Abstract
We investigate the role played by membrane composition on the interaction and self-assembly of β-amyloid peptide (AβP1–40) during pore formation in planar lipid membranes (PLMs). Incorporation studies showed that AβP does not interact with zwitterionic membranes made up of phosphatidylcholine, whereas the addition of cholesterol or ergosterol to the membranes leads to channel formation. Among the PLMs used, a higher propensity of AβP to form channels at low applied potential (±20 mV) was observed in 7-dehydrocholesterol and in oxidized cholesterol PLMs. These channels present long lifetimes, high-occurrence frequencies, and are voltage dependent. In particular, the AβP channel in oxidized cholesterol showed anion selectivity. Thus cholesterol (and sterols in general) could be considered as targets for AβP, which prevents the fibrillation process by increasing incorporation into membranes. Furthermore, by switching the channel selectivity versus anions, cholesterol helps to reduce the imbalance of the cellular ions, calcium included, induced by membrane depolarization, which could be one of the factors responsible for cytotoxicity in Alzheimer's disease.
INTRODUCTION
β-Amyloid peptide (AβP) is a 1–40 or 42-residue polypeptide, the proteolytic product of the amyloid precursor protein (APP), thought to be implicated in plaques, which are pathognomonic of Alzheimer's disease (AD). APP is a transmembrane glycoprotein, defined by a locus on chromosome 21 (Tanzi et al., 1987). AβP is an amphiphilic peptide with a hydrophilic N-terminal domain (residues 1–28) and a hydrophobic C-terminal (residues 29–40 (−42)), the latter corresponding to a part of the transmembrane domain of APP. Circular dichroism studies have shown that AβP changes its conformation from lipid-free AβP to bind to phospholipid vesicles. AβP consists of 48.9% random-coil, 23.5% β-sheet, and 1.7% α-helix. When reconstituted in phospholipid vesicles made up of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), both β-sheet and α-helix show an ∼7% increase, i.e., β-sheet increases to 31.2% and α-helix to 9.5% (Ji et al., 2002).
Previous studies have demonstrated that AβP decreases the resistance of the membrane by interacting with model membranes, indicating aqueous routes for the ions with a permeability ratio favorable for cations (Arispe et al., 1993, 1996; Lin et al., 1999). Moreover, AβP also increases calcium permeability, which has been postulated to be responsible for the toxic action of the peptides (Hardy and Higgins, 1992).
Recent studies have suggested the importance of cholesterol in AβP binding and incorporation in model membranes (Ji et al., 2002), although its role remains unclear. In fact, some authors found that membrane components, such as cholesterol and gangliosides, alter the affinity of AβP for phospholipid membranes. Moreover, cholesterol and gangliosides, once associated with membranes made up of phospholipids, lead to an increase in β-sheet content and/or AβP aggregation rate (Choo-Smith et al., 1997). On the other hand, other authors have shown that, when AβP was added to a 33% cholesterol-containing DMPC vesicle, the structure of AβP was drastically altered, i.e., the β-sheet structure decreased to zero whereas the α-helix increased to 58.8% (Ji et al., 2002).
In addition, alterations of the soluble cholesterol concentration and/or in cholesterol biosynthesis have been shown to affect the normal processing of APP, both in vivo and in vitro (Galbete et al., 2000).
Oxidized cholesterol (OxCh), whose main component is cholesterol, typically contains other products characteristic of aged cholesterol, such as 7-dehydrocholesterol (7-DHC), which also can be found under physiological conditions (Weiner et al., 1972). In particular, 7-DHC is abundant in Smith-Lemli-Opitz syndrome, mainly due to the inability of cells to convert this sterol into cholesterol. However, it is useful to emphasize that a number of oxidation products of cholesterol are toxic to organ cultures of rabbit aorta (MacDougall et al., 1965). Furthermore, there is evidence of cholesterol-rich domains in both eukaryotic plasma membranes, such as brain and blood vessels and aged membrane plaque (Brown and London, 1998). For these reasons we used planar lipid membranes (PLMs) of POPC, the principal lipid component of cellular membranes, enriched with cholesterol or ergosterol or membranes of pure sterols such as 7-DHC or OxCh, each of which to some extent could be considered a model of aged membranes useful for clarifying some aspects of AD.
Channel formation during incorporation of AβP (1–40) into different planar lipid membranes made up of either phosphatidylserine and phosphatidylethanolamine (DOPS:DOPE), palmitoyl-oleoyl-phosphatidylcholine (POPC) or POPC:cholesterol (Ch) or POPC:ergosterol (Erg), or else made up of pure 7-DHC, or OxCh was evaluated following current variation.
METHODS
Channel activities were recorded in lipid bilayer membranes composed of DOPS:DOPE (50:50, w:w) or POPC (Avanti Polar Lipid, Alabaster, AL), or POPC:Ch (10, 20, 30% mol), or POPC:Erg (39% mol), or 7-DHC (Sigma, St. Louis, MO) or OxCh in 1% of n-decane (Fluka, Buchs, Switzerland). The OxCh was obtained following Ti Tien's method (1966). Bilayers were formed over a 200 μm hole in a teflon partition separating two teflon chambers that held symmetrical KCl 50 mM solutions, pH = 7, temperature 23 ± 1C°. The salts used in the experiments were of analytical grade.
The AβP (Sigma) was added to the cis side of the membrane, at a final concentration of 5 × 10−8 M; the cis side was positive whereas the trans side was negative.
In single-channel experiments, the membrane current was monitored with an oscilloscope and recorded on a chart recorder for data analysis by hand. The cis and trans chambers were connected to the amplifier head stage by Ag/AgCl electrodes in series with a voltage source and a highly sensitive current amplifier. The single-channel instrumentation had a time resolution of 1–10 ms depending on the magnitude of the single-channel conductance. The polarity of the voltage was defined according to the side where AβP was added (the cis-side). A trans-negative potential (indicated by a minus sign) means that a negative potential was applied to the trans side, the compartment opposite the one where AβP was added.
Data analysis
The single-channel data were obtained from at least three experiments with >100 single channels (openings and closings) for each series performed on different days. A histogram of the current amplitude distribution for each experiment was constructed and fitted by a Gaussian distribution function (GraphPad Prism version 3.0; GraphPad Software, San Diego, CA).
The distribution of single-channel lifetime was fitted with a single- or two-exponential function (GraphPad Prism version 3.0; GraphPad Software). Results are expressed as mean ± SD unless otherwise specified.
RESULTS
As previously stated, in this study we used PLMs of different compositions to evaluate the ability of AβP (1–40) to incorporate and form channels. The stability of the PLM was tested by applying a voltage of ±120 mV for 10–15 min under stirring, and monitoring a constant value of conductance and capacitance.
The incorporation of AβP (1–40) into lipid bilayers leads to nonrandom discrete current jumps that fluctuate between conductive and nonconductive states, compatible with channel-type opening and closure with different conductance levels, lifetimes, and occurrence frequencies, except in PLMs made up of POPC. In fact, in a large number of experiments carried out in different periods over the year, lasting >24 h and applying different voltages, no interaction was observed between AβP and POPC membranes. It is interesting to note that, for fixed experimental conditions, the applied voltage to trigger channel formation depends on the membrane composition. In this study, although many applied potentials were investigated, only those potentials that led to channel formation are reported.
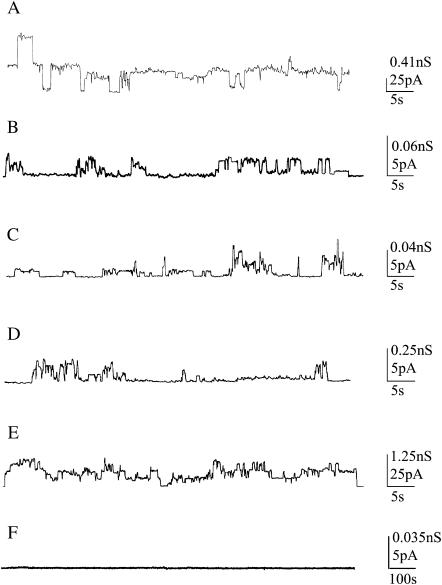
Fig. 1 shows examples of chart recordings of AβP channel formation in PLMs at the lowest applied potential for all membranes used except for the POPC membrane, where the highest applied voltage utilized is reported.
FIGURE 1.
Examples of chart recordings of AβP (1–40) channel formation in each PLM used. (A) DOPS:DOPE membrane, applied voltage V = 60 mV; (B) POPC:Ch membrane, applied voltage V = 80 mV; (C) POPC:Erg membrane, applied voltage V = 120 mV; (D) 7-DHC membrane, applied voltage V = 20 mV; (E) OxCh membrane, applied voltage V = 20 mV; (F) POPC membrane, applied voltage V = 140 mV. Each trace represents a fragment of the recording of the activity obtained in individual experiments.
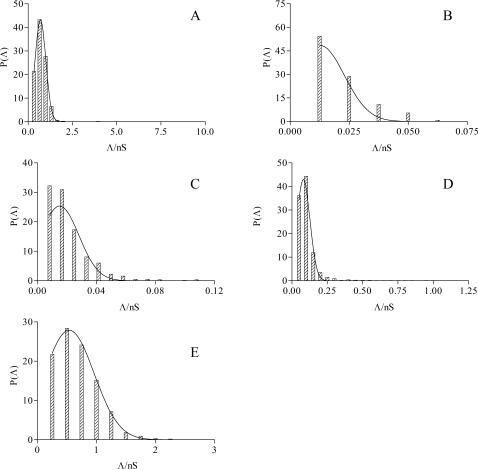
All single channels were used to calculate the channel amplitudes. Current amplitudes analyzed at different applied voltages, however, revealed the existence of one main conductance level. A histogram of amplitude distribution was constructed and fitted by a Gaussian distribution function, giving the central value of the single-channel conductance ±SD (Stipani et al., 2001; Gallucci et al., 2003) (Fig. 2).
FIGURE 2.
Amplitude histograms of AβP (1–40) channel conductance for the examples reported in Fig. 1.
Table 1 reports the central value of the single-channel conductance (Λc) ± SD, the occurrence frequency (number of channels in 60 s) ±SD, the total number of channels (Nt), and the different applied voltages (where the lower value represents the threshold potential) for all the membranes tested.
TABLE 1.
The mean conductance fitted by Gaussian distribution (Λc), the occurrence (channels/min), the fitted lifetimes (see text) of the single-channel events (s), the significant F-test (P) of the lifetimes, and the total number (Nt) of AβP (1–40) channels in different PLMs at different applied potentials
| PLM | V (mV) | Λc (nS) ± SD | Occurrence ± SD | τ1(s) | τ2(s) | P | Nt |
|---|---|---|---|---|---|---|---|
| DOPS:DOPE | 60 | 0.700 ± 0.30 | 5.62 ± 0.14 | 1.29 | – | – | 1650 |
| POPC:Ch | 80 | 0.013 ± 0.01 | 0.50 ± 0.04 | 0.99 | – | – | 129 |
| 100 | 0.018 ± 0.01 | 1.22 ± 0.12 | 0.20 | 2.60 | – | 108 | |
| 120 | 0.016 ± 0.01 | 2.97 ± 0.30 | 2.45 | – | 0.13 | 107 | |
| POPC:Erg | 120 | 0.015 ± 0.00 | 3.13 ± 0.12 | 3.80 | – | 0.65 | 631 |
| 7-DHC | 40 | 0.062 ± 0.04 | 7.91 ± 0.41 | 4.01 | – | – | 372 |
| 20 | 0.081 ± 0.04 | 3.68 ± 0.11 | 4.54 | – | 0.79 | 1164 | |
| −20 | 0.090 ± 0.03 | 4.42 ± 0.15 | 4.35 | – | 0.65 | 508 | |
| −40 | 0.040 ± 0.03 | 2.40 ± 0.18 | 2.81 | – | 0.58 | 176 | |
| OxCh | 120 | 0.072 ± 0.03 | 4.23 ± 0.15 | 1.44 | 8.90 | – | 748 |
| 100 | 0.095 ± 0.04 | 3.23 ± 0.20 | 1.98 | – | 0.23 | 272 | |
| 80 | 0.100 ± 0.06 | 1.82 ± 0.08 | 2.57 | – | – | 464 | |
| 60 | 0.120 ± 0.06 | 1.51 ± 0.05 | 0.16 | 3.33 | – | 724 | |
| 40 | 0.160 ± 0.10 | 2.32 ± 0.09 | 0.40 | 5.26 | – | 611 | |
| 20 | 0.540 ± 0.42 | 2.97 ± 0.06 | 0.40 | 3.02 | – | 2080 | |
| −20 | 0.980 ± 0.43 | 4.68 ± 0.17 | 1.71 | – | – | 791 | |
| −40 | 0.180 ± 0.10 | 3.33 ± 0.12 | 4.00 | – | 0.34 | 751 | |
| −60 | 0.170 ± 0.08 | 3.10 ± 0.09 | 3.70 | – | 0.12 | 1071 | |
| −80 | 0.110 ± 0.06 | 3.82 ± 0.22 | 1.65 | 12.38 | – | 284 | |
| −100 | 0.102 ± 0.07 | 2.99 ± 0.25 | 2.14 | – | 0.14 | 140 | |
| −120 | 0.063 ± 0.05 | 7.94 ± 0.44 | 2.25 | – | 0.12 | 329 |
The results obtained with DOPS:DOPE membranes confirm the previous observations of other authors (Arispe et al., 1993).
As mentioned above, AβP does not form channels in POPC membranes; however, the addition of not <30% of cholesterol to the POPC solution used to form the membrane gave rise to channel activity, whose Λc was lower than that in DOPS:DOPE membranes and increased with the applied voltage; moreover, the occurrence frequency seemed to be dependent on the applied voltage, suggesting that membrane insertion, channel formation, and/or channel activity are modulated by voltage. Applied voltages lower than 80 mV did not induce channel activity, whereas negative applied voltages determined breakage of the membrane.
To test the specificity of sterols on the incorporation and channel formation of AβP (1–40), membranes of POPC:Erg (39% mol) were used. Similarly to cholesterol, ergosterol gives rise to channel formation, although a higher potential is required. The Λc and occurrence frequency values are similar to those of POPC:Ch PLMs for the same applied potential.
Another sterol tested for its ability to form membrane was 7-DHC: in this case channels were formed at a threshold potential of ±20 mV, a very low potential compared to POPC:Ch or POPC:Erg membranes. The central conductance seems to be dependent on the applied voltage, decreasing as the voltage is increased, whereas the occurrence frequency proved to be higher than in POPC mixed membranes and was sensitive to the applied voltage. However, in the presence of AβP, applied voltages >±40 mV destabilize this membrane.
Another model membrane utilized to study the incorporation of AβP was OxCh PLM. It is interesting to note that this membrane bears a wide range of applied voltages when AβP is incorporated.
In this case, the minimum potential that triggers channel activity is the same as that for the 7-DHC membrane (±20 mV), but the Λc value, for the same applied voltage, is higher. Furthermore, it can be also noted that the channel conductance shows an asymmetric behavior, being higher at negative than at positive voltages and, irrespective of the sign of the voltage, a marked voltage dependence in the range from ±20 mV to ±40 mV is manifested. The occurrence frequency shows higher values at negative than at positive voltages and is no different from that found in 7-DHC for the same applied voltages.
Another parameter used to characterize a channel is its lifetime. The single-channel current recordings, with a conspicuous number of channels, were analyzed to obtain cumulative open-state lifetime distributions.
The distribution of the open times has been found to follow a single-exponential or a two-exponential function. The formula used is:
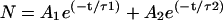 |
where N is the number of channels that remain open for a time equal to or greater than a certain time t, A1 and A2 are the zero-time amplitudes, and τ1 and τ2 are related to the fast and slow components of the time constant, respectively. The single-exponential distribution is included in the formula (A2 = 0). The results shown in Table 1 indicate that analysis of open-time distributions: 1), gives a statistically significant better description (P < 0.05) for the single-exponential function in five cases (i.e., AβP channels formed in DOPS:DOPE (+60 mV), in POPC:Ch (+80 mV), in 7-DHC (+40 mV), and in OxCh (+80 and −20 mV); 2), seems to prefer two time-constant components in six other cases (i.e., AβP channels formed in POPC:Ch (+100 mV) and in OxCh (+20, +40, +60, +120, −80 mV); and 3), do not clearly distinguish between single and two exponentials in all of the other 10 cases.
Irrespective of the presence of one or two time constants, the values were higher in 7-DHC and OxCh, indicating that AβP channels are more stable in sterol membranes.
Another parameter that characterizes a channel is ion selectivity. We performed experiments to determine the ion selectivity of the AβP channel in OxCh membranes by means of reversal potential and I-V relationship at different transmembrane potentials under asymmetrical solution conditions.
When the membrane conductance reached a virtually stable value after the addition of AβP in the cis chamber, the KCl concentration there was raised to 100 mM by the addition of concentrated salt solution.
The reversal potential was determined by changing the holding potential by ±2 mV step-by-step, and the potential at which the current reached zero was taken as the reversal potential for the open channel. The mean reversal potential was 10.3 mV.
The permeability ratio was calculated using the following equation:
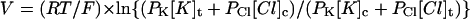 |
where [X]t and [X]c are the concentrations of the ion species X in the trans and cis compartments, respectively; R, T, and F have their usual meanings. 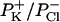 was 0.26.
was 0.26.
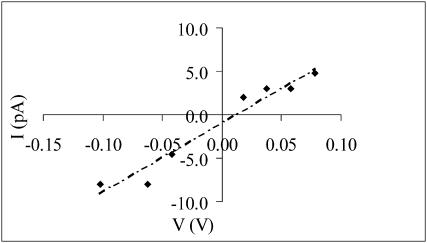
Approximately the same result was obtained with the I-V curve (Fig. 3), where the measured amplitude of the channel events at each membrane potential was used. In fact the reversal potential was 11.25 mV; the selective ratio 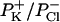 was 0.21.
was 0.21.
FIGURE 3.
AβP (1–40) channel selectivity in OxCh PLMs. The amplitude of the AβP (1–40) channel current (pA) is plotted as a function of the transmembrane potential (V). Each point along the I-V curve represents the mean value of at least three readings at the amplitude of the current at the potential indicated. Conductance was determined by linear regression of the current values from +60 to −120 mV and was 0.08 nS in asymmetrical solutions (100/50 mM KCl cis/trans). Intercept was 11.25 mV and was used to calculate PK/PCl.
DISCUSSION
It is a consolidated belief among researchers that abnormal aggregation of peptides in the brain may cause neurodegenerative disorders, such as AD, Parkinson's or prion disease. However, other examples of degenerative disorder with abnormal aggregation of protein have been described, such as BRI in familial British dementia, and progressive supranuclear palsy.
It is, however, worth noting that a common denominator of all peptides undergoing aggregation is the formation of channels constituting large ion pathways permeable to ions. In fact, prions, human calcitonin, and AβP peptides form channels when interacting with receptive membranes.
According to the amyloid hypothesis, the accumulation of AβP in the brain is the event leading to AD, through an imbalance of ionic homeostasis such that this disease could be speculatively considered a “disionic disease”.
Cholesterol is an integral component of all eukaryotic cell membranes and is essential for normal cellular functions (Howland et al., 1998). This finding seems to be in line with some functions of cholesterol such as the raft-like transport of proteins or the incorporation of mycoplasma into membranes (Gatfield and Pieters, 2000).
One characteristic of the cholesterol molecule is the rigid planar ring that probably manages all the interactions with proteins; examples of cholesterol-dependent proteins include prominin, synaptophysin, platelet-derived growth factor receptor, hemolysin, prions (Roper et al., 2000; Thiele et al., 2000; Liu et al., 2000; Song et al., 1996; Sanghera and Pinheiro, 2002), porins (Freitag et al., 1982; Popp et al., 1995; Micelli et al., 2002), magainin-2 (Gallucci et al., 2003).
After synthesis, cholesterol is delivered to different organelles and plasma membranes whose content reach the highest levels (Yeagle, 1985).
In particular, in brain synaptic plasma membranes in young people, ∼87% of total plasma membrane cholesterol is contained in the cytofacial leaflet, whereas during the aging process, the distribution of cholesterol tends to level off in the cytofacial and exofacial leaflet (Shinitzky, 1993).
However, in young people, AβP can be degraded through low-density lipoprotein (LRP), but during the aging process, LRP reduces by ∼45% (Christie et al., 1996). This decrease in LRP will impair the degradation pathway of AβP with the result that aggregations and plaques may form (Ji et al., 2002).
Our results, obtained in DOPS:DOPE membranes, confirm the previous finding obtained by other authors (Arispe et al., 1993) on the ability of AβP (1–40) to form channels. On the other hand, AβP (1–40) does not incorporate in the POPC PLMs probably due to its tendency to induce β-sheet structures (Ji et al., 2002; Curtain et al., 2003). It is interesting that this pattern is also followed by other peptides undergoing fibrillation, such as human calcitonin (Stipani et al., 2001).
However, we show that AβP (1–40) interacts and forms channel with POPC containing cholesterol or ergosterol (30 and 39% mol, respectively), with 7-DHC and with OxCh PLMs. These results are indicative of the role played by membrane composition in α-helix structure induction necessary to incorporate into membranes. These results are consistent with reports by Ji et al. (2002), in which the addition of 33% cholesterol to DOPC membranes turns β-sheet AβP (1–40) into α-helix structures. Our findings support and extend the emerging concept that cholesterol at a certain concentration can easily incorporate AβP (1–40), subtracting it from fibrillation. An interesting aspect is channel formation in membranes containing sterols and their product of oxidation.
The applied potential across the membrane may be important in driving the peptide into the membrane. It has been found that the incorporation of AβP (1–40) shows a threshold potential depending on the membrane structure where OxCh = 7-DHC < POPC:Ch < POPC:Erg < DOPS:DOPE. In the latter case, besides the applied voltage (+60 mV), the surface potential (−120 mV)—calculated according to the McLaughlin (1977) method—must also be considered. The same pattern is followed by the channel occurrence and to some extent by their lifetimes. However, incorporation into cholesterol-containing membranes seems to be driven by hydrophobic interaction, as expected by the high hydrophobic amino acid content in the C-terminus of AβP (1–40), thus partially reducing the energy imposed by the applied potential. There is a different channel conductance in OxCh and 7-DHC membranes for the same applied voltage; this can be tentatively explained by an increased number of oligomers of AβP forming channels.
Furthermore, in OxCh membranes, AβP (1–40) channel conductance shows voltage dependence characteristic of that found in palmitoyl-oleoyl-phosphatidylethanolamine:palmitoyl-oleoyl-phosphatidylserine:palmitoyl-oleoyl-phosphatidylcholine (5:3:2, by volume) membranes (Kourie et al., 2001).
By means of conductance value, and assuming a water-filled channel with an ion mobility similar to that of the bulk solution, knowing the thickness of the bilayer and the equivalent conductivity of the ions, it is possible to calculate the channel diameter. In our case, in OxCh membranes (average thickness 4 nm) (Tien, 1974) and at applied voltages of −20 and 60 mV, the channel diameter was ∼27.6 and 15.0 Å, respectively. By applying the same calculation in DOPS:DOPE membranes, with an average thickness of 5 nm (Tien and Ottowa, 2001) and an effective voltage (= applied voltage plus surface voltage) of −60 mV, the channel diameter was ∼26.0 Å. It is worth underlining, however, that the calculation of pore area by means of conductance, assuming the channel to be a water-filled hole, is not a straightforward rule (Smart et al., 1997). Considering the experimental error, these results seem to be not far from the values found in the theoretical calculation of a channel formed by six and four units, respectively, by Durell et al. (1994).
All these results support the claim that the membrane structure is responsible for different biophysical properties of the AβP (1–40) channel. This could be of a certain importance during the turnover of the plasma membranes during the life cycle.
In fact, during the aging process, when the homogeneous distribution and the presence of oxidation products of cholesterol occurs, it can be speculated that the preeminent hydrophobic interaction increases the number of channels, with ion selectivity toward anions that will hyperpolarize the membrane, thus moving the membrane potential far from the threshold of membrane excitability. In this condition, the contribution to calcium conductance is presumed to be very small. This is important because it has been hypothesized that the increased Ca2+ permeability through AβP (1–40) channels determines degeneration of neurons (Arispe et al., 1993). In particular, by means of atomic force microscopy in cultured endothelial cells, it has been observed that AβP induces morphological changes, attributed to a disorder of Ca2+ homeostasis (Hartmann et al., 1994).
Our results seem to be in line with the observation that cholesterol protects PC12 cells from AβP toxicity and inhibits the effect of AβP on cellular Ca2+ signals (Zhou and Richardson, 1996; Zhu et al., 2000). It has been reported that AβP (1–42), the neurotoxic peptide, causes long-lasting depolarization in human NHT neuronal cells (Blanchard et al., 2002a,b), through activation of type-I metabotropic glutamate receptors. The AβP(1–42)-induced hyperpolarization of these membranes has been found to antagonize membrane depolarization.
As mentioned above, the unifying motif characterizing fibrillating peptides such as AβP (1–40) and human calcitonin seems to be the content of β-sheet conformations, and the presence of Lys and Phe amino acids in the central region of the peptide molecule (Kim and Lee, 2003). With their electrostatic and hydrophobic interaction, these peptides could undergo fibrillation both in solution and in membranes containing POPC; by contrast, the addition of components that increase α-helix formation by favoring insertion into membranes is likely to be a protective mechanism against fibrillation, because the balance shifts toward clearance.
The results obtained in this study lead to the conclusion that by avidly incorporating the AβP (1–40) peptide, cholesterol may inhibit its tendency to fibrillate, thus reducing its cytotoxic effect.
In conclusion, considering the numerous genetical, biochemical, physiological, and environmental factors involved in the epigenetic and homeostatic processes that control AD, the path that research and clinical experimentation can undertake are multiple, but the correlation with some of the most ubiquitous compounds in the membrane (i.e., sterols) may be one of the most promising in the control of AβP clearance.
Acknowledgments
This work was supported by a grant from the Regione Puglia (No. 3603) Italy.
References
- Arispe, N., H. B. Pollard, and E. Rojas. 1993. Alzheimer disease amyloid β-protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc. Natl. Acad. Sci. USA. 90:567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe, N., H. B. Pollard, and E. Rojas. 1996. Zn2+ interaction with Alzheimer amyloid beta protein calcium channels. Proc. Natl. Acad. Sci. USA. 93:1700–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard, B. J., V. L. Thomas, and V. M. Ingram. 2002a. Mechanism of membrane depolarization caused by the Alzheimer Aβ1–42 peptide. 293:1197–1203. [DOI] [PubMed]
- Blanchard, B. J., B. R. Stockwell, and V. M. Ingram. 2002b. Eliminating membrane depolarization caused by the Alzheimer peptide Aβ(1–42, aggr.). Biochem. Biophys. Res. Commun. 293:1204–1208. [DOI] [PubMed] [Google Scholar]
- Brown, D. A., and E. London. 1998. Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol. 164:103–114. [DOI] [PubMed] [Google Scholar]
- Choo-Smith, L., W. Garzon-Rodriguez, C. G. Glabe, and W. K. Surewicz. 1997. Acceleration of amyloid fibril formation by specific binding of Aβ-(1–40) peptide to ganglioside-containing membrane vesicles. J. Biol. Chem. 27:22987–22990. [DOI] [PubMed] [Google Scholar]
- Christie, R. H., H. Chung, G. W. Rebeck, D. K. Strickland, and B. T. Hyman. 1996. Expression of the very low-density lipoprotein receptor (VLDL-r), an apolipoprotein-E receptor, in the central nervous system and in Alzheimer's disease. J. Neuropathol. Exp. Neurol. 53:491–498. [DOI] [PubMed] [Google Scholar]
- Curtain, C. C., F. E. Ali, D. G. Smith, A. J. Bush, C. L. Masters, and K. J. Barnham. 2003. Metal ions, pH, and cholesterol regulate the interactions of Alzheimer's disease amyloid-β peptide with membrane lipid. J. Biol. Chem. 278:2977–2982. [DOI] [PubMed] [Google Scholar]
- Durell, S. R., H. R. Guy, N. Arispe, E. Rojas, and H. B. Pollard. 1994. Theoretical models of the ion channel structure of amyloid β-protein. Biophys. J. 67:2137–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag, H., W. Neupert, and R. Benz. 1982. Biosynthesis of mitochondrial porin and insertion into the outer mitochondrial membrane of Neurospora crassa. Eur. J. Biochem. 123:629–636. [DOI] [PubMed] [Google Scholar]
- Galbete, J. L., T. R. Martin, E. Peressini, P. Modena, R. Bianchi, and G. Forloni. 2000. Cholesterol decreases secretion of the secreted form of amyloid precursor protein by interfering with glycosylation in the protein secretory pathway. Biochem. J. 348:307–313. [PMC free article] [PubMed] [Google Scholar]
- Gallucci, E., D. Meleleo, S. Micelli, and V. Picciarelli. 2003. Magainin 2 channel formation in planar lipid membranes: the role of lipid polar groups and ergosterol. Eur. Biophys. J. 32:22–32. [DOI] [PubMed] [Google Scholar]
- Gatfield, J., and J. Pieters. 2000. Essential role for cholesterol in entry of mycobacteria into macrophages. Science. 288:1647–1650. [DOI] [PubMed] [Google Scholar]
- Hardy, J. A., and G. A. Higgins. 1992. Alzheimer's disease: the amyloid cascade hypothesis. Science. 256:184–185. [DOI] [PubMed] [Google Scholar]
- Hartmann, H., A. Eckert, and W. E. Muller. 1994. Apolipoprotein E and cholesterol affect neuronal calcium signalling: the possible relationship to β-amyloid neurotoxicity. Biochem. Biophys. Res. Commun. 200:1185–1192. [DOI] [PubMed] [Google Scholar]
- Howland, D. S., S. P. Trusko, M. J. Savage, A. G. Reaumem, D. M. Lang, J. D. Hirsch, N. Maeda, R. Siman, B. D. Greenberg, R. W. Scott, and D. G. Flood. 1998. Modulation of secreted β-amyloid precursor protein and amyloid β-peptide in brain by cholesterol. J. Biol. Chem. 273:16576–16582. [DOI] [PubMed] [Google Scholar]
- Ji, S. R., Y. Wu, and S. F. Sui. 2002. Cholesterol is an important factor affecting the membrane insertion of beta-amyloid peptide (Aβ 1–40), which may potentially inhibit the fibril formation. J. Biol. Chem. 277:6273–6279. [DOI] [PubMed] [Google Scholar]
- Kim, J. E., and M. Lee. 2003. Fullerene inhibits β-amyloid peptide aggregation. Biochem. Biophys. Res. Commun. 303:576–579. [DOI] [PubMed] [Google Scholar]
- Kourie, J. I., C. L. Henry, and P. Farrelly. 2001. Diversity of amyloid-β protein fragment [1–40]-formed channels. Cell. Mol. Neurobiol. 21:255–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, H., Y. J. Zhu, and R. Lal. 1999. Amyloid beta protein (1–40) forms calcium-permeable Zn2+-sensitive channels in reconstituted lipid vesicles. Biochemistry. 38:11189–11196. [DOI] [PubMed] [Google Scholar]
- Liu, P., P. Wang, P. Michaely, M. Zhu, and R. G. Anderson. 2000. Presence of oxidized cholesterol in caveolae uncouples active platelet-derived growth factor receptors from tyrosine kinase substrates. J. Biol. Chem. 275:31648–31654. [DOI] [PubMed] [Google Scholar]
- MacDougall, J. D., S. Biswas, and R. P. Cook. 1965. The effects of certain C27 steroids on organ cultures of rabbit aorta. Br. J. Exp. Pathol. 46:549–553. [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, S. 1977. Electrostatic potential at membrane-solution interfaces. Curr. Top. Membr. Transport. 9:71–143. [Google Scholar]
- Micelli, S., E. Gallucci, D. Meleleo, V. Stipani, and V. Picciarelli. 2002. Mitochondrial porin incorporation into black lipid membranes: ionic and gating contribution to the total current. Bioelectrochemistry. 57:97–106. [DOI] [PubMed] [Google Scholar]
- Popp, B., A. Schmid, and R. Benz. 1995. Role of sterols in the functional reconstitution of water-soluble mitochondrial porins from different organisms. Biochemistry. 34:3352–3361. [DOI] [PubMed] [Google Scholar]
- Roper, K., D. Corbeil, and W. B. Huttner. 2000. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat. Cell Biol. 2:582–592. [DOI] [PubMed] [Google Scholar]
- Sanghera, N., and T. J. Pinheiro. 2002. Binding of prion protein to lipid membranes and implications for prion conversion. J. Mol. Biol. 315:1241–1256. [DOI] [PubMed] [Google Scholar]
- Shinitzky, M. 1993. Biomembranes: Structural and Functional Aspects. M. Shinitzky, editor. John Wiley & Sons, New York. 1–82.
- Smart, O. S., J. Breed, G. R. Smith, and M. S. Sansom. 1997. A novel method for structure-based prediction of ion channel conductance properties. Biophys. J. 72:1109–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, L., M. R. Hobaugh, C. Shustak, S. Cheley, H. Bayley, and J. E. Gouaux. 1996. Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science. 274:1859–1865. [DOI] [PubMed] [Google Scholar]
- Stipani, V., E. Gallucci, S. Micelli, V. Picciarelli, and R. Benz. 2001. Channel formation by salmon and human calcitonin in black lipid membranes. Biophys. J. 81:3332–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi, R. E., J. F. Gusella, P. C. Watkins, A. P. Bruns, P. St.George-Hyslop, M. L. Van Keuren, D. Patterson, S. Pagan, D. M. Kurnit, and L. Neve. 1987. Amyloid-β protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 235:880–884. [DOI] [PubMed] [Google Scholar]
- Thiele, C., M. J. Hannah, F. Fahrenholz, and W. B. Huttner. 2000. Cholesterol binds to synaptophysin and is required for biogenesis of synaptic vesicles. Nat. Cell Biol. 2:42–49. [DOI] [PubMed] [Google Scholar]
- Tien, T. H., and A. Ottowa. 2001. The lipid bilayer concept and its experimental realization: from soap bubbles, kitchen sink, to bilayer lipid membranes. J. Membr. Sci. 189:83–117. [Google Scholar]
- Tien, T. H. 1974. Bilayer Lipid Membranes (BLM): Theory and Practice. Marcel Dekker, New York.
- Tien, T. H., S. Carbone, and E. A. Dawidowicz. 1966. Procedure for preparation of oxidized cholesterol membrane solution. Nature. 212:718–719. [Google Scholar]
- Weiner, N. D., P. Noomnont, and A. Felmeister. 1972. Autoxidation of cholesterol in aqueous dispersions and in monomolecular films. J. Lipid Res. 13:253–255. [PubMed] [Google Scholar]
- Yeagle, P. L. 1985. Cholesterol and the cell membrane. Biochim. Biophys. Acta. 822:267–287. [DOI] [PubMed] [Google Scholar]
- Zhou, Y., and J. S. Richardson. 1996. Cholesterol protects PC12 cells from β-amyloid induced calcium disordering and cytotoxicity. Neuroreport. 7:2487–2490. [DOI] [PubMed] [Google Scholar]
- Zhu, Y. J., H. Lin, and R. Lal. 2000. Fresh and nonfibrillar amyloid-β protein(1–40) induces rapid cellular degeneration in aged human fibroblasts: evidence for AβP-channel-mediated cellular toxicity. FASEB. 14:1244–1254. [DOI] [PubMed] [Google Scholar]