extract of Pasteurella multocida SP-72 serotype D. Infect. Immun. 46:429-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawara, M., N. Inagaki, K. Tsujimura, Y. Takai, M. Sekimata, M. H. Ha, S. Imajoh-Ohmi, S. Hirai, S. Ohno, H. Sugiura, T. Yamauchi, and M. Inagaki. 1995. Differential targeting of protein kinase C and CaM kinase II signalings to vimentin. J. Cell Biol. 131:1055-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohnishi, T., Y. Horiguchi, M. Masuda, N. Sugimoto, and M. Matsuda. 1998. Pasteurella multocida toxin and Bordetella bronchiseptica dermonecrotizing toxin elicit similar effects on cultured cells by different mechanisms. J. Vet. Med. Sci. 60:301-305. [DOI] [PubMed] [Google Scholar]
- 26.Oswald, E., M. Sugai, A. Labigne, H. C. Wu, C. Fiorentini, P. Boquet, and A. D. O'Brien. 1994. Cytotoxic necrotizing factor type 2 produced by virulent Escherichia coli modifies the small GTP-binding proteins Rho involved in assembly of actin stress fibers. Proc. Natl. Acad. Sci. USA 91:3814-3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen, S. K. 1990. The complete nucleotide sequence of the Pasteurella multocida toxin gene and evidence for a transcriptional repressor, TxaR. Mol. Microbiol. 4:821-830. [DOI] [PubMed] [Google Scholar]
- 28.Pullinger, G. D., R. Sowdhamini, and A. J. Lax. 2001. Localization of functional domains of the mitogenic toxin of Pasteurella multocida. Infect. Immun. 69:7839-7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozengurt, E., T. Higgins, N. Chanter, A. J. Lax, and J. M. Staddon. 1990. Pasteurella multocida toxin: potent mitogen for cultured fibroblasts. Proc. Natl. Acad. Sci. USA 87:123-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt, G., P. Sehr, M. Wilm, J. Selzer, M. Mann, and K. Aktories. 1997. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature 387:725-729. [DOI] [PubMed] [Google Scholar]
- 31.Seo, B., E. W. Choy, S. Maudsley, W. E. Miller, B. A. Wilson, and L. M. Luttrell. 2000. Pasteurella multocida toxin stimulates mitogen-activated protein kinase via Gq/11-dependent transactivation of the epidermal growth factor receptor. J. Biol. Chem. 275:2239-2245. [DOI] [PubMed] [Google Scholar]
- 32.Staddon, J. M., C. J. Barker, A. C. Murphy, N. Chanter, A. J. Lax, R. H. Michell, and E. Rozengurt. 1991. Pasteurella multocida toxin, a potent mitogen, increases inositol 1,4,5-triphosphate and mobilizes Ca2+ in Swiss 3T3 cells. J. Biol. Chem. 266:4840-4847. [PubMed] [Google Scholar]
- 33.Staddon, J. M., N. Chanter, A. J. Lax, T. E. Higgins, and E. Rozengurt. 1990. Pasteurella multocida toxin, a potent mitogen, stimulates protein kinase C-dependent and -independent protein phosphorylation in Swiss 3T3 cells. J. Biol. Chem. 265:11841-11848. [PubMed] [Google Scholar]
- 34.Sterner-Kock, A., B. Lanske, S. Übershär, and M. J. Atkinson. 1995. Effects of the Pasteurella multocida toxin on osteoblastic cells in vitro. Vet. Pathol. 32:274-279. [DOI] [PubMed] [Google Scholar]
- 35.Walker, K. E., and A. A. Weiss. 1994. Characterization of the dermonecrotic toxin in members of the genus Bordetella. Infect. Immun. 62:3817-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson, B. A., X. Zhu, M. Ho, and L. Lu. 1997. Pasteurella multocida toxin activates the inositol triphosphate signaling pathway in Xenopus oocytes via Gqα-coupled phospholipase C-β1. J. Biol. Chem. 272:1268-1275. [DOI] [PubMed] [Google Scholar]
- 37.Yasui, Y., M. Amano, K. Nagata, N. Inagaki, H. Nakamura, H. Saya, K. Kaibuchi, and M. Inagaki. 1998. Roles of Rho-associated kinase in cytokinesis; mutations in Rho-associated kinase phosphorylation sites impair cytokinetic segregation of glial filaments. J. Cell Biol. 143:1249-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zywietz, A., A. Gohla, M. Schmelz, G. Schultz, and S. Offermanns. 2001. Pleiotropic effects of Pasteurella multocida toxin are mediated by Gq-dependent and -independent mechanisms: involvement of Gq but not G11. J. Biol. Chem. 276:3840-3845. [DOI] [PubMed] [Google Scholar]