Abstract
MscL is a bacterial mechanosensitive channel that is activated directly by membrane stretch. Although the gene has been cloned and the crystal structure of the closed channel has been defined, how membrane tension causes conformational changes in MscL remains largely unknown. To identify the site where MscL senses membrane tension, we examined the function of the mutants generated by random and scanning mutagenesis. In vitro (patch-clamp) and in vivo (hypoosmotic-shock) experiments showed that when a hydrophilic amino acid replaces one of the hydrophobic residues that are thought to make contact with the membrane lipid near the periplasmic end of the M1 or M2 transmembrane domain, MscL loses the ability to open in response to membrane tension. Hydrophilic (asparagine) substitution of the other residues in the lipid-protein interface did not impair the channel's mechanosensitivity. These observations suggest that the disturbance of the hydrophobic interaction between the membrane lipid and the periplasmic rim of the channel's funnel impairs the function of MscL.
INTRODUCTION
Virtually all eukaryotic and prokaryotic cells possess mechanosensitive channels that detect mechanical force acting on the cell. Organisms detect sound, touch, gravity, and pressure through activation or inactivation of the mechanosensitive channels. Cells that are not directly involved in the sensory transduction also have mechanosensitive channels to monitor cell swelling and deformation. When bacterial cells are subjected to hypoosmotic shock, they avoid cell lysis by opening MscS (mechanosensitive channel of small conductance) and MscL (mechanosensitive channel of large conductance) (Levina et al., 1999; Batiza et al., 2002). Escherichia coli MscL (EcoMscL) is a homopentamer of 136 amino acids with two transmembrane α-helices, M1 and M2 (Sukharev et al., 1994, 1999). The crystal structure of Mycobacterium tuberculosis MscL (TbMscL) shows that M1 helices line the pore of the closed channel and form a pore constriction near the cytoplasmic end when in the closed state (Fig. 1; Chang et al., 1998). The surface toward the membrane lipid is occupied largely by M2 helices and partly by the periplasmic end of M1 helices. These transmembrane α-helices are estimated to expand and tilt to form a pore of ∼30 Å in diameter when in the open state (Sukharev et al., 1993; Cruickshank et al., 1997; Häse et al., 1995; Betanzos et al., 2002; Perozo et al., 2002a,b). Expansion of the transmembrane domain subsequently leads to opening of the second gate formed by the amino-terminal segment (Sukharev et al., 2001).
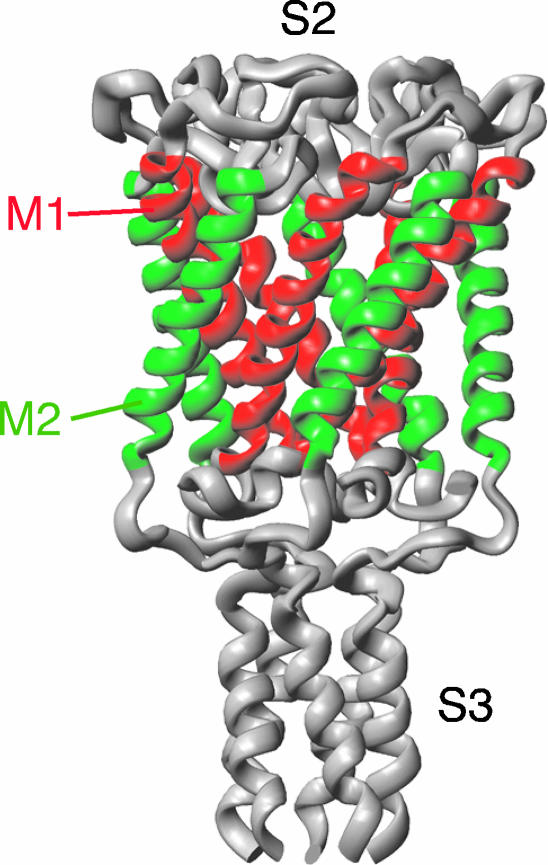
FIGURE 1.
Crystal structure of TbMscL shown as a ribbon diagram (Chang et al., 1998). M1 and M2 transmembrane α-helices are shown in red and green, respectively.
The finding that MscL retains mechanosensitivity in its purified and reconstituted form in liposomes (Sukharev et al., 1993; Häse et al., 1995) indicates that MscL opens directly by the tension of membrane lipid. Therefore, the gating of MscL is a consequence of the change in the balance between the work by the membrane lipid tension that expands MscL and the energy of intramolecular interactions that keeps MscL in the closed conformation. A clue to understanding the origin of the latter force has been obtained through screening for gain-of-function mutants; the isolated mutants with a mutation of the glycine residues along one face of the cytoplasmic half of M1 showed a decreased threshold for channel opening (Ou et al., 1998). By replacing one of the glycines (Gly-22) with all 19 other amino acids or by chemically charging residue 22, the gating threshold was found to decrease with the hydrophilicity of residue 22 (Yoshimura et al., 1999, 2001). Based on these findings, it has been proposed that hydrophobic interaction at the cytoplasmic part of M1 (“hydrophobic lock”) stabilizes the closed-state conformation and this interaction is unlocked when MscL opens (Ou et al., 1998; Yoshimura et al., 1999, 2001; Blount and Moe, 1999; Moe et al., 2000). Introduction of a hydrophilic amino acid at residue 22 is thought to destabilize the hydrophobic interaction and allow the channel to gate with weak stimulus. Since proteolytic digestion of the periplasmic loop, which connects M1 and M2 (S2 in Fig. 1), cause a significant increase in MscL sensitivity to membrane tension (Ajouz et al., 2000), the periplasmic loop may also contribute to keep MscL in the closed state.
Much less is known about the interaction between MscL and membrane lipid. Pressure in the lipid bilayer acting on membrane proteins is not uniform across the bilayer: interactions between headgroups and those between acyl chains produce repulsive force whereas cohesive force acts at the polar-apolar interface (Marsh, 1996; Cantor, 1997; Gullingsrud and Schulten, 2003). In support of the idea that MscL directly senses the pressure of the membrane lipid, alteration of the pressure profile by using phospholipids with shorter acyl chains dramatically changes the mechanosensitivity of MscL (Perozo et al., 2002b). The finding that asymmetric incorporation of conical lipids to one monolayer of the lipid bilayer is sufficient to open MscL (Perozo et al., 2002b) indicates that the asymmetry of the pressure profile, or the asymmetrical reception of pressure by MscL, may cause the channel opening.
In the present study, we approached the problem of where and how MscL senses membrane tension by isolating mutants that do not open in response to membrane stretch. To isolate loss-of-function mutants, we introduced random mutations in mscL and looked for mutants that complemented lethal (open-pore) mutation at Gly-22. This screening revealed that hydrophilic substitution of one of the hydrophobic residues facing membrane lipid results in loss of MscL's ability to open by membrane stretch. To completely map the residues that impair channel function on hydrophilic substitution, each residue in the lipid-protein interface was replaced one by one with asparagine. Asparagine was used here because 1), substitution with asparagine is one of the severest mutations (I41N MscL, see Results); 2), asparagine is electrically neutral; and 3), asparagine substitution of the residues in the lipid-protein interface has been shown not to affect channel function of a voltage-gated channel, Shaker K+ channel (Monks et al., 1999). The asparagine scanning mutagenesis identified a band of hydrophobic residues at the periplasmic border of the lipid-protein interface that were essential for MscL function.
MATERIALS AND METHODS
Strains
Escherichia coli PB104 (ΔmscL) (Blount et al., 1996) and MJF455 (ΔmscL, ΔmscS) (Levina et al., 1999) were used to host MscL expression in patch-clamp experiments and hypoosmotic-shock experiments, respectively. E. coli DH5α was used for molecular biology.
Molecular biology
Wild-type (Sukharev et al., 1994), G22D, G22K, or G22N (Yoshimura et al., 1999) mscL was cloned in vector pB10b (Ou et al., 1998), which has an ampicillin resistance gene and the lacUV5 promoter.
Random mutagenesis on the open reading frame of G22D, G22K, or G22N mscL was performed with error-prone polymerase chain reaction (PCR) (GeneMorph, Stratagene, Cedar Creek, TX) using BglII-tagged 5′ primer and XhoI-tagged 3′ primer (Blount et al., 1999). The amount of template was adjusted so that 0–3 mutations occurred in the open reading frame. The PCR product was cut with BglII and XhoI, ligated into pB10b, and transformed into PB104 by electroporation. Transformants were incubated overnight on Luria-Bertani (LB) agar plates containing 100 μg/ml ampicillin and 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG). Site-directed mutagenesis was performed by megaprimer PCR (Yoshimura et al., 1999) or by the DpnI-based method (Quick Change Site-directed Mutagenesis Kit, Stratagene). The mutated product was cut with BglII and XhoI, and then ligated into fresh vector pB10b. The DNA sequence was determined by reading from both the 5′ and 3′ ends of the open reading frame.
Electrophysiology
The channel activity of MscL was examined by the patch-clamp method as described previously (Yoshimura et al., 1999). After incubation of log-phase PB104 cells for 1.5 h in the presence of cephalexin, MscL expression was induced by adding 1 mM IPTG for 10 min. Cells were digested by lysozyme for 4 min and spheroplasts were collected by centrifugation.
Channel current was obtained from inside-out patches from spheroplasts. Pipette solution contained 200 mM KCl, 90 mM MgCl2, 10 mM CaCl2, and 5 mM Hepes (pH 6.0). Bath solution contained 0.3 M sucrose in addition to pipette solution. The pipette potential was held +20 mV relative to the bath. The current was amplified with an amplifier (Axopatch 200B, Axon, Foster City, CA) and the data acquired and stored in a personal computer. The threshold of MscL gating was expressed as the ratio of the pressure required to gate MscL relative to that to gate MscS (Blount et al., 1996).
Protein assay
Membrane protein was purified using a method based on that of Blount et al. (1999). Cells in log-phase growth were incubated in the presence of 1 mM IPTG for 2 h. Cells were then collected by centrifugation and resuspended in sonication buffer that contained 50 mM K-phosphate (pH 7.2), 5 mM MgSO4, 1 mM DTT, and 1.25 mM Pefabloc SC (Roche, Basel, Switzerland). After the cell suspension was sonicated (Sonifier 450, Branson Ultrasonics, Danbury, CT), cell debris was removed by centrifugation (6000 × g, 10 min). The supernatant was centrifuged at 100,000 × g for 1 h, and the pellet was resuspended in 150 mM NaCl, 50 mM Tris-HCl (pH 8.0), and 1% octyl-β-D-glucopyranoside. Insoluble particles were removed by centrifugation (100,000 × g, 1 h). The protein was electrophoresed on a 12% sodium dodecyl sulfate polyacrylamide gel (Bis-Tris Gel, Invitrogen, Carlsbad, CA). An antibody that recognizes the carboxyl terminus of MscL (Blount et al., 1996) was used to detect MscL by Western blot.
In the cross-linking experiment, the membrane pellet was resuspended in 100 mM NaCl and 30 mM Na phosphate, pH 7.5. Disuccinimidyl suberate (DSS; 0.1 mM final concentration) was added; the sample was incubated for 1 h at room temperature and the reaction was terminated by adding Tris (50 mM final concentration; pH 8.0). The cross-linked protein was electrophoresed as described above.
Hypoosmotic shock and growth assay
Survival rate after hypoosmotic shock was examined using a method based on that of Levina et al. (1999). MJF455 cells, which do not have mscS and mscL, were used to express MscL. Expression was induced by adding IPTG (1 mM) to cells grown to OD600 = 0.14 in minimal medium supplemented with 500 mM NaCl and ampicillin. After incubation for 1 h, the cells were diluted 1:20 in the prewarmed minimal medium with or without 500 mM NaCl. After 5 min, diluted cells were spread on an LB plate with ampicillin and 1 mM IPTG in triplicate. The survival rate was calculated (ndown/ncontrol) from the number of colony-forming units of the cells that experienced osmotic downshock (ndown) and those that did not experience the downshock (ncontrol). The osmotic shock experiment was repeated three times on each mutant.
RESULTS
Second site suppressor mutations in the toxic G22X MscL
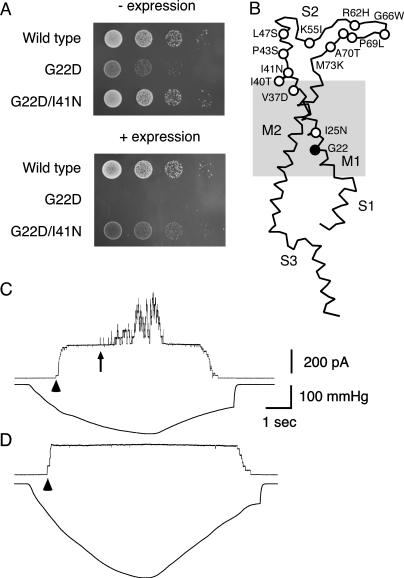
As a first step to screen out loss-of-function MscL mutants, we introduced random mutations into MscL that have a hydrophilic amino acid (Asn, Asp, or Lys) in place of Gly-22 (Yoshimura et al., 1999). When expressed, these G22X MscLs (X = D, K, or N) are lethal to cells (Fig. 2 A, second row) because of the loss of solute from the cell that occurs with the spontaneous opening of MscL (Ou et al., 1998; Yoshimura et al., 1999). We found, however, that some cells having the mutagenized G22X MscL still survived even when expression was induced on agar plates containing IPTG. The DNA sequence of mscL from 51 clones was determined. While about three-fifths of the isolated mutants contained either a nonsense mutation or multiple mutations, we finally isolated 12 independent mutants, each with a single amino acid substitution (this screening did not reach saturation while four mutants were hit multiple times in independent mutagenesis reactions). These mutants allowed cells to form colonies when the expression was induced (for example, G22D/I41N; Fig. 2 A). The sites of the single amino acid substitution were found only at the periplasmic end of M1, the periplasmic loop S2, and Ile-25 (Fig. 2 B) whereas nonsense mutation and multiple mutations occurred randomly throughout the entire open reading frame.
FIGURE 2.
Complementation of lethal mutation at residue 22. (A) The growth on agar plates of cells that expressed wild-type, G22D, and G22D/I41N MscL without (top) and with (bottom) induced expression. Growth of 5 μl of each 10-fold dilution (10−3–10−6) of cells is shown. (B) Sites of mutation shown in a single subunit. (C and D) Channel openings of wild-type (C) and G22D/I41N (D) MscL. The upper trace shows the current record when the pipette was held 20 mV positive to the bath. The lower trace illustrates the negative pressure applied to the patch membrane. The arrowhead and arrow indicate the beginning of MscS and MscL opening, respectively.
The channel activity of the MscL mutants was examined by applying negative pressure through a patch pipette to the inside-out membrane patch of giant spheroplasts expressing MscL. When an increasingly negative pressure was applied, wild-type MscL began to open at a pressure 1.69 ± 0.13 times (n = 7) that of the threshold of MscS (Fig. 2 C). There were 41 ± 10 channels in the patch membrane used in our experiments; however, even with a pressure up to 3.2 times that of the MscS threshold, no channel activity was observed in cells expressing G22D/I41N MscL (Fig. 2 D). We examined four to eight spheroplasts from each mutant MscL; however, up until the time of membrane lysis none displayed channel opening.
Mutant MscL that does not open in vitro or in vivo
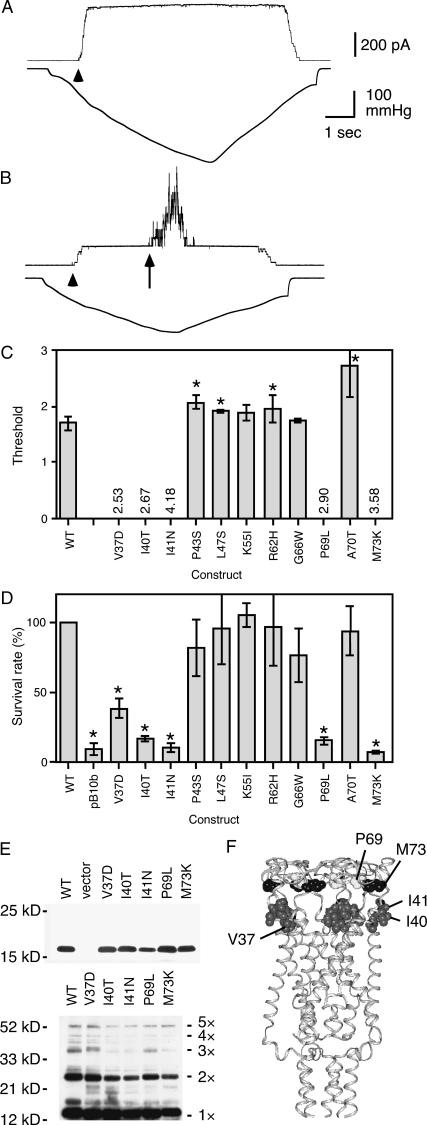
Because complementation of the Gly-22 mutation and loss of channel activity may be a combined effect between Gly-22 mutation and a second mutation and because a loss of spontaneous opening does not necessarily imply that the second mutation alone can impair the mechanosensitivity of MscL, we constructed mutants that did not have a residue 22 mutation. All mutants except I25N MscL were examined by patch clamping. In the absence of Gly-22 mutation, mutants showed various degrees of mechanosensitivity. I41N MscL did not open even when pressure four times that of the MscS threshold was applied (Fig. 3 A). Channel openings were not observed during subsequent application of pressure up to the point of membrane lysis. In four other mutants (V37D, I40T, P69L, and M73K) with similar phenotype, no channel activity was observed in most spheroplasts; however, in some patches (<20%) opening of a single MscL channel with a high threshold was observed. Since the patch membrane infrequently tolerated pressure more than 2.5 times that of the threshold of MscS (irrespective of what MscL was expressed), we hereafter regard each MscL showing no activity up to pressure 2.5 (/MscS) as an MscL that does not open in patch-clamp experiments (these mutants are the same ones that did not show MscL activity up to membrane lysis in >80% of patch). The highest pressure (point of membrane lysis) without any indication of MscL activity is indicated as numerals in Fig. 3 C. Other mutants retained the ability to open with a normal or high threshold (Fig. 3 B; threshold shown as bars in Fig. 3 C).
FIGURE 3.
Function of mutant MscL in vitro and in vivo. (A and B) Application of negative pressure to the membrane of cells expressing I41N (A) and P43S MscL (B). The arrowhead and arrow indicate the beginning of MscS and MscL opening, respectively. (C) Threshold (mean and standard deviation of four to seven spheroplasts) of single-mutation MscL expressed as the ratio to the threshold of MscS. The asterisk indicates that the threshold is significantly different from wild type (p < 0.05). Numerical values indicate the highest pressure examined for which MscL did not open. (D) The survival rate of cells expressing MscL and having empty vector (pB10b) (mean and SD of three experiments) when osmotic downshock was applied. The asterisk indicates that the survival rate is significantly different from wild type (p < 0.05). (E) Western blot showing that mutant MscL is present in the cell membrane (top). Cross-linking by DSS shows that the channels are pentameric (bottom). (F) The position of loss-of-function mutation in EcoMscL.
To probe the opening of mutant MscL in vivo, the cells (mscL− mscS− strain) expressing mutant MscL were challenged by osmotic downshock. Cells initially grown in a minimal medium supplemented with 500 mM NaCl were diluted into a minimal medium without additive NaCl (Levina et al., 1999). The survival rate of cells harboring an empty vector (pB10b) was significantly lower than that of cells expressing wild-type MscL (Fig. 3 D) (Levina et al., 1999). Thus, survival increases probably due to osmolyte release through MscL channels that open as a result of cell swelling. The mutants showed a survival rate that tended to depend on the threshold of MscL as determined by the patch clamp (Fig. 3, C and D). Importantly, all mutants with severe defects in opening under patch clamp (V37D, I40T, I41N, P69L, and M73K) were also defective at rescuing cells from hypoosmotic shock, although the defect was less severe in V37D MscL. Hypoosmotic shock of 500 mM NaCl in LB medium (Batiza et al., 2002) also yielded similar results. These observations indicate that V37D, I40T, I41N, P69L, and M73K MscL have severe defects in MscL opening in vivo as well as in vitro. Despite the defect of MscL activities, Western blot analysis using an antibody against the carboxyl-terminal segment of MscL (Blount et al., 1996) showed that these mutant MscLs are expressed in the cell membrane (Fig. 3 E, top). Cross-linking of the subunits by DSS indicated that MscLs are in pentameric form (Fig. 3 E, bottom).
Asparagine scanning mutagenesis of the residues in the lipid-protein interface region
The modeled structure of EcoMscL (Sukharev et al., 2001) and its molecular environment as assessed by electron paramagnetic resonance spectroscopy (EPR, Perozo et al., 2002b) indicate that Val-37, Ile-40, and Ile-41 form a cluster at the periplasmic end of M1 that interacts with membrane lipid (Fig. 3 F). Since hydrophilic substitution of these hydrophobic residues results in loss of function, we surmised that hydrophobic interaction between these residues and the lipid may be essential to the mechanical gating of MscL. We next examined whether, among the residues in the lipid-protein interface, these three residues are the only ones that cause loss of function upon hydrophilic substitution. To answer this question, we substituted each residue facing membrane lipids, one by one, with asparagine (see Introduction). This thorough mapping was performed to complement the lack of saturation of the screening of random mutants described above and more specifically to assess the effect of hydrophilic substitution. The effect of introducing an electrically neutral amino acid is restricted more to a change in hydrophilicity than would be the case if substituted with a charged amino acid. In addition, random substitution of a single nucleotide does not produce a full set of mutants with all possible single amino acid substitutions (for example, the only possible hydrophilic amino acid that can be converted from valine is aspartic acid).
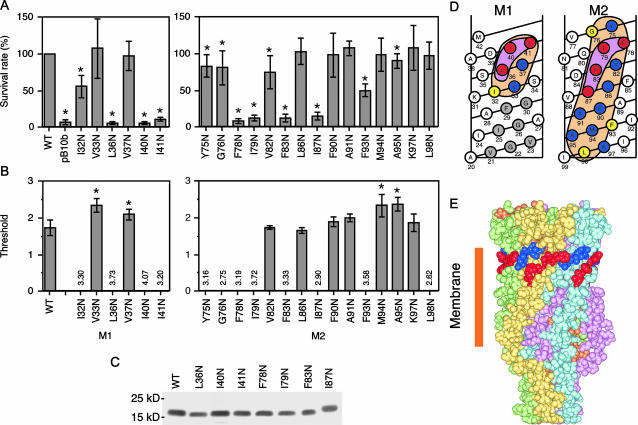
Using the EcoMscL structure model (Sukharev et al., 2001) and EPR data (Perozo et al., 2002b), 21 residues that interact with membrane lipid were identified (see Fig. 4 D, orange area). One at a time, each of these residues was substituted with asparagine and the construct was expressed for testing by hypotonic shock. Cells harboring expression vector containing L36N, I40N, I41N, F78N, I79N, F83N, or I87N mscL showed survival rate as low as that of cells harboring empty vector (Fig. 4 A). Despite the lack of indication of MscL activity, Western blot showed that MscL was present in the membrane (Fig. 4 C). In the patch-clamp experiments, 12 mutants were found to show no MscL activity when a negative pressure was applied to the inside-out membrane patch (Fig. 4 B). Other asparagine mutants retained the ability to open with normal or high thresholds. All seven mutants that failed to rescue the cell from hypotonic shock did not show channel activity under patch clamping. Most of the other five mutants that did not open in patch-clamp experiments (I32N, Y75N, G76N, and F93N) had decreased survival. Here, we define a “high-impact” residue as one whose asparagine substitution resulted in loss of activity both in vivo and in vitro. Interestingly, the high-impact residues line up in a characteristic Γ shape at the periplasmic end of M1 and M2 when mapped on a net diagram of the transmembrane α-helices (Fig. 4 D). These residues form a band near the periplasmic boundary of the transmembrane domain, i. e., the outer rim of the channel's funnel (Fig. 4 E).
FIGURE 4.
Asparagine scanning mutagenesis of the residues in the lipid-protein interface. (A) Effect of hypoosmotic shock on cells expressing mutant MscL. The asterisk indicates that the survival rate is significantly different from wild type (p < 0.05). (B) Threshold of MscL gating as determined by the patch clamp. Numerical values indicate the highest pressure examined at which MscL opening was not observed. (C) Western blot showing mutant MscL is present in the membrane. (D) The impact (severeness of function loss) of asparagine substitution shown on a net diagram of M1 and M2 α-helices. High-impact residues (see text) are indicated by red. Moderate-impact residues, asparagine substitution of which resulted in loss of function only in patch-clamp experiments, are shown in yellow. The residues for which function was not affected by asparagine replacement are indicated by blue. Gray residues represent site of gain-of-function mutation (the current study and Ou et al., 1998). I25N and F29N are gain-of-function mutants found in this study. No other mutants examined in this study showed gain-of-function phenotype. (E) High-impact residues at the end of M1 (blue) and M2 (red).
DISCUSSION
The results of complementation testing of G22X mutations indicate that the function of MscL is severely impaired in vitro (in patch-clamp experiments) and in vivo (in hypoosmotic-shock experiments) when mutation of V37D, I40T, I41N, P69L, or M73K occurs. The structural model of EcoMscL shows that Val-37, Ile-40, and Ile-41 form a face toward the outside of the transmembrane domain (Fig. 3 F) (Sukharev et al., 2001). Pro-69 and Met-73, on the other hand, are located in the periplasmic loop S2 (Fig. 3 F). The data from the EPR experiments indicate that, during transition from the closed to the intermediate state, Val-37, Ile-40, and Ile-41 obtain the highest degree of lipid accessibility among the residues in M1 (Perozo et al., 2002b). Furthermore, the accessibility to water is close to zero. These structural data clearly indicate that Val-37, Ile-40, and Ile-41 significantly interact with lipids during channel opening. Since substitution of Val-37, Ile-40, and Ile-41 with a hydrophilic amino acid causes a defect in the channel protein's ability to open by membrane tension, it is most likely that the hydrophobic interaction between these residues and the lipid is essential to the function of MscL.
The results of the random mutagenesis experiment indicate that the hydrophilic substitution at the periplasmic end of M1 causes a severe defect in MscL function. Recently, Maurer and Dougherty (2003) also isolated hydrophilic mutants at these residues through a distinct screening protocol. However, whether changes to this part are the only ones that cause loss of function remained unclear. Moreover, it is probable that the defect is due to introduction of charge rather than change in hydrophilicity (see discussion on V37D mutant below). Mapping the whole lipid-protein interface by asparagine scanning revealed that changes in another cluster of residues, at the periplasmic end of M2, cause loss of function. The high-impact residues at the M1 and M2 ends formed a Γ shape and changes in the residues outside this part did not result in any severe defect in MscL function (Fig. 4 D). Thus, the high-impact residues are restricted to the area where the protein interacts with membrane lipid near its surface (Fig. 4 E). Since it has been estimated that negative pressure from the lipid occurs only at the apolar-polar interface (Marsh, 1996; Cantor, 1997; Gullingsrud and Schulten, 2003), this area consisting of three residues at the end of M1 (Leu-36, Ile-40, and Ile-41) and four residues at the end of M2 (Phe-78, Ile-79, Phe-83, and Ile-87) probably receives the membrane tension (stretch) from the lipid through hydrophobic interaction. Since the lipid molecules are dynamic in the normal and tangential direction of the bilayer (Wiener and White, 1992) and since the hydrophobic interaction is weak whereas MscL needs a large tension, these clustered and highly hydrophobic residues are possibly needed to reinforce the hydrophobic interaction. This hydrophobic interaction may be hampered by asparagine substitution; if so, then the area highlighted by asparagine mutagenesis may be the putative mechanosensor of MscL. We surmise that the double mutants with G22X mutation do not show the spontaneous opening seen with G22X mutants because the negative pressure that MscL experiences at the periplasmic ends of M1 and M2 is decreased by the hydrophilic substitution.
The residues in the high-impact sites are Ile, Leu, and Phe. Ile is present at four residues out of seven. Sequence comparison among MscL of numerous species of bacteria (Maurer et al., 2000; Sukharev et al., 2001) shows that these three amino acids and Val are found almost exclusively at these positions. On the other hand, other hydrophobic amino acids (Ala, Met, and Trp) and some hydrophilic amino acids (Asn, Arg, Gly, Lys, Ser, and Thr) frequently appear at other positions in the lipid-protein interface. Interestingly, the order of hydrophobicity of amino acids (according to Kyte and Doolittle, 1982) starts with Ile, which is followed by Val, Leu, Phe, and the other 16 amino acids. This sequence conservation of the most hydrophobic amino acids in the high-impact residues suggests the importance of the hydrophobicity of these residues in the function of MscL.
In contrast to MscL, Shaker K+ channel, a voltage-gated ion channel, operates normally when a hydrophilic amino acid is introduced in the lipid-protein interface: substitution with asparagine of one of the hydrophobic residues (Ile, Leu, Phe, and Val) that face membrane lipid does not affect channel function (Monks et al., 1999). Unlike the case with voltage dependent channels, the loss of function in MscL may be because the lipid-protein interaction is essential to the function.
Since V37N MscL functioned normally while V37D did not, the loss of function in V37D MscL is due to the negative charge of aspartic acid rather than the loss of hydrophobic interaction. The negative charge of Asp-37 may interact with the headgroup of membrane lipid and disturb the interaction between lipid and channel protein. This finding suggests that using electrically neutral amino acids is essential to the assessment of the hydrophobic interaction between lipid and protein. However, full justification that the main effect of asparagine introduction in the lipid-protein interface is the change in hydrophobicity may require a complementary experiment in which the residues are replaced with other amino acids as was done with the G22X mutation (Yoshimura et al., 1999).
Several gain-of-function mutants (Ou et al., 1998) and loss-of-function mutants (P69L or M73K) have been isolated in the periplasmic loop. The change in the function may be due to a change in the folding and/or stiffness of the loop since the proteolytic digestion of the loop has been shown to increase the mechanosensitivity (Ajouz et al., 2000). Taking these findings into account, the data from loss-of-function mutants should be handled with care. Nevertheless, the random mutagenesis data of ours and Maurer's (Maurer and Dougherty, 2003), the information from the crystal structure and EPR experiment, and the pressure profile of membrane lipid are all in favor of the importance of the hydrophobic interaction at the M1 and M2 ends. For instance, the loss of function by the mutation at the periplasmic ends could be because of a change in the structure (e.g., in the periplasmic loop) rather than the decrease in the lipid-protein interaction. Although this possibility cannot be ruled out, we suspect that it is not very likely since a single hydrophilic substitution of the residue that faces membrane lipid has not been thought to affect the structure of the channel protein (Monks et al., 1999). Another possibility is that hydrophilic substitutions at the ends of helices, which are assumed to submerge into membrane during gating, prevent tilting of the helices (Betanzos et al., 2002; Perozo et al., 2002a). Hydrophilic substitution at the periplasmic ends of M2 does not have great effect on tilting possibly because existing charges are present nearby (K101, R104, and K105). The exact mechanism of how the hydrophilic substitution affects the MscL function should be examined in the future by experimentation or by computer simulation.
In this study, we examined the function of MscL by directly recording the current through the membrane and also by evaluating the survival rate upon hypoosmotic shock. No mutant that failed to rescue cells from hypoosmotic shock showed channel activity under patch clamp. However, some mutant MscL that did not show channel opening under patch clamp rescued the cell; in most of these cases, the survival was lower than that of cells expressing wild-type MscL. This discrepancy may be because 1), MscL opens more readily under the ionic and electrical condition in vivo than in vitro; 2), the native membrane tolerates higher tension than the membrane of spheroplasts; and/or 3), opening of a very small number of MscLs is enough to rescue the cell from hypoosmotic shock. Although we used preparations close to the native condition, it will be worthwhile to examine the function of MscL mutants by reconsitution into liposomes to assess the effect of the length of the hydrocarbon chain and to avoid possible effects of other proteins that may influence MscL activity. These experiments are now under way.
Previous studies showed that substitution of Val-21, Gly-22, Val-23, Gly-26, or Gly-30 with a hydrophilic amino acid decreases the gating threshold and results in growth defects (Fig. 4 D) (Yoshimura et al., 1999, 2001). During this study, we found that cells expressing I25N or F29N MscL also did not grow when the expression was induced (data not shown). Taken together, these findings provide further support to the idea that the hydrophobic environment within the cytoplasmic half of M1 (“hydrophobic lock”) stabilizes the closed-state conformation (Ou et al., 1998; Yoshimura et al., 1999, 2001; Blount and Moe, 1999; Moe et al., 2000). Thus, the tilting of M1 during gating (Betanzos et al., 2002; Perozo et al., 2002a) may occur by holding the cytoplasmic ends of M1 together and by pulling the periplasmic ends of M1 apart from the molecular fivefold axis.
To summarize, we located residues essential to the function of MscL through the combination of forward and reverse genetics. By generating random mutations and screening mutants for the phenotype of interest (forward genetics), we obtained a clue to the location and the type of mutation that impairs the channel's function. A thorough map of the residues that cause loss of function on hydrophilic substitution was drawn using a method of reverse genetics, i.e., asparagine scanning mutagenesis. This strategy would be effective to examine other types of mechanosensitive channels that are directly activated by membrane stretch.
Acknowledgments
We thank Sergei Sukharev for the gift of antibody and Ian Booth for the gift of MJF455. We are grateful to Richard Weisburd for critically reading the manuscript.
This work was supported by a grant-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (to M.S.), and a grant from the Japan Space Forum (to M.S.).
References
- Ajouz, B., C. Berrier, M. Besnard, B. Martinac, and A. Ghazi. 2000. Contributions of the different extramembranous domains of the mechanosensitive ion channel MscL to its response to membrane tension. J. Biol. Chem. 275:1015–1022. [DOI] [PubMed] [Google Scholar]
- Batiza, A. F., M. M.-C. Kuo, K. Yoshimura, and C. Kung. 2002. Gating the bacterial mechanosensitive channel MscL in vivo. Proc. Natl. Acad. Sci. USA. 99:5643–5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betanzos, M., C.-S. Chiang, R. Guy, and S. Sukharev. 2002. A large iris-like expansion of a mechanosensitive channel protein induced by membrane tension. Nat. Struct. Biol. 9:704–710. [DOI] [PubMed] [Google Scholar]
- Blount, P., and P. Moe. 1999. Bacterial mechanosensitive channels: integrating physiology, structure and function. Trends Microbiol. 7:420–424. [DOI] [PubMed] [Google Scholar]
- Blount, P., S. Sukharev, P. Moe, B. Martinac, and C. Kung. 1999. Mechanosensitive channels of bacteria. Methods Enzymol. 294:458–482. [DOI] [PubMed] [Google Scholar]
- Blount, P., S. I. Sukharev, M. J. Schroeder, S. K. Nagle, and C. Kung. 1996. Single residue substitutions that change the gating properties of a mechanosensitive channel in Escherichia coli. Proc. Natl. Acad. Sci. USA. 93:11652–11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor, R. S. 1997. Lateral pressures in cell membranes: a mechanism for modulation of protein function. J. Phys. Chem. B. 101:1723–1725. [Google Scholar]
- Chang, G., R. H. Spencer, A. T. Lee, M. T. Barclay, and D. C. Rees. 1998. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 282:2220–2226. [DOI] [PubMed] [Google Scholar]
- Cruickshank, C., R. Minchin, A. Le Dain, and B. Martinac. 1997. Estimation of the pore size of the large-conductance mechanosensitive ion channel of Escherichia coli. Biophys. J. 73:1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullingsrud, J., and K. Schulten. 2003. Gating of MscL studied by steered molecular dynamics. Biophys. J. 85:2087–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häse, C. C., A. C. Le Dain, and B. Martinac. 1995. Purification and functional reconstitution of the recombinant large mechanosensitive ion channel (MscL) of Escherichia coli. J. Biol. Chem. 270:18329–18334. [DOI] [PubMed] [Google Scholar]
- Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105–132. [DOI] [PubMed] [Google Scholar]
- Levina, N., S. Totemeyer, N. R. Stokes, P. Louis, M. A. Jones, and I. Booth. 1999. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 18:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, D. 1996. Lateral pressure in membranes. Biochim. Biophys. Acta. 1286:183–223. [DOI] [PubMed] [Google Scholar]
- Maurer, J. A., and D. A. Dougherty. 2003. Generation and evaluation of a large mutational library from the Escherichia coli mechanosensitive channel of large conductance, MscL: implications for channel gating and evolutionary design. J. Biol. Chem. 278:21076–21082. [DOI] [PubMed] [Google Scholar]
- Maurer, J. A., D. E. Elmore, H. A. Lester, and D. A. Dougherty. 2000. Comparing and contrasting Escherichia coli and Mycobacterium tuberculosis mechanosensitive channels (MscL). New gain of function mutations in the loop region. J. Biol. Chem. 275:22238–22244. [DOI] [PubMed] [Google Scholar]
- Moe, P., G. Levin, and P. Blount. 2000. Correlating a protein structure with function of a bacterial mechanosensitive channel. J. Biol. Chem. 275:31121–31127. [DOI] [PubMed] [Google Scholar]
- Monks, S. A., D. J. Needleman, and C. Miller. 1999. Helical structure and packing orientation of the S2 segment in the Shaker K+ channel. J. Gen. Physiol. 113:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou, X., P. Blount, R. Hoffman, and C. Kung. 1998. One face of a transmembrane helix is crucial in mechanosensitive channel gating. Proc. Natl. Acad. Sci. USA. 95:11471–11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo, E., D. M. Cortes, P. Sompornpisut, A. Kloda, and B. Martinac. 2002a. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature. 418:942–948. [DOI] [PubMed] [Google Scholar]
- Perozo, E., A. Kloda, D. M. Cortes, and B. Martinac. 2002b. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat. Struct. Biol. 9:696–703. [DOI] [PubMed] [Google Scholar]
- Sukharev, S. I., M. Betanzos, C. S. Chiang, and H. R. Guy. 2001. The gating mechanism of the large mechanosensitive channel MscL. Nature. 409:720–724. [DOI] [PubMed] [Google Scholar]
- Sukharev, S. I., P. Blount, B. Martinac, F. R. Blattner, and C. Kung. 1994. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature. 368:265–268. [DOI] [PubMed] [Google Scholar]
- Sukharev, S. I., B. Martinac, V. Y. Arshavsky, and C. Kung. 1993. Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys. J. 65:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev, S. I., M. J. Schroeder, and D. R. McCaslin. 1999. Stoichiometry of the large conductance bacterial mechanosensitive channel of E. coli. A biochemical study. J. Membr. Biol. 171:183–193. [DOI] [PubMed] [Google Scholar]
- Wiener, M. C., and S. H. White. 1992. Structure of a fluid dioleoylphosphatidylcholine bilayer determined by joint refinement of x-ray and neutron diffraction data. III. Complete data. Biophys. J. 61:434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura, K., A. Batiza, and C. Kung. 2001. Chemically charging the pore constriction opens the mechanosensitive channel MscL. Biophys. J. 80:2198–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura, K., A. Batiza, M. Schroeder, P. Blount, and C. Kung. 1999. Hydrophilicity of a single residue within MscL correlates with increased channel mechanosensitivity. Biophys. J. 77:1960–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]