Abstract
KATP channels assemble from four regulatory SUR1 and four pore-forming Kir6.2 subunits. At the single-channel current level, ATP-dependent gating transitions between the active burst and the inactive interburst conformations underlie inhibition of the KATP channel by intracellular ATP. Previously, we identified a slow gating mutation, T171A in the Kir6.2 subunit, which dramatically reduces rates of burst to interburst transitions in Kir6.2ΔC26 channels without SUR1 in the absence of ATP. Here, we constructed all possible mutations at position 171 in Kir6.2ΔC26 channels without SUR1. Only four substitutions, 171A, 171F, 171H, and 171S, gave rise to functional channels, each increasing Ki,ATP for ATP inhibition by >55-fold and slowing gating to the interburst by >35-fold. Moreover, we investigated the role of individual Kir6.2 subunits in the gating by comparing burst to interburst transition rates of channels constructed from different combinations of slow 171A and fast T171 “wild-type” subunits. The relationship between gating transition rate and number of slow subunits is exponential, which excludes independent gating models where any one subunit is sufficient for inhibition gating. Rather, our results support mechanisms where four ATP sites independently can control a single gate formed by the concerted action of all four Kir6.2 subunit inner helices of the KATP channel.
INTRODUCTION
The KATP channel mechanism by which ATP site occupancy couples to inhibition gate closure is at the heart of the signal transduction coordinating cell physiology throughout our bodies (Aguilar-Bryan et al., 2001; Ashcroft, 1988). Elevated energy metabolism, signaled by an increase in the ATP/ADP ratio, inhibits channel activity, which triggers cell electrical excitability typically leading to Ca2+ influx. In the β-cell of the endocrine pancreas, this signaling stimulates insulin secretion in response to high blood glucose levels. In other cells of the body, the KATP channel plays similar roles governing Ca2+ influx. In heart and smooth muscle, the signal flow helps regulate contraction and relaxation (Alekseev et al., 1998; Deutsch et al., 1994; Fan and Makielski, 1999; Noma, 1983; Terzic et al., 1994). In the nervous system, the signaling regulates neurotransmitter secretion (Amoroso et al., 1990; Ashcroft, 1988; Lee et al., 1996; Miki et al., 2001; Schmid-Antomarchi et al., 1990), as it does in the pancreas. Knowledge of inhibition gating of the KATP channel by ATP is therefore critical to our understanding of the signaling mechanisms coupling energy metabolism and cell excitability that regulate muscle contraction and vesicle exocytosis.
Understanding how the KATP channel transduces changes in the ATP signal of energy metabolism into changes in membrane excitability has been greatly advanced over the last two decades. Noma (1983) first demonstrated KATP channel activity and revealed its burst gating behavior where rapid interconversions between an open and closed state are interrupted by long-lived closed states. Indeed, KATP channel activity occurs in bursts of brief openings that alternate with briefer closings, and these active burst episodes are separated by long-lived inactive interburst intervals (Alekseev et al., 1998; Ashcroft et al., 1984; Babenko et al., 1999a; Cook and Hales, 1984; Drain et al., 1998; Gillis et al., 1989; Nichols et al., 1991; Qin et al., 1989; Trapp et al., 1998). In the context of ATP inhibition gating, the KATP channel has two major gating conformations, an active burst state (open to potassium ion flow) and an inactive interburst state (closed to potassium ion flow) controlled by the movements of a gate closely associated with the transmembrane pore. The briefer closings within the active burst state are likely due to an additional independent gate involving the filter within the extracellular half of the transmembrane pore, which is unaffected by intracellular ATP (Fan and Makielski, 1999; Proks et al., 2001). The transition from the active burst to the inactive interburst state occurs at a relatively slow rate in the absence of ligand (ligand-independent gating) or at a greatly accelerated rate in the presence of intracellular ATP (ATP-dependent gating; Alekseev et al., 1998; Babenko et al., 1999a; Drain et al., 1998; Li et al., 2000, 2002; Nichols et al., 1991; Qin et al., 1989; Trapp et al., 1998; Tucker et al., 1998). When ATP binds to an active channel, it can cause inhibition gate closure. ATP also binds to the inactive interburst state. When ATP binds to the inactive interburst state, bound ATP further stabilizes the already shut gate of the interburst state, prolonging its life. Although ligand-independent and ATP-dependent gating to the interburst state differ in rate and by the presence of bound ATP, the two processes likely share the same mechanisms of pore occlusion (Drain et al., 1998; Li et al., 2000, 2002; Trapp et al., 1998; Tucker et al., 1998).
At the molecular level, the KATP channel is assembled from four regulatory SURx subunits and four pore-forming Kir6.x subunits (Aguilar-Bryan et al., 1995; Clement et al., 1997; Inagaki et al., 1995, 1997; Shyng and Nichols, 1997). The SURx subunits mediate regulation of KATP channel activity by inhibitory sulfonylurea, and stimulatory ADP and potassium channel opener ligands (Babenko et al., 1999b,c, 2000; Gribble et al., 1997; Nichols et al., 1996; Shyng et al., 1997b), whereas the Kir6.x subunits mediate inhibition gating by inhibitory ATP (Drain et al., 1998; John et al., 1998; Tucker et al., 1997, 1998). The ATP inhibition gating can be dissected into component gating and ATP binding mechanisms. The ATP binding mechanism is mainly determined by residues of both the proximal and distal cytoplasmic C-terminus of Kir6.2, with residues of the cytoplasmic N-terminus also involved (Tucker et al., 1997, 1998; Drain et al., 1998; Takano et al., 1998; Trapp et al., 1998; Koster et al., 1999; Babenko et al., 1999c; Proks et al., 1999; Reimann et al., 1999; Tanabe et al., 1999, 2000; Li et al., 2000; MacGregor et al., 2002; Vanoye et al., 2002). Not only do the cytoplasmic N- and C-termini of Kir6.2 each function in the inhibition gating by ATP, but the termini might directly interact during the gating transitions (Jones et al., 2001; Tucker and Ashcroft, 1999). Importantly, molecular approaches using the candidate site mutation G334D (Drain et al., 1998) have provided evidence that four identical noncooperative ATP binding sites, one per Kir6.2 subunit, operate during KATP channel inhibition by ATP (Drain et al., 1998; Li et al., 1999; Markworth et al., 2000). There is no study, however, on the number of inhibition gates and their relationship to the four Kir6.2 subunits.
To better understand the ATP-dependent inhibition gating mechanism, it is fundamental to know not only the number of ATP sites but also the number of inhibition gates at work in the channel protein. Here, we investigated the inhibition gate mechanism of the transmembrane pore, the number of inhibition gates in a single KATP channel, and whether Kir6.2 subunits work independently or cooperatively in the gating. We used a previously characterized mutation, T171A, located at the cytoplasmic mouth of the transmembrane pore, expressed in truncated Kir6.2ΔC26 channels without SUR1 (Drain et al., 1998). Unlike the full-length wild-type Kir6.2, the truncated Kir6.2ΔC26 efficiently expresses channels at the plasma membrane in the absence of SUR1 (Tucker et al., 1997; Zerangue et al., 1999; Ma et al., 2001; Sharma et al., 1999). The truncated channels exhibit 10-fold less ATP sensitivity (Tucker et al., 1997); however, the burst-interburst gating kinetics remain sensitive to ATP, similar to wild-type channels with SUR1. Importantly, the burst conformation is destabilized, and the interburst conformations stabilized by ATP bound to Kir6.2ΔC26 channels without SUR1 (Drain et al., 1998; Li et al., 2002). A preliminary account of these findings was reported (Li et al., 2003).
MATERIALS AND METHODS
Mutagenesis
The truncated Kir6.2ΔC26 channel of Tucker et al. (1997) was used as the wild-type or parent channel. The substitutions at position 171 in Kir6.2ΔC26 background were constructed by a saturation mutagenesis technique (Reidharr-Olson et al., 1991), using polymerase chain reaction (PCR) overlap extension and silent sites, as described previously (Li et al., 2000). The N160D (Shyng and Nichols, 1997) and N160D/T171A double mutants were obtained by using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). All the mutants were confirmed by DNA sequencing.
Oocyte expression and electrophysiology
Preparation and injection of Xenopus oocytes, patch pipette fabrication, solutions, and inside-out patch excised recording techniques were as described (Drain et al., 1994, 1998). Briefly, Ki,ATP for ATP inhibition of 171 mutant channels was obtained using different ATP concentrations applied to the patches by constant perfusion of the cytoplasmic face of patches using a Biologic RSC-160 nine-sewer pipe syringe-pressurized system (Molecular Kinetics, Pullman, WA). Recordings were always begun within 1 min after excision with the patch pipette partially inserted into one of the sewer pipes. ATP was added as the magnesium salt to minimize rundown (Trube and Hescheler, 1984), and no other ligands were added until after the ATP dose response data were obtained (Drain et al., 1998; Li et al., 2000). Experiments showing rundown, characterized by a sudden significant decrease in PO were discarded. Patch-clamp currents were amplified with Axopatch 200A (Axon Instruments, Foster City, CA) or EPC-9 (HEKA Elektronik, Lambrecht/Pfalz, Germany) instruments, low-pass filtered with an eight-pole Bessel filter (Frequency Devices, Haverhill, MA) at a corner frequency of 2 or 4 kHz, and sampled at 20 kHz using HEKA PULSE v.8.0 (HEKA Elektronik). For the stoichiometry experiments, single-channel currents were recorded with the inside-out patch configuration with the following pipette and bath solutions (Guo et al., 2003), unless indicated otherwise: 100 mM KCl, 5 mM K2EDTA, and 10 mM K2HPO4/KH2PO4 (in a ratio maintaining pH 7.6). The wild-type/mutant subunit stoichiometry was determined by sensitivity to block by 100 μM spermine at +80 mV (Sigma, St. Louis, MO; Shyng et al., 1997a; Markworth et al., 2000). We first recorded the single-channel currents without spermine at −80 mV to obtain burst times. On the same channel we then bath-applied 100 μM spermine. In each condition, we recorded several cycles of the single-channel currents at −80 mV where there was little or no block and stationarity of channel activity was checked, followed by +80 mV where there was significant blocking of outward currents by spermine if present. The PO in the presence and in the absence of spermine at +80 mV was determined by dividing the open current level area by the total area of the single-channel current amplitude histograms. Fractional spermine sensitivity was given by the PO at +80 mV in the presence of spermine normalized to the PO at +80 mV in the absence of spermine.
Data analysis
Analysis and display were done using TAC v.4.0 (Bruxton, Seattle, WA), IGOR Pro v.4.0.8 (WaveMetrics, Lake Oswego, OR), and Illustrator v.9.0 (Adobe Systems, San Jose, CA). Dose-response measurements were fit to the Hill equation, I/Imax = 1/(1 + ([ATP]/Ki,ATP)αH), where [ATP] is the concentration of ATP, I/Imax the fractional current at the indicated [ATP] relative to that in the same solution in the absence of added ATP (Imax was defined as the average of measurements taken before and after current measurements in the presence of [ATP]), Ki,ATP the [ATP] at which inhibition is half-maximal, and αH the slope factor, or Hill coefficient, as before (Drain et al., 1998). We found αH = 1.0 ± 0.2 for the wild-type channel and all 171 mutant channels. Single-channel current events were detected using the time of the half-amplitude of transitions between current levels with TAC v.4.0 (Bruxton). Durations were corrected for missed events during construction of duration histograms based on the filter corner frequency of the recording by the method of Colquhoun and Sigworth (1995). Duration analysis was done with TACfit v.4.0 (Bruxton), which uses the transformations of Sigworth and Sine (1987) to construct and fit duration histograms. For the burst duration analysis, a burst criterion of 1.6 ms (four times the intraburst closed time mean) was used. Student's t-test showed for each of the four mutant channels, 171S, 171A, 171F, and 171H, that the Ki,ATP, PO, and mean burst times values were significantly different from the respective values of the parent wild-type Kir6.2ΔC26/T171 channels (P < 0.001). Stoichiometry of single channels arising from coinjection of cRNAs (1:1 fast wild-type T171/slow mutant 171A) was also classified solely by their mean burst times using k-means clustering statistics (Hartigan and Wong, 1979), which showed the same five stoichiometry classes determined by spermine sensitivities. Box plots (Tukey, 1977) were constructed using IGOR Pro v4.0.8.
RESULTS
The threonine at 171 of Kir6.2 is positioned at the cytoplasmic end of the inner helix or M2 transmembrane segment, just below the so-called bundle crossing, based on primary protein sequence homology to bacterial K channels whose structures have been highly resolved (Doyle et al., 1998; Yellen, 2002). The homology places the T171 of Kir6.2 at the cytoplasmic end of the transmembrane pore. Experimental (Loussouarn et al., 2000, 2001) and modeling (Capener et al., 2000) results on the structure of the KATP channels are consistent with this view. Previously, we extensively characterized the gating kinetics of the Kir6.2ΔC26 background without SUR1 and the 171A point mutation in that background, to show that the 171 region of Kir6.2 plays an important role in both ATP-dependent and ligand-independent gating of the KATP channel (Drain et al., 1998; Li et al., 2000). Others had shown similar results (Tucker et al., 1998). Although limited to only one substitution, the functional results, together with the structural considerations, suggest that the 171 region of Kir6.2 plays a direct role in gate closure of the KATP channel, distinct from the ATP binding step that couples to the gating step. Here, we expand the characterization of T171 in the gating mechanism.
T171 is indispensable for wild-type KATP channel gating
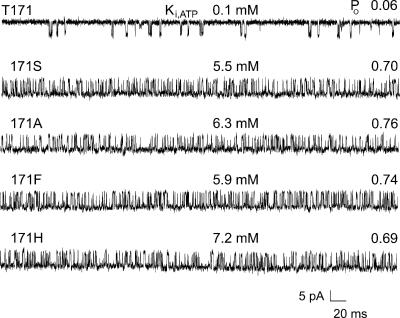
The mutation 171A acts in large part by dramatically slowing the rate at which the ATP-dependent inhibition gate closes (Fig. 1). The gating step defects are easily observed in the Kir6.2Δ26 background without SUR1 and ATP, so we used these conditions. Throughout this study, we refer to Kir6.2ΔC26 channels expressed without SUR1 as wild type. Even in the absence of ATP, Kir6.2Δ26 without SUR1 forms channels that exhibit low PO (<0.1) and short mean burst time (<2 ms), which result from fast gate movements as the channel transits from the burst to interburst gating conformations. Substitution of T171A in the Kir6.2Δ26 channel without SUR1 results in dramatically higher PO (>0.6) and long mean burst times (≥60 ms) in the absence of ATP. Of all 19 substitutions constructed at 171, only four gave rise to functional channels. The single-channel current records clearly show that all four substitutions, 171S, 171A, 171F, and 171H, dramatically slow gating from the burst to interburst gating conformations, compared to their wild-type parent. The substitutions increased PO in the absence of ATP by 11.5–12.7-fold and the Ki,ATP for inhibition by 55–72-fold. The results indicate that threonine at position 171 is indispensable for wild-type gating of the channel.
FIGURE 1.
Substitutions at T171 dramatically slow gating from the active burst to the inactive interburst in truncated Kir6.2 channels without SUR1. Of all possible substitutions constructed only four, S, A, F, and H, allowed detectable channel expression. Each of these substitutions substantially increased Ki,ATP by 55-fold or more and importantly increased PO by 11-fold or more. Representative single-channel records are shown with the single-letter abbreviation for the amino acid residue at position 171 of Kir6.2 indicated. The top trace is from a channel with the wild-type T at position 171. These control channels had a relatively low Ki for ATP inhibition of 0.1 mM and a low PO in the absence of ATP of 0.06. For both Ki,ATP and PO values of each channel, n ≥ 5, with mutant values significantly different from wild-type values, P < 0.001.
All substitutions at 171 dramatically slow gating to the interburst
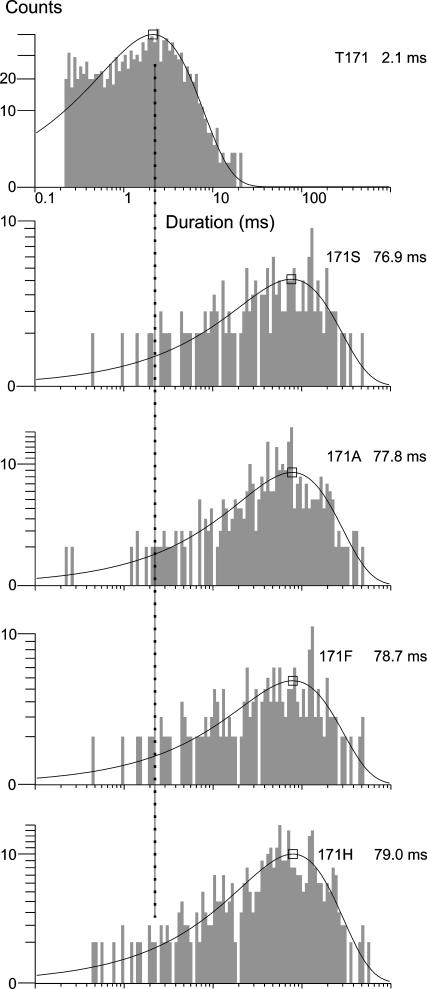
So far the results indicate that each of the four functional substitutions at position 171 slow the gating from the burst to the interburst state, speed the gating from the interburst to the burst, or both. To distinguish and further quantify the changes in gating for each of the four substitutions, we fit the measured burst and interburst times to exponential functions. We found that the burst times increased from 2.1 to 76.9 ms or more by each of the substitutions, compared to wild type (Fig. 2). The results indicate a >35-fold slowing in the gating of the channel from the burst to the interburst and explain at least in part the increase in the Ki,ATP by the substitutions, consistent with no replacement, not even serine, substituting well for threonine. From the burst times alone, however, we cannot know whether the substitutions only act by slowing the burst to interburst gating transition or whether the interburst to burst gating transition is speeded as well. The latter would indicate that the mutations also destabilize the otherwise long-lived interburst closed conformation of the cytoplasmic gate of the KATP channel. We therefore tested for additional effects on interburst times.
FIGURE 2.
Burst times are substantially increased by all 171 substitutions that express. The dramatic disruption of gating suggests that T171 is required for wild-type gate closure and that no other amino acid residue at the position substitutes well for threonine. The dashed line indicates the value of the wild-type mean burst time in each of the duration histograms. For mean burst time values of each channel, n ≥ 4, with mutant values significantly different from wild-type values, P < 0.001.
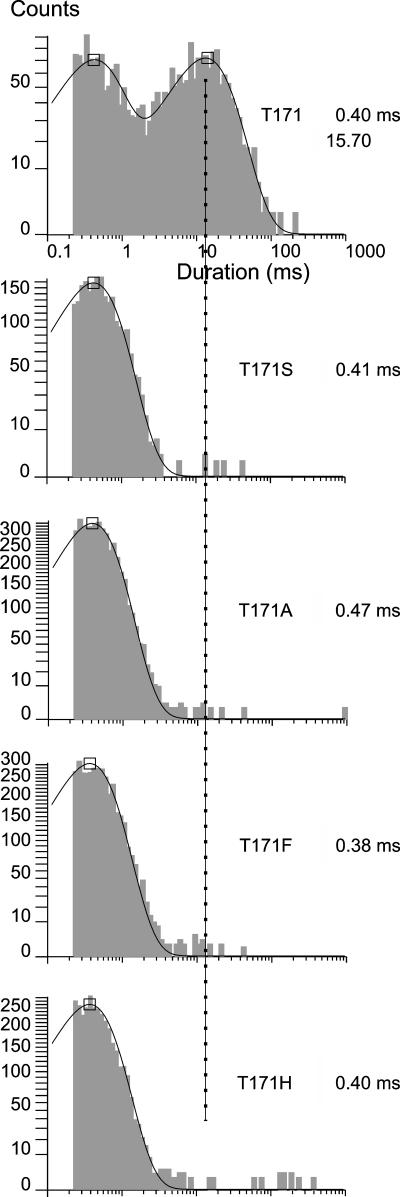
All substitutions at 171 nearly eliminate interburst events
Fig. 3 shows that each of the 171 substitutions nearly if not completely eliminate the interburst times that are normally prominent in the wild-type Kir6.2ΔC26 channel. In the single-channel current records, the 171 mutant channels appear to burst all but continuously. The kinetic analysis shows significant numbers of only the short-lived intraburst closed durations and indicate either nearly complete loss of transitions to the wild-type inactive interburst conformation or destabilization of the conformation to the point that they are indistinguishable from the very short-lived intraburst closed conformation. The results show that although all four substitutions at position 171 dramatically slow burst to interburst gating compared to the wild-type threonine residue at the position, the substitutions have no effect on intraburst kinetics determined by the selectivity filter gate.
FIGURE 3.
Closed time distributions of 171 substitutions show nearly complete loss of long-lived interburst closed times, with little or no effect on the short-lived intraburst closed times. The dashed line indicates the value of the wild-type mean long-lived closed time of 15.7 ms in each of the duration histograms. Note that the relatively few long-lived closed events are consistent with but cannot differentiate between the 171 substitutions either greatly slowing gate closure or dramatically disrupting its shut state stability.
Gate stoichiometry of the KATP channel
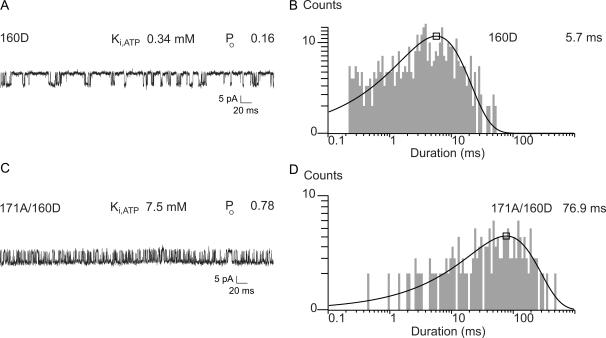
We also used gating kinetic analysis to investigate how many cytoplasmic gates function in the KATP channel and the relationship between the gate and the four Kir6.2 subunits. It is possible that each subunit undergoes a conformational change that is sufficient to cause the gating to occur. In this independent model with four gates, the gating rate constant will be the sum of the individual rate constants contributed by each of the four subunits. Alternatively, all four Kir6.2 subunits might undergo concerted conformational changes. In the concerted model, the gating rate constant will be exponentially related to the sum of the energetic contributions of each of the four subunits. To distinguish these possibilities, we studied how the rate of burst to interburst gating changes with increasing number of slow subunits in the channel. For these experiments we used the 171A mutant as a slow subunit and T171 wild type as a fast subunit. To independently know the number of slow and fast subunits in a given channel, we first marked 171A mutant subunits with the N160D mutation. Previous work demonstrated that the 160D mutation is nearly without effect on the Ki,ATP, increasing it by only threefold, yet the mutation dramatically increases block by intracellular spermine (Shyng et al., 1997a). To further show that 160D had relatively little effect on the gating, we also determined its effect on PO and burst times of KirΔC26 channels. For example, Fig. 4 shows that N160D in the Kir6.2ΔC26 background slowed gating to the interburst by only 2.7-fold, compared to the >35-fold slowing observed for the 171 substitutions. Accordingly, the 171A/160D double mutant channels show the dramatically extended burst times typical of the 171A mutation alone.
FIGURE 4.
The N160D subunit marker of strong spermine block only slightly slows gating to the inactive interburst. (A) Single-channel record of a representative Kir6.2ΔC26/160D channel. (B) Duration histogram of the burst times shows the relatively mild effect of the 160D mutation on gating. (C) Single-channel record of a representative Kir6.2ΔC26/171A/160D double mutant channel. (D) Duration histogram of the burst times shows a dramatic increase in the burst times of the Kir6.2ΔC26/171A/160D double mutant channel. The slowing of gating by all our T171 mutations, as shown here by 171A, is dramatic compared to that of 160D. In these experiments, both the 160D and 171A mutations were in all four subunits. The effect of T171A predominantly determines both Ki,ATP and burst to interburst gating rates in the channels.
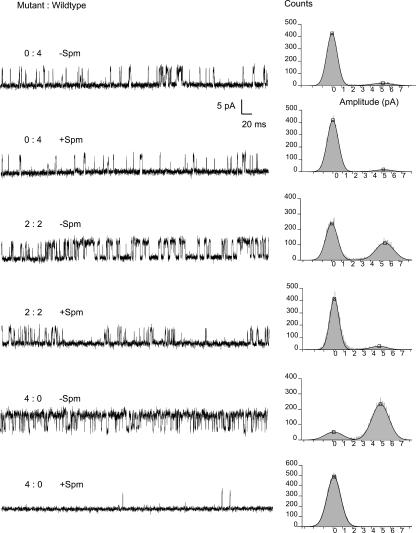
The 100-μM spermine bath applied to inside-out excised patches at +80 mV was optimal to classify the channels into the five slow/fast subunit stoichiometry classes. Fig. 5 shows the single-channel records and current amplitude histograms of single channels with the indicated ratios of wild-type and 160D/171A mutant subunits. In these experiments, we first recorded the single-channel currents without spermine at −80 mV to obtain burst times. To the same channel we then bath applied 100-μM spermine and recorded several cycles of single-channel currents at −80 mV where there was little blocking by spermine, followed by +80 mV where there was significant blocking of outward currents. The PO in the presence and in the absence of spermine at +80 mV was determined by dividing the open current level area by the total area of the single-channel current amplitude histograms. Fractional spermine sensitivity was given by the PO in the presence of spermine normalized to the PO in the absence of PO. The spermine sensitivities, observed as the normalized PO in the presence of spermine, binned into five classes. One class was completely refractory to 100-μM spermine, which was also true for homotetrameric wild-type Kir6.2ΔC26/T171 channels. Three classes of single channels were found with increasing partial sensitivity to spermine, and represent 3:1, 2:2, 1:3 wild-type/mutant heterotetrameric channels, respectively. The fifth class was completely blocked by the spermine, which was also true for homotetrameric mutant Kir6.2ΔC26/171A channels. The frequency of channels in each of the five classes was predictable from binomial distributions given equal and independent expression and assembly of two types of subunit into tetramer channels. Because the 160D and 171A mutations simply by construction are in the same subunit, the spermine sensitivity indicates 171A subunit stoichiometry, as well as 160D subunit stoichiometry.
FIGURE 5.
Representative single-channel currents and outward current block by spermine. Stoichiometry of mutant/wild-type (160D/171A:N160/T171) subunits was determined by the distribution of open probability with and without 100-uM spermine at different mutant to wild-type cRNA ratios. Note that sensitivity to outward current block by intracellular spermine increases with the number of mutant subunits, due to the presence of the 160D mutation. In the presence of spermine, sensitivities were found that correlated with experiments using a known 0:4 subunit ratio (only mutant cRNA injected, completely blocked) and known 4:0 subunit ratio (only wild-type cRNA injected, little or no block). Three major sensitivities between these extremes were also found, consistent with their being from channels 1:3, 2:2, and 3:1 subunit ratios. Only the 0:4, 2:2, and 4:0 subunit single-channel currents are shown.
Exponential relationship between gating rate and the number of 171A subunits
For the main stoichiometry experiments, we varied the slow and fast subunit ratios by coinjecting cRNA in a 1:1 ratio into Xenopus oocytes. For each single channel, we first characterized the gating rate to the interburst in the absence of ATP at −80 mV and then determined the subunit stoichiometry by its spermine sensitivity. The mean burst times of channels with differing T171:171A subunit ratios varied exponentially, as shown in 1. Accordingly, one, two, or three slow 171A subunits in an otherwise wild-type channel dramatically increased the mean burst time, by over twofold, 12-fold, and 28-fold, respectively, where the mean burst time of homotetrameric mutant channels was increased by over 40-fold, compared to wild type. Independent models predict that one, two, or three slow 171A subunits in an otherwise wild-type channel would only modestly increase the mean burst times, by 1.4-fold, twofold, and fourfold. Initially we used spermine sensitivity to place the 96 single channels recorded into the five slow/fast subunit stoichiometry classes, as mentioned. By simple inspection and k-means clustering analysis (Hartigan and Wong, 1979), we later found that the burst time means alone can be used to place the channels into the correct stoichiometry classes.
TABLE 1.
Mean burst times of single channels from coinjection of 1:1 slow/fast subunit cRNAs
| 4 fast | 1 slow:3 fast | 2 slow:2 fast | 3 slow:1 fast | 4 slow |
|---|---|---|---|---|
| 1.6 | 4.7 | 12.3 | 28.1 | 80.1 |
| 2.3 | 4.5 | 13.1 | 22.8 | 61.8 |
| 2.1 | 3.8 | 12.1 | 32.1 | 77.2 |
| 1.8 | 4.3 | 11.5 | 23.5 | 84.3 |
| 2.2 | 4.9 | 12.9 | 29.1 | 102.0 |
| 2.4 | 4.3 | 13.8 | 26.0 | |
| 4.8 | 13.4 | 45.2 | ||
| 5.0 | 11.8 | 25.5 | ||
| 4.5 | 10.9 | 26.1 | ||
| 3.9 | 11.9 | 33.0 | ||
| 4.9 | 11.9 | 35.8 | ||
| 3.7 | 13.0 | 41.1 | ||
| 3.3 | 12.1 | 37.2 | ||
| 5.1 | 13.9 | 31.2 | ||
| 4.8 | 12.2 | 31.0 | ||
| 5.6 | 14.1 | 26.2 | ||
| 4.8 | 11.4 | 23.1 | ||
| 5.7 | 10.9 | 21.3 | ||
| 4.9 | 11.3 | 22.5 | ||
| 5.9 | 12.7 | 24.7 | ||
| 6.2 | 9.1 | 20.4 | ||
| 5.2 | 10.5 | 21.6 | ||
| 4.0 | 14.3 | 30.1 | ||
| 4.8 | 13.3 | 28.2 | ||
| 5.6 | 12.6 | |||
| 5.3 | 12.3 | |||
| 10.8 | ||||
| 15.3 | ||||
| 2.0 ± 0.12 | 4.8 ± 0.3 | 12.4 | 28.5 ± 1.81 | 80.8 ± 6.4 |
| 11.6 | ||||
| 11.8 | ||||
| 9.9 | ||||
| 13.0 | ||||
| 12.6 | ||||
| 10.0 | ||||
| 12.2 ± 0.67 |
All values are in milliseconds. “Fast” designates the Kir6.2ΔC26/T171 wild-type subunit. “Slow” designates the Kir6.2ΔC26/171A mutant subunit. At the bottom of each column of values is the mean of means ± SE. Each mean of means is significantly different from each of the other mean of means, P < 0.001.
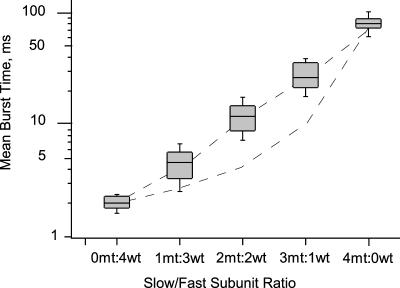
Fig. 6 summarizes the mean burst time data, together with predicted values from concerted and independent gating models. The concerted model is represented by the dashed line going straight through the experimental data in the semilog plot. The independent model is represented by the lower dashed line falling below the experimental data. The mean burst times are grouped according to subunit stoichiometry. Within each slow/fast subunit stoichiometry class the mean burst times are shown as box plots. The mean burst times increase exponentially with increasing slow/fast subunit stoichiometry. The gating kinetics therefore fit the concerted model predictions and fail to fit independent models. The results indicate a single gate that works by a highly cooperative mechanism involving all four subunits.
FIGURE 6.
Exponential distribution of mean burst times with increasing slow/fast subunit ratio. Mean burst time data are shown as box plots on a semilog scale. For each box plot, the central shaded box shows the middle half of the data between the 25th and 75th percentiles. The horizontal within the box indicates the median of the data. The whiskers indicate the remainder of the data. The straight dashed line represents the concerted model predictions, whereas the curved dashed lines represent the independent model predictions on the semilog plot.
DISCUSSION
Threonine at 171 of Kir6.2 is indispensable for wild-type ATP inhibition
All four substitutions that express at position 171 of Kir6.2 dramatically decreased ATP-dependent and ligand-independent gating. Thus, no other residue substituted well, suggesting that threonine at position 171 of Kir6.2 plays a unique role in closure of the KATP channel gate. Even the most highly related serine substitution resulted in a 55-fold increase in Ki,ATP and 37-fold slowing of ligand-independent gating. The result suggests that proper closure of the cytoplasmic gate requires the methyl group of threonine directly in steric and hydrophobic interactions or indirectly by positioning its hydroxyl or carbonyl moiety for electrostatic interactions.
Concerted action of all four Kir6.2 subunits in one inhibition gate
Our approach was to collect hundreds of burst times for each single-channel record, fit them to exponentials where the time constant is equal to the duration mean, and then determine the spermine sensitivity of the channel. We then grouped the duration mean of each channel according to its spermine sensitivity into the five slow/fast subunit stoichiometry classes. As we began to analyze our results, however, we noticed that the mean burst duration values themselves were clustering into the same groups determined by spermine sensitivity. We confirmed using k-means clustering statistics that the burst duration means alone can also be used to identify subunit stoichiometry. The ability to classify the channels by the duration means likely results from a combination of properties including the dramatic slowing of the gating rate by the 171A mutation, the concerted nature of the gating mechanism, and the population sampling afforded by hundreds of burst events fitted for each duration mean. Because the burst to interburst gating transition is speeded by ATP binding, and the interburst to burst gating transition is slowed by bound ATP, the concerted conformational change likely underlies KATP channel inhibition gating by ATP. Moreover, the T171X mutations dramatically slow the burst to interburst gating transitions and increase Ki,ATP.
The 171 region as part of the cytoplasmic gate of the KATP channel
Mutations from positions 160–171 in Kir6.2 so far have the most dramatic disruptions in the burst-interburst gating. Mutations at residues 171 (Drain et al., 1998; Tucker et al., 1998) and 166 (Trapp et al., 1998) dramatically disrupt both ATP-dependent and ATP-independent gating from the burst to the interburst. Mutations at 160 have by comparison mild (<fivefold) but significant effects on both ATP-dependent gating, as shown previously (Shyng et al., 1997a), and ATP-independent gating, as shown here. Evidently, mutation of successive positions from the middle of M2 to its cytoplasmic end results in increasingly greater disruption of the gating (Loussouarn et al., 2000, 2001). These results taken together suggest that the 171 region or cytoplasmic half of the inner helix of Kir6.2 plays a major role in the closure of a cytoplasmic inhibition gate. The main chain at position T171 likely lines the pore and could participate in pore occlusion, whereas the side chain likely points away from the pore and could couple the gate to ATP-bound cytoplasmic C-terminal domains. Such highly specialized roles are consistent with no other residue, not even serine, substituting well for threonine at this position.
Linkage and ATP binding domains in the cytoplasmic C-terminus of Kir6.2
The mutations of Kir6.2, more distal than 171A in the cytoplasmic C-terminus, dramatically alter ATP-dependent gating but have little or no effect on ligand-independent gating. The results suggest that the more distal C-terminal segments do not play a direct role in gate closure of the KATP channel but rather function in ATP binding or its linkage to gate closure. The distal mutations so far cluster into the 182 (Li et al., 2000), 185 (Tucker et al., 1997, 1998), 201 (Ribalet et al., 2003), and 334 (Drain et al., 1998) regions of Kir6.2. Mutations at each of these positions can disrupt ATP-dependent gating by 100-fold or more and yet can have no detectable change in the ATP-independent gating transitions. Positively charged substitutions at 182, occasionally, can dramatically alter ATP-independent gating kinetics, which suggests that the 182 region at least when positively charged can link conditionally to the inhibition gate of the KATP channel (Li et al., 2000). The neighboring 185 region of Kir6.2 plays a role in the ATP site rather than the inhibition gate of the KATP channel (Tucker et al., 1997, 1998; Tanabe et al., 1999, 2000). Mutations in the 334 region also profoundly disrupt ATP-dependent gating with no change in ligand-independent gating (Drain et al., 1998). The 334 region includes an ATP binding site motif found in P-type ATPases (McIntosh et al., 1996), which is supported by molecular modeling of the inhibitory ATP site of Kir6.2 (Trapp et al., 2003).
Four independent ATP sites and one inhibition gate that opens and closes by the concerted action of four Kir6.2 subunits
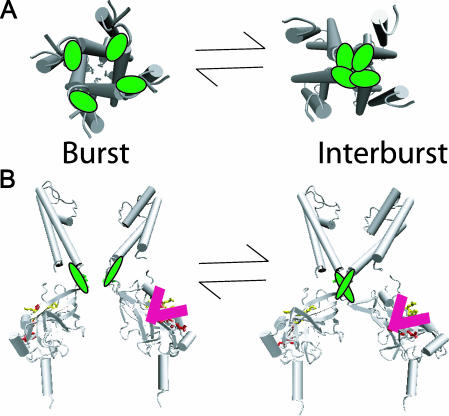
Additional results on the 334 region have shown that this distal region of each Kir6.2 subunit likely forms one independent ATP site per subunit (Drain et al., 1998, 2001; Li et al., 1999; Markworth et al., 2000). Four ATP sites, each working independently in its subunit, contrasts sharply with the one single inhibition gate working by the highly cooperative action of all four subunits shown here. Fig. 7 depicts the concerted movements of the inhibition gate of the KATP channel in the cytoplasmic half of the inner helix lining the transmembrane pore. The structural model is based on the crystal structures of the bacterial inward rectifier potassium channels, KcsA (Doyle et al., 1998), MthK (Jiang et al., 2002a,b), KirBac1.1 (Kuo et al., 2003), and the mouse Kir3.1 channels (Nishida and MacKinnon, 2002). The crystallized region of Kir3.1 has nearly 50% identity with the cytoplasmic C-terminus of Kir6.2, which includes the 182, 185, and 334 regions. The high degree of identity suggests that, with few exceptions, secondary and tertiary structures will be tightly conserved between the C-termini of the two proteins. In the upper panel, the concerted action of the four inner helices of the Kir6.2 subunits is depicted, where in one step the four T171 (green) residues in a single step move together at the cytoplasmic mouth of the transmembrane pore to obstruct K+ ion flow (see below). In the lower panel, a membrane cross sectional view is depicted, where only two opposing subunits of the four are shown. In the model, the single inhibition gate involving the T171 residues is immediately above the four cytoplasmic ATP sites, likely formed by the 182, 185, and 334 regions of the cytoplasmic C-terminal domains, as discussed above. The model suggests that linkage between the transmembrane pore domain and the cytoplasmic ATP sites might involve direct interactions between the domains.
FIGURE 7.
Concerted action of all four Kir6.2 subunits underlies burst gate closure. (A) The view from the cytoplasm depicts one-step occlusion of the pore. The gating results from highly cooperative tertiary conformational changes involving T171 determinants (green) at the cytoplasmic end of the inner helix of each subunit. (B) The membrane cross sectional view depicts the topological relation between T171 residues (green) and the candidate inhibitory ATP site residues I182, K185, R201, and G334 (red). Only two subunits are shown in the panel for clarity. Occupancy of one of the ATP sites of the cytoplasmic C-terminal domain of a single Kir6.2 subunit can cause concerted conformational changes in all four Kir6.2 subunits, to shut the intracellular M2 burst gate. Conformational changes accompanying ATP binding to any one of the four subunits are independent, and yet each independent conformational change is coupled to a concerted conformational change in which all four subunits act to close the intracellular M2 burst gate. The closed gating conformation of each subunit, however, does not couple to its site unless it is occupied by ATP. This ligand-dependent linkage, coupling conformations of occupied ATP site and closed intracellular M2 burst gate, loosens the constraint that conformational changes at the gate, and conformational changes at the site, always exhibit tight reciprocity, which explains much of what is known about ATP-dependent inhibition gating of the KATP channel.
Coupling four independent sites and one concerted inhibition gate
Given the finding of four independent inhibitory ATP sites, we were surprised to find a single gate that works by a cooperative mechanism involving all four subunits. The simpler model, in which each Kir6.2 subunit acts independently with its own gate coupled to its own ATP site, however, is excluded by the results reported here. Tenable models of inhibition gating of the KATP channel by ATP must now accommodate the curious coupling of four independent sites and a single gate arising from the concerted action of all four Kir6.2 subunits.
The conformational coupling between ATP binding within the cytoplasmic domain and gate closure within the transmembrane pore domain of each Kir6.2 subunit exhibits the properties of ligand-dependence and nonreciprocity. Previously, we proposed ligand-dependent linkage as a conditional linkage mechanism coupling ATP binding and gating domains of Kir6.2 to account for the effect of mutations in the cytoplasmic ATP binding domains of the subunit (Li et al., 2000). Ligand-dependent linkage also explains how the single inhibition gate operating by the concerted action of all four Kir6.2 subunits shown here can be controlled by ligand binding to any of four independent ATP sites. We propose a combined linkage and gating mechanism, in which the concerted tertiary conformational changes of the four gating domains cannot conformationally couple to unoccupied ATP site domains. In our model, ATP occupancy initiates a conformational change at the site that obligatorily couples to inhibition gate closure, but inhibition gate closure does not, in turn, initiate a conformational change that couples to the high affinity conformation of unoccupied sites. Otherwise, the ATP sites would be expected to exhibit cooperativity by reciprocal linkage to the gating domains, which is inconsistent with the Hill coefficient ∼1 for pancreatic KATP channels (Qin et al., 1989; Nichols et al., 1991; Markworth et al., 2000). In the pancreatic KATP channel, unoccupied sites evidently remain uncoupled from the cytoplasmic gate regardless of its open or closed state, whereas ATP binding at any of its intracellular sites always induces the ligand-dependent linkage mechanism that conformationally couples the binding to inhibition gate closure.
Concerted action of pore-forming subunits in cytoplasmic gate for all K channels
Concerted mechanisms, similar to the one identified here by using the 171A mutation in Kir6.2 of the KATP channel, have been previously identified and well studied in voltage-dependent Kv channels (Zagotta et al., 1994a,b; Schoppa and Sigworth, 1998a,b) where there is incisive support for an intracellular gate at the entrance of the inner vestibule that likely opens and closes at the S6 bundle crossing (Armstrong, 1966, 1975; del Camino et al., 2000; del Camino and Yellen, 2001; Holmgren et al., 1997, 1998; Liu et al., 1997; Hackos et al., 2002; Lu et al., 2002). Although the conformational changes involving the S4 voltage sensor in Kv channels are steeply voltage dependent, the final opening and closing transitions are largely voltage independent (Ledwell and Aldrich, 1999) consistent with the S6 bundle crossing gate being largely outside the membrane electric field. For the KATP channel, cysteine modification experiments like those to study Kv channels have provided evidence for gated access of methanethiosulfonate-2-aminoethyl (MTSEA) and methanethiosulfonate-ethyltrimethylammonium (MTSET) to the inner vestibule of Kir6.2 (Phillips et al., 2003). Additional results using cysteine modification and homology modeling are consistent with an ATP-sensitive inhibition gate of the KATP channel at the cytoplasmic end of the transmembrane pore, where F168 residues are at the point of closest approach, with T171 one helical turn immediately below, lining the pore (Capener et al., 2000; Loussouarn et al., 2000, 2001).
Other results, however, suggest that the inhibition gate might be nearer the filter at the extracellular end of the transmembrane pore (Proks et al., 2003, 2001; Xiao et al., 2003). We emphasize that the burst-interburst gating involving T171 is ATP dependent but voltage independent, whereas the intraburst gating between the open and fast closed state is voltage dependent and ATP independent (Fan and Makielski, 1999; Proks et al., 2001). This fits with T171 residues being outside the membrane electric field where they move in concert to open and close the KATP channel independent of voltage. The separable properties of the ATP-dependent burst gating and the voltage-dependent intraburst gating, together with the location of the filter within and T171 outside the membrane electric field, suggest that the two classes of gating mechanisms largely operate on two distinct gates. We therefore favor the T171 residues at a position either physically part of the inhibition gate closure point at the cytoplasmic mouth or allosterically required for its formation at or below the nearby bundle crossing. It remains possible that the concerted T171 movements are strictly coupled to a physical closure of the gate at the extracellular filter of the KATP channel (cf. Alagem et al., 2003) that somehow does not involve charge or dipole movements for ATP-dependent gating. Although more direct physical measurements are needed to define the exact part of Kir6.2 that forms the closure point of the ATP-sensitive gate, the results reported here indicate that the KATP channel has one inhibition gate and its movements require the concerted action of all four T171 regions of Kir6.2.
Acknowledgments
We thank Rick Aldrich for critical reading of the manuscript, Pei Tang and Michael Yonkunas for structural expertise, and Ray Frizzell and lab for excellent discussions and oocytes.
This work was supported by National Science Foundation grant MCB 9817116.
Abbreviations used: KATP, adenosine triphosphate-sensitive potassium; PO, open channel probability; Kir, inwardly rectifying potassium; SUR, sulfonylurea receptor.
References
- Aguilar-Bryan, L., J. Bryan, and M. Nakazaki. 2001. Of mice and men: KATP channels and insulin secretion. Recent Prog. Horm. Res. 56:47–68. [DOI] [PubMed] [Google Scholar]
- Aguilar-Bryan, L., C. G. Nichols, S. W. Weschler, J. P. T. Clement, A. E. Boyd 3rd, G. Gonzalez, H. Herrera-Sosa, K. Nguy, J. Bryan, and D. A. Nelson.1995. Cloning of the β cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 268:423–426. [DOI] [PubMed] [Google Scholar]
- Alagem, N., S. Yesylevskyy, and E. Reuveny. 2003. The pore helix is involved in stabilizing the open state of inwardly rectifying K+ channels. Biophys. J. 85:300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseev, A. E., P. A. Brady, and A. Terzic. 1998. Ligand-insensitive state of cardiac ATP-sensitive K+ channels. Basis for channel opening. J. Gen. Physiol. 111:381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoroso, S., H. Schmid-Antomarchi, M. Fosset, and M. Lazdunski. 1990. Glucose, sulfonylureas, and neurotransmitter release: role of ATP-sensitive K+ channels. Science. 247:852–854. [DOI] [PubMed] [Google Scholar]
- Armstrong, C. M. 1966. Time course of TEA+-induced anomalous rectification in squid giant axons. J. Gen. Physiol. 50:491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, C. M. 1975. Ionic pores, gates, and gating currents. Q. Rev. Biophys. 7:179–210. [DOI] [PubMed] [Google Scholar]
- Ashcroft, F. M. 1988. Adenosine 5′-triphosphate-sensitive potassium channels. Annu. Rev. Neurosci. 11:97–118. [DOI] [PubMed] [Google Scholar]
- Ashcroft, F. M., D. E. Harrison, and S. J. Ashcroft. 1984. Glucose induces closure of single potassium channels in isolated rat pancreatic β-cells. Nature. 312:446–448. [DOI] [PubMed] [Google Scholar]
- Babenko, A. P., G. Gonzalez, and J. Bryan. 1999a. The tolbutamide site of SUR1 and a mechanism for its functional coupling to KATP channel closure. FEBS Lett. 459:367–376. [DOI] [PubMed] [Google Scholar]
- Babenko, A. P., G. Gonzalez, and J. Bryan. 1999b. Two regions of sulfonylurea receptor specify the spontaneous bursting and ATP inhibition of KATP channel isoforms. J. Biol. Chem. 274:11587–11592. [DOI] [PubMed] [Google Scholar]
- Babenko, A. P., G. Gonzalez, and J. Bryan. 1999c. The N-terminus of Kir6.2 limits spontaneous bursting and modulates the ATP-inhibition of KATP channels. Biochem. Biophys. Res. Commun. 255:231–238. [DOI] [PubMed] [Google Scholar]
- Babenko, A. P., G. Gonzalez, and J. Bryan. 2000. Pharmaco-topology of sulfonylurea receptors. J. Biol. Chem. 275:717–720. [DOI] [PubMed] [Google Scholar]
- Colquhoun, D., and F. J. Sigworth. 1995. Fitting and statistical analysis of single-channel records. In Single-Channel Recording, 2nd ed. B. Sakmann and E. Neher, editors. Plenum Press, New York. 483–587.
- Capener, C. E., I. H. Shrivastava, K. M. Ranatunga, L. R. Forrest, G. R. Smith, and M. S. Sansom. 2000. Homology modeling and molecular dynamics simulation studies of an inward rectifier potassium channel. Biophys. J. 78:2929–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement, J. P. T., K. Kunjilwar, G. Gonzalez, M. Schwanstecher, U. Panten, L. Aguilar-Bryan, and J. Bryan. 1997. Association and stoichiometry of KATP channel subunits. Neuron. 18:827–838. [DOI] [PubMed] [Google Scholar]
- Cook, D. L., and C. N. Hales. 1984. Intracellular ATP directly blocks K+ channels in pancreatic β-cells. Nature. 311:271–273. [DOI] [PubMed] [Google Scholar]
- del Camino, D., M. Holmgren, Y. Liu, and G. Yellen. 2000. Blocker protection in the pore of a voltage-gated K+ channel and its structural implications. Nature. 403:321–325. [DOI] [PubMed] [Google Scholar]
- del Camino, D., and G. Yellen. 2001. Tight steric closure at the intracellular activation gate of a voltage-gated K+ channel. Neuron. 32:649–656. [DOI] [PubMed] [Google Scholar]
- Deutsch, N., S. Matsuoka, and J. N. Weiss. 1994. Surface charge and properties of cardiac ATP-sensitive K+ channels. J. Gen. Physiol. 104:773–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, D. A., J. Morais-Cabral, R. A. Pfuetzner, A. Kuo, J. M. Gulbis, S. L. Cohen, B. T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- Drain, P., A. E. Dubin, and R. W. Aldrich. 1994. Regulation of Shaker K+ channel inactivation gating by the cAMP-dependent protein kinase. Neuron. 12:1097–1109. [DOI] [PubMed] [Google Scholar]
- Drain, P., L. Li, and X. Geng. 2001. Incremental stabilization of the shut inhibition gate of the KATP channel by simultaneous occupation of up to four independent sites by ATP. Biophys. J. 80:626a.11159431 [Google Scholar]
- Drain, P., L. Li, and J. Wang. 1998. KATP channel inhibition by ATP requires distinct functional domains of the cytoplasmic C terminus of the pore-forming subunit. Proc. Natl. Acad. Sci. USA. 95:13953–13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, Z., and J. C. Makielski. 1999. Phosphoinositides decrease ATP sensitivity of the cardiac ATP-sensitive K channel. J. Gen. Physiol. 114:251–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis, K. D., W. M. Gee, A. Hammoud, M. L. McDaniel, L. C. Falke, and S. Misler. 1989. Effects of sulfonamides on a metabolite-regulated ATPi-sensitive K+ channel in rat pancreatic β-cells. Am. J. Physiol. 257:C1119–C1127. [DOI] [PubMed] [Google Scholar]
- Gribble, F. M., S. J. Tucker, and F. M. Ashcroft. 1997. The essential role of the Walker A motifs of SUR1 in KATP channel activation by Mg-ADP and diazoxide. EMBO J. 16:1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, D., Y. Ramu, A. M. Klem, and Z. Lu. 2003. Mechanism of rectification in inward-rectifier K+ channels. J. Gen. Physiol. 121:261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackos, D. H., T. H. Chang, and K. J. Swartz. 2002. Scanning the intracellular S6 activation gate in the Shaker K+ channel. J. Gen. Physiol. 119:521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartigan, J. A., and M. A. Wong. 1979. A k-means clustering algorithm. Applied Statistics. 28:100–108. [Google Scholar]
- Holmgren, M., K. S. Shin, and G. Yellen. 1998. The activation gate of a voltage-gated K+ channel can be trapped in the open state by an intersubunit metal bridge. Neuron. 21:617–621. [DOI] [PubMed] [Google Scholar]
- Holmgren, M., P. L. Smith, and G. Yellen. 1997. Trapping of organic blockers by closing of voltage-dependent K+ channels: evidence for a trap door mechanism of activation gating. J. Gen. Physiol. 109:527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki, N., T. Gonoi, J. P. T. Clement, N. Namba, J. Inazawa, G. Gonzalez, L. Aguilar-Bryan, S. Seino, and J. Bryan. 1995. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 270:1166–1170. [DOI] [PubMed] [Google Scholar]
- Inagaki, N., T. Gonoi, and S. Seino. 1997. Subunit stoichiometry of the pancreatic β-cell ATP-sensitive K+ channel. FEBS Lett. 409:232–236. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, M. Cadene, B. T. Chait, and R. MacKinnon. 2002a. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 417:515–522. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, M. Cadene, B. T. Chait, and R. MacKinnon. 2002b. The open pore conformation of potassium channels. Nature. 417:523–526. [DOI] [PubMed] [Google Scholar]
- John, S. A., J. R. Monck, J. N. Weiss, and B. Ribalet. 1998. The sulphonylurea receptor SUR1 regulates ATP-sensitive mouse Kir6.2 K channels linked to the green fluorescent protein in human embryonic kidney cells. J. Physiol. 510:333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, P. A., S. J. Tucker, and F. M. Ashcroft. 2001. Multiple sites of interaction between the intracellular domains of an inwardly rectifying potassium channel, Kir6.2. FEBS Lett. 508:85–89. [DOI] [PubMed] [Google Scholar]
- Koster, J. C., Q. Sha, S.-L. Shyng, and C. G. Nichols. 1999. ATP inhibition of KATP channels: control of nucleotide sensitivity by the N-terminal domain of the Kir6.2 subunit. J. Physiol. 515:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, A., J. M. Gulbis, J. F. Antcliff, T. Rahman, E. D. Lowe, J. Zimmer, J. Cuthbertson, F. M. Ashcroft, T. Ezaki, and D. A. Doyle. 2003. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 300:1922–1926. [DOI] [PubMed] [Google Scholar]
- Ledwell, J. L., and R. W. Aldrich. 1999. Mutations in the S4 region isolate the final voltage-dependent cooperative step in potassium channel activation. J. Gen. Physiol. 113:389–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K., A. K. Dixon, I. C. Rowe, M. L. Ashford, and P. J. Richardson. 1996. The high-affinity sulphonylurea receptor regulates KATP channels in nerve terminals of the rat motor cortex. J. Neurochem. 66:2562–2571. [DOI] [PubMed] [Google Scholar]
- Li, L., X. Geng, and P. Drain. 2002. Open state destabilization by ATP occupancy is mechanism speeding burst exit underlying KATP channel inhibition by ATP. J. Gen. Physiol. 119:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L., X. Geng, and P. Drain. 2003. Concerted gating separable from independent binding sites underlying KATP channel inhibition by ATP. Biophys. J. 84:81a. [Google Scholar]
- Li, L., J. Wang, and P. Drain. 1999. Multiple independent components and subunit interactions in the ATP-dependent inhibition gating of the KATP channel. Biophys. J. 76:A77. [Google Scholar]
- Li, L., J. Wang, and P. Drain. 2000. The I182 region of Kir6.2 is closely associated with ligand binding in KATP channel inhibition by ATP. Biophys. ET J. 79:841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., M. Holmgren, M. E. Jurman, and G. Yellen. 1997. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 19:175–184. [DOI] [PubMed] [Google Scholar]
- Loussouarn, G., E. N. Makhina, T. Rose, and C. G. Nichols. 2000. Structure and dynamics of the pore of inwardly rectifying KATP channels. J. Biol. Chem. 275:1137–1144. [DOI] [PubMed] [Google Scholar]
- Loussouarn, G., L. R. Phillips, R. Masia, T. Rose, and C. G. Nichols. 2001. Flexibility of the Kir6.2 inward rectifier K+ channel pore. Proc. Natl. Acad. Sci. USA. 98:4227–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Z., A. M. Klem, and Y. Ramu. 2002. Coupling between voltage sensors and activation gate in voltage-gated K+ channels. J. Gen. Physiol. 120:663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, D., N. Zerangue, Y. F. Lin, A. Collins, M. Yu, Y. N. Jan, and L. Y. Jan. 2001. Role of export signals in controlling surface potassium channel numbers. Science. 291:316–319. [DOI] [PubMed] [Google Scholar]
- MacGregor, G. G., K. Dong, C. G. Vanoye, L. Tang, G. Giebisch, and S. C. Hebert. 2002. Nucleotides and phospholipids compete for binding to the C terminus of KATP channels. Proc. Natl. Acad. Sci. USA. 99:2726–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markworth, E., C. Schwanstecher, and M. Schwanstecher. 2000. ATP4− mediates closure of pancreatic beta-cell ATP-sensitive potassium channels by interaction with 1 of 4 identical sites. Diabetes. 49:1413–1418. [DOI] [PubMed] [Google Scholar]
- McIntosh, D. B., D. G. Woolley, B. Vilsen, and J. P. Andersen. 1996. Mutagenesis of segment 487Phe-Ser-Arg-Asp-Arg-Lys492 of sarcoplasmic reticulum Ca2+-ATPase produces pumps defective in ATP-binding. J. Biol. Chem. 271:25778–25789. [DOI] [PubMed] [Google Scholar]
- Miki, T., B. Liss, K. Minami, T. Shiuchi, A. Saraya, Y. Kashima, M. Horiuchi, F. M. Ashcroft, Y. Minokoshi, J. Roeper, and S. Seino. 2001. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat. Neurosci. 4:507–512. [DOI] [PubMed] [Google Scholar]
- Nichols, C. G., W. J. Lederer, and M. B. Cannell. 1991. ATP dependence of KATP channel kinetics in isolated membrane patches from rat ventricle. Biophys. J. 60:1164–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, C. G., S.-L. Shyng, A. Nestorowicz, B. Glaser, J. P. T. Clement, G. Gonzalez, L. Aguilar-Bryan, M. A. Permutt, and J. Bryan. 1996. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science. 272:1785–1787. [DOI] [PubMed] [Google Scholar]
- Nishida, M., and R. MacKinnon. 2002. Structural basis of inward rectification: cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 A resolution. Cell. 111:957–965. [DOI] [PubMed] [Google Scholar]
- Noma, A. 1983. ATP-regulated K+ channels in cardiac muscle. Nature. 305:147–148. [DOI] [PubMed] [Google Scholar]
- Phillips, L. R., D. Enkvetchakul, and C. G. Nichols. 2003. Gating dependence of inner pore access in inward rectifier K+ channels. Neuron. 37:953–962. [DOI] [PubMed] [Google Scholar]
- Proks, P., J. F. Antcliff, and F. M. Ashcroft. 2003. The ligand-sensitive gate of a potassium channel lies close to the selectivity filter. EMBO Rep. 4:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proks, P., C. E. Capener, P. Jones, and F. M. Ashcroft. 2001. Mutations within the P-loop of Kir6.2 modulate the intraburst kinetics of the ATP-sensitive potassium channel. J. Gen. Physiol. 118:341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proks, P., F. M. Gribble, R. Adhikari, S. J. Tucker, and F. M. Ashcroft. 1999. Involvement of the N-terminus of Kir6.2 in the inhibition of the KATP channel by ATP. J. Physiol. 514:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, D. Y., M. Takano, and A. Noma. 1989. Kinetics of ATP-sensitive K+ channel revealed with oil-gate concentration jump method. Am. J. Physiol. 257:H1624–H1633. [DOI] [PubMed] [Google Scholar]
- Reidharr-Olson, J. F., J. U. Bowie, R. M. Breyer, J. C. Hu, K. L. Knight, W. A. Lin, M. C. Mossing, K. R. Shoemaker, and R. T. Sauer. 1991. Random mutagenesis of protein sequences using oligonucleotide cassette. Methods Enzymol. 208:564–586. [DOI] [PubMed] [Google Scholar]
- Reimann, F., S. J. Tucker, P. Proks, and F. M. Ashcroft. 1999. Involvement of the N-terminus of Kir6.2 in coupling to the sulphonylurea receptor. J. Physiol. 518:325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribalet, B., S. A. Scott, and J. N. Weiss. 2003. Molecular basis for Kir6.2 channel inhibition by adenine nucleotides. Biophys. J. 84:266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Antomarchi, H., S. Amoroso, M. Fosset, and M. Lazdunski. 1990. K+ channel openers activate brain sulfonylurea-sensitive K+ channels and block neurosecretion. Proc. Natl. Acad. Sci. USA. 87:3489–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa, N. E., and F. J. Sigworth. 1998a. Activation of Shaker potassium channels. II. Kinetics of the V2 mutant channel. J. Gen. Physiol. 111:295–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa, N. E., and F. J. Sigworth. 1998b. Activation of Shaker potassium channels. III. An activation gating model for wild-type and V2 mutant channels. J. Gen. Physiol. 111:313–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, N., A. Crane, J. P. T. Clement, G. Gonzalez, A. P. Babenko, J. Bryan, and L. Aguilar-Bryan. 1999. The C terminus of SUR1 is required for trafficking of KATP channels. J. Biol. Chem. 274:20628–20632. [DOI] [PubMed] [Google Scholar]
- Shyng, S.-L., T. Ferrigni, and C. G. Nichols. 1997a. Control of rectification and gating of cloned KATP channels by the Kir6.2 subunit. J. Gen. Physiol. 110:141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng, S., T. Ferrigni, and C. G. Nichols. 1997b. Regulation of KATP channel activity by diazoxide and MgADP. Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J. Gen. Physiol. 110:643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng, S.-L., and C. G. Nichols. 1997. Octameric stoichiometry of the KATP channel complex. J. Gen. Physiol. 110:655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth, F. J., and S. M. Sine. 1987. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys. J. 48:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano, M., L. H. Xie, H. Otani, and M. Horie. 1998. Cytoplasmic terminus domains of Kir6.x confer different nucleotide-dependent gating on the ATP-sensitive K+ channel. J. Physiol. 512:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe, K., S. J. Tucker, F. M. Ashcroft, P. Proks, N. Kioka, T. Amachi, and K. Ueda. 2000. Direct photoaffinity labeling of Kir6.2 by [γ-32P]-ATP-[γ]4-azidoanilide. Biochem. Biophys. Res. Commun. 272:316–319. [DOI] [PubMed] [Google Scholar]
- Tanabe, K., S. J. Tucker, M. Matsuo, P. Proks, F. M. Ashcroft, S. Seino, T. Amachi, and K. Ueda. 1999. Direct photoaffinity labeling of the Kir6.2 subunit of the ATP-sensitive K+ channel by 8-azido-ATP. J. Biol. Chem. 274:3931–3933. [DOI] [PubMed] [Google Scholar]
- Terzic, A., I. Findlay, Y. Hosoya, and Y. Kurachi. 1994. Dualistic behavior of ATP-sensitive K+ channels toward intracellular nucleoside diphosphates. Neuron. 12:1049–1058. [DOI] [PubMed] [Google Scholar]
- Trapp, S., S. Haider, P. Jones, M. S. Sansom, and F. M. Ashcroft. 2003. Identification of residues contributing to the ATP binding site of Kir6.2. EMBO J. 22:2903–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp, S., P. Proks, S. J. Tucker, and F. M. Ashcroft. 1998. Molecular analysis of ATP-sensitive K channel gating and implications for channel inhibition by ATP. J. Gen. Physiol. 112:333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trube, G., and J. Hescheler. 1984. Inward rectifying channels in isolated patches of the heart cell membrane: ATP-dependence and comparison with cell-attached patches. Pflugers Arch. 401:178–184. [DOI] [PubMed] [Google Scholar]
- Tucker, S. J., and F. M. Ashcroft. 1999. Mapping of the physical interaction between the intracellular domains of an inwardly rectifying potassium channel, Kir6.2. J. Biol. Chem. 274:33393–33397. [DOI] [PubMed] [Google Scholar]
- Tucker, S. J., F. M. Gribble, P. Proks, S. Trapp, T. J. Ryder, T. Haug, F. Reimann, and F. M. Ashcroft. 1998. Molecular determinants of KATP channel inhibition by ATP. EMBO J. 17:3290–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker, S. J. F., F. M. Gribble, C. Zhao, S. Trapp, and F. M. Ashcroft. 1997. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 387:179–183. [DOI] [PubMed] [Google Scholar]
- Tukey, J. W. 1977. Exploratory Data Analysis. Addison-Wesley Publication Co., Reading, MA.
- Vanoye, C. G., G. G. MacGregor, D. Dong, L. Tang, A. S. Buschmann, A. E. Hall, M. Lu, G. Giebisch, and S. C. Hebert. 2002. The carboxyl termini of KATP channels bind nucleotides. J. Biol. Chem. 277:23260–23270. [DOI] [PubMed] [Google Scholar]
- Xiao, J., X. G. Zhen, and J. Yang. 2003. Localization of PIP2 activation gate in inward rectifier K+ channels. Nat. Neurosci. 6:811–818. [DOI] [PubMed] [Google Scholar]
- Yellen, G. 2002. The voltage-gated potassium channels and their relatives. Nature. 419:35–42. [DOI] [PubMed] [Google Scholar]
- Zagotta, W. N., T. Hoshi, J. Dittman, and R. W. Aldrich. 1994a. Shaker potassium channel gating. II: transitions in the activation pathway. J. Gen. Physiol. 103:279–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta, W. N., T. Hoshi, and R. W. Aldrich. 1994b. Shaker potassium channel gating. III: evaluation of kinetic models for activation. J. Gen. Physiol. 103:321–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerangue, N., B. Schwappach, Y. N. Jan, and L. Y. Jan. 1999. A new trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron. 22:537–548. [DOI] [PubMed] [Google Scholar]