Abstract
Phospholamban (PLB) is a 52-amino acid integral membrane protein that regulates the flow of Ca2+ ions in cardiac muscle cells. In the present study, the transmembrane domain of PLB (24–52) was incorporated into phospholipid bilayers prepared from 1-palmitoyl-2-oleoyl-sn-glycero-phosphocholine (POPC). Solid-state 31P and 2H NMR experiments were carried out to study the behavior of POPC bilayers in the presence of the hydrophobic peptide PLB at temperatures ranging from 30°C to 60°C. The PLB peptide concentration varied from 0 mol % to 6 mol % with respect to POPC. Solid-state 31P NMR spectroscopy is a valuable technique to study the different phases formed by phospholipid membranes. 31P NMR results suggest that the transmembrane protein phospholamban is incorporated successfully into the bilayer and the effects are observed in the lipid lamellar phase. Simulations of the 31P NMR spectra were carried out to reveal the formation of different vesicle sizes upon PLB insertion. The bilayer vesicles fragmented into smaller sizes by increasing the concentration of PLB with respect to POPC. Finally, molecular order parameters (SCD) were calculated by performing 2H solid-state NMR studies on deuterated POPC (sn-1 chain) phospholipid bilayers when the PLB peptide was inserted into the membrane.
INTRODUCTION
Methods for understanding interactions between proteins and lipids are essential for elucidating biological structure-function relationships. Peptide-lipid interactions can affect both protein and bilayer structure (Bloom et al., 1991; Lemmon and Engelman, 1994a; Watts, 1993). Previous studies have suggested that these interactions can serve several regulatory roles such as controlling the membrane-association of lytic peptides, modulating membrane-protein activity, promoting peptide aggregation, segregating proteins within the membrane, determining protein sorting during secretion recognition (Arora and Tamm, 2001; Dempsey et al., 1986; Lemmon and Engelman, 1994b; Watts, 1981). Three typical models of biological membranes are planar lipid bilayers, vesicles (liposomes) and monolayers. According to Singer-Nicholson's model of a cell membrane, a lipid bilayer resembles a biomembrane closer than a monolayer (Singer and Nicolson, 1972). Liposomes or phospholipid dispersions are commonly used to study membrane structure upon peptide insertion (Epand, 1998; Liu et al., 2001). In an aqueous solution, phospholipids self-assemble to form lipid bilayers rather than micelles. The reason is that phosphatidylcholines have two acyl chains that are more or less parallel to one another. The overall shape of a phospholipid molecule is approximately rectangular, so these molecules are too bulky to fit in the interior of micelles. The well-defined synthetic membranes are used as model systems to mimic the properties of biological membranes. Interestingly, the lipid bilayer in biological membranes is generally in the liquid-crystalline phase where the axis of symmetry of the acyl chain motion is perpendicular to the plane formed by the polar headgroup.
High-resolution NMR techniques are now routinely employed to study the structure of complex macromolecules in solution (Brunner et al., 2000; Cavagnero et al., 1999; Ottiger and Bax, 1999; Vold et al., 1997). An alternative approach to solution structural studies of membrane macromolecules is the determination of their structural and dynamic properties using solid-state NMR spectroscopy. Solid-state NMR spectroscopy has been widely used to study the structure and dynamics of peptides, including those that associate with membranes or with biomineral surfaces (Barre et al., 2003; Cross, 1997; Long et al., 1998, 2001; Marassi et al., 1999; Marassi and Opella, 1998; Marcotte et al., 2003; Nakazawa and Asakura, 2003; Nicholson and Cross, 1989; Shaw et al., 2000; Watts, 1998). For membrane-bound peptides, solid-state NMR spectroscopy has the ability to probe lipid bilayers in the presence of a peptide, which can be poised in a biologically relevant liquid-crystalline state. Membrane proteins reconstituted into synthetic phospholipid bilayers simulate the biological membrane better than detergent micelles such as sodium dodecyl sulfate (Morrow and Grant, 2000; Rigby et al., 1996; Sharpe et al., 2002a,b).
Phospholamban (PLB) is a small transmembrane peptide (52 amino acids) that interacts with the Ca2+-ATPase pump and lowers its affinity for Ca2+ (Simmerman et al., 1986, 1996; Simmerman and Jones, 1998; Stokes, 1997; Yao et al., 2001). It consists of three domains: residues 1–20 (hydrophilic cytoplasmic domain), residues 21–30 (hinge segment), and residues 31–52 (hydrophobic α-helical membrane-spanning region). Because of its biological importance and its relatively small size, PLB has been the benchmark used in many theoretical and experimental studies of membrane protein structure and assemblies. Fujii and co-workers elucidated the complete PLB primary structure by amino acid sequencing (Fujii et al., 1987). They established that the molecular mass of the PLB monomer was 6082 Da and determined that PLB is a pentamer consisting of five identical subunits.
Determining the structure of PLB and its interactions with the lipid bilayer is central for understanding its regulatory role (Mascioni et al., 2002a,b; Ying et al., 2000). The transmembrane segment of PLB (24–52) has been synthesized using solid-phase peptide synthesis and purified according to the modified method reported recently by our group (Tiburu et al., 2003). In the present study, the transmembrane domain of PLB (24–52) was incorporated into phospholipid bilayers prepared from 1-palmitoyl-2-oleoyl-sn-glycero-phosphatidylcholine (POPC). Solid-state NMR spectroscopy has been used to monitor the interactions between the lipid bilayers and PLB, by exploiting the 31P nuclei as a natural spin reporter on the headgroup of POPC. Solid-state 31P NMR spectroscopy is a valuable technique to study the different phases formed by model phospholipid membranes (Cullis and de Kruijff, 1979; Seelig, 1978). The 31P NMR line shapes have distinct characteristics for different lipid phases such as the gel and liquid crystalline lamellar phases, the inverted hexagonal phase, and isotropic phases such as small vesicles or micelles (Smith and Ekiel, 1984). The low chain melting point of POPC makes it possible to examine PLB in membranes at a physiologically relevant temperature utilizing solid-state NMR spectroscopy. In the present paper, we are focusing on three main points: 1), lipid-peptide interactions of PLB incorporated into POPC bilayers utilizing 31P NMR spectroscopy; 2), perturbations of the phospholipid bilayers using POPC-d31 as a NMR probe; and 3), the effects of various concentrations of PLB and temperature on the dynamic properties of the phospholipid bilayers.
MATERIALS AND METHODS
Materials
9-Fluorenylmethoxycarbonyl (Fmoc)-amino acids and other chemicals for peptide synthesis were purchased from Applied Biosystems (Foster City, CA). POPC and POPC-d31 were purchased from Avanti Polar Lipids (Alabaster, AL). Prior to use, phospholipids were dissolved in chloroform and stored at −20°C. Chloroform, hexafluoro-2-propanol, formic acid, and 2,2,2 trifluoroethanol (TFE) were purchased from Sigma-Aldrich (Milwaukee, WI). High performance liquid chromatography-grade acetonitrile and 2-propanol were obtained from Pharmco (Brookfield, CT) and were filtered through a 0.22-μm nylon membrane before use. Water was purified using a Nanopure reverse osmosis system (Millipore, Bedford, MA). N-[2-hydroxyethyl]piperazine-N′-2-ethane sulfonic acid (HEPES) and EDTA were obtained from Sigma-Aldrich.
Synthesis and purification of PLB
PLB was synthesized according to the recently published procedure (Tiburu et al., 2003). In brief, PLB was synthesized using modified Fmoc-based solid-phase methods with an ABI 433A peptide synthesizer (Applied Biosystems, Foster City, CA). The sequence of the synthesized transmembrane segment of PLB (24–52) is ARQNLQNLFINFCLILICLLLICIIVMLL. The crude peptide was purified on an Amersham Pharmacia Biotech AKTA explorer 10S high performance liquid chromatograph controlled by Unicorn (version 3) system software. A C4 semipreparative polymer supported column (259VHP82215) was acquired from Grace Vydac (Hesperia, CA). Columns were equilibrated with 95% solvent A and 5% solvent B. Solvent A consisted of H2O and solvent B was 38% MeCN, 57% IPA, and 5% H2O. Elution of the peptide was achieved with a linear gradient to a final solvent composition of 93% solvent B. The purified peptide was lyophilized and characterized by matrix-assisted laser desorption ionization time-of-flight mass spectrometry.
NMR sample preparation
The POPC-rich bilayer samples, containing various mol % of peptide to phospholipid, were prepared following a slightly modified protocol given by Rigby and co-workers. (1996). POPC (76 mg) and PLB were dissolved in CHCl3 and TFE, respectively, and added to a 12 × 75-mm test tube. The solvents were removed under a steady stream of N2 gas for ∼15–20 min. The test tube was placed in a vacuum dessicator overnight to remove any residual solvents. The peptide/lipid mixture was resuspended in 190 μL HEPES buffer (5 mM EDTA, 20 mM NaCl, and 30 mM HEPES, pH 7.0) by heating in a water bath at 50°C along with slight frequent sample agitation to avoid frothing the mixture. After all the phospholipids were fully dissolved, the sample was transferred to a NMR sample tube. POPC-d31 (4 mg) was added to the samples when conducting the 2H NMR experiments.
NMR spectroscopy
31P NMR spectra were recorded on a Bruker Avance 500-MHz solid-state NMR spectrometer operating at 202.4 MHz using a Bruker double resonance 5-mm round coil static probe (Bruker, Billerica, MA). The 31P NMR spectra were recorded with 1H decoupling using a 4-μs π/2 pulse for 31P and a 5-s recycle delay. For the 31P NMR spectra 1024 scans were taken and the free induction decay was processed using 100 Hz of line broadening. The spectral width was set to 150 ppm. 2H NMR spectra were recorded on the same NMR spectrometer operating at 76.77 MHz using the same 5-mm static round coil NMR probe. The quadrupolar echo pulse sequence was employed using quadrature detection with complete phase cycling of the pulse pairs (Davis et al., 1976). The 90° pulse length was 3 μs, the interpulse delay was 20 μs, the recycle delay was 0.4 s, and the spectral width was set to 100 kHz. A total of 12,288 transients was averaged for each spectrum and processed using 200 Hz line broadening. The sample was held at the desired temperature for 10 min prior to signal acquisition.
NMR data analysis
Simulation of 31P NMR spectra was carried out using the software program called DMFIT (Massiot et al., 2002). This program permits the fitting or modeling of experimental 1D and 2D spectra to a sum of lines or contributions characterized by their corresponding NMR parameters. The spectral fittings were conducted using a minimum number of species. Static chemical shift anisotropy spectral patterns were considered for all the species. Lorentzian broadening was used for all simulations.
Powder-type 2H NMR spectra of multilamellar dispersions of POPC-d31 were numerically deconvoluted (dePaked) using the algorithm of McCabe and Wassall (1995, 1997). The spectra were deconvoluted such that the bilayer normal was perpendicular with respect to the direction of the static magnetic field. The quadrupolar splittings were directly measured from the dePaked spectra and converted into order parameters according to the following expression (Dave et al., 2003; Huster et al., 2002):
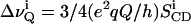 |
where 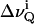 is the quadrupolar splitting for a deuteron attached to the ith carbon, e2qQ/h is the quadrupolar splitting constant (168 kHz for deuterons in C-2H bonds), and
is the quadrupolar splitting for a deuteron attached to the ith carbon, e2qQ/h is the quadrupolar splitting constant (168 kHz for deuterons in C-2H bonds), and 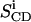 is the chain order parameter for a deuteron attached to the ith carbon of the acyl chain of POPC. The 2H nuclei attached to the terminal methyl carbons were assigned carbon number 15. The remaining 2H assignments were made in decreasing order along the phospholipid acyl chain. Thus, the corresponding order parameters for the individual C-D methylene groups and the terminal methyl groups of the acyl chains were directly evaluated from the quadrupole splittings of the dePaked 2H NMR spectra. The 2H peaks in the NMR spectra were assigned based upon the dynamic properties of the individual CD3 and CD2 groups. The quadrupole splittings of the CD3 methyl groups at the end of the acyl chains are the smallest and closest to 0 kHz because they rotate at the fastest frequency. The next smallest splitting was assigned to the 2H attached to C-14 and so forth along the acyl chain. The quadrupole splittings for the deuterons in the plateau region were estimated by integration of the last broad peak according to the literature (Huster et al., 1998). The order parameters calculated for the CD3 quadrupole splitting were multiplied by 3 according to the literature (Dufourc et al., 1984; Stockson et al., 1976).
is the chain order parameter for a deuteron attached to the ith carbon of the acyl chain of POPC. The 2H nuclei attached to the terminal methyl carbons were assigned carbon number 15. The remaining 2H assignments were made in decreasing order along the phospholipid acyl chain. Thus, the corresponding order parameters for the individual C-D methylene groups and the terminal methyl groups of the acyl chains were directly evaluated from the quadrupole splittings of the dePaked 2H NMR spectra. The 2H peaks in the NMR spectra were assigned based upon the dynamic properties of the individual CD3 and CD2 groups. The quadrupole splittings of the CD3 methyl groups at the end of the acyl chains are the smallest and closest to 0 kHz because they rotate at the fastest frequency. The next smallest splitting was assigned to the 2H attached to C-14 and so forth along the acyl chain. The quadrupole splittings for the deuterons in the plateau region were estimated by integration of the last broad peak according to the literature (Huster et al., 1998). The order parameters calculated for the CD3 quadrupole splitting were multiplied by 3 according to the literature (Dufourc et al., 1984; Stockson et al., 1976).
RESULTS AND DISCUSSION
31P NMR study of PLB incorporated into POPC bilayer
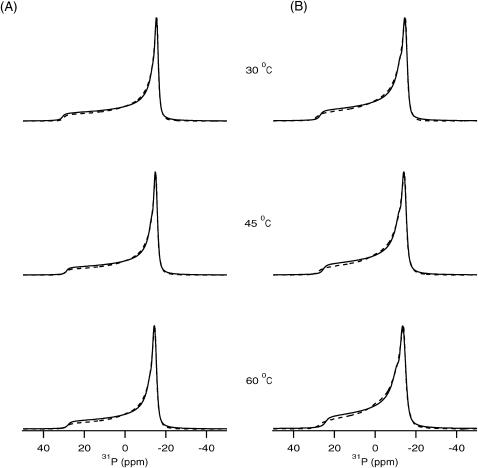
31P NMR spectroscopic measurements were performed to determine the nature of the various lamellar and nonlamellar phase transitions exhibited by the transmembrane segment of PLB incorporated into POPC phospholipid bilayers. The static 31P NMR spectra of the POPC phospholipid bilayer with and without 1 mol % of the hydrophobic segment of PLB are shown in Fig. 1, A and B, respectively. The 31P powder-pattern NMR spectra were recorded at temperatures ranging from 30°C to 60°C. The motionally averaged powder-pattern spectra are characteristic of phospholipid bilayers in the liquid crystalline phase (Lα) and are expected for POPC at a temperature well above its chain melting transition temperature of −3°C (Seelig, 1978). The spectra in the absence and in the presence of 1 mol % PLB showed a similar powder lineshape, both with η = 0 (axial symmetry). The spectra indicate that the lipid bilayers remain in the Lα phase even after addition of 1 mol % phospholamban with respect to POPC, and do not form isotropic or inverse hexagonal phases with high curvatures. In the Lα phase, a POPC bilayer has been determined to have a hydrophobic thickness of ∼27 Å (Harzer and Bechinger, 2000; Nezil and Bloom, 1992). The entire thickness of the POPC phospholipid bilayer is ∼50–54 Å (Huber et al., 2002). If the hydrophobic region of PLB (24–52) is 100% α-helical then it would be ∼43 Å in length (Jones et al., 1994). This indicates that the POPC bilayer is a close match in thickness to the transmembrane segment of PLB and hydrophobic mismatch is not a serious problem under these conditions. The peptide is incorporated into the bilayer without distorting the lipid structure. Hydrophobic mismatch for model peptides KK(LA)15KK (45 Å hydrophobic length) have been studied in detail using 31P and 15N NMR spectroscopy (Harzer and Bechinger, 2000). Those results clearly suggest that the POPC bilayer is a perfect match for hydrophobic peptides with a 36- to 45-Å length. A dioleoyl phosphophatidyl choline (DOPC) bilayer has been used previously for incorporation of AFA-PLB into a mechanically oriented membrane system (Mascioni et al., 2002a). The 31P NMR spectra of POPC samples containing no PLB and with 1 mol % PLB were found to possess chemical shift anisotropy (CSA; in this paper, CSA is σll − σ⊥) widths of 44 ppm and 41 ppm, respectively (Seelig, 1978; Sharpe et al., 2002a,b). A somewhat smaller (∼3 ppm) 31P CSA width is detected for the membrane-bound sample in our study as seen from the lineshape simulations. Similarly, in both cases by increasing the sample temperature, the CSA width decreases, indicating that the molecular motion in the phospholipid bilayer increases with temperature.
FIGURE 1.
31P NMR spectra of POPC phospholipid bilayers investigated as a function of temperature. (A) In the absence of PLB, spectra are shown for pure POPC bilayers. (B) 31P spectra are shown for the POPC bilayer in the presence of 1 mol % PLB with respect to POPC. The solid-line spectra represent the experimental results and the dotted-line spectra represent best-fit simulated spectra corresponding to the experimental spectra.
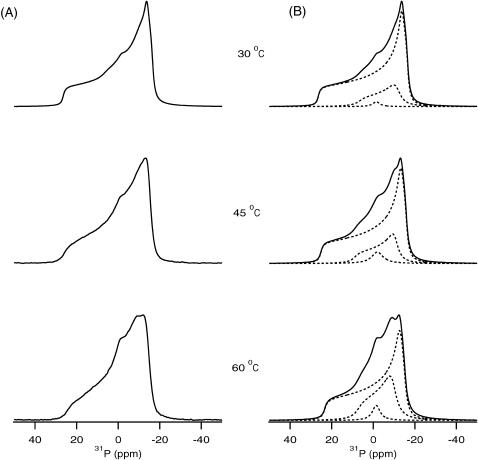
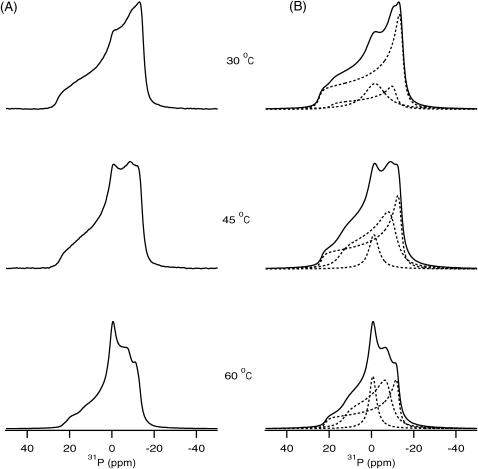
Higher concentrations of PLB were also incorporated into POPC bilayers to probe the peptide-lipid interactions. Fig. 2, A and B, show the experimental and simulated 31P NMR spectra of POPC bilayer samples containing 4 mol % PLB with respect to phospholipid. Similarly, Fig. 3, A and B, represent the experimental and simulated 31P NMR spectra of POPC bilayer samples containing 6 mol % PLB with respect to phospholipid. The results clearly indicate that PLB interacts with the headgroups of the POPC bilayer. This study was performed at temperatures ranging from 30°C to 60°C. The unoriented multilamellar liposome samples containing 0–6 mol % peptide were all found to produce 31P NMR powder-pattern spectra representing POPC in the Lα phase even at higher temperatures (60°C). The membrane remains in the lamellar phase upon binding of PLB even at the highest peptide concentration (6 mol %) studied. The overall CSA spectral width is ∼3 ppm smaller when compared to the pure POPC membrane. This can be attributed to a faster rotation of the lipids when the membrane-associated PLB peptide partially disrupts the hydrogen bonding network between the lipid headgroups. At higher concentrations of PLB, the spectra represent super positions of the different lamellar phases. Previously, the presence of two different anisotropic or lamellar phases was observed in the spectral simulations of 31P NMR spectra of cardiotoxin incorporated into 1,2-dimyristoyl-sn-glycero-3-phosphocholine bilayers (Auger, 1997). Also, Strandberg and co-workers reported the presence of three different lamellar phases upon incorporation of the peptide KK(LA)8KK into DOPC bilayers utilizing 31P NMR spectroscopy (Strandberg et al., 2001). To fully understand the peptide-lipid interactions from 31P NMR, spectral simulations have been carried out as explained in the materials and method section (Figs. 2 B and 3 B). It is important to note here that the 31P chemical shift of phosphodiester, such as is found in membrane lipids, depends upon the molecular motions and orientation of the group with respect to the magnetic field of the spectrometer (Smith and Ekiel, 1984). The orientation of the headgroup depends upon the chemistry, hydration, hydrogen bonding, and charge interactions of each phospholipid headgroup. 31P NMR spectra are very sensitive to the rate of motions of lipids in the liquid crystalline phase, which usually have motional rates in the fast-limit region. Burnell and co-workers were able to simulate experimental spectra of DOPC vesicles of different sizes at various temperatures and viscosities of the medium (Burnell et al., 1980). The general results from these studies indicate that the spectral lineshape and CSA width are dependent upon the size of the vesicles. The CSA spectral width decreases with decreasing vesicle size, and isotropic lines are observed for very small vesicles. The formation of micelles or small vesicles by fragmentation of the bilayers and the observation of different phases has been observed previously on other membrane proteins utilizing 31P NMR spectra obtained from the phospholipids of mechanically oriented bilayers (Hori et al., 1999; Hallock et al., 2003; Henzler Wildman et al., 2003). The structural results gleaned from both aligned and unoriented solid-state NMR samples are important because the morphological membrane structure is different for the two methods.
FIGURE 2.
31P NMR spectra of POPC phospholipid bilayers in the presence of 4 mol % PLB with respect to POPC investigated as a function of temperature. (A) Experimental spectra. (B) The dotted-line spectra represent the simulated bilayer species having different vesicle sizes possessing different CSA widths. The solid-line spectra are the sum of the dotted-line spectra and represent the best-fit simulated spectra corresponding to the experimental spectra.
FIGURE 3.
31P NMR spectra of POPC phospholipid bilayers in the presence of 6 mol % PLB with respect to the phospholipid POPC bilayers investigated as a function of temperature. (A) Experimental spectra. (B) The dotted-line spectra represent the simulated bilayer species having different vesicle sizes possessing different CSA widths. The solid-line spectra are the sum of the dotted-line spectra and represent the best-fit simulated spectra corresponding to the experimental spectra.
The 31P NMR in Figs. 2 A and 3 A at higher concentrations of PLB clearly indicates that the presence of an isotropic peak and a shallow high-field shoulder (near to σ⊥). The spectral simulations reveal the presence of different species of POPC bilayers (Figs. 2 B and 3 B). The interpretation of the 31P NMR spectra with the isotropic components is not certain (Pinheiro et al., 1997). In some studies, they have been attributed to vesicular or micellar structures on or within the bilayers, possibly associated with different phases (Cullis and de Kruijff, 1979; de Kruijff and Cullis, 1980). Others have attributed them to the presence of smaller diameter vesicles induced upon protein binding (Pinheiro and Watts, 1994a,b). Alternatively, the lineshapes may arise from a different 31P species, which undergoes molecular motion that yields isotropic chemical shift transitions. Despite the nonbilayer spectral component being broader from lipid-peptide complexes when compared to peptide-free bilayers, there is no indication of a well-defined hexagonal HII phase. The spectral simulation of 31P NMR lineshape clearly indicates the presence of two different lamellar phases and one isotropic phase. The presence of two lamellar phases having different CSA width values also indicates the presence of multilamellar vesicles (MLVs) of different sizes forming different microdomains. The presence of an isotropic component indicates the existence of very small size vesicles like micelles. Interestingly, spectral simulations suggest the presence of different species at higher PLB concentrations, when compared to 1 mol % PLB in which case only one species is present (Fig. 1). The 31P NMR spectral lineshapes clearly indicate (Figs. 1–3) that the phospholipid bilayers are disrupted by increasing the concentration of PLB. The presence of one species having a similar large CSA width (41 ppm) was observed for the control and 1 mol % PLB sample and suggests the presence of large size MLVs. The smaller CSA width obtained for the other lamellar phases when 4% and 6 mol % PLB was added to the POPC bilayer suggests the presence of smaller size vesicles (Burnell et al., 1980). The large size vesicles can be fragmented to form microdomains composed of small size vesicles. The diameter of the vesicles can be calculated from the CSA width for different MLVs species (Burnell et al., 1980). At 1 mol % PLB with respect to POPC, larger MLVs with an approximate diameter of 25,000 Å are formed. When 4 mol % PLB was embedded into the bilayers two different size vesicles were formed with approximate diameters of 20,000 and 1500 Å. Additionally, the isotropic components suggest the presence of very small vesicles having diameters <1000 Å. Analysis of the 6 mol % PLB/POPC data indicates that large size vesicles fragmented and formed the components possessing vesicles with a diameter of ∼10,000 and ∼2500 Å, and one isotropic component with a diameter <1000 Å. The contributions of each component were calculated by integrating the area of the individual species and comparing it with the entire CSA width of the experimental spectrum. These results indicate that when 4 mol % PLB was incorporated into POPC, contributions from component I (large CSA width), component II (small CSA width), and component III (isotropic species) are ∼82%, ∼17%, and ∼1%, respectively, at 30°C. In addition to that, contributions from component I decreased from 82% to 67% and contributions from component II and component III increased from 17% to 26% and 1% to 7%, respectively, when the temperature was increased from 30°C to 60°C. Interestingly, at 30°C the sample containing 6 mol % PLB/POPC was found to have contributions from component I, component II, and component III of ∼67%, ∼25%, and ∼8%, respectively. Contributions from component I decreased from 67% to 39% and increased for component II from 25% to 44% and for component III from 8% to 17% by increasing the temperature from 30°C to 60°C. A comparison of Figs. 2 and 3 indicates that the contribution from the anisotropic phases having smaller CSA widths increases as more PLB is incorporated into the phospholipid bilayers. One can see clearly from Figs. 2 and 3 that upon increasing the temperature the contributions from the different species change. At higher temperatures, molecular motions increase and small vesicles rotate faster. Also, lateral diffusion influences the 31P NMR lineshape of phospholipid bilayer (Burnell et al., 1980; Cullis and de Kruijff, 1979; Smith and Ekiel, 1984). Another possibility is that by increasing the amount of PLB incorporated into the POPC bilayer, the lateral diffusion rate of the lipids at higher temperature increases, resulting in a decrease in the CSA width of the 31P NMR spectra.
2H NMR study of PLB incorporated into POPC bilayer
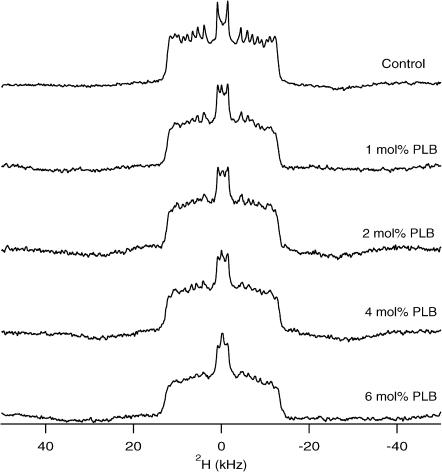
The effect of PLB on the order and dynamics of the acyl chains of the POPC bilayer have been studied using POPC-d31 (deuterated palmitoyl acyl chain). The 2H NMR spectra of a dispersion of POPC-d31 in the absence and in the presence of PLB at 35°C are shown in Fig. 4. Several conclusions can be immediately drawn from the 2H NMR lineshapes in Fig. 4. First, the spectra are characteristic of axially symmetric motions of the phospholipids about the bilayer normal and the spectra consist mostly of overlapping doublet resonances that result from the different CD2 segments of the acyl chain. The central doublet corresponds to the terminal CD3 group. Secondly, the spectral width is a measure of the fluidity of the lipid bilayer. The range observed in the POPC/POPC-d31 is typical for acyl chains in a liquid-crystalline bilayer (Lafleur et al., 1989; Seelig and Seelig, 1974; Seelig and Niederberger, 1974). In Fig. 4, the spectral width of POPC-d31 marginally decreases by increasing the PLB concentrations when compared to the control spectrum of pure POPC/POPC-d31. The marginal decrease in the spectral width suggests that the presence of the PLB peptide disorders the acyl chains to some extent for all the different PLB/POPC concentrations. It also reveals that the POPC bilayer is still in the liquid-crystalline Lα phase. This supports our 31P NMR results as discussed earlier. The spectral resolution deteriorates as the concentration of PLB increases, manifested by the disappearance of the sharp edges of the peaks. This suggests intermediate-timescale motions of the lipids induced by the PLB peptide. The changes in the spectral resolution of the 2H NMR spectra confirm that PLB interacts with the acyl chains of the lipid bilayers. Interesting features of the 2H NMR spectra are the appearance of an isotropic peak in the presence of PLB the intensity of which increases with the amount of PLB. This indicates that the large vesicles are fragmented into smaller size vesicles by increasing the amount of PLB in the POPC bilayers. These results agree with our 31P NMR data.
FIGURE 4.
2H NMR powder spectra of PLB at various concentrations incorporated into POPC/POPC-d31 phospholipid bilayers at 35°C. 5.55 mol % POPC-d31 was doped into the POPC sample. The concentration of PLB with respect to POPC is noted on the right side of each spectrum.
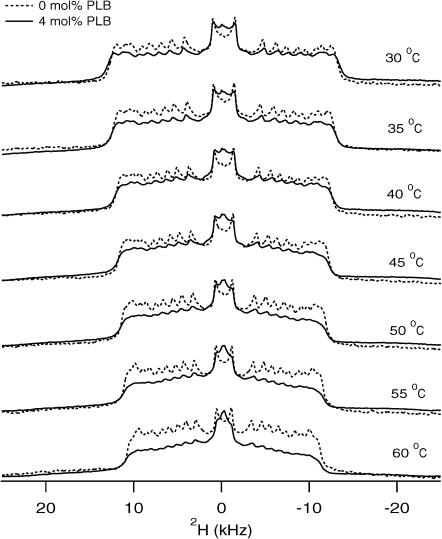
Fig. 5 shows the 2H NMR spectra of POPC-d31 samples in the presence (4 mol %, dotted line) and in the absence (pure POPC/POPC-d31 bilayers, solid line) of PLB obtained over a temperature ranging from 30°C to 60°C. The shape of the 2H NMR spectra of the control POPC/POPC-d31 is a typical 2H phospholipid bilayer lineshape (Lα phase), and looks very similar to those previously reported (Huber et al., 2002; Lafleur et al., 1989; Nezil and Bloom, 1992). As the temperature is raised, the spectra retain their overall lineshape in both cases with and without PLB. The spectral features become narrower due to increased mobility by raising the temperature. The 2H NMR spectra with and without 4 mol % PLB did not undergo any substantial changes in the spectral breadth or quadrupolar splitting. Interestingly, the isotropic component observed by the addition of 4 mol % PLB indicates the formation of small vesicles due to the fragmentation of large vesicles throughout the temperature range from 30°C to 60°C. The intensity of the isotropic peak increases as the temperature increases, indicating an increase in the rapid tumbling of small vesicles and/or an increase in lateral diffusion of the phospholipids (Davis, 1979).
FIGURE 5.
Temperature-dependent 2H NMR spectra of PLB incorporated into POPC/POPC-d31 phospholipid bilayers. The solid-line spectra represent POPC/POPC-d31 sample prepared in the absence of PLB, whereas the dotted-line spectra represent samples prepared with 4 mol % PLB with respect to POPC. The temperature at which each spectrum was taken is noted on the right side of that spectrum.
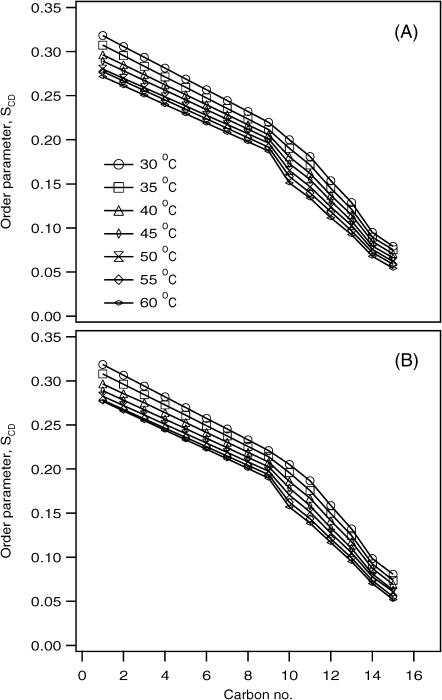
The smoothed segmental C-D bond order parameters (SCD) were calculated by dePaking the powder spectra represented in Fig. 5 for the POPC-d31 as a function of temperature (Fig. 6). The SCD order parameters depend upon several averaging modes provided by intramolecular, intermolecular, and collective motions (Sanders and Schwonek, 1992; Seelig and Seelig, 1974; Seelig and Niederberger, 1974). The segmental SCD order parameter describes local orientation or dynamic perturbations of the C-D bond vector from its standard state due to perturbations of the POPC phospholipid conformations or dynamics as a result of the addition of PLB. The magnitude of the order parameters (SCD ∼0.20–0.30) indicates that the phospholipid bilayers are in the liquid-crystalline phase (Huster et al., 1998; Lafleur et al., 1989). A characteristic profile of decreasing order parameters with increasing distance from the glycerol backbone was obtained both for the pure bilayer and for the PLB-bound bilayer. These values are comparable with the results previously determined using labeled POPC by NMR, and also recently calculated by using molecular dynamic simulations (Huber et al., 2002; Seelig and Seelig, 1974). The data indicates that there is more disorder and motion in the center and at the end of the acyl chain when compared to the headgroup region in the presence of PLB. Interestingly, the order parameter profile obtained from the sample in the presence of PLB (4 mol %) closely resembles the order parameter profile of pure POPC bilayers. This indicates that the POPC bilayer acyl chains are not significantly perturbed by the addition of PLB at these concentrations. Also, the order parameter profiles indicate (for both cases) that by increasing the temperature, the SCD values decrease. This is due to a combination of the increase in the mobility of the acyl chains, rapid tumbling of vesicles, and possible increases in lateral diffusion of the POPC phospholipids.
FIGURE 6.
Temperature-dependent smoothed acyl chain (POPC-d31) orientational order SCD profiles calculated from the dePaked spectra of Fig. 5. (A) Pure POPC bilayer. (B) 4 mol % PLB embedded into the POPC bilayer.
CONCLUSIONS
In the present study, the 31P NMR spectra indicated that the hydrophobic segment of phospholamban was incorporated successfully into the fully hydrated dispersed POPC phospholipid bilayers. The spectral simulations of 31P NMR spectra of samples containing higher concentrations of PLB indicate the presence of different sizes of vesicles. The large phospholipid bilayer vesicles fragmented into smaller vesicles by increasing the concentration of PLB with respect to POPC. Interestingly, the contribution from smaller vesicles increased as the temperature increased. The data indicate that small vesicles are tumbling faster at higher temperatures. Another possibility is that by increasing the amount of PLB incorporated into the POPC bilayer, the lateral diffusion rate of the lipids increases at higher temperatures. In addition to the 31P NMR studies, 2H NMR spectroscopic studies were carried out using POPC-d31 to understand the lipid-peptide interactions in the hydrophobic region of the phospholipid bilayers. The data suggest that smaller size vesicles are formed by the addition of 4 mol % PLB into the bilayers and support our 31P NMR spectral study. The segmental SCD order parameter indicates that there are no significant changes in the ordering of the acyl chains of the phospholipid bilayers. It suggests that the incorporation of PLB into the POPC bilayers did not significantly perturb the acyl chains of the phospholipid bilayers at these concentrations. In the present paper, solid-state NMR spectroscopic studies were carried out to better understand the lipid-peptide interactions from the membrane perspective utilizing unoriented POPC lipid bilayers. The unoriented phospholipid bilayer samples used in the study with PLB can be used for a variety of magic angle spinning experiments such as REDOR (rotational-echo double-resonance). These experiments can reveal unique structural information on PLB with respect to the membrane. Future solid-state NMR experiments specifically designed to investigate the spatial position and orientation of the PLB with respect to the membrane utilizing site-specific 2H-labeled and 15N-labeled PLB samples mechanically aligned on glass plates are in progress.
Acknowledgments
This work was supported by an American Heart Association Scientist Development grant (0130396N) and a National Institutes of Health grant (GM60259-01). The 500-MHz wide-bore NMR spectrometer was obtained from a National Science Foundation grant (10116333).
References
- Arora, A., and L. K. Tamm. 2001. Biophysical approaches to membrane protein structure determination. Curr. Opin. Struct. Biol. 11:540–547. [DOI] [PubMed] [Google Scholar]
- Auger, M. 1997. Membrane structure and dynamics as viewed by solid-state NMR spectroscopy. Biophys. Chem. 68:233–241. [DOI] [PubMed] [Google Scholar]
- Barre, P., O. Zschoring, K. Arnold, and D. Huster. 2003. Structural and dynamical changes of the bindin B18 peptide upon binding to lipid membranes. Biochemistry. 42:8377–8386. [DOI] [PubMed] [Google Scholar]
- Bloom, M., E. Evans, and O. G. Mouritsen. 1991. Physical properties of the fluid lipid-bilayer component of cell membrane: a perspective. Q. Rev. Biophys. 24:293–397. [DOI] [PubMed] [Google Scholar]
- Brunner, E., J. Ogle, M. Wenzler, and H. R. Kalbitzer. 2000. Molecular alignment of proteins in bicellar solutions: Quantitative evaluation of effects induced in 2D COSY spectra. Biochem. Biophys. Res. Commun. 272:694–698. [DOI] [PubMed] [Google Scholar]
- Burnell, E. E., P. R. Cullis, and B. De Kruijff. 1980. Effects of tumbling and lateral diffusion on phosphatidylcholine model membrane 31P NMR line shapes. Biochim. Biophys. Acta. 1980:63–69. [DOI] [PubMed] [Google Scholar]
- Cavagnero, S., H. J. Dyson, and P. E. Wright. 1999. Improved low pH bicelle system for orienting macromolecules over a wide temperature range. J. Biomol. NMR. 13:387–391. [DOI] [PubMed] [Google Scholar]
- Cross, T. A. 1997. Solid-state nuclear magnetic resonance characterization of gramicidin channel structure. Methods Enzymol. 289:672–696. [DOI] [PubMed] [Google Scholar]
- Cullis, P. R., and B. de Kruijff. 1979. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim. Biophys. Acta. 559:399–420. [DOI] [PubMed] [Google Scholar]
- Dave, P. C., E. K. Tiburu, N. A. Nusair, and G. A. Lorigan. 2003. Calculating order parameter profiles utilizing magnetically aligned phospholipid bilayers for 2H NMR studies. Solid State Nucl. Magn. Reson. 24:328–339. [DOI] [PubMed] [Google Scholar]
- Davis, J. H. 1979. Deuterium magnetic resonance study of the gel and liquid crystalline phases of dipalmitoyl phosphatidylcholine. Biophys. J. 27:339–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, J. H., K. R. Jeffrey, M. Bloom, and M. I. Valic. 1976. Quadrupolar echo deuteron magnetic resonance spectroscopy in ordered hydrocarbon chains. Chem. Phys. Lett. 42:390–394. [Google Scholar]
- de Kruijff, B., and P. R. Cullis. 1980. Cytochrome-c specifically induces non-bilayer structures in cardiolipin-containing model membranes. Biochim. Biophys. Acta. 902:477–490. [DOI] [PubMed] [Google Scholar]
- Dempsey, C. E., N. J. P. Ryba, and A. Watts. 1986. Evidence from deuterium nuclear magnetic resonance for the temperature dependent reversible self association of erythrocyte band-3 in dimyristoylphosphatidylcholine bilayers. Biochemistry. 25:2180–2187. [DOI] [PubMed] [Google Scholar]
- Dufourc, E. J., E. J. Parish, S. Chitrakorn, and I. C. P. Smith. 1984. Structural and dynamical details of cholesterol-lipid interaction as revealed by deuterium NMR. Biochemistry. 23:6062–6071. [Google Scholar]
- Epand, R. M. 1998. Lipid polymorphism and protein-lipid interactions. Biochim. Biophys. Acta. 1376:353–368. [DOI] [PubMed] [Google Scholar]
- Fujii, J., A. Ueno, K. Kitano, S. Tanaka, M. Kadoma, and M. Tada. 1987. Characterization of structural unit of phospholamban by amino acid sequencing and electropherotic analysis. Biochem. Biophys. Res. Commun. 138:1044–1050. [DOI] [PubMed] [Google Scholar]
- Hallock, K. J., D. K. Lee, and A. Ramamoorthy. 2003. MSI-78, an analogue of the magainin antimicrobial peptides, disrupts lipid structure via positive curvature strain. Biophys. J. 84:3052–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzer, U., and B. Bechinger. 2000. Alignment of lysine-anchored membrane peptides under conditions of hydrophobic mismatch: a CD, 15N and 31P solid-state NMR spectroscopy investigation. Biochemistry. 39:13106–13114. [DOI] [PubMed] [Google Scholar]
- Henzler Wildman, K. A., D. K. Lee, and A. Ramamoorthy. 2003. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry. 42:6545–6558. [DOI] [PubMed] [Google Scholar]
- Hori, Y., M. Demura, T. Niidome, H. Aoyagi, and T. Asakura. 1999. Orientational behavior of phospholipid membranes with mastoparan studied by 31P solid state NMR. FEBS Lett. 455:228–232. [DOI] [PubMed] [Google Scholar]
- Huber, T., K. Rajamoorthi, V. F. Kurze, K. Bayer, and M. F. Brown. 2002. Structure of docosahexaenoic acid-containing phospholipid bilayers as studied by 2H NMR and molecular dynamics simulations. J. Am. Chem. Soc. 124:298–309. [DOI] [PubMed] [Google Scholar]
- Huster, D., K. Arnold, and K. Garwrisch. 1998. Influence of docosahexaenoic acid and cholesterol on lateral lipid organization. Biochemistry. 37:17299–17308. [DOI] [PubMed] [Google Scholar]
- Huster, D., X. Yao, K. Jakes, and M. Hong. 2002. Conformational changes of colicin Ia channel-forming domain upon membrane binding: a solid-state NMR study. Biochim. Biophys. Acta. 1561:159–170. [DOI] [PubMed] [Google Scholar]
- Jones, D. T., W. R. Taylor, and J. M. Thornton. 1994. A model recognition approach to the prediction of all-helical membrane protein structure and topology. Biochemistry. 33:3038–3049. [DOI] [PubMed] [Google Scholar]
- Lafleur, M., B. Fine, E. Sternin, P. R. Cullis, and M. Bloom. 1989. Smoothed orientational order profile of lipid bilayers by 2H-nuclear magnetic resonance. Biophys. J. 56:1037–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon, M. A., and D. M. Engelman. 1994a. Specificity and promiscuity in membrane helix interactions. FEBS Lett. 346:17–20. [DOI] [PubMed] [Google Scholar]
- Lemmon, M. A., and D. M. Engelman. 1994b. Specificity and promiscuity in membrane helix interactions. Q. Rev. Biophys. 27:157–218. [DOI] [PubMed] [Google Scholar]
- Liu, F., R. N. A. H. Lewis, R. S. Hodges, and R. N. McElhaney. 2001. A differential scanning calorimetric and 31P NMR spectroscopic study of the effect of transmembrane a-helical peptides on the lamellar-reversed hexagonal phase transition of phosphatidylethanolamine model membranes. Biochemistry. 40:760–768. [DOI] [PubMed] [Google Scholar]
- Long, J. R., J. L. Dindot, H. Zebroski, S. Kiihne, R. H. Clark, A. A. Campbell, P. S. Stayton, and G. P. Drobny. 1998. A peptide that inhibits hydroxyapatite growth is in an extended conformation on the crystal surface. Proc. Natl. Acad. Sci. USA. 95:12083–12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, J. R., W. J. Shaw, P. S. Stayton, and G. P. Drobny. 2001. Structure and dynamics of hydrated statherin on hydroxyapatite as determined by solid-state NMR. Biochemistry. 40:15451–15455. [DOI] [PubMed] [Google Scholar]
- Marassi, F. M., C. Ma, H. Gratkowski, S. K. Straus, K. Strebel, M. Orblatt-Montal, M. Montal, and S. J. Opella. 1999. Correlation of the structural and functional domains in the membrane protein Vpu from HIV-1. Proc. Natl. Acad. Sci. USA. 96:14336–14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marassi, F. M., and S. J. Opella. 1998. NMR structural studies of membrane proteins. Curr. Opin. Struct. Biol. 8:640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte, I., E. J. Dufourc, M. Ouellet, and M. Auger. 2003. Interaction of the neuropeptide Met-Enkephalin with zwitterionic and negatively charged bicelles as viewed by 31P and 2H solid-state NMR. Biophys. J. 85:328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascioni, A., C. Karim, J. Zamoon, D. D. Thomas, and G. Vegila. 2002a. Solid-state NMR and rigid body molecular dynamics to determine domain orientations of monomeric phospholamban. J. Am. Chem. Soc. 124:9392–9393. [DOI] [PubMed] [Google Scholar]
- Mascioni, A., C. Karim, G. Barany, D. D. Thomas, and G. Vegila. 2002b. Structure and orientation of sacrolipin in lipid environments. Biochemistry. 41:475–482. [DOI] [PubMed] [Google Scholar]
- Massiot, D., F. Fayon, M. Capron, I. King, S. Le Calve, B. Alonso, J. O. Durand, B. Bujoli, Z. Gan, and G. Hoatson. 2002. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 40:70–76. [Google Scholar]
- McCabe, M. A., and S. R. Wassall. 1995. Fast-Fourier-transform dePaking. J. Magn. Reson. B. 106:80–82. [Google Scholar]
- McCabe, M. A., and S. R. Wassall. 1997. Rapid deconvolution of NMR powder spectra by weighted fast Fourier transformation. Solid State Nucl. Magn. Reson. 10:53–61. [DOI] [PubMed] [Google Scholar]
- Morrow, M. R., and C. W. M. Grant. 2000. The EGF-receptor transmembrane domain: peptide-peptide interactions in fluid bilayer membranes. Biophys. J. 79:2024–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa, Y., and T. Asakura. 2003. Structure determination of a peptide model of the repeated helical domain in Samia cynthia ricini silk fibroin before spinning by a combination of advanced solid-state NMR methods. J. Am. Chem. Soc. 125:7230–7237. [DOI] [PubMed] [Google Scholar]
- Nezil, F. A., and M. Bloom. 1992. Combined influence of cholesterol and synthetic amphiphilic peptides upon bilayer thickness in model membranes. Biophys. J. 61:1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, L. K., and T. A. Cross. 1989. Gramicidin cation channel: an experimental determination of the right-handed helix sense and verification of beta-type hydrogen bonding. Biochemistry. 28:9379–9385. [DOI] [PubMed] [Google Scholar]
- Ottiger, M., and A. Bax. 1999. Bicelle-based liquid crystals for NMR-measurement of dipolar couplings at acidic and basic pH values. J. Biomol. NMR. 13:187–191. [DOI] [PubMed] [Google Scholar]
- Pinheiro, T. J. T., M. J. Duer, and A. Watts. 1997. Phospholipid headgroup dynamics in DOPC-d5-cytochrome c complexes as revealed by 2H and 31P NMR: the effects of a peripheral protein on collective lipid fluctuations. Solid State Nucl. Magn. Reson. 8:55–64. [DOI] [PubMed] [Google Scholar]
- Pinheiro, T. J. T., and A. Watts. 1994a. Lipid specificity in the interaction of cytochrome-c with anionic phospholipid-bilayers revealed by solid-state P-31 NMR. Biochemistry. 33:2451–2458. [DOI] [PubMed] [Google Scholar]
- Pinheiro, T. J. T., and A. Watts. 1994b. Resolution of individual lipids in mixed phospholipid membranes and specific lipid-cytochrome c interactions by magic-angle spinning solid-state P-31 NMR. Biochemistry. 33:2459–2467. [DOI] [PubMed] [Google Scholar]
- Rigby, A. C., K. R. Barber, G. S. Shaw, and C. W. M. Grant. 1996. Transmembrane region of the epidermal growth factor receptor: behavior and interactions via 2H NMR. Biochemistry. 35:12591–12601. [DOI] [PubMed] [Google Scholar]
- Sanders, C. R., and J. P. Schwonek. 1992. Characterization of magnetically orientable bilayers in mixtures of dihexanoylphosphatidylcholine and dimyristoylphosphatidylcholine by solid-state NMR. Biochemistry. 31:8898–8905. [DOI] [PubMed] [Google Scholar]
- Seelig, J. 1978. 31P Nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim. Biophys. Acta. 515:105–140. [DOI] [PubMed] [Google Scholar]
- Seelig, J., and W. Niederberger. 1974. Deuterium-labeled lipids as structural probes in liquid crystalline bilayers. A deuterium magnetic resonance. J. Am. Chem. Soc. 96:2069–2072. [Google Scholar]
- Seelig, A., and J. Seelig. 1974. The dynamic structure of fatty acyl chains in a phosphatidylcholine bilayer measured by deuterium magnetic resonance. Biochemistry. 13:4839–4845. [DOI] [PubMed] [Google Scholar]
- Sharpe, S., K. R. Barber, C. W. M. Grant, D. Goodyear, and M. R. Morrow. 2002a. Organization of model helical peptides in lipid bilayers: insight into the behavior of single-span protein transmembrane domains. Biophys. J. 83:345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe, S., K. R. Barber, C. W. M. Grant, and M. R. Morrow. 2002b. Evidence of a tendency to self-association of the transmembrane domain of ErbB-2 in fluid phospholipid bilayers. Biochemistry. 41:2341–2352. [DOI] [PubMed] [Google Scholar]
- Shaw, W. J., J. R. Long, J. L. Dindot, A. A. Campbell, P. S. Stayton, and G. P. Drobny. 2000. Determination of statherin N-terminal peptide conformation on hydroxyapatite crystals. J. Am. Chem. Soc. 122:1709–1716. [Google Scholar]
- Simmerman, H. K. B., J. H. Collins, J. L. Theibert, A. D. Wegner, and L. R. Jones. 1986. Sequence analysis of phospholamban: identification of phosphorylation sites and two major structural domains. J. Biol. Chem. 261:13333–13341. [PubMed] [Google Scholar]
- Simmerman, H. K. B., and L. R. Jones. 1998. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol. Rev. 78:921–947. [DOI] [PubMed] [Google Scholar]
- Simmerman, H. K. B., Y. M. Kobayashi, J. M. Autry, and L. R. Jones. 1996. A leucine zipper stabilizes the pentameric membrane domain of phospholamban and forms a coiled-coil pore structure. J. Biol. Chem. 271:5941–5946. [DOI] [PubMed] [Google Scholar]
- Singer, S. L., and G. L. Nicolson. 1972. The fluid mosaic model of the structure of cell membranes. Science. 175:720–731. [DOI] [PubMed] [Google Scholar]
- Smith, I. C. P., and I. H. Ekiel. 1984. Phosphorus-31 NMR of phospholipids in membranes, Chapter 5. In Phosphorus-31 NMR. D. G. Gorenstein, editor. Academic Press, New York. 447–475.
- Stockson, G. W., C. F. Polnaszek, A. P. Tulloch, F. Hasan, and I. C. P. Hasan. 1976. Molecular motion and order in single bilayer vesicles and multilamellar dispersions of egg lecithin and lecithin-cholesterol mixtures. A deuterium nuclear magnetic resonance study of specifically labeled lipids. Biochemistry. 15:954–966. [DOI] [PubMed] [Google Scholar]
- Stokes, D. L. 1997. Keeping calcium in its place: Ca2+-ATPase and phospholamban. Curr. Opin. Struct. Biol. 7:550–558. [DOI] [PubMed] [Google Scholar]
- Strandberg, E., T. Sparrman, and G. Lindblom. 2001. Phase diagrams of systems with cationic a-helical membrane-spanning model peptides and dioleoylphosphatidylcholine. Adv. Colloid Interface Sci. 89– 90:239–261. [DOI] [PubMed] [Google Scholar]
- Tiburu, E. K., P. C. Dave, J. F. Vanlerberghe, T. B. Cardon, R. E. Minto, and G. A. Lorigan. 2003. An improved synthetic and purification procedure for the hydrophobic segment of the transmembrane peptide phospholamban. Anal. Biochem. 318:146–151. [DOI] [PubMed] [Google Scholar]
- Vold, R. R., R. S. Prosser, and A. J. Deese. 1997. Isotropic solutions of phospholipid bicelles: a new membrane mimetic for high-resolution NMR studies of polypeptides. J. Biomol. NMR. 9:329–335. [DOI] [PubMed] [Google Scholar]
- Watts, A. 1981. Protein-lipid interactions: do the spectroscopists now agree? Nature. 294:512–513. [DOI] [PubMed] [Google Scholar]
- Watts, A. 1993. Magnetic resonance studies of phospholipid-protein interactions in bilayers. In Phospholipids Handbook. G. Cevc, editor. Marcel Dekker, New York. 687–744.
- Watts, A. 1998. Solid-state NMR approaches for studying the interaction of peptides and proteins with membranes. Biochim. Biophys. Acta. 1376:297–318. [DOI] [PubMed] [Google Scholar]
- Yao, Q., L. T. L. Chen, J. Li, K. Brungardt, T. C. Squier, and D. J. Bigelow. 2001. Oligomeric interactions between phospholamban molecules regulate Ca-ATPase activity in functionally reconstituted membranes. Biochemistry. 40:6406–6413. [DOI] [PubMed] [Google Scholar]
- Ying, W., S. E. Irvine, R. A. Beekman, D. J. Siminovitch, and S. O. Smith. 2000. Deuterium NMR reveals helix packing interactions in phospholamban. J. Am. Chem. Soc. 122:11125–11128. [Google Scholar]