Abstract
The ROMK (Kir1.1) family of epithelial K channels can be inactivated by a combination of low internal pH and low external K, such that alkalization does not reopen the channels unless external K is elevated. Previous work suggested that this inactivation results from an allosteric interaction between an inner pH gate and an outer K sensor, and could be described by a simple three-state kinetic model. In the present study, we report that a sustained depolarization slowly inactivated (half-time = 10–15 min) ROMK channels that had been engineered for increased affinity to internal polyamines. Furthermore, this inactivation occurred at external [K] ≤1 mM in ROMK mutants whose inner pH gate was constitutively open (ROMK2-K61M mutation). Both pH and voltage inactivation depended on external K in a manner reminiscent of C-type inactivation, but having a much slower time course. Replacement of ROMK extracellular loop residues by Kir2.1 homologous residues attenuated or abolished this inactivation. These results are consistent with the hypothesis that there are (at least) two separate closure processes in these channels: an inner pH-regulated gate, and an outer (inactivation) gate, where the latter is modulated by both voltage and external [K].
INTRODUCTION
ROMK (Kir1.1), expressed on the surface of Xenopus oocytes, displays kinetic and permeation properties similar to the native small K channel that mediates K secretion in the mammalian cortical collecting tubule and K recycling in the mammalian thick ascending limb of the loop of Henle (Palmer et al., 1997). When expressed in oocytes, ROMK is a weak inward rectifier with an inward single-channel conductance of between 10 and 30 pS, depending on extracellular potassium (Chepilko et al., 1995). At an internal pH near the pH of cortical collecting tubule cells, ROMK has a high open probability (P = .9) that is punctuated by brief (1.5 ± 0.6 ms) flickery closures (Choe et al., 1997).
Intracellular acidification shuts down ROMK by a cooperative process with an apparent Hill coefficient of ∼3 (Leipziger et al., 2000; McNicholas et al., 1998; Sackin et al., 2001). At the single-channel level, closure is characterized by the de novo appearance of long (several-minute) closures, interrupted by bursts of occasional openings that eventually disappear (McNicholas et al., 1998; Choe et al., 1997). Single-channel conductance remains invariant during acidification, except for occasional discrete subconductance states immediately before complete closure (McNicholas et al., 1998; MacGregor et al., 1998; Choe et al., 1997). These previous observations are consistent with the hypothesis that ROMK pH sensitivity arises from closure of a single gate, probably near the putative pH sensor at the cytoplasmic side of the first transmembrane helix: K61 in ROMK2 (Kir1.1b) and K80 in ROMK1(Kir1.1a) (Fakler et al., 1996; Choe et al., 1997).
ROMK is not only regulated by internal pH but also by external K at a concentration that is found normally in the late distal nephron (Doi et al., 1996; Sackin et al., 2001, 2003; Schulte et al., 2001). Furthermore, there is an interaction between internal pH sensitivity and external K, in which low external K stabilizes the closed state that results from internal acidification. This interaction has been described by a simple three-state model (Schulte et al., 2001; Sackin et al., 2001, 2003).
However, the structural basis for the coupling between internal pH and external K is not known. If this coupling were mediated by a single pH gate at the cytoplasmic end of the permeation path, the effect of external K would have to be communicated across most of the channel protein.
An alternative hypothesis is that the coupling between K and pH is mediated by two separate gates in series. In this model, the inner gate is pH-sensitive, and the outer gate is primarily K-sensitive. One problem with this model is that it has so far been impossible to show closure of the putative outer gate independent of closure of the pH-sensitive gate. In this paper we demonstrate that the channels that lack the internal pH-sensing mechanism can be inactivated by membrane depolarization, possibly through closure of an outer, K-dependent gate.
METHODS
Chimeras and mutants
The rationale of this study was to use applied membrane voltage to trigger inactivation of ROMK by a process that was independent of internal pH. Previous studies with ROMK indicated that outward current associated with sustained depolarization actually prevented inactivation when channels were exposed to low external K (see Fig. 3 of Sackin et al. (2001)). To avoid this complication, the ROMK channels of the present study (Fig. 1) were modified to pass negligible outward current. Since ROMK rectification is determined by both residues in the C-terminus and an Asp in the inner transmembrane helix (residue N152 in Kir1.1b) (Yang et al., 1995; Taglialatela et al., 1994), the constructs contained either an altered C-terminus (C13 chimera) or the N152D point mutation. Chimeras of Kir1.1 (ROMK) and Kir2.1 (IRK1) were fabricated using the overlap extension method (Horton et al., 1989).
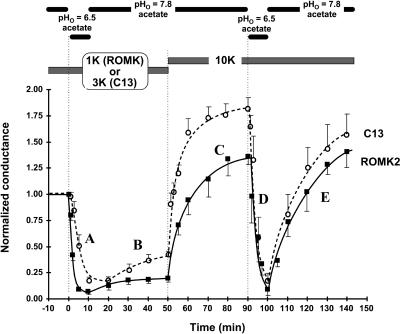
FIGURE 3.
ROMK and the C13 chimera have a similar K dependence of pH inactivation. Permeant acetate buffers were used to control the internal pH of oocytes expressing either ROMK2 (solid line, filled squares) or the C13 chimera (dashed line, open circles). Internal pH was controlled using an acetate buffer system. The calibration for this buffer was pHi = 0.595 × pHo + 2.4. Oocytes were first preincubated in either 1 mM K (ROMK) or 3 mM K (C13) at pHo = 7.8 in acetate solutions (pHi = 7.04), and then exposed to pHo = 6.5 (pHi = 6.3), followed by a return to pHo = 7.8 acetate solution. After 40 min, external K was raised to 10 mM (still at pHo = 7.8). The data are normalized to the initial conductance for each oocyte.
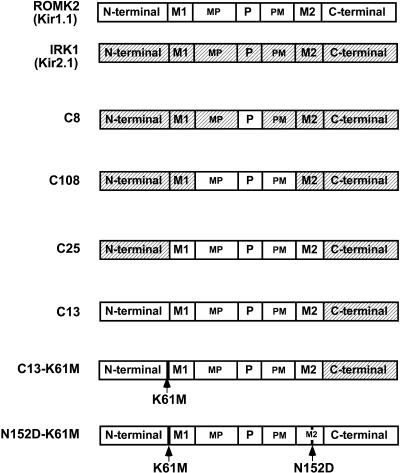
FIGURE 1.
Chimeras used to study the K dependence of slow inactivation in the Kir1.1 family. Both ROMK and IRK (shaded) consist of four identical subunits surrounding a central pore. Based on hydropathy analysis, we designated seven homologous regions of both the ROMK and IRK subunits: the N-terminus, which is mostly cytoplasmic and includes K61; the first (or outer) transmembrane segment (M1); the MP region which lies between M1 and the pore region; the P, or pore, region (also referred to as H5); the PM region, which lies between the P region and the second (or inner) transmembrane segment (M2); and finally the C-terminus, which is mostly cytoplasmic.
Both C13 and ROMK2-N152D retained a sensitivity to internal pH and could still be inactivated when placed in low external K. Additional chimeras were constructed to examine the importance of specific regions in the inactivation process. The complete set of constructs is summarized in Fig. 1. Note that all chimeras having an N-terminus derived from IRK1 would necessarily have the K61M mutation and be insensitive to internal pH.
Expression of channels
Plasmids were linearized with Not I restriction enzyme and transcribed in vitro with T7 RNA polymerase in the presence of the GpppG cap using mMESSAGE mMACHINE kit (Ambion, Austin, TX). Synthetic cRNA was dissolved in water and stored at −70°C before use. Stage V-VI oocytes were obtained by partial ovariectomy of female Xenopus laevis (NASCO, Ft. Atkinson, WI), anesthetized with tricaine methanesulfonate (1.5 g/L, adjusted to pH 7.0). Oocytes were defolliculated by incubation in OR2 solution (82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, and 5 mM HEPES, adjusted to pH 7.5 with NaOH) containing 2 mg/ml collagenase type II and 2 mg/ml hyaluronidase type II (Sigma Chemical, St. Louis, MO) for 90 min and (if necessary) another 90 min in a fresh enzyme solution at 23°C. Oocytes were injected with 0.5–1 ng of cRNA and incubated at 19°C in 2× diluted Leibovitz medium (Life Technologies, Grand Island, NY) for 1–4 days before measurements were made.
Two-electrode voltage clamp
Whole-cell conductances were measured for the chimeras and mutants of Fig. 1 with intact oocytes, using a two-electrode voltage clamp. All of the constructs in Fig. 1 rectified more strongly than ROMK, so that holding potentials more positive than EK produced very little outward current. This is illustrated in Fig. 2, which compares the whole-cell rectification properties of ROMK and the C13 chimera.
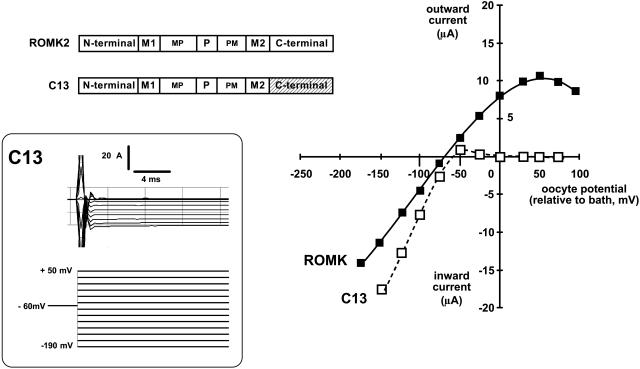
FIGURE 2.
The C13 chimera is a strongly rectifying variant of ROMK (Kir1.1). Whole-cell current voltage relations from oocytes expressing either wild-type ROMK (solid line, filled squares) or the C13 chimera (dashed line, open squares). The C13 chimera was formed by replacing the C-terminus of ROMK with the homologous region from IRK1 (Kir2.1). Results were obtained with the two-electrode voltage clamp on oocytes bathed in 10 mM external K where internal pH was controlled with permeant acetate buffers (see Methods). Inset shows typical C13 currents generated by the voltage protocol around the resting potential of −60 mV.
The internal pH of intact oocytes was controlled with permeant buffers as previously described (Doi et al., 1996; Leipziger et al., 2000; Choe et al., 1997). The relation between intracellular and extracellular pH was calculated from a previous calibration with ROMK oocytes: pHi = 0.595 × pHo + 2.4 (Choe et al., 1997).
To produce inactivation, channels at the oocyte membrane were subjected to sustained depolarizations either by clamping the membrane at an absolute value of +50 mV or at a voltage 70 mV more positive than the resting potential. In either case, there was no outward current through the channels during the depolarization. Throughout the experimental protocols, whole-cell conductance was measured periodically using 13 voltage steps of 20-ms duration to generate momentary inward current for construction of current-voltage relations.
Channel activity was assayed by measurement of whole-cell inward conductance since single-channel conductance and channel open probability are approximately independent of voltage over the range examined. In this protocol, channels that have become inactivated (rather than just blocked by polyamines) would pass neither outward nor inward current during generation of the I-V curves. Therefore, progressive inactivation of membrane channels would appear as a progressive decrease in measured inward conductance.
Solutions
Since the oocyte remains intact throughout the two-electrode voltage clamp, oocyte internal pH was controlled with permeant acetate buffers (Doi et al., 1996; Leipziger et al., 2000; Choe et al., 1997). Except for the experiment of Fig. 3 in which internal pH was changed, all oocytes were bathed in KCl and NaAcetate solutions that were pH-adjusted to 7.8 with NaOH. Under these conditions internal pH would be ∼7.04. Approximate osmolarities of the external bathing solutions were between 205 and 215 mOsm/L.
For potassium concentrations between 0.1 mM and 25 mM, the sum of KCl and NaCl was maintained at 50 mM, and combined with 55 mM NaAcetate, 1 mM MgCl2, 2 mM CaCl2. and 5 mM HEPES. In the 75-mM K solution, K was derived from 50 mM KCl plus 25 mM KAcetate, and combined with 30 mM NaAcetate, 1 mM MgCl2, 2 mM CaCl2. and 5 mM HEPES. In the 100-mM K solution, K was derived from 45 mM KCl plus 55 mM KAcetate, and combined with 1 mM MgCl2, 2 mM CaCl2. and 5 mM HEPES. In this way there was always 55 mM acetate in the solutions to maintain a constant internal pH. Variations in external [Na] had no effect on K-channel conductance or K-channel inactivation.
Normalization
Inherent differences in expression efficiencies of the different chimeras and mutants used in this study warrants normalization of conductances to the maximum conductance for individual protocols. In all figures depicting time course of conductance, whole-cell conductance was normalized to the conductance after 1-h incubation in the indicated external K. Error bars denote mean ± SE (for five oocytes, unless otherwise indicated).
RESULTS
Rationale
Previous studies indicated that inactivation of ROMK channels by low extracellular K required an intact pH-sensing apparatus (Schulte et al., 1999, 2001; Sackin et al., 2001, 2003). The goal of the present work was to determine if the channels could inactivate independently of internal pH. Specifically, we found that when a sustained depolarization was applied to a strongly rectifying mutant or chimera of ROMK, the channels would slowly inactivate in a K-dependent manner, analogous to the inactivation observed during internal acidification with low external K.
The C13 chimera and the ROMK2-N152D mutant contain IRK1 residues that confer strong rectification via a heightened affinity for internal polyamines and magnesium, as previously reported (Kubo and Murata, 2001; Taglialatela et al., 1994; Yang et al., 1995). Hence, all of the ROMK derivatives used in this study, C13, C13-K61M, N152D, N152D-K61M, C25, C108, and C8 (as well as wild type IRK1), have negligible outward current during depolarization because of block by internal Mg and/or polyamines.
C13 and ROMK have similar patterns of inactivation
Despite dramatically different degrees of inward rectification (Fig. 2), both ROMK and C13 exhibited similar K-dependent inactivation in intact oocytes after transient acidification with permeant buffers (Fig. 3). Since changes in internal pH do not alter single-channel conductance (McNicholas et al., 1998; Choe et al., 1997), whole-cell conductance provides a good measure of channel activity. As indicated in Fig. 3 A, internal acidification closed the inner pH gates of both ROMK and C13, causing a decrease in whole-cell conductance. This protocol of acidification with low external [K] inactivated both ROMK and C13, and channel activity could not be fully recovered by realkalization (Fig. 3 B). However, when external [K] was elevated, channel activity recovered completely (Fig. 3 C), indicating that the initial conductance decrease was not a result of channel “rundown.” In contrast, the same changes of internal pH produced completely reversible conductance changes when the external solution contained 10 mM external K (Fig. 3, D and E).
An important difference in the response of ROMK and C13 to the inactivation process is that the K “set point” for inactivation was somewhat higher in C13 than in ROMK. This is consistent with the apparent absence of C13 current at 1 mM external K, even at normal pH (Dahlmann et al., 2004). In contrast with C13, inward ROMK currents were visible at all external [K] ≥ 100 μM.
Depolarization inactivates a strongly rectifying chimera of ROMK
Since ROMK and C13 have similar pH-dependent inactivation, the strong inward rectifier (C13) was used to study voltage inactivation because this chimera would have no outward current during depolarization. Effects of ion depletion and/or accumulation in the submembrane compartments were therefore eliminated.
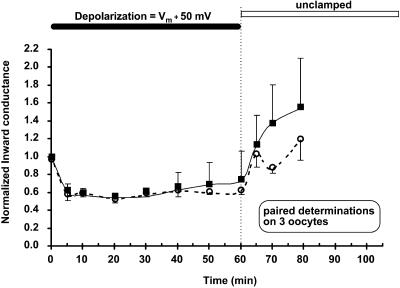
In these experiments, a sustained depolarization 100 mV more positive than the resting potential was applied to the oocyte. Internal pH was maximally alkaline to maintain an open pH gate. Channel activity was monitored by brief (20-ms) periodic pulses of inward current throughout the depolarization. As indicated in Fig. 4, a sustained depolarization produced a slow inactivation of C13, whose magnitude depended on external [K]. We do not believe that that inactivation is caused by the oocyte losing K because the zero-current membrane potential remained close to the estimated K Nernst potential throughout the inactivation period.
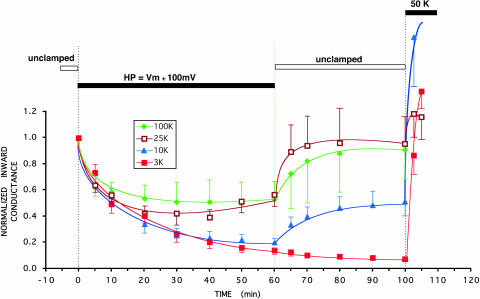
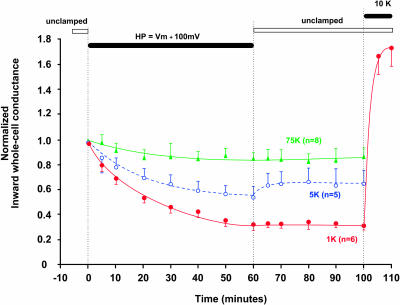
FIGURE 4.
A sustained positive holding potential deactivates the strongly rectifying C13 in a manner that depends on external K (indicated). The solid bar indicates a holding potential maintained at 100 mV more positive than the resting potential. There was no outward current during this period. The average resting potentials (Vm) were approximately Nernstian: −82.6 ± 1 mV for 3 mM external K, −55.1 ± 1.4 mV for 10 mM external K, −35.2 ± 1.1 mV for 25 mM external K, and −4.1 ± 1 mV for 100 mM external K. During the period of two-electrode voltage clamp with intact oocytes, internal pH was maintained at 7.04 by external acetate buffer at pHo = 7.8. Inward conductance was measured periodically using 20-ms voltage steps to generate momentary inward current.
In 3 mM external K, a 100 mV depolarization above resting potential inactivated C13 channels by 85 ± 3% (n = 8). This was the lowest external [K] at which inward conductance could still be measured. In 10 mM external K, a similar 100 mV depolarization above resting potential inactivated the K current by 80 ± 4% (n = 6) after 1 h. With either 25 mM or 100 mM external K, a 100 mV depolarization above resting potential inactivated 45 ± 10% of C13-mediated current after 1 h (Fig. 4).
The half-times for depolarization inactivation in the strongly rectifying C13 derivative of ROMK were between 10 and 15 min. The inactivation in Fig. 4 was somewhat slower than the pH-induced inactivation of Fig. 3, but the difference in the time courses may be even greater since the pH response may be slowed by a delay in the equilibration of pH across the oocyte membrane. Slow inactivation reversed completely for [K] ≥ 25 mM, partially for [K] = 10 mM, and not at all for K ≤ 3 mM.
We also addressed the possibility that the pulse protocol used for inward conductance measurements was itself selectively affecting inactivation by passing more inward K current at higher external [K]. This was systematically investigated in Fig. 5, in which inward conductance was determined using two different inward 20-ms pulse protocols on each oocyte. In the first protocol the command potential was stepped between 0 and −50 mV and in the second, the voltage range was expanded to between 0 and −200 mV. The inactivation produced by the sustained depolarization (Vm + 50 mV) was unaffected by the type of pulse protocol (Fig. 5). Given the results of Fig. 5, we have no reason to believe that the measurement of inward conductance would have any effect on the inactivation of other strongly rectifying mutants used in this study.
FIGURE 5.
Paired comparison of inward conductance measurements using two different pulse protocols. Depolarization inactivation in C13 produced by a sustained depolarization 50 mV more positive than the resting potential of −4.1 ± 1 mV in 100 mM external K. Filled squares, solid line: K conductance was measured using five 20-ms command potentials between 0 and −50 mV. After recovery, inward conductance was again measured (open circles, dashed line) in each oocyte using ten 20-ms command potentials between 0 and −200 mV. Two-electrode voltage clamp with intact oocytes. Internal pH maintained at 7.04 by external acetate buffer at pHo = 7.8.
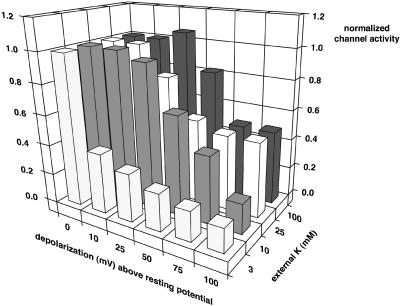
In general, inactivation of C13, a strongly rectifying derivative of ROMK, is a complex function of both the applied depolarization and the external [K]. Fig. 6 illustrates the level of channel activity (as determined from whole-cell conductance) after 1 h of applied depolarization (to various voltages above the resting potential). Oocytes were incubated for 1 h in the specified external K before application of depolarization. This provided a conductance at zero depolarization for each external [K] to which all subsequent conductances were normalized. At 3 mM external K, depolarizations as small as 10 mV were effective at inactivating the channels. At higher external [K], larger depolarizations were required. The maximum inactivation observed at 75–100 mV was also dependent on external K.
FIGURE 6.
Effect of both depolarization and external [K] on inactivation of the C13 chimera as measured by whole-cell conductance (bar height) after 60 min at the indicated depolarization and external K. The average resting potentials for each external K were −82.6 ± 1 mV at 3 mM K, −55.1 ± 1.4 mV at 10 mM K, −35.2 ± 1.1 at 25 mM K, −21.2 ± 1.1 at 50 mM K, −7.9 ± 1 mV at 75 mM K, and −4.8 ± 1 mV at 100 mM K. All conductances were normalized to the whole-cell conductance after 1-h incubation in the indicated external K. These were defined as unity (leftmost bars in the zero-depolarization row). Error bars on channel activity have been omitted for clarity.
Slow inactivation is seen in the absence of the pH sensor
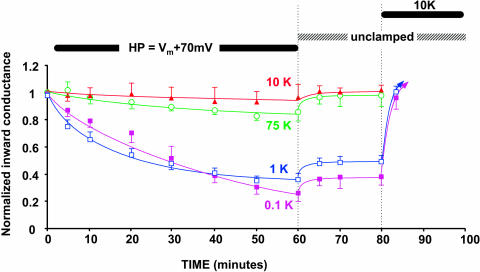
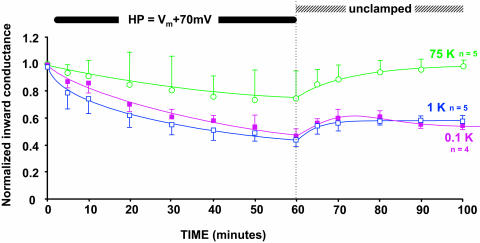
Since it is possible that depolarization might be directly affecting the pH gate rather than an independent inactivation mechanism, we used a similar voltage protocol with a mutant of the C13 chimera that has no internal pH sensor (C13-K61M). These results are shown in Fig. 7, where the applied depolarization was 70 mV positive to the resting potential.
FIGURE 7.
An applied depolarization inactivates C13-K61M, a strongly rectifying, pH-insensitive mutant of Kir1.1 as indicated by a progressive decline in macroscopic inward conductance. The solid bar denotes application of a sustained voltage 70 mV more positive than the resting potentials (Vm), which were −129.6 ± 2 mV for 0.1 mM external K, −103.8 ±1 mV for 1 mM external K, −50.5 ± 3 mV for 10 mM external K, and −9.2 ± 2 mV for 75 mM external K. Internal pH was maintained at 7.04 by external acetate buffer at pHo = 7.8. Inward conductance was measured periodically using 20-ms voltage steps to generate momentary inward current during the sustained depolarization.
Removal of the pH sensor allowed measurement of inward currents at lower extracellular K than was possible with C13 (Dahlmann et al., 2004). However, voltage inactivation of C13-K61M no longer occurred at 10 mM external K. Inactivation now required external [K] to be ≤1 mM (Fig. 7). Despite this shift in K sensitivity, the time course of inactivation was similar for both C13-K61M (Fig. 7) and C13 (Fig. 4). In both channel types, about an hour of sustained depolarization was required for maximal inactivation. Similar to the C13 chimera, external K <1 mM prevented reversal of C13-K61M inactivation upon release of the depolarization (unclamped condition, Fig. 7). Nonetheless, elevation of external [K] at the end of the protocol restored channel activity in both C13 (Fig. 4) and C13-K61M (Fig. 7), suggesting that the inactivation process does not permanently remove channels from the membrane.
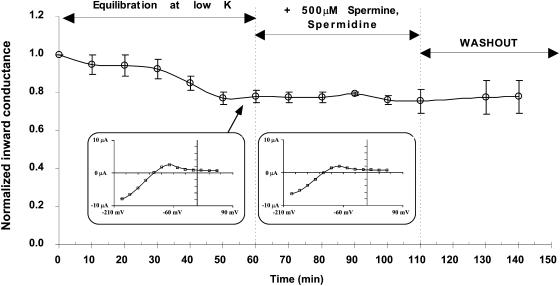
Slow inactivation is not a result of block by external polyamines
It is possible that depolarization forces internal polyamines through the pore (Guo and Lu, 2000), where they might accumulate in an unstirred extracellular space, blocking the channel during the measurement of inward conductance. To test this possibility, spermine and spermidine were added directly to the external solution at concentrations as high as 500 μM, and inward conductance measured periodically over the next hour. To favor optimal block by external polyamines, 1 mM [K] was used in the external solution, and the effect was tested on C13-K61M because this mutant still exhibits sizable inward currents at low external K. Under these conditions, external polyamines had no effect on macroscopic inward conductance (Fig. 8). Although we did not specifically test the effect of external polyamines on the other strongly rectifying derivatives of ROMK, we have no reason to believe that the result would be any different.
FIGURE 8.
Effect of external spermine + spermidine on inward conductance of the chimera/mutant C13-K61M. Oocytes were initially equilibrated in 1 mM external K until a steady state was achieved. At this point, spermidine and spermine were added to the external solution. The oocyte remained unclamped throughout the experiment and inward conductance was periodically measured. A typical I-V curve for this mutant in low external K is shown in the inset. There was no significant difference after addition of external polyamines. Internal pH was maintained at 7.04 by external acetate buffer at pHo = 7.8.
Depolarization produces slow inactivation in strong rectifiers with the N152D mutation
Previous work has indicated that the point mutation N152D greatly increases the sensitivity of ROMK2 to internal polyamines (Yang et al., 1995), similar to modification of the C-terminal region (chimera C13). Therefore, ROMK2-N152D and its pH-insensitive version ROMK2-N152D-K61M were subjected to sustained depolarizations similar to the protocol for C13 and C13-K61M.
The results for ROMK2-N152D are illustrated in Fig. 9 for different external [K]. The time course of inactivation was similar to what was found with C13 for the same applied voltage, although at each external [K], the extent of inactivation was slightly less for N152D than for the C13 chimera, and there was less reversibility of inactivation when the depolarization was discontinued (Fig. 9).
FIGURE 9.
An applied depolarization inactivates N152D, a strongly rectifying, pH-insensitive mutant of Kir1.1, as indicated by a progressive decline in macroscopic inward conductance. The solid bar denotes application of a sustained voltage 100 mV more positive than the resting potentials (Vm), which were −107 ± 2 mV for 1 mM external K, −71.9 ± 2 mV for 10 mM external K, and −8.9 ± 2 mV for 75 mM external K. Internal pH was maintained at 7.04 by external acetate buffer at pHo = 7.8. Inward conductance was measured periodically using 20-ms voltage steps to generate momentary inward current during the sustained depolarization.
The effect of depolarization was also examined in ROMK2-N152D-K61M because this mutant lacks pH sensitivity. As with the pH-insensitive C13-K61M, slow inactivation occurred whenever external [K] was ≤1 mM, but was prevented by high external [K] (Fig. 10). In all cases, there was a slight conductance increase associated with termination of the depolarization. As with C13-K61M, maintaining external [K] below 1 mM prevented recovery from inactivation during release of the depolarization (Fig. 10, unclamped).
FIGURE 10.
An applied depolarization inactivates N152D-K61M, a strongly rectifying, pH-insensitive mutant of Kir1.1, as indicated by a progressive decline in macroscopic inward conductance. The solid bar denotes the time during which voltage was sustained at 70 mV more positive than the resting potentials (Vm) which were −131 ± 5 mV for 0.1 mM external K, −105.9 ± 2 mV for 1 mM external K, and −10.6 ± 2 mV for 75 mM external K. Internal pH was maintained at 7.04 by external acetate buffer at pHo = 7.8. Inward conductance was measured periodically using 20-ms voltage steps to generate momentary inward current during the sustained depolarization.
The magnitude of slow inactivation in low external K is structure-dependent
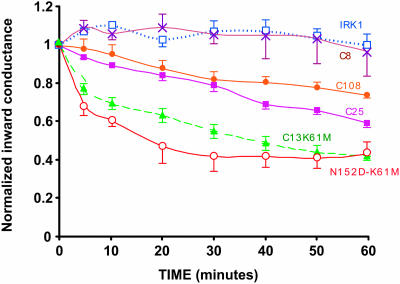
At a constant external [K] of 1 mM, the same depolarization resulted in different levels of inactivation that depended on the relative ROMK/IRK chimeric structure. In general, the more ROMK regions in the subunits, the greater the inactivation. IRK1 (Kir2.1) had no significant voltage-induced inactivation. Replacement of the pore region with that of ROMK (C8) was not sufficient to produce inactivation (Fig. 11). However, there was significant inactivation when the pore region and the two adjacent extracellular domains MP and PM were both derived from ROMK (C108). Further inclusion of ROMK M1 and M2 transmembrane segments (C25) slightly increased the level of inactivation after 1 h (Fig. 11). The largest inactivation occurred when the N-terminal region and the extracellular loop were both derived from ROMK (C13-K61M and N152D-K61M in Figs. 1 and 11).
FIGURE 11.
Slow inactivation of ROMK-IRK chimeras is structure-dependent. A depolarization (Vm + 70 mV) was applied for 60 min beginning at time zero, with external [K] at 1 mM in all cases. Positive voltage produced no outward current in these strong inward rectifiers. The labels on each curve refer to the chimeras described in Fig. 1. During the two-electrode voltage clamp period with intact oocytes, internal pH was maintained at 7.04 by external acetate buffer at pHo = 7.8. Inward conductance was measured periodically using 20-ms voltage steps to generate momentary inward current during the sustained depolarization. All of the channels in this figure lack the pH sensor at K61.
DISCUSSION
Previous work has indicated that the combination of low internal pH and low external K inactivates ROMK (Kir1.1) but not IRK1 (Kir2.1). This process was described by a three-state linear scheme in which inactivation occurs only after closure of the pH gate (Schulte et al., 2001; Sackin et al., 2003). Reentry into the active state from the inactivated state required both elevation of internal pH and elevation of external K (Schulte et al., 2001; Sackin et al., 2003).
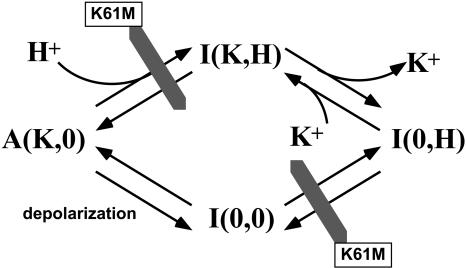
Results of the present studies with sustained depolarization suggest that, under some conditions, positive membrane voltage can inactivate ROMK-type channels whose pH gates are constitutively open (K61M mutation), implying that an alternative pathway exists betweeen the active and inactive states that might bypass the pH closed state. This is discussed below, with reference to Fig. 12.
FIGURE 12.
Four-state model for the interaction between external K and internal pH. This model is consistent with the slow inactivation of ROMK (Kir1.1) by depolarization. The inactivated I(K,H) state is reached from the active A(K,0) state by protonation of the pH sensor (Kir1.1b-K61). The mutation K61M blocks this pathway as well as the transition between I(0,0) and I(0,H). The further removal of external K drives the system from the pH-closed state, I(K,H), to the inactivated I(0,H) state. Elevation of external K shifts the equilibrium from I(0,H) to I(K,H). A fourth state, I(0,0), can be reached by depolarization without protonation.
Since inactivation occurred in strongly rectifying mutants that lacked pH sensors (C13-K61M and N152D-K61M), we do not believe that voltage was producing inactivation by altering the local [H+]. Furthermore, voltage did not seem to be producing inactivation by altering local [K], since oocyte resting potential remained unchanged throughout the inactivation process.
Although our protocol for evaluating inactivation requires channels that have a high polyamine affinity, we do not believe that polyamines per se produce slow inactivation. First of all, inactivation was assayed in the negative voltage range (inward current) where positively charged polyamines would be prevented from entering the pore. Second, the susceptibility of particular chimeras and mutants to slow inactivation depends on specific residues in the extracellular loop of the channel protein (Fig. 11) that are far removed from any of the putative polyamine binding sites in Kir.
We also investigated the possibility that a sustained depolarization might force internal polyamines through the pore into an extracellular region from which they might produce inactivation by blocking from the outside. To test this possibility, spermine and spermidine were placed directly in the external solutions at concentrations as high as 500 μM. The complete lack of effect of these polyamines after 1 h argues against the idea that slow inactivation is caused by any type of external polyamine block.
Finally, the slow inactivation described in this study is not related to the hyperpolarization-induced fast inactivation reported for Kir2.1 (Shieh and Lee, 2001; Shieh, 2000). Although both types of inactivation are sensitive to external [K], they have completely different voltage sensitivities and depend on different residues (e.g., fast inactivation specifically requires Kir2.1-R148 (Shieh et al., 1999)). A fast inactivation has also been reported in depolarized oocytes and COS-7 cells expressing ROMK2 (Riochet et al., 2001). However, this inactivation was essentially complete within 300 ms after the start of the depolarization, which contrasts with the slow (60-min) inactivation reported in the present study.
Voltage inactivation of ROMK derivatives depends on low extracellular K
Both voltage and pH dependent inactivation (Fig. 3) of the strongly rectifying derivatives of ROMK exhibit a marked dependence on external K (Figs. 3, 4, 6, 7, 9, and 10), with high external K preventing the inactivation process. This is similar to the K dependence of pH inactivation on external K previously reported (Sackin et al., 2003). Although we do not understand exactly how depolarization shuts down active channels (see next section), low external K may decrease K binding within the permeation path, favoring a more stable closed state. As shown in the three-dimensional plot of Fig. 6, when external K is <10 mM, the channels are very sensitive to depolarization-induced inactivation.
We do not believe that the pulse protocol used to measure inward conductance contributes to the K dependence of the inactivation process. When several different measurement protocols were used with high external [K], the magnitude of the 20-ms inward command voltage (and hence the amount of brief inward K current) had no discernable effect on the time course of inactivation or recovery in a paired study (Fig. 5).
The dependence of inactivation on external K is reminiscent of the well-documented K sensitivity of C-type inactivation (Baukrowitz and Yellen, 1995; Lopez-Barneo et al., 1993; Pardo et al., 1992; Consiglio et al., 2003; Wood and Korn, 2000).
Hence, it is possible that the slow inactivation reported in our experiments also involves some type of conformational change similar to what has been proposed at the outer mouth of excitable channels during C-type inactivation (Baukrowitz and Yellen, 1995, 1996; Liu et al., 1996; Yellen, 1998; Sigworth, 2001; Basso et al., 1998; Eghbali et al., 2002; Kiss et al., 1999). Alternatively, the K dependence of ROMK inactivation might arise from long-range coupling between intracellular and extracellular domains of the channel, analogous to what has been reported for Kv1.4 (Li et al., 2003).
Some of the K dependence of depolarization-induced inactivation might also involve interactions between external K and the pH sensor. Channels like C13-K61M (Fig. 7), that lacked a functional pH sensor, required a lower concentration of external K for inactivation than did C13 channels (Fig. 4) that had an intact pH sensor (cf. Figs. 4 and 7). This could reflect the increased stability of the open state reported in mutant Kir1.1 channels after removal of pH sensitivity (Schulte et al., 2001).
A possible mechanism for slow voltage inactivation of ROMK derivatives
Elevation of external K appeared to reverse the inactivation produced by either prolonged acidification or prolonged depolarization. This suggests that slow inactivation involves gating of channels at the membrane surface rather than removal and reinsertion of channels from the cytoplasm. However, the unusually slow time course of the inactivation process raises the possibility that membrane recycling could be partly involved in the inactivation process. In theory, none of the experiments reported in this study can rule out endocytosis as a mechanism of inactivation. Our principal argument against membrane recycling is that slow inactivation depends on specific structural regions of the channel (e.g., the extracellular loop) that would not be expected to be critical for membrane trafficking. For instance, the C108 chimera differs from IRK1 only in its extracellular loop (Fig. 1), yet it inactivates with depolarization, whereas IRK1 does not (Fig. 11).
The slow inactivation of Kir1.1 derivatives seems to require both K depletion within the pore and some effect of depolarization itself on protein conformation and/or K binding within the channel. Depolarization alone was insufficient to inactivate ROMK channels that are conducting K. This was previously demonstrated during application of a sustained positive voltage to wild-type ROMK (dashed line, Fig. 3 of Sackin et al., 2001).
On the other hand, K depletion (in the absence of depolarization or acidification) was also insufficient to produce inactivation. This was demonstrated by Schulte et al. (2001), using ROMK excised inside-out patches. In these studies, complete removal of internal K was unable to produce inactivation, even when channels were exposed to zero external K (Schulte et al., 2001).
In the original inactivation scheme, inactivation of ROMK channels by low external K required that the channel first be closed by low internal pH. There is a single active state A(K,0), corresponding to bound external K but unbound internal H. Binding of internal protons produced the pH-closed state I(K,H). The inactivated state I(0,H) is reached by K removal from the pH-closed state I(K,H). The finding that sustained depolarization also produced slow inactivation in channels that lacked functional pH sensors (K61M mutants) implies that inactivation can occur independently of the pH sensor, and is consistent with inclusion of a fourth state (Fig. 12) in the original inactivation scheme. Since the voltage-dependent and pH-dependent processes seem to interact with external K in similar ways, we favor placement of this fourth state, closed I(0,0), in parallel with the pH-closed state as shown in Fig. 12.
The structural basis for the interaction between depolarization, K depletion, and pH is not yet understood; however, the dependence of inactivation on low [K] suggests that depolarization may be affecting K binding, either within the channel or at the outer mouth of the channel. This is consistent with specific ROMK extracellular residues (MP, P, and PM in Fig. 1) being absolutely required for the slow inactivation process (Fig. 11). The strongly rectifying derivatives of ROMK that possess the extracellular loop of ROMK (i.e., C108, C25, C13, C13-K61M, N152D, and N152D-K61M) would have a weaker affinity for external K than IRK1 and C8, making them easier to inactivate during a sustained depolarization. Depolarization-induced polyamine block would foster this inactivation by preventing cytoplasmic K from reaching K-binding sites within the pore.
Studies with KcsA have implied that K near the extracellular mouth of the channel is first partially dehydrated before becoming fully dehydrated within the selectivity filter (Zhou et al., 2001). A sustained depolarization, in combination with low external [K] could interfere with this process, thereby producing a slow inactivation. Since K occupancy of the channel and the structure of the permeation path itself is affected by external K (Zhou and MacKinnon, 2003), it is plausible that the combination of low K and depolarization alters protein conformation to produce a stable inactive state. Detailed studies of inward rectification in Kir2.1 suggest that internal polyamine block during positive voltages may change the binding characteristics of K within the selectivity filter and displace K ions toward the extracellular side (Guo et al., 2003). Furthermore, there are reports that depolarization does affect K-dependent, C-type inactivation, possibly by increasing the rate at which the last K ion leaves multi-ion K channels (Baukrowitz and Yellen, 1996).
In summary, a sustained depolarization produced a very slow (τ1/2 = 10–15 min) inactivation of strongly rectifying Kir1.1 derivatives. The inactivation also occurred in pH-insensitive channels (Kir1.1b-K61M), implying the existence of an inactivated state that could be reached by depolarization, independent of the pH sensor. Entry and exit from this inactivated state depended on external [K], suggesting that K depletion at the exterior of the pore (or within the pore) facilitates the inactivation process. This process may involve structural rearrangement of the selectivity filter itself.
Acknowledgments
This work was supported by National Institutes of Health grants DK46950 (H. Sackin) and DK27847 (L. G. Palmer).
References
- Basso, C., P. Labarca, E. Stefani, O. Alvarez, and R. Latorre. 1998. Pore accessibility during C-type inactivation in Shaker K channels. FEBS Lett. 429:375–380. [DOI] [PubMed] [Google Scholar]
- Baukrowitz, T., and G. Yellen. 1995. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 15:951–960. [DOI] [PubMed] [Google Scholar]
- Baukrowitz, T., and G. Yellen. 1996. Use-dependent blockers and exit rate of the last ion from the multi-ion pore of a K channel. Science. 271:653–656. [DOI] [PubMed] [Google Scholar]
- Chepilko, S., H. Zhou, H. Sackin, and L. G. Palmer. 1995. Permeation and gating properties of a cloned renal K+ channel. Am. J. Physiol. 268:C389–C401. [DOI] [PubMed] [Google Scholar]
- Choe, H., H. Zhou, L. G. Palmer, and H. Sackin. 1997. A conserved cytoplasmic region of ROMK modulates pH sensitivity, conductance, and gating. Am. J. Physiol. 273:F516–F529. [DOI] [PubMed] [Google Scholar]
- Consiglio, J., P. Andalib, and S. Korn. 2003. Influence of pore residues on permeation properties in the Kv2.1 potassium channel. Evidence for a selective functional interaction of K with the outer vestibule. J. Gen. Physiol. 121:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlmann, A., M. Li, Z. Gao, D. McGarrigle, H. Sackin, and L. G. Palmer. 2004. Regulation of Kir channels by intracellular pH and extracellular K: mechanisms of coupling. J. Gen. Physiol. In press. [DOI] [PMC free article] [PubMed]
- Doi, T., B. Fakler, J. H. Schultz, U. Schulte, U. Brandle, S. Weidemann, H. P. Zenner, F. Lang, and J. P. Ruppersberg. 1996. Extracellular K+ and intracellular pH allosterically regulate renal Kir1.1 channels. J. Biol. Chem. 271:17261–17266. [DOI] [PubMed] [Google Scholar]
- Eghbali, M., R. Olcese, M. Zarei, L. Toro, and E. Stefani. 2002. External pore collapse as an inactivation mechanism ofr Kv4.3 K channels. J. Membr. Biol. 188:73–86. [DOI] [PubMed] [Google Scholar]
- Fakler, B., J. H. Schultz, J. Yang, U. Schulte, U. Brandle, H. P. Zenner, L. Y. Jan, and J. P. Ruppersberg. 1996. Identification of a titratable lysine residue that determines sensitivity of kidney potassium channels (ROMK) to intracellular pH. EMBO J. 15:4093–4099. [PMC free article] [PubMed] [Google Scholar]
- Guo, D., and Z. Lu. 2000. Mechanism of cGMP-gated channel block by intracellular polyamines. J. Gen. Physiol. 115:783–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, D., Y. Ramu, A. M. Klem, and Z. Lu. 2003. Mechanism of rectification in inward-rectifier K channels. J. Gen. Physiol. 121:261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 77:61–68. [DOI] [PubMed] [Google Scholar]
- Kiss, L., J. LoTurco, and S. Korn. 1999. Contribution of the selectivity filter to inactivation in potassium channels. Biophys. J. 76:253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo, Y., and Y. Murata. 2001. Control of rectification and permeation by two distinct sites after the second transmembrane region in Kir2.1 K channel. J. Physiol. 531:645–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipziger, J., G. MacGregor, G. Cooper, J. Xu, S. Hebert, and G. Giebisch. 2000. PKA site mutations of ROMK2 channels shift the pH dependence to more alkaline values. Am. J. Physiol. 279:F919–F926. [DOI] [PubMed] [Google Scholar]
- Li, X., G. Bett, X. Jiang, V. Bondarenko, M. Morales, and R. Rasmuson. 2003. Regulation of N- and C-type inactivation of Kv1.4 by pHo and K: evidence of transmembrane communication. Am. J. Physiol. Heart Circ. Physiol. 284:H71–H80. [DOI] [PubMed] [Google Scholar]
- Liu, Y., M. E. Jurman, and G. Yellen. 1996. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron. 16:859–867. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo, J., T. Hoshi, S. Heinemann, and R. W. Aldrich. 1993. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1:61–71. [PubMed] [Google Scholar]
- MacGregor, G. G., J. Z. Xu, C. M. McNicholas, G. Giebisch, and S. C. Hebert. 1998. Partially active channels produced by PKA site mutation of the cloned renal K+ channel, ROMK2 (kir1.2). Am. J. Physiol. 275:F415–F422. [DOI] [PubMed] [Google Scholar]
- McNicholas, C. M., G. G. MacGregor, L. D. Islas, Y. Yang, S. C. Hebert, and G. Giebisch. 1998. pH-dependent modulation of the cloned renal K+ channel, ROMK. Am. J. Physiol. 275:F972–F981. [DOI] [PubMed] [Google Scholar]
- Palmer, L. G., H. Choe, and G. Frindt. 1997. Is the secretory K channel in the rat CCT ROMK? Am. J. Physiol. 273:F404–F410. [DOI] [PubMed] [Google Scholar]
- Pardo, L. A., S. H. Heinemann, H. Terlau, U. Ludewig, C. Lorra, O. Pongs, and W. Stuhmer. 1992. Extracellular K+ specifically modulates a rat brain K+ channel. Proc. Natl. Acad. Sci. USA. 89:2466–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riochet, D., R. Mohammad-Panah, S. Hebert, G. MacGregor, I. Baro, G. Guihard, and D. Escande. 2001. Inactivating properties of recombinant ROMK2 channels expressed in mammalian cells. Biochem. Biophys. Res. Commun. 286:376–380. [DOI] [PubMed] [Google Scholar]
- Sackin, H., S. Syn, L. G. Palmer, H. Choe, and E. Walters. 2001. Regulation of ROMK by extracellular cations. Biophys. J. 80:683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackin, H., A. Vasilyev, L. G. Palmer, and M. Krambis. 2003. Permeant cations and blockers modulate pH gating of ROMK channels. Biophys. J. 84:910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte, U., H. Hahn, M. Konrad, N. Jeck, C. Derst, K. Wild, S. Weidemann, J. Ruppersberg, B. Fakler, and J. Ludwig. 1999. pH gating of ROMK (Kir1.1) channels: control by an Arg-Lys-Arg triad disrupted in antenatal Bartter syndrome. Proc. Natl. Acad. Sci. USA. 96:15298–15303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte, U., S. Weidemann, J. Ludwig, J. Ruppersberg, and B. Fakler. 2001. K-dependent gating of Kir1.1 channels is linked to pH gating through a conformational change in the pore. J. Physiol. 534:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh, R. 2000. Mechanisms for the time-dependent decay of inward currents through cloned Kir2.1 channels expressed in Xenopus oocytes. J. Physiol. 526:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh, R., J. Chang, and C. C. Kuo. 1999. K binding sites and interactions between permeating K ions at the external pore mouth of an inward rectifier K channel (Kir2.1). J. Biol. Chem. 274:17424–17430. [DOI] [PubMed] [Google Scholar]
- Shieh, R. C., and Y. L. Lee. 2001. Ammonium ions induce inactivation of Kir2.1 potassium channels expressed in Xenopus oocytes. J. Physiol. 535:359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth, F. 2001. Potassium channel mechanics. Neuron. 32:555–556. [DOI] [PubMed] [Google Scholar]
- Taglialatela, M., B. A. Wible, R. Caporaso, and A. M. Brown. 1994. Specification of pore properties by the carboxyl terminus of inwardly rectifying K+ channels. Science. 264:844–847. [DOI] [PubMed] [Google Scholar]
- Wood, M., and S. Korn. 2000. Two mechanisms of K-dependent potentiation in Kv2.1 potassium channels. Biophys. J. 79:2535–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J., Y. N. Jan, and L. Y. Jan. 1995. Control of rectification and permeation by residues in two distinct domains in an inward rectifier K+ channel. Neuron. 14:1047–1054. [DOI] [PubMed] [Google Scholar]
- Yellen, G. 1998. The moving parts of voltage-gated ion channels. Q. Rev. Biophys. 31:239–295. [DOI] [PubMed] [Google Scholar]
- Zhou, Y., and R. MacKinnon. 2003. The occupancy of ions in the K selectivity filter: charge balance and coupling of ion binding to a protein conformational change underlie high conduction rates. J. Mol. Biol. 333:965–975. [DOI] [PubMed] [Google Scholar]
- Zhou, Y., J. Morais-Cabral, A. Kaufman, and R. MacKinnon. 2001. Chemistry of ion coordination and hydration revealed by a K channel Fab complex at 2.0Å resolution. Nature. 414:43–48. [DOI] [PubMed] [Google Scholar]