Abstract
The conformational transition from the native state in water (“β-state”) to a state containing a considerable amount of α-helices (“α-state”) was studied for the protein β-lactoglobulin (BLG), from bovine milk, in several colloidal solutions containing mixed micelles or spontaneous vesicles. These aggregates were formed in the bicationic system containing the surfactant dodecyltrimethylammonium chloride (DTAC) and the lipid didodecyldimethylammonium bromide (DDAB). The β→α transition in BLG, investigated by far-ultraviolet circular dichroism spectroscopy, is induced to the same protein α-state by pure and mixed DDAB/DTAC micelles or vesicles. This implies a similar interaction mechanism of BLG with DDAB or DTAC, once the colloidal aggregates are formed. In premicelle DTAC solutions, the fraction of α-helix is lower and increases with the DTAC concentration. DDAB and DTAC also promote conformational changes in the protein tertiary structure that expose the tryptophans to a less constrained environment. These unfolding transitions were investigated by near-ultraviolet circular dichroism and steady-state fluorescence spectroscopies. In equilibrium conditions, it was found that higher DTAC (and, probably, DDAB) concentrations are needed to induce the β→α transition than to unfold the protein. β-Lactoglobulin may therefore be considered as a model for protein–surfactant and protein–lipid interactions.
INTRODUCTION
Conformational transitions in the secondary/tertiary structures of proteins may lead to misfolding and consequent self-association or aggregation in cells, causing the so called “conformational diseases” (Carrell and Lomas, 1997). The risk of aggregation and self-association is greatly increased in proteins with high conformational lability (i.e., that are inherently able to undergo radical changes in their conformation). Examples are the prion proteins, which can cause diseases such as the human Creutzfeldt-Jakob's disease and the bovine spongioform encephalopathy (Carrell and Lomas, 1997; Prusiner, 1997). These disorders are caused by a conformational change where the protein α-helical content decreases whereas the amount of β-sheet increases (Prusiner, 1997). The α-helix to β-sheet transition (α→β transition) or its reverse (β→α transition) may therefore be important to understand the folding and biological function of a variety of proteins, and as such have been the subject of considerable studies (see, e.g., Kuwata et al., 1998 and references therein).
β-Lactoglobulin (BLG), a small (162 amino acid residues) globular protein present in large amounts in the milk of several mammals, has been frequently chosen as a convenient model for clarifying the mechanism of the α→β transition in proteins because this transition occurs during its own folding process (Shiraki et al., 1995; Hamada et al., 1995; Hamada and Goto, 1997). Despite abundant studies on this protein, its biological role is not well established yet (Sawyer and Kontopidis, 2000).
BLG is a predominantly β-sheet protein mainly composed by a central β-barrel formed by eight antiparallel β-strands (named βA–βH, from the N-terminal end of the protein) shaped into a flattened cone, or calyx (Brownlow et al., 1997). Outside the calyx there is one further β-strand (βI), one α-helix, and two (Brownlow et al., 1997) or four (Kuwata et al., 1998) short 310-helices. Above pH ≈ 3 (but depending also on protein concentration, ionic strength, and temperature) BLG exists as a dimer in water (Verheul et al., 1999), where the protein monomers, retaining their native conformation, are binded noncovalently mainly through their βI-strands (Brownlow et al., 1997).
Contrary to the β-sheet prevalence in the native state of BLG in water (N-state or β-state), secondary structure predictions support a marked α-helical preference for a significant number of residues (Shiraki et al., 1995; Hamada et al., 1995). Furthermore, heteronuclear NMR measurements in the presence of 2,2,2-trifluoroethanol (TFE) show that a considerable part of the residues in BLG are structured in α-helices, instead of the native β-strands (Kuwata et al., 1998). This nonnative α-state has also been called the “TFE-state” (Kuwata et al., 1998) because this β→α transition has been widely studied in the presence of TFE.
The interaction of BLG with phospholipid bilayers has also proven to increase the protein α-helical content (Brown et al., 1983). Therefore, studies of the transition between the native and α-helical states, in the presence of phospholipids, may have a significant physiological importance to understand the interaction of BLG with biological membranes.
Spontaneous vesicles prepared from aqueous mixtures of double or single-chained synthetic amphiphiles have attracted a great interest in the last years as models of biological membranes (Jung et al., 2001 and references therein). These vesicles are quite stable when compared with “conventional” (sonicated) liposomes, and their size, charge, or permeability can be readily adjusted by varying the relative amounts and/or chain lengths of the two component surfactants (Kaler et al., 1989). Spontaneous vesicles were obtained in the bicationic amphiphile system containing the double-tailed (lamellar-forming) didodecyldimethylammonium bromide (DDAB) and the single-tailed (micelle-forming) dodecyltrimethylammonium chloride (DTAC) (Viseu et al., 2000a), for which a phase diagram of the vesicle-micelle region was defined (Viseu et al., 2000b).
This paper investigates the behavior of bovine BLG in several colloidal systems, such as micelles of pure DTAC, micelles and vesicles of DDAB-DTAC, and DDAB vesicles. In DTAC and/or DDAB solutions, BLG shows a transition from its native β-state in water to a nonnative α-state. This β→α (or secondary structure) transition, studied herein by far-ultraviolet (UV) circular dichroism (CD) spectroscopy in equilibrium conditions, seems to have striking similarities to the one that has been described in the presence of many alcohols, fluorinated or not: methanol, ethanol, isopropanol, TFE, 1,1,1,3,3,3-hexafluoro-2-propanol, etc. (see, e.g., Dufour and Haertlé, 1990; Hirota et al., 1997; Uversky et al., 1997; Ragona et al., 1999; Mendieta et al., 1999; Kauffmann et al., 2001). Therefore, we also analyze herein, for comparison, the behavior of BLG in the presence of a few alcohol-water mixtures.
Changes in the protein tertiary structure (corresponding to an unfolding transition) that accompany the β→α transition were investigated by equilibrium near-UV CD and steady-state fluorescence techniques, which are sensitive to the tryptophan (Trp) solvent environment.
MATERIALS AND METHODS
Materials
The protein β-lactoglobulin from bovine milk (a mixture of variants A and B) was purchased from Sigma (St. Louis, MO), with ≈90% purity as determined by polyacrylamide gel electrophoresis. DDAB was obtained from Fluka (Buchs, Switzerland) with purity ≥98%, whereas DTAC was purchased from TCI (Tokyo Kasei, Japan) as an ion-pair chromatographic reagent with purity ≥98%. The alcohols n-propanol and n-butanol were pro analysi reagents from Merck (Darmstadt, Germany), with 99.5% purity, whereas absolute ethanol with 99.5% purity (spectroscopic grade) was obtained from Panreac (Barcelona, Spain). Guanidine hydrochloride (GnHCl) was obtained from Gibco Life Sciences (Gaithersburg, MD). All compounds were used as purchased, without further purification. Except for GnHCl solutions, where a 0.1 M phosphate buffer (NaH2PO4/Na2HPO4) was used, all other samples were prepared with freshly bidistilled water, without the use of buffers. In the final solutions, the measured pH was ≈6.0–6.5.
Sample preparation
β-Lactoglobulin solutions
Concentrated aqueous solutions of BLG (18 mg mL−1, or ≈1 mM in monomeric protein) were stocked in the freeze, and used later on for preparing the final solutions in water, water-surfactant, or water-alcohol mixtures. The final solutions, with protein concentrations ranging from 10 to 100 μM, were kept in the refrigerator and used within two days after preparation.
Colloidal aggregates
As described in Table 1 different colloidal media were prepared, b–f, in order of increasing complexity. Because DTAC is highly soluble in water, the dynamics of aggregate formation in media b and c are fast, and so these solutions were easily obtained. Medium d (mixed micelles) was prepared by dissolving the adequate amount of the sparingly soluble DDAB in a concentrated DTAC aqueous solution (Viseu et al., 2000a); the final solution was clear. Medium e (mixed vesicles) was obtained by conveniently diluting medium d to induce the spontaneous micelles → vesicles transition (Viseu et al., 2000a,b); the final solution was turbid. The dynamics of vesicle formation are much slower than those for equilibration of pure DTAC micelles, but in <≈1 h these aggregates were almost completely formed (M. I. Viseu, unpublished kinetic results). Finally, metastable vesicles of pure DDAB were obtained in medium f after a vigorous emulsification of the surfactant in water; the solution was left to equilibrate for at least one day (Viseu et al., 2000a).
TABLE 1.
Characteristics of the colloidal media investigated: DDAB and DTAC concentrations and aggregates formed
| Medium | Aggregates | Composition |
|---|---|---|
| a | – | Water |
| b | Monomers | 8 mM DTAC |
| c | Micelles | 40 mM DTAC |
| d | Mixed micelles | 5 mM DDAB; 20 mM DTAC |
| e | Mixed vesicles (spontaneous) | 2 mM DDAB; 8 mM DTAC |
| f | Vesicles (metastable) | 2 mM DDAB |
Alcohol-water mixtures
These mixtures were prepared in a % volume basis of the alcohol, covering the whole miscibility range of the alcohol and water components: 0–100% (v/v) for ethanol-water and propanol-water solutions, and ≈0–8% (v/v) for butanol-water mixtures.
Spectral measurements
All spectral measurements were performed at a controlled temperature of 25.0 ± 0.2°C.
UV-visible absorption spectra
The absorption spectra were obtained on a Jasco V-560 UV-visible absorption spectrometer (Easton, MD), using quartz cells with an optical path length of 1 cm for the near-UV wavelength region, ≈230–350 nm. Background light scattering (or turbidity) is very critical in samples containing vesicles (see Fig. 1 below). It was corrected by subtracting from the sample spectra a scattering function of the type s = a + b/λc (Castanho et al., 1997); the three parameters a, b, and c were evaluated by an iterative fitting procedure (M. I. Viseu, manuscript in preparation) after subtracting from each sample spectrum the corresponding spectrum of BLG in water. In pure or mixed micelles, turbidity could be corrected by subtracting from the sample spectrum the corresponding solvent spectrum. All solutions used for CD or fluorescence measurements were checked by absorption to calibrate (or confirm) the protein concentration, using a molar extinction coefficient ɛ of 17,600 M−1 cm−1, for the monomeric BLG in water at 280 nm (Collini et al., 2000).
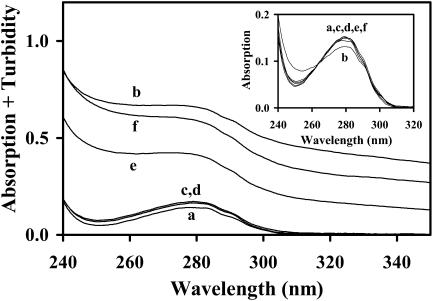
FIGURE 1.
Near-UV absorption spectra of BLG in the colloidal media a–f (see Table 1 for characterization). The inset shows the absorption spectra after correction for turbidity.
Circular dichroism spectra
The CD spectra were obtained on a Jasco J-720 spectropolarimeter (Easton, MD). The BLG secondary structure was followed in the wavelength range of the peptide bond absorption, in the far-UV (≈190–260 nm), using a protein concentration of ≈10 μM (in monomer) and an optical path length of 2 mm. The tertiary structure was observed in the wavelength range of the aromatic amino acid absorption, in the near-UV (≈250–330 nm), with a BLG monomeric concentration of ≈40–100 μM and a path length of 1 cm. A mean of five spectra was averaged for each solution. The baseline was corrected by subtracting from each sample spectrum the corresponding solvent spectrum. The results were expressed in the graphs as molar ellipticity [θ] (per monomer unit of the protein).
Evaluation of secondary structure content
The fraction of each main secondary structure element of BLG (α-helix, β-sheet, β-turn, random coil, etc.) was evaluated from far-UV CD spectra by means of the program package Dicroprot 2000, downloaded from the Internet (http://lamar.colostate.edu/). This package contains several distinct deconvolution algorithms: SELCON3 (Sreerama and Woody, 1993; Sreerama et al., 1999), K2D (Andrade et al., 1993; Merelo et al., 1994), VARSELECT (Compton and Johnson, 1986; Manavalan and Johnson, 1987), CONTIN (Provencher and Glöckner, 1981; Provencher, 1982). For some unknown reason, however, the results obtained by CONTIN did not show appreciable changes for the different CD spectra, and so could not be used herein.
Steady-state fluorescence spectra
Fluorescence spectra were obtained with a Perkin-Elmer LS-50B luminescence spectrometer (Fremont, CA), using a quartz fluorescence cell and a BLG monomeric concentration of ≈10 μM. The protein was excited in the near-UV, at the wavelengths corresponding to the main peaks of the CD spectrum, 293 and 285 nm. All other operating conditions (photomultiplier voltage, excitation and emission slits, etc.) were kept constant in all emission runs to obtain relative fluorescence yields. There was no need to correct the emission spectra for background scattered light because the “scattering peaks” did not superimpose on the emission bands significantly.
Treatment of transition data
Equilibrium transitions induced in BLG by DTAC or GnHCl were fitted to a two-state process, according to the following equations (Fersht, 1999):
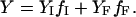 |
(1) |
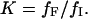 |
(2) |
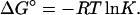 |
(3) |
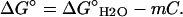 |
(4) |
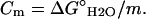 |
(5) |
In these equations, Y is the measured value of the spectroscopic property (e.g., the molar ellipticity at 222 nm), I and F the initial and final BLG conformational states, f the molar fraction of each state, K the equilibrium constant, ΔG° the standard free energy of the transition from I to F, ΔG°H2O the standard free energy of the transition in water, m a constant of proportionality (the observed “slope” of the transition), C the concentration of the “denaturing” agent (DTAC or GnHCl), and Cm its concentration at the midtransition point (where 50% of the protein molecules are in each conformational state, for which ΔG° = 0).
RESULTS AND DISCUSSION
Absorption spectra of β-lactoglobulin in colloidal solutions
The amino acid residue responsible for the major absorbance of proteins in the near-UV is Trp, with a maximum at ≈280 nm (Cantor and Schimmel, 1998; Lakowicz, 1999).
Absorption spectra of BLG in the colloidal media a–f are presented in Fig. 1. The high turbidity of samples e and f is due to the large dimensions of the vesicles contained herein (Viseu et al., 2000a), which are comparable to the wavelength of the incident light. Protein solutions are usually clear in medium c (pure micelles), but present some turbidity in medium d (mixed micelles). After correcting the scattered light in media c–f (see the Materials and Methods section), all the absorption spectra fall on the same curve, over the spectrum in pure water (medium a), regardless of the kind of colloidal aggregates present (inset). The BLG concentration in media c–f was then calibrated using its molar extinction coefficient (ɛ) in water, at 280 nm.
BLG solutions in medium b (premicellar DTAC) were usually also quite turbid (see, e.g., Fig. 1) probably due to the formation of large complexes (aggregates) between BLG and DTAC monomers. Indeed, turbidity increased with the protein concentration, whereas the corresponding pure BLG (or DTAC) aqueous solutions were clear. Possibly due to the high polydispersity of BLG-DTAC complexes the same scattering function could not correct spectrum b, but the same ɛ value was assumed for this medium. We should note that aggregation between BLG and surfactant ions is not uncommon: for example, BLG-SDS (sodium dodecylsulfate) complexes, formed through an ionic interaction, have also been found at low, premicellar, SDS concentrations (Jones and Wilkinson, 1976).
Secondary structure of BLG
The far-UV CD spectra characterize the secondary structure of proteins due to the peptide bond absorption (Kelly and Price, 1997; Cantor and Schimmel, 1998); whereas the molar ellipticities at 222 nm ([θ]222) and 208 nm ([θ]208) (or the ratio [θ]208/[θ]222) have been typically used to characterize their α-helix content.
Media a–f
The far-UV CD spectral changes observed for BLG when going successively from water to premicellar DTAC, and then to DTAC micelles (media a→b→c), i.e., at increasing DTAC concentrations (Fig. 2 A and inset), clearly correspond to an increase in the protein α-helical content.
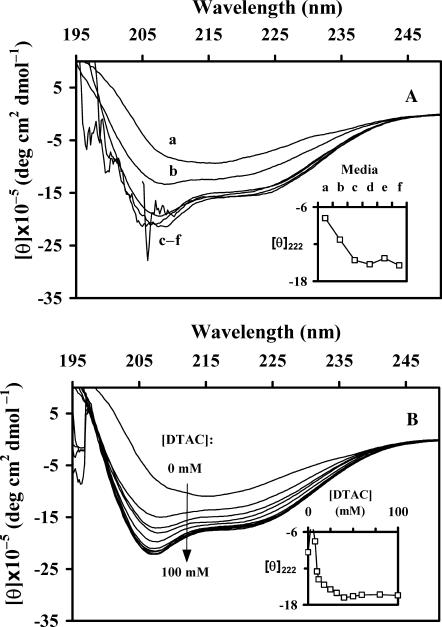
FIGURE 2.
Far-UV CD spectra of BLG: (A) in media a–f (see Table 1); (B) in DTAC aqueous solutions, as a function of the DTAC concentration. The ordinate scales represent the molar ellipticities [θ] per monomeric protein. The insets show the evolution of [θ]222 as a function of the medium (A) or the DTAC concentration (B).
Samples d–f containing the sparingly soluble surfactant DDAB are highly turbid, and so there is a low signal/noise ratio below ≈210 nm in the corresponding CD spectra (Fig. 2 A). This fact prevents more quantitative calculations in media d–f. Apart from the noise, however, these spectra are nearly superimposed to the one in pure DTAC micelles (medium c), showing that the secondary structure of BLG is the same in media c–f. Consequently, more detailed (and quantitative) studies were performed only in DTAC solutions, by varying the concentration of this surfactant in both the premicelle and micelle regions.
DTAC solutions
The evolution of [θ]222 for BLG in aqueous DTAC solutions (inset of Fig. 2 B, where the spectra of a few, turbid, solutions were omitted) show that the spectral changes are due to an increase in the protein α-helical content, when [DTAC] increases. A spectral stabilization is achieved for [DTAC] > ≈30 mM, nearly above the critical micelle concentration (CMC = 22.4−22.5 mM; Viseu et al., 2000b).
Instead of the direct reading of [θ]222, which only accounts for the relative changes in the protein α-helical content, a more quantitative evaluation of the secondary structure content can be performed with adequate programs, which generally give the fraction of α-helixes, β-strands, random coils, turns, etc. In this work, the CD spectra of BLG as a function of [DTAC] were analyzed using the program package Dicroprot 2000 (see the Materials and Methods section).
Fig. 3 A illustrates the BLG α-helical content, for which the CD spectra are more sensitive (Cantor and Schimmel, 1998) and therefore the deconvolution is more reliable. Even though the dispersion of the values is high (possibly due mainly to the specific protein database(s) used by each algorithm), the trend shown by the curves follows the same pattern: it corresponds to an increase in the BLG α-helical content, when [DTAC] increases.
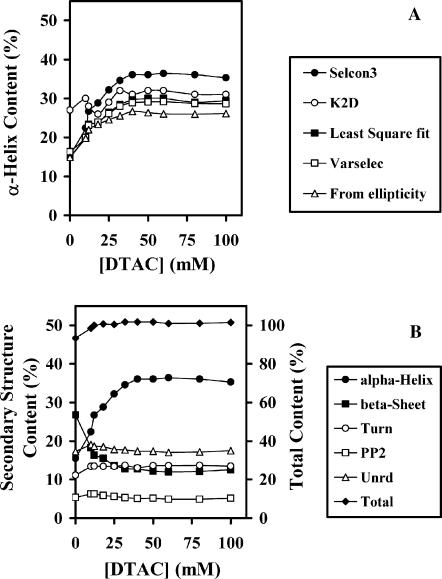
FIGURE 3.
Secondary structure content of BLG, evaluated from far-UV CD spectra, as a function of the DTAC concentration. (A) Fractional content in α-helixes, obtained with several programs from Dicroprot 2000. (B) Fractional content in α-helixes, β-strands, turns, poly(Pro)II, and unordered fraction (scale at left), and total secondary structure content (scale at right), obtained with the program SELCON3, from Dicroprot 2000.
The fraction of other secondary structure elements (β-strands, random coil, turns, etc.) may also be evaluated by most of these programs. Fig. 3 B illustrates the results given by the program SELCON3, based on a self-consistent algorithm. From the whole Dicroprot package, this program is the one that gave the more stable (and nearest to unity) sum of the fractions of all the secondary structure elements (the sum is the higher curve, read on the right scale of the figure). We see that, when [DTAC] increases, the BLG α-helical content increases mostly at the expense of its β-sheet content. This means that DTAC induces a β-sheet→α-helix transition (or β→α transition) in the protein.
In the “Media a–f” section, a similar β→α transition was found in solutions containing the double-chained surfactant DDAB, i.e., in media d–f. The main difference observed is that, at low DTAC concentrations (in medium b and, generally, in premicelle media) the fraction of α-helix attained is lower than in media c–f.
Alcohol-water solutions
The behavior of BLG in DTAC and/or DDAB solutions shows striking similarities to the one observed in the presence of several alcohols (or other organic solvents), which has been widely described in the literature (see, e.g., Dufour and Haertlé, 1990; Hirota et al., 1997; Uversky et al., 1997; Mendieta et al., 1999; Kauffmann et al., 2001). For example, in the presence of the fluorinated alcohol TFE it was found that a considerable part of the protein residues (mostly those on the N-terminal end) have an unusually high propensity to form nonnative α-helices, instead of the native β-strands (Kuwata et al., 1998).
However, the equilibrium “α-state” of BLG may not be the same in the presence of the surfactant or alcohol. Consequently, we found relevant to compare the β→α transition of BLG in the present colloidal systems with that of BLG in a few alcohol-water mixtures (Figs. 4 and 5).
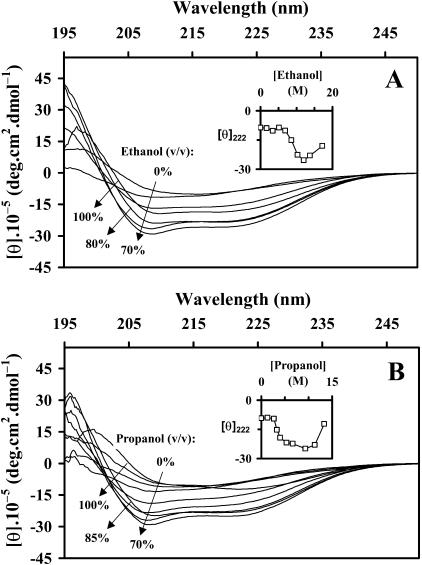
FIGURE 4.
Far-UV CD spectra of BLG: (A) in ethanol-water and (B) in propanol-water mixtures. The insets show the evolution of [θ]222 with the alcohol content. To compare with the DTAC-water data, the abscissa scales in the insets were converted to molar units.
FIGURE 5.
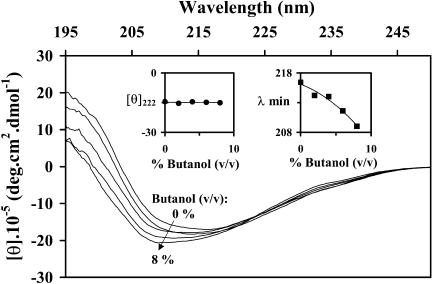
Far-UV CD spectra of BLG in butanol-water mixtures. The insets show the evolution of [θ]222 and of the wavelength at the minimum of the band (λmin) with the alcohol content.
The far-UV CD spectral evolution of BLG in ethanol-water (Fig. 4 A) and n-propanol-water solutions (Fig. 4 B) presents a similar, but less regular, pattern than the one observed in the presence of DTAC (Fig. 2 B), showing evidence of an increase in the fraction of α-helix with the alcohol content. Deconvolution with SELCON3 (results not presented herein) also showed that the increase in the α-helix fraction is correlated with the decrease in the β-sheet content, but the trend is not so regular as it was in DTAC–water solutions. Also, as seen in Fig. 6 below, the β→α transition occurs at much higher contents of ethanol or n-propanol (molar region) than those found for DTAC (millimolar region).
FIGURE 6.
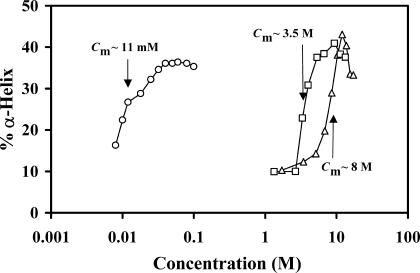
Comparison of the α-helical content of BLG, evaluated with the program SELCON3, in the solvent systems ethanol-water (▵), propanol-water (□), and DTAC-water (○), as a function of the “denaturant” (alcohol or surfactant) concentration. “Denaturant” concentrations at the midtransition point, Cm, are indicated. A logarithmic abscissa scale was used because of the quite different concentration regimes where the β→α transition occurs, for DTAC and the alcohols.
The CD spectra presented in Fig. 4 show an inversion of the trend at alcohol contents above ≈70% (v/v), as if there was another transition on the protein secondary structure (from α-helix to β-sheet) at high alcohol contents. This might be an apparent effect, probably due to BLG aggregation and/or precipitation in the alcohol solvent. Indeed, BLG hardly dissolves in almost pure ethanol (with a minimum of water), and only after a drop of concentrated HCl is added (Gonçalves da Silva et al., 2003).
Unexpectedly, the far-UV CD spectra of BLG in n-butanol-water mixtures up to 8% butanol (v/v) (Fig. 5) show a different pattern from the one observed in the presence of DTAC, ethanol, or n-propanol: the minimum ellipticity shifts to lower wavelengths with almost no change in [θ]222 (insets). This pattern does not correspond to an increase of the protein α-helical content, and so could not be adequately quantified by the Dicroprot package. Interestingly, the far-UV CD spectrum of a distorted β-strand (a portion of the strand that lacks some H-bonds) shows a strong negative band between ≈182–189 nm (Sreerama et al., 1999). Therefore, it seems possible that the CD spectral blue shifts and increased (negative) intensity induced by n-butanol are due to some H-bond disruption and consequent partial distortion of the β-strands or β-sheets—but far from a complete distortion of the main β-structure.
Curiously, a similar spectral evolution (a blue shift and a slight increase in the band intensity, without appreciable change in [θ]222) was also found when heating BLG to 80°C (Qi et al., 1997) or to 86°C (Manderson et al., 1999b). Qi et al. (1997) interpreted the spectral changes as due to a direct conversion of regular (β-sheet, α-helix) into irregular (unordered) structure, when the temperature increases; whereas Manderson et al. (1999b) explain the same effect by the loss of H-bonding between the strands βI, at the dimer interface, due to a decreased dimer/monomer ratio at the higher temperature.
Comparison of results
The “denaturant” concentrations at the point of midtransition, Cm (i.e., for equal concentrations of the β- and α-states), compared in Fig. 6 for the systems DTAC-water, ethanol-water, and propanol-water, show that the ability of the alcohols to promote the β→α transition in BLG increases with the number of carbon atoms, i.e., ethanol < n-propanol. The surfactant DTAC, with a much longer carbon chain (C12), still has a higher capacity than propanol to induce the transition. With n-butanol, the β→α transition was not observed.
On the other hand, the double-chained DDAB (2C12) is only sparingly soluble in water: its monomer solubility is ≈10−5 M (Evans and Wennerström, 1994). At higher concentrations, it does not form micelles in pure water, but forms lamellae or vesicles, and therefore its water emulsions are turbid. It would not be easy to undergo a similar investigation by far-UV CD measurements with this surfactant (see, e.g., the high noise of curve f in Fig. 2 A). But in the “Media a–f” section, from the superposition of the far-UV CD spectra, we concluded that the secondary structure of BLG was the same in media c–f. This means that, for example, 2 mM DDAB (vesicles, medium f) induce the β→α transition in BLG to the same extent as 40 mM DTAC (micelles, medium c), i.e., the more hydrophobic DDAB has a greater capacity than DTAC to promote the transition.
In summary, the ability of the denaturants to induce the β→α transition in β-lactoglobulin correlates with their hydrophobic character, following the order: ethanol < n-propanol ≪ DTAC ≪ DDAB.
A similar behavior has been reported in the literature: e.g., the ability to induce the β→α transition in BLG was found to increase with the hydrophobic character of the denaturant alcohol, in the order: methanol < ethanol < isopropanol < TFE < hexafluoroisopropanol (Hirota et al., 1997). The same effect has, alternatively, been explained by the decrease of the solvent dielectric constant, either using alcohols (Dufour et al., 1993) or alcohols and other organic solvents (dimethylformamide, dioxane) as “denaturants” (Uversky et al., 1997).
Another observation concerns the extent of the β→α transition, i.e., the final (equilibrium) α-state attained. Fig. 6 shows that the maximum fraction of α-helix obtained with DTAC is ≈36–37%, whereas it is ≈41% for propanol and ≈43% for ethanol. This means that the final BLG “α-state” is not exactly the same in all cases. Even though DTAC and DDAB induce the transition at much lower concentrations than ethanol and propanol, it is in alcohol solutions that the protein achieves a higher α-helical content. An even stronger effect has been described with TFE, which induces in BLG the so-called “TFE-state,” containing 14 helical segments (i.e., an α-helical content of ≈61%) as estimated by NMR measurements (Kuwata et al., 1998).
It is important to discuss the different colloidal media used herein. In solutions containing micelles or vesicles, the BLG β→α transition occurs up to the same extent. This is a strong indication that BLG interacts with all these colloidal aggregates in a similar fashion. The intermolecular forces involved between the protein and the aggregates should be of a hydrophobic nature (because the capacity of promoting the transition increases with the “denaturant” hydrophobic character), as well as electrostatic (because the surfactants are cationic and BLG is negatively charged at pH ≈ 6.0−6.5, above its isoelectric point of 5.2). Consequently, BLG must be inserted within the aggregate and/or located near its interface, interacting both with the surfactant headgroup and tail(s). An increase in the BLG α-helical content has also been observed on its interaction with phospholipid bilayers (Brown et al., 1983). Our results therefore suggest that the micelles and vesicles investigated herein, with the incorporated β-lactoglobulin, can be useful models of biological membranes.
Tertiary structure of BLG
Near-UV CD measurements
The near-UV CD spectra of a protein characterize its tertiary structure mainly because of constrained asymmetries in the environment of the aromatic amino acids (Kelly and Price, 1997; Cantor and Schimmel, 1998). The two peaks at ≈293 and ≈285 nm in the spectrum of native BLG in water (Fig. 7) are mainly due to tryptophan (Trp-19 and/or Trp-61) absorbance.
FIGURE 7.
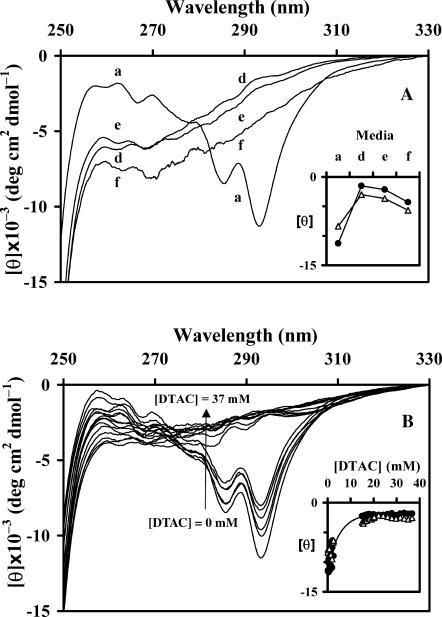
Near-UV CD spectra of BLG: (A) in water (a) and in media d–f (see Table 1); (B) in DTAC aqueous solutions, as a function of the DTAC concentration. The insets show the evolution of [θ]293 (•) and [θ]285 (▵) as a function of the medium (A) or the DTAC concentration (B).
Media a–f
Important changes are seen in the spectra when going from water to mixed micelles (media a→d), but only a small variation is observed thereafter, in media d→f (Fig. 7 A and inset). (This slight decrease in [θ]293 and [θ]285, instead of the expected stabilization seen in the far-UV, might result from the difficulty in correcting the turbidity of these solutions.) These data mean that, in media d–f, BLG undergoes a conformational transition affecting its tertiary structure. The loss of the Trp peaks in the spectrum indicates that the environment of these residues (or, at least, of one of them) became less constrained than in native BLG. Consequently, the protein has suffered an unfolding transition, i.e., it has changed from a folded conformation (its native state) to a more expanded one (a set of equilibrium, partially unfolded, conformations) (Fersht, 1999).
DTAC solutions
More detailed studies, performed at varying DTAC concentrations up to ≈40 mM, clearly indicate the occurrence of an unfolding transition of the protein when [DTAC] increases above ≈15 mM (Fig. 7 B and inset).
However, sample turbidity due to BLG-DTAC complexes prevented the recording of CD spectra in solutions with [DTAC] ≈ 2–15 mM. This problem is more critical in the near-UV (rather than the far-UV) because higher protein concentrations are used herein. Sample turbidity (and/or the accumulation of an intermediate between the BLG native- and α-states, as proposed in the section entitled “Evolution of secondary and tertiary structures of BLG in DTAC solutions” below) may cause the absence of an isodichroic point in the CD spectra. Turbidity also leads to the poor definition of the rising portion of the curves in the inset of Fig. 7 B. Because fluorescence has a higher sensitivity to probe the Trp environment (i.e., much lower BLG concentrations are needed for fluorescence than for near-UV CD measurements), this technique was used to “fill the gap” of DTAC concentrations in the above representation.
Emission spectra
Steady-state fluorescence spectroscopy is an adequate technique to follow tertiary structure (or folding/unfolding) transitions in proteins because the tryptophan emission is highly sensitive to changes in the solvent polarity and/or local environment along the transition (Lakowicz, 1999).
Media a–f
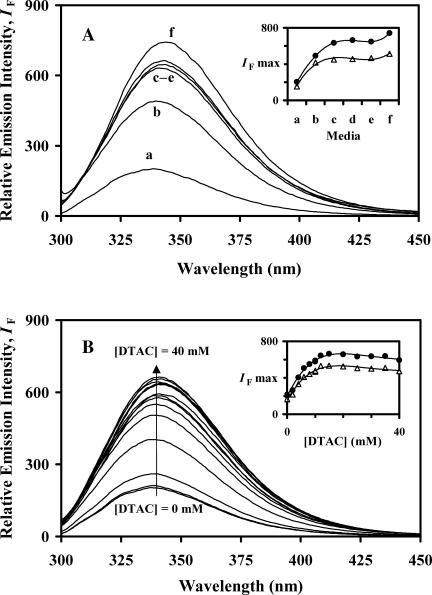
Fluorescence emission spectra of BLG in media a–f were obtained either with selective excitation of Trp at the longest wavelength peak observed in the near-UV CD spectra, at 293 nm (Fig. 8 A), or with excitation at 285 nm, the other main Trp CD peak where tyrosine (Tyr) also absorbs (spectra not shown). The relative maximum emission intensities, represented as a function of the media a→f (inset), show a common trend, possibly because both of them correspond to the Trp emission after its own direct excitation or after energy transfer from Tyr. This trend may be described by a strong increase in intensity when going from pure water to DTAC micelles (media a→b→c); a stabilization in media c→e; and, finally, another (much smaller) increase in intensity on going to medium f. A general, small, red shift of ≈5 nm (from ≈338 to ≈343 nm, in media a→f) accompanies these changes.
FIGURE 8.
Fluorescence emission spectra of BLG, with excitation at 293 nm: (A) in media a–f (see Table 1); (B) in DTAC aqueous solutions, as a function of the DTAC concentration. The insets show the maximum emission intensity, for excitation at 293 (•) and 285 nm (▵), as a function of the medium (A) or the DTAC concentration (B).
These data show evidence of an unfolding transition in media b–f: its extent, being smaller in medium b (but increasing with [DTAC]), becomes approximately constant in media c→f. This means that the unfolding transition is independent of the colloidal aggregates present in solution, pure or mixed micelles, or vesicles. So, it was studied in more detail in aqueous DTAC solutions, in the premicelle and micelle regions.
DTAC solutions
At increasing DTAC concentrations up to 40 mM (media a→c, Fig. 8 B), the same general effect as found previously is observed: a considerable increase in the Trp emission intensity, but only a slight spectral red shift (from ≈338 to ≈342 nm). Both effects tend to stabilize with the micelle formation, >22.5 mM.
Discussion of tertiary structure changes
To interpret near-UV CD and fluorescence data by relating them to the environment of the individual Trp residues in bovine BLG (Trp-19 and Trp-61) is not straightforward because both tryptophans can, in principle, contribute to the spectra.
In the native state, the indole group of Trp-19 is located inside the calyx (Brownlow et al., 1997), which is the main hydrophobic cavity where ligands such as retinol can bind (Cho et al., 1994). On the other hand, the indole group of Trp-61 protrudes into the protein exterior surface, adjacent to strand βI (Brownlow et al., 1997), and so this residue is believed to play a significant role in dimer bonding (Renard et al., 1998). The water accessibility to Trp-19 is, accordingly, much lower than that to Trp-61 (Qin et al., 1998).
Near-UV CD data
On the basis of the similarity of the near-UV CD spectra of bovine and equine β-lactoglobulins, where the latter does not contain Trp-61 (Ikeguchi et al., 1997), it is generally assumed that Trp-19 is the residue mainly responsible for these spectra. With this simplified assumption, the spectral data observed in the presence of DTAC (or DDAB) would point to a less constrained Trp-19 than in the native protein; this could mean that the calyx structure (or β-barrel) of BLG has partially loosened.
Fluorescence data
On the other hand, by comparing the fluorescence of wild-type BLG with that of a W19A mutant (without Trp-19), at pH 8, Cho et al. (1994) proved that Trp-19 contributes ≈80% to the total native protein fluorescence. This can be due to the location of Trp-61 near a disulfide bond (Cys-66–Cys-160), which may quench its emission (Palazolo et al., 2000), and/or to the self-quenching of Trp-61 by the nearby Trp-61 of the other monomer, in the BLG dimeric form (Renard et al., 1998).
The positions of the two Trps should therefore explain the trends observed in the fluorescence spectrum of the native BLG: a low quantum yield (because Trp-61 is quenched) and a (relatively) blue-shifted emission (because Trp-19 is in a hydrophobic environment). However, the position of the emission maximum, at ≈338 nm, is not characteristic of a completely nonpolar medium (Lakowicz, 1999), suggesting some contribution to the spectrum of the more surface-exposed Trp-61.
In the presence of DTAC or DDAB, the emission spectra greatly increased in intensity. This effect is more probably due to the changing environment of Trp-61, should it be highly quenched in the native protein. Indeed, in the unfolded state, Trp-61 could be more distant from its quenchers, the disulfide bond and/or Trp-61 of the other BLG subunit (in the latter case, because of a decreased dimer/monomer ratio, likely induced by the colloidal aggregates). On the other hand, the small (but consistent) red shift seems to indicate a “more polar” Trp environment than in the native state; this might indeed happen with Trp-19 if the calyx structure of BLG has partially loosened (as suggested by the near-UV CD data). Alternatively, the red shift might simply be due to the increased contribution of Trp-61 (which has a more red-shifted emission than Trp-19) to the fluorescence spectra, in the colloidal media. We favor the latter hypothesis because the width of the emission band at half-height also increased with [DTAC] (data not shown), suggesting the contribution of both Trp residues to the spectra.
Comparison with other unfolding agents
The stabilization of the maximum wavelength and emission quantum yield in the colloidal media c–f suggests that BLG attains the same partially unfolded state in all these aggregates, micelles, or vesicles.
However, this state is quite different from the one induced in the protein by ≈4 M (or higher) GnHCl, where the emission maximum shifts to ≈355 nm (spectra not shown). This wavelength is characteristic of a complete exposure of tryptophanyl residues to water (Lakshmikanth and Krishnamoorthy, 1999) and means that the protein is totally unfolded in the presence of this denaturant. Near-UV CD spectra confirm these findings, whereas far-UV CD data further indicate the absence of any chiral structure (α-helix, β-sheet, etc.) of the peptide bond. Our own results with GnHCl are compared in Table 2 with those obtained with DTAC. The values of ΔG°H2O show that the totally unfolded state attained in the presence of GnHCl has a substantially higher free energy (referred to water) than the “α-state” stabilized in the presence of DTAC. On the other hand, the quite different m values obtained mean that the transition is much more “cooperative” in the presence of DTAC (it is completed in a much smaller range of concentrations) rather than in the presence of GnHCl.
TABLE 2.
“Denaturant” concentrations at the midtransition point (Cm), and slope (m), intercept (ΔG°H2O), and squared correlation coefficient (r2) of the free energy versus “denaturant” concentration (Eq. 4), for the conformational transitions of β-lactoglobulin induced by DTAC and GnHCl assuming a two-state equilibrium
| Transitions induced by DTAC
| ||||
|---|---|---|---|---|
| Spectroscopic signal | Cm (mM) | m (kcal mol−1 M−1) | ΔG°H2O (kcal mol−1) | r2 |
| [θ]222 | 10.6 | −66 | 0.7 | 0.966 |
| [θ]293 | 3.2 | −530 | 1.7 | 0.803 |
| IF max | 5.8 | −240 | 1.4 | 0.941 |
| Transitions induced by GnHCl
| ||||
| Spectroscopic signal | Cm (M) | m (kcal mol−1 M−1) | ΔG°H2O (kcal mol−1) | r2 |
| [θ]222 | 2.8 | −1.2 | 3.2 | 0.975 |
| [θ]293 | 2.7 | −4.2 | 11.2 | 0.969 |
| IF max | 2.9 | −2.1 | 6.0 | 0.985 |
Denaturation of BLG by urea follows a similar pattern to the one by GnHCl, with the Trp emission also shifted to ≈355 nm (D'Alfonso et al., 2002).
A less drastic unfolding of BLG is induced by high hydrostatic pressure (600 MPa), applied for ≈1 h at 50°C (Yang et al., 2001). Curiously, these authors report changes in CD and Trp emission spectra similar to those induced by DTAC or DDAB. The “α-state” obtained by high-pressure treatment of this protein is very stable, and it is considered by Yang et al. (2001) as being a “molten globule state.”
An even less drastic change of the BLG tertiary structure seems to be produced when the protein is heated up to ≈80–90°C, where red shifts of ≈6–7 nm are seen in the Trp emission spectra (Manderson et al., 1999a). These changes accompany the partial distortion of the protein secondary β-structure, apparently without the formation of new α-helices (see “Alcohol-water solutions” section).
Evolution of secondary and tertiary structures of BLG in DTAC solutions
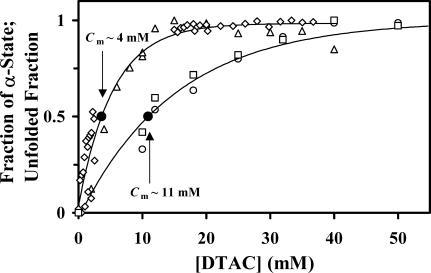
To compare the effect of DTAC in both the secondary and tertiary structures of BLG in the same graph (Fig. 9), the fraction of “α-state” fα and the “unfolding” fraction fU were evaluated by the equation:
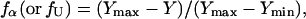 |
(6) |
where max and min refer to the maximum and minimum values of the investigated spectroscopic property Y, in the range of DTAC concentrations studied.
FIGURE 9.
Comparison of the effect induced by DTAC on the secondary and tertiary structures of BLG. The “fraction of α-state” was calculated from the parameters [θ]222 (□) or the α-helix content evaluated by SELCON3 (○); whereas the protein “unfolded fraction” was calculated from [θ]293 (⋄) or the maximum emission intensity with excitation at 293 nm (▵). The DTAC concentrations at the midtransition points, Cm, are indicated (see also Table 2).
The DTAC concentrations at the point of midtransition are also indicated: Cm ≈ 4 and 11 mM, respectively, for tertiary and secondary structure changes (see Table 2 for values calculated by Eq. 5). These results show that the conformational transition seen in the presence of DTAC involves changes in both the secondary and tertiary structures of BLG, but, in equilibrium conditions, the changes in the tertiary structure (unfolding transition) precede those in the secondary structure (β→α transition). This means, for example, that DTAC concentrations around 4 mM are able to unfold the protein considerably (≈50%), but change much less (≈20%) its secondary structure, from the β- to the α-state. This result probably indicates the accumulation of an intermediate between the native- and α-states of BLG, which may be considerably unfolded but still retaining much of the protein β-strands. Intermediates such as a molten globule state have been proposed in the literature to explain the noncoincidence of the midtransition points in the secondary and tertiary structures of BLG (see, e.g., Uversky et al., 1997). Other possibilities include a native monomer, which could be the intermediate between the native dimer (that exists at neutral pH, in water) and the final partially unfolded state stabilized by the surfactants (α-state). A native monomer intermediate has also been proposed in the literature for the BLG denaturation by urea (Hamada and Dobson, 2002). With these data, however, it is not possible to ascertain yet the nature of such an intermediate.
CONCLUDING REMARKS
β-Lactoglobulin presents a native state in water formed mainly by β-strands (“β-state”). Due to the high helical propensity of some residues included in β-strands, a conformational transition involving the protein secondary structure (β→α transition) occurs in the presence of millimolar concentrations of the cationic surfactants DTAC and DDAB. This transition was studied herein in equilibrium conditions using far-UV CD spectroscopy, and it was found that the same equilibrium “α-state” is attained in these surfactant solutions regardless of the type of colloidal aggregates (pure or mixed micelles, or vesicles) present. This implies a similar interaction mechanism of BLG with DDAB or DTAC, once the colloidal aggregates are formed.
Far-UV CD spectra of BLG in DTAC-water solutions confirm that the fraction of the protein α-helix increases mainly at the expense of its β-sheet content. Ethanol and n-propanol also promote the β→α transition, and the effect increases strongly with the hydrophobic character of the “denaturing” agent, surfactant, or alcohol.
DDAB and DTAC further induce tertiary structure changes in BLG, which correspond to a partial unfolding of the protein. Near-UV CD and steady-state fluorescence spectroscopies were used to study these conformational changes, which expose one or both Trps to a less constrained environment. In equilibrium conditions, the unfolding changes precede the β→α transition, suggesting the presence of an intermediate between the native protein and the α-state. A further understanding of the mechanism of these conformational transitions (the sequence of events in the BLG tertiary/secondary structure changes) was sought with complementary investigations on its unfolding/refolding kinetics, in the presence of DTAC, which are currently being developed in our laboratory using stopped-flow/fluorescence measurements.
Acknowledgments
The authors are most grateful to Professor J. Pessoa for providing the CD spectrometer and to Ms. C. Tavares for performing preliminary experimental work. M.I.V. is indebted to Drs. S. Andrade and E. Melo for providing references and participating in useful discussions, and to Dr. D. Roccatano of the University of L'Aquila, Italy, for fruitful discussions online.
Project POCTI/2000/QUI/35398, funded by Fundação para a Ciência e a Tecnologia and 3° Quadro Comunitário de Apoio (Fundo Europeu de Desenvolvimento Regional), supported this work. T.C. acknowledges a grant through the same project.
References
- Andrade, M. A., P. Chacón, J. J. Merelo, and F. Morán. 1993. Evaluation of secondary structure of proteins from UV circular dichroism spectra using an unsupervised learning neural network. Prot. Eng. 6:383–390. [DOI] [PubMed] [Google Scholar]
- Brown, E. M., R. J. Carroll, P. E. Pfeffer, and J. Sampugna. 1983. Complex formation in sonicated mixtures of β-lactoglobulin and phosphatidylcholine. Lipids. 18:111–118. [Google Scholar]
- Brownlow, S., J. H. M. Cabral, R. Cooper, D. R. Flower, S. J. Yewdall, I. Policarpov, T. North, and L. Sawyer. 1997. Bovine β-lactoglobulin at 1.8 Å resolution: still an enigmatic lipocalin. Structure. 5:481–495. [DOI] [PubMed] [Google Scholar]
- Cantor, C. R., and P. R. Schimmel. 1998. Biophysical Chemistry. Part II. Techniques for the Study of Biological Structure and Function, 11th ed. Freeman and Co., New York.
- Carrell, R. W., and D. A. Lomas. 1997. Conformational disease. Lancet. 350:134–138. [DOI] [PubMed] [Google Scholar]
- Castanho, M. A. R. B., N. C. Santos, and L. M. S. Loura. 1997. Separating the turbidity spectra of vesicles from the absorption spectra of membrane probes and other chromophores. Eur. Biophys. J. 39:253–259. [Google Scholar]
- Cho, Y., C. A. Batt, and L. Sawyer. 1994. Probing the retinol-binding site of bovine β-lactoglobulin. J. Biol. Chem. 269:11102–11107. [PubMed] [Google Scholar]
- Collini, M., L. D'Alfonso, and G. Baldini. 2000. New insight on β-lactoglobulin binding sites by 1-anilinonaphthalene-8-sulfonate fluorescence decay. Protein Science. 9:1968–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton, L. A., and W. C. Johnson, Jr. 1986. Analysis of protein circular dichroism spectra for secondary structure using a simple matrix multiplication. Anal. Biochem. 155:155–167. [DOI] [PubMed] [Google Scholar]
- D'Alfonso, L., M. Collini, and G. Baldini. 2002. Does β-lactoglobulin denaturation occur via an intermediate state? Biochemistry. 41:326–333. [DOI] [PubMed] [Google Scholar]
- Dufour, E., and T. Haertlé. 1990. Alcohol-induced changes of β-lactoglobulin: retinol-binding stoichiometry. Protein Eng. 4:185–190. [DOI] [PubMed] [Google Scholar]
- Dufour, E., C. Bertrand-Harb, and T. Haertlé. 1993. Reversible effects of medium dielectric constant on structural transformation of β-lactoglobulin and its retinol binding. Biopolymers. 33:589–598. [DOI] [PubMed] [Google Scholar]
- Evans, D. F., and H. Wennerström. 1994. The Colloidal Domain. Where Physics, Chemistry, Biology, and Technology Meet. VCH Publishers Inc., New York.
- Fersht, A. 1999. Structure and Mechanism in Protein Science. A Guide to Enzyme Catalysis and Protein Folding. Freeman and Co., New York.
- Gonçalves da Silva, A. M., R. I. S. Romão, S. M. Andrade, and S. M. B. Costa.2003. Incorporation of β-lactoglobulin in monolayers of dioctadecyldimethylammonium bromide studied by Brewster angle microscopy. Colloids and Surfaces B. 30:259–272. [Google Scholar]
- Hamada, D., and C. M. Dobson. 2002. A kinetic study of β-lactoglobulin amyloid fibril formation promoted by urea. Protein Science. 11:2417–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada, D., and Y. Goto. 1997. The equilibrium intermediate of β-lactoglobulin with non-native α-helical structure. J. Mol. Biol. 269:479–487. [DOI] [PubMed] [Google Scholar]
- Hamada, D., Y. Kuroda, T. Tanaka, and Y. Goto. 1995. High helical propensity of the peptide fragments derived from β-lactoglobulin, a predominantly β-sheet protein. J. Mol. Biol. 254:737–746. [DOI] [PubMed] [Google Scholar]
- Hirota, N., K. Mizuno, and Y. Goto. 1997. Cooperative α-helix formation of β-lactoglobulin and melittin induced by hexafluoroisopropanol. Protein Science. 6:416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeguchi, M., S. Kato, A. Shimizu, and S. Sugai. 1997. Molten globule state of equine β-lactoglobulin. Proteins: Structure, Function, and Genetics. 27:567–575. [DOI] [PubMed] [Google Scholar]
- Jones, M. N., and A. Wilkinson. 1976. The interaction between β-lactoglobulin and sodium n-dodecyl sulphate. Biochem. J. 153:713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, H. T., B. Coldren, J. A. Zasadzinski, D. J. Iampietro, and E. W. Kaler. 2001. The origins of stability of spontaneous vesicles. Proc. Natl. Acad. Sci. USA. 98:1353–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler, E. W., A. K. Murthy, B. E. Rodriguez, and J. A. N. Zasadzinski. 1989. Spontaneous vesicle formation in aqueous mixtures of single-tailed surfactants. Science. 245:1371–1374. [DOI] [PubMed] [Google Scholar]
- Kauffmann, E., N. C. Darnton, R. H. Austin, C. Batt, and K. Gerwert. 2001. Lifetimes of intermediates in the β-sheet to α-helix transition of β-lactoglobulin by using a diffusional IR mixer. Proc. Natl. Acad. Sci. USA. 98:6646–6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, S. M., and N. C. Price. 1997. The application of circular dichroism to studies of protein folding and unfolding. Biochim. Biophys. Acta. 1338:161–185. [DOI] [PubMed] [Google Scholar]
- Kuwata, K., M. Hoshino, S. Era, C. A. Batt, and Y. Goto. 1998. α→β Transition of β-lactoglobulin as evidenced by heteronuclear NMR. J. Mol. Biol. 283:731–739. [DOI] [PubMed] [Google Scholar]
- Lakowicz, J. R. 1999. Principles of Fluorescence Spectroscopy, 2nd Ed. Kluwer Academic/Plenum Publishers, New York.
- Lakshmikanth, G. S., and G. Krishnamoorthy. 1999. Solvent-exposed tryptophans probe the dynamics at protein surfaces. Biophys. J. 77:1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavalan, P., and W. C. Johnson, Jr. 1987. Variable selection method improves the prediction of protein secondary structure from circular dichroism spectra. Anal. Biochem. 167:76–85. [DOI] [PubMed] [Google Scholar]
- Manderson, G. A., M. J. Hardman, and L. K. Creamer. 1999a. Effect of heat treatment on bovine β-lactoglobulin A, B, and C explored using thiol availability and fluorescence. J. Agric. Food Chem. 47:3617–3627. [DOI] [PubMed] [Google Scholar]
- Manderson, G. A., L. K. Creamer, and M. J. Hardman. 1999b. Effect of heat treatment on the circular dichroism spectra of bovine β-lactoglobulin A, B, and C. J. Agric. Food Chem. 47:4557–4567. [DOI] [PubMed] [Google Scholar]
- Mendieta, J., H. Folqué, and R. Tauler. 1999. Two-phase induction of the nonnactive α-helical form of β-lactoglobulin in the presence of trifluoroethanol. Biophys. J. 76:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merelo, J. J., M. A. Andrade, A. Prieto, and F. Morán. 1994. Proteinotopic feature maps. Neurocomputing. 6:443–454. [Google Scholar]
- Palazolo, G., F. Rodríguez, B. Farruggia, G. Picó, and N. Delorenzi. 2000. Heat treatment of β-lactoglobulin: structural changes studied by partitioning and fluorescence. J. Agric. Food Chem. 48:3817–3822. [DOI] [PubMed] [Google Scholar]
- Provencher, S. W., and J. Glöckner. 1981. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 20:33–37. [DOI] [PubMed] [Google Scholar]
- Provencher, S. W. 1982. CONTIN: a general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 27:229–242. [Google Scholar]
- Prusiner, S. B. 1997. Prion diseases and the BSE crisis. Science. 278:245–251. [DOI] [PubMed] [Google Scholar]
- Qi, X. L., C. Holt, D. McNulty, D. T. Clarke, S. Brownlow, and G. R. Jones. 1997. Effect of temperature on the secondary structure of β-lactoglobulin at pH 6.7, as determined by CD and IR spectroscopy: a test of the molten globule hypothesis. Biochem. J. 324:341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, B. Y., M. C. Bewley, L. K. Creamer, H. M. Baker, E. N. Baker, and G. B. Jameson. 1998. Structural basis of the Tanford transition of bovine β-lactoglobulin. Biochemistry. 37:14014–14023. [DOI] [PubMed] [Google Scholar]
- Ragona, L., L. Confalonieri, L. Zetta, K. G. De Kruif, S. Mammi, E. Peggion, R. Longhi, and H. Molinari. 1999. Equilibrium unfolding CD studies of bovine β-lactoglobulin and its 14–52 fragment at acidic pH. Biopolymers. 49:441–450. [DOI] [PubMed] [Google Scholar]
- Renard, D., J. Lefebvre, M. C. A. Griffin, and W. G. Griffin. 1998. Effects of pH and salt environment on the association of β-lactoglobulin revealed by intrinsic fluorescence studies. Int. J. Biol. Macromolecules. 22:41–49. [DOI] [PubMed] [Google Scholar]
- Sawyer, L., and G. Kontopidis. 2000. The core lipocalin, bovine β-lactoglobulin. Biochim. Biophys. Acta. 1482:136–148. [DOI] [PubMed] [Google Scholar]
- Shiraki, K., K. Nishikawa, and Y. Goto. 1995. Trifluoroethanol-induced stabilization of the α-helical structure of β-lactoglobulin: implication for non-hierarchical protein folding. J. Mol. Biol. 245:180–194. [DOI] [PubMed] [Google Scholar]
- Sreerama, N., and R. W. Woody. 1993. A self-consistent method for the analysis of protein secondary structure from circular dichroism. Anal. Biochem. 209:32–44. [DOI] [PubMed] [Google Scholar]
- Sreerama, N., S. Yu. Venyaminov, and R. W. Woody. 1999. Estimation of the number of α-helical and β-strand segments in proteins using circular dichroism spectroscopy. Protein Science. 8:370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky, V. N., N. V. Narizhneva, S. O. Kirschstein, S. Winter, and G. Löber. 1997. Conformational transitions provoked by organic solvents in β-lactoglobulin: can a molten globule like intermediate be induced by the decrease in dielectric constant? Folding & Design. 2:163–172. [DOI] [PubMed] [Google Scholar]
- Verheul, M., J. S. Pedersen, S. P. F. M. Roefs, and K. G. De Kruif. 1999. Association behavior of native β-lactoglobulin. Biopolymers. 49:11–20. [DOI] [PubMed] [Google Scholar]
- Viseu, M. I., K. Edwards, C. S. Campos, and S. M. B. Costa. 2000a. Spontaneous vesicles formed in aqueous mixtures of two cationic amphiphiles. Langmuir. 16: 2105–2114. [Google Scholar]
- Viseu, M. I., M. M. Velázquez, C. S. Campos, I. García-Mateos, and S. M. B. Costa. 2000b. Structural transitions in a bicationic amphiphile system studied by light-scattering, conductivity, and surface tension measurements. Langmuir. 16:4882–4889. [Google Scholar]
- Yang, J., A. K. Dunker, J. R. Powers, S. Clark, and B. G. Swanson. 2001. β-Lactoglobulin molten globule induced by high pressure. J. Agric. Food Chem. 49:3236–3243. [DOI] [PubMed] [Google Scholar]