Abstract
Simple lipid binary systems are intensively used to understand the formation of domains in biological membranes. The size of individual domains present in the gel/fluid coexistence region of single supported bilayers, determined by atomic force microscopy (AFM), generally exceeds by two to three orders of magnitude that estimated from multibilayers membranes by indirect spectroscopic methods. In this article, the topography of equimolar dimyristoylphosphatidylcholine/distearoylphosphatidylcholine (DMPC/DSPC) multibilayers, made of two superimposed bilayers supported on mica surface, was studied by AFM in a temperature range from room temperature to 45°C. In the gel/fluid phase coexistence region the size of domains, between ∼100 nm and a few micrometers, was of the same order for the first bilayer facing the mica and the top bilayer facing the buffer. The gel to fluid phase separation temperature of the first bilayer, however, could be increased by up to 8°C, most likely as a function of the buffer layer thickness that separated it from the mica. Topography of the top bilayer revealed the presence of lipids in ripple phase up to 38–40°C. Above this temperature, a pattern characteristic of the coexistence of fluid and gel domains was observed. These data show that difference in the size of lipid domains given by AFM and spectroscopy can hardly be attributed to the use of multibilayers models in spectroscopy experiments. They also provide a direct evidence for metastable ripple phase transformation into a gel/fluid phase separated structure upon heating.
INTRODUCTION
Evidence for an organization of biological membranes in in-plane microdomains has now been accumulated for more than two decades (Morrisett et al., 1975; Jain and White, 1977; Karnovsky et al., 1982; Yechiel and Edidin, 1987). Lipid-lipid interactions are at the origin of the lipid domains that include rafts, a category of microdomains enriched in sphingolipids and cholesterol (Simons and Ikonen, 1997). Such lipid domains play a crucial role in the expression and regulation of membrane functions and in many pathological processes (reviewed in Tocanne et al., 1994; Jacobson et al., 1995; Brown and London, 1998; Simons and Ehehalt, 2002). They are formed when two immiscible lipid phases are present. Bilayers made of simple lipid binary systems have been intensively used as models to understand the formation of lipid domains in biological membranes. The dimyristoylphosphatidylcholine/distearoylphosphatidylcholine (DMPC/DSPC) mixture is the most thoroughly studied two-component, two-phase lipid bilayer. Differential scanning calorimetry (Mabrey and Sturtevant, 1976), freeze-fracture electron microscopy (Luna and McConnell, 1978), Raman spectroscopy (Mendelsohn and Maisano, 1978), dilatometry (Wilkinson and Nagle, 1979), neutron scattering (Knoll et al., 1981), fluorescence recovery after photobleaching (FRAP) (Vaz et al., 1989), electron spin resonance (ESR) (Sankaram et al., 1992), NMR (Sankaram and Thompson, 1992), Fourier transform infrared spectroscopy (Brumm et al., 1996), and fluorescence energy transfer (Leidy et al., 2001) have been used to characterize the dynamics and thermodynamic parameters of DMPC/DSPC bilayers and to estimate the size of microdomains formed in the gel/fluid coexistence region. Phase properties and lateral distribution of components of this system have also been investigated theoretically (Ipsen and Mouritsen, 1988; Jorgensen et al., 1993; Sugar et al., 1999).
By giving a direct access, at a mesoscopic scale, to the topography of lipid domains in supported bilayers (Radmacher et al., 1992; Shao et al., 1996), atomic force microscopy (AFM) has revealed domains up to 106–107 molecules in various two-component, two-phase lipid bilayers (Dufrêne, et al., 1997; Czajkowsky et al., 1998; Giocondi et al., 2001a; Rinia et al., 2001; Milhiet et al., 2002; see, however, Gliss et al., 1998), including DMPC/DSPC equimolar mixture (Giocondi et al., 2001b). This domain size range is from two to three orders of magnitude larger than that estimated from electron spin resonance and fluorescence spectroscopy experiments and from modeling (Sankaram et al., 1992; Jørgensen et al., 1993; Sugar et al., 1999). Most of the spectroscopy experiments on DMPC/DSPC mixtures have been performed on freestanding multilamellar vesicles (MLVs) and supported multilayers (Mendelsohn and Maisano, 1978; Almeida et al., 1992; Schram et al., 1996; Leidy et al., 2001). Thus differences between membrane models might be at the origin of the different domains size. Recently it was proposed that the upper bilayer of double supported DMPC/DSPC bilayers made by vesicle fusion behaved more similarly to freestanding bilayers (Leidy et al., 2002). Defining the parameters that govern the size, shape, and stability of domains in lipid bilayers is an important step in the understanding of biomembranes lipid domains. Accordingly, we have used AFM to examine the temperature-dependent topographical characteristics of membrane domains in DMPC/DSPC multibilayers.
MATERIALS AND METHODS
Multilamellar vesicles (MLV) were prepared under argon, at 75°C in phosphate buffered saline (PBS), pH 7.4, from chloroform/methanol 2:1 (v/v) stock solutions (10 mM) of DMPC and DSPC (Sigma-Aldrich, Saint Quentin, France) dried under nitrogen gas. Purity of the phospholipids was checked by thin-layer chromatography (TLC), and the phospholipid concentration was determined according to Mrsny et al. (1986). Small unilamellar vesicles (SUV) were prepared at 75°C by sonication, under argon, of MLV. To form supported multilayers, SUV in excess (4 mM phospholipid concentration) were deposited on a freshly cleaved mica disk (1/2-inch diameter, Goodfellow, Huntingdon, England), inserted in a 13 mm holder for swinney syringe (Millipore, Bedford, MA), and incubated at 75°C for 2 h in a water bath (Giocondi et al., 2001a,b). At the end of the incubation the samples, always maintained in an aqueous environment, were slowly returned to room temperature, kept overnight, then carefully rinsed six times with PBS to remove the SUV in excess and the loosely adsorbed bilayers. The mica support was glued onto a glass coverslip with Super Glue 3 (Loctite, Newington, CT) before mounting on a homemade temperature regulated holder screwed on the stage of an inverted microscope (Zeiss, Le Pecq, France) coupled to a Bioscope (Digital Instruments, Santa Barbara, CA) or on the Peltier stage of a Pico SPM II (Molecular Imaging, Phoenix, AZ). Formation of single or multiple bilayers on the mica support depends on incubation conditions (Leidy et al., 2002), being favored by maintaining overnight the sample at room temperature before washing vesicles in excess. AFM was used to identify multibilayers from the depth of defects or holes in the samples. For the Bioscope, temperature of the solution bathing the bilayers was measured with a thermocouple probe (YSI 500, YSI, Yellow Springs, OH). Silicon nitride cantilevers, with 0.01 and 0.03 N/m nominal spring constant (Park Scientific Instruments, Sunnyvale, CA), were used in the experiments that were run in contact mode. The scanning force was adjusted to below 0.3 nN and readjusted for drift during image acquisition. The scan rate was adjusted between 1 and 3.5 Hz, according to the scan size.
RESULTS
Topography of multibilayers at room temperature
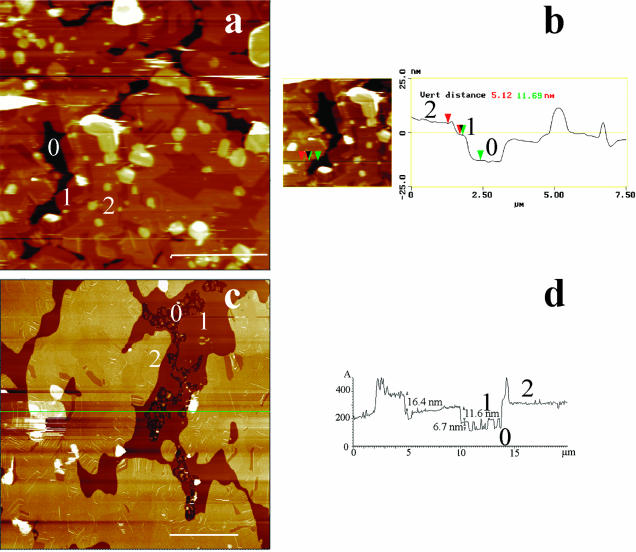
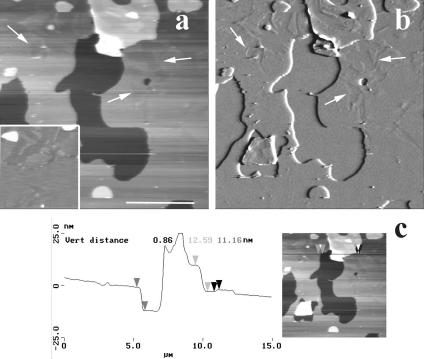
Fusion at a high temperature, in the miscible state, of an excess of DMPC/DSPC vesicles in phosphate buffered saline followed by a slow return to room temperature (∼23°C) resulted in formation of supported multibilayers. Fig. 1 is a low magnification of DMPC/DSPC multibilayers imaged at room temperature in PBS. The topography of large scans (height mode, Fig. 1, a and c) revealed a heterogeneous surface made of three principal levels, here designed by numbers 0, 1, and 2, contaminated by round-shaped pieces of bilayers, up to a few micrometers in size, that appeared as bright patches. AFM estimate of the number of bilayers that constituted the sample relies on the thickness determination of the superimposed layers revealed by the imaging. Incomplete coverage of the surface by a bilayer or the presence of holes and defects in the bilayers are generally used for these determinations (Czajkowsky et al., 1995; Giocondi et al., 2001b). Alternatively, scanning a bilayer in gel phase at high force allows creating such defects (Mou et al., 1994; Leidy et al., 2002). Sample lighter top level 2 was 16–18 nm above the darker 0 surface taken as a reference, whereas level 1 could take a value of either ∼11 or ∼6 nm (Fig. 1, b and d). The thickness of contaminating round-shaped bilayers pieces lightly adsorbed on the top bilayer was 12–17 nm. These observations strongly suggested that the samples were made of at least two superimposed phospholipid bilayers covering the mica surface (Fang and Yang, 1996; Leidy et al., 2002).
FIGURE 1.
Low-magnification AFM imaging of the surface of DMPC/DSPC (1:1) multibilayers in PBS buffer, at room temperature. Except for one sample, low magnification height images (10–20-μm scans) identified three distinct height levels, denoted 0, 1, and 2 (a and c). b is a virtual section of an electronic zoom of a, whereas d is a virtual section of b at the green line position. (Bars) 5 μm.
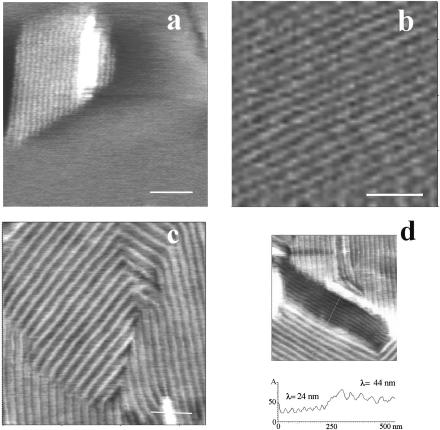
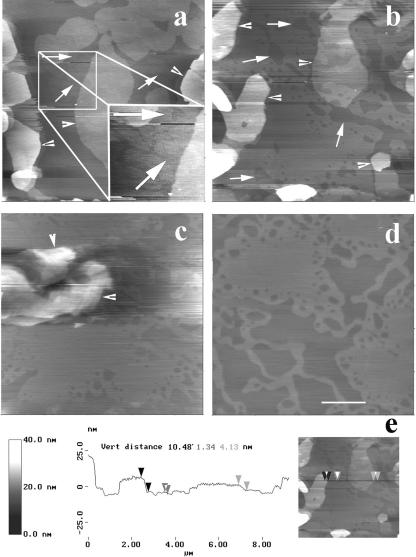
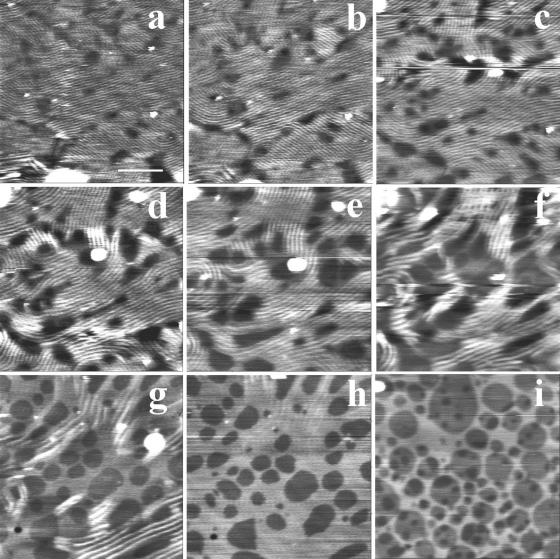
Upon scanning at intermediate magnification (4–5 μm), angular ripple phase (Pβ′) domains (Tardieu et al., 1973) with a modulation wavelength λ1 of ∼24 nm (amplitude ∼0.3 nm), a value that corresponds to the metastable ripple phase for one-component bilayers (Tenchov et al., 1989), were observed at the samples top surface (level 2). They either protruded from a smooth flat matrix (Fig. 2, a and b) or were surrounded by lipids in a ripple phase of higher wavelength λ2 (Fig. 2, d and e). The apparent thickness of these λ2 regions was about twice that of λ1 regions (Fig. 2 e). Examination of the upper level topography at a higher magnification using smaller scan sizes confirmed the absence of long range mesoscopic structural organization in the matrix surrounding protruding λ1 ripples (Fig. 3 a) that form straight-edged angular domains of well-aligned and regular structures (Fig. 3, b and d). The λ2 domains were also made of regular and aligned ripples (Fig. 3 c). Ridge spacings of ∼34, ∼44, and ∼64 nm were the most frequently encountered. More complex arrangements associating several ripple structures on small membrane fragments were also visualized (Fig. 2). On the other hand, we did not observe in our samples the 12.5–15 nm ripple periodicity recently described in comparable mixtures (Leidy et al., 2002; Kaasgaard et al., 2003). The surface of the first bilayer, close to the mica, could be flat and featureless (Fig. 4 a), show either small filamentous structures ∼10 nm in diameter, up to 250 nm in length, and eventually branched, protruding by ∼0.1–0.3 nm from the matrix (Fig. 4 b), or wavelike structures with 20–35 nm ridge spacing and of ∼0.5 nm amplitude (Fig. 4 c).
FIGURE 2.
Intermediate magnification imaging of the surface of DMPC/DSPC (1:1) multibilayers in PBS buffer. Using these scan sizes (4–5 μm), the geometrical structures detected in Fig. 1 appear to be made of phospholipids in ripple phase. a and b and c and d correspond to height and deflection images, respectively, showing that λ1 ripples can be surrounded by either smooth regions or by ripples of higher wavelength. e is a virtual section of c at the green line position. (Bars) 1 μm.
FIGURE 3.
Details of ripples structure from high resolution scans (height images). (Bars) 200 nm.
FIGURE 4.
High resolution images of the topography at room temperature of the first bilayer close to the mica. Height images. (Bar) 200 nm.
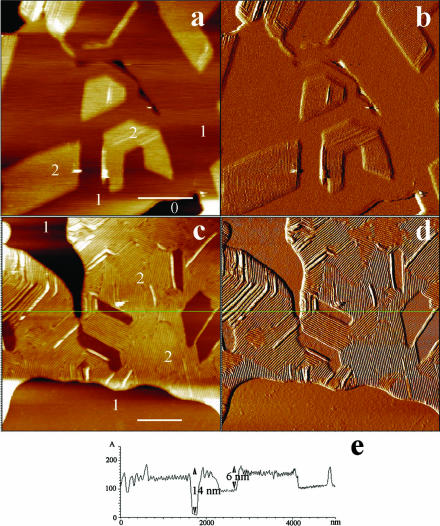
In contrast with these data, the fourth sample examined was devoid of angular domains and covered by only a few membrane patches (Fig. 5). Examination of its surface did not reveal any ripple structure, neither in the height (Fig. 5 a) nor in the deflection mode (Fig. 5 b). Rather, the presence of flat, darker domains (height difference of ∼1.0 nm) of mesoscopic to microscopic size, some of them pointed by arrows (see also inset in Fig. 5 a), evoked a gel/gel phase separation as expected from the DMPC/DSPC phase diagram (Knoll et al., 1981). Thickness determination across the edges of defects gave a value close to 11 nm (11.3 ± 0.5 nm) (Fig. 5 c).
FIGURE 5.
Gel-gel phase separation in DMPC/DSPC (1:1) multibilayers at room temperature. The surface of upper bilayer is essentially smooth but has two height levels separated by ∼1 nm. Arrows point to some of these domains that are more easily discernable in the inset of a. a and b correspond to height and deflection images, respectively. c is a virtual section of a. (Bar) 5 μm.
The large differences between AFM and spectroscopic methods about domains size concern the fluid-gel coexistence region. Accordingly, we have examined the temperature-dependent behavior of our multibilayers samples.
Temperature dependence of multilayers surfaces
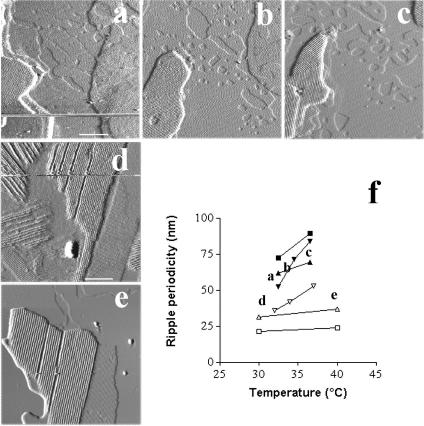
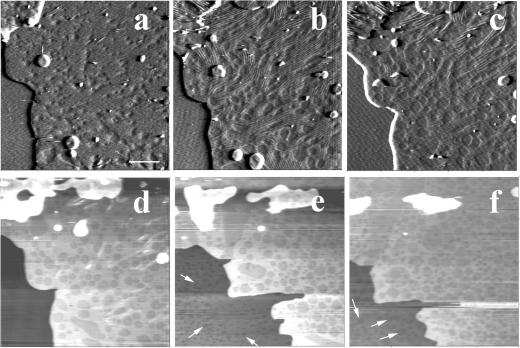
Heating up to 45°C, the limit of our homemade temperature regulation system, the five samples showing surface ripple domains at room temperature revealed two marked changes in the samples topography, i.e., the progressive disappearance of ripple domains (marked by arrowheads in Fig. 6) and the formation of large fluid phase domains, of a size between several hundred nanometers and a few micrometers (Fig. 6, a–d) with a measured height difference of ∼1.3 nm between the phases. Interestingly, formation of domains in the first bilayer close to the mica, as pointed by arrows, became visible between 29°C (Fig. 6, a and inset, arrows) and 31°C, according to the samples. Ripple forming domains lying on the top of the multibilayers were observed up to 40°C (Figs. 6 c and 7 e). Selecting domains that were imaged at different temperatures, the ripple periodicity was found to increase when raising temperature. This increase could be marked as illustrated in Fig. 7, a–c, where the modulation wavelength of three well identified domains raised from 37 to 42 and 53 nm when imaging at 32, 34, and 37°C, respectively. We also observed more limited evolution, i.e., between 10 and 20% variation in ridge spacing for a 10°C increase in temperature (Fig. 7, d and e). These data are summarized in Fig. 7 f.
FIGURE 6.
Temperature dependence of ripple domains in samples 1–3. a–d are height images of the same zone at 30, 34, 39, and 45°C, respectively. Arrows point at gel/fluid phase separation domains in the bilayer underneath the ripple domains bilayer. To better visualize the occurrence of the gel/fluid phase separation at 30°C, an electronic zoom applied to the region selected by a white frame is presented in the inset. Arrowheads designate ripple phase domains. e is a virtual section of b. (Bar) 2 μm.
FIGURE 7.
Ripples modulation wavelength increases with temperature. Imaging of the same domains at different temperatures allows determining the evolution of the ridge spacing. a–c are deflection images of a first selected region at 32, 34, and 37°C, respectively. d and e correspond to deflection images obtained at 30 and 40°C, respectively, on another sample. The graph presented in f summarizes the evolution of the ripple wavelength from five different regions selected in three different samples. (Bars) a–c, 1 μm; d and e, 500 nm.
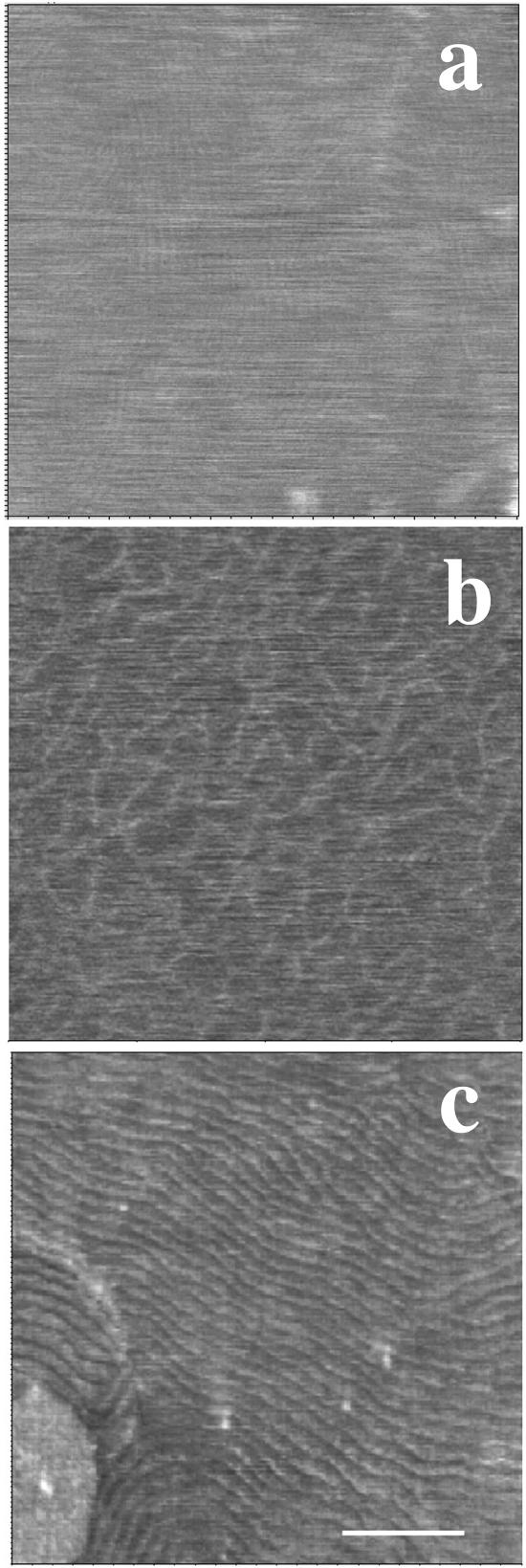
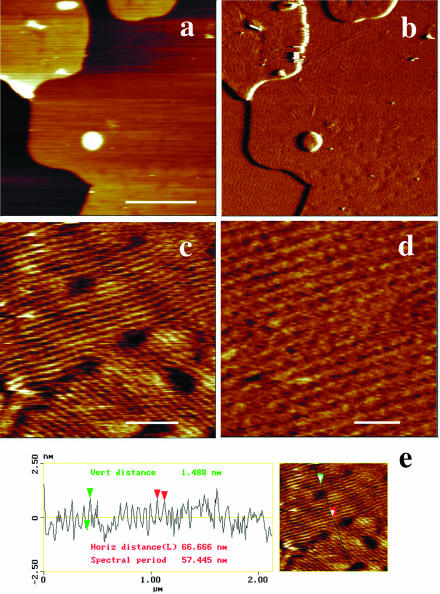
Examination of the temperature-dependent surface topography of sample four revealed a markedly different behavior. Upon heating, the surface of the top bilayer underwent profound modifications at 27°C. At low magnification (Fig. 8, a and b), the sample surface was essentially made of periodic corrugations, characteristic of the Pβ′ ripple phase. At a higher magnification, AFM height images (Fig. 8, c and d) showed that the ripple pattern was not organized as regularly as that imaged in Figs. 2 and 3. The ripple periodicity was 57 nm with an apparent amplitude of ∼1.5 nm (Fig. 8 e). Numerous defects forming pit-like structures with a depth up to 2.5 nm were observed. Upon further heating, low magnification images (Fig. 9, a–f) revealed the presence at 33°C of both wavy structures of large amplitude and more flat, likely fluid phase domains, which apparently grew from the defect zones and were aligned with the ripples. At 36°C, wavy structures have practically disappeared, and the surface of the multibilayers resembled that of bilayers under gel/fluid phase separation with fluid domains up to micron size surrounded by gel phase domains. Upon further temperature increase, fluid domains started to form (at ∼37°C in the experiment shown) in the matrix supporting the top bilayer (Fig. 9, arrows). This demonstrated that the bilayer imaged was in fact standing on another single DMPC/DSPC bilayer. The gel to fluid transition temperature of the first bilayer of this sample four was shifted up to 8°C upward as compared with the other samples. Heating to 45°C increased the area occupied by fluid domains in both the top bilayer and the first bilayer closest to the mica surface. As shown by Fig. 9 f, the size of domains present on the first and the top bilayers facing the buffer was in the same range (0.36 ± 0.16 μm vs. 0.47 ± 0.26 μm, respectively), varying from ∼100 nm up to 1 μm. Higher magnification imaging of the same zone at various temperatures (Fig. 10) confirmed the increase in ripple periodicity when raising the temperature (compare Fig. 10, a and e). Upon further increase in temperature, ripples progressively disappeared while darker domains formed ∼1.3 nm below the level of the light matrix. This indicated these darker domains likely correspond to lipids in the fluid phase. As noticed above, the fluid phase domains grew from ripple defect zones, preferentially in the direction of the ripples. The fluid domains then tended to round shape when the ripples dissipate. At 38°C ripples are no longer present, and the surface presented the characteristic aspect of a disconnected Lα phase within a gel matrix. These observations strongly suggest that upon heating, DMPC/DSPC bilayers in Pβ′ phase can transform into a Lβ′/Lα phase separated mixture.
FIGURE 8.
Temperature induced ripple phase formation from gel/gel phase separation. At 27°C, the sample four topography markedly changes with the replacement of gel/gel phase separation by a ripple phase. (a and b) height (z scale = 40 nm) and deflection images, respectively. (Bar) 2.5 μm. c and d are height images at higher magnification (bars, 500 and 200 nm, respectively; corresponding z scales are 5 and 4 nm). (e) Virtual section of c.
FIGURE 9.
Low magnification imaging of sample four temperature dependence. a–c are deflection images obtained at 30, 33, and 35°C, and d–f are height images obtained at 35, 42, and 45°C, respectively. Arrows point at the phase separation in the first bilayer facing the mica. (Bar) 2 μm.
FIGURE 10.
Temperature induced fluid/gel phase separation from ripple phase. a–i are height images recorded at 28, 29, 31, 32, 33, 34, 35, 38, and 45°C, respectively. (Bar) 1 μm.
DISCUSSION
These experiments demonstrate that, in supported multibilayers made of DMPC/DSPC equimolar mixture, the size of lipid domains in the gel/fluid coexistence region is comparable in the first bilayer facing the mica and in the upper bilayer facing the buffer. They indicate that, most likely as a function of the thickness of the buffer layer at the mica surface, the gel-fluid phase-separation temperature of this first bilayer can be close to that of freestanding bilayers or can be increased by up to 8°C. In the temperature range from room temperature to 32.5°C, the data confirm the existence of ripple phase domains in the upper bilayer (Leidy et al., 2002). They show that in DMPC/DSPC equimolar mixture the ripple phase domains persist up to 38–40°C. Finally, they provide direct evidence for metastable ripple phase transforming into gel/fluid phase separated mixture upon heating.
Multibilayers are made of two superimposed bilayers
Formation of supported multibilayers made of phospholipids by vesicle fusion followed by their characterization by AFM was reported earlier. Fang and Yang (1996), using vesicle fusion onto a preformed supported bilayer, obtained double dipalmitoylphosphatidylcholine (DPPC) bilayers, with respective thicknesses of 6 and 7 nm for the first and the floating bilayer. Recently, Leidy et al. (2002), using vesicle fusion on mica, also obtained double dipalmitoylphosphatidylcholine bilayers, the height difference between the second and the first bilayer being 9–10 nm, giving a floating bilayer surface 15–17 nm above the substrate. This value compares with the 16–18 nm of the samples top level determined at room temperature in our experiments in which, when accessible, the surface of the first bilayer was ∼6 nm from the mica support. A value of 5.5 nm was previously reported for supported single DMPC/DSPC 1:1 bilayer on mica (Giocondi et al., 2001b). Taking into account the presence of a buffer layer between the support and the bilayer, such values are in reasonable agreement with results of specular reflection of neutrons (Johnson et al., 1991) and x-ray diffraction (Wack and Webb, 1989) on pure DMPC and DSPC samples. Accordingly, the upper part of samples had 10–12 nm apparent thickness. Examination of the samples surface confirmed the existence of a ripple phase at room temperature (Fang and Yang, 1996; Leidy et al., 2002) capable to account for the extra thickness of a single bilayer. Evidence for such a ripple phase was obtained independently by the presence of a pretransition peak centered at 16°C in the DSC thermogram of the initial DMPC/DSPC MLV suspension (data not shown), in accordance with Mabrey and Sturtevant data (1976). Taken together, these data suggest that multibilayers samples obtained in our experiments were most likely made of double bilayers, although the presence of two superimposed and perfectly coupled bilayers with a lamellar periodicity close to 6 nm can not be totally excluded.
Size of domains in multibilayers
Heating samples above 40°C gave AFM images characteristic of gel/fluid phase separation with smooth gel domains protruding from a smooth DMPC enriched matrix by ∼1.3 nm, a value within the range of those reported for phase-separated supported bilayers made of various phospholipid mixtures (Giocondi et al., 2001b; Milhiet et al., 2003). The size of domains at the multibilayer surface was comprised between ∼100 nm and a few micrometers. This is a range also commonly encountered in lipid phase-separated supported single bilayers examined by AFM, including DMPC/DSPC mixture (Dufrêne et al., 1997; Czajkowsky et al., 1998; Giocondi et al., 2001a,b; Rinia et al., 2001; Milhiet et al., 2002). This strongly suggests that the presence of the mica support had, if any, a limited effect on the size of lipid domains in supported bilayers. However, the domains size can be affected by the sample preparation procedures. For instance, it can vary by at least two orders of magnitude according to the thermal history of the samples (Milhiet et al., 2003). Multibilayers offer the possibility to get access to the first bilayer facing the mica and to the upper bilayer facing the buffer on the same image. Comparison of domains present in both bilayers provided direct evidence that, in the gel/fluid coexistence region, mica had little influence on their size. Accordingly, the 2–4 orders of magnitude that separate the spectroscopy estimated and AFM determined size of microdomains in DMPC/DSPC mixtures can hardly be attributed to the use of different membrane models, i.e., supported single bilayer versus multibilayers. It is noticeable that recent fluorescence microscopy data on giant unilamellar liposomes made of comparable phospholipid mixtures also suggest the existence of very large domains (Korlach et al., 1999).
Structural properties of samples at room temperature
In accordance with freeze fracture electron microscopy (Luna and McConnell, 1978) and recent AFM studies (Leidy et al., 2002), the surface of DMPC/DSPC 1:1 multibilayers at room temperature was characterized by the presence of Pβ′ ripple domains for five out of six samples. As expected, domains with different ridge spacings, from λ1 of ∼24 nm to λ2 wavelengths close to ∼3/2 λ1, 2 λ1, and ∼5/2 λ1, coexisted in the bilayers (Luna and McConnell, 1978; Copeland and McConnel, 1980; Meyer, 1996). On the other hand, we did not visualize the stable λ1/2 ripples reported by Leidy et al. (2002), even in the high resolution images like those presented in Fig. 3. This could be explained by the different protocols used for samples preparations: Leidy and co-workers made their bilayers at 24°C, whereas, to insure a supported bilayer composition that reflects the phospholipid mixture composition, we performed vesicle fusion at a temperature above the DSPC gel-fluid phase transition temperature. This was followed by a slow return to room temperature, a process known to favor the formation of metastable ripples (Meyer, 1996; Meyer and Richter, 2001). As pointed out by Hentschel and Rustichelli (1991), the preparation techniques and history of the lipid samples can account for different characteristics of the Pβ′ phase. Although treated identically and prepared from the same phospholipid batches, sample four behaved differently with the observation of a gel-gel phase separation at room temperature (see below). This observation supports the view that the DMPC/DSPC system is peritectic (presence of gel/gel coexistence) rather than monotectic (absence of gel/gel coexistence), a debated topic in the literature (Knoll et al., 1981; Sankaram and Thompson, 1992; Sankaram et al., 1992).
Temperature-dependent behavior of ripples
Whereas for all samples the topography of the upper bilayer showed the coexistence of gel and fluid domains when raising the temperature above 40°C, the experiments strongly suggest that DMPC/DSPC bilayers in metastable Pβ′ phase can transform into a Lβ′/Lα phase separated mixture upon heating. The melting of the metastable ripple phase presented many similarities with the process described recently for the stable ripple phase (Kaasgaard et al., 2003). Thus melting occurred at the defects forming pit-like structure likely corresponding to a higher degree of lipid disorder. The fluid phase was preferentially growing in the direction of the ripples to give first elongated domains. These domains then rounded when the anisotropic line tension disappeared due to the ripples transformation into a Lβ′ phase devoid of long-range order. It cannot be excluded that this gel/fluid phase separated mixture was not a thermodynamically stable structure. Its lifetime under continuous scanning, however, exceeded 2 h. During this process, the ridge spacing had practically doubled (from 57 to 121 nm). A comparable relative increase in periodicity was measured in other samples. On the other hand, the ridge spacing increase of the ripple domains that melt at higher temperatures, around 40°C, was more limited, between 10 and 20%. Focused new experimental series, on supported double bilayers made of binary mixtures, are required to understand the relationships between temperature and ripple periodicity (and stability), a debated topic in the literature (Kaasgaard et al., 2003).
Temperature-dependent behavior of the first bilayer
Varying the temperature confirmed that the presence of the solid support exerted an effect on the gel to fluid transition temperature of the first supported bilayer. As compared to free standing DMPC/DSPC 1:1 bilayers (Knoll et al., 1981; Sankaram et al., 1992), the support induced upward shift in transition temperature varied from less than 2°C, which agrees with a previous report (Giocondi et al., 2001b), up to 8°C for sample four. A comparable temperature shift associated with a broadening of the transition was recently reported from AFM experiments on supported DMPC bilayer pieces (Tokumasu et al., 2002). DSC experiments have also shown that the main transition temperature of dipalmitoylphosphatidylcholine bilayers can be shifted upward by more than 5°C upon adsorption onto mica chips (Yang and Appleyard, 2000). This strongly suggests that the shift in gel-fluid phase transition temperature of the lipids present in the first bilayer facing mica depends on the thickness and the composition of the sandwiched fluid layer. The thinner the aqueous film, the higher the temperature shift according to a mechanism that likely involves the inhibitory effect of the solid surface on membrane fluctuations. This hypothesis was supported by images of the topography at room temperature of the first bilayer showing variations from flat featureless surfaces to undulated wave-like structures. The lateral stress on the first adsorbed phosphatidylcholine bilayer, however, prevented the formation of the Pβ′ ripple phase for aqueous film thickness up to 3 nm (Johnson et al., 1991; Fragneto et al., 2001) and led to the coexistence of gel and fluid domains above the solidus phase line (Giocondi et al., 2001b).
In the absence of a polymer cushion, the aqueous film thickness was found to vary between a few angstroms and more than 4 nm (Tamm and McConnell, 1985; Bayerl and Bloom, 1990; Johnson et al., 1991; Mou et al., 1994; Fragneto et al., 2001; Milhiet et al., 2002). This thickness is so far poorly controlled but is markedly affected by the cleanness and hydrophilicity of the support surface (McGuiggan and Israelachvili, 1990; Sackmann, 1996; Charitat et al., 1999). This suggests that variability in mica sheets hydrophilicity might be at the origin of the variability in samples behavior. It is worth noting that the gel to fluid transition temperature of sample four's first bilayer presented the most marked shift toward higher temperatures, likely linked to a particularly thin film of buffer. Presence of a gel/gel phase separation rather than a ripple phase in the corresponding upper bilayer at room temperature indicated that the physical constraints on the first bilayer were to some extent transmitted to this upper bilayer. Fluctuation of the upper bilayer upon heating (Fragneto et al., 2001) would relax the interbilayer constraints and allow the formation of a ripple phase like in other samples.
Ripples presence and domains size estimate
Calculations in DMPC/DSPC mixtures of the size of domains from FRAP (Almeida et al., 1992) and electron spin resonance experiments (Sankaram et al., 1992) were based on the estimates of the fraction of the lipid in the fluid state from a phase diagram that ignored the presence of the Pβ′ phase in the phases coexistence region. FRAP experimental data are commonly interpreted using the free-volume diffusion model in which gel domains form immobile circular obstacles to diffusion and are surrounded by a few layers of lipids of higher order than the bulk fluid lipids, defining a coherence length ξ (Almeida et al., 1992). When ripples are present, estimating the size of ordered domains from the analysis of the diffusion properties of an inserted probe encounters unsolved difficulties. Thus, equations for isotropic mixtures of phase domains are not applicable. For this reason, Owicki and McConnell (1980) derived an expression for diffusion in the solid-liquid coexistence region corresponding to a DMPC/cholesterol mixture presenting ripple structures. This system was modeled as alternating parallel bands of solid and fluid phases lipids, with anisotropic diffusion in two dimensions, in the direction parallel and perpendicular to the ripple phase bands. Determination of diffusion rates from pure DMPC multibilayers in Pβ′ phase, however, revealed a more complex behavior than that predicted from the DMPC/cholesterol mixture. Thus, in ripple phase, fast diffusion along bands of partially disordered material followed a simple one-dimensional diffusion model (Schneider et al., 1983). This suggests that, in the gel-fluid coexistence region of the DMPC/DSPC phase diagram in fact occupied by ripples, estimate of the size of domains from the analysis of the diffusion properties of an inserted probe can hardly be achieved using available models.
Acknowledgments
We are grateful to Prof. Grégoire Porte and to Prof. Vladimir Lormon for stimulating discussions concerning these results.
We also thank “La Région Languedoc-Roussillon” for its financial support.
References
- Almeida, P. F. F., W. L. C. Vaz, and T. E. Thompson. 1992. Lateral diffusion and percolation in two-phase, two component lipid bilayers. Topology of the solid-phase domains in-plane and across the lipid bilayer. Biochemistry. 31:7198–7210. [DOI] [PubMed] [Google Scholar]
- Bayerl, T. M., and M. Bloom. 1990. Physical properties of single phospholipid bilayers adsorbed to micro glass beads. Biophys. J. 58:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, D. A., and E. London. 1998. Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol. 164:103–114. [DOI] [PubMed] [Google Scholar]
- Brumm, T., K. Jorgensen, O. G. Mouritsen, and T. M. Bayerl. 1996. The effect of increasing membrane curvature on the phase transition and mixing behavior of a dimyristoyl-sn-glycero-3-phosphatidylcholine/distearoyl-sn-glycero-3-phosphatidylcholine lipid mixture as studied by Fourier transform infrared spectroscopy and differential scanning calorimetry. Biophys. J. 70:1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charitat, T., E. Bellet-Amalric, G. Fragneto, and F. Graner. 1999. Adsorbed and free bilayers at the solid-liquid interface. Eur. Phys. J. B. 8:583–593. [Google Scholar]
- Copeland, B. R., and H. M. McConnel. 1980. The rippled structure in bilayer membranes of phosphatidylcholine and binary mixtures of phosphatidylcholine and cholesterol. Biochim. Biophys. Acta. 599:95–109. [DOI] [PubMed] [Google Scholar]
- Czajkowsky, D. M., M. J. Allen, V. Elings, and Z. Shao. 1998. Direct visualization of surface charge in aqueous solution. Ultramicroscopy. 74:1–5. [DOI] [PubMed] [Google Scholar]
- Czajkowsky, D. M., C. Huang, and Z. Shao. 1995. Ripple phase in asymmetric unilamellar bilayers with saturated and unsaturated phospholipids. Biochemistry. 34:12501–12505. [DOI] [PubMed] [Google Scholar]
- Dufrêne, Y. F., W. R. Barger, J. B. Green, and G. U. Lee. 1997. Nanometer scale surface properties of mixed phospholipid monolayers and bilayers. Langmuir. 13:4779–4784. [Google Scholar]
- Fang, Y., and J. Yang. 1996. Role of the bilayer-bilayer interaction on the ripple structure of supported bilayers in solution. J. Phys. Chem. 100:15614–15619. [Google Scholar]
- Fragneto, G., T. Charitat, F. Graner, K. Mecke, L. Perino-Gallice, and E. Bellet-Amalric. 2001. A fluid floating bilayer. Europhys. Lett. 53:100–106. [Google Scholar]
- Giocondi, M.-C., V. Vié, E. Lesniewska, P. E. Milhiet, M. Zinke-Allmang, and C. Le Grimellec. 2001a. Imaging the phase topology and growth of domains in supported lipid bilayers. Langmuir. 17:1653–1659. [Google Scholar]
- Giocondi, M.-C., L. Pacheco, P. E. Milhiet, and C. Le Grimellec. 2001b. Temperature dependence of the topology of supported dimyristoyl-distearoyl phosphatidylcholine bilayers. Ultramicroscopy. 86:151–157. [DOI] [PubMed] [Google Scholar]
- Gliss, C., H. Clausen-Schaumann, R. Günther, S. Odenbach, O. Randl, and T. M. Bayer. 1998. Direct detection of domains in phospholipid bilayers by grazing incidence diffraction of neutrons and atomic force microscopy. Biophys. J. 74:2443–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel, M. P., and F. Rustichelli. 1991. Structure of the ripple phase Pβ′ in hydrated phosphatidylcholine multimembranes. Phys. Rev. Lett. 66:903–906. [DOI] [PubMed] [Google Scholar]
- Ipsen, J. H., and O. G. Mouritsen. 1988. Modelling the phase equilibria in two-component membranes of phospholipids with different acyl-chain lengths. Biochim. Biophys. Acta. 944:121–134. [DOI] [PubMed] [Google Scholar]
- Jacobson, K., E. D. Sheets, and R. Simon. 1995. Revisiting the fluid mosaic model of membranes. Science. 268:1441–1442. [DOI] [PubMed] [Google Scholar]
- Jain, M. K., and H. White. 1977. Long range order in biomembranes. Adv. Lipid Res. 15:1–60. [DOI] [PubMed] [Google Scholar]
- Johnson, S. J., T. M. Bayerl, D. C. McDermott, G. W. Adam, A. R. Rennie, R. K. Thomas, and E. Sackmann. 1991. Structure of an absorbed dimyristoylphosphatidylcholine bilayer measured with specular reflection of neutrons. Biophys. J. 59:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen, K., M. M. Sperotto, O. G. Mouritsen, J. H. Ipsen, and M. J. Zuckermann. 1993. Phase equilibria and local structure in binary lipid bilayers. Biochim. Biophys. Acta. 1152:135–145. [DOI] [PubMed] [Google Scholar]
- Kaasgaard, T., C. Leidy, J. H. Crowe, O. G. Mouritsen, and K. Jorgensen. 2003. Temperature-controlled structure and kinetics of ripple phases in one and two-component supported lipid bilayers. Biophys. J. 85:350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky, M. J., A. M. Kleinfeld, R. L. Hoover, and R. D. Klausner. 1982. The concept of lipid domains in membranes. J. Cell Biol. 94:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll, W., K. Ibel, and E. Sackmann. 1981. Small-angle neutron scattering study of lipid phase diagrams by the contrast variation method. Biochemistry. 20:6379–6383. [DOI] [PubMed] [Google Scholar]
- Korlach, J., P. Schwille, W. W. Webb, and G. W. Feigenson. 1999. Characterization of lipid bilayer phases by confocal microscopy and fluorescence correlation spectroscopy. Proc. Natl. Acad. Sci. USA. 96:8461–8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidy, C., T. Kaasgaard, J. H. Crowe, O. G. Mouritsen, and K. Jorgensen. 2002. Ripples and the formation of anisotropic lipid domains: imaging two-component supported double bilayers by atomic force microscopy. Biophys. J. 83:2625–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidy, C., W. F. Wolkers, K. Jorgensen, O. G. Mouritsen, and J. H. Crowe. 2001. Lateral organization and domain formation in a two-component lipid membrane system. Biophys. J. 80:1819–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna, E., and H. M. McConnell. 1978. Multiple phase equilibria in binary mixtures of phospholipids. Biochim. Biophys. Acta. 509:462–473. [DOI] [PubMed] [Google Scholar]
- Mabrey, S., and J. M. Sturtevant. 1976. Investigation of phase transitions of lipids and lipid mixtures by high sensitivity differential scanning calorimetry. Proc. Natl. Acad. Sci. USA. 73:3862–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuiggan, P. M., and J. N. Israelachvili. 1990. Adhesion and short-range forces between surface. Part II: effects of surface lattice mismatch. J. Mater. Res. 5:2232–2243. [Google Scholar]
- Mendelsohn, R., and J. Maisano. 1978. Use of deuterated phospholipids in Raman spectroscopic studies of membrane structure. I. Multilayers of dimyristoylphosphatidylcholine (and its -d54 derivative) with distearoylphosphatidylcholine. Biochim. Biophys. Acta. 506:192–201. [DOI] [PubMed] [Google Scholar]
- Meyer, H. W. 1996. Pretransition ripples in bilayers of dipalmitoylphosphatidylcholine: undulation or periodic segments? A freeze-fracture study. Biochim. Biophys. Acta. 1302:138–144. [DOI] [PubMed] [Google Scholar]
- Meyer, H. W., and W. Richter. 2001. Freeze-fracture studies on lipids and membranes. Micron. 32:615–644. [DOI] [PubMed] [Google Scholar]
- Milhiet, P. E., M.-C. Giocondi, and C. Le Grimellec. 2002. Cholesterol is not crucial for the existence of microdomains in kidney brush-border membrane models. J. Biol. Chem. 277:875–878. [DOI] [PubMed] [Google Scholar]
- Milhiet, P. E., M.-C. Giocondi, and C. Le Grimellec. 2003. AFM imaging of lipids domains in model membranes. http://www.thescientificworld.com. 3:59–74. [DOI] [PMC free article] [PubMed]
- Morrisett, J. D., H. J. Pownall, R. T. Plumlee, L. C. Smith, Z. E. Zehner, M. Esfahani, and S. J. Wakil. 1975. Multiple thermotropic phase transitions in Escherichia coli membranes and membrane lipids. J. Biol. Chem. 250:6969–6976. [PubMed] [Google Scholar]
- Mou, J., J. Yang, and Z. Shao. 1994. Tris(hydroxymethyl)aminomethane induced a ripple phase in supported unilamellar phospholipid bilayer. Biochemistry. 33:4439–4443. [DOI] [PubMed] [Google Scholar]
- Mrsny, R. J., J. J. Volwerk, and O. H. Griffith. 1986. A simplified procedure for lipid phosphorus analysis shows that digestion rates vary with phospholipid structure. Chem. Phys. Lipids. 39:185–191. [DOI] [PubMed] [Google Scholar]
- Owicki, J., and H. M. McConnell. 1980. Lateral diffusion in inhomogeneous membranes. Model membranes containing cholesterol. Biophys. J. 30:383–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmacher, M., R. W. Tillmann, M. Fritz, and H. E. Gaub. 1992. From molecules to cells: imaging soft samples with the atomic force microscope. Science. 257:1900–1905. [DOI] [PubMed] [Google Scholar]
- Rinia, H. A., M. M. E. Snel, J. P. J. M. van der Eerden, and B. de Kruijff. 2001. Visualizing detergent resistant domains in model membranes with atomic force microscopy. FEBS Lett. 501:92–96. [DOI] [PubMed] [Google Scholar]
- Sackmann, E. 1996. Supported membranes: scientific and practical applications. Science. 271:43–47. [DOI] [PubMed] [Google Scholar]
- Sankaram, M. B., D. Marsh, and T. E. Thompson. 1992. Determination of fluid and gel domain sizes in two-component, two-phase lipid bilayers: an electron spin resonance spin label study. Biophys. J. 63:340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaram, M. B., and T. E. Thompson. 1992. Deuterium magnetic resonance study of phase equilibria and membrane thickness in binary phospholipid mixed bilayers. Biochemistry. 31:8258–8268. [DOI] [PubMed] [Google Scholar]
- Schneider, M. B., W. K. Chan, and W. W. Webb. 1983. Fast diffusion along defects and corrugations in phospholipid Pβ′ liquid crystals. Biophys. J. 43:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram, V., H.-N. Lin, and T. E. Thompson. 1996. Topology of gel phase domains and lipid mixing properties in phase-separated two-component phosphatidylcholine bilayers. Biophys. J. 71:1811–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, Z., J. Mou, D. M. Czajkowsky, J. Yang, and J.-Y. Yuan. 1996. Biological atomic force microscopy: what is achieved and what is needed. Adv. Physics. 45:1–86. [Google Scholar]
- Simons, K., and R. Ehehalt. 2002. Cholesterol, lipid rafts and disease. J. Clin. Invest. 110:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. [DOI] [PubMed] [Google Scholar]
- Sugar, I. P., T. E. Thompson, and R. L. Biltonen. 1999. Monte Carlo simulation of two-component bilayers: DMPC/DSPC mixtures. Biophys. J. 76:2099–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm, L. K., and H. M. McConnell. 1985. Supported phospholipid bilayers. Biophys. J. 47:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu, A., V. Luzzati, and F. C. Reman. 1973. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J. Mol. Biol. 75:711–733. [DOI] [PubMed] [Google Scholar]
- Tenchov, B. B., H. Yao, and I. Hatta. 1989. Time resolved x-ray diffraction and calorimetric studies at low scan rates. I. Fully hydrated dipalmitoylphosphatidylcholine (DPPC) and DPPC/water/ethanol phases. Biophys. J. 56:757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocanne, J.-F., L. Dupou-Cézanne, and A. Lopez. 1994. Lateral diffusion of lipids in model and natural membranes. Prog. Lipid Res. 33:203–237. [DOI] [PubMed] [Google Scholar]
- Tokumasu, F., A. J. Jin, and J. A. Dvorak. 2002. Lipid membrane phase behaviour elucidated in real time by controlled environment atomic force microscopy. J. Electron. Microsc. (Tokyo). 51:1–9. [DOI] [PubMed] [Google Scholar]
- Vaz, W. L. C., E. C. C. Melo, and T. E. Thompson. 1989. Translational diffusion and fluid domain connectivity in two component, two-phase phospholipid bilayers. Biophys. J. 56:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wack, D. C., and W. W. Webb. 1989. Synchrotron x-ray study of the modulated lamellar Pβ′ in the lecithin-water system. Phys. Rev. A. 40:2712–2730. [DOI] [PubMed] [Google Scholar]
- Wilkinson, D. A., and J. F. Nagle. 1979. Dilatometric study of binary mixtures of phosphatidylcholines. Biochemistry. 18:4244–4249. [DOI] [PubMed] [Google Scholar]
- Yang, J., and J. Appleyard. 2000. The main phase-transition of mica-supported phosphatidylcholine membranes. J. Phys. Chem. B. 104:8097–8100. [Google Scholar]
- Yechiel, E., and M. Edidin. 1987. Micrometer-scale domains in fibroblast plasma membranes. J. Cell Biol. 105:755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]