Abstract
The capsular polysaccharide of Neisseria meningitidis group B is an autoantigen, whereas noncapsular antigens are highly variable. These factors present formidable challenges for development of a broadly protective and safe group B vaccine. Mice and guinea pigs were sequentially immunized with three doses of micovesicles or outer membrane vesicles prepared from three meningococcal strains that were each antigenically heterologous with respect to the two major porin proteins, PorA and PorB, and the group capsular polysaccharide. The resulting antisera conferred passive protection against meningococcal group B bacteremia in infant rats and elicited complement-mediated bactericidal activity against genetically diverse group B strains that were either homologous or heterologous with respect to PorA of the strains used to prepare the vaccine. By using knockout strains, a portion of the bactericidal antibody was directed against the highly conserved protein, neisserial surface protein A (NspA). Further, an anti-NspA monoclonal antibody elicited by the sequential immunization was highly bactericidal against strains that were previously shown to be resistant to bacteriolysis by anti-NspA antibodies produced by immunization with recombinant NspA. Sequential immunization with heterologous vesicle preparations offers a novel approach to eliciting broadly protective immunity against N. meningitidis strains. An NspA-based vaccine prepared from protein expressed by Neisseria also may be more effective than the corresponding recombinant protein made in Escherichia coli.
Neisseria meningitidis is a major cause of bacterial meningitis and septicemia in children and young adults. Meningococcal strains can be subdivided into capsular groups based on immunologically and chemically distinctive capsular polysaccharides. Serum antibody to the capsular polysaccharide confers protection against disease. Effective capsular polysaccharide-based vaccines have been developed for the prevention of disease caused by meningococcal strains from groups A, C, Y, and W-135. However, there is no vaccine capable of eliciting broadly protective antibodies to group B strains (reviewed in reference 14). The lack of a group B vaccine is a serious public health limitation since these strains account for approximately one-third of meningococcal disease in North America (28) and up to 80% in northern Europe (6).
The group B capsular polysaccharide is identical to human polysialic acid and, therefore, is an autoantigen (7), as well as a poor immunogen (13, 37, 39). A chemically modified derivative of group B polysaccharide in which N-propionyl groups are substituted for N-acetyl groups has been shown to be more immunogenic and to elict bactercidal antibodies. However, a subset of the antibodies elicited by the modified polysaccharide react with N-acetyl group B polysaccharide and bind to host tissues (11, 12). Whether or not these autoreactive antibodies are harmful is not known, but these observations raise safety concerns that will be difficult to resolve before this vaccine can be used widely in humans.
Alternative approaches to the development of a group B vaccine include the use of noncapsular antigens such as pilin, the opacity proteins Opa and Opc, iron-regulated proteins, porin proteins (i.e., PorA), or outer membrane protein vesicles (OMV) containing a single or multiple PorA variants (reviewed in reference 14). In general, these candidate vaccines do not provide broad protection against group B strains as a result of variable expression and/or antigenic variation of the epitopes accessible on the surface of the bacteria from different strains.
Analyses of genomic sequence data from group A and B strains of N. meningitidis, as well as a strain of N. gonorrhea, have revealed many previously unidentified genes that are predicted to encode novel conserved proteins, some of which appear to be potential vaccine candidates (24, 26). Neisserial protein A (NspA), which originally was discovered with a monoclonal antibody, is also a highly conserved protein and is under investigation as a vaccine candidate (16, 17, 19, 21). Some of these proteins would be expected to be present in membrane vesicles prepared from N. meningitidis strains. However, repeated immunization with OMV prepared from a single meningococcal strain elicits strain-specific bactericidal antibodies that are primarily directed at PorA (22, 27, 36) and, to a lesser extent, Opc (27). Our hypothesis in the present study was that sequential immunization with vesicles prepared from three meningococcal strains that were each heterologous with respect to PorA, PorB, and capsule would focus the immune response to conserved proteins that normally are poorly immunogenic when repeated injections are given with vesicles prepared from one strain or multiple strains.
MATERIALS AND METHODS
Bacterial strains.
The 20 N. meningitidis strains chosen for the present study (Table 1) were selected to represent diverse PorA VR types. Ten of the strains have VR types homologous to those of the three vaccine strains and 10 have heterologous VR types. VR sequence types for strains 8047, NMB, RM1090, and Z1092 were determined by I. Feavers, National Institute for Biological Standards and Control (NIBSC) (United Kingdom). The VR types of strains CU385, H44/76, M986, S3032, and S3446 were inferred from DNA sequences obtained from GenBank (accession numbers U92935, X52995, U92942, X57178, and U92919, respectively). The VR types of the remaining strains were inferred from DNA sequences done by the Institute for Genome Research, Rockville, Md. (24).
TABLE 1.
Summary of N. meningitidis strains
| Straina | Country | Yr | Serologic classificationb | PorA VR designation (sequence)c | Immunotype | NspA reactivityd |
|---|---|---|---|---|---|---|
| Homologous | ||||||
| 1000 | Russia | 1989 | B:NT:5 | 5-1,10-4 | NT | ++ |
| 8047 | United States | 1978 | B:2b:5,2 | 5-1,2-2 | 3,7,9 | ++ |
| BZ198e | The Netherlands | 1986 | B:NT:4 | 7-2,4 | NT | ++ |
| BZ232f | The Netherlands | 1964 | B:5,2 | 5-2,2-2 | 2,5 | V |
| BZ83 | The Netherlands | 1984 | B:NT:5,10 | 5-2,10 | 3,7 | + |
| M986 | United States | 1963 | B:2a:5,2 | 5,2 | 3,7,9 | − |
| NGP165 | Norway | 1974 | B:NT:5,2 | 5,2 | 3,7 | − |
| NMB | United States | 1982 | B:2b:5,2 | 5-1,2-2 | 2 | + |
| RM1090e | United States | Pre-1995 | C:2a:5,2 | 5-1,2 | 3,7,9 | ++ |
| Z1092e | West Germany | 1964 | A:4,21:10 | 5-2,10 | 10 | + |
| Heterologous | ||||||
| BZ147 | The Netherlands | 1963 | B:NT:NST | 18-2,1-2 | 3,7,9 | − |
| CU385 | Cuba | 1980 | B:4,7:19,15 | 19,15 | 3,7,9 | ++ |
| H44/76 | Norway | 1976 | B:15:7,16 | 7,16 | 3,7 | − |
| MC58 | United Kingdom | 1985 | B:15:7,16 | 7,16-2 | 3,7,9 | V |
| NG3/88 | Norway | 1988 | B:8:1 | 7-1,1 | 3,7 | ++ |
| NGH15 | Norway | 1988 | B:8:15 | 19,15-2 | NT | + |
| NGH38 | Norway | 1988 | B:NT:3 | 18-1,3 | 2,5 | + |
| S3032 | United States | 1973 | B:19,7:12,16 | 12,16 | 3,7,9 | ++ |
| S3446 | United States | 1972 | B:19,14:23,14 | 23,14 | 3,7,9 | ++ |
| SWZ107 | Switzerland | 1986 | B:4:14 | 22-1,14 | 3,7,9 | − |
Homologous strains are considered to have PorA VR types homologous to that of one of the vaccine strains. The remaining strains are considered to be heterologous. In order to be consistent, strains that have related but not identical VR types (e.g. 7, 7-1, or 7-2) are defined as heterologous, since even one amino acid difference can result in differences in susceptibility to bactericidal activity (see Results for an example).
NT, nonserotypeable; NST, nonserosubtypeable (with available MAbs).
Based on the proposed PorA VR-type designation nomenclature of Sacchi et al. (30).
As determined by flow cytometry with the anti-NspA MAb AL12. Results were scored as follows: −, <50% of total fluorescent events have an intensity above the background level when tested at 100 μg of MAb/ml; +, ≥50% of total fluorescent events have an intensity above the background level at 100 μg/ml; ++, ≥50% of total fluorescent events have an intensity above the background level at 10 μg/ml; V, subcultures were variable, ranging from − to ++ as described elsewhere (21).
Vaccine strain.
Strain BZ232 was used only in the rat protection assay.
The NspA mutants of strain MC58ΔNspA, in which the nspA gene was inactivated, was a gift from J. Abu-Bobie, Chiron Corp., Siena, Italy. The PorA-deficient MC58 mutant was selected by using a high-inoculum bactericidal assay (21) that included human complement and the anti-PorA monoclonal antibody (MAb) MN14C11.6 (obtained from the National Institute of Biological Standards and Control, Potters Bar, United Kingdom) that is specific for the P1.7 serosubtype. Lack of PorA expression was confirmed by whole-cell enzyme-linked immunosorbent assay (ELISA) (21) and Western blots of outer membrane proteins prepared from the PorA deficient strain and resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels as described below.
Vesicle preparations.
The meningococcal strain, which was frozen at −80°C in aqueous 2% skim milk (wt/vol), was subcultured on a commercial chocolate agar plate (Remel, Laztakas, Kans.). After overnight growth at 37°C in 4% CO2, several colonies were selected to inoculate ∼7 ml of sterile Mueller-Hinton broth to an optical density at 620 nm (OD620) of 0.1. The culture was incubated at 37°C and 4% CO2 with rocking until the OD620 reached 0.6 to 0.8 (2 to 3 h). Two to three 7-ml starter cultures were then used to inoculate 500 ml of Mueller-Hinton broth. The larger culture was grown to an OD620 of 0.9 to 1.0 at 37°C with vigorous shaking. Phenol was added to the culture to a final concentration of 0.5% (wt/vol), and the mixture was left at 4°C overnight to inactivate the bacteria. The cells were then pelleted by centrifugation (11,000 × g) for 30 min at 4°C. The cell pellets were frozen at −20°C until used for preparation of OMV (strain Z1092 OMV only). Microvesicles (MV) were harvested from the phenol-treated cell-free culture supernatant by adding solid ammonium sulfate (390 g/liter, final concentration) slowly with stirring. (Note that we use the term MV to differentiate between blebs, which are outermembrane protrusions that are still attached to the bacterium, versus blebbing outermembrane that has been released into the medium as an MV [3]). After the ammonium sulfate was added and completely dissolved, the mixture was left at 4°C overnight. The precipitate containing MV was collected by centrifugation at 11,000 × g for 30 min. The pellet was resuspended in 20 ml of phosphate-buffered saline (PBS) and centrifuged again at 16,000 × g for 15 min at 4°C. The low-speed pellet was discarded and the MV, which remained in the supernatant, were collected by centrifugation at 100,000 × g for 2 h at 4°C. The final MV-containing pellet was resuspended in 5 ml of water (MV vaccine preparation).
OMV were prepared by the method of Zollinger et al. (39). The frozen cell pellet was resuspended in 10 ml of 0.05 M Tris-HCl buffer (pH 7.4) containing 0.15 M NaCl and 0.01 M EDTA and heated to 56°C for 30 min, followed by cooling on ice. The cell suspension was then sonicated on ice with several 15-s bursts by using a sonifier fitted with a microtip (Branson, Danbury, Conn.). Cell debris was removed by centrifugation at 16,000 × g for 15 min, and the OMV in the supernatant were obtained by ultracentrifugation at 100,000 × g for 2 h at 4°C. The OMV pellet was resuspended in 2 ml of water (OMV vaccine preparation). The protein concentrations of the MV and OMV preparations were determined by Dc protein assay (Bio-Rad, Richmond, Calif.). The OMV and MV vaccine preparations were stored at −20°C until used for immunization.
Characterization of LOS and capsular polysaccharide content of MV and OMV preparations.
The lipooligosaccharide (LOS) content in each MV and OMV preparation was determined by using a commercial Limulus amebocyte lysate (LAL) assay (BioWhittaker, Inc., Walkersville, Md.) performed according to the manufacturer's directions. LOS standards included E. coli O111:B4 endotoxin provided with the LAL kit and LOS purified by phenol extraction (1) from each vaccine strain (mass uncorrected for water content). The results for estimating the LOS content of MV and OMV preparations by using the respective vaccine strains and E. coli LOS standards were similar.
The amount of capsular polysaccharide in each of the vesicle vaccines was determined by competitive ELISA (2) after treatment of the vesicles with 10 mM EDTA to release free polysaccharide, performed as previously described (19). The groups A-, B-, and C-specific MAbs used in the assays included a group A MAb from NIBSC, 2-1-B (29), and C2/730 (9), respectively. Purified capsular polysaccharides were used as standards in the competition assays (polysaccharide from groups A and C were a the gift of Aventis Pasteur [Swiftwater, Pa.], and group B was prepared in our laboratory by procedures described by Yang and Jennings [38]).
SDS-PAGE and Western blots.
The MV and OMV preparations were analyzed by SDS-15% PAGE as described by Laemmli (15) employing a Mini-Protean II electrophoresis apparatus (Bio-Rad). Samples were suspended in SDS sample buffer (0.06 M Tris-HCl [pH 6.8] 10% [vol/vol] glycerol, 2% [wt/vol] SDS, 5% [vol/vol] 2-mercaptoethanol, 10 μg of bromophenol blue/ml) and heated to 100°C for 5 min before being loaded directly onto the gel. The Western blots were performed as previously described (19).
Immunization.
MV or OMV preparations were diluted in PBS and mixed with an equal volume of aluminum phosphate (1.0% Alhydrogel [wt/vol; Superfos Biosector, Frederikssund, Denmark] that had been incubated with PBS buffer for at least 3 h). For the first injection, mice were immunized subcutaneously (s.c.) with 100 μl containing 5 μg of total protein of MV prepared from meningococcal strain RM1090 or a mixture of equal parts of MV prepared from strains RM1090 and BZ198, and OMV prepared from strain Z1092. At 3- to 4-week intervals two subsequent booster doses (5 μg/mouse, given s.c.) were given of either the mixture of vesicles or MV prepared from meningococcal strain BZ198, followed by OMV prepared from meningococcal strain Z1092. The sequential immunization with vesicles prepared from three different meningococcal strains, each differing with respect to capsular group, PorB serotype, PorA serosubtype, and LOS immunotypes, constitutes “sequential immunization,” whereas three doses of an equal mixture of the three vesicle preparations constitute the “mixture immunization.” In a second experiment, groups of guinea pigs were given either sequential immunizations or the mixture immunization as described for the mice, the only difference being that the total dose of protein for each injection was 25 μg for the guinea pigs instead of 5 μg used in the mice. As a negative control, groups of animals in both experiments were given three injections of 25 or 5 μg, respectively, of MV prepared from E. coli and adsorbed to aluminum phosphate as described above for the Neisserial vesicles.
Complement-dependent bactericidal antibody activity.
The bactericidal assay was performed as previously described (19). The assay used log-phase broth-grown bacteria. The complement source was human serum from a healthy adult with no detectable intrinsic bactericidal activity or group B anticapsular antibody when tested by ELISA (31). Serum bactericidal titers were defined as the serum dilution (or antibody concentration) resulting in a 50% decrease in CFU per ml after 60 min of incubation of bacteria in the reaction mixture compared to control CFU per ml at time zero. Typically, bacteria incubated with the negative control antibody and complement showed a 150 to 200% increase in CFU/ml during 60 min of incubation.
Detection of anti-LOS antibody activity.
LOS was prepared from each vaccine strain by the method of Apicella et al. (1) for use as solid-phase antigens in an ELISA and to prepare LOS affinity columns. The anti-LOS ELISA was performed as described by Plested et al. (25). Monoclonal immunotyping reagents were used as positive controls and included the MAbs 9-2-L379, 17-1-L1, 2-1-L8, and 14-1-L10 (32). The LOS affinity columns were prepared as described by Shenep et al. (33) with the following modifications. LOS was conjugated to bovine serum albumin (BSA) through the carboxylic acid group of the terminal 2-keto-3-deoxyoctulosonic acid moiety as described by Brett et al. (5). Briefly, LOS (1 mg) was combined with BSA (2 mg) in 100 mM MES buffer (pH 5.0). EDC [1-ethyl-3-(3-dimethylaminopropyl) carbodiimide-HCl [Pierce Chemical, Rockford, Ill.]; 100 μl of a 10-mg/ml solution in water] was added with stirring, followed by incubation at ambient temperature for 2 h. An equal mixture of the three LOS-BSA conjugates (1 mg of LOS-BSA conjugate per ml of hydrated gel) was coupled to CNBr-activated agarose beads (Sigma) in sodium carbonate buffer (0.1 M, pH 8.0) by overnight incubation at ambient temperature. Unreacted sites were blocked by adding 1 mM ethanolamine (Sigma) in carbonate buffer. After the column was washed with carbonate buffer, the matrix was equilibrated with PBS buffer containing 1% (wt/vol) BSA (PBS-BSA).
Mouse or guinea pig antiserum pools were added to the columns. The columns were washed with PBS-BSA, and the antibody containing fractions passing through the column were identified by whole-cell ELISA (21) by using the nonencapsulated strain M7 (35) as the antigen. Fractions were combined, concentrated by ultrafiltration (Microcon; Millipore Corp., Bedford, Mass.) and adjusted to the same anti-M7 titer as the unabsorbed antiserum pool by dilution with PBS-BSA buffer.
Anti-rNspA antibody ELISA.
An ELISA was used to measure serum antibody titers to NspA as previously described (21). The solid-phase antigen consisted of rNspA-containing MV prepared from E. coli or, as a control, vesicles from the same E. coli strain lacking rNspA expression (19). The titer was defined as the serum dilution giving an OD405 of 0.5 after a 30-min incubation with substrate.
Preparation of MAbs.
Female CD1 mice (Charles River, Hollister, Calif.) were vaccinated sequentially with MV or OMV from strains RM1090, BZ198, and Z1092 as described above. The mice were given three 100-μl injections, each separated by 3 weeks, containing 5 μg of protein. The first two doses were given s.c., together with aluminum phosphate (0.5% [wt/vol]), and the final dose was given without adjuvant and administered intraperitoneally (i.p.). Three days later, the animals were sacrificed, and their spleen cells were fused with myeloma cells (P3X63-AG8.653) at a ratio of 1 spleen cell to 1.7 myeloma cell. After 2 weeks of incubation in hypoxanthine-aminopterin-thymidine selective medium, hybridoma supernatants were screened for antibody binding activity by whole-cell ELISA with encapsulated group B strains 1000 and CU385 as the target antigen as previously described (21). Hybridoma cells lines expressing antibodies that were reactive with both group B strains by ELISA were then tested in bactericidal assay as described above against group B strain 8047.
Passive animal protection.
The ability of antiserum to confer passive protection against N. meningitidis group B bacteremia was tested in infant rats challenged i.p. by using a method described previously (21). In brief, infant pups (6 to 7 days old) from litters of outbred Wistar rats (Charles River, Hollister, Calif.) were randomly redistributed to the nursing mothers. At time zero, groups of five to six animals were given antisera or antibodies diluted in PBS containing 1% BSA (wt/vol) by i.p. injection. Two hours later, the animals were challenged i.p. with ca. 5 × 103 CFU of group B strain 8047, M986, or BZ232. At 18 h after the bacterial challenge, blood specimens were obtained, and aliquots of 1, 10, and 100 μl of blood were plated onto chocolate agar as previously described (21).
RESULTS
MV and OMV used for immunization.
MV were prepared from three N. meningitidis strains: RM1090 (C:2a:5-1,2), BZ198 (B:NT:7-2,4), and Z1092 (A:4,21:5-2,10). These strains were primarily selected based on diversity of their respective capsular groups, PorB serotypes and PorA serosubtypes. Subsequently, they also were found to have heterologous LOS immunotypes. Two of the strains were also high (BZ198) or medium (RM1090) producers of NspA, as measured by SDS-PAGE and Western blot of OMV and naturally blebbed MV (See below). For strains BZ198 and RM1090, MV were used for immunization since they are enriched for outer membrane (23). Of the limited number of group A strains in our collection, none produced large amounts of MV. Therefore, for the group A strain Z1092, an OMV preparation was used for immunization instead of MV.
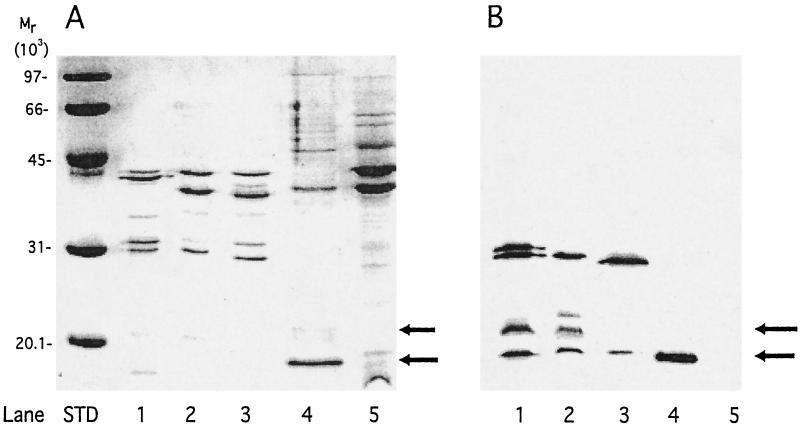
Figure 1A shows a Coomassie blue-stained SDS-15% PAGE gel of the proteins present in the vesicles used for immunization (lanes 1 to 3). As is typical of N. meningitidis MV or OMV, all three vesicle preparations consisted mainly of five major outer membrane proteins, including PorA (∼43 kDa), PorB (∼38 to 43 kDa), RmpM (∼36 kDa), and Opa/Opc (∼29 to 33 kDa). A band that may represent a partially unfolded form of NspA (∼21 kDa) is visible by Coomassie blue staining in the MV prepared from the group C and B strains RM1090 and BZ198, respectively (lanes 1 and 2). For comparison, lane 4 contains MV prepared from E. coli BL21(DE3) transformed with plasmid pGMS1.0 containing nspA and expressing rNspA (unfolded ∼18 kDa, partially unfolded ∼21 kDa; lane 4) and, in lane 5, a 10-fold larger amount of MV from the same E. coli stain containing the vector without nspA. Figure 1B shows a Western blot of the same vesicle preparations as those shown in Fig. 1A developed with polyclonal antisera prepared to purified HisTag-rNspA (19). The results show that all three vesicle preparations (lanes 1 to 3) and rNspA (lane 4) contain bands at ∼18 kDa that react with anti-HisTag NspA. Additional bands corresponding to partially unfolded NspA having an apparent mass of ∼21 kDa also are visible for MV prepared from strains RM1090 and BZ198 (lanes 1 and 2). Also, the anti-HisTag NspA antibody appears to be reactive with proteins in the MV and OMV preparations, with apparent masses of ∼33 kDa that may correspond to Opa proteins that are known to contain several segments having the same amino acid sequences as segments in NspA (19). Based on the results of SDS-PAGE, MV from BZ198 and RM1090 contain the highest relative amount of NspA and OMV from strain Z1092 contains the lowest amount of NspA.
FIG. 1.
(A) Coomassie brilliant blue R250-stained SDS-PAGE gel (15% polyacrylamide). Lanes: 1, RM1090 MV; 2, BZ198 MV; 3, Z1092 OMV; 4, rNspA MV prepared from E. coli BL21(DE3) containing plasmid pGMS1.0; 5, MV prepared from E. coli strain BL21(DE3) containing control plasmid pSK(+) without nspA (10-fold-greater amount of protein loaded). (B) Binding of anti-HisTag-NspA mouse antiserum to the same protein samples shown in panel A as determined by Western blotting.
The MV and OMV also contained LOS and capsular polysaccharide. The amount of LOS in each preparation was determined by using an LAL assay with purified LOS from each vaccine strain as the standard. The amount of LOS measured was 0.3 μg/μg of protein for strain RM1090 MV, 0.2 μg/μg of protein for strain BZ198 MV, and 1.4 μg/μg of protein for strain Z1092 OMV. For comparison, the deoxycholate-extracted OMV used in the Norwegian vesicle vaccine is reported to contain between 0.04 and 0.12 μg of LOS/μg of protein (8).
Capsular polysaccharide was determined by an inhibition ELISA performed on soluble extracts of MV or OMV treated with EDTA to release polysaccharide, as previously described (19). The capsular polysaccharide contents of MV prepared from group C strain RM1090 and group B strain BZ198 and of OMV from group A strain Z1092 were 0.6, 0.003, and 0.009 μg/μg of protein, respectively.
Bactericidal activity of polyclonal antisera prepared in mice and guinea pigs.
Groups of mice (n = 7 to 10) and guinea pigs (n = 3 to 8) were immunized at 0 and 3 weeks with MV prepared from strains RM1090 and BZ198, respectively, and were boosted at 6 weeks with OMV prepared from strain Z1092. Control animals were given three injections of either an equal mixture of MV and OMV prepared from each of the three strains or, as a negative control, MV prepared from E. coli. Mice received a dose containing 5 μg of protein, and guinea pigs received a dose of 25 μg for each injection. Although the animals immunized with the mixture of vesicles received one-third the amount of each respective vesicle preparation with each dose, over the three-dose schedule the animals in this group received the same total amount of each vesicle preparation as that given to animals assigned to the sequential immunization protocol. Each vesicle preparation was given with aluminum phosphate (0.5% [wt/vol]). Serum samples were obtained 2 to 3 weeks after the third injection and were pooled and assayed for bactericidal activity using as a complement source normal human serum that lacked endogenous bactericidal activity and had no detectable group B anticapsular antibody as measured by ELISA. The 19 strains tested (Table 1) included 9 strains that expressed PorA homologous to 1 of the 3 vaccine strains as determined by VR type and 10 strains that expressed heterologous PorA. The negative control antisera from mice or guinea pigs immunized with E. coli MV were negative for bactericidal activity with all 19 strains tested (titers of <1:4). The positive control, a murine anticapsular MAb (SEAM 12; immunoglobulin G2a [IgG2a]) (11), killed all 17 group B strains at similar respective concentrations of antibody. Positive controls for the group A (Z1092) and group C (RM1090) test strains, consisted of dilutions of a human serum pool obtained from adults immunized with meningococcal polysaccharide vaccine (Menomune; Aventis Pasteur, Swiftwater, Pa.), and a murine group C anticapsular MAb (C2/735) (9), respectively.
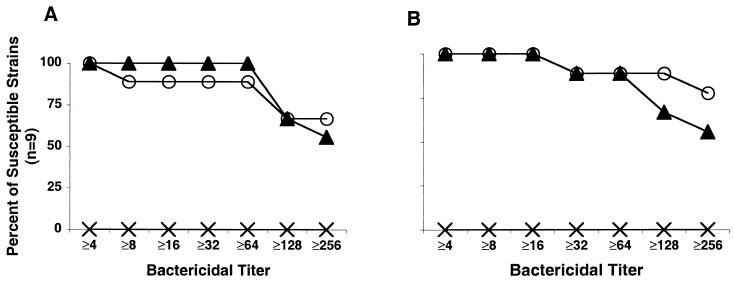
Figure 2A shows the reverse cumulative distributions of the bactericidal titers of pooled mouse antisera tested against nine N. meningitidis strains with homologous PorA to that of one of the three vaccine strains. Figure 2B shows the corresponding data for the guinea pig antisera. The complement-mediated bactericidal titers of the antiserum pools against each of the strains tested is summarized in Table 2. Antisera prepared from mice immunized with meningococcal vesicles, either by sequential immunization or with three doses of a mixture of vesicles, were bactericidal (titer of ≥1:8) against nine of nine or eight of nine of the homologous strains tested, respectively. The results for the corresponding guinea pig sera were similar (nine of nine strains tested for both sequential and mixture immunization). For many of the strains the titers in the antisera after the sequential or mixture immunization schedules were 1:128 or greater.
FIG. 2.
Reverse cumulative bactericidal titers of antiserum from mice (A) and guinea pigs (B) immunized sequentially (▴) or with three doses of a mixture of vesicles (○) against homologous VR type strains. The corresponding titers for the antisera pool from control animals immunized with three doses of MV prepared from E. coli are indicated by “×”.
TABLE 2.
Complement-mediated bactericidal titers of pooled mouse and guinea pig antisera against homologous and heterologous strainsa
| Homologous strain | Bactericidal activity (1/titerb)
|
Heterologous strain | Bactericidal activity (1/titerb)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mouse
|
Guinea pig
|
Mouse
|
Guinea pig
|
||||||
| Sequential | Mix | Sequential | Mix | Sequential | Mix | Sequential | Mix | ||
| 1000 | 128 | 6 | 64 | 16 | BZ147 | 40 | 51 | 9 | >128 |
| 8047 | 300 | 125 | >128 | >128 | CU385 | 128 | <4 | 12 | 4 |
| BZ198 | 220 | 1000 | 40 | 16 | H44/76 | >128 | 21 | 64 | 8 |
| BZ83 | 109 | 205 | 24 | >128 | MC58 | 8 | <4 | >128 | <4 |
| M986 | 101 | 133 | 128 | >256 | NG3/88 | 4 | <4 | 9 | 5 |
| NGP165 | 120 | 90 | 64 | >256 | NGH15 | >128 | <4 | >128 | 92 |
| NMB | 441 | 141 | >256 | >256 | NGH38 | <4 | <4 | >128 | <4 |
| RM1090 | >128 | >128 | >1024 | >1024 | S3032 | 400 | 230 | 32 | <4 |
| Z1092 | >128 | >128 | >1024 | >1024 | S3446 | <4 | <4 | 24 | 4 |
| SWZ107 | 40 | <4 | <4 | <4 | |||||
For definitions of homologous and heterologous strains, see Table 1 and the text.
That is the dilution of serum that results in 50% killing of bacteria after 60 min of incubation in the presence of human complement.
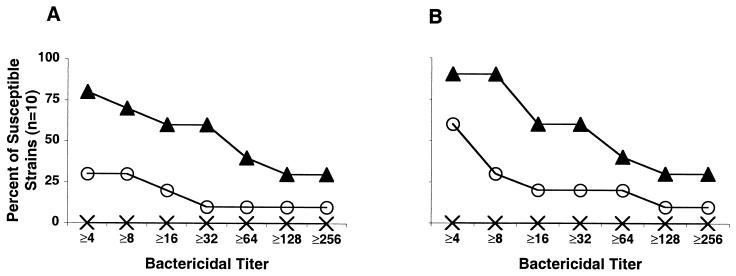
Figure 3 shows the corresponding reverse cumulative distributions of the bactericidal titers measured against 10 strains expressing heterologous PorA to those contained in the three vaccine strains. Antiserum from the mice given sequential immunization killed 7 of 10 strains tested at titers of 1:8 or greater compared to 3 of 10 for mice given three injections of the mixture of vesicles (Fig. 3A, P < 0.08 by Fisher exact test). The corresponding results for the guinea pig antisera were 9 of 10 strains compared to 3 of 10, respectively (Fig. 3B, P < 0.01). For both the mouse and the guinea pig antisera, more heterologous strains were killed at higher titers with the antisera from the animals given sequential immunization than the antisera from the animals given three injections of the mixture.
FIG. 3.
Reverse cummulative bactericidal titers of antiserum from mice (A) and guinea pigs (B) immunized sequentially (▴) or with three doses of a mixture of vesicles (○) against heterologous serosubtype strains. The corresponding titers for the antisera pool from control animals immunized with three doses of MV prepared from E. coli are indicated by “×”.
Although not shown in Fig. 2 and 3, we also measured bactericidal activity of pooled antisera from mice and guinea pigs immunized with three or two injections, respectively, of an OMV vaccine prepared by the Norwegian Institute of Public Health, Oslo (4). The mouse antiserum had titers >1:1,000 against the group B strain H44/76 (B:15:7,16), which was used to prepare the vaccine, as well as to a second group B strain, S3032 (B:19,7:12,16), which also is PorA VR2 type 16. In contrast, there was no detectable bactericidal activity (titers of <1:4) against the remaining 16 of 17 strains. The exception was strain MC58 (B:15:7,16-2), which had a titer of 1:5. MC58 expresses PorA with a VR1 sequence identical to that of H44/76 and a VR2 sequence that differs by a single amino acid from VR2 expressed by the H44/76 vaccine strain (VR2 type 16-2 for MC58 versus 16 for H44/76) (18). The results with antiserum prepared from guinea pigs immunized with two doses of the Norwegian vaccine were similarly limited to strains having type as that of the vaccine strain, except that the guinea pig antiserum also had high bactericidal titers (≥1:128) against strains MC58 and NG3/88 (VR type 7-1,1) that have similar VR1 sequences.
Lack of contribution of anti-LOS or anticapsular antibody to serum bactericidal activity.
The vesicle preparations used for immunization contained LOS and therefore had the potential to elicit anti-LOS antibodies, which could have contributed to the observed bactericidal activity. To determine the possible contribution of anti-LOS antibodies, we purified LOS from each of the three N. meningitidis strains used to prepare the vesicle vaccines as described in Materials and Methods. The resulting LOS preparations were used as solid-phase antigens in an ELISA to measure anti-LOS antibody titers and to prepare LOS-absorbent columns. Immunotyping MAbs 9-2-L379 (strongly reactive with LOS from strain RM1090 and weakly with LOS from strain BZ198) and 2-14-L10 (strongly reactive with LOS from strain Z1092) were used as positive controls in the anti-LOS antibody ELISA. Although the vesicles used for immunization of the animals contained relatively large amounts of LOS (0.2 to 1.4 μg/μg of protein), the anti-LOS titers of the pooled guinea pig antisera to each of the three LOS antigens were less than 1:400 irrespective of the immunization protocol used. In mice, the highest anti-LOS titers in the animals given the sequential immunization schedule were against LOS prepared from immunizing strain BZ198 (titer of 1:900). The respective titers to LOS prepared from strains RM1090 and Z1092 were <1:100 and 1:150. To determine the possible contribution of the LOS-reactive antibody to the bactericidal activity of the serum pool from animals given the sequential immunization, we absorbed LOS-reactive antibody by using affinity columns containing LOS from each of the three immunizing strains. Although the LOS column absorptions removed ≥90% of the antibody reactive with LOS (anti-LOS titers of ≤1:100), there was no significant change in the bactericidal titers against a representative heterologous N. meningitidis strain, S3032 (B:19,7:12,16:L3,7) (bactericidal titers of 1:259 for the LOS-absorbed antiserum and 1:234 for the unabsorbed serum). Similarly, the LOS absorption did not decrease the bactericidal activity of the corresponding guinea pig antiserum. Taken together, the data indicate that anti-LOS antibodies do not contribute to the observed bactericidal activity, although this possibility cannot be completely excluded for all of the strains.
The vesicles used for immunization also contained small amounts of group A and B capsular polysaccharide and larger amounts of group C polysaccharide (see above). For group A and B polysaccharides, there were no detectable serum anticapsular antibody titers in the mouse or guinea pig antisera (serum antibody titers by ELISA of <1:100). There also was no detectable group C anticapsular antibody in the guinea pig antiserum pools (titers of <1:75) compared to a titer of >1:2,000 in control antiserum from guinea pigs given two doses of a group C polysaccharide protein conjugate (D. M. Granoff, I. Aaberge, B. Haneberg, J. Holst, and H. Raff, Abstr. 11th Int. Pathogenic Neisseria Conf., p. 61, 1998). The guinea pig antisera also were negative for group C anticapsular antibody (<0.4 μg/ml) when tested by a radioantigen binding assay (10). However, the antiserum pools from mice given the sequential or mixture immunization had group C anticapsular antibody titers of 1:100 and 1:500, respectively, as measured by ELISA. The only group C strain tested for bactericidal activity with these mouse antiserum pools was the vaccine strain, RM1090, which was included in the group of the nine strains tested with homologous PorA VR types. The bactericidal titers of both pools against this strain were >1:1,000. At a dilution of 1:1,000, it is unlikely that anticapsular antibody contributed to the observed bactericidal activity, given the relatively low anticapsular antibody titers measured by ELISA (estimated to be <4 μg/ml in undiluted sera).
Of the 19 strains tested for susceptibility to bactericidal activity, 17 were capsular group B strains. None of the antisera from guinea pigs or mice given the sequential or mixture immunization had detectable group B anticapsular antibody by ELISA. To be certain that group B anticapsular antibodies were not contributing to the observed bactericidal activity, we tested the bactericidal activity of antisera prepared from the mice or guinea pigs against a subset of homologous and heterologous group B strains in the presence or absence of 50 μg/ml of group B polysaccharide. This concentration of soluble polysaccharides was chosen because it is 50-fold higher than that needed to inhibit >90% of the binding of a panel of group B anticapsular antibodies (2). There was no significant decrease in the respective bactericidal titers measured with or without the inhibitor (data not shown). Thus, group B anticapsular antibodies also do not contribute to the serum bactericidal antibody elicited by the sequential immunization.
Role of anti-NspA antibodies in bactericidal activity.
The strains used to prepare the vesicle vaccines were expressed the highly conserved protein NspA. In an ELISA with E. coli MV containing rNspA as the solid-phase antigen, antiserum from guinea pigs given sequential vesicle immunization had a fivefold-higher anti-NspA titer (1:76,000) than that of animals immunized with a mixture of vesicles (1:16,000). The corresponding titers in the mouse antisera were 1:48,000 and 1:9,000, respectively. The titers of each antiserum pool measured against the corresponding E. coli MV without rNspA were <1:100.
To determine the possible role of anti-NspA antibodies in the serum bactericidal activity induced by the sequential immunization, we measured the bactericidal titers against group B strain MC58, which has a PorA serosubtype heterologous to those of the three strains used to prepare the vesicle vaccines, and to a mutant strain of MC58 in which the gene encoding NspA had been inactivated (21). To determine the possible contribution of anti-PorA antibodies, we also measured bactericidal activity against an MC58 strain that no longer expressed PorA (selected with an anti-PorA P1.7 antibody MN14C11.6 as described earlier [21]). The results obtained with guinea pig antisera are summarized in Table 3. A similar experiment with the antisera from mice was not done because of a lack of bactericidal activity against this strain. The bactericidal titer of the antiserum from guinea pigs given the sequential immunization as measured against the MC58 parent strain was similar to that of the PorA-deficient MC58 strain (1:290 versus 1:484, a difference within the experimental error of the assay). In contrast, there was an ∼10-fold decrease in the bactericidal titer measured against the MC58 NspA knockout strain (titer of 1:32). These results show that a portion of the bactericidal antibody against a strain with a heterologous PorA serosubtype is directed against NspA. In this experiment, the antisera from guinea pigs given a mixture of vesicles or those immunized with vesicles prepared from E. coli lacked bactericidal activity against any of the MC58 variant strains.
TABLE 3.
Complement-mediated bactericidal activity of guinea pig antisera measured against strain MC58 and two mutant strains derived from MC58
| Antiserum or MAb | Bactericidal activity (titer or MAb concn [μg/ml])a
|
||
|---|---|---|---|
| Parent | ΔNspA | PorA-Negative | |
| Sequential immunization | 1:290 | 1:32 | 1:484 |
| Mixed immunization | <1:4 | <1:4 | <1:4 |
| E. coli vesicle immunization | <1:4 | <1:4 | <1:4 |
| Anti-PorA P1.7 MAb | 1:3,000 | 1:2,200 | <1:4 |
| Anti-NspA 14C7 MAb | 204 | >820 | 182 |
| Anticapsular group B MAb | 150 | 75 | 75 |
That is, the concentration of antibody or dilution of polyclonal antiserum required to give 50% killing after 60 min of incubation with human complement compared to that of controls in the absence of antibody or in the presence of antibody and heat-inactivated complement. Typically, the CFU/milliliter at 60 min is 150 to 200% the CFU/milliliter at time zero.
As a control for variation in susceptibility to complement-mediated bacteriolysis of the mutants, all three strains were killed by similar concentrations of an anticapsular MAb. Also, the MC58 parent and mutant MC58ΔNspA stains showed similar susceptibility to a bactericidal anti-PorA MAb (anti-P1.7), whereas the MC58 strain that no longer expressed PorA was completely resistant. Finally, the MC58 parent and PorA-deficient strain showed similar susceptibility to a bactericidal anti-NspA MAb (14C7; see below), whereas the MC58ΔNspA mutant was resistant to this MAb.
MAbs prepared from mice sequentially immunized with MV and OMV.
A CD1 mouse given sequential immunization with MV and OMV was sacrificed 3 days after the third dose, and the spleen cells were fused with myeloma cells as described previously (21). Hybridoma supernatants were screened for antibody binding activity by a whole-bacterial-cell ELISA with two encapsulated group B strains (1000 and CU385) that are additionally relatively resistant to complement-mediated bacteriolysis by anti-rNspA antibodies (21). Supernatants that were found to be positive by ELISA were retested for bactericidal activity against group B strain 8047. One of the positive hybridoma cell lines, designated 14C7, produced an IgG3 antibody that bound by ELISA to strain BZ198 but did not bind to an isogenic mutant in which the NspA gene had been inactivated. This MAb also bound by ELISA to rNspA expressed from plasmid pGMS1.0 in E. coli but not to membranes from the same E. coli strain without nspA. Therefore, the MAb 14C7 is specific for NspA.
Table 4 summarizes the bactericidal activity of MAb 14C7 as measured against 10 group B strains. Six of these ten strains were selected based on being resistant in previous studies to complement-mediated bactericidal activity induced by polyclonal or monoclonal antibodies prepared to rNspA (19, 21). The remaining four strains (1000, 8047, BZ198, and S3446) were known to be susceptible to complement-mediated lysis by anti-rNspA antibody (19, 21). In the presence of human complement, MAb 14C7 was bactericidal for these four known susceptible strains, as well as for four of the strains (BZ232, CU385, MC58, and M986) previously found to be resistant. For the two strains not killed by 14C7 (NG3/88 and NGP165), the MAb was bacteriostatic (>50%, but <100% survival after 1 h of incubation with antibody and complement compared to the CFU/ml at time zero). For the strains killed by MAb 14C7, the concentrations required for 50% bacteriolysis were, on average, 10-fold lower than those required by anti-NspA MAbs previously isolated from mice immunized with rNspA (representative data are shown in Table 4 for AL12, an IgG2a MAb).
TABLE 4.
Complement-mediated bactericidal activity of two anti-NspA MAbs
| Strain | Bactericidal activity (μg/ml)a
|
|
|---|---|---|
| AL12 (Ig2a)b | 14C7 (IgG3)b | |
| 1000c | 32 | 4 |
| 8047c | 40 | 2 |
| BZ198c | 30 | 2 |
| S3446c | 80 | 7 |
| BZ232 | >600 | 94 |
| CU385 | >600 | 45 |
| M986 | >600 | 50 |
| MC58 | >600 | 204 |
| NG3/88 | >600 | (120) |
| NGP165 | >600 | (70) |
| BZ198ΔNspA | >600 | >60 |
See Table 3, footnote a.
The MAb 14C7 is a subclass IgG3 MAb and was prone to loss of activity both during purification and as a purified antibody. Therefore, for uniformity, both the 14C7 and the AL12 MAbs used in the assay were precipitated from culture supernatant by ammonium sulfate (50%) and resuspended and dialyzed in PBS buffer containing 1% (wt/vol) BSA. Note that the previously reported concentrations of AL12 for bacteriolysis were slightly lower since purified antibody was used in the experiments. Values in parentheses were static.
Strains selected based on previous data that they were susceptible to complement-mediated anti-NspA bactericidal antibody. The remaining strains were selected based on data showing resistance to anti-NspA bactericidal activity.
Passive protective activity in infant rat model.
Anti-NspA MAb 14C7 also was more active than anti-rNspA MAb AL12 in the ability to confer passive protection of infant rats challenged with encapsulated group B strains. For these experiments we selected two group B challenge strains, BZ232 and M986, which in previous experiments were moderately or completely resistant, respectively, to passive protection by monoclonal or polyclonal antibodies prepared to recombinant NspA (19, 21). In experiment 1 (Table 5), 25 μg of MAb 14C7/rat given i.p. at time zero gave better protection against development of meningococcal bacteremia in infant rats caused by strain BZ232 than a dose of 25 or 100 μg of MAb AL12 prepared against rNspA/rat (P < 0.03).
TABLE 5.
Passive protection of infant rats challenged with N. meningitidis group B strains
| Expt no. (group B challenge strain) | Pretreatment MAb (immunoglobulin subclass) or antiseruma | Dose (μg) per rat or serum dilution | Blood culture at 18 h
|
|
|---|---|---|---|---|
| No. positive/total no. | CFU/mld | |||
| 1 (BZ232) | Irrelevant (G1) | 100 | 7/7 | 29 |
| Anticapsular MAb (G2b) | 25 | 1/7 | 0.002 | |
| Anti-rNspA MAb AL12 (G2a) | 100 | 7/7b | 23 | |
| 25 | 7/7b | 58 | ||
| Anti-NspA MAb 14C7 (G3) | 25 | 3/7b | 0.4 | |
| 5 | 7/7 | 42 | ||
| 1 | 7/7 | 64 | ||
| 2 (M986) | Irrelevant (G1) | 100 | 7/7 | 75 |
| Anticapsular MAb (G2b) | 25 | 4/7 | 0.02 | |
| Anti-rNspA MAb AL12 (G2a) | 100 | 7/7 | 296 | |
| 25 | 7/7 | 355 | ||
| Anti-NspA MAb 14C7 (IgG3) | 25 | 5/7 | 5 | |
| 5 | 7/7 | 381 | ||
| 1 | 7/7 | 169 | ||
| 3 (M986) | Anticapsular MAb (G2b) | 20 | 1/6 | 0.002 |
| Anti-E. coli MV (guinea pig) | 1:5 | 6/6c | 630 | |
| Anti-NspA 14C7 (G3) | 10 | 5/7 | 5 | |
| Anti-N. meningitidis vesicle (sequential guinea pig) | 1:25 | 1/6c | 0.001 | |
Five- to seven-day-old infant rats were treated i.p. with different doses of control or anti-NspA MAbs or, in experiment 3, dilutions of antiserum prepared in guinea pigs given sequential immunizations with MV, and OMV. Two hours later, the animals were challenged i.p. In experiment 1, the animals were given with 6 × 103 CFU of strain BZ232. In experiments 2 and 3, the challenges were with 3.5 × 103 CFU and 4.8 × 103 CFU of strain M986, respectively. Quantitative blood cultures were obtained 18 h later. For calculation of the geometric mean CFU/milliliter, animals with sterile cultures (100 μl of blood) were assigned values of 1 CFU/ml.
P < 0.03, comparing 14 of 14 animals with bacteremia in the combined groups pretreated with 100 or 25 μg of MAb AL12 per rat versus 3 of 7 animals with bacteremia treated with 25 μg of MAb 14C7. The difference between the respective geometric means of the CFU/milliliter valves in experiment 1 was also significant (P = 0.01).
P < 0.02 by Fisher exact test. In experiments 2 and 3, the differences in the respective geometric mean CFU/milliliter values between animals pretreated with AL12 or 14C7 (experiment 2) or between animals treated with 14C7 and anti-E. coli MV antisera (experiment 3) are not significant (0.05 < P ≤ 0.12). In experiments 1 and 2, the respective geometric means of the CFU/milliliter values of animals given the irrelevant MAb are not significantly different from those given the corresponding dose of AL12 (P ≥ 0.2).
Geometric mean value/103.
In experiment 2, there also was a trend for MAb 14C7 to give better protection against strain M986 than AL12 (0.05 < P < 0.12; Table 5, footnote c). In experiment 3, a similar trend was observed for MAb 14C7 to give partial protection against M986, compared to the respective geometric mean CFU/milliliter in blood from animals pretreated with control antiserum prepared to E. coli proteins (0.05 < P < 0.10; Table 5). In experiment 3, pretreatment of rats with a 1:25 dilution of antiserum from guinea pigs immunized sequentially with MV or OMV was protective against this strain (P < 0.02).
Possible role of other antigens in eliciting protective antibodies.
In addition to MAb 14C7, we isolated several other MAbs that were broadly reactive with diverse meningococcal strains when tested in a whole-bacterial-cell ELISA. However, these MAbs bound poorly to live cells in a flow cytometry assay (21) and were not bactericidal (data not shown). The MAbs were reactive with a 36-kDa outer membrane protein in Western blots and in immunoprecipitation experiments (data not shown). Based on the estimated mass of the target protein and its ubiquitous presence in all Neisseria strains tested, these MAbs may be specific for reduction modifiable protein (RmpM). These putative anti-RmpM MAbs did not enhance or inhibit bactericidal activity when combined with anti-NspA or anti-PorA MAbs (data not shown). Therefore, it is unlikely that these antibodies contributed to the bactericidal activity in the polyclonal antisera from animals given the sequential immunization.
DISCUSSION
Immunization with vesicle vaccines prepared from a single meningococcal strain is known to elicit bactericidal antibody responses that are directed primarily against surface-exposed loops of PorA (36) and, to a lesser extent, Opc (27). For example, mice immunized in the present study with three doses of OMV vaccine prepared at the National Institute of Public Health (NIPH; Oslo, Norway) from group B strain H4476 developed serum bactericidal antibody titers of >1:1,000 when measured against strain H44/76 or against a second group B strain with the same PorA VR2 type. However, the titer against group B strain MC58, which has a single-amino-acid difference in the loop 4 sequence (VR2) of PorA compared to that of strain H4476 (18), was only 1:5. In contrast, antiserum from guinea pigs immunized with two doses of the Norwegian vaccine was highly bactericidal against both strains H44/76 and MC58 and against other strains having the homologous PorA VR types but not against strains having different VR types. The different results with strain MC58 between mice and guinea pigs immunized with NIPH vesicle vaccine underscore the potential for animal species differences to influence strain-specific bactericidal results.
In the present study, both guinea pigs and mice immunized with three injections of a mixture of vesicles prepared from three N. meningitidis strains developed high serum bactericidal antibody responses when measured against the three strains used to prepare the vaccines, as well as to six other test strains with homologous PorA VR types (Fig. 2). However, these antisera had a more-limited bactericidal activity against a panel of group B strains with heterologous PorA VR types (Fig. 3). These results were anticipated since the animals given three injections of the mixture would be expected to have serum bactericidal antibody responses directed primarily at antigenic domains on loops 1 and/or 4 of the PorA molecules from each of the three vaccine strains. Further, these antibodies would be anticipated to react poorly with most PorA molecules with heterologous VR types.
The most important finding in our study was the broad spectrum of bactericidal activity found in sera from both mice and guinea pigs immunized sequentially with vesicles prepared from three strains with heterologous PorA, PorB, and capsular groups. Although the animals given the sequential immunization schedule received only a single dose of each of the vesicles containing a particular PorA, high serum bactericidal titers (i.e., ≥32) were present against most group B strains with either homologous or heterologous PorA VR types (Fig. 2 and 3, respectively). The targets of the bactericidal antibodies in these sera likely include PorA and a number of other antigens, some of which remain undefined.
In designing the present study, our hypothesis was that a portion of the antibody responses of animals given the sequential immunization would be directed, in part, at relatively conserved antigenic domains that are relatively poorly immunogenic when multiple doses of vesicles are given from one strain or a mixture of strains. Consistent with this hypothesis was our finding that the animals given the sequential immunization schedule had fivefold-greater serum antibody titers to NspA than that of the control animals immunized with three injections of the mixture of the vesicles. Also, the bactericidal titer of antiserum from sequentially immunized guinea pigs decreased ∼10-fold when measured against a mutant strain MC58 that was deficient in NspA expression compared to the corresponding titer measured against the parent strain that expressed NspA (Table 4). NspA is a previously described highly conserved membrane protein that is capable of eliciting serum bactericidal antibodies in experimental animals (17). However, the protein is present in relatively low copy numbers (17, 19) and is reported to be poorly immunogenic in humans recovering from meningococcal disease (J. L. Farrant, J. S. Kroll, B. R. Brodeur, and D. Martin, Abstr. 11th Int. Pathogenic Neisseria Conf., p. 208, 1998). Conceivably, in the present study, the use of MV vaccines prepared from two strains expressing relatively large amounts of NspA may have contributed to the relatively high anti-NspA antibody responses of the mice or guinea pigs given the sequential immunization.
Finally, we also produced an anti-NspA hybridoma cell line from the spleen of a mouse given the sequential immunization, and the MAb expressed (14C7) appeared to have superior bactericidal activity (Table 4) and passive protective activity against bacteremia in infant rats (Table 5) than that of polyclonal or MAbs prepared by us previously in mice immunized with recombinant NspA (21). Although we cannot exclude the possibility that greater functional activity of anti-NspA MAb 14C7 is a result of it being a different IgG subclass (IgG3) than that of the AL12 MAb prepared to rNspA (IgG2a), it seems more likely that the superior functional activity of the 14C7 MAb is a result of reactivity with different NspA epitopes expressed in neisserial vesicles than that of antibodies made in response to the recombinant protein.
While anti-NspA antibodies contribute to the broad-spectrum bactericidal activity elicited in response to the sequential immunization, it is likely that antibodies to other conserved antigens are also important. For example, the guinea pig antisera from animals given the sequential immunization retained a significant bactericidal titer (≥1:32) against strains deficient in NspA and/or PorA (Table 3). The identity of these other antigenic targets remains unknown and will require further investigation.
Sequential vaccination with vesicle vaccines shows promise as an approach to elicit broad-based immunity to N. meningitidis. However, the vaccine preparations used in the present study contained capsular polysaccharide and relatively large amounts of LOS that are undesirable in a vaccine intended for use in humans. The results of preliminary studies in mice and guinea pigs with vesicle vaccines for sequential immunization that were treated with deoxycholate as described for the preparation of the Norwegian vesicle vaccine (8), and which would be more suitable for use in humans, showed poor anti-NspA antibody responses and poor serum bactericidal responses against heterologous VR type strains (unpublished data). Thus, for a vaccine intended for humans, alternative approaches to prepare vesicles are needed to minimize or eliminate unwanted components. Similarly, although the presence of LOS or group B capsular polysaccharide in the vaccines used in the present study did not elicit significant serum antibody responses that contributed to the observed broad-spectrum serum bactericidal activity, their presence in the vesicle preparations could have been important in maintaining “native” epitopes in other antigens such as NspA, PorA, or other as-yet-unidentified antigens. The LOS present in the vaccine preparations may also have had an adjuvant effect (34). These questions also will need to be addressed before considering testing the sequential immunization approach in humans.
Acknowledgments
This work was supported by grants RO1 AI45642 and AI46464 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
We thank S. Lee (Children's Hospital Oakland Research Institute [CHORI]) for vesicle preparation and in vivo protection studies; K. Alter Lewis (CHORI) for purifying LOS, preparing the LOS affinity column, and absorbing anti-LOS antibody from the antiserum pools; and A. Dave (CHORI) for performing flow cytometry experiments. We also thank I. Feavers and S. Hardy of the National Institute of Biological Standards and Control (United Kingdom) for kindly performing DNA sequencing of PorA genes. The NIPH vesicle vaccine was a gift from the NIPH, Oslo, Norway. The anti-LOS immunotype MAbs were a gift from W. Zollinger, Walter Reed Army Institute of Research, Washington, D.C.
Editor: D. L. Burns
REFERENCES
- 1.Apicella, M. A., J. M. Griffiss, and H. Schneider. 1997. Isolation and characterization of lipopolysaccharides, lipooligosaccharides, and lipid A, p. 123-133. In V. L. Clark and P. M. Bavoil (ed.), Bacterial pathogenesis. Academic Press, Inc., San Diego, Calif.
- 2.Azmi, F. H., A. H. Lucas, H. L. Spiegelberg, and D. M. Granoff. 1995. Human immunoglobulin M paraproteins cross-reactive with Neisseria meningitidis group B polysaccharide and fetal brain. Infect. Immun. 63:1906-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beveridge, T. J. 1999. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 181:4725-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjune, G., J. K. Gronnesby, E. A. Hoiby, O. Closs, and H. Nokleby. 1991. Results of an efficacy trial with an outer membrane vesicle vaccine against systemic serogroup B meningococcal disease in Norway. NIPH Ann. 14:125-132. [PubMed] [Google Scholar]
- 5.Brett, P. J., I. M. Feavers, and B. M. Charalambous. 2001. Identification of peptides that mimic N. meningitidis LOS epitopes via the use of combinatorial phage-display libraries, p. 181-197. In A. J. Pollard and M. C. J. Maiden (ed.), Meningococcal vaccines: methods and protocols. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 6.Cartwright, K., N. Noah, and H. Peltola. 2001. Meningococcal disease in Europe: epidemiology, mortality, and prevention with conjugate vaccines. Report of a European advisory board meeting, Vienna, Austria, 6 to 8 October, 2000. Vaccine 19:4347-4356. [DOI] [PubMed] [Google Scholar]
- 7.Finne, J., D. Bitter-Suermann, C. Goridis, and U. Finne. 1987. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J. Immunol. 138:4402-4407. [PubMed] [Google Scholar]
- 8.Frasch, C. E., L. van Alphen, J. Holst, J. T. Poolman, and E. Rosenqvist. 2001. Outer membrane protein vesicle vaccines for meningococcal disease, p. 81-107. In A. J. Pollard and M. C. J. Maiden (ed.), Meningococcal vaccines: methods and protocols. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 9.Garcia-Ojeda, P. A., M. E. Monser, L. J. Rubinstein, H. J. Jennings, and K. E. Stein. 2000. Murine immune response to Neisseria meningitidis group C capsular polysaccharide: analysis of monoclonal antibodies generated in response to a thymus-independent antigen and a thymus-dependent toxoid conjugate vaccine. Infect. Immun. 68:239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotschlich, E. C., M. Rey, R. Triau, and K. J. Sparks. 1972. Quantitative determination of the human immune response to immunization with meningococcal vaccines. J. Clin. Investig. 51:89-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granoff, D. M., A. Bartoloni, S. Ricci, E. Gallo, D. Rosa, N. Ravenscroft, V. Guarnieri, R. C. Seid, A. Shan, W. R. Usinger, S. Tan, Y. E. McHugh, and G. R. Moe. 1998. Bactericidal monoclonal antibodies that define unique meningococcal B polysaccharide epitopes that do not cross-react with human polysialic acid. J. Immunol. 160:5028-5036. [PubMed] [Google Scholar]
- 12.Hayrinen, J., H. Jennings, H. V. Raff, G. Rougon, N. Hanai, R. Gerardy-Schahn, and J. Finne. 1995. Antibodies to polysialic acid and its N-propyl derivative: binding properties and interaction with human embryonal brain glycopeptides. J. Infect. Dis. 171:1481-1490. [DOI] [PubMed] [Google Scholar]
- 13.Jennings, H. J., and C. Lugowski. 1981. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J. Immunol. 127:1011-1018. [PubMed] [Google Scholar]
- 14.Jodar, L., I. M. Feavers, D. Salisbury, and D. M. Granoff. 2002. Development of vaccines against meningococcal disease. Lancet 359:1499-1508. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 16.Martin, D., B. R. Brodeur, J. Hamel, F. Couture, U. de Alwis, Z. Lian, S. Martin, D. Andrews, and R. W. Ellis. 2000. Candidate Neisseria meningitidis NspA vaccine. J. Biotechnol. 83:27-31. [DOI] [PubMed] [Google Scholar]
- 17.Martin, D., N. Cadieux, J. Hamel, and B. R. Brodeur. 1997. Highly conserved Neisseria meningitidis surface protein confers protection against experimental infection. J. Exp. Med. 185:1173-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGuinness, B. T., I. N. Clarke, P. R. Lambden, A. K. Barlow, J. T. Poolman, D. M. Jones, and J. E. Heckels. 1991. Point mutation in meningococcal porA gene associated with increased endemic disease. Lancet 337:514-517. [DOI] [PubMed] [Google Scholar]
- 19.Moe, G. R., S. Tan, and D. M. Granoff. 1999. Differences in surface expression of NspA among Neisseria meningitidis group B strains. Infect. Immun. 67:5664-5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moe, G. R., S. Tan, and D. M. Granoff. 1999. Molecular mimetics of polysaccharide epitopes as vaccine candidates for prevention of Neisseria meningitidis serogroup B disease. FEMS Immunol. Med. Microbiol. 26:209-226. [DOI] [PubMed] [Google Scholar]
- 21.Moe, G. R., P. Zuno-Mitchell, S. S. Lee, A. H. Lucas, and D. M. Granoff. 2001. Functional activity of antineisserial surface protein A monoclonal antibodies against strains of Neisseria meningitidis serogroup B. Infect. Immun. 69:3762-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morley, S. L., M. J. Cole, C. A. Ison, M. A. Camaraza, F. Sotolongo, N. Anwar, I. Cuevas, M. Carbonero, H. C. Campa, G. Sierra, and M. Levin. 2001. Immunogenicity of a serogroup B meningococcal vaccine against multiple Neisseria meningitidis strains in infants. Pediatr. Infect. Dis. J. 20:1054-1061. [DOI] [PubMed] [Google Scholar]
- 23.Peppler, M. S., and C. E. Frasch. 1982. Protection against group B Neisseria meningitidis disease: effect of serogroup B polysaccharide and polymyxin B on immunogenicity of serotype protein preparations. Infect. Immun. 37:264-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliani, B. Arico, M. Comanducci, G. T. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J. Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816-1820. [DOI] [PubMed] [Google Scholar]
- 25.Plested, J. S., K. Makepeace, M. P. Jennings, M. A. Gidney, S. Lacelle, J. Brisson, A. D. Cox, A. Martin, A. G. Bird, C. M. Tang, F. M. Mackinnon, J. C. Richards, and E. R. Moxon. 1999. Conservation and accessibility of an inner core lipopolysaccharide epitope of Neisseria meningitidis. Infect. Immun. 67:5417-5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poolman, J., and F. X. Berthet. 2001. Alternative vaccine strategies to prevent serogroup B meningococcal diseases. Vaccine 20(Suppl. 1):S24-S26. [DOI] [PubMed] [Google Scholar]
- 27.Rosenqvist, E., E. A. Hoiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Ronnild, G. Bjune, and H. Nokleby. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 63:4642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, L. Lefkowitz, M. L. Cartter, R. Danila, P. Cieslak, K. A. Shutt, T. Popovic, A. Schuchat, L. H. Harrison, and A. L. Reingold. 1999. The changing epidemiology of meningococcal disease in the United States, 1992-1996. J. Infect. Dis. 180:1894-1901. [DOI] [PubMed] [Google Scholar]
- 29.Rougon, G., C. Dubois, N. Buckley, J. L. Magnani, and W. Zollinger. 1986. A monoclonal antibody against meningococcus group B polysaccharides distinguishes embryonic from adult N-CAM. J. Cell Biol. 103:2429-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacchi, C. T., A. M. Whitney, T. Popovic, D. S. Beall, M. W. Reeves, B. D. Plikaytis, N. E. Rosenstein, B. A. Perkins, M. L. Tondella, and L. W. Mayer. 2000. Diversity and prevalence of PorA types in Neisseria meningitidis serogroup B in the United States, 1992-1998. J. Infect. Dis. 182:1169-1176. [DOI] [PubMed] [Google Scholar]
- 31.Santos, G. F., R. R. Deck, J. Donnelly, W. Blackwelder, and D. M. Granoff. 2001. Importance of complement source in measuring meningococcal bactericidal titers. Clin. Diagn. Lab Immunol. 8:616-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scholten, R. J., B. Kuipers, H. A. Valkenburg, J. Dankert, W. D. Zollinger, and J. T. Poolman. 1994. Lipo-oligosaccharide immunotyping of Neisseria meningitidis by a whole-cell ELISA with monoclonal antibodies. J. Med. Microbiol. 41:236-243. [DOI] [PubMed] [Google Scholar]
- 33.Shenep, J. L., R. S. Munson, Jr., and D. M. Granoff. 1982. Human antibody responses to lipopolysaccharide after meningitis due to Haemophilus influenzae type b. J. Infect. Dis. 145:181-190. [DOI] [PubMed] [Google Scholar]
- 34.Steeghs, L., B. Kuipers, H. J. Hamstra, G. Kersten, L. van Alphen, and P. van der Ley. 1999. Immunogenicity of outer membrane proteins in a lipopolysaccharide-deficient mutant of Neisseria meningitidis: influence of adjuvants on the immune response. Infect. Immun. 67:4988-4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stephens, D. S., J. S. Swartley, S. Kathariou, and S. A. Morse. 1991. Insertion of Tn916 in Neisseria meningitidis resulting in loss of group B capsular polysaccharide. Infect. Immun. 59:4097-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tappero, J. W., R. Lagos, A. M. Ballesteros, B. Plikaytis, D. Williams, J. Dykes, L. L. Gheesling, G. M. Carlone, E. A. Hoiby, J. Holst, H. Nokleby, E. Rosenqvist, G. Sierra, C. Campa, F. Sotolongo, J. Vega, J. Garcia, P. Herrera, J. T. Poolman, and B. A. Perkins. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281:1520-1527. [DOI] [PubMed] [Google Scholar]
- 37.Wyle, F. A., M. S. Artenstein, B. L. Brandt, E. C. Tramont, D. L. Kasper, P. L. Altieri, S. L. Berman, and J. P. Lowenthal. 1972. Immunologic response of man to group B meningococcal polysaccharide vaccines. J. Infect. Dis. 126:514-521. [DOI] [PubMed] [Google Scholar]
- 38.Yang, Q., and H. J. Jennings. 2001. Purification of capsular polysaccharide, p. 41-47. In A. J. Pollard and M. C. J. Maiden (ed.), Meningococcal vaccines: methods and protocols. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 39.Zollinger, W. D., R. E. Mandrell, J. M. Griffiss, P. Altieri, and S. Berman. 1979. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J. Clin. Investig. 63:836-848. [DOI] [PMC free article] [PubMed] [Google Scholar]