Abstract
Elevated concentrations of intracellular calcium in erythrocytes increase membrane order and susceptibility to secretory phospholipase A2. We hypothesize that calcium aids the formation of domains of ordered lipids within erythrocyte membranes by interacting directly with the inner leaflet of the cell membrane. The interface of these domains with regions of more fluid lipids may create an environment with weakened neighbor-neighbor interactions that would facilitate phospholipid migration into the active site of bound secretory phospholipase A2. This hypothesis was investigated by determining the effects of seven other divalent ions on erythrocyte membrane properties. Changes in membrane order were assessed with steady-state fluorescence spectroscopy and two-photon microscopy with an environment-sensitive probe, laurdan. Each ion increased apparent membrane order in model membranes and in erythrocytes when introduced with an ionophore, suggesting that direct binding to the inner face of the membrane accounts for the effects of calcium on membrane fluidity. Furthermore, the degree to which ions affected membrane properties correlated with the ionic radius and electronegativity of the ions. Lastly, erythrocytes became more susceptible to enzyme hydrolysis in the presence of elevated intracellular levels of nickel and manganese, but not magnesium. These differences appeared related to the ability of the ions to induce a transition in erythrocyte shape.
INTRODUCTION
The physiological and pathological importance of secretory phospholipase A2 (sPLA2) involves, in part, its ability to hydrolyze susceptible cell membranes. One potential function of the enzyme is to act extracellularly to destroy the membranes of apoptotic and necrotic cells (Atsumi et al., 1997; Nielson et al., 2000). In addition, it is suspected that sPLA2 aids in host defense against bacteria, implicated by its presence in tears and its ability to hydrolyze the membranes of gram-positive bacteria (Beers et al., 2002).
Not all cell membranes are susceptible to hydrolysis by sPLA2. Healthy cells resist attack by the enzyme; however, phospholipids in apoptotic and diseased or damaged cells are vulnerable (Atsumi et al., 1997; Judd et al., 2003; Nielson et al., 2000; Wilson et al., 1999). Apparently, this increased level of hydrolysis is a consequence of differences in membrane physical properties between healthy and damaged cells (Smith et al., 2001; Wilson et al., 1999). Commonly, high levels of intracellular calcium introduced by the cellular damage appear responsible for these differences. Properties causing enhanced susceptibility to sPLA2 have been investigated most thoroughly using erythrocytes as a simple model of biological membranes. Candidates include changes in cell shape, exposure of phosphatidylserine on the outer leaflet of the cell membrane, and alterations in apparent membrane fluidity (Harris et al., 2001; Smith et al., 2001). Among these, an increase in membrane phospholipid order appears to be of primary importance in enhancing the ability of sPLA2 to hydrolyze erythrocyte phospholipids (Best et al., 2002; Harris et al., 2001; Smith et al., 2001).
Two-photon microscopy imaging techniques have shown that this observed alteration in membrane order actually represents an increase in the heterogeneity of membrane properties. Specifically, domains of differential fluidity proliferate in the membrane (Smith et al., 2001). This observation has been reinforced by temperature studies suggesting that membrane order per se is not responsible for increased cell susceptibility to sPLA2; rather, it seems that the formation of less fluid phospholipid domains within a sea of more fluid lipids increases the action of sPLA2 toward the cells (Best et al., 2002).
Although some attention has been focused on how changes in membrane fluidity might enhance the susceptibility of the membrane to sPLA2, no investigations have yet explored how a sustained elevation in intracellular calcium causes such changes. The focus of this project, then, is to investigate possible mechanisms linking changes in membrane fluidity to increased concentration of intracellular calcium. Previous studies with artificial membranes have shown that divalent cations can alter bilayer phase properties through direct interactions (Binder et al., 2001; Binder and Zschornig, 2002; Hauser, 1991). We hypothesize that the same applies to erythrocyte membranes and that calcium therefore alters membrane fluidity by direct interaction between the cation and the inner leaflet of the cell membrane. If this hypothesis is correct, many divalent ions should produce a change in membrane order in erythrocytes similar to the increase in membrane order induced by calcium. An alternative hypothesis is that calcium acts by interacting with calcium-binding proteins within the membrane of the cell that in turn alter the membrane either directly or through a chain of biochemical events. Should this alternative hypothesis be correct, we would expect specificity for calcium; few other ions would produce membrane phospholipid order changes since most divalent cations do not activate calcium-binding proteins.
These hypotheses were investigated by measuring the effects of seven other divalent cations on the membranes of intact erythrocytes. Multilamellar vesicles (MLV) composed of dipalmitoylphosphatidylcholine (DPPC) and dipalmitoylphosphatidylglycerol (DPPG) were used to verify that the divalent ions interacted with membranes and caused the same effects as calcium. Ions were introduced into intact erythrocytes using an ionophore (ionomycin), and changes in membrane properties were assessed using steady-state fluorescence spectroscopy and two-photon microscopy with the environment-sensitive fluorescent probe laurdan. Finally, we explored the relevance of the results to membrane hydrolysis by sPLA2.
MATERIALS AND METHODS
Materials
MLV were prepared from stock solutions of DPPC and DPPG obtained from Avanti Polar Lipids (Alabaster, AL). DPPC and DPPG, dissolved in chloroform, were mixed at a ratio of 91:9 mol % and dried under a stream of nitrogen gas. Sufficient low-calcium, low-magnesium cell medium (LCCM; NaCl = 134 mM, KCl = 6.2 mM, Hepes = 18.0 mM, and glucose = 13.6 mM, pH 7.4, 37°C, suspended in deionized water) was added to the dried lipids to bring the lipid concentration to 10 mM. The suspension was converted to MLV by heating and vigorous mixing using standard procedures (Harris et al., 2002).
Human erythrocytes were obtained from healthy individuals at the Brigham Young University Health Center. Blood samples were stored in EDTA vacutainers overnight at 4°C. Control experiments comparing fresh blood with samples stored overnight demonstrated that storage conditions did not influence the results (Smith et al., 2001). Erythrocytes were isolated by centrifugation, washed, and suspended to original hematocrit in LCCM.
Acrylodan-labeled fatty acid binding protein (ADIFAB) and laurdan were obtained from Molecular Probes (Eugene, OR). Ionomycin was purchased from Calbiochem (La Jolla, CA). All other reagents were from standard sources. Snake venom sPLA2 (monomeric aspartate 49 from Agkistrodon piscivorus piscivorus) was isolated according to published procedures (Maraganore et al., 1984). ADIFAB (200 μg/ml) and sPLA2 (100 μg/ml) were dissolved in 50 mM KCl with 3 mM NaN3 as a preservative and stored at 4°C. Laurdan (250 μM) and ionomycin (120 μM) were dissolved in dimethylsulfoxide (DMSO). BaCl2, CdCl2, CaCl2, CoCl2, MgCl2, MnCl2, NiSO4, Sr(NO3)2, (Na)2SO4, and NaNO3 were dissolved at 1.0 M stock concentrations in deionized water. Control experiments verified that addition of these ions to LCCM at the concentrations used in this study (up to 2 mM) did not alter the pH by more than 0.01 units.
Fluorescence spectroscopy
Measurements with fluorescent probes were acquired with a Fluoromax (Jobin Yvon, Edison, NJ) photon-counting spectrofluorometer. Gentle continuous stirring with a magnetic stir bar maintained sample homogeneity. Temperature was held constant at 37°C. Fluorescence intensity was measured at multiple excitation and emission wavelengths, obtained by rapid sluing of monochromator mirrors using control software provided with the spectrofluorometer. Bandpass was set at 4.25 nm for all experiments. Samples were suspended in LCCM (2 ml final volume) in 1 cm quartz sample cells and equilibrated in the spectrofluorometer sample chambers for at least 5 min to achieve temperature stability. The final concentration of phospholipids as MLV was 100 μM; erythrocytes were suspended at a final density of 3–4 × 106 cells/ml.
Laurdan generalized polarization
Membrane order was evaluated using laurdan, a fluorescent probe sensitive to the presence and mobility of water molecules trapped within the lipid bilayer (Parasassi et al., 1991, 1993, 1997). Laurdan (2.5 μM final concentration) was added directly to samples of MLV or erythrocytes prepared for fluorescence experiments as described above. Fluorescence emission was then monitored as a function of time at dual wavelengths (excitation = 350 nm, emission = 435 and 500 nM) for a minimum of 10 min after the addition of laurdan to allow sufficient time for the probe to incorporate into the membrane. Light scatter was evaluated from baseline emission intensity before addition of laurdan in both model systems and determined to contribute insignificantly to the fluorescence intensity at either wavelength. After incubation with laurdan, the desired ionic solution or an equivalent volume of LCCM (to account for dilution artifacts) was added to either the MLV or erythrocyte solution at appropriate concentrations.
For experiments with MLV, various doses of each ion were added sequentially to the same sample. Fluorescence was measured continuously after each addition, and the next dose was not added until the signal was stable (∼5 min). This pattern was continued until the final concentration of ion in the MLV sample solution was equal to 10.0 mM. Fresh samples of MLV were used for each ion and for multiple replicates of each experiment.
With erythrocytes, addition of the first (and only) dose of ion was followed by 10 min incubation, addition of 0.3 μM ionomycin (final concentration) or control vehicle (DMSO), and 10 min further incubation. Laurdan fluorescence was monitored continuously throughout the experiment. Each ion concentration represents a new sample of erythrocytes, and each replicate represents blood from a different donor. Some experiments were repeated in the absence of molecular oxygen. In this case, LCCM was degassed under vacuum before use. Erythrocyte samples were further bubbled with nitrogen for 45 min, and experiments were conducted under a nitrogen atmosphere at ambient pressure.
Experiments with erythrocytes were repeated with either sodium sulfate or sodium nitrate replacing the divalent ion at the same concentration. These controls verified that results were not due to the anion in the salt (data not shown). Likewise, control experiments were conducted with ionomycin added in the absence of divalent cation to identify any contributions to laurdan fluorescence caused directly by the ionophore. Results were quantified by calculating the generalized polarization (GP) as described by Parasassi et al. (1991). The value of GP obtained from the ionophore controls was subtracted from the data to isolate the effect of introduction of the cations into the cells. In some experiments, a linear positive baseline in the value of laurdan GP was observed. This baseline has also been seen commonly in previous studies in our lab. It appeared to have no effect on the responses measured, and it was therefore subtracted from the data when it occurred (see legend to Fig. 2).
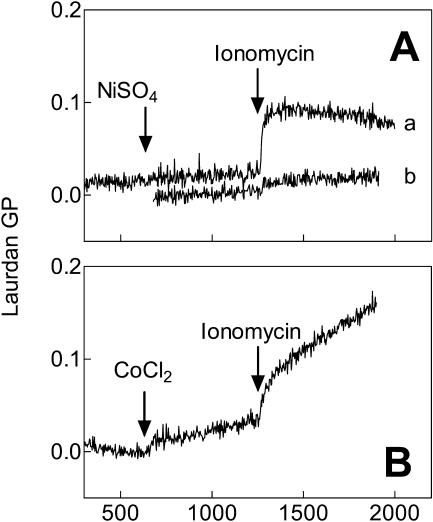
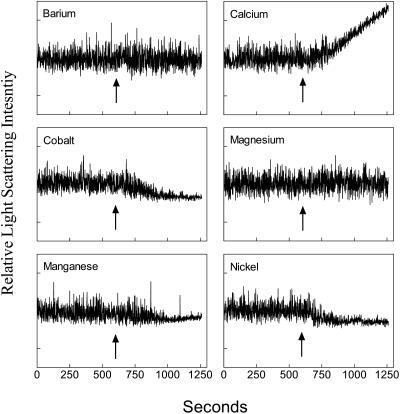
FIGURE 2.
Time course of the effects of nickel (panel A) or cobalt (panel B) on laurdan GP in human erythrocytes before and after addition of ionomycin. Arrows indicate the time of addition of NiSO4 (curve a, 0.6 mM) or CoCl2 (0.5 mM) and ionomycin (also added in the absence of divalent cation as a control in curve b). A linear baseline with a slope of 5.4 × 10−5 GP units s−1 was subtracted from curve a (see Materials and Methods). Figure shows data collected from a single sample. Comparable responses were observed in at least five replicate experiments in each case as summarized in Fig. 3.
Membrane susceptibility to sPLA2
Susceptibility of erythrocytes to sPLA2 was assayed using ADIFAB to monitor the release of free fatty acid after the addition of sPLA2 (Wilson et al., 1997). Erythrocyte samples were prepared for fluorescence spectroscopy as described above. A low concentration of CaCl2 was added to the LCCM (final concentration, 50 μM). This inclusion of a small concentration of calcium was necessary since calcium is an obligatory cofactor for sPLA2 function (Jain et al., 1982). As explained in the results, this amount of calcium caused minimal changes to membrane susceptibility upon addition of ionomycin. Fluorescence emission was monitored throughout the time course at dual wavelengths (excitation = 390 nm, emission = 432 and 505 nM) (Richieri and Kleinfeld, 1995). ADIFAB (1 μg/ml), the test ion, and ionomycin (or control vehicle, DMSO) were added sequentially during data acquisition with a 5-min equilibration period between each addition. Samples were then equilibrated 10 min after ionomycin followed by addition of sPLA2 (final concentration, 3.3 μg/ml or 10 μg/ml). Hydrolysis was quantified by calculating the GP from the data as explained previously (Smith et al., 2001).
Rayleigh light scattering
Light scattering was assessed in samples of erythrocytes prepared and treated as for fluorescence spectroscopy. In this case, no fluorescent probes were included in the experiments, and monochromator settings were 500 nm excitation and 510 nm emission.
Two-photon microscopy
Two-photon excitation images were collected on an Axiovert 35 inverted microscope (Zeiss, Thornwood, NY), with a Zeiss 20X LD-Achroplan (0.4 N.A., air) using a titanium-sapphire laser excitation source (Coherent, Palo Alto, CA) tuned to 770 nM and pumped by a frequency-doubled Nd:vanadate laser (Coherent, Palo Alto, CA) as described previously (Parasassi et al., 1997). Dual images were collected simultaneously with a beam-splitter, interference filters (Ealing 490 and Ealing 440), and two detectors for calculation of GP (Parasassi et al., 1997).
Washed erythrocytes were suspended in 2 ml LCCM in a temperature-controlled microscopy sample dish (Bioptechs, Butler, PA) at a final concentration of ∼2 × 106 cells per ml and warmed to 37°C. Laurdan (0.25 μM final concentration) was added, and the solution incubated for a minimum of 10 min before acquisition of the first image. The test ion was added at the indicated concentration and a second image of the same field was obtained. Ionomycin (0.3 μM final concentration) was added after an additional incubation of at least 5 min. Images were then acquired over several minutes after mixing of the cells with ionomycin.
RESULTS
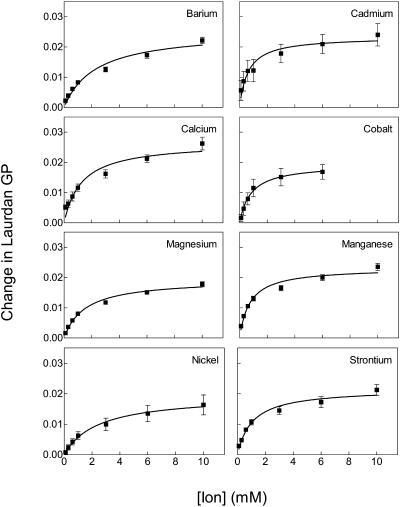
Fig. 1 illustrates the change in laurdan GP as increasing concentrations of BaCl2, CdCl2, CaCl2, CoCl2, MgCl2, MnCl2, NiSO4, or Sr(NO3)2 were added to MLV labeled with laurdan. An increase in the value of GP indicates a reduction in interactions between laurdan and water molecules (Parasassi et al., 1991). In phospholipid bilayers, this effect has generally been interpreted to represent an ordering of the lipids in the region of the glycerol backbone (Chong and Wong, 1993; Harris et al., 2002; Parasassi et al., 1991). Throughout this article, the terms “order” and “fluidity” refer specifically to these changes in solvent interactions with laurdan. The maximum change in laurdan GP was similar for each ion, indicating that all bound to the membrane. In contrast, differences were observed in the apparent potency of the various ions. Nevertheless, these differences were minor since the apparent dissociation constant values all resided within the relatively narrow range of 0.8–3.2 mM.
FIGURE 1.
Divalent ions bind to artificial bilayers and increase membrane order (increase laurdan GP). The figure shows changes in GP of MLV labeled with laurdan after fluorescence had reached steady state after addition of increasing doses of BaCl2, CdCl2, CaCl2, CoCl2, MgCl2, MnCl2, NiSO4, and Sr(NO3)2. GP was calculated from the intensity of light at 435 and 500 nm as described in Materials and Methods. Curves were obtained by nonlinear regression with the following equation: Y = (A × X)/(B + X). Error bars represent mean ± SE (n = 5).
Having established that these seven divalent ions bind to phospholipid bilayers and cause changes similar to calcium, we examined their effects on the outer and inner surfaces of erythrocyte membranes. Fig. 2 A shows the effect of nickel on intact erythrocytes labeled with laurdan. The ion had no effect on laurdan GP when added directly to the sample. This result argued either that the ion did not bind to the outer leaflet of the membrane or that any binding did not alter membrane properties detected by laurdan. However, upon addition of ionomycin, the value of laurdan GP immediately rose, suggesting that the membrane lipids had become more ordered by entrance of nickel into the cell. The response to ionomycin alone in the absence of divalent cation is shown in curve b. This small effect of the ionophore directly on laurdan fluorescence has been observed previously (Best et al., 2002; Smith et al., 2001) and was subtracted from summaries of the data (i.e., as in Fig. 3).
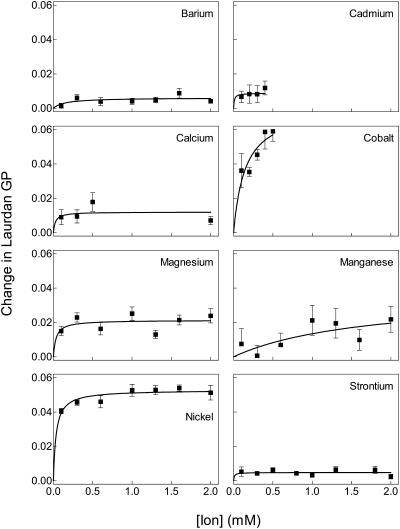
FIGURE 3.
Divalent ions increase laurdan GP in erythrocyte membranes when the ion enters the cell. Figure illustrates changes in laurdan GP of erythrocytes upon addition of ionomycin in the presence of the indicated concentrations of BaCl2, CdCl2, CaCl2, CoCl2, MgCl2, MnCl2, NiSO4, or Sr(NO3)2 obtained as shown in Fig. 2. The small response observed in the presence of ionomycin alone (no divalent ions, see example in Fig. 2) was quantified and subtracted from the data before analysis. Data were then fit by nonlinear regression as explained in Fig. 1. Error bars represent mean ± SE (n = 4−8).
Cobalt (Fig. 2 B), in contrast, appeared to increase membrane order without entering the cell since it raised the laurdan GP value in the absence of ionophore. Nevertheless, the laurdan GP was further increased upon the addition of ionomycin. Cobalt was the only ion that had an obvious effect on membrane order before entering the cell, though fluorescence microscopy images described below suggested that other ions might cause small changes in membrane order by binding to the outer leaflet of the cell membrane as well.
Fig. 3 depicts the steady-state fluorescence GP after addition of various concentrations of ion and ionomycin to intact erythrocytes. Erythrocyte samples from four to eight people were tested to obtain average GP values at each concentration of ion. Each ion increased membrane order in the erythrocyte samples, though the degree of GP changes, as well as potency of the change, varied among the ions. Barium and strontium had relatively little effect on erythrocyte membranes whereas cobalt and nickel induced the largest changes. Cadmium and cobalt were not tested at higher concentrations of ion because of limited solubility in erythrocytes (cadmium forms a precipitate with sulfur-containing proteins) and optical interference (cobalt absorbs blue/green light).
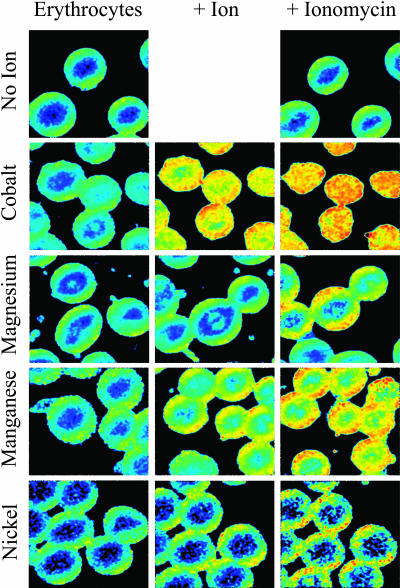
Fig. 4 visually confirms that the addition of divalent ion and ionomycin increased the value of laurdan GP in erythrocyte membranes. These images, obtained by two-photon scanning microscopy, indicated that changes in GP represented expansion of domains of ordered lipids. Consistent with the result shown in Fig. 2 B, cobalt had a prominent effect on membrane order before the addition of ionomycin. Furthermore, some ions apparently altered the shape of the erythrocytes.
FIGURE 4.
Two-photon microscopy images of the distribution of laurdan GP in the presence or absence of a divalent ion or ionomycin. Columns depict the treatments. (Erythrocytes) Only laurdan and red blood cells have been added to the sample dish. (+ Ion) The ion indicated on the left has been added to the sample (2 mM final concentration) and allowed to equilibrate with the cells. (+ Ionomycin) Image obtained 10 min after ionomycin addition. Data were quantified by calculating the laurdan GP of each image. False color indicates the GP values, with blue being the lowest and red the highest (most ordered).
As reported previously, changes in erythrocyte shape can be detected in a bulk sample by monitoring light scattering from a stirred suspension (Hoffman, 1987; Smith et al., 2001). The normal disc shape of erythrocytes causes them to form aggregates (rouleaux) which scatter light irregularly leading to a large amount of noise in the signal. Upon addition of ionomycin in the presence of calcium, the noise diminishes as the cells become round (spherocytes) and disperse from the rouleaux. Calcium influx also causes a shedding of microvesicles from erythrocytes, which elevates the intensity of light scattering due to the increase in the number of particles (Smith et al., 2001). Fig. 5 demonstrates that calcium, cobalt, nickel, and manganese caused changes in erythrocyte shape based on the decrease in light scatter noise upon mixing with ionomycin. No response was observed with the other ions (cadmium and strontium not shown, but identical to barium and magnesium). Interestingly, calcium was the only ion to also cause a release of microvesicles (i.e., the rise in light scatter intensity, Fig. 5).
FIGURE 5.
Rayleigh light scattering by erythrocytes treated with divalent cations and ionomycin. Samples contained 0.6 mM BaCl2, CaCl2, CoCl2, MgCl2, MnCl2, or NiSO4. Ionomycin was added at the arrow.
To determine whether the changes in membrane lipid order induced by the various ions also increased erythrocyte susceptibility to sPLA2, intact erythrocytes were treated with nickel, magnesium, or manganese in the presence or absence of ionomycin. A low dose of calcium (50 μM compared to the usual 1.6 mM) was also included in the experiments to function as a cofactor for sPLA2 activity. The data of Fig. 3 indicated that this dose would be low enough to not contribute to changes in membrane order initiated by the test ion. The other divalent cations tested in Fig. 3 were not included in this set of experiments because previous studies (Dam-Mieras et al., 1975; Yu et al., 1993, 1998) or control experiments with sPLA2 and artificial substrate demonstrated that they inhibited the enzyme directly.
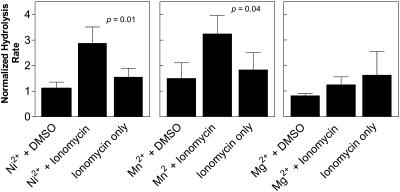
Fig. 6 shows the maximum rate of hydrolysis under various treatment conditions calculated from first-order derivatives of the original hydrolysis time course data. The rate was normalized to the control hydrolysis rate obtained when the cells were exposed to sPLA2 without nickel, magnesium, or manganese and without ionomycin. The purpose of this normalization was to account for variation among blood donors. Addition of nickel, magnesium, or manganese to erythrocytes in the absence of ionomycin did not significantly increase the rate or the amount of membrane hydrolysis by sPLA2. When ionomycin was included, however, the rate of catalysis by sPLA2 was elevated in the presence of nickel and manganese, but not in the presence of magnesium.
FIGURE 6.
Effect of elevated intracellular levels of nickel, manganese, or magnesium on the susceptibility of erythrocytes to hydrolysis by sPLA2. The maximum rate of hydrolysis was quantified from reaction time courses in the presence or absence of 0.3 mM NiSO4, MnCl2, or MgCl2 with or without ionomycin as explained in Materials and Methods. Sample replicates indicate separate blood donors. Rates for each condition were normalized to the corresponding control (no divalent cation or ionomycin) from the same blood sample. Statistical significance was determined using a one sample t-test comparing each condition to a null hypothetical value of 1.0. Error bars represent mean ± SE (n = 3−13).
DISCUSSION
Previous experiments indicated that calcium accumulation in the cytoplasm of erythrocytes induces alterations of membrane structure (Smith et al., 2001). Among these alterations, the most important for augmented susceptibility to hydrolysis by sPLA2 appears to be an increase in the number of boundaries between regions of ordered and disordered lipids (Best et al., 2002; Smith et al., 2001). There have been considerable efforts made to understand other effects of calcium on erythrocyte membranes such as the transition in cell shape from the normal biconcave disk to the rounded spherocyte, phospholipid flip-flop, and microvesicle shedding (e.g., Comfurius et al., 1990; Dekkers et al., 2002; Hoffman, 1987; Middelkoop et al., 1989; Schroit and Zwaal, 1991; Zhou et al., 1997; Allan and Thomas, 1981; Bucki et al., 1998; Gedde et al., 1997; Gimsa, 1998; Mohandas and Chasis, 1993; Muller et al., 1981; Pasquet et al., 1998; Sheetz and Singer, 1977; Shukla et al., 1978; Williamson et al., 1992; Xu et al., 1991). However, attempts to explain the changes in membrane fluidity that accompany these responses have been minimal. The only information previously available indicated that the increase in membrane order detected by laurdan does not require microvesicle release or phospholipid flip-flop (Smith et al., 2001). Accordingly, we have addressed this void by testing the hypothesis that the alteration in membrane fluidity is induced by direct interaction of calcium with the inner leaflet of the cell membrane rather than by an action on specific calcium-binding proteins. If this hypothesis is correct, a broad range of divalent ions would produce changes similar to calcium on erythrocyte membranes upon introduction into the cells.
An important first step to meet the objective of this study was to identify a set of divalent cations that adsorb to membranes and cause physical changes similar to calcium when they bind. To allow simple interpretation of the data, the experimental model we selected consisted of artificial bilayers that were homogeneous with respect to phase properties and contained a small amount of negative charge to promote ion binding (91:9 mixture of DPPC and DPPG). Based on the results of previous investigations, it was reasonable to expect that the ions would all bind to these membranes (e.g., Garidel et al., 2000). These bilayers were then labeled with laurdan, and fluorescent measurements were used to verify that each of these ions was able to interact with the membranes in a manner reminiscent of calcium (Fig. 2). Experiments were conducted below the phase transition temperature of the vesicles to observe changes in membrane order without the complication of the phase transition. Nevertheless, replication of the experiments at a temperature above the transition (42°C) revealed that binding of the ions could be observed equally well for membranes in either the gel or liquid crystalline phases (data not shown).
The ability of calcium to alter the order of erythrocyte membrane lipids requires that the ion enter the cell (Smith et al., 2001). Experimentally, we have used the ionophore ionomycin to accomplish this purpose. Previous investigations have demonstrated that ionomycin is also effective at transporting barium, cadmium, cobalt, magnesium, manganese, nickel, and strontium, as well as other divalent ions not used in this study (Erdahl et al., 2000). An important issue is whether differences seen in the relative potency of the different divalent ions with erythrocytes (Fig. 3) might reflect differences in affinity of the ions for ionomycin (Erdahl et al., 2000). We took steps to minimize such effects by waiting until steady state had been achieved after addition of ionomycin before calculating the change in laurdan GP (10 min). Furthermore, no correlation was found between the affinities of the ions for ionomycin and the different potencies observed in our study. Nevertheless, to avoid these complications, we have chosen not to focus on the potency of the ions with respect to erythrocytes, but instead to evaluate the maximum effect of each ion on the erythrocyte membrane once changes reached a steady state.
Our results show that each ion increased membrane order in erythrocytes as predicted by our hypothesis (Figs. 3 and 4). In contrast to the results obtained with MLV, the maximum effect of each ion on erythrocytes varied significantly. Nevertheless, differences in the behavior of the ions between artificial and biological membranes are not surprising when one considers the vast distinctions in composition, structure, and complexity between the two.
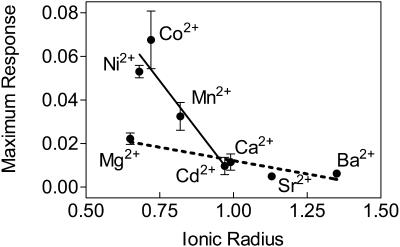
If the membrane alterations caused by calcium and the other ions involve direct interactions with membrane lipids as we have hypothesized, it seems likely that the propensity for each ion to alter the membrane would depend on fundamental ionic properties. Two such properties appear to correlate with the effects of the ions. The first is ionic radius. Fig. 7 displays the relationship between ionic radius and maximum effect of the ions on membrane order. The divalent ions have been categorized as alkaline earth metal ions (calcium, barium, strontium, magnesium) or transition metal ions (cobalt, cadmium, nickel, and manganese). Linear regression revealed a strong relationship between the ionic radius and the effect the ion exerted on the cell membrane when we considered the ions in each grouping separately. The alkaline earth metals are represented by the dashed line in Fig. 7 (r2 = 0.57, p = 0.0002, n = 19); the solid line depicts the linear regression through the transition metal ions (r2 = 0.67, p < 0.0001, n = 18).
FIGURE 7.
Relationships between the ionic radius of each ion and the maximum effect exerted by that ion on erythrocyte membrane order after the addition of ionomycin. Dashed line represents linear regression for the alkaline earth metals (r2 = 0.57, p = 0.0002). Solid line represents linear regression for the transition metals (r2 = 0.67, p < 0.0001). Error bars represent mean ± SE (n = 3−6).
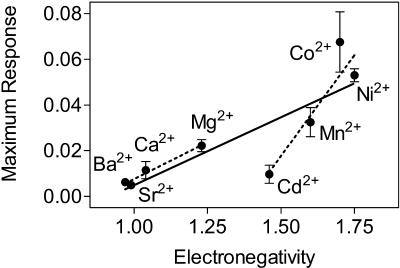
The second property considered was electronegativity (Fig. 8). Linear regression provided strong evidence that electronegativity correlated with the maximum change in membrane order caused by entry of the divalent cation into the cells (r2 = 0.57, p < 0.0001, n = 37). As emphasized by the dashed lines, it appeared that, again, separate relationships exist for the alkaline earth metal ions (r2 = 0.64, p < 0.0001, n = 19) and for the transition metal ions (r2 = 0.66, p < 0.0001, n = 18).
FIGURE 8.
The relationship between the electronegativity of each ion compared to the maximum effect exerted by that ion on erythrocyte membrane order after the addition of ionomycin. Electronegativity values were obtained from Little and Jones (1960). The dashed lines represent linear regression for the alkaline earth metals (r2 = 0.64, p < 0.0001) and transition metal ions (r2 = 0.66, p < 0.0001) considered separately. The solid line represents linear regression through all of the ions without separating them into categories (r2 = 0.57, p < 0.0001). Error bars represent mean ± SE (n = 3−6).
We also analyzed the data by multiple regression including the effects of both ionic radius and electronegativity together in the analysis. In this case, a significant relationship between these two variables and laurdan GP was observed (p = 2 × 10−7). The effect of electronegativity dominated the analysis, suggesting that it is the major contributor (p = 0.0007 vs. 0.16). Nevertheless, these two parameters accounted for only 58% of the variation in the data, indicating that other factors might also be involved. Based on the appearance of the data in Figs. 7 and 8, it seems likely that these other factors relate to differences in behavior between alkaline earth and transition metal ions. Alkaline earth metal ions more readily bind “hard” ligands such as phosphates, carboxylates, and hydroxyl groups (McDaniel and Douglas, 1965). Cobalt(II), nickel(II), cadmium(II), and manganese(II) ions, on the other hand, more readily complex with “soft” ligands such as sulfides and nitrogen donors (McDaniel and Douglas, 1965). In addition, these transition metal ions have unique coordination geometry (i.e., their ability to bind in either tetrahedral or octahedral conformations), incomplete d-electron shells, and a tendency to form coordinate covalent bonds (McDaniel and Douglas, 1965). These properties may also allow the transition metal ions to interact more strongly with erythrocyte membranes than would be explained by their ionic radii or electronegativities.
Interestingly, cobalt can be further differentiated from the other ions tested, as it seemed to have the extreme effect on membrane order, even before entry into the cell. It is possible that the high efficacy of cobalt is due to chemical reduction of surrounding components, converting the ion from cobalt(II) to cobalt(III). This occurs when cobalt(II) is exposed to strong ligand field effects in complexes with good electron pair donors such as nitrogen or sulfur and would thus allow cobalt(III) to be irreversibly bound to various components in the cell membrane, including those that might be found on the outer surface. In addition to molecular oxygen, cobalt(II) complexed by strong field ligands is capable of being oxidized by cellular components such as disulfides and water. We repeated the experiments testing the addition of cobalt on erythrocyte membranes with samples from which molecular oxygen had been removed. No differences in the response to cobalt were observed (i.e., the data, not shown, were identical to Fig. 2 B).
Two-photon scanning electron images confirmed, visually, the changes seen in erythrocytes upon introduction of positively charged ions. Furthermore, the two-photon images indicated that domains of fluid or less-fluid phospholipids are expanded when the ion enters the cell instead of the more uniform changes observed when erythrocytes are simply exposed to lower temperatures (Best et al., 2002). This observation is similar to that reported for calcium entry into erythrocytes (Best et al., 2002).
Another interesting result of the two-photon images is that some ions other than calcium caused the red blood cells to change shape. Light-scattering experiments were conducted to verify the shape changes observed in the two-photon images. Decreases in the fluctuations in the intensity of light scatter (Hoffman, 1987; Smith et al., 2001) indicate that shape change was caused by the entry of calcium, cobalt, nickel, or manganese. This result verified the assertion of previous studies that introduction of certain divalent cations besides calcium (but not magnesium) into erythrocytes cause them to change shape (Sheetz, 1977). In contrast, only calcium caused a release of microvesicles. This observation is not surprising, since microvesicle release is dependent upon calcium-activated proteins such as potassium channels and proteases (Allan and Thomas, 1981; Smith et al., 2001).
Although the calcium-induced erythrocyte shape transition is well documented (Smith et al., 1981), the mechanism by which calcium causes erythrocytes to change shape has not been established. This change is not because of osmotic pressure. The maximum divalent ion concentration was 2 mM, which would contribute, at the most, 6 mOsM to the total osmotic pressure of ∼300 mOsM. Furthermore, if osmotic gradients were involved, all of the ions would have caused the cells to deform.
To further test the hypothesis that the changes in membrane order induced by divalent cation influx are involved in modulating the level of membrane hydrolysis by sPLA2, we examined the effects of uptake of the different ions on the activity of the enzyme toward erythrocytes. Measuring the susceptibility of erythrocytes exposed to ionomycin and divalent ion was complicated by the calcium dependence of sPLA2; calcium functions as a cofactor for sPLA2 activity (Jain et al., 1982). Because of this, it became necessary to determine a concentration of calcium and sPLA2 that would allow for sufficient activation of sPLA2 without also significantly altering membrane order. Additional difficulties arose when divalent ions were added to the erythrocyte solution. Some of the ions directly inhibited sPLA2 activity, presumably by competing with calcium for the calcium-binding site on sPLA2. Nickel, magnesium, and manganese, however, did not directly inhibit sPLA2, and were therefore chosen as our experimental ions to test whether ions other than calcium can increase erythrocyte membrane susceptibility to sPLA2.
Exposure of erythrocytes to calcium at low levels, and to nickel or manganese with ionomycin, amplified the maximum rate of hydrolysis of the erythrocyte membrane by sPLA2. This increase in membrane hydrolysis caused by intracellular nickel and manganese was similar to the enhancement of membrane hydrolysis caused by calcium influx into the cell. Therefore, the result supports the idea that there is a relationship between membrane order and the degree to which that membrane is vulnerable to enzymatic attack (Best et al., 2002; Smith et al., 2001).
Surprisingly, magnesium did not increase cell susceptibility to sPLA2. This led us to consider other relationships beyond membrane order that might be causing erythrocyte susceptibility to sPLA2. One property that distinguishes magnesium from manganese and nickel is that magnesium did not cause an erythrocyte shape transition (Figs. 4 and 5). Although previous investigations argued that the cell shape, per se, is not the determining factor in susceptibility (Harris et al., 2001), it may be that a parallel link between the two phenomena exists. For example, changes in cell membrane properties more subtle than those detected by laurdan may facilitate the shape transition and also be required to allow the bilayer to become susceptible to hydrolysis by sPLA2.
In summary, the results presented in this article address the question of how calcium influx increases erythrocyte membrane order. Specifically, we conclude that calcium alters cell membrane properties by interacting directly with the inner face of the bilayer. These effects on membrane order were not calcium-specific, but instead were achieved with barium, cadmium, cobalt, magnesium, manganese, nickel, and strontium. Thus, any hypothesis requiring specificity for calcium ion is excluded by these findings. Furthermore, the observation that some of these ions also induced a shape transition in erythrocytes and promoted susceptibility of the bilayer to sPLA2 suggests a potential relationship among these events. Clarifying that relationship will be the subject of future investigations.
Acknowledgments
Two-photon scanning microscopy experiments were performed at the Laboratory for Fluorescence Dynamics, Urbana, IL; the authors express gratitude to Drs. Theodore Hazlett and Enrico Gratton for providing technical assistance and access to the facility for these experiments.
This work was supported by a grant from the National Science Foundation (MCB 9904597).
References
- Allan, D., and P. Thomas. 1981. Ca2+-induced biochemical changes in human erythrocytes and their relation to microvesiculation. Biochem. J. 198:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsumi, G., M. Murakami, M. Tajima, S. Shimbara, N. Hara, and I. Kudo. 1997. The perturbed membrane of cells undergoing apoptosis is susceptible to type II secretory phospholipase A2 to liberate arachidonic acid. Biochim. Biophys. Acta. 1349:43–54. [DOI] [PubMed] [Google Scholar]
- Beers, S. A., A. G. Buckland, R. S. Koduri, W. Cho, M. H. Gelb, and D. C. Wilton. 2002. The antibacterial properties of secreted phospholipases A2: a major physiological role for the group IIA enzyme that depends on the very high pI of the enzyme to allow penetration of the bacterial cell wall. J. Biol. Chem. 277:1788–1793. [DOI] [PubMed] [Google Scholar]
- Best, K., A. Ohran, A. Hawes, T. L. Hazlett, E. Gratton, A. M. Judd, and J. D. Bell. 2002. Relationship between erythrocyte membrane phase properties and susceptibility to secretory phospholipase A2. Biochemistry. 41:13982–13988. [DOI] [PubMed] [Google Scholar]
- Binder, H., K. Arnold, A. S. Ulrich, and O. Zschornig. 2001. Interaction of Zn2+ with phospholipid membranes. Biophys. Chem. 90:57–74. [DOI] [PubMed] [Google Scholar]
- Binder, H., and O. Zschornig. 2002. The effect of metal cations on the phase behavior and hydration characteristics of phospholipid membranes. Chem. Phys. Lipids. 115:39–61. [DOI] [PubMed] [Google Scholar]
- Bucki, R., C. Bachelot-Loza, A. Zachowski, F. Giraud, and J. C. Sulpice. 1998. Calcium induces phospholipid redistribution and microvesicle release in human erythrocyte membranes by independent pathways. Biochemistry. 37:15383–15391. [DOI] [PubMed] [Google Scholar]
- Chong, P. L., and P. T. Wong. 1993. Interactions of Laurdan with phosphatidylcholine liposomes: a high pressure FTIR study. Biochim. Biophys. Acta. 1149:260–266. [DOI] [PubMed] [Google Scholar]
- Comfurius, P., J. M. Senden, R. H. Tilly, A. J. Schroit, E. M. Bevers, and R. F. Zwaal. 1990. Loss of membrane phospholipid asymmetry in platelets and red cells may be associated with calcium-induced shedding of plasma membrane and inhibition of aminophospholipid translocase. Biochim. Biophys. Acta. 1026:153–160. [DOI] [PubMed] [Google Scholar]
- Dam-Mieras, M. C., A. J. Slotboom, W. A. Pieterson, and G. H. de Haas. 1975. The interaction of phospholipase A2 with micellar interfaces. The role of the N-terminal region. Biochemistry. 14:5387–5394. [DOI] [PubMed] [Google Scholar]
- Dekkers, D. W., P. Comfurius, E. M. Bevers, and R. F. Zwaal. 2002. Comparison between Ca2+-induced scrambling of various fluorescently labelled lipid analogues in red blood cells. Biochem. J. 362:741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdahl, W. L., C. J. Chapman, R. W. Taylor, and D. R. Pfeiffer. 2000. Ionomycin, a carboxylic acid ionophore, transports Pb(2+) with high selectivity. J. Biol. Chem. 275:7071–7079. [DOI] [PubMed] [Google Scholar]
- Garidel, P., A. Blume, and W. Hubner. 2000. A Fourier transform infrared spectroscopic study of the interaction of alkaline earth cations with the negatively charged phospholipid 1, 2- dimyristoyl-sn-glycero-3-phosphoglycerol. Biochim. Biophys. Acta. 1466:245–259. [DOI] [PubMed] [Google Scholar]
- Gedde, M. M., D. K. Davis, and W. H. Huestis. 1997. Cytoplasmic pH and human erythrocyte shape. Biophys. J. 72:1234–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimsa, J. 1998. A possible molecular mechanism governing human erythrocyte shape. Biophys. J. 75:568–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, F. M., K. B. Best, and J. D. Bell. 2002. Use of laurdan fluorescence intensity and polarization to distinguish between changes in membrane fluidity and phospholipid order. Biochim. Biophys. Acta. 1565:123–128. [DOI] [PubMed] [Google Scholar]
- Harris, F. M., S. K. Smith, and J. D. Bell. 2001. Physical properties of erythrocyte ghosts that determine susceptibility to secretory phospholipase A2. J. Biol. Chem. 276:22722–22731. [DOI] [PubMed] [Google Scholar]
- Hauser, H. 1991. Effect of inorganic cations on phase transitions. Chem. Phys. Lipids. 57:309–325. [DOI] [PubMed] [Google Scholar]
- Hoffman, J. F. 1987. On the mechanism and measurement of shape transformations of constant volume of human red blood cells. Blood Cells. 12:565–588. [PubMed] [Google Scholar]
- Jain, M. K., M. R. Egmond, H. M. Verheij, R. Apitz-Castro, R. Dijkman, and G. H. de Haas. 1982. Interaction of phospholipase A2 and phospholipid bilayers. Biochim. Biophys. Acta. 688:341–348. [DOI] [PubMed] [Google Scholar]
- Judd, A. M., K. B. Best, K. Christensen, G. M. Rodgers, and J. D. Bell. 2003. Alterations in sensitivity to calcium and enzymatic hydrolysis of membranes from sickle cell disease and trait erythrocytes. Am. J. Hematol. 72:162–169. [DOI] [PubMed] [Google Scholar]
- Little, E. J., and M. M. Jones. 1960. A complete table of electronegativities. J. Chem. Educ. 37:231–233. [Google Scholar]
- Maraganore, J. M., G. Merutka, W. Cho, W. Welches, F. J. Kezdy, and R. L. Heinrikson. 1984. A new class of phospholipase A2 with lysine in place of aspartate 49. Functional consequences for calcium and substrate binding. J. Biol. Chem. 259:13839–13843. [PubMed] [Google Scholar]
- McDaniel, D., and B. E. Douglas. 1965. Concepts and Models of Inorganic Chemistry. Blaisdell Publishing, New York.
- Middelkoop, E., A. Coppens, M. Llanillo, E. E. Van der Hoek, A. J. Slotboom, B. H. Lubin, J. A. Op den Kamp, L. L. Van Deenen, and B. Roelofsen. 1989. Aminophospholipid translocase in the plasma membrane of Friend erythroleukemic cells can induce an asymmetric topology for phosphatidylserine but not for phosphatidylethanolamine. Biochim. Biophys. Acta. 978:241–248. [DOI] [PubMed] [Google Scholar]
- Mohandas, N., and J. A. Chasis. 1993. Red blood cell deformability, membrane material properties and shape: regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin. Hematol. 30:171–192. [PubMed] [Google Scholar]
- Muller, H., U. Schmidt, and H. U. Lutz. 1981. On the mechanism of red blood cell shape change and release of spectrin-free vesicles. Acta Biol. Med. Ger. 40:413–417. [PubMed] [Google Scholar]
- Nielson, K. H., C. A. Olsen, D. V. Allred, K. L. O'Neill, G. F. Burton, and J. D. Bell. 2000. Susceptibility of S49 lymphoma cell membranes to hydrolysis by secretory phospholipase A(2) during early phase of apoptosis. Biochim. Biophys. Acta. 1484:163–174. [DOI] [PubMed] [Google Scholar]
- Parasassi, T., G. De Stasio, G. Ravagnan, R. M. Rusch, and E. Gratton. 1991. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 60:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi, T., E. Gratton, W. M. Yu, P. Wilson, and M. Levi. 1997. Two-photon fluorescence microscopy of laurdan generalized polarization domains in model and natural membranes. Biophys. J. 72:2413–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasassi, T., G. Ravagnan, R. M. Rusch, and E. Gratton. 1993. Modulation and dynamics of phase properties in phospholipid mixtures detected by Laurdan fluorescence. Photochem. Photobiol. 57:403–410. [DOI] [PubMed] [Google Scholar]
- Pasquet, J. M., J. Dachary-Prigent, and A. T. Nurden. 1998. Microvesicle release is associated with extensive protein tyrosine dephosphorylation in platelets stimulated by A23187 or a mixture of thrombin and collagen. Biochem. J. 333:591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richieri, G. V., and A. M. Kleinfeld. 1995. Continuous measurement of phospholipase A2 activity using the fluorescent probe ADIFAB. Anal. Biochem. 229:256–263. [DOI] [PubMed] [Google Scholar]
- Schroit, A. J., and R. F. Zwaal. 1991. Transbilayer movement of phospholipids in red cell and platelet membranes. Biochim. Biophys. Acta. 1071:313–329. [DOI] [PubMed] [Google Scholar]
- Sheetz, M. P. 1977. Cation effects on cell shape. Prog. Clin. Biol. Res. 17:559–567. [PubMed] [Google Scholar]
- Sheetz, M. P., and S. J. Singer. 1977. On the mechanism of ATP-induced shape changes in human erythrocyte membranes. I. The role of the spectrin complex. J. Cell Biol. 73:638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla, S. D., J. Berriman, R. Coleman, J. B. Finean, and R. H. Michell. 1978. Membrane protein segregation during release of microvesicles from human erythrocytes. FEBS Lett. 90:289–292. [DOI] [PubMed] [Google Scholar]
- Smith, B. D., P. L. La Celle, G. E. Siefring, Jr., L. Lowe-Krentz, and L. Lorand. 1981. Effects of the calcium-mediated enzymatic cross-linking of membrane proteins on cellular deformability. J. Membr. Biol. 61:75–80. [DOI] [PubMed] [Google Scholar]
- Smith, S. K., A. R. Farnbach, F. M. Harris, A. C. Hawes, L. R. Jackson, A. M. Judd, R. S. Vest, S. Sanchez, and J. D. Bell. 2001. Mechanisms by which intracellular calcium induces susceptibility to secretory phospholipase A2 in human erythrocytes. J. Biol. Chem. 276:22732–22741. [DOI] [PubMed] [Google Scholar]
- Williamson, P., A. Kulick, A. Zachowski, R. A. Schlegel, and P. F. Devaux. 1992. Ca2+ induces transbilayer redistribution of all major phospholipids in human erythrocytes. Biochemistry. 31:6355–6360. [DOI] [PubMed] [Google Scholar]
- Wilson, H. A., W. Huang, J. B. Waldrip, A. M. Judd, L. P. Vernon, and J. D. Bell. 1997. Mechanisms by which thionin induces susceptibility of S49 cell membranes to extracellular phospholipase A2. Biochim. Biophys. Acta. 1349:142–156. [DOI] [PubMed] [Google Scholar]
- Wilson, H. A., J. B. Waldrip, K. H. Nielson, A. M. Judd, S. K. Han, W. Cho, P. J. Sims, and J. D. Bell. 1999. Mechanisms by which elevated intracellular calcium induces S49 cell membranes to become susceptible to the action of secretory phospholipase A2. J. Biol. Chem. 274:11494–11504. [DOI] [PubMed] [Google Scholar]
- Xu, Y. H., Z. Y. Lu, A. D. Conigrave, M. E. Auland, and B. D. Roufogalis. 1991. Association of vanadate-sensitive Mg(2+)-ATPase and shape change in intact red blood cells. J. Cell. Biochem. 46:284–290. [DOI] [PubMed] [Google Scholar]
- Yu, B. Z., O. G. Berg, and M. K. Jain. 1993. The divalent cation is obligatory for the binding of ligands to the catalytic site of secreted phospholipase A2. Biochemistry. 32:6485–6492. [DOI] [PubMed] [Google Scholar]
- Yu, B. Z., J. Rogers, G. R. Nicol, K. H. Theopold, K. Seshadri, S. Vishweshwara, and M. K. Jain. 1998. Catalytic significance of the specificity of divalent cations as KS* and kcat* cofactors for secreted phospholipase A2. Biochemistry. 37:12576–12587. [DOI] [PubMed] [Google Scholar]
- Zhou, Q., J. Zhao, J. G. Stout, R. A. Luhm, T. Wiedmer, and P. J. Sims. 1997. Molecular cloning of human plasma membrane phospholipid scramblase. A protein mediating transbilayer movement of plasma membrane phospholipids. J. Biol. Chem. 272:18240–18244. [DOI] [PubMed] [Google Scholar]