Abstract
Heteromeric KCNQ2/3 potassium channels are thought to underlie the M-current, a subthreshold potassium current involved in the regulation of neuronal excitability. KCNQ channel subunits are structurally unique, but it is unknown whether these structural differences result in unique conduction properties. Heterologously expressed KCNQ2/3 channels showed a permeation sequence of 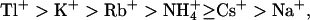 while showing a conduction sequence of
while showing a conduction sequence of 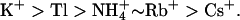 A differential contribution of component subunits to the properties of heteromeric KCNQ2/3 channels was demonstrated by studying homomeric KCNQ2 and KCNQ3 channels, which displayed contrasting ionic selectivities. KCNQ2/3 channels did not exhibit an anomalous mole-fraction effect in mixtures of K+ and Rb+. However, extreme voltage-dependence of block by external Cs+ was indicative of multi-ion pore behavior. Block of KCNQ2/3 channels by external Ba2+ ions was voltage-independent, demonstrating unusual ionic occupation of the outer pore. Selectivity properties and block of KCNQ2 were altered by mutation of outer pore residues in a manner consistent with the presence of multiple ion-binding sites. KCNQ2/3 channel deactivation kinetics were slowed exclusively by Rb+, whereas activation of KCNQ2/3 channels was altered by a variety of external permeant ions. These data indicate that KCNQ2/3 channels are multi-ion pores which exhibit distinctive mechanisms of ion conduction and gating.
A differential contribution of component subunits to the properties of heteromeric KCNQ2/3 channels was demonstrated by studying homomeric KCNQ2 and KCNQ3 channels, which displayed contrasting ionic selectivities. KCNQ2/3 channels did not exhibit an anomalous mole-fraction effect in mixtures of K+ and Rb+. However, extreme voltage-dependence of block by external Cs+ was indicative of multi-ion pore behavior. Block of KCNQ2/3 channels by external Ba2+ ions was voltage-independent, demonstrating unusual ionic occupation of the outer pore. Selectivity properties and block of KCNQ2 were altered by mutation of outer pore residues in a manner consistent with the presence of multiple ion-binding sites. KCNQ2/3 channel deactivation kinetics were slowed exclusively by Rb+, whereas activation of KCNQ2/3 channels was altered by a variety of external permeant ions. These data indicate that KCNQ2/3 channels are multi-ion pores which exhibit distinctive mechanisms of ion conduction and gating.
INTRODUCTION
The KCNQ family of voltage-gated potassium channels contains five members to date, of which KCNQ channels 2–5 have been shown to be expressed in neuronal cells (Tinel et al., 1998; Yang et al., 1998; Kharkovets et al., 2000; Lerche et al., 2000; Schroeder et al., 2000). Mutation of KCNQ channels has been implicated in a range of inherited diseases and pharmacological modulation of these channels is likely to be of significant therapeutic importance (Robbins, 2001). KCNQ2 and KCNQ3 channels are thought to be important in the regulation of neuronal excitability and mutation of these channels is associated with a form of human neonatal epilepsy (Biervert et al., 1998; Charlier et al., 1998; Singh et al., 1998). Heteromeric KCNQ2 and KCNQ3 channels have been proposed to underlie the neuronalM-current, a subthreshold, non-inactivating potassium current which is an important determinant of membrane excitability in many neuronal cells (Brown and Adams, 1980; Marrion, 1997; Wang et al., 1998).
KCNQ channels possess primary structures that are distinct from those of other voltage-gated potassium channels (Kv channels) (Wei et al., 1996; Coetzee et al., 1999; Robbins, 2001). Although they possess a putatively six-transmembrane topology and a superficially similar selectivity filter region, other functionally important structural features conserved within the Kv channel family are different in KCNQ channels. For example, the transmembrane and P-loop domains of KCNQ channels show marked differences from those of other Kv channels and KCNQ channels lack regions such as the conserved N-terminal T1-domain of Kv channels (Brown and Yu, 2000; Schroeder et al., 2000; Choe, 2002). In addition, KCNQ channels possess relatively short intracellular N-termini and relatively long intracellular C-termini compared to other potassium channels. The mechanisms of KCNQ channel formation, ion conduction, gating, and modulation are therefore likely to be different from those of other Kv channels. However, despite their physiological importance, information on the functional properties of KCNQ channels and the relationship of these properties to the structure of the channels involved is limited. Such information has relevance to the etiology of neuronal disease and the therapeutic potential of drugs that are active at KCNQ channels, as well as empirical importance to the understanding of the structure and function of potassium channels in general.
This study was therefore undertaken to characterize the ionic permeation, conduction, and gating properties of KCNQ2/KCNQ3 channels and to determine whether these structurally unique channels show biophysical features displayed by other potassium channels.
MATERIALS AND METHODS
Materials
Reagents were obtained as follows: standard chemicals (Sigma-Aldrich, Poole, UK), calcium chloride (Fluka, Gillingham, UK), Superfect transfection reagent (Qiagen, Crawley, UK), fetal calf serum (Harlan Sera-lab, Loughborough, UK), DMEM, MEM, penicillin/streptomycin, and nerve growth factor (Gibco-BRL, Paisley, UK). All salts used for electrophysiological solutions were obtained at the highest available purity.
Molecular biology
Rat KCNQ2 (Splice form B, Pan et al., 2001; otherwise, similar to GenBank accession number AF087453) and rat KCNQ3 (GenBank accession number AF091247) constructs were kindly provided by David McKinnon (State University of New York at Stony Brook, NY). KCNQ2 and KCNQ3 inserts were subcloned independently into the plasmid vector pcDNA3.1 (Invitrogen, Paisley, UK). Site-directed mutagenesis was carried out using the QuikChange-XL Mutagenesis Kit (Stratagene, Limerick, Ireland). All sequences were confirmed by dye-termination sequencing (Department of Biochemistry, University of Oxford, UK).
Tissue culture and transfection
HEK-293 cells (ECACC, Salisbury, UK) and HEK-293T cells (also known as tsA-201 cells, an SV40 virus-transformed HEK-293 cell line known to give rise to enhanced expression of certain plasmid DNA, which were obtained from Prof. David Brown, University College London, UK and Dr. Galen Flynn, University of Washington, Seattle) were grown at 37°C and 5% CO2. The culture medium was MEM for HEK-293 cells, and DMEM for HEK-293T cells, supplemented with 10% fetal calf serum and 0.2% penicillin/streptomycin. Cells were split twice weekly when confluent and were plated in 35-mm dishes (Falcon Primaria, distributed by Fahrenheit, Miltozn Keynes, UK) using a reduced serum content (3%) medium in preparation for transfection. Cells were transiently transfected 24 h after plating using Superfect transfection reagent (Qiagen). Transfected cells were identified for recording by visualization of cotransfected green fluorescent marker EGFP (Clontech, Oxford, UK). Coexpression experiments were performed in HEK-293 cells, whereas homomeric channels were expressed in HEK-293T cells (due to low expression levels of these channels in HEK-293 cells). A total of 2 μg of DNA was used for each plate of cells, using equal molarities of KCNQ2 and KCNQ3 for coexpression experiments and a channel/marker ratio of 10:1. For expression of homomeric channels, the channel/marker ratio was 2:1. For coexpression studies, cells were used for recording within 24 h of transfection, whereas homomeric channel expression was studied 2–4 days after transfection. Superior cervical ganglion neurons were obtained from 16- to 20-day-old Sprague-Dawley rats and cultured as described previously (Beech et al., 1991). Neurons were grown in 35-mm dishes (Falcon Primaria) and were used for recording after 1–2 days in culture.
Electrophysiology
Whole-cell voltage-clamp recordings were made from single, uncoupled cells at room temperature (20–24°C) using an Axopatch (Union City, CA) 200B amplifier. Fire-polished electrodes (3–4 MΩ) pulled from borosilicate glass and coated in “Sticky Wax” (Kerr, Romulus, MI) contained K-acetate (110 mM), KCl (10 mM), KOH (30 mM), MgCl2 (1.5 mM), HEPES (10 mM), EGTA (10 mM), CaCl2 (4.98 mM, calculated free [Ca2+] = 80 nM), and Na2ATP (3 mM), at pH 7.2 with HCl. The bathing external solution was constantly perfused (∼8–10 min). To determine the permeation sequence, the external solution contained N-methyl-D-glucamine (NMDG) (135 mM), acetic acid (135 mM), X-acetate (15 mM), Mg-acetate (5 mM), Ca-acetate (0.1 mM), d-glucose (22 mM), and HEPES (10 mM), at pH 7.2 with acetic acid, where X was the test cation. For experiments using 150 mM X and for investigating the anomalous mole-fraction effect and block by Cs+, NMDG and acetic acid were replaced with equimolar quantities of X-acetate. Activity coefficients used (relative to K+) were Tl+ (0.94), Rb+ (1.0), 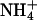 (0.93), Na+ (0.99), and Cs+ (1.0) (Robinson and Stokes, 1959). Acetate salts were used to avoid the problem of TlCl precipitation. For experiments investigating block by tetraethylammonium (TEA) and Ba2+ ions, the external solution contained NaCl (144 mM), KCl (5.85 mM), CaCl2 (2.52 mM), MgCl2 (1.2 mM), HEPES (10 mM), and D-glucose (22 mM), at pH 7.2 with HCl. NaCl was replaced with appropriate equimolar quantities of TEACl in the above solution. To isolate the M-current from other voltage-dependent currents when recording from neurons, tetrodotoxin (1 μM), 4-aminopyridine (1 mM), and 3,4-diaminopyridine (1 mM) were added to the external solution. In addition, a deactivating protocol was used to isolate the non-inactivating M-current (Constanti and Brown, 1981). A 3 M KCl or K-acetate saturated agar bridge was used as a reference bath ground. Initial offset potentials were corrected for before recording. Junction potential shifts associated with solution changes varied by <2 mV and were not adjusted for. Currents were measured with capacitance- and series-resistance compensation (>90%), filtered at 1 kHz using an 8-pole Bessel filter and sampled at 5 kHz. Only cells with negligible leak current were used for experiments and currents were therefore not leak-subtracted. Only cells with negligible current rundown after equilibration of whole-cell recording conditions were used for experiments. Voltage pulses were applied at 10-s intervals in all cases.
(0.93), Na+ (0.99), and Cs+ (1.0) (Robinson and Stokes, 1959). Acetate salts were used to avoid the problem of TlCl precipitation. For experiments investigating block by tetraethylammonium (TEA) and Ba2+ ions, the external solution contained NaCl (144 mM), KCl (5.85 mM), CaCl2 (2.52 mM), MgCl2 (1.2 mM), HEPES (10 mM), and D-glucose (22 mM), at pH 7.2 with HCl. NaCl was replaced with appropriate equimolar quantities of TEACl in the above solution. To isolate the M-current from other voltage-dependent currents when recording from neurons, tetrodotoxin (1 μM), 4-aminopyridine (1 mM), and 3,4-diaminopyridine (1 mM) were added to the external solution. In addition, a deactivating protocol was used to isolate the non-inactivating M-current (Constanti and Brown, 1981). A 3 M KCl or K-acetate saturated agar bridge was used as a reference bath ground. Initial offset potentials were corrected for before recording. Junction potential shifts associated with solution changes varied by <2 mV and were not adjusted for. Currents were measured with capacitance- and series-resistance compensation (>90%), filtered at 1 kHz using an 8-pole Bessel filter and sampled at 5 kHz. Only cells with negligible leak current were used for experiments and currents were therefore not leak-subtracted. Only cells with negligible current rundown after equilibration of whole-cell recording conditions were used for experiments. Voltage pulses were applied at 10-s intervals in all cases.
Data analysis
The permeability relative to K+ (PX/PK) of external monovalent cations was calculated from the Goldman-Hodgkin-Katz equation
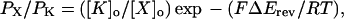 |
where ΔErev is the shift in reversal potential when K+ is replaced with a test cation X, and F, R, and T have their usual thermodynamic definitions (Goldman, 1943; Hodgkin and Katz, 1949).
The voltage-dependence of channel activation was estimated from measurements of tail currents. The data were fit with the sum of two independent Boltzmann distributions (except in one case, where a single Boltzmann was required),
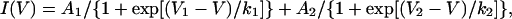 |
where I(V) is the normalized tail current amplitude at a test potential V; V1 and V2 are the half-activation potentials; k1 and k2 are slope constants related to the apparent equivalent charge involved in channel gating; and A1 and A2 are the maximal amplitudes of each Boltzmann distribution (B1 and B2).
Data were analyzed using PulseFit (HEKA Elektronik, Lambrecht, Germany) and Origin 6.0 (Microcal Software, Northampton, MA). All values are expressed as mean ± SE. Where error bars are not visible, they lie within symbols. Significance of results was compared using an appropriatet-test and considered to be significant if p < 0.05.
RESULTS
Sensitivity to external TEA
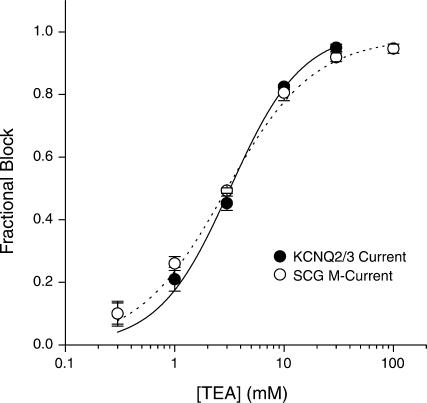
Coexpression of KCNQ2 and KCNQ3 potassium channel subunits has previously been shown to result in the formation of heteromeric channels (Wang et al., 1998; Cooper et al., 2000; Shapiro et al., 2000). The sensitivity of expressed KCNQ2/3 currents to externally applied TEA showed an IC50 of 3.2 mM and a Hill slope of 1.3 (Fig. 1). This sensitivity to TEA is intermediate to that found for homomeric KCNQ2 and homomeric KCNQ3 channels and is consistent with the formation of heteromeric KCNQ2/3 channels (Wang et al., 1998; Hadley et al., 2000). The sensitivity of expressed KCNQ2/3 channels was similar to that found for the model native M-current in rat SCG neurons, which showed an IC50 of 2.9 mM and a Hill slope of 1.1 (Fig. 1). In both current types, there was a lack of either highly TEA-sensitive or -insensitive current components. The highest concentration of TEA used (100 mM) inhibited SCG M-current by 94.7 ± 1.5% (n = 4). This indicates an absence of certain channel subunit combinations (see Discussion). These results also suggest that both cell types predominantly expressed a single population of channels with a fixed stoichiometry. The subsequent biophysical characterization of expressed KCNQ2/3 currents therefore corresponds to a single population of heteromeric KCNQ2/3 channels, which on the basis of external TEA sensitivity is comparable with the M-current found in SCG neurons.
FIGURE 1.
TEA sensitivity of heteromeric KCNQ2/3 channels and native SCG M-current. Sensitivity of currents arising from the coexpression of KCNQ2 and KCNQ3 subunits was described by a single Hill equation with an IC50 of 3.2 mM and a slope of 1.3. Sensitivity of native neuronal SCGM-current was also described by a single Hill equation with an IC50 of 2.9 mM and a slope of 1.1. Fractional block was calculated from the decrease in amplitude of deactivation relaxations, which were evoked by holding cells at a potential of −30 mV and stepping to −50 mV for 1.5 s.
Permeation and conduction properties
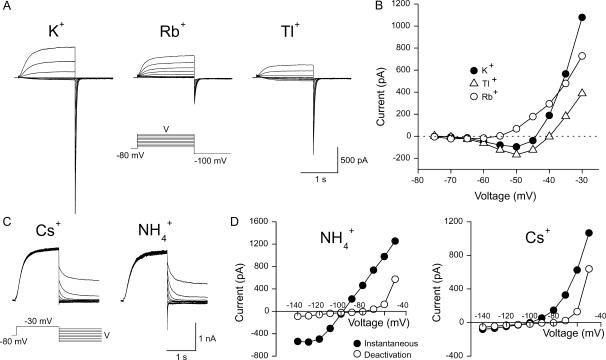
To determine the permeability of KCNQ2/3 channels to monovalent cations, whole-cell current relaxations were recorded under bi-ionic conditions when extracellular K+ (15 mM) was replaced with a test cation (Fig. 2, A–D). The mean Erevs for ions tested under these conditions were K+ = −44.1 ± 0.8 mV (n = 10), Tl+ = −36.7 ± 1.9 mV (n = 5), Rb+ = −52.9 ± 1.6 mV (n = 5), 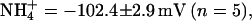 and Cs+ = −107.2 ± 2.6 mV (n = 5). Inward currents were not observed for Na+ or NMDG+ under these conditions, even at potentials as negative as −140 mV (n = 3). The mean ΔErevs obtained when 15 mM K+ was replaced with 15 mM test cation were Tl+ = +7.7 ± 1.6 mV (n = 5), Rb+ = −8.5 ± 1.4 mV (n = 5),
and Cs+ = −107.2 ± 2.6 mV (n = 5). Inward currents were not observed for Na+ or NMDG+ under these conditions, even at potentials as negative as −140 mV (n = 3). The mean ΔErevs obtained when 15 mM K+ was replaced with 15 mM test cation were Tl+ = +7.7 ± 1.6 mV (n = 5), Rb+ = −8.5 ± 1.4 mV (n = 5), 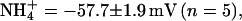 and Cs+ = −62.5 ± 2.0 mV (n = 5). Relative ionic permeability in 15 mM external cation calculated from the Goldman-Hodgkin-Katz equation (see Materials and Methods) was therefore
and Cs+ = −62.5 ± 2.0 mV (n = 5). Relative ionic permeability in 15 mM external cation calculated from the Goldman-Hodgkin-Katz equation (see Materials and Methods) was therefore 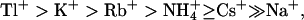 NMDG+ (see Table 1).
NMDG+ (see Table 1).
FIGURE 2.
Permeation properties: KCNQ2/3 currents recorded in the presence of different external cations (15 mM). (A) To measure Erevs in the presence of K+, Tl+, and Rb+, cells were whole-cell voltage-clamped at −80 mV and current was activated by depolarizing voltage steps (1.5 s) in +5 mV increments. Tail currents were produced by a subsequent 1-s voltage step to −100 mV. (B) Current-voltage relationship for the cell shown in A in the presence of 15 mM external K+, Rb+, or Tl+. The amplitude of activation relaxations was measured as the difference between the current level 3 ms after the start of the depolarizing voltage step and the current level at 10 ms before the end of the depolarizing voltage step. The dashed line shows the zero current level. The Erev for ions tested on this cell were K+ (−44.2 mV), Rb+ (−55.5 mV), and Tl+ (−39.6 mV). (C) To measure Erevs in the presence of 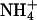 and Cs+, as well as Na+ and NMDG+, cells were voltage-clamped at −80 mV and KCNQ2/3 channels activated by a depolarizing voltage step to −30 mV for 1.5 s. Deactivation relaxations were then produced by subsequent hyperpolarizing voltage steps (1.5 s) in −10 mV increments. (D) Current-voltage relationships for the cell shown in C. Solid circles denote the instantaneous current level 3 ms after the start of the hyperpolarizing voltage step and open circles denote the steady-state current level 10 ms before the end of the hyperpolarizing voltage step. The intersection of these current traces defined the Erev for each ion. The Erev for ions tested on this cell were K+ (−44.4 mV, not shown), Cs+ (−107.7 mV), and
and Cs+, as well as Na+ and NMDG+, cells were voltage-clamped at −80 mV and KCNQ2/3 channels activated by a depolarizing voltage step to −30 mV for 1.5 s. Deactivation relaxations were then produced by subsequent hyperpolarizing voltage steps (1.5 s) in −10 mV increments. (D) Current-voltage relationships for the cell shown in C. Solid circles denote the instantaneous current level 3 ms after the start of the hyperpolarizing voltage step and open circles denote the steady-state current level 10 ms before the end of the hyperpolarizing voltage step. The intersection of these current traces defined the Erev for each ion. The Erev for ions tested on this cell were K+ (−44.4 mV, not shown), Cs+ (−107.7 mV), and 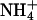 (−99.2 mV).
(−99.2 mV).
TABLE 1.
Permeability and conductance series of KCNQ2/3 channels
| Ion (X) | PX/PK | GX/GK | n |
|---|---|---|---|
| Tl+ | 1.37 ± 0.09 | 0.59 ± 0.04 | 5 |
| Rb+ | 0.71 ± 0.04 | 0.29 ± 0.04 | 5 |
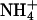 |
0.10 ± 0.007 | 0.30 ± 0.05 | 5 |
| Cs+ | 0.086 ± 0.007 | 0.018 ± 0.005 | 5 |
| Na+ | <0.005 | – | 3 |
Shifts in reversal potential were measured under bi-ionic conditions, with 15 mM X in the external solution and 150 mM K+ in the internal solution. In the case of Na+, 150 mM Na+ was included in the external solution. The permeability of each ion X relative to K+ was calculated from the Goldman-Hodgkin-Katz equation. Relative conductance was determined from the slope of the instantaneous current-voltage relationship for each ion X relative to K+ in the same cell.
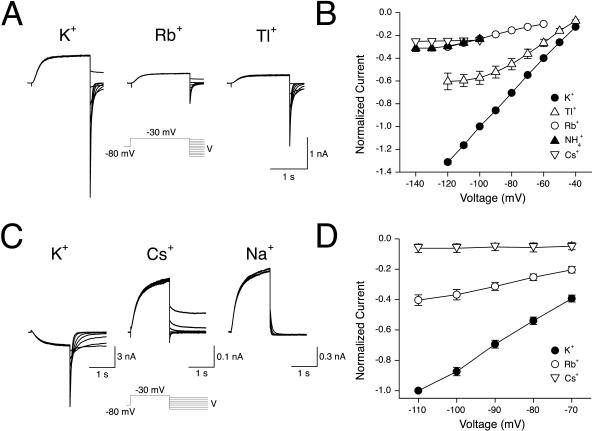
Channel conductance reflects the rate at which permeant ions traverse the channel pore. Relative conductances of K+, Tl+, Rb+, 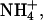 and Cs+ were estimated from measurements of the slope of instantaneous current-voltage relationships in the presence of each ion (15 mM; Fig. 3, A and B). The instantaneous current-voltage relationships for inward current in the presence of external K+, Rb+, and Cs+ were found to be approximately linear across the voltage range studied whereas inward currents in the presence of external Tl+ and
and Cs+ were estimated from measurements of the slope of instantaneous current-voltage relationships in the presence of each ion (15 mM; Fig. 3, A and B). The instantaneous current-voltage relationships for inward current in the presence of external K+, Rb+, and Cs+ were found to be approximately linear across the voltage range studied whereas inward currents in the presence of external Tl+ and 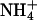 were both found to exhibit nonlinear instantaneous current-voltage relationships (Fig. 3 B). In the case of these ions, the slope conductance was defined as the maximum slope at potentials negative to the reversal potential for that ion in each cell. In 15 mM external test cation, the relative slope conductance of ions through KCNQ2/3 channels was found to be
were both found to exhibit nonlinear instantaneous current-voltage relationships (Fig. 3 B). In the case of these ions, the slope conductance was defined as the maximum slope at potentials negative to the reversal potential for that ion in each cell. In 15 mM external test cation, the relative slope conductance of ions through KCNQ2/3 channels was found to be 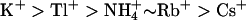 (Table 1). The conductance of Cs+ and Rb+ relative to K+ was also assessed under the higher ionic conditions of 150 mM X (Fig. 3, C and D). The value ofGCs/GK under these conditions was found to be 0.022 ± 0.007 (n = 3), whereas GRb/GK = 0.30 ± 0.04 (n = 6). In addition, relative Rb+ conductance was assessed under conditions of 5.85 mM X. Under these conditions, GRb/GK = 0.27 ± 0.03 (n = 5). These values were not significantly different from those found in 15 mM Cs+ or Rb+ (see Table 1; p > 0.05, unpaired t-test). The relative conductance values of Rb+ and Cs+ were therefore not dependent on the concentration of these ions over the range studied. To further test whether KCNQ2/3 channels were permeable to Na+, cells were bathed in 150 mM external cation. Again, no inward currents were observed in 150 mM external Na+, even at potentials as negative as −140 mV (Fig. 3 C). The permeability ratio for Na+, shown in Table 1, is based on a ΔErev of >−140 mV when 150 mM K+ was replaced with 150 mM Na+ as described above. The Erev in the presence of 150 mM external K+ was found to be −3.0 ± 0.8 mV (n = 6), whereas that in 150 mM external Cs+ was −67.2 ± 1.8 mV (n = 9). The relative ionic permeability of Cs+ was not altered by the concentration of external Cs+. Under these higher ionic conditions, the relative permeability of Cs+ was found to be equal to 0.08, similar to that found in 15 mM Cs+ (see Table 1).
(Table 1). The conductance of Cs+ and Rb+ relative to K+ was also assessed under the higher ionic conditions of 150 mM X (Fig. 3, C and D). The value ofGCs/GK under these conditions was found to be 0.022 ± 0.007 (n = 3), whereas GRb/GK = 0.30 ± 0.04 (n = 6). In addition, relative Rb+ conductance was assessed under conditions of 5.85 mM X. Under these conditions, GRb/GK = 0.27 ± 0.03 (n = 5). These values were not significantly different from those found in 15 mM Cs+ or Rb+ (see Table 1; p > 0.05, unpaired t-test). The relative conductance values of Rb+ and Cs+ were therefore not dependent on the concentration of these ions over the range studied. To further test whether KCNQ2/3 channels were permeable to Na+, cells were bathed in 150 mM external cation. Again, no inward currents were observed in 150 mM external Na+, even at potentials as negative as −140 mV (Fig. 3 C). The permeability ratio for Na+, shown in Table 1, is based on a ΔErev of >−140 mV when 150 mM K+ was replaced with 150 mM Na+ as described above. The Erev in the presence of 150 mM external K+ was found to be −3.0 ± 0.8 mV (n = 6), whereas that in 150 mM external Cs+ was −67.2 ± 1.8 mV (n = 9). The relative ionic permeability of Cs+ was not altered by the concentration of external Cs+. Under these higher ionic conditions, the relative permeability of Cs+ was found to be equal to 0.08, similar to that found in 15 mM Cs+ (see Table 1).
FIGURE 3.
Conduction properties: KCNQ2/3 current-voltage relationships for cells bathed in different concentrations of external cations. (A) Cells exposed to 15 mM external permeant cation were whole-cell voltage-clamped at −80 mV and a depolarizing voltage pulse to −30 mV was applied (1.5 s) to activate expressed currents. From this potential, the cells were stepped to potentials ranging from −40 to −120 mV. (B) Mean normalized instantaneous current-voltage relationships for cells bathed in 15 mM K+, Rb+, Tl+, Cs+, and 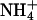 (n = 4–5). Instantaneous currents were defined as the difference between the current level at the end of the depolarizing voltage step and the current level 3 ms after the start of the hyperpolarizing voltage step. Currents were normalized to the current level at −100 mV in 15 mM K+. (C) Cells exposed to 150 mM external permeant cation were voltage-clamped at −80 mV and a depolarizing pulse to −30 mV was applied (1.5 s) to activate expressed currents. From this potential the voltage was stepped to potentials ranging from −40 mV to −100 mV (K+, Cs+) or −80 to −140 mV (Na+). (D) Mean instantaneous current-voltage relationships from cells bathed in 150 mM K+, Rb+, or Cs+ (n = 3–6). Instantaneous currents were defined as in B.
(n = 4–5). Instantaneous currents were defined as the difference between the current level at the end of the depolarizing voltage step and the current level 3 ms after the start of the hyperpolarizing voltage step. Currents were normalized to the current level at −100 mV in 15 mM K+. (C) Cells exposed to 150 mM external permeant cation were voltage-clamped at −80 mV and a depolarizing pulse to −30 mV was applied (1.5 s) to activate expressed currents. From this potential the voltage was stepped to potentials ranging from −40 mV to −100 mV (K+, Cs+) or −80 to −140 mV (Na+). (D) Mean instantaneous current-voltage relationships from cells bathed in 150 mM K+, Rb+, or Cs+ (n = 3–6). Instantaneous currents were defined as in B.
Component subunit selectivity
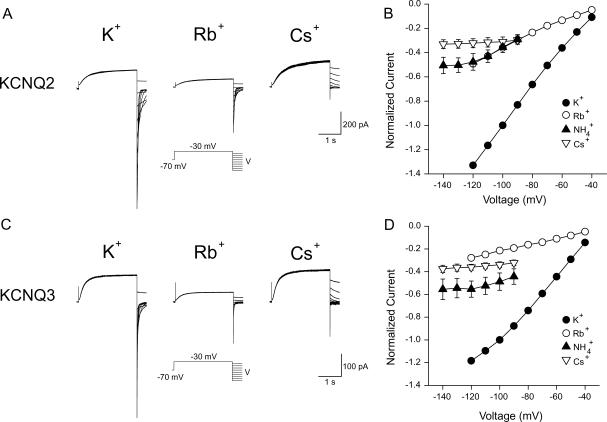
The respective contribution of the individual component subunits KCNQ2 and KCNQ3 to the overall selectivity properties of heteromeric KCNQ2/3 channels was assessed by expressing these subunits individually in HEK-293T cells. Currents arising from the expression of homomeric KCNQ2 and KCNQ3 channels are shown in Fig. 4, A and C. Whole-cell current relaxations were recorded under bi-ionic conditions when extracellular K+ (15 mM) was replaced with a test cation. For KCNQ2, the mean Erevs of ions tested under these conditions were K+ = −43.9 ± 0.4 mV (n = 8), Rb+ = −47.9 ± 0.3 mV (n = 7), 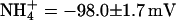 (n = 5), and Cs+ = −99.5 ± 2.9 mV (n = 6). For KCNQ3, the mean Erevs of ions tested were K+ = −43.3 ± 0.6 mV (n = 8), Rb+ = −50.3 ± 0.7 mV (n = 8),
(n = 5), and Cs+ = −99.5 ± 2.9 mV (n = 6). For KCNQ3, the mean Erevs of ions tested were K+ = −43.3 ± 0.6 mV (n = 8), Rb+ = −50.3 ± 0.7 mV (n = 8), 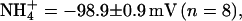 and Cs+ = −89.3 ± 1.3 mV (n = 8). KCNQ2 channels showed a permeation sequence of
and Cs+ = −89.3 ± 1.3 mV (n = 8). KCNQ2 channels showed a permeation sequence of 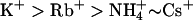 . In contrast, KCNQ3 channels showed a permeation sequence of:
. In contrast, KCNQ3 channels showed a permeation sequence of: 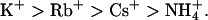 The conduction sequences and relative conductance values for various ions were also different for these two channel types. KCNQ2 channels showed a conduction sequence of
The conduction sequences and relative conductance values for various ions were also different for these two channel types. KCNQ2 channels showed a conduction sequence of 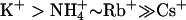 (Fig. 4 B), whereas KCNQ3 channels showed a conduction sequence of
(Fig. 4 B), whereas KCNQ3 channels showed a conduction sequence of 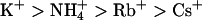 (Fig. 4 D). Compared to homomeric KCNQ3 channels, homomeric KCNQ2 channels showed a significantly higher relative Rb+ permeability and higher relative Rb+ and
(Fig. 4 D). Compared to homomeric KCNQ3 channels, homomeric KCNQ2 channels showed a significantly higher relative Rb+ permeability and higher relative Rb+ and 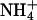 conductances, although showing lower relative Cs+ permeability and relative Cs+ conductance. These results are summarized in Table 2. Except in the case of relative Rb+ conductance, the selectivity properties of heteromeric KCNQ2/3 channels were not a simple intermediate of the properties of the respective subunits. Based on relative permeability, heteromeric KCNQ2/3 channels showed a higher K+ selectivity than either of the respective component subunits (Tables 1 and 2). KCNQ2/3 channels were also more selective for K+ over Cs+ than either of the respective component subunits in terms of relative conductance. The rectification properties of the two contributing subunit channel types were also different. KCNQ2 showed a weak inward rectification in both K+ and Rb+ (Fig. 4 B), whereas KCNQ3 showed an outward rectification in K+ which was absent in the presence of Rb+ (Fig. 4 D).
conductances, although showing lower relative Cs+ permeability and relative Cs+ conductance. These results are summarized in Table 2. Except in the case of relative Rb+ conductance, the selectivity properties of heteromeric KCNQ2/3 channels were not a simple intermediate of the properties of the respective subunits. Based on relative permeability, heteromeric KCNQ2/3 channels showed a higher K+ selectivity than either of the respective component subunits (Tables 1 and 2). KCNQ2/3 channels were also more selective for K+ over Cs+ than either of the respective component subunits in terms of relative conductance. The rectification properties of the two contributing subunit channel types were also different. KCNQ2 showed a weak inward rectification in both K+ and Rb+ (Fig. 4 B), whereas KCNQ3 showed an outward rectification in K+ which was absent in the presence of Rb+ (Fig. 4 D).
FIGURE 4.
Ionic conduction properties of homomeric KCNQ2 and KCNQ3 channels. (A and C) Cells were voltage-clamped at −70 mV and a depolarizing voltage pulse to −30 mV was applied (3.0 s) to activate expressed currents. From this potential, cells were stepped to voltages ranging from −40 mV to −120 mV (15 mM K+, Rb+) or −140 mV (15 mM Cs+). (B and D) Mean instantaneous current-voltage relationships for cells bathed in 15 mM K+, Rb+, Cs+, or 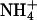 (n = 4–8). Instantaneous currents were measured as in Fig. 3. Currents were normalized to the current level at −100 mV in 15 mM K+.
(n = 4–8). Instantaneous currents were measured as in Fig. 3. Currents were normalized to the current level at −100 mV in 15 mM K+.
TABLE 2.
Ionic selectivity of homomeric KCNQ2/3 channels
| Ion
|
||||
|---|---|---|---|---|
| Rb+ | Cs+ | 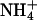 |
||
| PX/PK | Q2 | 0.88 ± 0.006 (6) | 0.12 ± 0.01 (6) | 0.12 ± 0.01 (5) |
| Q2(K283M) | 0.91 ± 0.007 (4) | 0.12 ± 0.01 (8) | 0.13 ± 0.004 (5) | |
| Q2(K283R) | 1.11 ± 0.09 (4)* | 0.13 ± 0.01 (4) | 0.13 ± 0.01 (4) | |
| Q2(T276S) | 0.99 ± 0.02 (4)* | 0.16 ± 0.01 (4)* | 0.15 ± 0.004 (4)* | |
| Q3 | 0.77 ± 0.02 (8)* | 0.17 ± 0.01 (8)* | 0.11 ± 0.004 (8) | |
| GX/GK | Q2 | 0.39 ± 0.02 (4) | 0.036 ± 0.007 (4) | 0.46 ± 0.04 (4) |
| Q2(K283M) | 0.49 ± 0.004 (4)* | 0.036 ± 0.01 (7) | 0.44 ± 0.07 (4) | |
| Q2(K283R) | 0.34 ± 0.007) (4) | 0.084 ± 0.01 (5)* | 0.43 ± 0.05 (4) | |
| Q2(T276S) | 0.36 ± 0.02 (4) | 0.065 ± 0.01 (4)* | 0.60 ± 0.06 (4)* | |
| Q3 | 0.20 ± 0.02 (8)* | 0.10 ± 0.02 (8)* | 0.30 ± 0.03 (8)* | |
Experiments were conducted under identical conditions to those used for deriving values for heteromeric channels shown in Table 1. The number of cells from which values were obtained is indicated in parentheses.
Significantly different from the corresponding value for wild-type KCNQ2 (p < 0.05, t-test).
Anomalous mole-fraction effect
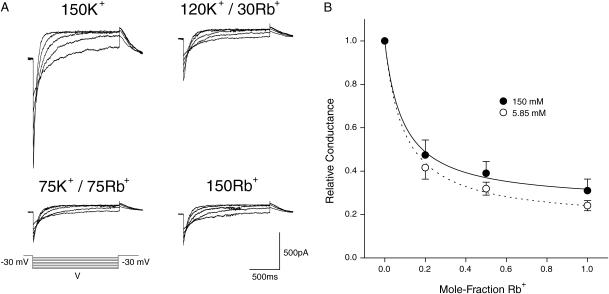
Many potassium and calcium channels display an anomalous mole-fraction effect (AMFE) in mixtures of permeant ions, which is often interpreted as evidence that they are multi-ion pores (Hille and Schwarz, 1978). To investigate whether this effect occurs in KCNQ2/3 channels, the inward slope conductance of KCNQ2/3 channels was measured in mixtures of external K+ and Rb+. This measure of conductance obviates the need to account for changes in reversal potential during titration of K+ with Rb+. The conductance of many potassium channels saturates at high concentrations of K+ (Eisenman et al., 1986; Heginbotham and MacKinnon, 1993; Lu and MacKinnon, 1994; Block and Jones, 1996; Stampe et al., 1998). The M-current native to amphibian neurons has been shown to exhibit concentration-dependent conductance saturation with an apparent KM of 71 mM K+ (Block and Jones, 1996). A total concentration of 150 mM permeant cation was therefore maintained in the external solution, as this would be predicted to increase the probability of multiple permeant ions occupying the pore simultaneously. Under these conditions, there was no evidence of a conductance minimum in mixtures of K+ and Rb+ (Fig. 5, A and B). Instead, the observed slope conductances (G) were well fit by the algebraic sum of the K+ conductance (GK) and the Rb+ conductance (GRb), assuming that these values are simple saturatable functions of the competitive binding of K+ and Rb+ with different apparent binding affinities, AK and ARb, respectively:
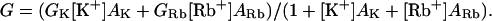 |
FIGURE 5.
KCNQ2/3 currents lack an anomalous mole-fraction effect in mixtures of K+ and Rb+ ions. (A) Cells were voltage-clamped at −80 mV and a depolarizing pulse to −30 mV was applied (1.5 s) to activate expressed currents in different mixtures of K+ and Rb+. From this potential the voltage was stepped to potentials ranging from −70 mV to −120 mV to induce inward tail currents. (B) Mean relative inward slope conductance plotted as a function of the mole-fraction of Rb+, where [K+] + [Rb+] = 150 mM (solid circles, n = 4) or 5.85 mM (open circles, n = 4). Lines represent the fit of a competitive binding model (see text) to the observed mean data for 150 mM (solid line) and 5.85 mM (dotted line).
The ratio of apparent binding affinity of K+ and Rb+ (ARb/AK) obtained from this analysis was found to be equal to 11.9. A lack of anomalous mole-fraction behavior could be due to the lack of a suitable “vacancy” within the channel pore (Hille and Schwarz, 1978). We therefore investigated whether an AMFE existed under conditions of different permeant ion concentration. Considering the previously determined KM for K+ saturation of M-current (71 mM; Block and Jones, 1996) and the value for ARb/AK derived above, we used conditions of 5.85 mM K+/Rb+, as this concentration would be predicted to give submaximal saturation by Rb+. Again, no AMFE was observed in mixtures of K+ and Rb+ under these conditions (Fig. 5 B). The ratio ARb/AK under conditions of 5.85 mM external K+/Rb+ was found to be equal to 11.5 according to the above binding model. The ratios of apparent affinities and the values for relative conductance in each mixture of K+ and Rb+ were not significantly different between 5.85 mM and 150 mM total concentration of K+ and Rb+ (t-test, p > 0.05).
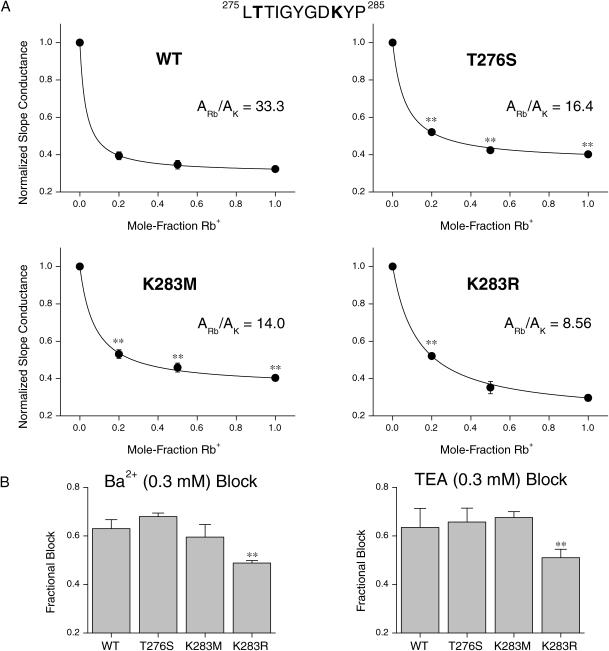
Alteration of KCNQ2 channel ionic properties by mutations within the outer pore
The effect of mutations within the outer pore were evaluated for homomeric KCNQ2 currents, to investigate structural aspects of channel selectivity. KCNQ2 current lacked an AMFE in mixtures of K+ and Rb+ ions (Fig. 6 A). The apparent relative binding affinity of K+ and Rb+ for KCNQ2 channels obtained from this analysis was 1:33.3 (Fig. 6 A), higher than the value determined for heteromeric KCNQ2/3 channels.
FIGURE 6.
Mutation of pore residues affects relative conductance and ionic affinity of KCNQ2 channels. (A) Mean relative inward slope conductance plotted as a function of the mole-fraction of Rb+ (5.85 mM total). Lines represent the fit of a competitive binding model (see text) to the observed mean data. Currents were elicited by a protocol similar to that used in Fig. 5 A, using 3.0-s depolarizing pulses to activate wild-type (n = 4–7), T276S (n = 4–5), K283M (n = 4–6), and K283R (n = 4) KCNQ2 currents. (B) Block of wild-type and mutant KCNQ2 currents by external Ba2+ and TEA (n = 4 for each current type). Block was assessed by the fractional reduction of currents elicited by a 3.0-s depolarizing step from a holding potential of −70 mV.
The identity of the residue at the Shaker 441 locus has been shown to affect the occurrence of an AMFE in Shaker channels. Introduction of a serine residue at this locus in Shaker (T441S) and other potassium channel types causes the AMFE to become more pronounced (Yool and Schwarz, 1996; Lacombe and Thibaud, 1998). A similar mutation was therefore imposed on the KCNQ2 subunit (T276S), in an effort to introduce an AMFE in homomeric KCNQ2 channels incorporating this mutation. This mutation did not lead to the appearance of an AMFE (Fig. 6 A). It did, however, decrease the apparent relative affinity of Rb+ ions for the channel, in addition to increasing the relative slope conductance of Rb+ ions (under conditions of 15 mM Rb+; Fig. 6 A). Mutations at the locus equivalent to T276 of KCNQ2 have also been demonstrated to alter the ionic selectivity of potassium channels, including Shaker (Becker et al., 1996; Yool and Schwarz, 1996; Lacombe and Thibaud, 1998; Choe et al., 2000). The selectivity properties of homomeric KCNQ2 (T276S) channels were therefore investigated. The T276S mutation was found to increase the relative permeability and conductance of Rb+, Cs+, and 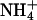 ions (Table 2 and Fig. 6 A).
ions (Table 2 and Fig. 6 A).
The lysine residue at position 283 of the KCNQ2 subunit is conserved in all KCNQ family members (GYGDK). This basic lysine is unique to KCNQ subunits, being a neutral residue in all other Kv channels. The majority of inward rectifier potassium channels possess a basic residue (arginine) at the analogous position. The identity of the residue at this locus has been demonstrated to dramatically affect the properties of inward rectifier potassium channels (Doring et al., 1998; Krapivinsky et al., 1998). One effect of mutations at this locus is to alter the occurrence of an AMFE (Wischmeyer et al., 2000). Channels incorporating mutations at this locus, to give the corresponding residue found in Shaker (i.e., K283M) and the residue found in the majority of inward rectifiers (K283R), were therefore investigated in homomeric KCNQ2 channels. The mutation K283M did not lead to the appearance of an AMFE, but did lead to a decrease in the apparent relative affinity for Rb+ ions (Fig. 6 A). This mutation increased the apparent relative slope conductance of Rb+ ions, without any significant effect on the relative permeability of Rb+ ions or the relative permeability or conductance of Cs+ or 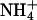 ions (Fig. 6 A and Table 2). The K283R mutation did not lead to the appearance of an AMFE and did not cause any significant change in the relative slope conductance of Rb+ ions (Fig. 6 A and Table 2). However, this mutation did lead to a decrease in the apparent relative affinity of Rb+ ions for the KCNQ2 channel (Fig. 6 A). This mutation also increased the relative permeability of Rb+ ions and the relative slope conductance of Cs+ ions (Table 2).
ions (Fig. 6 A and Table 2). The K283R mutation did not lead to the appearance of an AMFE and did not cause any significant change in the relative slope conductance of Rb+ ions (Fig. 6 A and Table 2). However, this mutation did lead to a decrease in the apparent relative affinity of Rb+ ions for the KCNQ2 channel (Fig. 6 A). This mutation also increased the relative permeability of Rb+ ions and the relative slope conductance of Cs+ ions (Table 2).
Alteration of channel affinity for external permeant ions might also be expected to give rise to changes in the affinity for external blocking ions. We therefore tested the sensitivity of wild-type and mutant KCNQ2 channels to the commonly used potassium channel blockers Ba2+ and TEA. The mutations K283M and T276S had no significant effect on sensitivity to external Ba2+ (Fig. 6 B). In contrast, the K283R mutation resulted in a reduction of sensitivity (p < 0.05, t-test). The latter effect is consistent with observations for inward rectifier potassium channels, where the presence of a basic arginine at this locus results in exclusion of cationic blockers from the outer pore (Sabirov et al., 1997; Doring et al., 1998; Krapivinsky et al., 1998). These results may be an indication that the less basic lysine residue present in KCNQ channels does not contribute to the structure of the KCNQ channel outer pore in a similar way to that for inward rectifier channels. Residue K283 lies directly adjacent to the site previously shown to confer high affinity block of Shaker and KCNQ2 channels by external TEA (MacKinnon and Yellen, 1990; Hadley et al., 2000). Mutations at the K283 locus of KCNQ2 might therefore be expected to alter the affinity of TEA binding. The K283M mutation had no effect on sensitivity to TEA, whereas the K283R mutation decreased sensitivity (Fig. 6 B; p < 0.05, t-test).
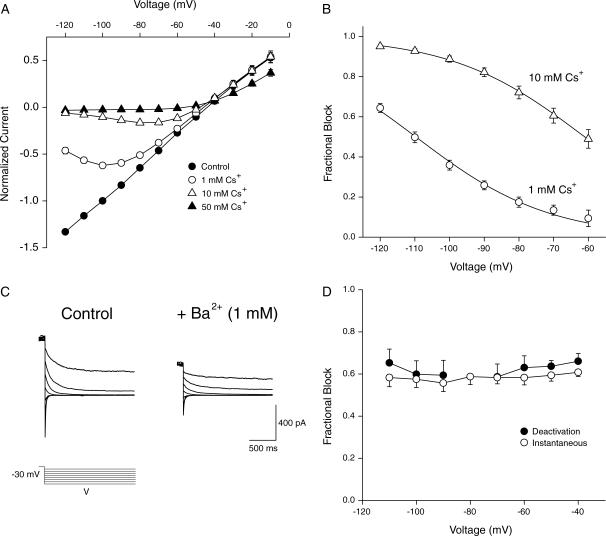
Block by external cesium
Another phenomenon which is cited as evidence for the multi-ion pore behavior of potassium channels is block by permeant cations which show apparent effective valencies (∂) > 1 that are dependent on the concentration of the blocking ion (Hille and Schwarz, 1978; Perez-Cornejo and Begenisich, 1994). Addition of Cs+ to the external solution produced a block of current through KCNQ2/3 channels that was both concentration- and voltage-dependent (Fig. 7 A). The voltage-dependence of this block was quantitatively assessed using the relation
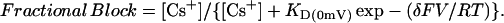 |
FIGURE 7.
Block of KCNQ2/3 current by external Cs+ and Ba2+ ions. (A) Normalized instantaneous current-voltage relationship in the presence of different concentrations of external Cs+, for cells bathed in 15 mM K+ (n = 4). Cells were whole-cell voltage-clamped at −80 mV and a depolarizing voltage pulse to 0 mV was applied for 1.5 s. From this potential the voltage was stepped to potentials ranging from −10 mV to −120 mV. (B) Modeling the observed mean fractional block of conductance by Cs+ with a Woodhull model (see text). Conductance was calculated as Ii/(V–Erev), where Ii was the instantaneous current level measured 3 ms after the start of the hyperpolarizing voltage step. Shown are fractional block by 1 mM Cs+ and 10 mM Cs+. Solid lines are the best fits of the model used to the observed mean data, with values in 1 mM Cs+ of KD(0 mV) = 290 mM and δ = 1.33. Values in 10 mM Cs+ were KD(0 mV) = 240 mM and δ = 1.31. (C) Block of KCNQ2/3 current by external Ba2+ ions (1 mM). KCNQ2/3 deactivation currents were produced by stepping cells from a holding potential of −30 mV to potentials ranging from −40 to −110 mV in −10-mV increments. Tail currents are omitted for clarity. (D) Fractional block of KCNQ2/3 deactivation relaxations and instantaneous currents (n = 5).
The above equation is a form of the more general equation developed to describe the block of sodium channels by protons (Woodhull, 1973; Block and Jones, 1996). The effective valency of the monovalent blocking ion (δ) represents the apparent fraction of the membrane electric field that the ion must traverse to reach its binding site within the channel. Block of current through KCNQ2/3 channels by external Cs+ was assessed by measuring the fractional decrease of instantaneous conductances. The solid lines in Fig. 7 B are the best fits of the above model to the observed mean data. The best fits of the mean data from four cells gave values in 1 mM Cs+ of KD(0 mV) = 294 ± 22 mM and δ = 1.33 ± 0.02. In 10 mM Cs+ these values were found to be KD(0 mV) = 235 ± 70 mM and δ = 1.31 ± 0.08. The voltage-dependence of Cs+ block and the apparent affinity of Cs+ for KCNQ2/3 channels were therefore not dependent on the concentration of Cs+ used. The fractional block of deactivation relaxations by Cs+ was also assessed. This method was undertaken to directly compare results with those obtained for the M-current found in amphibian sympathetic neurons (Block and Jones, 1996). The best fits of the above model to data analyzed in this way gave values in 1 mM Cs+ of KD(0 mV) = 120 ± 30 mM and δ = 1.18 ± 0.06. In 10 mM Cs+ these values were found to be KD(0 mV) = 100 ± 21 mM and δ = 1.15 ± 0.08. Again, the voltage-dependence of block by Cs+ and the apparent affinity of Cs+ for the channel were not affected by the concentration of Cs+ used. The voltage-dependence of Cs+ block of KCNQ2/3 channels was found to be slightly greater than that reported for theM-current studied under similar ionic conditions, although the affinity values were similar (Block and Jones, 1996).
Block by external barium
External Ba2+ ions are known to block many potassium channels by binding within the outer pore (Jiang and MacKinnon, 2000). Fig. 7, C and D, show block of KCNQ2/3 current by externally applied Ba2+ ions. Block of both instantaneous currents and deactivation relaxations was voltage-independent over the range studied (Fig. 7 D). This is an unusual effect, as many other potassium channels show voltage-dependent block (Armstrong et al., 1982; Hurst et al., 1995; Harris et al., 1998).
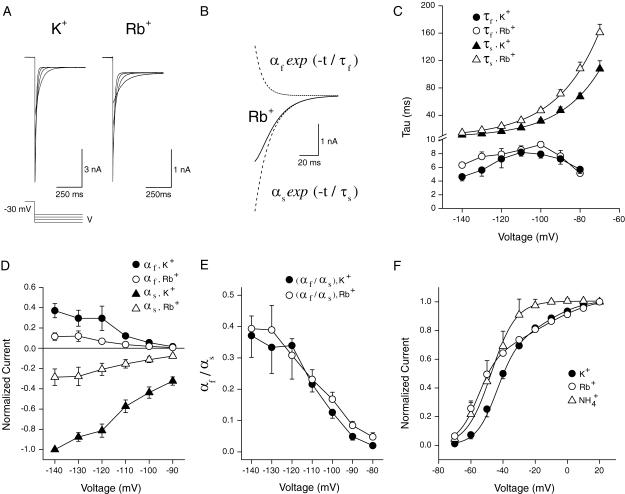
Effect of permeant ions on channel gating
Many potassium channels show a change in deactivation kinetics when K+ is replaced by another permeant ion (Matteson and Swenson, 1986). In contrast, there was no significant change in the deactivation time constants of KCNQ2/3 inward tail currents when Tl+, Cs+, or 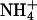 were used instead of K+ as the permeant ion (when tail currents were fit with a single exponential decay; see Table 3), although
were used instead of K+ as the permeant ion (when tail currents were fit with a single exponential decay; see Table 3), although 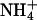 ions did cause an increase in the rate of deactivation of outward current (carried by K+) at −80 mV. However, replacement of K+ with Rb+ led to a slowing of the deactivation kinetics of inward current through KCNQ2/3 channels over the voltage range studied (p < 0.05, paired t-test). A more detailed examination of the effect of Rb+ on the deactivation kinetics of KCNQ2/3 channels is shown in Fig. 8. On closer inspection, tail currents produced by hyperpolarizing voltage steps were observed to deviate from a simple exponential decay. Instead, they displayed a sigmoidal time course at potentials <−70 mV (Fig. 8, A and B), with increasing sigmoidicity at more negative potentials. Deactivation tail currents were therefore fitted with the sum of two exponential functions of opposing amplitudes (Fig. 8 B; see also Pusch et al., 1998, 2000), as in the equation
ions did cause an increase in the rate of deactivation of outward current (carried by K+) at −80 mV. However, replacement of K+ with Rb+ led to a slowing of the deactivation kinetics of inward current through KCNQ2/3 channels over the voltage range studied (p < 0.05, paired t-test). A more detailed examination of the effect of Rb+ on the deactivation kinetics of KCNQ2/3 channels is shown in Fig. 8. On closer inspection, tail currents produced by hyperpolarizing voltage steps were observed to deviate from a simple exponential decay. Instead, they displayed a sigmoidal time course at potentials <−70 mV (Fig. 8, A and B), with increasing sigmoidicity at more negative potentials. Deactivation tail currents were therefore fitted with the sum of two exponential functions of opposing amplitudes (Fig. 8 B; see also Pusch et al., 1998, 2000), as in the equation
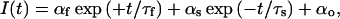 |
with a slow time constant of deactivation, τs; a fast time constant, τf; their respective amplitudes, αs and αf; and a steady-state current, αo. Greater values of αf relative to αs therefore lead to a more pronounced sigmoidal appearance of the tail current. This appearance of the deactivation tail current is in contrast to deactivation relaxations at more depolarized potentials (>−70 mV), which are described by two exponentials of similar direction (Wang et al., 1998). Fig. 8 C shows the voltage-dependence of τs and τf in the presence of either K+ or Rb+ as the permeant ion. Exchange of K+ with Rb+ led to an increase in the value of the deactivation time constant, τs, over the voltage range studied. The observed values of τs were voltage-dependent and showed an exponential distribution (Fig. 8 C), as previously described for both KCNQ2/3 current and M-current (Wang et al., 1998). The voltage-dependence of deactivation was unaffected by Rb+ (Fig. 8 C). The fast time constant τf showed a weak, bell-shaped voltage-dependence (Fig. 8 C). The exchange of K+ for Rb+ had no significant effect on the values or voltage-dependence of τf, except at hyperpolarized potentials, where a slight increase in τf was observed in Rb+ (at −140 mV, p < 0.05; paired t-test). The amplitude of both current components was decreased when K+ was exchanged with Rb+ (Fig. 8 D). The relative amplitude of the current components described by the time constants τs and τf can be expressed as the ratio αf/αs. This ratio was observed to be voltage-dependent, becoming greater at more negative potentials (Fig. 8 E). This indicates a difference in the voltage sensitivity of the processes giving rise to these components. The exchange of K+ with Rb+ had no significant effect on this ratio, except at more depolarized potentials where a slight increase was observed in Rb+ (at −90 mV and −80 mV, p < 0.05; paired t-test). The slowing of deactivation tail currents in the presence of Rb+ was therefore mainly due to an increase of the deactivation rate constant, τs.
TABLE 3.
Effect of permeant ions on KCNQ2/3 deactivation kinetics
|
τ(ms)
| |||
|---|---|---|---|
| Ion | −80 mV | −100 mV | −140 mV |
| K+ | 71 ± 5 (11) | 29.0 ± 1.3 (8) | 11.2 ± 0.7 (11) |
| Tl+ | 87 ± 7 (4) | 30.3 ± 1.4 (5) | 11.9 ± 1.0 (4) |
| Rb+ | 107 ± 7 (5)* | 40.9 ± 3.6 (8)* | 16.4 ± 1.0 (4)* |
| Cs+ | 68 ± 4 (4) | — | 12.1 ± 0.8 (6) |
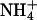 |
48 ± 4 (4)* | — | 12.1 ± 0.9 (5) |
Deactivation time constants (τ) for KCNQ2/3 current in different external cations (15 mM). Deactivation relaxations were evoked by stepping from a holding potential of −30 mV to the potentials indicated. The absence of measurements for Cs+ and 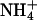 at −100 mV is due to the proximity of this potential to the Erev for these ions. Relaxations were fit by a single exponential over the period 3–500 ms. The number of cells from which values were obtained is indicated in parentheses.
at −100 mV is due to the proximity of this potential to the Erev for these ions. Relaxations were fit by a single exponential over the period 3–500 ms. The number of cells from which values were obtained is indicated in parentheses.
Significantly different from the corresponding value in K+ (p < 0.05,t-test).
FIGURE 8.
Effect of external permeant ions on KCNQ2/3 channel gating. (A) Deactivation tail currents in the presence of 15 mM K+ or Rb+. Cells were clamped at −80 mV and currents were activated by a depolarizing voltage step to −30 mV (1.5 s duration). Tail currents were then produced by stepping the voltage to potentials ranging from −70 mV to −140 mV. For clarity, only tail currents to −80, −100, −120, and −140 mV are shown. (B) The solid line is an expanded view of an Rb+ tail current at −120 mV. The dashed lines show each of the two exponential functions whose sum models the observed current. (C) Voltage-dependence of tail current component time courses. Values of τs in K+ and Rb+ and values of τf in K+ and Rb+ are shown plotted against the pulse voltage (n = 5). The voltage-dependence of τs in K+ and Rb+ was described by a single exponential function in each case, with slopes of e-fold in 20.8 ± 2 mV (K+) and 21.8 ± 2 mV (Rb+). (D) Voltage-dependence of tail current component amplitudes. Values of αs in K+ and Rb+ and values of αf in K+ and Rb+ are shown plotted against the pulse voltage (n = 5). (E) Voltage-dependence of the ratio of current component amplitudes. Values of αf/αs in K+ and Rb+ are shown plotted against the pulse voltage (n = 5). The period 3–500 ms after the hyperpolarizing voltage step was used to fit exponentials to the observed tail currents. Currents were then extrapolated back to time zero after the hyperpolarizing voltage step to calculate the full amplitude of the tail current. (F) Effect of external permeant ions on the voltage-dependence of KCNQ2/3 channel activation. Outward currents in the presence of 15 mM permeant cation were activated by a similar voltage protocol to that shown in Fig. 2 A. Normalized tail current amplitude is shown plotted against the prepulse voltage. Tail current amplitude was assessed at −100 mV (K+, Tl+, Rb+) or −80 mV (Cs+, 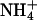 ). Tail current amplitudes were normalized to the amplitude of tail currents produced after a prepulse voltage step to +20 mV. Solid curves are fits of Boltzmann distributions (see Materials and Methods) to the observed mean data in K+, Rb+, and
). Tail current amplitudes were normalized to the amplitude of tail currents produced after a prepulse voltage step to +20 mV. Solid curves are fits of Boltzmann distributions (see Materials and Methods) to the observed mean data in K+, Rb+, and 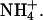 Data in the presence of Tl+ and Cs+ overlap that for K+ and are omitted for clarity.
Data in the presence of Tl+ and Cs+ overlap that for K+ and are omitted for clarity.
The voltage-dependence of KCNQ2/3 channel activation was also assessed in different external ions. Depolarizing voltage steps were applied to activate outward currents (carried by K+) through expressed channels and activation curves were constructed from subsequent tail currents (Fig. 8 F). The sum of two Boltzmann distributions (B1 and B2) were required to fit the data in most cases. This has been observed previously for Shaker and KCNQ2/3 potassium channels (Stefani et al., 1994; Castaldo et al., 2002). One dominant component with steeper voltage-dependence activating at more negative potentials (B1) and one less pronounced component with shallower voltage-dependence activating at more depolarized potentials (B2) were observed. The activation parameters for KCNQ2/3 channels bathed in different external cations are shown in Table 4. Some ions (Tl+ and Cs+) had no significant effect on the half-activation voltages of KCNQ2/3 channels. However, a hyperpolarizing shift in the half-activation voltage V1 was observed on replacement of K+ with Rb+. Rb+ ions also caused a significant decrease in the slope of the B2 component (i.e., an increase of k2). Decreases in the slope of the B1 component (i.e., an increase of k1) were also observed when K+ was replaced with Tl+ or 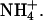 The ratio of the maximal amplitudes of B1 and B2 (i.e., A2/A1) was not significantly altered by the presence of the different ions tested, except in the case of
The ratio of the maximal amplitudes of B1 and B2 (i.e., A2/A1) was not significantly altered by the presence of the different ions tested, except in the case of 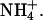 In the presence of
In the presence of 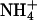 ions there was an apparent loss of the B2 component of channel activation, which caused a hyperpolarizing shift in the overall activation curve compared to K+.
ions there was an apparent loss of the B2 component of channel activation, which caused a hyperpolarizing shift in the overall activation curve compared to K+.
TABLE 4.
Effect of external permeant ions on the activation parameters of KCNQ2/3 channels
| Ion | V1 (mV) | V2 (mV) | k1 (mV) | k2 (mV) | A2/A1 | n |
|---|---|---|---|---|---|---|
| K+ | −46.3 ± 2.4 | −19.8 ± 1.7 | 5.6 ± 0.1 | 12.2 ± 0.7 | 0.63 ± 0.07 | 6 |
| Tl+ | −50.1 ± 0.7 | −18.5 ± 2.2 | 6.2 ± 0.1* | 13.6 ± 0.5 | 0.75 ± 0.03 | 4 |
| Rb+ | −56.7 ± 1.4* | −19.3 ± 2.2 | 5.8 ± 0.4 | 17.0 ± 1.2* | 0.80 ± 0.07 | 4 |
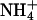 |
−46.9 ± 4.0 | — | 8.5 ± 1.0* | — | 0.0* | 4 |
| Cs+ | −44.5 ± 1.6 | −23.2 ± 6.8 | 5.3 ± 0.4 | 10.8 ± 0.8 | 0.50 ± 0.20 | 4 |
Estimated parameters from fits of the sum of two Boltzmann distributions to KCNQ2/3 tail currents after activating prepulses (see Materials and Methods). Activation of currents in the presence of 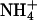 were best described by a single Boltzmann distribution. The value A2/A1 represents the ratio of the maximal amplitudes of each Boltzmann distribution (B1 and B2). Tail currents were measured at −100 mV (K+, Tl+, and Rb+) or −80 mV (
were best described by a single Boltzmann distribution. The value A2/A1 represents the ratio of the maximal amplitudes of each Boltzmann distribution (B1 and B2). Tail currents were measured at −100 mV (K+, Tl+, and Rb+) or −80 mV (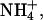 Cs+).
Cs+).
Significantly different to the corresponding value in K+ (p < 0.05, t-test).
DISCUSSION
Sensitivity to external TEA
KCNQ2-5 subunits are expressed in neuronal cells (Tinel et al., 1998; Yang et al., 1998; Kharkovets et al., 2000; Lerche et al., 2000; Schroeder et al., 2000). The sensitivity of KCNQ channels to external TEA differs widely according to their subunit composition (Wang et al., 1998; Hadley et al., 2000; Schroeder et al., 2000). Sensitivity to external TEA is therefore a useful indicator of which KCNQ subunits might contribute to functionally expressed currents. The sensitivity to external TEA of currents arising from the coexpression of KCNQ2 and KCNQ3 subunits in this study was consistent with the functional expression of predominantly heteromeric KCNQ2/3 channels with a fixed subunit stoichiometry. The sensitivity of native SCG M-current to external TEA mirrored the sensitivity of these heterologously expressed KCNQ2/3 channels. It has been reported previously that KCNQ5 subunits are expressed in SCG neurons (Schroeder et al., 2000; Hadley et al., 2003). Homomeric KCNQ5 channels, heteromeric KCNQ3/5 channels, and homomeric KCNQ3 channels have been reported to be relatively insensitive to external TEA, exhibiting IC50 values of 71 mM, ∼200 mM, and 224 mM, respectively (Schroeder et al., 2000; Shapiro et al., 2000). Our data showed ∼95% block of SCG M-current by 100 mM TEA. This indicates a relative lack (<10% of total current) of functionally expressed TEA-insensitive channels formed from KCNQ3 and KCNQ5 subunits in SCG neurons (consistent with Hadley et al., 2003).
Selectivity of KCNQ2/KCNQ3 channels for monovalent cations
KCNQ2/3 channels displayed an Eisenman Type IV permeability series (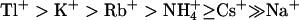 ) that is typical of many potassium channels (Table 5) (Hille, 2001). The relative conductance series based on slope conductances (
) that is typical of many potassium channels (Table 5) (Hille, 2001). The relative conductance series based on slope conductances (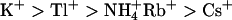 ) was different from the relative permeation series. Heteromeric KCNQ2/3 channels displayed ionic conduction properties that were distinct from other previously characterized potassium channels (Table 5). The low relative conductance of Rb+ through KCNQ2/3 channels is in contrast to other KCNQ channels. For example, KCNQ1 channels show an Rb+ conductance that is greater than the conductance of K+ (Pusch et al, 2000) and KCNQ5 channels exhibit a relative Rb+ conductance close to that of K+ (Schroeder et al., 2000). The uniquely conserved pore lysine of KCNQ channels (K283 of KCNQ2) was shown in this study to affect the relative conductance of Rb+ ions, with introduction of a Shaker-like methionine at this locus in KCNQ2 resulting in an increase of relative Rb+ conductance to levels near those seen for Shaker channels (Heginbotham and MacKinnon, 1993; Table 2). The effects of the T276S mutation on ion permeation and conduction indicate that this residue may contribute to stabilization of an ion at the intracellular end of the selectivity filter in KCNQ2 channels. This is consistent with involvement of the equivalent residue (T441) in intracellular ionic block of Shaker (Yellen et al., 1991).
) was different from the relative permeation series. Heteromeric KCNQ2/3 channels displayed ionic conduction properties that were distinct from other previously characterized potassium channels (Table 5). The low relative conductance of Rb+ through KCNQ2/3 channels is in contrast to other KCNQ channels. For example, KCNQ1 channels show an Rb+ conductance that is greater than the conductance of K+ (Pusch et al, 2000) and KCNQ5 channels exhibit a relative Rb+ conductance close to that of K+ (Schroeder et al., 2000). The uniquely conserved pore lysine of KCNQ channels (K283 of KCNQ2) was shown in this study to affect the relative conductance of Rb+ ions, with introduction of a Shaker-like methionine at this locus in KCNQ2 resulting in an increase of relative Rb+ conductance to levels near those seen for Shaker channels (Heginbotham and MacKinnon, 1993; Table 2). The effects of the T276S mutation on ion permeation and conduction indicate that this residue may contribute to stabilization of an ion at the intracellular end of the selectivity filter in KCNQ2 channels. This is consistent with involvement of the equivalent residue (T441) in intracellular ionic block of Shaker (Yellen et al., 1991).
TABLE 5.
Comparison of potassium channel selectivity
|
PX/PK
|
GX/GK
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tl+ | Rb+ | 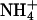 |
Cs+ | Tl+ | Rb+ | 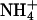 |
Cs+ | ||
| KCNQ2/3 | 1.37 | 0.71 | 0.10 | 0.086 | 0.59 | 0.24 | 0.30 | 0.018 | This study |
| IM (rat SCG) | 1.5 | 0.8 | 0.1 | 0.2 | 0.28 | 0.11 | 0.23 | 0.1 | Cloues and Marrion (1996) |
| IKCa | 1.3 | 0.7 | 0.1 | — | 0.41 | 0.07 | 0.18 | — | Eisenman et al. (1986) |
| Shaker | — | 0.66 | 0.09 | 0.06 | — | 0.50 | 0.75 | 0.01 | Heginbotham and MacKinnon (1993) |
| KcsA | 3.2 | 0.78 | 0.2 | — | 0.29 | 0.42 | 0.43 | — | LeMasurier et al. (2001) |
| Kir1.1b | 2.58 | 0.42 | 0.09 | — | 0.52 | 0.38 | 1.62 | — | Choe et al. (2000) |
| Kir2.1 | 1.49 | 0.49 | 0.10 | — | 0.94 | 0.10 | 0.59 | — | Choe et al. (2000) |
Mechanism of ion conduction in KCNQ2/KCNQ3 channels
Potassium channels are considered to conduct ions by a mechanism which is facilitated by the simultaneous occupation of the channel pore by multiple K+ ions (Hille and Schwarz, 1978; Doyle et al., 1998; Morais-Cabral et al., 2001). One functional characteristic that is indicative of a conduction mechanism involving multiple ions within the channel pore is the AMFE. Most potassium channels display an AMFE when bathed in a solution containing a mixture of permeant ions (Hille and Schwarz, 1978; Eisenman et al., 1986; Wagoner and Oxford, 1987; Heginbotham and MacKinnon, 1993; Block and Jones, 1997). Unusually, the M-current native to sympathetic neurons does not show this effect under a range of different ionic conditions (Block and Jones, 1996; Cloues and Marrion, 1996). We have shown that this effect is also absent for currents arising from the expression of heteromeric KCNQ2/3 channels and homomeric KCNQ2 channels in mixtures of K+ and Rb+ ions. The related KCNQ1 channel and the KCNQ1/KCNE1 complex have also previously been shown to lack an AMFE in mixtures of K+ and Rb+ (Pusch et al., 2000). This may therefore be a feature of KCNQ channels in general. Absence of this effect is not in itself evidence against the existence of multi-ion pore behavior in these channels. It may indicate that the number or nature (i.e., affinity or position) of binding sites for permeant ions differ from those of other potassium channels, such that interaction between permeant ions either does not occur, or does not alter the rate-limiting steps of ion conduction. In support of this, the ratio GRb/GK (and the ratio GCs/GK) of KCNQ2/3 channels did not vary significantly over the concentration range 5.85 mM−150 mM of monovalent cation. This is in contrast to the KcsA potassium channel, where the conductance of K+ and Rb+ show markedly different concentration-dependence (LeMasurier et al., 2001; Morais-Cabral et al., 2001).
Another effect that has been attributed to the multi-ion nature of potassium channels is block by permeant cations which shows an extreme voltage-dependence (δ > 1) that is dependent on the concentration of blocking ion. This effect has been shown to occur for a variety of potassium channel types including Shaker and delayed rectifiers (Adelman and French, 1978; Hille and Schwarz, 1978; Perez-Cornejo and Begenisich, 1994; Block and Jones, 1996). This effect has been rationalized by invoking ion-ion interactions within the pore, meaning that the blocking ion experiences both the effect of the membrane field and the effect of permeant ions within the pore. Block of inward current through KCNQ2/3 channels by extracellular Cs+ was observed to be strongly voltage-dependent. However, although the apparent value of δ was >1, it was not dependent on the concentration of Cs+, even though the magnitude of block by Cs+ was concentration-dependent. This result differs from observations in other potassium channels, where the voltage-dependence of block was found to be dependent on the concentration of permeant blocking or conducting ion (Perez-Cornejo and Begenisich, 1994; Block and Jones, 1996).
Block of KCNQ2/3 current by external Ba2+ was found to be voltage-independent. This is in contrast to other potassium channels including Shaker, which show voltage-dependent block (Armstrong et al., 1982; Hurst et al., 1995; Harris et al., 1998). Voltage-independent block suggests that the binding site for Ba2+ resides in an external region of KCNQ2/3 channels that is outside the majority of the membrane field. This is an indication that Ba2+ may not occupy the inner selectivity filter site as it does in other potassium channels (Jiang and MacKinnon, 2000) and provides further evidence that the nature of ion binding sites within the pore of KCNQ2/3 channels is distinct from other potassium channels.
Effect of permeant ions on gating of KCNQ2/3 channels
The nature of the permeant ion has been shown to alter the deactivation-gating characteristics of many potassium channel types (Swenson and Armstrong, 1981; Matteson and Swenson, 1986; Demo and Yellen, 1992; Block and Jones, 1997). In contrast, the deactivation kinetics of inward current through KCNQ2/3 channels were not significantly affected by replacement of K+ with Tl+, Cs+, or 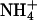 . However, replacement of K+ with Rb+ led to a slowing of KCNQ2/3 current deactivation kinetics. The specific effect of Rb+ on deactivation kinetics could be due to occupation by Rb+ of a site within the pore that is not occupied by the other monovalent cations tested and that is close to sites responsible for deactivation gating. In support of this, the predominant locations of Rb+ within the crystal structure of the model potassium channel KcsA differ from those of K+ (Morais-Cabral et al., 2001).
. However, replacement of K+ with Rb+ led to a slowing of KCNQ2/3 current deactivation kinetics. The specific effect of Rb+ on deactivation kinetics could be due to occupation by Rb+ of a site within the pore that is not occupied by the other monovalent cations tested and that is close to sites responsible for deactivation gating. In support of this, the predominant locations of Rb+ within the crystal structure of the model potassium channel KcsA differ from those of K+ (Morais-Cabral et al., 2001).
The sigmoid nature of KCNQ2/3 deactivation tail currents at negative potentials is similar to that observed for heteromeric KCNQ1/KCNE1 channels (Pusch et al., 1998, 2000). Sigmoid outward tail currents were also observed in external Cs+ and 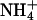 at −80 mV, but in these cases the fast component inducing sigmoidicity was reversed (i.e., outward; unpublished observations). This suggested that ionic block was not responsible for the sigmoid appearance of these tail currents. In addition, no time-dependent inactivation was apparent during the depolarizing pulse preceding deactivation. These results suggest that sigmoidicity may be derived from the presence of multiple open states within the gating scheme of KCNQ2/3 channels. This would be consistent with observations of single KCNQ2/3 channels and M-channels from SCG neurons, which have been demonstrated to exhibit multiple kinetically distinct open states (Selyanko and Brown, 1999; Selyanko et al., 2001). However, it is also possible that recovery from rapid inactivation or closed-state inactivation underlies the sigmoidicity of tail currents. The effect of Rb+ on the deactivation kinetics of KCNQ2/3 channels is also similar in some ways to its effect on heteromeric KCNQ1/KCNE1 channels (Pusch et al., 2000). Rb+ was found to have no effect on the deactivation kinetics of KCNQ1 channels, whereas it caused an increase in the values of τs, τf, and αf/αs for KCNQ1/KCNE1 channels (Pusch et al., 2000).
at −80 mV, but in these cases the fast component inducing sigmoidicity was reversed (i.e., outward; unpublished observations). This suggested that ionic block was not responsible for the sigmoid appearance of these tail currents. In addition, no time-dependent inactivation was apparent during the depolarizing pulse preceding deactivation. These results suggest that sigmoidicity may be derived from the presence of multiple open states within the gating scheme of KCNQ2/3 channels. This would be consistent with observations of single KCNQ2/3 channels and M-channels from SCG neurons, which have been demonstrated to exhibit multiple kinetically distinct open states (Selyanko and Brown, 1999; Selyanko et al., 2001). However, it is also possible that recovery from rapid inactivation or closed-state inactivation underlies the sigmoidicity of tail currents. The effect of Rb+ on the deactivation kinetics of KCNQ2/3 channels is also similar in some ways to its effect on heteromeric KCNQ1/KCNE1 channels (Pusch et al., 2000). Rb+ was found to have no effect on the deactivation kinetics of KCNQ1 channels, whereas it caused an increase in the values of τs, τf, and αf/αs for KCNQ1/KCNE1 channels (Pusch et al., 2000).
Comparison of KCNQ2/3 current with M-current
KCNQ2/3 channels have been proposed to underlie the neuronal M-current on the basis of their similar pharmacology and modulation by muscarinic receptor activation (Wang et al., 1998). However, these properties are not capable of distinguishing between different combinations of KCNQ subunits and do not exclude the involvement of other potassium channel types. Recent results using a dominant-negative KCNQ3 construct suggest that KCNQ channel subunits do contribute to the channels which underlie the neuronal SCG M-current (Selyanko et al., 2002). However, the possibility has remained that other KCNQ subunits or other potassium channel subunits might contribute to heteromeric channels underlying this native current, perhaps in combination with auxiliary subunits (Lerche et al., 2000; Schroeder et al., 2000; Tinel et al., 2000; Robbins, 2001; Sogaard et al., 2001). Inward current through KCNQ2/3 channels and neuronal M-current both display a lack of anomalous mole-fraction behavior. However, some marked differences exist between the two current types in terms of their permeation and conduction characteristics (Table 5). These include markedly different relative Cs+ permeability and conductance, as well as different relative Rb+ and Tl+ conductances. The effect of Rb+ on deactivation kinetics was also different between the two current types. Deactivation kinetics of M-current are independent of the identity of the conducting ion (Cloues and Marrion, 1996). In contrast, Rb+ ions slowed the deactivation kinetics of KCNQ2/3 channels. Also, in contrast to previous results for SCG M-current (Cloues and Marrion, 1996), block of KCNQ2/3 channels by external Ba2+ ions did not show any significant dependence on the direction of current flow. However, comparison with results for mammalian M-current is hampered by the requirement for use of isolating blockers such as TEA in previous studies (Cloues and Marrion, 1996). This may have increased the contribution of TEA-insensitive current (e.g., KCNQ3/5) present in these neurons. Differences between the conduction and gating properties of KCNQ2/3 channels shown here and previous studies of neuronal M-current may therefore constitute further evidence for the presence of other contributing potassium channel subunits or auxiliary subunits within neurons.
In summary, KCNQ2/3 channels display relative permeation properties that are similar to a wide range of other potassium channels, while showing conduction properties and effects of ions on gating that are distinctive. The selectivity properties of these heteromeric channels are differentially affected by the respective contributing KCNQ2 and KCNQ3 subunits in a generally nonadditive manner. KCNQ2/3 channels lack some characteristics that are shown by many other potassium channels and that are commonly interpreted as consequences of multi-ion occupancy. However, the balance of data suggests that KCNQ2/3 channels do form multi-ion pores. Given the novel primary structure of KCNQ channels, these characteristics suggest that these channels may display distinctive mechanisms of ion conduction through the channel pore.
Acknowledgments
We thank Dr. David McKinnon (State University of New York at Stony Brook, NY) for providing KCNQ2 and KCNQ3 constructs. We also thank Prof. David Brown (University College London, London, UK) and Dr. Galen Flynn (University of Washington, Seattle, WA) for providing HEK-293T cells.
This work was supported by the United Kingdom Medical Research Council.
References
- Adelman, W. J., Jr., and R. J. French. 1978. Blocking of the squid axon potassium channel by external cesium ions. J. Physiol. 276:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, C. M., R. P. Swenson, Jr., and S. R. Taylor. 1982. Block of squid axon K channels by internally and externally applied barium ions. J. Gen. Physiol. 80:663–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, D., I. Dreyer, S. Hoth, J. D. Reid, H. Busch, M. Lehnen, K. Palme, and R. Hedrich. 1996. Changes in voltage activation, Cs+ sensitivity, and ion permeability in H5 mutants of the plant K+ channel KAT1. Proc. Natl. Acad. Sci. USA. 93:8123–8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech, D. J., L. Bernheim, A. Mathie, and B. Hille. 1991. Intracellular Ca2+ buffers disrupt muscarinic suppression of Ca2+ current and M current in rat sympathetic neurons. Proc. Natl. Acad. Sci. USA. 88:652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biervert, C., B. C. Schroeder, C. Kubisch, S. F. Berkovic, P. Propping, T. J. Jentsch, and O. K. Steinlein. 1998. A potassium channel mutation in neonatal human epilepsy. Science. 279:403–406. [DOI] [PubMed] [Google Scholar]
- Block, B. M., and S. W. Jones. 1996. Ion permeation and block of M-type and delayed rectifier potassium channels. J. Gen. Physiol. 107:473–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block, B. M., and S. W. Jones. 1997. Delayed rectifier current of bullfrog sympathetic neurons: ion-ion competition, asymmetrical block and effects of ions on gating. J. Physiol. 499:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, D. A., and P. R. Adams. 1980. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 283:673–676. [DOI] [PubMed] [Google Scholar]
- Brown, B. S., and S. P. Yu. 2000. Modulation and genetic identification of the M channel. Prog. Biophys. Mol. Biol. 73:135–166. [DOI] [PubMed] [Google Scholar]
- Castaldo, P., E. Miraglia del Giudice, G. Coppola, A. Pascotto, L. Annunziato, and M. Taglialatela. 2002. Benign familial neonatal convulsions caused by altered gating of KCNQ2/3 potassium channels. J. Neurosci. 22:RC199:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier, C., N. A. Singh, S. G. Ryan, T. B. Lewis, B. E. Reus, R. J. Leach, and M. Leppert. 1998. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat. Genet. 18:53–55. [DOI] [PubMed] [Google Scholar]
- Choe, S. 2002. Potassium channel structures. Nat. Rev. Neurosci. 3:115–121. [DOI] [PubMed] [Google Scholar]
- Choe, H., H. Sackin, and L. G. Palmer. 2000. Permeation properties of inward-rectifier potassium channels and their molecular determinants. J. Gen. Physiol. 115:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloues, R., and N. V. Marrion. 1996. Conduction properties of theM-channel in rat sympathetic neurons. Biophys. J. 70:806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee, W. A., Y. Amarillo, J. Chiu, A. Chow, D. Lau, T. McCormack, H. Moreno, M. S. Nadal, A. Ozaita, D. Pountney, M. Saganich, E. Vega-Saenz de Miera, and B. Rudy.E. Vega-Saenz de Miera, and B. Rudy.1999. Molecular diversity of K+ channels. Ann. N. Y. Acad. Sci. 868:233–285. [DOI] [PubMed] [Google Scholar]
- Constanti, A., and D. A. Brown. 1981. M-currents in voltage-clamped mammalian sympathetic neurones. Neurosci. Lett. 24:289–294. [DOI] [PubMed] [Google Scholar]
- Cooper, E. C., K. D. Aldape, A. Abosch, N. M. Barbaro, M. S. Berger, W. S. Peacock, Y. N. Jan, and L. Y. Jan. 2000. Colocalization and coassembly of two human brain M-type potassium channels subunits that are mutated in epilepsy. Proc. Natl. Acad. Sci. USA. 97:4914–4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demo, S. D., and G. Yellen. 1992. Ion effects on gating of the Ca2+-activated K+ channel correlate with occupancy of the pore. Biophys. J. 61:639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doring, F., C. Derst, E. Wischmeyer, C. Karschin, R. Schneggenburger, J. Daut, and A. Karschin. 1998. The epithelial inward rectifier channel Kir7.1 displays unusual K+ permeation properties. J. Neurosci. 18:8625–8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, D. A., J. Morais-Cabral, R. A. Pfuetzner, A. Kuo, J. M. Gulbis, S. L. Cohen, B. T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- Eisenman, G., R. Latorre, and C. Miller. 1986. Multi-ion conduction in the high-conductance Ca2+-activated K+ channel from skeletal muscle. Biophys. J. 50:1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman, D. E. 1943. Potential, impedance, and rectification in membranes. J. Gen. Physiol. 27:37–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley, J. K., M. Noda, A. A. Selyanko, I. C. Wood, F. C. Abogadie, and D. A. Brown. 2000. Differential tetraethylammonium sensitivity of KCNQ1–4 potassium channels. Br. J. Pharmacol. 129:413–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley, J. K., G. M. Passmore, L. Tatulian, M. Al-Qatari, F. Ye, A. D. Wickenden, and D. A. Brown. 2003. Stoichiometry of expressed KCNQ2/KCNQ3 potassium channels and subunit composition of native ganglionic M channels deduced from block by tetraethylammonium. J. Neurosci. 23:5012–5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, R. E., H. P. Larsson, and E. Y. Isacoff. 1998. A permanent ion binding site located between two gates of the Shaker K+ channel. Biophys. J. 74:1808–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham, L., and R. MacKinnon. 1993. Conduction properties of the cloned Shaker K+ channel. Biophys. J. 65:2089–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille, B. 2001. Ion Channels of Excitable Membranes, 3rd Ed. Sinauer Associates, Sunderland, MA.
- Hille, B., and W. Schwarz. 1978. Potassium channels as multi-ion single-file pores. J. Gen. Physiol. 72:409–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin, A. L., and B. Katz. 1949. The effect of Na ions on the electrical activity of the giant axon of the squid. J. Physiol. 108:37–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst, R. S., R. Latorre, L. Toro, and E. Stefani. 1995. External barium block of Shaker potassium channels: evidence for two binding sites. J. Gen. Physiol. 106:1069–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y., and R. MacKinnon. 2000. The barium site in a potassium channel by x-ray crystallography. J. Gen. Physiol. 115:269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharkovets, T., J. P. Hardelin, S. Saffiedine, M. Schweizer, A. El-Amraoui, C. Petit, and T. J. Jentsch. 2000. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc. Natl. Acad. Sci. USA. 97:4333–4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky, G., I. Medina, L. Eng, L. Krapivinsky, Y. Yang, and D. E. Clapham. 1998. A novel inward rectifier K+ channel with unique pore properties. Neuron. 20:995–1005. [DOI] [PubMed] [Google Scholar]
- Lacombe, B., and J. B. Thibaud. 1998. Evidence for a multi-ion pore behavior in the plant potassium channel KAT1. J. Membr. Biol. 166:91–100. [DOI] [PubMed] [Google Scholar]
- LeMasurier, M., L. Heginbotham, and C. Miller. 2001. KcsA: It's a potassium channel. J. Gen. Physiol. 118:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerche, C., C. R. Scherer, G. Seebohm, C. Derst, A. D. Wei, A. E. Busch, and K. Steinmeyer. 2000. Molecular cloning and functional expression of KCNQ5, a potassium channel subunit that may contribute to neuronal M-current diversity. J. Biol. Chem. 275:22395–22400. [DOI] [PubMed] [Google Scholar]
- Lu, Z., and R. MacKinnon. 1994. A conductance maximum observed in an inward-rectifier potassium channel. J. Gen. Physiol. 104:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon, R., and G. Yellen. 1990. Mutations affecting TEA blockade and ion permeation in voltage-activated K+ channels. Science. 250:276–279. [DOI] [PubMed] [Google Scholar]
- Marrion, N. V. 1997. Control of M-current. Annu. Rev. Physiol. 59:483–504. [DOI] [PubMed] [Google Scholar]
- Matteson, D. R., and R. P. Swenson. 1986. External monovalent cations that impede the closing of K channels. J. Gen. Physiol. 87:795–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais-Cabral, J. H., Y. Zhou, and R. MacKinnon. 2001. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 414:37–42. [DOI] [PubMed] [Google Scholar]
- Pan, Z., A. A. Selyanko, J. K. Hadley, D. A. Brown, J. E. Dixon, and D. McKinnon. 2001. Alternative splicing of KCNQ2 potassium channel transcripts contributes to the functional diversity of M-currents. J. Physiol. 531:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Cornejo, P., and T. Begenisich. 1994. The multi-ion nature of the pore in Shaker K+ channels. Biophys. J. 66:1929–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch, M., L. Bertorello, and F. Conti. 2000. Gating and flickery block differentially affected by rubidium in homomeric KCNQ1 and heteromeric KCNQ1/KCNE1 potassium channels. Biophys. J. 78:211–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch, M., R. Magrassi, B. Wollnik, and F. Conti. 1998. Activation and inactivation of homomeric KvLQT1 potassium channels. Biophys. J. 75:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, J. 2001. KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol. Ther. 90:1–19. [DOI] [PubMed] [Google Scholar]
- Robinson, R. A., and R. H. Stokes. 1959. Electrolyte Solutions, 2nd Ed. Butterworth & Co., London, UK.
- Sabirov, R. Z., T. Tominaga, A. Miwa, Y. Okada, and S. Oiki. 1997. A conserved arginine residue in the pore region of an inward rectifier K channel (IRK1) as an external barrier for cationic blockers. J. Gen. Physiol. 110:665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, B. C., M. Hechenberger, F. Weinreich, C. Kubisch, and T. J. Jentsch. 2000. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J. Biol. Chem. 275:24089–24095. [DOI] [PubMed] [Google Scholar]
- Selyanko, A. A., and D. A. Brown. 1999. M-channel gating and simulation. Biophys. J. 77:701–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selyanko, A. A., P. Delmas, J. K. Hadley, L. Tatulian, I. C. Wood, M. Mistry, B. London, and D. A. Brown. 2002. Dominant-negative subunits reveal potassium channel families that contribute to M-like potassium currents. J. Neurosci. 22:RC212–RC216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selyanko, A. A., J. K. Hadley, and D. A. Brown. 2001. Properties of single M-type KCNQ2/KCNQ3 potassium channels expressed in mammalian cells. J. Physiol. 534:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro, M. S., J. P. Roche, E. J. Kaftan, H. Cruzblanca, K. Mackie, and B. Hille. 2000. Reconstitution of muscarinic modulation of the KCNQ2/KCNQ3 K+ channels that underlie the neuronal M current. J. Neurosci. 20:1710–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, N. A., C. Charlier, D. Stauffer, R. DuPont, R. J. Leach, and R. Melis. 1998. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat. Genet. 18:25–29. [DOI] [PubMed] [Google Scholar]
- Sogaard, R., T. Ljungstrom, K. A. Pedersen, S. P. Olesen, and B. S. Jensen. 2001. KCNQ4 channels expressed in mammalian cells: functional characteristics and pharmacology. Am. J. Physiol. Cell Physiol. 280:C859–C866. [DOI] [PubMed] [Google Scholar]
- Stampe, P., J. Arreola, P. Perez-Cornejo, and T. Begenisich. 1998. Nonindependent K+ movement through the pore in IRK1 potassium channels. J. Gen. Physiol. 112:475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani, E., L. Toro, E. Perozo, and F. Bezanilla. 1994. Gating of Shaker K+ channels: I. Ionic and gating currents. Biophys. J. 66:996–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson, R. P., Jr., and C. M. Armstrong. 1981. K+ channels close more slowly in the presence of external K+ and Rb+. Nature. 291:427–429. [DOI] [PubMed] [Google Scholar]
- Tinel, N., S. Diochot, I. Lauritzen, J. Barhanin, M. Lazdunski, and M. Borsotto. 2000. M-type KCNQ2-KCNQ3 potassium channels are modulated by the KCNE2 subunit. FEBS Lett. 480:137–141. [DOI] [PubMed] [Google Scholar]
- Tinel, N., I. Lauritzen, C. Chouabe, M. Lazdunski, and M. Borsotto. 1998. The KCNQ2 potassium channel: splice variants, functional and developmental expression. Brain localization and comparison with KCNQ3. FEBS Lett. 438:171–176. [DOI] [PubMed] [Google Scholar]
- Wagoner, P. K., and G. S. Oxford. 1987. Cation permeation through the voltage-dependent potassium channel in the squid axon. Characteristics and mechanisms. J. Gen. Physiol. 90:261–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H.-S., Z. Pang, W. Shi, B. S. Brown, R. S. Wymore, I. S. Cohen, J. E. Dixon, and D. McKinnon. 1998. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 282:1890–1893. [DOI] [PubMed] [Google Scholar]
- Wei, A., T. Jegla, and L. Salkoff. 1996. Eight potassium channel families revealed by the C. elegans genome project. Neuropharmacology. 35:805–829. [DOI] [PubMed] [Google Scholar]
- Wischmeyer, E., F. Doring, and A. Karschin. 2000. Stable cation coordination at a single outer pore residue defines permeation properties in Kir channels. FEBS Lett. 466:115–120. [DOI] [PubMed] [Google Scholar]
- Woodhull, A. M. 1973. Ionic blockage of sodium channels in nerve. J. Gen. Physiol. 61:687–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, W. P., P. C. Levesque, W. A. Little, M. L. Conder, P. Ramakrishnan, M. G. Neubauer, and M. A. Blanar. 1998. Functional expression of two KvLQT1-related potassium channels responsible for an inherited idiopathic epilepsy. J. Biol. Chem. 273:19419–19423. [DOI] [PubMed] [Google Scholar]
- Yellen, G., M. E. Jurman, T. Abramson, and R. MacKinnon. 1991. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science. 251:939–942. [DOI] [PubMed] [Google Scholar]
- Yool, A. J., and T. L. Schwarz. 1996. Anomalous mole fraction effect induced by mutation of the H5 pore region in the Shaker K+ channel. Biophys. J. 71:2467–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]