Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) protein is an epithelial receptor mediating the translocation of Salmonella enterica serovar Typhi to the gastric submucosa. Since the level of cell surface CFTR is directly related to the efficiency of serovar Typhi translocation, the goal of this study was to measure CFTR expression by the intestinal epithelium during infection. CFTR protein initially present in the epithelial cell cytoplasm was rapidly trafficked to the plasma membrane following exposure to live serovar Typhi or bacterial extracts. CFTR-dependent bacterial uptake by epithelial cells increased (>100-fold) following CFTR redistribution. The bacterial factor which triggers CFTR redistribution is heat and protease sensitive. These data suggest that serovar Typhi induces intestinal epithelial cells to increase membrane CFTR levels, leading to enhanced bacterial ingestion and submucosal translocation. This could be a key, early step in the infectious process leading to typhoid fever.
Salmonella enterica serovar Typhi causes typhoid or enteric fever and initiates infection by entering the enterocytes and specialized M cells of the small intestine, after which the bacterium is translocated to the intestinal submucosa and ingested by host macrophages. The mechanism by which serovar Typhi enters enterocytes is complex and involves intercellular adhesins (5) and intracellular signaling components (18) which modify the host cell cytoskeletal (6) and vacuolar (7) organization. This process is initiated when adhesins and signaling components are delivered into the host cell by the bacterium via the bacterial type III secretion apparatus (4). Since assembly of the type III secretion apparatus is stimulated by bacterium-epithelial cell contact (9), early interactions between serovar Typhi and the epithelial cell play an important role in the initiation of infection.
We have reported that the cystic fibrosis transmembrane conductance regulator (CFTR), an epithelial chloride channel, is a receptor for serovar Typhi (21). Lipopolysaccharide (LPS), the bacterial ligand for CFTR, colocalizes with the CFTR protein on the surface of epithelial cells (J. B. Lyczak, T. S. Zaidi, M. Grout, W. M. Bittner, I. Contreras, and G. B. Pier, Abstr. 101st Gen. Meet. Am. Soc. Microbiol. 2001, abstr. B-440, p. 139, 2001) and competitive inhibitors specific for CFTR (21) or LPS (15) significantly reduce bacterial entry into epithelial cells, as do mutations which affect the synthesis of CFTR or LPS. However, apical membrane expression of CFTR is generally low, in both the enterocytes of the gastrointestinal (GI) tract and the epithelial cells of the lungs, where CFTR serves as a receptor for Pseudomonas aeruginosa (22). CFTR is stored principally in the cytoplasm in subapical membrane vacuoles (28). Thus, apical membrane levels of CFTR must increase for efficient interaction with serovar Typhi. To evaluate the modulation of CFTR expression in enterocytes, we determined the effects of serovar Typhi cells and cell extracts on cell surface CFTR expression by using both intact mouse small intestine and cultured human epithelial cell lines. While serovar Typhi is a human pathogen, others (19) have shown that this microorganism demonstrates considerably less host specificity at the early stages of infection, making the mouse small intestine a useful model system. We report here that increased cell surface localization of the CFTR protein is induced on intestinal epithelium during serovar Typhi infection and that increased expression can also be induced by a water-soluble extract of whole bacterial cells. The active factor in the extract is heat and protease sensitive. Importantly, increased membrane localization of CFTR is correlated with an increase in invasion of epithelial cells by serovar Typhi but not by serovar Typhimurium, a related microorganism that invades epithelial cells in a CFTR-independent manner.
MATERIALS AND METHODS
Monoclonal antibodies.
The CFTR-specific monoclonal antibody CF3 (mouse immunoglobulin M [IgM]) was used for indirect immunofluorescent staining of CFTR protein (27). CF3 recognizes amino acids 103 to 117 in the first predicted extracellular loop of CFTR, binds to both human and mouse CFTR, and reacts with epithelial tissue taken from animals bearing wild-type CFTR genes but not with the epithelia of animals bearing mutations which eradicate CFTR protein production. CF3 reacts only with cell surface CFTR protein on intact epithelial cells; the cells must be permeabilized in order for CF3 to detect intracellular CFTR protein. Additionally, the CF3 antibody has never been found to bind directly to serovar Typhi. CF3 was purified from the cell culture supernatant of an Sp2/0 hybridoma by using an IgM-specific affinity column. Goat anti-mouse IgM antibody (mu chain specific; Sigma Chemical Co., St. Louis, Mo.) was covalently coupled to CNBr-activated Sepharose-4B (Pharmacia, Piscataway, N.J.) as instructed by the manufacturer. Hybridoma supernatants were loaded onto this resin by gravity flow, and the resin was washed extensively with binding buffer (0.45 M NaCl, 8.6 mM NaH2PO4, 11.4 mM Na2HPO4). IgM was then eluted from the column by alternating washes in pH 2.0 (0.1 M glycine, 0.4 M NaCl) and pH 8.0 (0.1 M Tris, 0.4 M NaCl) buffers. All eluants were collected as 1-ml fractions into tubes containing 100 μl of 1 M Tris (pH 8.0). The isotype- and species-matched irrelevant, negative control monoclonal antibody VF8 (specific for a capsular polysaccharide of Staphylococcus epidermidis) was obtained from mouse ascites fluid. VF8 does not bind to serovar Typhi cells or to human or mouse epithelial cells.
Salmonella-specific rabbit polyclonal antibody was purchased from Maine Biotechnology (Portland, Maine). This reagent is directed largely but not exclusively against the H and O antigens of Salmonella species.
Mice.
Female C57BL/6 mice, 5 to 7 weeks old (Harlan, Indianapolis, Ind.), were housed in microisolator cages and fed normal laboratory rodent chow and water. At 7 days prior to an experiment, the mice were switched to a liquid elemental diet (Peptamin; Nestle, Inc., Glendale, Calif.) containing 1 mg of gentamicin sulfate and 1 mg of streptomycin per ml. Gentamicin sulfate and streptomycin were also added to drinking water (at 1 mg/ml). At 2 days before an experiment, the mice were switched to liquid elemental diet and water, both lacking antibiotics. Mice were sacrificed by chloroform overdose, and 1-cm-long ligated loops were tied with a sterile suture by the method of Kohbata et al. (12). All animals were treated in accordance with the guidelines of the Harvard Medical Area Standing Committee on Animals.
Cell lines.
The human colon carcinoma cell line T84 was cultured at 37°C under 5% CO2 in a medium that was 45% (vol/vol) Ham's F-12 (Gibco, Grand Island, N.Y.), 45% Dulbecco modified Eagle medium (Gibco), and 10% fetal calf serum (Intergen, Purchase, N.Y.). CFT1-LCFSN is a stable transfectant of the CFT1 human airway epithelial cell line derived from a cystic fibrosis patient homozygous for the deletion of phenylalanine at position 508 (ΔF508 Cftr allele), resulting in inefficient trafficking and consequent low surface expression of the CFTR protein. LCFSN is complemented by stable transfection with a third, wild-type copy of the Cftr gene (17). LCFSN was cultured at 37°C under 5% CO2, in Ham's F-12 medium containing 10 μg of insulin/ml, 1 μM hydrocortisone, 3.75 μg of endothelial cell growth supplement/ml, 25 ng of epidermal growth factor/ml, 30 nM triiodo-l-thyronine, 5 μg of transferrin/ml, 10 ng of cholera toxin/ml, 150 μg of neomycin B/ml, and 100 U of penicillin G/ml. Since neomycin has been observed to affect CFTR expression, neomycin was always removed from the culture medium at least 3 days prior to an experiment. We have ensured that removal of neomycin for this period results in no detectable loss of the transfected copy of the Cftr gene.
The MDCK(GFP-CFTR) cell line (16) containing the cDNA for wild-type human CFTR linked to amino-terminal expression of green fluorescent protein (GFP) was used for confocal microscopic examination of the subcellular distribution of CFTR protein. MDCK(GFP-CFTR) cells were cultured in minimal essential medium with Earle's salts (Gibco) containing 10% fetal calf serum (Intergen), 150 μg of neomycin B/ml, and 100 U of penicillin G/ml.
Bacterial strains.
Ty2 is a wild-type, invasive strain of serovar Typhi. Serovar Typhi strains H245 and H193 were a kind gift of David Hone. H245 is equivalent to strain Ty2 and was tested to verify the results obtained with our isolate of Ty2. Strain H193 (also known as Quailes) is a clinical isolate of serovar Typhi that behaves as a wild-type invasive strain. Strain LT2 is a wild-type, invasive strain of serovar Typhimurium.
Cellular ingestion.
For ingestion experiments, Salmonella strains were grown overnight at 37°C in 8-ml portions of Luria-Bertani broth, with agitation. The following morning, 100 μl of the overnight culture was used to inoculate a fresh 8-ml portion of Luria-Bertani broth, and the second culture was grown at 37°C without agitation until an optical density at 650 nm of 0.35 to 0.40 was obtained (typically 3 to 4 h). The bacterial cells were immediately pelleted by centrifugation for 20 min at 1,000 × g, and the pellet was resuspended in F12 medium containing 10% fetal calf serum. The bacterial suspension was then adjusted to an appropriate concentration, and a portion of the suspension was diluted and plated on tryptic soy agar to ascertain the true concentration of bacteria in the suspension.
Preparation of bacterial extracts.
Bacterial extracts were prepared using a modification of an extraction protocol described previously (2). A 500-ml overnight broth culture of serovar Typhi (Ty2) in Luria-Bertani broth was carried out at 37°C, using minimal agitation (100 rpm in an orbital shaker-incubator). The bacteria were pelleted by centrifugation at 5,000 × g for 20 min, resuspended in 50 ml of distilled water, and vigorously shaken at 37°C for 2 h (300 rpm in an orbital shaker-incubator). The bacteria were again pelleted at 15,000 × g for 20 min, and the pellet was discarded. The supernatant was then cleared by one more centrifugation step at 15,000 × g for 20 min and two consecutive centrifugations at 100,000 × g for 1 h each. The final, cleared supernatant was lyophilized and stored dry at 4°C until use. Portions of the extract were cultured on tryptic soy agar to verify that no viable bacteria remained in the extract.
Destruction of LPS oligosaccharides in bacterial extracts.
Bacterial extracts were dissolved in water to 1 mg/ml, and KIO4 was added to 0.2 M. The mixture was then incubated at 37°C for 1 h and neutralized by the addition of 20 μl of polyethylene glycol per ml of reaction mixture. Sodium borohydride was then added to a concentration of 0.2 M, and the mixture was allowed to incubate overnight at 4°C to neutralize reactive aldehydes formed by periodate treatment. Lastly, the extract preparations were dialyzed with a 10,000-kDa-cutoff membrane (Pierce, Rockford, Ill.).
Destruction of protein in bacterial extracts.
Portions (1 mg) of bacterial extracts were dissolved in 1 ml of water and incubated for 1 h at 60°C with proteinase K conjugated to agar beads (containing approximately 5 U of proteinase K [Sigma]), after which the sample was centrifuged (10,000 × g for 10 min) to remove the proteinase-bead conjugate. The supernatant was again centrifuged and then transferred to a fresh vessel, avoiding the bottom 1/10 of the volume. Alternatively, protein in the bacterial extract was inactivated by heating the extract (dissolved in water to 1 mg/ml) for 20 min at 70°C.
Ligated intestinal-loop model of serovar Typhi intestinal translocation to the submucosa.
The ligated intestinal-loop model was used essentially as described previously (12, 21). In some cases, intestinal loops were initially treated by injecting bacterial extract (0.2 μg [dry weight]) into the lumen, incubating the loops at 37°C for 45 min, and then injecting the inoculum of live bacteria. The number of bacteria translocating to the intestinal submucosa under each set of conditions was compared by analysis of variance (ANOVA) and Fisher probable least significant differences (PLSD).
Modulation of CFTR protein expression by serovar Typhi infection of intestinal loops.
Ligated intestinal loops were injected with approximately 106 CFU of serovar Typhi in a volume of 50 μl and then incubated at 37°C under 5% CO2 for periods ranging from 5 min to 2 h. At the end of this incubation, the intestinal loops were cut open lengthwise and gently washed with phosphate-buffered saline (PBS) containing 1% bovine serum albumin. If the intestinal loops were to be used for fluorescent histological determination of CFTR expression, they were fixed by overnight incubation in 10% formalin at 4°C followed by embedding in paraffin. If the loops were to be used for flow cytometric determination of CFTR expression, the enterocytes were freed from the intestinal mucosa by gentle lavage and then stained and analyzed as described below.
Bacterial-extract treatment of epithelial cells to enhance CFTR surface expression.
Subconfluent cultures of the LCFSN and T84 cell lines were incubated with crude bacterial extract for 30 to 60 min at 37°C under 5% CO2. Each well was treated with 0.01 to 10 μg (dry weight) of bacterial extract. After incubation, cell surface CFTR expression by the cells was determined by fluorescence immunohistology.
Semiquantitative reverse transcription-PCR analysis of CFTR gene expression by mouse intestine.
Ligated intestinal loops were injected with approximately 106 CFU of serovar Typhi in a volume of 50 μl and then incubated at 37°C under 5% CO2 for periods ranging from 15 min to 2 h. Control intestinal loops (mock infected) were injected with an equal volume of sterile diluent. At the end of this incubation, total RNA was prepared using the RNEasy kit (Qiagen, Valencia, Calif.), and 3 μg of RNA was reverse transcribed using the components of the Superscript II preamplification kit (Gibco) and following the manufacturer's alternate protocol for cDNA synthesis from transcripts with a high G+C content. Each experiment included a duplicate reaction in which reverse transcriptase was omitted, to verify that the PCR products observed were not amplified from DNA contaminants in the RNA preparations.
Semiquantitative PCR was performed using cDNA products as substrate. The cDNA concentration was compared between samples by making several dilutions of each cDNA and then amplifying each dilution using a constant number of PCR cycles. Typically, the cDNA was diluted 1:100 and 1:1,000 and parallel PCR amplifications were carried out using 10, 5, and 2 μl of each dilution of the cDNA. A touchdown PCR protocol was used. The first 30 cycles were as follows: 94°C for 1 min, annealing temperature for 1 min (see below), and 72°C for 1 min. This was followed by an additional 30 cycles under the following conditions: 94°C for 1 min, 45°C for 1 min, and 72°C for 1 min. The reactions were completed with a 10-min incubation at 72°C. During the first 30 cycles, the annealing temperature started at 60°C and was decreased by 0.5°C per cycle over 30 cycles to a final annealing temperature of 45.5°C.
PCR amplifications were carried out in a 50-μl volume in a buffer containing 200 mM Tris-HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 50 μM each deoxynucleoside triphosphate, 1.25 U of Taq polymerase (Boehringer-Mannheim/Roche Applied Science, Indianapolis, Ind.), and 30 pmol of each of two gene-specific primers. The following murine CFTR-specific primers were obtained from Operon Technologies, Inc. (Alameda, Calif.): CFTRFOROUT (5′-ATTACTGGAGAAAGTACAACAAAG-3′), complementary to exon 9, and CFTRREVOUT (5′-AATACTTGTTCTTCAGTAAAAAC-3′), complementary to exon 12. These primers successfully amplified a product corresponding to the expected size of 540 bp.
A 20-μl volume of each 50-μl reaction mixture was electrophoresed in 1.8% agarose, stained in 0.5 μg of ethidium bromide per ml for 2 to 3 h, and photographed. The photograph was scanned at 150 dots per in. using VistaScan v.1.2.2 (UMAX Data Systems), and the density of each PCR product was determined using NIH Image software v.1.61. The density of each product was plotted against the dilution factor of the cDNA in the reaction mixture. Linear-regression analysis was used to determine the cDNA dilution corresponding to a PCR product density of zero (the cDNA “titer”).
Fluorescent immunohistological analysis of CFTR expression on intestinal epithelium.
Sections (5 μm) of intestinal tissue were deparaffinized in xylene and rehydrated in a reverse ethanol series. The samples were then blocked against nonspecific antibody binding by incubation in histology blocking buffer (PBS containing 1% bovine serum albumin and 2% normal goat serum [Sigma]) for 30 to 60 min at 4°C and then stained with the CFTR IgM monoclonal antibody CF3 and in some cases with Salmonella-specific rabbit polyclonal antibody, as described previously (30). The samples were then washed and incubated for 1 h on ice with fluorescein isothiocyanate-conjugated goat anti-mouse IgM (Sigma) or with phycoerythrin-conjugated goat anti-rabbit IgG (Sigma), diluted in histology blocking buffer. Since these sample were sectioned at a thickness of 5 μm and fixed, dehydrated, and rehydrated, the samples are effectively permeabilized, allowing staining of total (membrane and intracellular) CFTR protein.
Flow-cytometric analysis of CFTR expression in ligated intestinal loops.
Enterocyte suspensions were centrifuged at 200 × g for 20 s to pellet debris, and the supernatants (containing the enterocytes) were transferred to fresh tubes and incubated in histology blocking buffer on ice for 30 to 60 min to minimize nonspecific antibody binding. Blocked samples were incubated for 1 h on ice with CFTR-specific IgM monoclonal antibody CF3 diluted to 10 μg/ml in histology blocking buffer. Since the enterocytes were intact during the staining procedure, only cell surface CFTR protein would be detected by the CF3 antibody in this experiment. The samples were then washed and incubated for 1 h on ice with fluorescein isothiocyanate-conjugated goat anti-mouse IgM (Sigma) diluted in histology blocking buffer. Finally, the samples were washed and fixed in PBS containing 1% paraformaldehyde. Control samples were treated identically, except that the irrelevant, isotype-matched monoclonal antibody VF8 (also used at 10 μg/ml) was substituted for CF3. Samples were read on a FACScalibur flow cytometer (Becton Dickinson, Franklin Lakes, N.J.) and analyzed using the CELLQuest software package, v.3.1 (Becton Dickinson). Selected events (gated using forward and side scatter to disregard noncellular debris) were collected and analyzed statistically (using Kolmogorov-Smirnov statistics) for changes in the total level of CFTR detected.
Fluorescent immunohistological analysis of CFTR expression by epithelial cell lines.
Immediately following infection or bacterial extract treatment, epithelial cell monolayers were washed twice in ice-cold PBS and put on ice for the remainder of the staining procedure. For experiments aimed at measuring only cell surface CFTR protein, fluorescent immunostaining of CFTR was carried out on the live, intact cells using the same conditions described for immunostaining intestinal loops. The concentration of CFTR-specific monoclonal antibody CF3 was 10 μg/ml. This concentration of CF3 antibody was selected since it yields little or no signal in uninfected epithelial cells but a readily detectable signal in infected epithelial cells.
For detection of total cellular (intra- and extracellular) CFTR protein, infected and/or extract-treated cells were washed with PBS, incubated for 15 min on ice with PBS containing 1% paraformaldehyde, and then incubated for 15 min at 37°C in PBS containing 0.2% Tween 20. Fluorescent staining of CFTR protein was then carried out.
For inhibition of CFTR trafficking by Clostridium botulinum type A neutrotoxin (BoNT-A), the light chain of BoNT-A (List Biologicals, Vandell, Wash.) was activated by a 30-min incubation at 37°C with 5 mM dithiothreitol. Epithelial cells were incubated for 30 min at 37°C with 67 nM activated BoNT-A, 20 U of streptolysin O (Sigma), and 5 mM dithiothreitol. The cells were then infected with serovar Typhi as described above and stained for surface expression of CFTR protein.
Confocal microscopic evaluation of the subcellular distribution of GFP-CFTR fusion protein.
MDCK(GFP-CFTR) cells were seeded into glass-bottom culture dishes (MatTek, Ashland, Mass.). Cells at 50 to 70% confluence were incubated for 45 min at 37°C with 1 μg of bacterial extract/ml, washed with ice-cold PBS, and kept on ice until used for microscopy. The cells were examined under an Axiovert S100 microscope (Carl Zeiss, Inc., Thornwood, N.Y.) with a Bio-Rad (Hercules, Calif.) MRC 1024 krypton-argon laser. GFP-CFTR was scored as being mobilized to the plasma membrane if GFP fluorescence was concentrated at the periphery of the cell in all Z-sections observed and was verified by a second operator who was blinded to the identity of the samples.
RESULTS
Serovar Typhi infection of mouse small intestine leads to a rapid increase in cell surface CFTR expression.
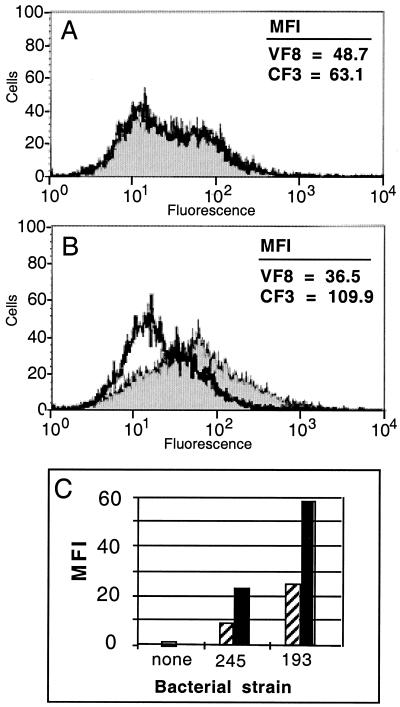
Previous work (21) had demonstrated a direct relationship between the expression or availability of CFTR protein on the epithelial surface and the efficiency with which serovar Typhi is translocated to the mouse intestinal submucosa, suggesting that changes in the expression or availability of CFTR during infection may influence the course of the early stages of infection. Therefore, we examined the level of CFTR protein on the plasma membrane of mouse intestinal enterocytes during serovar Typhi infection by using a mouse ligated intestinal-loop model. As shown in Fig. 1, infection of intestinal loops from wild-type C57BL/6 mice leads to an increase in cell surface CFTR protein expression by the enterocytes. Ligated intestinal loops from C57BL/6 mice were injected with wild-type serovar Typhi (strain Ty2) and incubated for 1 h. The enterocytes were then freed from the tissue by gentle lavage, stained with CFTR-specific (CF3) or negative control (VF8) antibodies, and analyzed by flow cytometry. The enterocytes were intact during the staining procedure, so that only cell surface CFTR is detected. The increased cell surface CFTR expression was extremely rapid, detectable by 15 min postinfection, and was verified using two other isolates of wild-type serovar Typhi (Fig. 1C). The experiment in Fig. 1C was conducted independently of that in Fig. 1A and B. Therefore, the absolute intensities of fluorescence in Fig. 1C are not directly comparable to those shown in Fig. 1A and B.
FIG. 1.
Measurement of cell surface CFTR expression on enterocytes obtained from ligated intestinal loops, before and after serovar Typhi infection. (A and B) CFTR-specific (CF3, filled histograms) or negative control (VF8, open histograms) antibodies were used for staining of live, intact epithelial cells followed by flow cytometric analysis. (A) Cell surface CFTR on uninfected enterocytes. (B) Cell surface CFTR on enterocytes after 1 h of infection with serovar Typhi. (C) Results of a similar experiment carried out with 15-min (hatched bars) and 1-h (solid bars) infection periods and using two other strains of serovar Typhi: H245 (245; identical to strain Ty2) and H193 (193). The values depicted represent the staining obtained with the CFTR-specific antibody CF3 corrected for background fluorescence obtained with the negative control antibody VF8. MFI, mean fluorescence intensity.
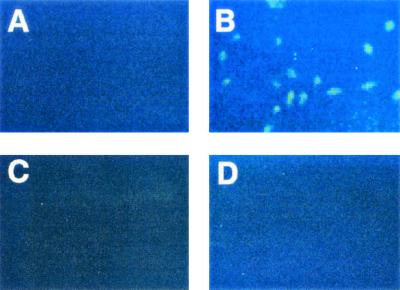
We have previously reported (30) that the level of CFTR protein on corneal epithelial cells is increased during infection with P. aeruginosa, another organism that uses epithelial cell CFTR as a receptor (22, 30). In the case of P. aeruginosa corneal infection, the increase in the level of CFTR does not require live P. aeruginosa and can be induced by sterile extracts of the bacterium. Therefore, we examined whether increased CFTR cell surface expression by cultured epithelial cells could also be triggered by sterile extracts of serovar Typhi. Cell extracts of serovar Typhi were prepared by the method of Breckenridge DiNovo (2), and tested for the ability to stimulate CFTR expression. Increased cell surface CFTR expression was detected on CFT1-LCFSN cells, which produce wild-type CFTR, following treatment of the cells with sterile extracts of serovar Typhi (Fig. 2B). It should be noted that the cells shown in Fig. 2A (untreated) are not actually CFTR negative; indeed, small amounts of cell surface CFTR can be detected without extract treatment if a higher concentration of the CFTR-specific primary antibody CF3 is used (data not shown).
FIG. 2.
Measurement of cell surface CFTR expression, by immunofluorescent staining, on the LCFSN epithelial cell line before and after treatment with serovar Typhi extract or LPS. The LCFSN cell line was cocultured for 1 h with sterile extracts, or purified LPS, of serovar Typhi and was then stained with CFTR-specific (A to C) or negative control (D) antibodies. (A) Untreated cells. (B and D) Serovar Typhi extract-treated cells. (C) Serovar Typhi LPS-treated cells. The epithelial cells were intact during the staining procedure, and so only cell surface CFTR is detected. Magnifications, ×100.
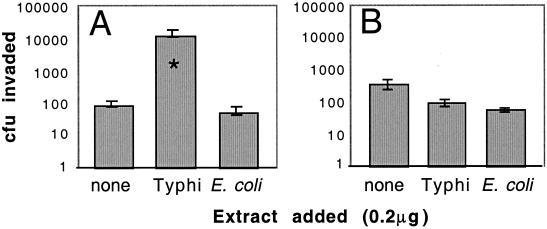
We next determined whether the observed increase in the level of membrane CFTR protein following extract treatment would result in an increase in CFTR-dependent bacterial uptake by epithelial cells. Therefore, we injected ligated intestinal loops with sterile serovar Typhi extract and subsequently inoculated them with live Salmonella. A sterile extract from Escherichia coli, which does not trigger an increase in CFTR expression, was used as a negative control. The results (Fig. 3A) show that serovar Typhi extract enhances translocation of live bacteria to the gastric submucosa approximately 100-fold relative to that in intestine not treated with extract or treated with the control extract. Treatment of the mouse intestine with bacterial extract potentially has many effects on the enterocytes in addition to stimulating CFTR trafficking. To assess the CFTR dependence of the effect of extract on bacterial invasion, we repeated this experiment using live serovar Typhimurium strain LT2. Similar to serovar Typhi strain Ty2, LT2 is a widely studied wild-type strain that invades mouse intestinal enterocytes efficiently. However, unlike serovar Typhi Ty2, serovar Typhimurium LT2 invades enterocytes in a CFTR-independent manner (21). Neither serovar Typhi extract nor E. coli extract affected the invasion by live serovar Typhimurium of the epithelial cells (Fig. 3B), indicating that enhanced invasion by serovar Typhi following extract treatment is not merely a result of nonspecific stimulation of GI tissues by the extract.
FIG. 3.
Translocation of wild-type S. enterica to the intestinal submucosa with and without pretreatment of intestine with sterile bacterial extract. Ligated intestinal loops were injected with sterile extracts (equivalent of 0.2 μg [dry weight]) of serovar Typhi, which triggers CFTR redistribution, or with negative control extracts of E. coli, which does not trigger CFTR redistribution. After incubation with extract, 2 × 104 CFU of live serovar Typhi strain Ty2 (A) or serovar Typhimurium strain LT2 (B) was injected into the intestinal lumen, and the number of bacteria was determined 2 h later by antibiotic treatment to kill extracellular/nontranslocated bacteria. Columns indicate means of seven replicate samples, and error bars indicate the standard errors. ∗, significantly different from “no extract” by ANOVA and Fisher PLSD (P = 0.01).
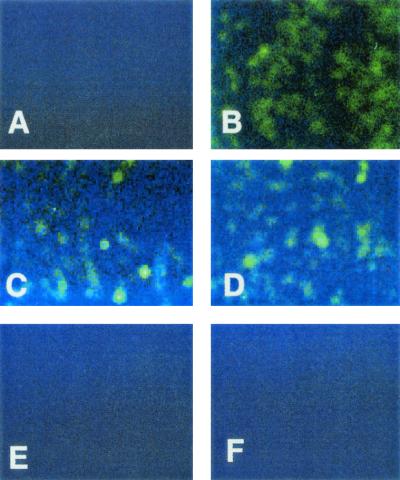
The epithelial cell mechanism for increasing the surface expression of CFTR protein in response to serovar Typhi infection was investigated next. Based on the fact that increased CFTR expression was first observed on enterocytes at the tips of villi, which are not the cells which typically synthesize CFTR in uninfected tissues (26), it was unlikely that the regulation of CFTR occurred at the transcriptional level. As expected, the results of semiquantitative RT-PCR of CFTR mRNA before and after serovar Typhi infection showed no significant difference in the quantity of CFTR mRNA in infected small intestine, compared to uninfected small intestine (P = 0.66 by ANOVA); (Table 1). Since the observed increase in CFTR expression was so rapid (15 min; [Fig. 1C]), it was likely that the increase in the amount of cell surface CFTR protein was due to a redistribution of preformed CFTR protein from intracellular vesicular stores (28) to the plasma membrane. If this was so, then the total amount of CFTR protein in the epithelial cells (membrane plus intracellular) should be comparable regardless of infection. As shown in Fig. 4A and B, cell surface CFTR protein (without epithelial cell permeabilization) showed a marked increase in staining intensity after serovar Typhi infection. However, when total cellular CFTR was detected (in cells that were detergent permeabilized after infection), a comparable level of staining was attained regardless of serovar Typhi infection (Fig. 4C and D). Thus, it appears that infection with serovar Typhi does not affect the total amount of CFTR present in the epithelial cells but instead changes its cellular distribution. To verify this, epithelial cells were treated with BoNT-A and then infected with serovar Typhi. BoNTs interfere with vesicle trafficking by cleaving proteins of the SNAP/SNARE family (13, 20), and, as shown in Fig. 4F, BoNT-A-treated epithelial cells did not increase cell surface CFTR expression on serovar Typhi infection.
TABLE 1.
Quantitation of CFTR mRNA by semiquantitative RT-PCR of mouse small intestine
| Infection status | n | Mean titera | SDb of titer |
|---|---|---|---|
| Uninfected | 7 | 174 | 104 |
| 15-min infected | 2 | 182 | NDc |
| 15-min mockd | 2 | 191 | ND |
| 1-h infected | 8 | 210 | 258 |
| 1-h mock | 5 | 151 | 244 |
| 2-h infected | 5 | 350 | 279 |
| 2-h mock | 3 | 231 | 210 |
Titer was determined by preparing cDNA from 3 μg of total RNA and then PCR amplifying different dilutions of the cDNA. The titer is the cDNA dilution at which no PCR product is detectable.
SD, standard deviation.
ND, not done.
mock, mock-infected intestinal loops were injected with diluent only instead of bacterial suspension.
FIG. 4.
Differential detection of cell surface CFTR protein and total cellular CFTR protein. (A and B) Cell surface CFTR expression was determined by immunofluorescent staining of intact LCFSN cells before (A) or after (B) infection with wild-type serovar Typhi. (C and D) Total cellular CFTR expression (membrane and intracellular) was determined by immunofluorescent staining of detergent-permeabilized cells before (C) or after (D) infection with wild-type serovar Typhi. (E) Sample similar to that in panel B but stained with an isotype-matched negative control antibody. (F) Epithelial cells were treated with BoNT-A, infected with serovar Typhi, and stained for cell surface CFTR expression (epithelial cells were not permeabilized prior to CFTR staining). Magnifications, ×100.
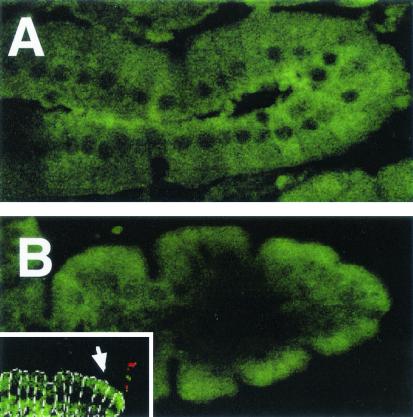
Confocal microscopy was used to examine the distribution of CFTR protein in the small intestine before and after infection. For this experiment, thin sections (5 μm) of fixed, paraffin-embedded tissue were used, allowing the staining reagents access to both cell surface and intracellular CFTR protein. As shown in Fig. 5A, CFTR protein is diffusely distributed throughout the cytoplasm of enterocytes in the uninfected intestine. However, after infection, CFTR is concentrated on and near the apical membranes of the enterocytes (Fig. 5B). Paraffin sections were then double stained for both CFTR protein and Salmonella bacteria to determine if regions of villi with membrane CFTR correlated with bacterium-epithelial cell contact. As shown in the inset in Fig. 5B, CFTR is mobilized to the apical membrane in regions of intestinal villi where bacterial cells are associated with the villus.
FIG. 5.
Confocal microscopic localization of fluorescently labeled CFTR protein in intact mouse small intestine. Ligated loops of mouse small intestine were left uninfected (A) or infected for 1 h with wild-type serovar Typhi (B) and were then paraffin embedded. Sections (5 μm) were then fluorescently stained (green) for CFTR protein. (Inset) Intestine infected for 1 h was double stained for CFTR (green) and serovar Typhi cells (red). In the inset, individual enterocytes are outlined in white dotted lines for clarity. The arrow marks a point of CFTR mobilization toward the apical membrane. Original magnifications, ×400. Note that the treatment of these tissue sections effectively permeabilized the enterocytes; therefore, the CFTR detected represents total (intracellular and cell surface) CFTR.
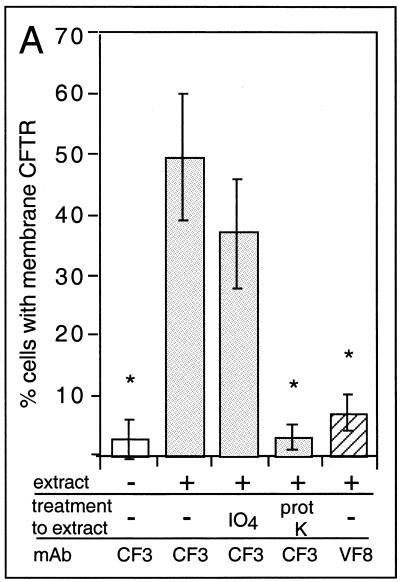
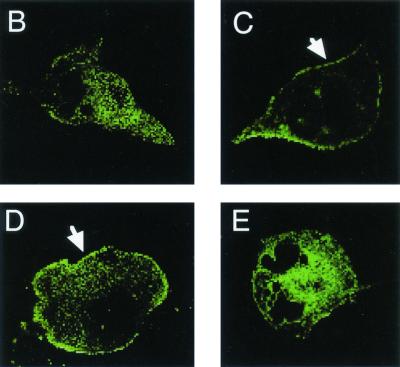
To determine what component of the bacterial extract was responsible for mobilizing CFTR protein to the epithelial cell surface, portions of extract were treated with either potassium periodate (to destroy carbohydrate in the extract) or proteinase K. The ability of whole extracts or treated extracts to modulate CFTR expression was then determined by two separate methods. First, cell surface CFTR expression was evaluated by indirect immunofluorescent staining of the LCFSN epithelial cell line by using the CF3 monoclonal antibody (Fig. 6A). Secondly, the subcellular distribution of CFTR was directly visualized using the MDCK(GFP-CFTR) cell line, which expresses a translational fusion of CFTR and GFP (Fig. 6B to E) (16). By both methods of detection, CFTR was visualized on the epithelial cell surface after treatment with whole extracts (Fig. 6A and C) and after treatment with periodate-treated extracts (Fig. 6A and D). However, CFTR was not mobilized to the epithelial cell surface after addition of proteinase K-treated extracts (Fig. 6A and E). Since periodate treatment should have destroyed any LPS oligosaccharides present in the bacterial extract, these data strongly suggest that the bacterial activity that mobilizes CFTR protein to the epithelial cell plasma membrane is protein and not LPS. This conclusion is further supported by the findings that purified LPS from serovar Typhi is incapable of mobilizing CFTR to the cell surface (Fig. 2C) and that the CFTR-mobilizing activity is inactivated by a 20-min incubation at 70°C (data not shown). It should be noted that Fig. 6B through E cannot be interpreted quantitatively, since this cell line demonstrates high cell-to-cell variability in expression of the CFTR-GFP fusion protein (8). These data can only be analyzed qualitatively as an indication of CFTR-GFP localization within the cells.
FIG. 6.
CFTR trafficking to the plasma membrane stimulated by extracts treated with proteinase K (prot K) or with potassium perio date (IO4) to selectively destroy proteins or carbohydrates, respectively. (A) Cell surface CFTR expression was determined by immunofluorescent staining of intact LCFSN cells as in Fig. 2. mAb, monoclonal antibody; ∗, significantly different from whole extract by ANOVA and Fisher PLSD (P ≤ 0.0001). (B through E) The subcellular localization of the CFTR protein was directly visualized by confocal microscopy of MDCK cells expressing a translational fusion of CFTR and GFP and treated with different preparations of bacterial extract. (B) Untreated. (C) Treated with whole-cell extract. (D) Treated with extract that was previously incubated with periodate. (E) Treated with extract that was previously incubated with proteinase K. Arrows highlight the surface accumulation of CFTR-GFP. Original magnifications (panels B through E), ×800.
DISCUSSION
The function of the CFTR protein has been intensely studied since genes encoding mutant forms of CFTR give rise to the autosomal recessive disorder cystic fibrosis (CF) (11). CFTR regulates the secretion of ions across epithelia in many tissues (25), and loss of CFTR function has dramatic negative effects on pancreatic function, nutrient absorption, the development of the male reproductive system, and the composition and consistency of skin and airway secretions (for a review, see reference 14). We have previously shown that in addition to these roles, the CFTR protein functions as a receptor for the bacterium P. aeruginosa (22-24), the primary cause of morbidity and mortality among CF patients as a result of chronic lung infection. Among individuals with wild-type Cftr alleles, binding of CFTR to P. aeruginosa leads to the removal of this bacterium from the airway, a process that does not occur in CF, which contributes to the high rate of infection of CF patients. Given that prior to modern medical management CF was typically lethal before 2 years of age (1), it is puzzling that mutations in the Cftr gene are maintained in certain human populations at high frequencies (e.g., 4 to 5% for the ΔF508 mutation in populations of Eastern European descent). A possible explanation for the high maintenance of a mutant allele among heterozygous carriers came from the discovery that the CFTR protein is also a receptor for S. enterica serovar Typhi (21). If at the earliest stages of serovar Typhi infection the CFTR protein promotes bacterial invasion, then heterozygotes with reduced levels of CFTR protein may have an increased resistance to infection and development of typhoid fever. Support of this hypothesis came from observations that transgenic mice heterozygous for the murine ΔF508 Cftr allele (resulting in reduced quantities of cell surface CFTR) translocate 86% fewer serovar Typhi organisms to the submucosa following inoculation into the GI lumen than do wild-type mice, and mice homozygous for the ΔF508 Cftr allele essentially translocate no serovar Typhi cells to the GI submucosa (21).
Given the established relationship between CFTR expression levels and efficiency of serovar Typhi ingestion, we were interested in whether CFTR expression levels are altered during the course of serovar Typhi infection. The data presented here show that serovar Typhi produces a factor(s) that is capable of triggering a rapid accumulation of preformed CFTR protein in the epithelial cell plasma membrane, where this protein then mediates bacterial uptake. The fact that this trafficking event can be triggered by sterile bacterial extracts distinguishes these results from the large body of existing work on host cell responses during infection, since many previous studies have demonstrated (29) that increased expression of host products is a consequence of, not a prelude to, bacterial ingestion. Nevertheless, the redistribution of CFTR protein during infection is not without precedent, for we have previously reported (30) that CFTR expression is increased on corneal epithelial cells during infection with P. aeruginosa. Similar to what is reported here, this increase in CFTR expression was subsequently shown to lack a requirement for live P. aeruginosa cells, since increases in CFTR levels could be observed after treatment of epithelial cells with sterile P. aeruginosa extract.
Invasion by live serovar Typhi of the intestinal epithelium was enhanced approximately 100-fold when the small intestine was treated with serovar Typhi extract prior to addition of live serovar Typhi cells. It is tempting to speculate that CFTR redistribution constitutes a bacterial virulence mechanism aimed at promoting its own uptake by the intestinal epithelium. Enhanced uptake was observed after treatment with serovar Typhi extract (Fig. 3A), which triggers CFTR redistribution, but was not observed when the intestine was treated with E. coli extract, which does not stimulate CFTR redistribution. Also, serovar Typhi extract has no effect on the invasion efficiency of serovar Typhimurium (Fig. 3B), which invades epithelial cells in a CFTR-independent fashion. These data suggest that the enhancement of invasion is due to membrane expression of the CFTR protein and not to the redistribution of some other plasma membrane component(s) that is also altered by extract treatment or to nonspecific activation of the intestinal enterocytes by the extract.
Transcription of the Cftr gene in the intestine has been studied using in situ hybridization to Cftr mRNA. The highest level of CFTR transcription typically occurs in cells at the base of the villi, near the crypts, with undetectable quantities of CFTR mRNA in the more mature enterocytes in the crypt neck region (26). In contrast to the results of these transcription studies, we found that surface expression of CFTR protein was increased primarily on enterocytes near the villus tips. While seemingly in conflict, these data are easily reconciled by the findings of Webster et al. (28), who reported that a large portion of the CFTR protein in epithelial cells is stored in cytoplasmic vesicles. Our results indicate that the stored CFTR protein can be stimulated to accumulate in the plasma membrane and that this can happen even in more mature enterocytes that no longer actively transcribe the Cftr gene. Indeed, the data we report clearly demonstrate that CFTR accumulation at the apical membrane is restricted to epithelial sites near points of bacterium-epithelial cell contact. Such increases in cell surface CFTR protein levels may affect many aspects of epithelial cell physiology such as chloride and bicarbonate transport, which may also be factors in the cellular uptake of serovar Typhi.
The extracts used in this study to stimulate increased CFTR expression were distilled-water washes of live bacterial cells. It is expected that the extracts consist largely of molecules adherent to the exterior of the bacterial cell, as well as some components of the outer membrane, such as LPS and outer membrane proteins, although our findings argue against a role for LPS in CFTR mobilization. We are currently fractionating the bacterial extracts to isolate and identify the CFTR-mobilizing activity. Preliminary fractionation of extracts indicates that this activity is present in the 90- to 250-kDa fraction.
The CFTR receptor probably participates in early interactions between the bacterium and the epithelial cell. This interaction is but one of many that occur early in the invasion process. Pili (10), flagella, and fimbriae (3) have also been shown to contribute to the initial interaction of this bacterium with host epithelial cells. Together, these early contacts probably serve to activate the bacterial type III secretion system (9), which must be triggered for the infectious process to proceed. However, without CFTR-mediated contacts between serovar Typhi and the intestinal epithelium, bacterial translocation to the intestinal submucosa is reduced, demonstrating a critical role for this epithelial cell receptor in bacterial invasion and perhaps providing a mechanism for increased resistance to development of typhoid fever in heterozygous carriers of mutant Cftr alleles.
Serovar Typhi interacts with CFTR via a ligand that is present on its LPS. One mutant strain of serovar Typhi that produces truncated LPS core is not capable of binding the CFTR protein and has a greatly reduced ability to enter epithelial cells, while another isogenic mutant producing a slightly larger LPS core interacts with CFTR normally and has an invasive phenotype (15). We are currently analyzing these LPS mutants in an attempt to identify the precise region of the LPS that serves as the CFTR-binding ligand. On wild-type serovar Typhi, this LPS-based ligand is blocked from interacting with its epithelial cell receptor. Alterations in the LPS, triggered by bacterium-epithelial cell contact, allow wild-type serovar Typhi LPS to bind its epithelial cell receptor (15).
In summary, we have shown that the CFTR protein, which serves as an epithelial cell receptor mediating the ingestion of serovar Typhi, is rapidly trafficked to the plasma membranes of epithelial cells following infection. This increase is brought about by a redistribution of performed CFTR protein from the cytoplasm to the plasma membrane, and not by de novo synthesis of CFTR protein. Thus, the early interactions between serovar Typhi and intestinal epithelial cells depend on alterations to both a bacterial ligand (LPS [15]) and the cellular localization of its host receptor (CFTR). A heat- and protease-sensitive factor produced by serovar Typhi is necessary for CFTR redistribution. CFTR redistribution is shown to promote invasion by Salmonella cells that utilize the CFTR receptor but has no effect on invasion by Salmonella cells that do not utilize this receptor. Efforts are under way to identify the active factor in the extract. Identification of the chemical properties of this factor and its mode of interaction with the gastric mucosal epithelium may provide targets for novel modalities aimed at manipulating or interfering with the bacterial invasion process.
Acknowledgments
We extend our appreciation to Martha Grout for extensive technical assistance, and we also thank David Hone for the gift of serovar Typhi strains H245 and H193.
This work was supported by NIH grant HL58398.
Editor: E. I. Tuomanen
REFERENCES
- 1.Andersen, D. H. 1938. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathologic study. Am. J. Dis. Child. 56:344-399. [Google Scholar]
- 2.Breckenridge DiNovo, B., R. Doan, R. B. Dyer, S. Baron, N. K. Herzog, and D. W. Niesel. 1996. Treatment of HeLa cells with bacterial water extracts inhibits Shigella flexneri invasion. FEMS Immunol. Med. Microbiol. 15:149-158. [DOI] [PubMed] [Google Scholar]
- 3.Dibb-Fuller, M. P., E. Allen-Vercoe, C. J. Thorns, and M. J. Woodward. 1999. Fimbriae- and flagella-mediated association with and invasion of cultured epithelial cells by Salmonella enteritidis. Microbiology 145:1023-1031. [DOI] [PubMed] [Google Scholar]
- 4.Finlay, B. B., J. Fry, E. P. Rock, and S. Falkow. 1989. Passage of Salmonella through polarized epithelial cells: role of the host and bacterium. J. Cell Sci. Suppl. 11:99-107. [DOI] [PubMed] [Google Scholar]
- 5.Finlay, B. B., F. Heffron, and S. Falkow. 1989. Epithelial cell surfaces induce Salmonella proteins required for bacterial adherence and invasion. Science 243:940-943. [DOI] [PubMed] [Google Scholar]
- 6.Francis, C. L., T. A. Ryan, B. D. Jones, S. J. Smith, and S. Falkow. 1993. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature 364:639-642. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-del Portillo, F., and B. B. Finlay. 1994. Salmonella invasion of nonphagocytic cells induces formation of macropinosomes in the host cell. Infect. Immun. 62:4641-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerceker, A. A., T. Zaidi, P. Marks, D. E. Golan, and G. B. Pier. 2000. Impact of heterogeneity within cultured cells on bacterial invasion: analysis of Pseudomonas aeruginosa and Salmonella enterica serovar Typhi entry into MDCK cells by using a green fluorescent protein-labeled cystic fibrosis transmembrane conductance regulator receptor. Infect. Immun. 68:861-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginocchio, C. C., S. B. Olmsted, C. L. Wells, and J. E. Galan. 1994. Contact with epithelial cells induces the formation of surface appendages on Salmonella typhimurium. Cell 76:717-724. [DOI] [PubMed] [Google Scholar]
- 10.Horiuchi, S., Y. Inagaki, N. Okamura, R. Nakaya, and N. Yamamoto. 1992. Type 1 pili enhance the invasion of Salmonella braenderup and Salmonella typhimurium to HeLa cells. Microbiol. Immunol. 36:593-602. [DOI] [PubMed] [Google Scholar]
- 11.Kerem, B., J. M. Rommens, J. A. Buchanan, D. Markiewicz, T. K. Cox, A. Chakravarti, M. Buchwald, and L. C. Tsui. 1989. Identification of the cystic fibrosis gene: genetic analysis. Science 245:1073-1080. [DOI] [PubMed] [Google Scholar]
- 12.Kohbata, S., H. Yokoyama, and E. Yabuuchi. 1986. Cytopathogenic effect of Salmonella typhi GIFU 10007 on M cells of murine ileal Peyer's patches in ligated ileal loops: an ultrastructural study. Microbiol. Immunol. 30:1225-1237. [DOI] [PubMed] [Google Scholar]
- 13.Low, S. H., S. J. Chapin, C. Wimmer, S. W. Whiteheart, L. G. Komuves, K. E. Mostov, and T. Weimbs. 1998. The SNARE machinery is involved in apical plasma membrane trafficking in MDCK cells. J. Cell Biol. 141:1503-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyczak, J. B., T. S. Zaidi, M. Grout, M. Bittner, I. Contreras, and G. B. Pier. 2001. Epithelial cell contact-induced alterations in Salmonella enterica serovar Typhi lipopolysaccharide are critical for bacterial internalization. Cell Microbiol. 3:763-772. [DOI] [PubMed] [Google Scholar]
- 16.Moyer, B. D., J. Loffing, E. M. Schwiebert, D. Loffing-Cueni, P. A. Halpin, K. H. Karlson, I. I. Ismailov, W. B. Guggino, G. M. Langford, and B. A. Stanton. 1998. Membrane trafficking of the cystic fibrosis gene product, cystic fibrosis transmembrane conductance regulator, tagged with green fluorescent protein in Madin-Darby canine kidney cells. J. Biol. Chem. 273:21759-21768. [DOI] [PubMed] [Google Scholar]
- 17.Olsen, J. C., L. G. Johnson, M. J. Stutts, B. Sarkadi, J. R. Yankaskas, R. Swanstrom, and R. C. Boucher. 1992. Correction of the apical membrane chloride permeability defect in polarized cystic fibrosis airway epithelia following retroviral-mediated gene transfer. Hum. Gene Ther. 3:253-266. [DOI] [PubMed] [Google Scholar]
- 18.Pace, J., M. J. Hayman, and J. E. Galan. 1993. Signal transduction and invasion of epithelial cells by S. typhimurium. Cell 72:505-514. [DOI] [PubMed] [Google Scholar]
- 19.Pascopella, L., B. Raupach, N. Ghori, D. Monack, S. Falkow, and P. L. Small. 1995. Host restriction phenotypes of Salmonella typhi and Salmonella gallinarum. Infect. Immun. 63:4329-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pellizzari, R., O. Rossetto, G. Schiavo, and C. Montecucco. 1999. Tetanus and botulinum neurotoxins: mechanism of action and therapeutic uses. Philos. Trans. R. Soc. Lond. Ser. B 354:259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pier, G. B., M. Grout, T. Zaidi, G. Meluleni, S. S. Mueschenborn, G. Banting, R. Ratcliff, M. J. Evans and W. H. Colledge. 1998. Salmonella typhi uses CFTR to enter intestinal epithelial cells. Nature 393:79-82. [DOI] [PubMed] [Google Scholar]
- 22.Pier, G. B., M. Grout, and T. S. Zaidi. 1997. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc. Natl. Acad. Sci. USA 94:12088-12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pier, G. B., M. Grout, T. S. Zaidi, and J. B. Goldberg. 1996. How mutant CFTR may contribute to Pseudomonas aeruginosa infection in cystic fibrosis. Am. J. Respir. Crit. Care Med. 154:S175-S182. [DOI] [PubMed] [Google Scholar]
- 24.Pier, G. B., M. Grout, T. S. Zaidi, J. C. Olsen, L. G. Johnson, J. R. Yankaskas, and J. B. Goldberg. 1996. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science 271:64-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwiebert, E. M., D. J. Benos, M. E. Egan, M. J. Stutts, and W. B. Guggino. 1999. CFTR is a conductance regulator as well as a chloride channel. Physiol. Rev. 79:S145-S166. [DOI] [PubMed] [Google Scholar]
- 26.Strong, T. V., K. Boehm, and F. S. Collins. 1994. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J. Clin. Investig. 93:347-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker, J., J. Watson, C. Holmes, A. Edelman, and G. Banting. 1995. Production and characterisation of monoclonal and polyclonal antibodies to different regions of the cystic fibrosis transmembrane conductance regulator (CFTR): detection of immunologically related proteins. J. Cell Sci. 108:2433-2444. [DOI] [PubMed] [Google Scholar]
- 28.Webster, P., L. Vanacore, A. C. Nairn, and C. R. Marino. 1994. Subcellular localization of CFTR to endosomes in a ductal epithelium. Am. J. Physiol. 267:C340-C348. [DOI] [PubMed] [Google Scholar]
- 29.Witthoft, T., L. Eckmann, J. M. Kim, and M. F. Kagnoff. 1998. Enteroinvasive bacteria directly activate expression of iNOS and NO production in human colon epithelial cells. Am. J. Physiol. 275:G564-G571. [DOI] [PubMed] [Google Scholar]
- 30.Zaidi, T. S., J. Lyczak, M. Preston, and G. B. Pier. 1999. Cystic fibrosis transmembrane conductance regulator-mediated corneal epithelial cell ingestion of Pseudomonas aeruginosa is a key component in the pathogenesis of experimental murine keratitis. Infect. Immun. 67:1481-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]